Abstract
Autophagy is an evolutionarily conserved stress response mechanism that often occurs in apoptosis-defective cancer cells and can protect against cell death. In this study, we investigated how apoptosis and autophagy affect each other in cancer cells in response to chemotherapeutic treatment. We found that specific ablation of the proapoptotic function of cytochrome c, a key regulator of mitochondria-mediated apoptosis, enhanced autophagy following chemotherapeutic treatment. Induction of autophagy required Beclin 1, and was associated with blockage of Beclin 1 cleavage by caspase 8 at two sites. To investigate the role of Beclin 1 cleavage in the suppression of autophagy and cell survival, a caspase-resistant mutant of Beclin 1 was knocked into HCT116 colon cancer cells. Beclin 1 mutant knock-in resulted in markedly increased autophagy and improved long-term cell survival after chemotherapeutic treatment, but without affecting apoptosis and caspase activation. Furthermore, Beclin 1 mutant tumors were significantly less responsive to chemotherapeutic treatment than wild-type tumors. These results demonstrate that chemotherapy-induced apoptosis inhibits autophagy at the execution stage subsequent to cytochrome c release through caspase 8-mediated cleavage of Beclin 1. If apoptosis fails to execute, autophagy is unleashed due to lack of Beclin 1 cleavage by caspases, and can contribute to cancer cell survival and therapeutic resistance. Therefore, Beclin 1 may be a useful target for inhibiting autophagy to sensitize chemotherapy.
Introduction
Apoptosis is a major cytotoxic mechanism of chemotherapy, and its deregulation contributes to therapeutic resistance (1, 2). Stress-induced apoptosis in mammalian cells often proceeds through the intrinsic pathway mediated by mitochondria and the Bcl-2 family proteins. Once cells are committed to apoptosis, a number of downstream events are triggered to execute cell death, including permeabilization of the mitochondrial outer membrane, release of several apoptogenic mitochondrial proteins such as cytochrome c, and activation of the caspase cascade (3, 4).
Autophagy, a catabolic degradation process, has recently emerged as an important stress response and cell death regulatory mechanism (5). It is characterized by the formation of double membrane autophagosomes, which enclose cytosolic materials and organelles, and then fuse with lysosomes, leading to degradation of their luminal content by lysosomal proteases (6). Autophagy is controlled by a group of evolutionarily conserved ATG (autophagy-related genes) genes, among which Beclin 1 (Bcl-2-interacting protein-1) is a key regulator of autophagy (7). Beclin 1 binds to VPS34, a class III phosphatidylinositol-3-OH kinase, to drive autophagosome formation (6). A number of studies indicate a functional role of Beclin 1 in cancer (7–9).
Apoptosis interconnects with autophagy in a number of ways (10). Morphological features of autophagy are often observed in dying cells in which caspases are not sufficiently activated (11). Autophagy can be inhibited by antiapoptotic Bcl-2 and Bcl-XL (12), which interact with Beclin 1 through its BH3 (Bcl-2 homology 3) domain (13). Several ATG proteins, including ATG5 and Beclin 1, can be cleaved by calpain or caspases that are activated during apoptosis (14, 15). Inhibition of autophagy is known to enhance chemotherapy-induced apoptosis, and autophagy modulating drugs are being tested in a number of combination chemotherapy trials (16). However, it has remained unclear how apoptosis and autophagy affect each other in chemotherapy-induced apoptosis, and whether autophagy is simply an innocent bystander that is suppressed during the execution of apoptosis (17).
In this study, we used a genetic approach to dissect when and how autophagy is suppressed during chemotherapy-induced apoptosis. Our results revealed autophagy suppression through caspase 8-mediated cleavage of Beclin 1, following cytochrome c release. Specific ablation of Beclin 1 cleavage restored autophagy in apoptotic cells, and led to enhanced cancer cell survival and therapeutic resistance.
Materials and Methods
Cell lines
Human colorectal cancer cell lines, including HCT116 (obtained before 2002), Caco2 (obtained in 2008), RKO (obtained in 2010), and LoVo (obtained before 2002), were from American Type Culture Collection. Cell lines were last tested and authenticated for absence of Mycoplasma, genotypes, drug response, and morphology in our laboratory in October, 2010.
Western blotting
Cells were lysed in the presence of a protease inhibitor cocktail (Roche Diagnostics) with RIPA buffer (for probing LC3 and p62), a mixture of 0.25 M sucrose, 10 mM HEPES (PH 7.4), and 1 mM EGTA (for probing endogenous Beclin 1), or 2× Laemmli sample buffer (for probing all other proteins). Western blotting was performed as previously described (18). Antibodies included those against N-terminal and C-terminal Beclin 1 (Sigma), caspase 8 (Cell Signaling Technology), GST (glutathione S-transferase; GE Healthcare), V5 (Invitrogen), cytochrome oxidase subunit IV (Cox IV; Invitrogen), α-tubulin (BD Biosciences), LC3 (19), and p62 (Novus Biologicals).
Analysis of apoptosis
After treatment, attached and floating cells were harvested at different time points. Apoptosis was analyzed by counting cells with condensed chromatin and micronucleation following nuclear staining with Hoechst 33258 (Invitrogen) (20). A minimum of 300 cells were analyzed for each sample. Annexin V staining was performed as previously described (21). Activation of caspases 3, 8, and 9 were analyzed by Western blotting. Long-term cell survival was determined by colony formation assay (22). Briefly, an equal number of treated cells were diluted and seeded in 12-well plates. As a control, untreated cells were also plated at 1/100 dilutions relative to treated cells. After 14 days, cell colonies were visualized by crystal violet staining and counted. Results of nuclear staining and colony formation assays were expressed as means ± SD of three independent experiments.
Analysis of autophagy
Cells were treated by CPT with (for LC3) or without (for p62) the lysosomal inhibitors E64d (10 µg/ml) and pepstatin A (10 µg/ml). LC3II accumulation and p62 degradation were analyzed by Western blotting. For analysis of GFP-LC3 puncta formation, cells were transfected with pEGFP-LC3 (19), prior to treatment to induce autophagy. GFP-LC3 punca signals were detected by confocal microscopy using a Zeiss LSM Pascal 5 and the Plan-Apochromat 63×/1.40 Oil DIC objective lenses. Images were acquired using the Zeiss LSM Image Browser software. GFP-LC3 punca signals were quantified by counting at least 300 cells for each sample, and expressed as means ± SD of three independent experiments. Autophagic vesicles, including autophagosomes and autolysosomes, were analyzed by transmission electron microscopy as described (19). EM sections were imaged using a JEOL JEM 1011 (Peabody) at 80 V fitted with a bottom mount AMT 2k digital camera (Advanced Microscopy Techniques).
Knock-in of mutant Beclin 1
The Beclin 1 targeting vector was constructed using the pUSER-rAAV (recombinant adeno-associated virus) system (23). Briefly, two 1-kb homologous arms flanking the fourth intron of Beclin 1 were inserted between two USER sites in the AAV shuttle vector pTK-Neo-USER. The coding sequence for Beclin 1 double mutant (D133A/D146A) was introduced into the right arm using the QuickChange XL Site-Directed Mutagenesis Kit (Agilent Technologies). For gene targeting, HCT116 cells were infected with the targeting rAAV, and selected by G418 (0.5 mg/ml; Mediatech) for 3 weeks. G418-resistant clones were pooled and screened by PCR for targeting events using the primer pairs listed in Table S1. To target the second allele, Neo flanked by two LoxP sites was excised from a heterozygous clone by infection with an adenovirus expressing Cre recombinase (Ad-Cre). The same targeting construct was used in the second round of gene targeting. After the second round, Neo was excised by Ad-Cre infection, and targeting was verified by sequencing of genomic DNA and Western blotting.
Xenograft tumor experiments
All animal experiments were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Xenograft tumors were established by subcutaneously injecting 3.5 × 106 WT, or 7.0 × 106 Beclin 1-KI HCT116 cells into both flanks of 5–6 week-old female Nu/Nu mice (Charles River). Mice were treated with CPT-11 (Irenotican; APP Pharmaceuticals) as previously described (24). Briefly, treatment was initiated on day 5 by intraperitoneal (i.p.) injection of 40 mg/kg of CPT-11 in 20 mM sodium citrate buffer (pH 6.0), and repeated on days 9, 13, 17, and 21. Tumor growth was monitored by calipers, and tumor volumes were calculated according to the formula ½ × length × width2.
Statistical analysis
Statistical analysis was performed using GraphPad Prism IV software. p values were calculated by student (t) test. Differences were considered significant if the probability of the difference occurring by chance was less than 5 in 100 (P<0.05).
Results
Induction of autophagy in apoptosis-defective cytochrome c-mutant-knock-in cells
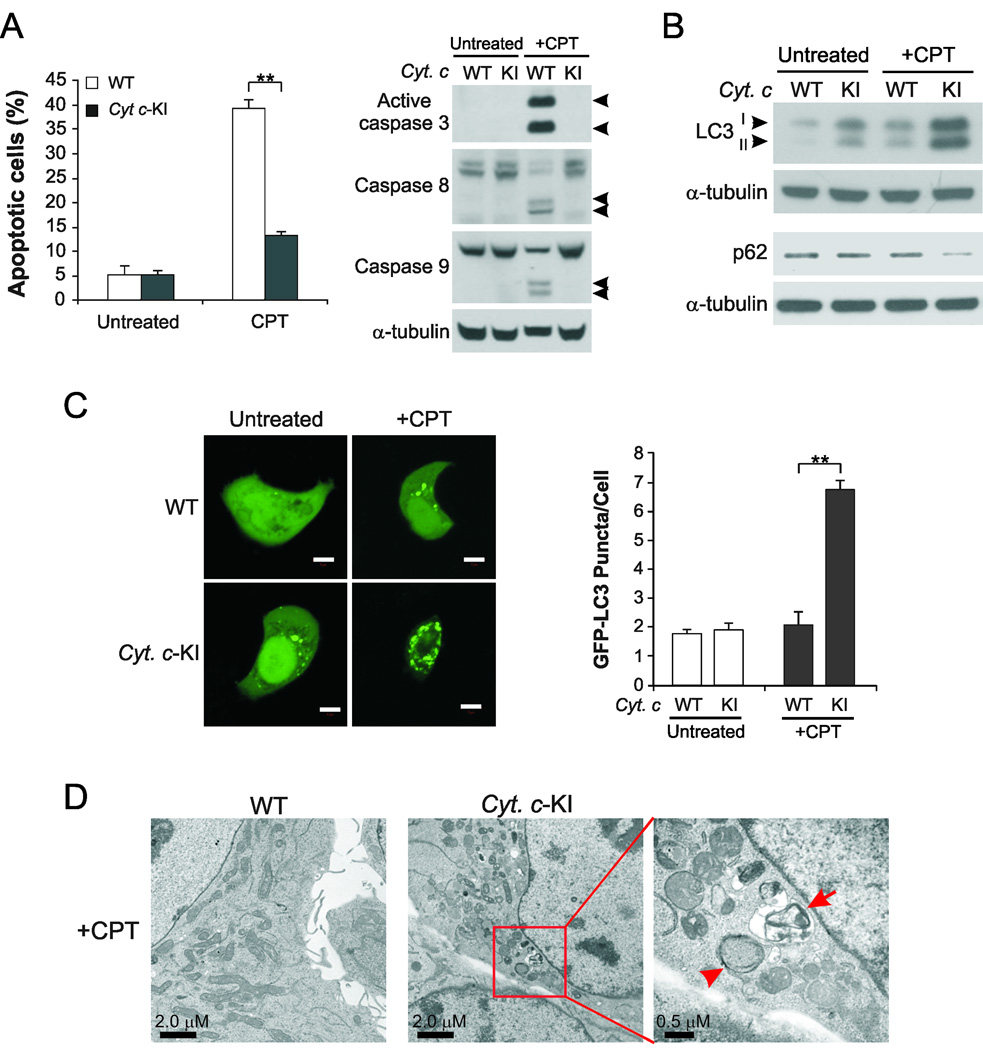
To determine how autophagy in apoptosis-defective cancer cells contributes to cell survival and therapeutic resistance, we dissected at which step autophagy occurs in apoptosis-defective cancer cells. The K72A mutant of cytochrome c (Cyt c), which disrupts its interaction with Apaf-1 but preserves its electron-transport function (25), was knocked into HCT116 colon cancer cells (26). WT and cytochrome c-knockin (Cyt c-KI) cells were treated with camptothecin (CPT), a DNA damaging agent whose derivatives are commonly used in cancer chemotherapy (27), to induce apoptosis. CPT-induced apoptosis was markedly reduced in Cyt c-KI cells (Fig. 1A, left panel), although the mutant cytochrome c was still released into the cytosol following CPT treatment (Fig. S1A). Activation of caspases 3, 8, and 9 were abrogated in Cyt c-KI cells compared to WT cells (Fig. 1A, right panel).
Fig. 1. Induction of autophagy in cytochrome c-mutant knock-in cells.
(A) WT and cytochrome c-mutant-knock-in (Cyt c-KI) HCT116 cells were treated with 500 nM camptothecin (CPT) for 48 hr. Left, apoptosis was analyzed by counting cells with condensed and fragmented nuclei following nuclear staining with Hoechst 33258. **p<0.01. Right, Western blot analysis of caspases 3, 8, and 9. Arrowheads indicate caspase cleavage fragments. (B) Western blot analysis of LC3II accumulation and p62 degradation in WT and Cyt c-KI HCT116 cells treated with CPT for 24 hr. (C) Confocal microscopic analysis of WT and Cyt c-KI HCT116 cells transfected with GFP-LC3, and then treated with 500 nM CPT for 24 hr. Left, representative confocal images. Scale bar: 5 µm. Right, quantification of GFP-LC3 puncta signals. (D) Transmission electron microscopic analysis of WT and Cyt c-KI HCT116 cells treated with 500 nM CPT for 24 hr. Arrowhead indicates an autophagosome with double membrane structure. Arrow indicates an autolysosome with a degraded organelle.
We then tested whether autophagy was induced in Cyt c-KI cells by analyzing commonly used autophagy markers, including LC3 and p62. The level of lipidated LC3II was increased in Cyt c-KI cells compared to WT cells following treatment with CPT along with the lysosomal inhibitors E64d and pepstatin A (Fig. 1B). LC3II accumulation was also detected in Cyt c-KI cells treated with CPT in the presence of chloroquine, another lysosomal inhibitor, but not in cells treated without a lysosomal inhibitor (Fig. S1B), indicating enhanced autophagosome formation, but not reduced lysosomal degradation in these cells (28). The level of p62, a protein that is degraded by autophagy, was reduced in CPT-treated Cyt c-KI cells than WT cells (Fig. 1B). Confocal microscopic analysis showed enhanced GFP-LC3 puncta formation in Cyt c-KI cells transfected with GFP-tagged LC3 and then treated with CPT, relative to WT cells (Fig. 1C). Furthermore, transmission electron microscopy detected frequent autophagic vesicles, including double membrane autophagosomes, as well as autolysosomes with engulfed organelle structures (29), in CPT-treated Cyt c-KI cells, but not in WT cells (Fig. 1D). These results demonstrate that autophagy can be induced when the initial execution step of apoptosis in Cyt c-KI cells is blocked.
Caspase-mediated Beclin 1 cleavage in apoptotic cells
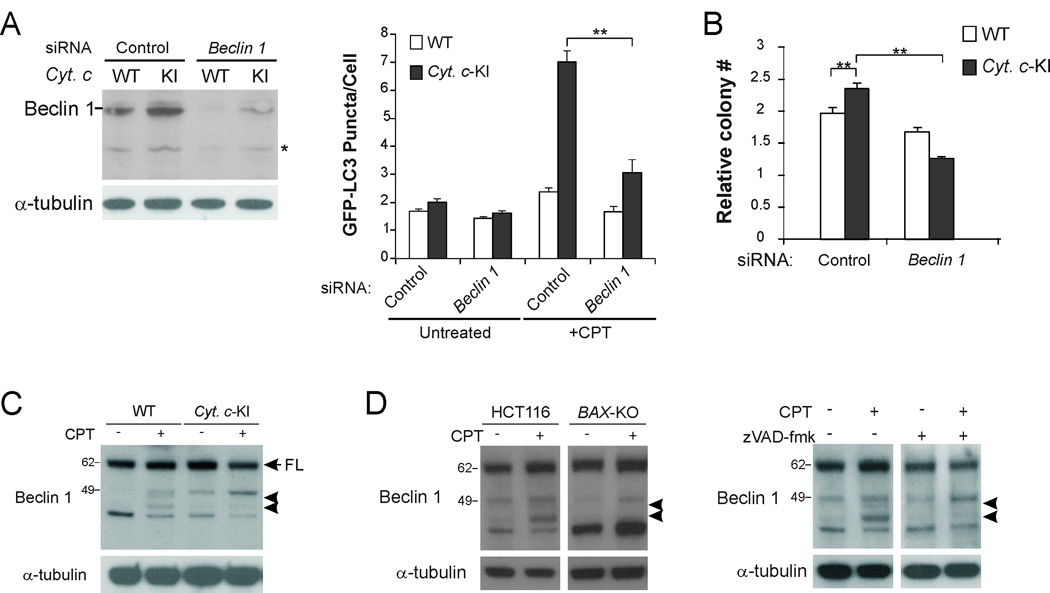
We then tested whether Beclin 1, a critical regulator of autophagosome formation (7), was involved in CPT-induced autophagy in Cyt c-KI cells. CPT-induced GFP-LC3 puncta formation in Cyt c-KI cells was significantly decreased after siRNA knockdown of Beclin 1 (p<0.01; Fig. 2A), indicating that autophagy in these cells was Beclin 1-dependent. While blockage of apoptosis in Cyt c-KI cells resulted in a slight increase in cell survival in a long-term colony formation assay, knockdown of Beclin 1 significantly reduced the survival of Cyt c-KI cells following CPT treatment (Fig. 2B), suggesting that Beclin 1-mediated autophagy contributes to cell survival.
Fig. 2. Inhibition of caspase-mediated Beclin 1 cleavage in Cyt c-KI cells.
(A) WT and Cyt c-KI HCT116 cells were transfected with Beclin 1 or control siRNA, followed by GFP-LC3 transfection, CPT (500 nM) treatment for 24 hr, and then confocal microscopic analysis. Left, Western blot analysis of Beclin 1 at 48 hr after siRNA transfection. * indicates non-specific bands. Right, quantification of GFP-LC3 puncta signals. **p<0.01. (B) Colony formation assay was used to compare the long-term survival of WT and Cyt c-KI HCT116 cells transfected with siRNA as in (A), and then treated with 500 nM CPT for 24 hr. Results are presented as ratios of colony numbers formed by treated cells vs. those of untreated cells plated at 1/100 dilutions relative to treated cells. ** p<0.01. (C) Western blot analysis of Beclin 1 in WT and Cyt c-KI HCT116 cells treated with 500 nM CPT for 24 hr. FL: full-length Beclin 1. Arrowheads indicate Beclin 1 cleavage fragments. (D) Left, Western blot analysis of Beclin 1 in WT and BAX-knockout (BAX-KO) HCT116 cells treated with 500 nM CPT for 24 hr. Right, Western blot analysis of Beclin 1 in WT HCT116 cells treated with 500 nM CPT for 24 hr, in the presence or absence of the pan-caspase inhibitor zVAD-fmk at 20 µM.
Although Beclin 1 expression was not significantly altered in Cyt c-KI cells compared to WT cells, two extra protein species were detected in WT HCT116 cells, but not in Cyt c-KI cells following CPT treatment (Fig. 2C). Two similar fragments were found in cells transfected with V5-tagged Beclin 1, and then subjected to CPT treatment and detection of exogenous Beclin 1 (Fig. S1C). Cyt c-dependent cleavage of Beclin 1 was also found in cells undergoing apoptosis induced by staurosporine (STS) or the BH3-only protein PUMA (Fig. S1D). Beclin 1 cleavage was observed in 3 additional colon cancer cell lines following CPT treatment (Fig. S1E). Furthermore, CPT-induced Beclin 1 cleavage was suppressed in BAX-knockout (BAX-KO) HCT116 cells (30), and in cells treated with CPT along with the pan-caspase inhibitor zVAD-fmk (Fig. 2D and S1E). Therefore, Beclin 1 is likely to be cleaved by a caspase(s) that is activated through the intrinsic apoptotic pathway in response to anticancer agents.
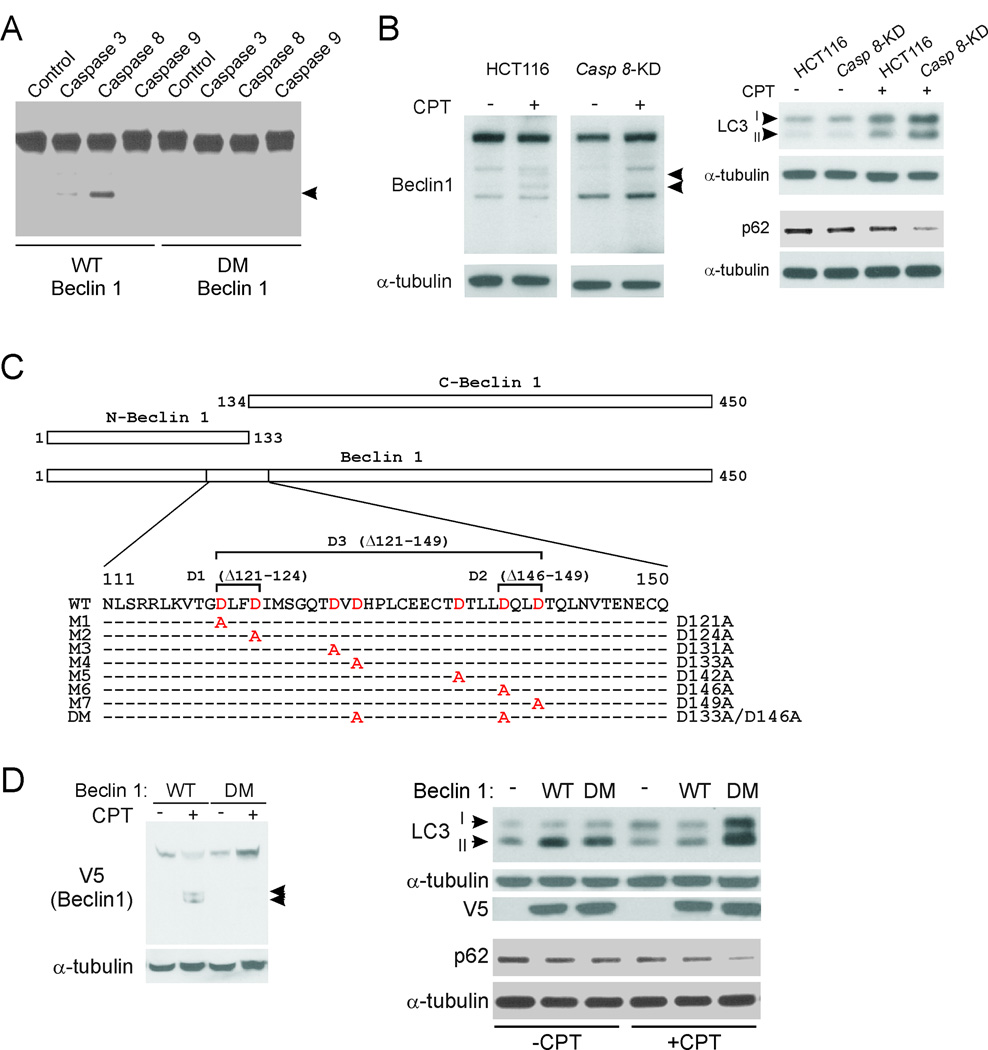
Cleavage of Beclin 1 at two sites by active caspase 8
To determine if and which caspase(s) can cleave Beclin 1, in vitro translated Beclin 1 was incubated with purified active caspase 3, 8, or 9. Beclin 1 could be strongly cleaved by active caspase 8, weakly by active caspase 3, but not by caspase 9 (Fig. 3A). This result was confirmed by using GST-tagged Beclin 1 (Fig. S2A). Only one fragment was detected in the cell-free assays, probably due to partial activities of purified caspases. To test whether caspase 8 was responsible for CPT-induced Beclin 1 cleavage, caspase 8 was knocked down by shRNA in HCT116 cells (Fig. S2B). Cleavage of both endogenous and transfected Beclin 1 was abrogated in caspase 8-knockdown (Casp 8-KD) cells treated with CPT or STS (Fig. 3B, left panel, S2C, S2D). Similar to Cyt c-KI cells, Casp 8-KD cells were more prone to autophagy induction than WT HCT116 in response to CPT treatment, as indicated by enhanced LC3II accumulation and p62 degradation (Fig. 3B, right panel), and GFP puncta signals (Fig. S2E). These data indicate that caspase 8 is the major mediator of Beclin 1 cleavage in chemotherapy-induced apoptosis.
Fig. 3. Cleavage of Beclin 1 by caspase 8 at two sites in CPT-induced apoptosis.
(A) In vitro translated WT and mutant Beclin 1 proteins were incubated with active caspase 3, 8, or 9 at 37°C for 1 hr, and then analyzed for Beclin 1 by Western blotting. Arrowhead indicates the caspase cleavage fragment. (B) Parental and stable caspase 8-knockdown (Casp 8-KD) HCT116 cells were treated with 500 nM CPT for 24 hr. Left, Western blot analysis of Beclin 1 expression. Right, Western blot analysis of LC3II accumulation and p62 degradation. (C) A schematic representation and amino acid sequences of Beclin 1 mutants analyzed. N-Beclin 1 and C-Beclin 1 correspond to two Beclin 1 fragments generated by caspase cleavage. (D) Left, WT HCT116 cells were transfected with V5-tagged WT or DM (double mutant; D133A/D146A) Beclin 1, and then treated with 500 nM CPT for 24 hr. Transfected Beclin 1 was analyzed by V5 Western blotting. Right, HCT116 cells were transfected with WT or DM Beclin 1, treated with 500 nM CPT for 24 hr, and then analyzed for LC3II accumulation and p62 degradation by Western blotting.
We then examined potential caspase cleavage sites of Beclin 1, focusing on residues 111–150 based on the size of the cleavage fragments (Fig. 3C). Seven aspartic acids (Ds), each of which could be a potential caspase cleavage site (31), were noted. A battery of Beclin 1 mutants, including deletions and alanine (A) replacements (Fig. 3C), were generated. Upon transfecting these mutants and treating cells with CPT, we found that deletion of the residues 121–149 (D3) completely blocked Beclin 1 cleavage, while the micro deletion of the residues 146–149 (D2) and two point mutants, D133A (M4) and D146A (M6), each partially blocked Beclin 1 cleavage (Fig. S3A and S3B), suggesting that both D133 and D146 contribute to Beclin 1 cleavage. Indeed, a double mutant (DM) with both sites altered (D133A/D146A) was completely resistant to cleavage by caspase 8 or caspase 3 in cell-free assays (Fig. 3A and S3C), or in CPT-treated HCT116 cells (Fig. 3D, left panel). Therefore, D133 and D146 of Beclin 1 are the two sites that are cleaved by caspases during apoptosis.
Specifically blocking Beclin 1 cleavage promotes autophagy in apoptotic cells
We then compared the autophagy-promoting activities of WT and DM Beclin 1, which differ only in their susceptibility to caspases, in cells undergoing apoptosis. Transfection of WT Beclin 1 significantly enhanced LC3II levels in untreated HCT116 cells, but was unable to do so in CPT-treated cells (Fig. 3D, right panel), reflecting inhibition of autophagy by caspase-mediated Beclin 1 cleavage. In contrast, transfection of DM Beclin 1 not only increased LC3II levels in untreated HCT116 cells, but also further enhanced LC3II accumulation and p62 degradation in CPT-treated cells (Fig. 3D, right panel). Therefore, specific blockage of caspase 8-mediated cleavage is sufficient to restore the autophagy-promoting function of Beclin 1 in cells that are undergoing apoptosis.
To further determine the effects of Beclin 1 cleavage by caspase 8, N- and C-terminal cleavage fragments of Beclin 1 were constructed (Fig. 3C), and transfected into HCT116 cells, followed by CPT treatment. Both Beclin 1 cleavage fragments lose much of the autophagy-promoting activity compared to the DM mutant in CPT-treated cells (Fig. S4A). Similar to WT Beclin 1, the transfected cleavage fragments did not affect CPT-induced apoptosis (Fig. S4B), and were exclusively cytosolic in both untreated and CPT-treated cells (Fig. S4C).
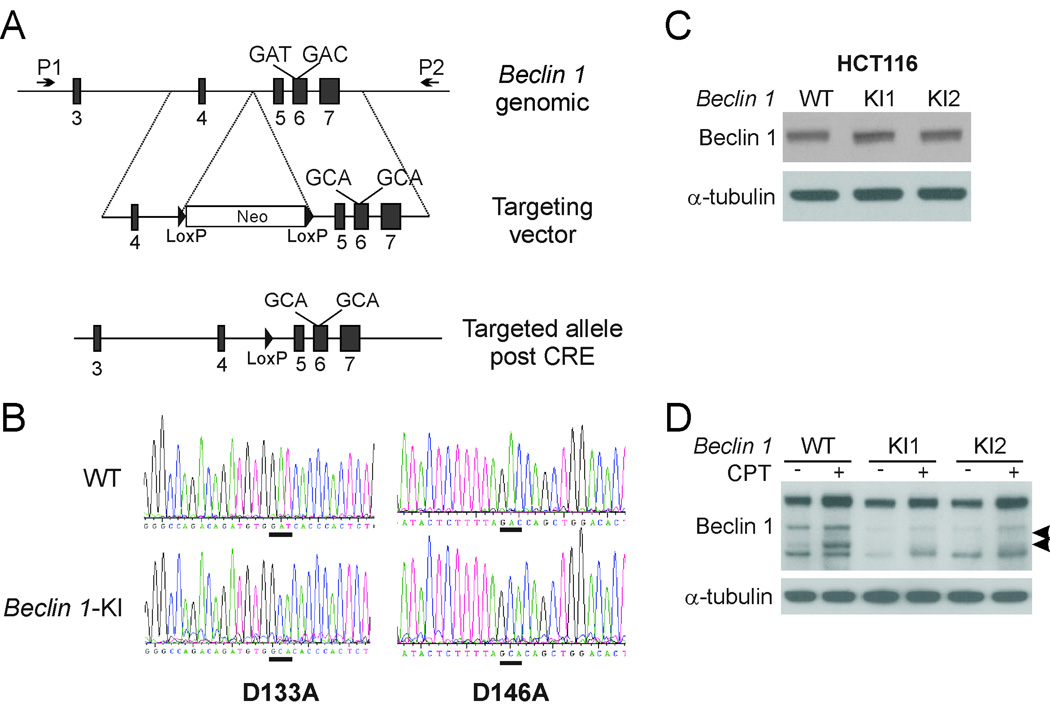
Blocking Beclin 1 cleavage in vivo induces autophagy in apoptotic cells
To determine the effects of blocking Beclin 1 cleavage in vivo, we replaced the WT Beclin 1 locus in HCT116 cells with the caspase-resistant DM mutant by homologous recombination (Fig. 4A). After two rounds of gene targeting, several desired recombinant clones were identified by genomic PCR analysis of drug-resistant clones. DNA sequencing revealed that the DM sequence was correctly targeted into both alleles of the Beclin 1 genomic locus (Fig. 4B). Mutant Beclin 1 was expressed at a similar level as WT protein (Fig. 4C). CPT-induced Beclin 1 cleavage observed in WT cells was abolished in Beclin 1-KI cells (Fig. 4D), indicating that endogenous Beclin 1 was cleaved at D133 and D146.
Fig. 4. Knock-in of Beclin 1 double mutant in HCT116 cells.
(A) A schematic representation of the Beclin 1 genomic locus and knock-in vector. P1 and P2 represent PCR primers for identifying knock-in clones. (B) DNA sequences of the targeted genomic region in WT and Beclin 1 knock-in (Beclin 1-KI) HCT116 cells, with WT and corresponding mutant sequences underlined. (C) Western blot analysis of Beclin 1 expression in WT and Beclin 1-KI HCT116 cells. (D) Western blot analysis of Beclin 1 in WT and Beclin 1-KI HCT116 cells treated with 500 nM CPT for 24 hr. Arrowheads indicate Beclin 1 cleavage fragments.
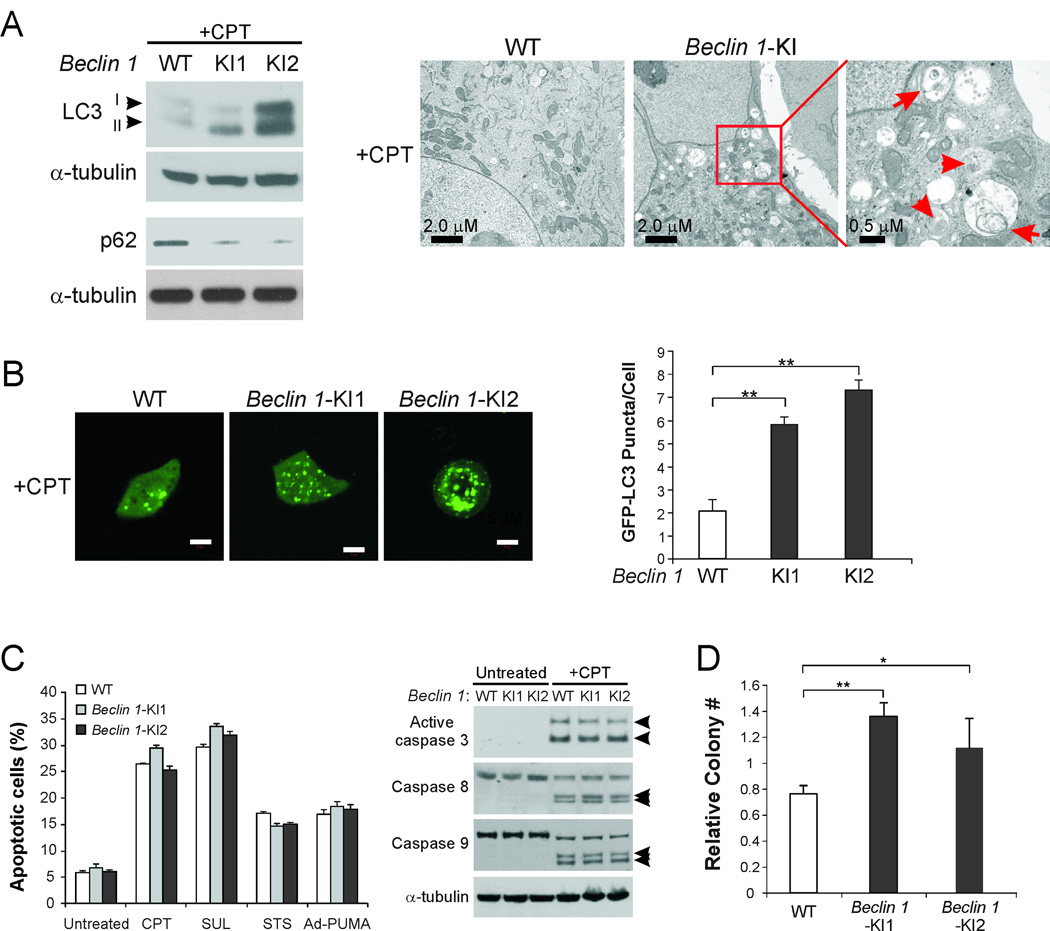
Similar to Cyt c-KI and Casp 8-KD cells, Beclin 1-KI cells were found to have higher levels of LC3II accumulation and p62 degradation than WT cells after CPT treatment (Fig. 5A, left panel). The increase in LC3II levels was due to enhanced autophagosome formation (Fig. S5A). Furthermore, GFP-LC3 puncta formation was significantly enhanced by 2–3 fold in CPT-treated Beclin 1-KI cells relative to WT cells (Fig. 5B). Transmission electron microscopy detected frequent autophagosome and autolysosome structures in CPT-treated Beclin 1-KI cells, while these structures were rarely found in WT cells (Fig. 5A, right panel). Autophagy was also enhanced in Beclin 1-KI cells in response to other treatments, including serum starvation, exposure to the mTOR inhibitor rapamycin, and amino acid starvation (Fig. S5B). Therefore, specifically blocking the cleavage of Beclin 1 in vivo recapitulated autophagy induction in apoptosis-defective cells, including Cyt c-KI (Fig. 1, B–D), Casp 8-KD (Fig. 3B), and BAX-KO cells (32).
Fig. 5. Induced autophagy, unchanged apoptosis, and improved survival of Beclin 1-KI cells.
(A) WT and Beclin 1-KI HCT116 cells were treated with 500 nM CPT for 24 hr. Left, Analysis of LC3II accumulation and p62 degradation by Western blotting. Right, Analysis of autophagy by transmission electron microscopy. Arrowheads indicate autophagosomes with double membrane structure. Arrows indicate autolysosomes with degraded organelles. (B) Confocal microscopic analysis of WT and Beclin 1-KI HCT116 cells transfected with GFP-LC3, and then treated with 500 nM CPT for 24 hr. Left, representative confocal images. Scale bar: 5 µm. Right, quantification of GFP-LC3 puncta signals. **p<0.01. (C) WT and Beclin 1-KI HCT116 cells were treated with the indicated apoptotic stimuli for 48 hr. Left, analysis of apoptosis by nuclear staining. Right, Western blot analysis of caspases 3, 8, and 9. CPT, 500 nM camptothecin; SUL, 120 µM sulindac sulfide; STS, 100 nM staurosporine; Ad-PUMA, infection with 10 MOI of adenovirus expressing PUMA. (D) Colony formation assay was used to compare the long-term survival of WT and Beclin 1-KI HCT116 cells treated with 500 nM CPT for 24 hr. Results are presented as ratios of colony numbers formed by treated cells vs. those of untreated cells plated at 1/100 dilutions relative to treated cells. *p<0.05; **p<0.01.
Blocking Beclin 1 cleavage does not affect apoptosis, but results in chemo resistance
WT and Beclin 1-KI HCT116 cells were then compared for apoptosis induction in response to several stimuli, including CPT, STS, PUMA expression, or sulindac sulfide (SUL), a nonsteroidal anti-inflammatory drug used in cancer chemoprevention (33). Analysis of apoptosis by nuclear staining, annexin V staining, or caspase activation showed that blocking Beclin 1 cleavage in vivo did not affect the execution of apoptosis induced by these stimuli (Fig. 5C and data not shown).
We then determined whether blocking Beclin 1 cleavage affects response to chemotherapy using colony formation assay. Following CPT treatment, Beclin 1-KI cells formed significantly more colonies compared to WT cells (Fig. 5D), suggesting that suppression of Beclin 1 cleavage is sufficient to protect against cell death. Therefore, Beclin 1 mutant knock-in, which specifically ablates caspase-mediated Beclin 1 cleavage, recapitulated protection against cell death seen in Cyt c-KI (Fig. 2B), and BAX-KO cells (30).
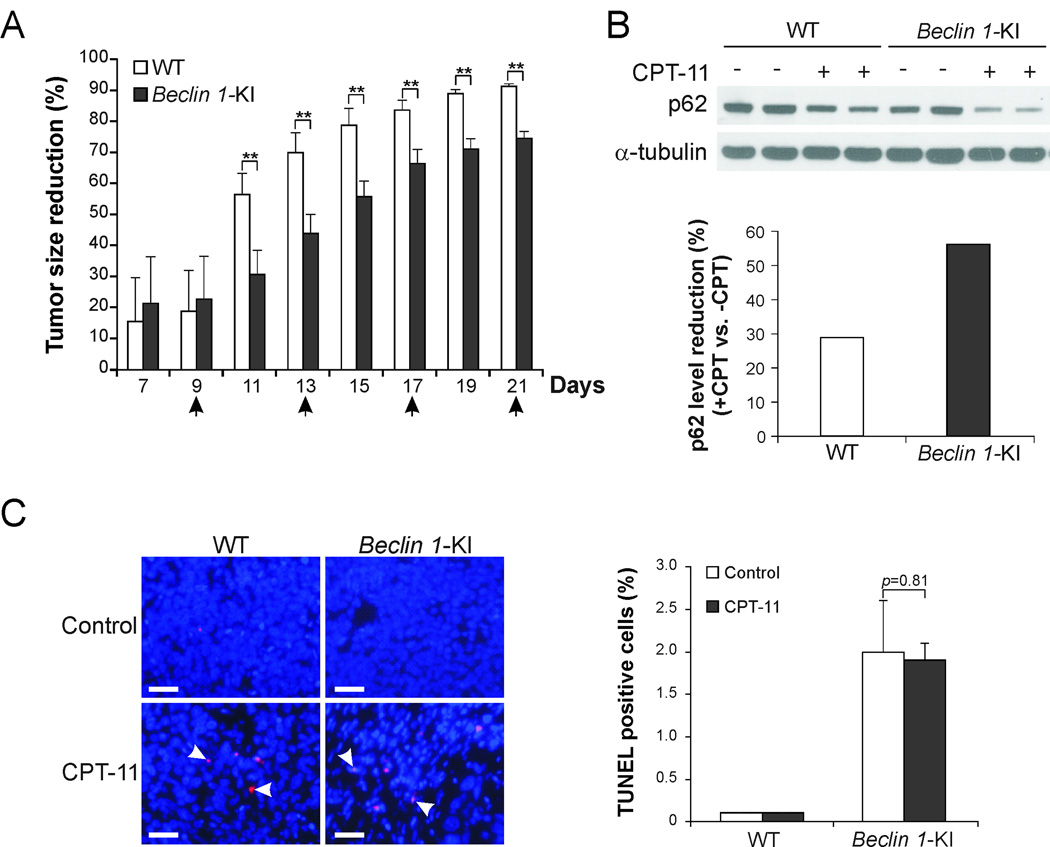
To investigate the effect of blocking Beclin 1 cleavage on chemotherapeutic response in vivo, tumors were established using WT and Beclin 1-KI HCT116 cells in nude mice, followed by CPT-11 treatment (24). Beclin 1-KI tumors were found to be significantly less responsive to CPT-11 treatment compared to WT tumors (Fig. 6A). The degradation of p62 was enhanced in Beclin 1-KI tumors compared to WT tumors (Fig. 6B). TUNEL staining revealed that the levels of apoptosis induction were similar in WT and Beclin 1-KI tumors following CPT-11 treatment (Fig. 6C). These observations suggest that autophagy induction in vivo as a result of blocking Beclin 1 cleavage can protect tumors from chemotherapy-induced cell death.
Fig. 6. In vivo therapeutic resistance of Beclin 1-KI tumors.
(A) Xenograft tumors established from WT and Beclin 1-KI HCT116 cells were treated with 40 mg/kg of irinotecan (CPT-11) by i.p. injection on days 5, 9, 13, 17, and 21, as indicated by arrows. Therapeutic effects were measured by calculating percentage decrease in tumor size of treated tumors compared to untreated tumors. **p<0.01. (B) Upper, Western blot analysis of p62 degradation in WT and Beclin 1-KI HCT116 tumors with or without CPT-11 treatment. Lower, quantification of p62 level reduction in CPT-11-treated tumors relative to untreated tumors using Image J software. (C) Apoptosis in WT and Beclin 1-KI HCT116 tumors with or without CPT-11 treatment was analyzed by TUNEL staining. Left, representative TUNEL staining pictures. Scale bar: 200 µm. Arrowheads indicate example TUNEL-positive cells. Right, quantification of TUNEL-positive cells, with 300 cells counted for each sample. Values in (A) and (C) were means ± SD (n = 6 in each group).
Discussion
Our results demonstrate that chemotherapy-induced apoptosis suppresses autophagy at the execution stage following cytochrome c release, at least in part through caspase 8-mediated Beclin 1 cleavage at D133 and D146. Caspase 8 not only plays a key role in death receptor-mediated apoptosis, but also contributes to the execution of DNA damage-induced apoptosis through the intrinsic pathway (22). Specifically blocking Beclin 1 cleavage by knocking in a caspase-resistant mutant of Beclin 1 recapitulated autophagy induction seen in apoptosis-deficient cells. On the other hand, autophagy induction in Beclin 1-KI cells did not affect apoptotic events, but protected against cell death. These results provide mechanistic insight into how chemotherapy-induced apoptosis suppresses autophagy, and explain why autophagy is generally observed in cells in which caspase activation is blocked.
Several recent studies using different cell types and stimuli also described caspase-mediated cleavage of ATG proteins in apoptosis (15). Cho et al. found caspase-dependent cleavage of Beclin 1 in Hela cells treated with a death receptor ligand (34). Luo and Rubinzstein reported caspase 3-mediated Beclin 1 cleavage at D149 in apoptosis induced by Bax overexpression (35). Wirawan et al. demonstrated caspase cleavage of Beclin 1 at D133 and D149 during apoptosis induced by IL-3 depletion in murine hematopoietic cells (36). ATG5 and ATG4D were also found to be cleaved by caspases in apoptotic cells (14, 37). Furthermore, caspase 8 has been implicated in autophagy suppression in mouse L929 fibroblast and human U937 monocytoid cells (38, 39), and also in proliferating T cells (40). Our knock-in data indicate that specific blockage of endogenous Beclin 1 cleavage alone is sufficient to restore autophagy in apoptotic cells, at least in colon cancer cells undergoing chemotherapy-induced apoptosis, and possibly in other scenarios as well. Differences in cell types and apoptotic stimuli may explain why D146 was required for Beclin 1 cleavage in our study, but D149 was necessary for Beclin 1 cleavage in other studies.
Autophagy occurring subsequent to cytochrome c release is likely to be trigged by mitochondrial outer membrane permeabilization (MOMP), and is therefore mitophagy, a recycle process in which mitochondria are captured and degraded (41). Although Beclin 1 can interact with antiapoptotic Bcl-2 family members through its BH3 domain, it does not seem to significantly affect apoptosis initiation and caspase activation. Nonetheless, Beclin 1-mediated autophagy may modulate chemotherapeutic response by removing terminally damaged mitochondria with MOMP, which may help to sustain energy metabolism of cancer cells, and allow them to tolerate and recover from the damages induced by chemotherapeutic agents. It has been shown that mitophagy mediated by ATG12 and metabolic changes mediated by glyceraldehyde 3-phosphate dehydrogenase (GAPDH) preserve the survival of staurosporine-treated cells in which cytochrome c release had occurred, but caspase activation was blocked (42). Therefore, autophagy may not simply be an innocent bystander (17), but rather an important cell survival pathway that has to be turned off for full execution of cell death.
A remaining question is how Beclin 1 cleavage during apoptosis affects its functions. Beclin 1 cleavage may not abolish its interaction with VPS34, which is mediated by a conserved region far apart from its cleavage sites (43). The cleavage of Beclin 1 also leaves its BH3 domain intact. Only a fraction of Beclin 1, as detected by Western blotting, was subject to caspase cleavage in CPT-treated cells, which seemed to suggest a gain of function by Beclin 1 cleavage fragments. However, both N- and C-terminal Beclin 1 cleavage fragments remained localized in the cytosol, and their overexpression did not significantly affect apoptosis, consistent with intact apoptosis induction in Beclin 1-KI cells. Therefore, caspase cleavage of Beclin 1 may serve primarily as an inhibitory mechanism of autophagy and cell survival.
Autophagy is a survival pathway utilized by cancer cells to tolerate metabolic stress. Most, if not all, anticancer agents can induce autophagy in cancer cells (16). Autophagy inhibitors such as hydroxychloroquine are being tested in a number of clinical trials as a chemo sensitizer (16). Our results suggest that Beclin 1, which is cleaved by caspases for switching off autophagy in chemotherapy-induced apoptosis, may be a useful target for more specific and effective pharmacological inhibition of autophagy.
Supplementary Material
Acknowledgements
We thank Dr. Monica E. Buchanan, Crissy Dudgeon, and Brian Leibowitz for critical reading and comments, and Xiaohong Wang for technical assistance.
Grant Support
This work is supported by NIH grants CA106348, CA121105, American Cancer Society grant RSG-07-156-01-CNE, and the American Lung Association/CHEST Foundation (L.Z.), NIH grants CA129829 and U01DK085570 (J.Y.), NIH grants CA132385, GM087798, and CA148629 (R.W.S), and the UPCI Cancer Center Support grant (CA047904).
References
- 1.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green DR, Kroemer G. Pharmacological manipulation of cell death: clinical applications in sight? J Clin Invest. 2005;115:2610–2617. doi: 10.1172/JCI26321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danial NN, Korsmeyer SJ. Cell death. Critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 4.Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–2933. [PubMed] [Google Scholar]
- 5.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Z, Klionsky DJ. Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol. 2010;22:124–131. doi: 10.1016/j.ceb.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinha S, Levine B. The autophagy effector Beclin 1: a novel BH3-only protein. Oncogene. 2008;27 Suppl 1:S137–S148. doi: 10.1038/onc.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qu X, Yu J, Bhagat G, et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 2003;112:1809–1820. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci U S A. 2003;100:15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 11.Degenhardt K, Mathew R, Beaudoin B, et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10:51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pattingre S, Tassa A, Qu X, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Oberstein A, Jeffrey PD, Shi Y. Crystal structure of the Bcl-XL-Beclin 1 peptide complex: Beclin 1 is a novel BH3-only protein. J Biol Chem. 2007;282:13123–13132. doi: 10.1074/jbc.M700492200. [DOI] [PubMed] [Google Scholar]
- 14.Yousefi S, Perozzo R, Schmid I, et al. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat Cell Biol. 2006;8:1124–1132. doi: 10.1038/ncb1482. [DOI] [PubMed] [Google Scholar]
- 15.Djavaheri-Mergny M, Maiuri MC, Kroemer G. Cross talk between apoptosis and autophagy by caspase-mediated cleavage of Beclin 1. Oncogene. 2010;29:1717–1719. doi: 10.1038/onc.2009.519. [DOI] [PubMed] [Google Scholar]
- 16.White E, DiPaola RS. The double-edged sword of autophagy modulation in cancer. Clin Cancer Res. 2009;15:5308–5316. doi: 10.1158/1078-0432.CCR-07-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest. 2005;115:2679–2688. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ming L, Wang P, Bank A, Yu J, Zhang L. PUMA dissociates Bax and BCL-XL to induce apoptosis in colon cancer cells. J Biol Chem. 2006;281:16034–16042. doi: 10.1074/jbc.M513587200. [DOI] [PubMed] [Google Scholar]
- 19.Gao W, Kang JH, Liao Y, et al. Biochemical isolation and characterization of the tubulovesicular LC3-positive autophagosomal compartment. J Biol Chem. 2010;285:1371–1383. doi: 10.1074/jbc.M109.054197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang P, Qiu W, Dudgeon C, et al. PUMA is directly activated by NF-kappaB and contributes to TNF-alpha-induced apoptosis. Cell Death Differ. 2009;16:1192–1202. doi: 10.1038/cdd.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu J, Wang P, Ming L, Wood MA, Zhang L. SMAC/Diablo mediates the proapoptotic function of PUMA by regulating PUMA-induced mitochondrial events. Oncogene. 2007;26:4189–4198. doi: 10.1038/sj.onc.1210196. [DOI] [PubMed] [Google Scholar]
- 22.Wang P, Yu J, Zhang L. The nuclear function of p53 is required for PUMA-mediated apoptosis induced by DNA damage. Proc Natl Acad Sci U S A. 2007;104:4054–4059. doi: 10.1073/pnas.0700020104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Guo C, Chen Y, et al. Epitope tagging of endogenous proteins for genome-wide ChIP-chip studies. Nat Methods. 2008;5:163–165. doi: 10.1038/nmeth1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolinsky K, Zhang YE, Dugan U, Heimbrook D, Packman K, Higgins B. Novel regimens of capecitabine alone and combined with irinotecan and bevacizumab in colorectal cancer xenografts. Anticancer Res. 2009;29:91–98. [PubMed] [Google Scholar]
- 25.Hao Z, Duncan GS, Chang CC, et al. Specific ablation of the apoptotic functions of cytochrome C reveals a differential requirement for cytochrome C and Apaf-1 in apoptosis. Cell. 2005;121:579–591. doi: 10.1016/j.cell.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 26.Wang P, Li H, Yu J, Zhang L. Manuscript in preparation. [Google Scholar]
- 27.Eng C. Toxic effects and their management: daily clinical challenges in the treatment of colorectal cancer. Nat Rev Clin Oncol. 2009;6:207–218. doi: 10.1038/nrclinonc.2009.16. [DOI] [PubMed] [Google Scholar]
- 28.Tanida I, Minematsu-Ikeguchi N, Ueno T, Kominami E. Lysosomal turnover, but not a cellular level, of endogenous LC3 is a marker for autophagy. Autophagy. 2005;1:84–91. doi: 10.4161/auto.1.2.1697. [DOI] [PubMed] [Google Scholar]
- 29.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L, Yu J, Park BH, Kinzler KW, Vogelstein B. Role of BAX in the apoptotic response to anticancer agents. Science. 2000;290:989–992. doi: 10.1126/science.290.5493.989. [DOI] [PubMed] [Google Scholar]
- 31.Li J, Yuan J. Caspases in apoptosis and beyond. Oncogene. 2008;27:6194–6206. doi: 10.1038/onc.2008.297. [DOI] [PubMed] [Google Scholar]
- 32.Ding WX, Ni HM, Gao W, et al. Linking of autophagy to ubiquitin-proteasome system is important for the regulation of endoplasmic reticulum stress and cell viability. Am J Pathol. 2007;171:513–524. doi: 10.2353/ajpath.2007.070188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiu W, Wang X, Leibowitz B, et al. Chemoprevention by nonsteroidal anti-inflammatory drugs eliminates oncogenic intestinal stem cells via SMAC-dependent apoptosis. Proc Natl Acad Sci U S A. 2010;107:20027–20032. doi: 10.1073/pnas.1010430107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho DH, Jo YK, Hwang JJ, Lee YM, Roh SA, Kim JC. Caspase-mediated cleavage of ATG6/Beclin-1 links apoptosis to autophagy in HeLa cells. Cancer Lett. 2009;274:95–100. doi: 10.1016/j.canlet.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Luo S, Rubinsztein DC. Apoptosis blocks Beclin 1-dependent autophagosome synthesis: an effect rescued by Bcl-xL. Cell Death Differ. 2010;17:268–277. doi: 10.1038/cdd.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wirawan E, Vande Walle L, Kersse K, Cornelis S, Claerhout S, Vanoverberghe I, Roelandt R, De Rycke R, Verspurten J, Declercq W, Agostinis P, Vanden Berghe T, Lippens S, Vandenabeele P. Caspase-mediated cleavage of Beclin-1 inactivates Beclin-1-induced autophagy and enhances apoptosis by promoting the release of proapoptotic factors from mitochondria. Cell Death and Disease. 2010;1:e18. doi: 10.1038/cddis.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Betin VM, Lane JD. Caspase cleavage of Atg4D stimulates GABARAP-L1 processing and triggers mitochondrial targeting and apoptosis. J Cell Sci. 2009;122:2554–2566. doi: 10.1242/jcs.046250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu L, Alva A, Su H, et al. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–1502. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]
- 39.Lee JS, Li Q, Lee JY, et al. FLIP-mediated autophagy regulation in cell death control. Nat Cell Biol. 2009;11:1355–1362. doi: 10.1038/ncb1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bell BD, Leverrier S, Weist BM, et al. FADD and caspase-8 control the outcome of autophagic signaling in proliferating T cells. Proc Natl Acad Sci U S A. 2008;105:16677–16682. doi: 10.1073/pnas.0808597105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mijaljica D, Prescott M, Devenish RJ. Different fates of mitochondria: alternative ways for degradation? Autophagy. 2007;3:4–9. doi: 10.4161/auto.3011. [DOI] [PubMed] [Google Scholar]
- 42.Colell A, Ricci JE, Tait S, et al. GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. Cell. 2007;129:983–997. doi: 10.1016/j.cell.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 43.Furuya N, Yu J, Byfield M, Pattingre S, Levine B. The evolutionarily conserved domain of Beclin 1 is required for Vps34 binding, autophagy and tumor suppressor function. Autophagy. 2005;1:46–52. doi: 10.4161/auto.1.1.1542. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.