Abstract
The interferon (IFN)-inducible IFI16 and AIM2 proteins act as innate immune sensors for cytosolic double-stranded DNA (dsDNA). Upon sensing dsDNA, the IFI16 protein induces the expression of IFN-β whereas the AIM2 protein forms an inflammasome, which promotes the secretion of IL-1β. Given that the knockdown of IFI16 expression in human diploid fibroblasts (HDFs) delays the onset of cellular senescence, we investigated the potential roles for the IFI16 and AIM2 proteins in cellular senescence. We found that increased IFI16 protein levels in old (versus young) HDFs were associated with the induction of IFN-β. In contrast, increased levels of the AIM2 protein in the senescent (versus old) HDFs were associated with increased production of IL-1β. The knockdown of type I IFN-receptor subunit-α, which reduced the basal levels of the IFI16, but not the AIM2, protein delayed the onset of cellular senescence. Accordingly, increased constitutive levels of IFI16 and AIM2 proteins in ataxia telangiectasia (AT) HDFs were associated with the activation of the IFN-signaling and increased levels of IL-1β. The IFN-β treatment of the young HDFs, which induced the expression of IFI16 and AIM2 proteins, activated a DNA-damage response and also increased basal levels of IL-1β. Interestingly, the knockdown of AIM2 expression in HDFs increased the basal levels of IFI16 protein and activated the IFN-signaling. In contrast, the knockdown of the IFI16 expression in HDFs decreased the basal and dsDNA-induced activation of the IFN-signaling. Collectively, our observations demonstrate differential roles for the IFI16 and AIM2 proteins in cellular senescence and associated secretory phenotype.
Keywords: Cytosolic DNA sensors, IFI16, IFNs, AIM2 inflammasome, Cellular senescence
Introduction
Cellular senescence refers to permanent cell cycle arrest in cells (1, 2). The cell cycle arrest is associated with the activation of a DNA damage response and the expression of interferon (IFN)-inducible genes. The onset of cellular senescence limits the proliferation of damaged cells, which have potential to develop cancers. However, the senescent cells secret proinflammatory cytokines and chemokines (2, 3). This phenotype of the senescent cells is termed senescence-associated secretory phenotype (SASP) (3). The SASP in human diploid fibroblasts (HDFs) is associated with higher levels of inflammatory cytokines, including the IL-1β, IL-6 and IL-8 (2, 3). Secretion of the inflammatory cytokines and chemokines by the senescent cells promotes the aging-associated inflammatory diseases (2). Currently, it is not known whether the innate immune sensors for cytosolic DNA play any role in cellular senescence-associated cell growth arrest and/or SASP.
With increasing passage, the human diploid fibroblasts (HDFs) in culture exhibit an increase in apoptotic markers and cell death (4). Furthermore, aging cells in culture also release “exosomes” (microvesicles), which contain proteins, small RNA (<100 base pair), and double stranded DNA (>1,000 bp) (5). These observations are consistent with the idea that fusion of exosomes with neighboring cells could deliver the contents (including the DNA) into the cytoplasm. Because cytosolic double-stranded DNA (dsDNA) in most cell types is sensed by the innate immune sensors as a danger signal (6, 7), the activation of an innate immune response results in the production of interferon-β (IFN-β) and proinflammatory cytokines, such as IL-1β.
Type-I IFNs (IFN-α/β) are multifunctional cytokines with the growth-inhibitory activities (8, 9). All cells produce low constitutive levels of the type-I IFNs in culture (10) and activation of interferon-regulated factors (IRFs) by the innate immune response can induce the IFN-β expression (11). Binding of type-I IFNs to cell surface receptor activates the receptor-associated Janus tyrosine kinases, Jak1 and Tyk2, which leads to the activating tyrosine phosphorylation of latent transcription factors that are termed STATs. The activated STATs, upon translocation into the nucleus, bind to the interferon stimulated response element (ISRE) and induce the transcription of a set of ISGs (8, 9).
Upon approaching cellular senescence (becoming old) in culture, HDFs express increased levels of IFN-β (12) and IFN-stimulated genes (ISGs) (13). HDFs from ataxia telangiectasia (AT) patients, who age prematurely (14), express increased levels of IFN-inducible proteins (15) and the knockdown of the ATM expression induces the expression of a set of IFN-inducible genes (16). Accordingly, the loss of type I IFN-signaling, which results in the lack of the expression of ISGs, is associated with immortalization of HDFs (17–20). Furthermore, prolonged IFN-β treatment of young HDFs induces a senescence-like phenotype (21). Although these observations are consistent with the activation of the innate immune responses in old HDFs, the molecular mechanisms that induce the expression of the IFNB gene remain largely unknown. Moreover, it remains unclear whether the activation of IFN-signaling, which results in the expression of certain ISGs, is required for senescence-associated cell cycle arrest and the secretory phenotype (SASP). The SASP in HDFs and epithelial cells is associated with increased production of proinflammatory cytokines, such as IL-6, IL-8, and IL-1 (3).
One family of the ISGs is the Ifi200-gene family, which encodes for structurally-related proteins (the p200-family proteins) (22-24). The p200-family proteins share at least one partially conserved repeat of 200-amino acid residue (or the HIN-200 domain), which contains two consecutive oligonucleotide/oligo-saccharide-binding folds (OB-folds) (24). The repeat is often found in proteins that bind either single or double-stranded DNA (24). Most p200-family proteins (except the murine p202 protein) also contain a homotypic protein-protein interaction pyrin domain (PYD) to recruit adaptor protein ASC, which recruits and activates the caspase-1 (24). The p200-protein family includes the IFI16 and AIM2 proteins. Interestingly, recent studies revealed that both IFI16 (6) and AIM2 (25–27) proteins can sense cytosolic dsDNA and initiate an innate immune response. The AIM2 protein, upon sensing dsDNA, forms an inflammasome (28), which through the activation of caspase-1, increases the secretion of proinflammatory cytokines, including IL-1β and IL-18, and induces cell death by pyroptosis (caspase-1-dependent death) in macrophages (25–28). Interestingly, the generation of Aim2-deficient mice revealed that the Aim2 protein is not needed for IFN-β production (29–31). Moreover, Aim2 protein appears to negatively regulate the IFN-β expression and the activation of IFN-inducible genes (32). In contrast to the AIM2 protein, upon sensing dsDNA, the IFI16 protein recruits stimulator of interferon genes (STING) protein to stimulate the expression of IFN-β through activation of IRF3 (6).
The expression of IFI16 gene is up-regulated by IFNs (α, β or γ) and the activated p53 (22, 23, 33). The IFI16 gene encodes for three isoforms (A, B, and C) of the protein (~80 kDa) due to alternative splicing (22, 23). Increased levels of IFI16 protein in old HDFs (lung fibroblast WI-38 and IMR-90) are associated with the onset of cellular senescence and the knockdown of the IFI16 expression in HDFs delays the onset of cellular senescence (13). Interestingly, the extent of sub-cellular localization of the IFI16 protein in the cytoplasm appears to depend on the cell type (23). Increased levels of IFI16 protein in cells negatively regulate cell proliferation in part by potentiating the p53/p21CIP1 and Rb/E2F-mediated inhibition of cell cycle progression (13, 23, 34), and inhibition of the hTERT activity (35). Moreover, increased levels of IFI16 protein in primary human umbilical vein endothelial cells up-regulate the expression of inflammatory cytokines through the activation of the transcriptional activity of NF-κB (36, 37). The IFI16 protein activates the NF-κB activity through down-regulation of the IκBα expression, a negative regulator of the NF-κB (36).
Many cells type are reported to express the AIM2 gene (38). However, it remains unclear which signaling pathways regulate the expression of the AIM2 gene. Notably, the AIM2 gene contains a microsatellite instability site thatresults in inactivation of the gene in ~ 47% of colorectal tumors withhigh microsatellite instability (39). Interestingly, a search of DNA sequence databases revealed that different isoforms of the human AIM2 protein may be expressed in cells and, depending upon the isoform of the AIM2 protein that is expressed, the sub-cellular localization of the protein may vary (24). For example, in HL-60 cells, the endogenous AIM2 protein is primarily detected in the nucleus (40). In contrast, the approach involving overexpression of the protein in transfected cells indicated a cytoplasmic localization of the AIM2 protein (25–27).
Given that reduced levels of IFI16 protein are associated with immortalization of HDFs (23) and both IFI16 and AIM2 proteins appear to initiate different innate immune responses upon sensing cytosolic dsDNA (6, 24), we investigated the role of these two proteins in cellular senescence of HDFs.
Materials and Methods
Cell Culture and Treatments
Human lung fibroblasts WI-38 (at population doubling or PDL 23) and IMR-90 (at PDL 26) were purchased from the American Type Culture Collection (ATCC; Manassas, VA). Human skin fibroblasts (AG03057 and AG02496A) from Ataxia-Telangiectasia (AT) individuals were obtained from the National Institute on Aging Cell Culture Repository (Coriell Institute for Medical Research, Camden, NJ). Human benign prostate hyperplasia cell line BPH-1 (41) was generously provided by Simon W. Hayward (Vanderbilt University Medical Center, Nashville, TN). All HDFs were maintained in culture (C02, 5%; Oxygen, 20%) as suggested by the supplier. Cultures of HDFs were split (1: 4) upon approaching confluence. Consequently, each cell passage was equivalent to ~2 PDLs. When 50–60% cells in culture at the PDL-50 appeared morphologically large and flat, we considered these cells old (13). Moreover, when the most (>95%) HDFs in culture lost the ability to divide and also stained positive for the senescence-associated acidic β-galactosidase, we considered them senescent (versus old).
When indicated, sub-confluent cultures of HDFs were treated with the human IFN-α (Universal interferon) or IFN-β (1,000 u/ml; from PBL Biomedical laboratories, Piscataway, NJ) for the indicated times. Additionally, when indicated, HDFs were treated with the indicated units of bleomycin (Sigma-Aldrich, St. Louis, MO). The BPH-1 cell line was treated with human IFN-γ (10 ng/ml; PBL Biomedical laboratories) for the indicated times.
Knockdown of the Expression of Genes
To knockdown the expression of the indicated genes in young WI-38 HDFs, sub-confluent cultures of cells (in a six well plate) were infected with lentivirus (purchased from Santa Cruz Biotech, Santa Cruz, CA) encoding shRNA to the IFI16 (sc-35633-V), AIM2 (sc-88166-V), IFNα/βRα (sc-35637-V), or ATM (sc-29761-V) gene. As a control, cells were infected with the lentivirus encoding a control shRNA (sc-108080). 24 hours after infections of HDFs, cells were selected in puromycin (1 μg/ml) for 3-5-days. Puromycin-resistant HDFs were pooled and cell cultures were maintained without the puromycin in the medium for several days before performing any experiments. Similarly, to knockdown the AIM2 expression in BPH-1 cell line, cells were either infected with the control virus or the virus expressing shRNA AIM2. 24 h after infections, cell were selected in puromycin (for a week) and resistant cells were pooled.
Plasmids and Expression Vectors
The pISRE-luc plasmid, which contains five copies of ISRE-responsive element, was purchased from Clontech Laboratories (Mountain View, CA). The IFI16-luc plasmid, which contains the promoter region (1.677 kb) of the IFI16 gene, has been described (33). The pCMV-IFI16 plasmid allowing the expression of the IFI16B protein has been described (34). The pCMV-AIM2 plasmid allowing the expression of FLAG-tagged full-length human AIM2 protein was purchased from Origene (Rockville, MD).
Transfections, Nucleofections and Reporter Assays
Sub-confluent cultures of HDFs were transfected with the indicated reporter plasmid (1.8 μg) along with the pRL-TK reporter plasmid (0.2 μg; as an internal control) using FuGene6 transfection reagent (Roche, Indianapolis, IN) as suggested by the supplier. 48 h after transfections of cells, the firefly luciferase and Renilla luciferase activities were assayed using dual-luciferase reporter assay kit (Promega, Madison, WI). The relative firefly luciferase activity is expressed as the ratio of the firefly luciferase/Renilla luciferase. Student’s t-test for paired samples was used to determine statistical significance of the reporter activity data. The differences were considered statistically significant at the P ≤ 0.05.
The young WI-38 HDFs were nucleofected with highly purified (endotoxin-free) pEGFP plasmid that is supplied with the Nucleofector-II device (Amaxa Biosystems, Germany). We used the kit R and program V-001 for nucleofections as suggested by the supplier. After nucleofections, cells were incubated for the indicated times to isolate total RNA or to prepare total cell extracts for further analyses.
Regular and Quantitative Real-time PCR
Total RNA was isolated using the Trizol reagent (Invitrogen, Carlsbad, CA, USA). cDNA was synthesized using primers that were supplied with the SuperScript First-strand Synthesis System for RT-PCR from Invitrogen (Carlsbad, CA, USA). Quantitative real-time TaqMan PCR technology (Applied Biosystems, Foster City, CA, USA) was used to compare the expression of the indicated genes. The PCR cycling program consisted of denaturing at 95°C for 10 min and 40 cycles at 95°C for 15 seconds, and annealing and elongation at 60°C for 1 min. The TaqMan assays for the IFI16 (assay Id #Hs00194216_m1), human AIM2 (Hs00175457_m1), human interferon-β (IFNB; Hs01077958_s1), and for the endogenous control β-actin (assay Id# Hs99999903_ml) were purchased from the Applied Biosystems and used as suggested by the supplier.
For regular PCR, the human IFNB1 primers (forward: 5’-gaatgggaggcttgaatactgcct-3’; reverse: 5’-tagcaaagatgttctggagcatctc-3’) were used. The conditions for regular PCR have been described (33).
Immunoblotting
Cell lysates were prepared in the radio-immunoprecipitation assay buffer (RIPA buffer; 50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate). The RIPA buffer was supplemented with protease inhibitor cocktail tablet (Mini Complete from Roche Diagnostics, Mannheim, Germany) and phosphates inhibitor cocktail 1 and 2 (Sigma, St Lois, MO). Equal amounts of protein were resolved by SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride (PVDF) membranes. Membranes were probed overnight at 4°C with the primary antibodies specific to the indicated proteins. Antibodies for IFI16 (sc-8023), IFNα/βRα (sc-7391), IFI35 (sc-100769), IFI44L (sc-101981), ASC (sc-22514-R), p53 (sc-126), and thioredoxin (sc-20146) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies for STING (ab82960) were purchased from Abcam (Cambridge, MA). Antibodies for p-IRF3 (#4947), IRF3 (#4962), PY-STAT1 (#9167), STAT1 (#9172), actin (#4970), Cleaved IL-1β (#2022), p-p53(Ser-15) (#9284), ATM (#2873), p-H2AX (# 9718), p-ATM (Ser-1981) (#4526), p-p53(Ser-392) (#9281), IκBα (#9242), Histone H3 (# 9715), p-TBK1 (#5483), and TBK1 (#3013) were purchased from Cell Signaling (Beverly, MA). Antibodies for caspase-1 (AH20082) were purchased from Invitrogen. Polyclonal antibodies to the human AIM2 protein were raised against the C-terminal peptide (GVHSTIKVIKAKKKT) in rabbits and the specificity of the antiserum was established using immunoblotting. Protein-antibody complexes were detected with horseradish-conjugated secondary antibodies by using an ECL chemiluminescence system (Amersham Biosciences, Buckinghamshire, United Kingdom).
The procedure to fractionate HDFs into the nuclear and cytoplasmic fractions has been described (39). The detection of IκBα protein primarily in the cytoplasmic fraction and the histone H3 in the nuclear fraction served as the quality control for the cell fractionations.
Immunoprecipitation and Western (IP-western) Assays
Cultures of human embryonic kidney cells (HEK-293) in 60 mm plates were transfected with pCMV-IFI16 plasmid (2 μg) alone or along with pCMV-AIM2 plasmid (2 μg) using the FuGene 6 transfection reagent as suggested by the supplier. 24–36 h after transfections, total lysates were prepared in immunoprecipitation buffer (20 mM HEPES, pH 7.0, 250 mM NaCl, and 1% NP-40) and lysates containing equal amounts of proteins (~500 μg) were incubated with an isotype antibody (2 μg/tube) or an antibody to the FLAG-tag supplied in the FLAG immunoprecipitation kit (Sigma-Aldrich). Immune complexes were collected by incubating with protein A/G-sepahrose beads (Thermo Fisher, Rockford, IL). The beads were washed 5-times with the washing buffer (20 mM HEPES, pH 7.0, 150 mM NaCl, and 1% NP-40), boiled with the 1X protein sample buffer, and analyzed by immunoblotting.
Senescence-associated β-galactosidase Assays
These assays were performed essentially as described by us (13). In brief, HDFs in culture were fixed using the in situ β-galactosidase staining kit (cat. # 200384; Stratagene, La Jolla, CA, USA). The fixed cells were incubated 4–6 h at 37° C with the staining solution. HDFs, which stained blue, were considered positive for the activity of acidic SA-β-gal and were counted and photographed.
Results
Differential Expressions and Roles for the IFI16 and AIM2 Proteins during Cellular Senescence of HDFs
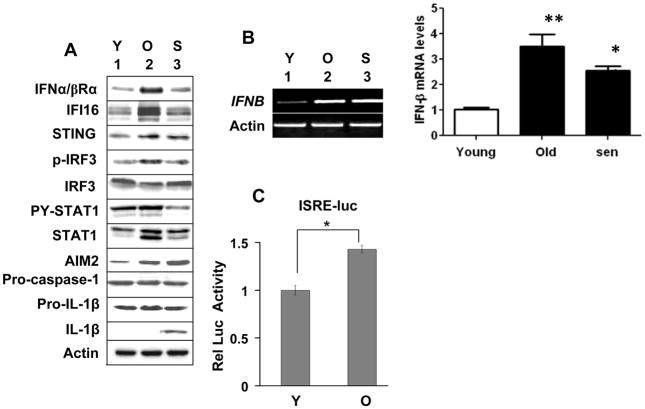
A recent study noted that upon sensing cytosolic dsDNA the IFN-inducible IFI16 protein recruits the STING protein to stimulate the expression of IFN-β through the activation of IRF3 (6). Moreover, studies involving the Aim2-deficient mice indicated that the Aim2 protein negatively regulates the expression of IFN-β (32). Therefore, to explore the role of IFI16 and AIM2 proteins in cellular senescence, we decided to compare steady-state levels of IFI16 and AIM2 proteins (and their predicted downstream effector proteins, such as STING, IRF3, and STAT1) in young, old, and senescent populations of HDFs. We chose human WI-38 lung fibroblasts for our studies because we had identified a potential role for the IFI16 protein in cellular senescence-associated cell growth arrest in these cells (13). As shown in Fig. 1, the young WI-38 HDFs (PD ~30) expressed low, but detectable, levels of both IFI16 and AIM2 proteins. Consistent with our previous observations (13), old WI-38 HDFs (PD ~50) expressed much higher levels of the IFI16 protein as compared to young HDFs (Fig. 1A). Interestingly, the increase in the IFI16 protein levels was associated with increases in the levels of the IFNα/βRα subunit, STING protein, and the activating phosphorylation of the IRF3 and STAT1 proteins. Furthermore, the senescent HDFs (PD ~56; >95% cells staining for senescence-associated β-galactosidase; SA-β-Gal; ref. 13) had lower levels of IFI16 protein than old or young HDFs. We also tested whether the AIM2 protein levels also increase when cells become old. In contrast to our expectations, the AIM2 protein levels were higher in the senescent HDFs than the old HDFs and the levels were inversely correlated with the levels of p-STAT1, STAT1 and IFI16 proteins. Notably, the increases in the AIM2 protein levels were associated with the cleavage of the pro-IL-1β to IL-1β (Fig. 1A).
FIGURE 1.
Differential expression and roles for the IFI16 and AIM2 proteins during cellular senescence of HDFs. A, Total cell extracts prepared from young (Y), old (O), or senescent (S) WI-38 HDFs were analyzed by immunoblotting using antibodies specific to the indicated proteins. B, Total RNA isolated from young (Y), old (O), or senescent (S) WI-38 HDFs were analyzed by semi-quantitative PCR (left panel) or quantitative real-time PCR (right panel) for the indicated genes. The ratio of the test gene to actin mRNA was calculated in units (one unit being the ratio of the test gene to actin mRNA). The relative steady-state levels of IFNB mRNA in young HDFs are indicated as 1. Results are mean values of triplicate experiments and error bars represent standard deviation (*p <0.05; ** p <0.01). C, Sub-confluent cultures of young (Y) or old (O) WI-38 HDFs were infected with Cignal Lenti ISRE Reporter (Luc) virus as suggested by the supplier. The infected cells were harvested after 40-44 h to assays for the firefly and Renilla luciferase activitiesas described in methods.Normalized relative firefly luciferase activity is shown.
The above observations that increased levels of IFI16 protein in old WI-38 HDFs are associated with increased levels of STAT1 and its activated form (the p-STAT1) prompted us to compare steady-state levels of IFN-β mRNA. As shown in Fig. 1B, steady-state levels of IFN-β mRNA were higher in old as well as senescent WI-38 HDFs as compared to the young HDFs. Furthermore, quantitative real-time PCR indicated that the levels of IFN-β mRNA were higher in old HDFs than senescent cells (Fig. 1B). This observation that increased levels of IFN-β mRNA in senescent cells did not correlate with the activation of STAT1 protein is consistent with the increased levels of type I IFN receptor (the IFNα/βRα subunit) in the old HDFs as compared to senescent HDFs, thus, probably accounting for the increased levels of STAT1 protein and its activation in old (versus senescent) HDFs. We also compared the activity of an IFN-responsive reporter (the ISRE-luc) between the young and old HDFs. As shown in Fig. 1C, the activity of the reporter was ~ 40% higher in old versus young HDFs. Consistent with this observation, levels of several mRNAs corresponding to the known IFN-inducible genes (including the CXCL10, IFI27, OAS1, and Mx1) were higher (>5-fold) in old versus young HDFs (data not shown). Collectively, these observations demonstrate differential expression and roles for the IFI16 and AIM2 proteins during the onset of the cellular senescence and associated production of proinflammatory cytokines, such as IL-1β.
The IFN-signaling in HDFs is Required to Maintain the Constitutive Expression of the IFI16 Protein and the Onset of Cellular Senescence
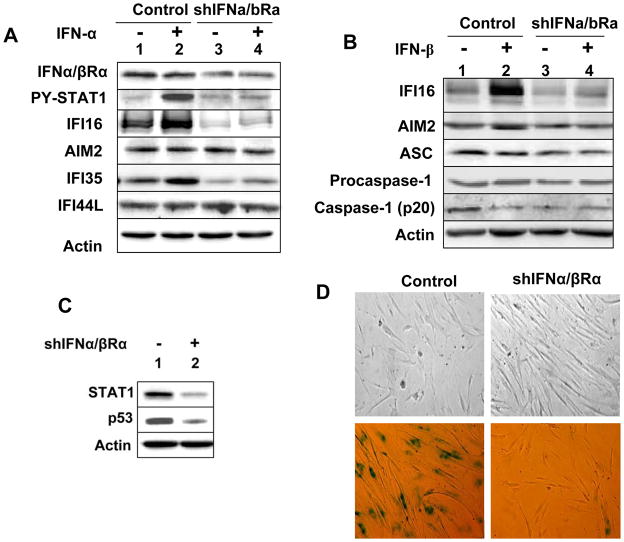
Studies indicate that the loss of type I IFN-signaling, which results in the lack of the expression of ISGs, is associated with immortalization of HDFs (19, 20). Moreover, the knockdown of IFI16 expression in WI-38 HDFs delays the onset of cellular senescence (13). Therefore, our above observations that increased levels of IFI16 protein in old WI-38 HDFs are associated with the induction of the expression of IFN-β and IFN-inducible genes prompted us to investigate whether the type-I IFN-signaling regulates the expression of the IFI16 gene and cellular senescence in these cells. As shown in Fig. 2A, the knockdown of IFNα/βRα subunit expression in WI-38 HDFs significantly reduced basal and IFN-α-induced levels of the IFI16 protein and IFN-inducible IFI35 protein. However, levels of the AIM2 and IFI44L proteins did not decrease. Similarly, the knockdown also reduced basal and IFN-β-induced levels of IFI16 protein (Fig. 2B). Notably, the knockdown also reduced basal levels of adaptor protein ASC and cleaved IL-1β, but not procaspase-1. Additionally, the basal levels of p53 also decreased in the knocked down HDFs (Fig. 2C). Consistent with these observations, the knockdown of IFNα/βRα subunit expression decreased the number of SA-β-gal-positive cells (Fig. 2D), which is indicative of the onset of cellular senescence in HDFs (13). Collectively, these observations demonstrated that the type I IFN-signaling is required to maintain the basal and the IFN-induced levels of certain IFN-inducible proteins, including the IFI16 protein, but not the AIM2 protein, and the onset of cellular senescence in WI-38 HDFs. Additionally, these observations revealed differential regulation of the IFI16 and AIM2 expression by IFN-signaling.
FIGURE 2.
The knockdown of the IFNα/βRα subunit expression in HDFs decreases IFI16 expression and delays the onset of a senescence phenotype. A, Sub-confluent cultures of young WI-38 HDFs infected with control lentivirus (control) or virus encoding for shIFNa/bRa RNA (shIFNa/bRa) were either left untreated or treated with IFN-α for 18 h. After the treatment, total cell extracts were prepared and analyzed by immunoblotting using antibodies specific to the indicated proteins. B, Control or shIFNa/bRa HDFs described in panel A were either left untreated or treated with IFN-β for 18 h. Total cell extracts were analyzed by immunoblotting. C, Total cell extracts from control or shIFNa/bRa HDFs were analyzed by immunoblotting for the indicated proteins. D, Phase contrast photographs indicating morphological changes (top two panels) and differences in the number of SA-β-gal-positive cells (bottom two panels) between control HDFs and HDFs after the knockdown of the IFNa/bRa subunit expression.
Activation of DNA-damage Response in HDFs Differentially Regulates the Expression of the IFI16 and AIM2 Proteins
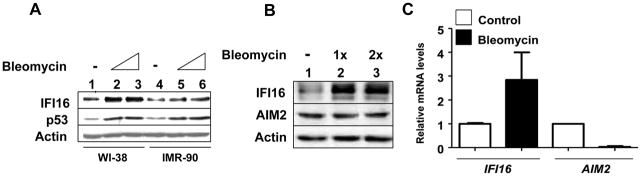
Cellular senescence in HDFs is associated with activation of DNA-damage response (21). Moreover, the activation of p53 in response to DNA-damage activates the transcription of the IFI16 gene (33). Because prolonged IFN-treatment of HDFs activates the DNA-damage response and p53 (21), we also compared levels of IFI16 protein in young WI-38 and IMR-90 HDFs after bleomycin treatment, a mimetic of double strand DNA break. As shown in Fig. 3A, the treatment increased levels of IFI16 protein and the increase was associated with increased levels of p53 (which indicates the activation of DNA-damage response). However, bleomycin treatment of young WI-38 HDFs did not increase levels of the AIM2 protein (Fig. 3B). Consistent with these observations, treatment of young WI-38 HDFs with bleomycin increased steady-state levels of IFI16 mRNA (Fig. 3C). However, levels of the AIM2 mRNA decreased, thus, indicating a negative regulation of the AIM2 expression by the activation of DNA-damage response. Together, these observations support the idea that the activation of DNA-damage response in young HDFs differentially regulates the expression of IFI16 and AIM2 proteins.
FIGURE 3.
DNA-damage response also contributes to the constitutive levels of the IFI16 protein. A, Sub-confluent cultures of young WI-38 or IMR-90 HDFs were either left untreated (lanes 1 and 4) or treated with increasing units of bleomycin (lanes 2, 3, 5 and 6) for 24 h. After the treatment, total cell extracts were analyzed by immunoblotting for the indicated proteins. B, Sub-confluent cultures of young WI-38 were either left untreated (lanes 1) or treated with increasing units of bleomycin (lanes 2 and 3) for 24 h. After the treatment, total cell extracts were analyzed by immunoblotting for the indicated proteins. C, Sub-confluent cultures of young WI-38 were either left untreated or treated with bleomycin for 24 h. After the treatment, total RNA was analyzed by quantitative real-time PCR for IFI16 and AIM2 mRNA levels. The ratio between the test gene (the IFI16 or AIM2) mRNA levels to actin mRNA was calculated in units (one unit being the ratio of the test gene to actin mRNA). The relative steady-state levels of IFI16 mRNA in control HDFs are indicated as 1.
Constitutively Increased Levels of the IFI16 and AIM2 Proteins in AT HDFs are Associated with the Activation of the IFN-signaling and the Production of IL-1β
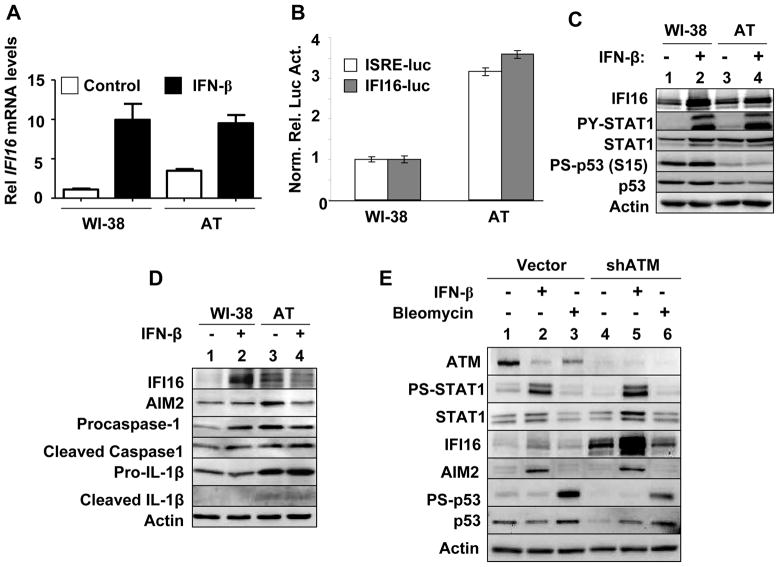
HDFs from AT patients express increased levels of IFN-inducible proteins (15) and the knockdown of the ATM expression in HeLa cells induces the expression of IFN-inducible genes (16). Therefore, our above observations that increased levels of IFI16 protein in old HDFs are associated with the activation of IFN-signaling (Fig. 1) encouraged us to compare the steady-state levels of IFI16 mRNA and protein between WI-38 and AT (AG03057) HDFs. As shown in Fig. 4A, basal levels of IFI16 mRNA were higher in the young AT than WI-38 HDFs. Consistent with this observation, the activities of the IFI16-luc and ISRE-luc-reporters were ~3-fold higher in the young AT than WI-38 HDFs (Fig. 4B). Accordingly, the basal levels of IFI16 protein were higher in AT than WI-38 HDFs (Fig. 4C, compare lane 3 with 1). Importantly, the increased basal levels of the IFI16 protein in AT HDFs were associated with elevated basal levels of STAT1 and the activated p-STAT1 (Fig. 4C). We also compared the basal and IFN-induced levels of the AIM2 protein between the young AT and WI-38 HDFs. As shown in Fig. 4D, the basal levels of AIM2 protein were higher in AT than WI-38 HDFs (compare lane 3 with 1). Importantly, the increased basal levels of the AIM2 protein in AT HDFs were associated with elevated basal levels of procaspase-1, cleaved caspase-1, pro-IL-1β, and IL-1β. To further investigate the role of the ATM in the regulation of the IFI16 and AIM2 genes expression, we knockdown the expression of ATM in young WI-38 HDFs. As shown in Fig. 4E, the knockdown increased basal and IFN-β-induced levels of IFI16 protein. Notably, the induced, but not the basal, levels of IFI16 protein in the ATM knocked down HDFs were associated with increases in the steady-state levels of STAT1 and p-STAT1. These observations revealed that the ATM negatively regulates the expression of the IFI16 gene in WI-38 HDFs. Moreover, these observations indicated that the knockdown of ATM (or mutation in the ATM gene) potentiates the IFN-signaling and the expression of the IFN-inducible proteins, including the IFI16 protein. Interestingly, no measurable differences were noted between the basal or IFN-β-induced levels of the AIM2 protein between control and WI-38 HDFs with the reduced ATM expression. Together, these observations indicated that the constitutively increased levels of the IFI16 and AIM2 proteins in AT HDFs are associated with the activation of IFN-signaling and the production of IL-1β.
FIGURE 4.
Constitutively increased levels of the IFI16 protein in ataxia telangiectasia (AT) HDFs are associated with the activation of IFN-signaling. A, Sub-confluent cultures of young WI-38 or AT HDFs were either left untreated or treated with IFN-β for 18 h. After the treatment, total RNA was extracted and analyzed by quantitative real-time PCR for steady-state levels of IFI16 mRNA. The ratio between the test gene the IFI16 mRNA levels to actin mRNA was calculated in units (one unit being the ratio of the test gene to actin mRNA). The relative steady-state levels of IFI16 mRNA in WI-38 control HDFs are indicated as 1. B, Sub-confluent culture of young WI-38 or AT HDFs was transfected with infected with either ISRE-luc or IFI-16-luc reporter plasmid (1.8 μg) along with a second pRL-TK reporter (0.2 μg). The transfected cells were harvested after 40-44 h to assays for the firefly and Renilla luciferase activitiesas described in methods.Normalized relative firefly luciferase activity in WI-38 cells is shown as 1. C, Sub-confluent cultures of young WI-38 or AT HDFs were either left untreated (lanes 1 and 3) or treated with IFN-β (1000 u/ml) for 18 h. After the treatment, total cell extracts were analyzed by immunoblotting for the indicated proteins. D, Sub-confluent cultures of young WI-38 or AT HDFs were either left untreated (lanes 1 and 3) or treated with IFN-β (1000 u/ml) for 18 h. After the treatment, total cell extracts were analyzed by immunoblotting for the indicated proteins. E, Sub-confluent cultures of young WI-38 HDFs that were infected with control virus (Vector) or shATM virus (shATM) were left untreated (lanes 1 and 4), treated with IFN-β (lanes 2 and 5), or bleomycin (lanes 3 and 6) for 18 h. After the treatment, total cell extracts were analyzed by immunoblotting for the indicated proteins.
Prolonged IFN-β Treatment of Young HDFs Activates DNA-damage Response and Increases the Production of IL-1β
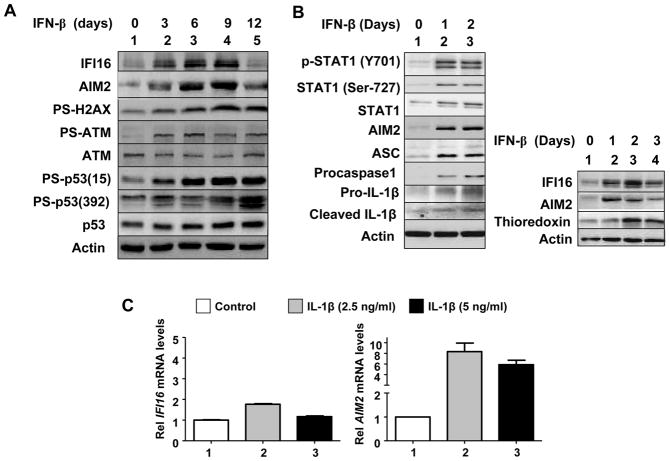
Prolonged IFN-β treatment of IMR-90 HDFs increases the reactive oxygen species (ROS), activates DNA-damage response (as determined by phosphorylation of H2AX, ATM, and p53), and induces a senescence-like phenotype (21). Therefore, we tested whether prolonged constitutive activation of IFN-signaling in young WI-38 HDFs induces a DNA-damage response. As shown in Fig. 5A, prolonged IFN-β treatment of HDFs, which increased steady-state levels of both IFI16 and AIM2 proteins, activated a DNA-damage response (the ATM/p53 pathway) as determined by phosphorylation of H2AX, ATM, and p53 proteins.
FIGURE 5.
Prolonged IFN-β treatment of young HDFs increases levels of IFI16, AIM2, and proinflammatory cytokine IL-1β. A and B, For treatment of young WI-38 HDFs with IFN-β, cultures at ~20% confluence were treated with IFN-β for the indicated days (medium was changed after every two days and fresh IFN-β was added). After the treatment, HDFs were harvested and total cell lysates were analyzed by immunoblotting for the levels of the indicated proteins. C, Cultures of young WI-38 were either left untreated or treated with the indicated amount of the human recombinant IL-β for 18 h. After the treatment, total RNA was extracted and analyzed by quantitative real-time PCR for steady-state levels of IFI16 (left panel) or AIM2 (right panel) mRNA. The ratio between the test gene (the IFI16 or AIM2) mRNA levels to actin mRNA was calculated in units (one unit being the ratio of the test gene to actin mRNA). The relative steady-state levels of IFI16 or AIM2 mRNA in WI-38 control HDFs are indicated as 1.
The presence of inflammasome component proteins, such as AIM2, ASC, procaspase-1, and pro-IL-1β, has been predicted in non-myeloid cells (43). However, their expression has not been demonstrated in HDFs. Therefore, we explored whether inflammasome proteins are expressed in the young WI-38 HDFs and whether the IFN-β treatment affects their expression and/or activation. As shown in Fig. 5B, the IFN-β treatment of young WI-38 HDFs increased steady-state levels of AIM2, adaptor protein ASC, procaspase-1, and pro-IL-1β (Fig. 5B, left panel). Interestingly, the treatment also increased levels of the cleaved IL-1β, a proinflammatory cytokine, which is indicative of activation of an inflammasome. Moreover, the treatment also increased levels of thioredoxin (Fig. 5B, right panel), indicative of the generation of ROS by the IFN-β treatment. Together, these observations support the idea that a prolonged IFN-β treatment of WI-38 HDFs results in ROS production, activation of DNA-damage response and an inflammasome activity (as determined by increases in the IL-1β levels within the cells).
Given that levels of the AIM2 protein in the senescent WI-38 HDFs were inversely correlated with the levels of IFI16 protein and production of IL-1β (Fig. 1), the above observation that a prolonged IFN-β treatment of young WI-38 HDFs increased the levels of IL-1β prompted us to test whether IL-1β could differentially regulate the expression of IFI16 and AIM2 genes in HDFs, which could account for differential expression of these two genes between the old and senescent HDFs (Fig. 1). As shown in Fig. 5C, treatment of young HDFs with IL-1β only moderately increased steady-state levels of the IFI16 mRNA. In contrast, the treatment increased levels of the AIM2 mRNA robustly (>6-fold). Together, these observations demonstrate that a prolonged activation of IFN-signaling in HDFs can activate a DNA-damage response (the ATM/p53 pathway) and the production of proinflammatory cytokine IL-1β, which in turn can differentially regulate the expression of the IFI16 and AIM2 genes.
IFI16 Protein is Primarily Detected in the Cytoplasm of Old or IFN-β-treated HDFs and dsDNA Induces the IFN-β Expression
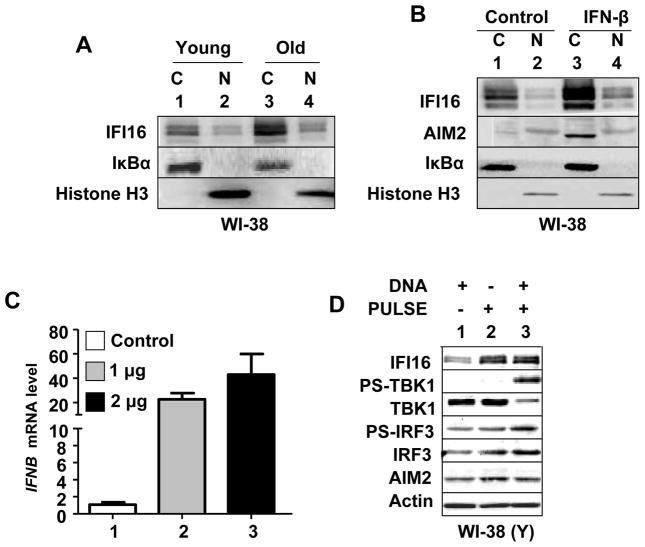
Studies indicate that the extent of the cytoplasmic and nuclear localization of the endogenous IFI16 protein varies among various cell types (23). Therefore, we investigated the sub-cellular localization of the IFI16 protein in young versus old and IFN-β treated WI-38 HDFs. As shown in Fig. 6A, the bulk of the IFI16 proteins (all three isoforms) were detected primarily in the cytoplasm of the young HDFs and only a fraction was detectable in the nucleus. Similarly, increased levels of IFI16 proteins in old HDFs were detected primarily in the cytoplasm and only a fraction in the nucleus. Furthermore, IFN-β-induced levels of IFI16 proteins in young WI-38 HDFs were detected primarily in the cytoplasm (Fig. 6B). As expected (24), the bulk of the AIM2 protein was detected primarily in the cytoplasm after IFN-β treatment of WI-38 HDFs (Fig. 6B). However, a fraction was detectable in the nucleus.
FIGURE 6.
IFI16 protein is primarily detected in the cytoplasm of old or IFN-β treated HDFs and nucleofection of young HDFs with dsDNA induces the IFN-β expression. A, Cultures of young (PD ~30) proliferating or old (PD ~50) WI-38 HDFs were harvested and cells were subjected to the nuclear and cytoplasmic fractionation. The fractions containing equal amounts of proteins were analyzed by immunoblotting using antibodies specific to the indicated proteins. B, Cultures of young proliferating WI-38 HDFs were either left untreated or treated with IFN-β for 24 h. After the treatment, cells were subjected to the nuclear and cytoplasmic fractionation. The fractions containing approximately equal amounts of proteins were analyzed by immunoblotting. C, Young proliferating (PD ~30) WI-38 HDFs were nucleofected without DNA (control) or with the indicated amounts of plasmid DNA (GFP plasmid) as described in methods. After 18 h of nucleofection, total RNA was extracted and analyzed by quantitative real-time PCR for steady-state levels of IFNB mRNA. The ratio between the IFNB mRNA levels to actin mRNA was calculated in units (one unit being the ratio of the IFNB mRNA to actin mRNA). The relative steady-state levels of IFNB mRNA in control HDFs are indicated as 1. D, Young proliferating WI-38 HDFs were either left without nucleofection or nucleofected without DNA or nucleofected with 2 μg of plasmid DNA. After 18 h of nucleofection, total cell lysates were analyzed by immunoblotting for the indicated proteins.
IFI16 protein is a cytosolic sensor of dsDNA in macrophages and upon sensing of dsDNA the IFI16 protein induces the expression of IFN-β through the activation of TBK1 and IRF3 (6). Therefore, our above observations that the IFI16 protein is primarily localized in the cytoplasm of WI-38 HDFs (Fig. 6A and B) encouraged us to test whether nucleofection of dsDNA into HDFs induces the expression of IFN-β. As shown in Fig. 6C, nucleofection of dsDNA plasmid into young WI-38 HDFs increased steady-state levels of IFN-β mRNA. Importantly, the increase was associated with the activation of TBK1 and IRF3 (Fig. 6D). Additionally, the increase in the IFN-β expression was associated with increases in IFI16, but not AIM2, protein levels. Together, these observations support the idea that increased cytoplasmic levels of the IFI16 protein in old HDFs upon sensing cytosolic dsDNA induce the expression of IFN-β through the activation of TBK1 and IRF3.
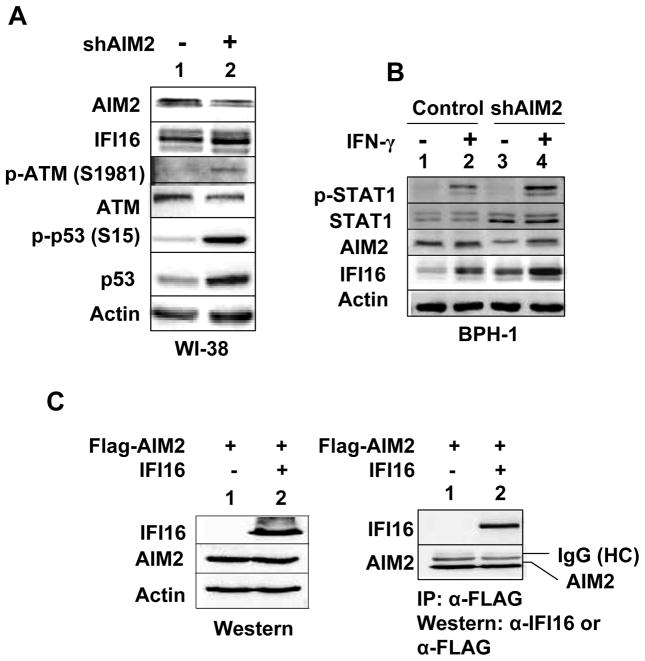
The Knockdown of the AIM2 Expression in Cells Activates the DNA-damage Response, IFN-signaling, and Increases IFI16 Protein Levels
Aim2-deficiency in the murine immune cells increases the expression of IFN-β and IFN-inducible genes (32). Therefore, to explore the potential role of the AIM2 protein in cellular senescence, we chose to knockdown its expression in young HDFs. In several attempts, infection of WI-38 cells with the shAIM2 lentivirus, but not control virus, resulted in accumulation of large flat cells in cultures, indicating the induction of cellular senescence (data not shown). Accordingly, we noted increased steady-state levels of IFI16 protein (Fig. 7A). Moreover, the increase was associated with the activation of a DNA-damage response (the ATM/p53 pathway). Because the knockdown of AIM2 expression in WI-38 HDFs resulted in cell growth arrest, we chose to knockdown the AIM2 expression in benign prostate hyperplasia (BPH-1) cell line. We chose this cell line because basal levels of the AIM2 protein are detectable in this immortalized cell line. As shown in Fig. 7B, the knockdown of AIM2 expression in BPH-1 cells increased steady-state levels of STAT1 and potentiated IFN-γ-induced activation of STAT1. Importantly, the knockdown increased basal as well as the IFN-γ-induced levels of the IFI16 protein. Together, these observations support the idea that the reduced levels of the AIM2 protein in WI-38 and BPH-1 cell line increase the steady-state levels of IFI16 protein through the activation of the IFN-signaling and a DNA-damage response (the ATM/p53 pathway).
FIGURE 7.
Knockdown of the AIM2 expression in cells increases the IFI16 protein levels. A, Total cell extracts prepared from WI-38 HDFs that were infected with control virus or shAIM2 virus were analyzed by immunoblotting for the indicated proteins. B, Sub-confluent cultures of the human benign prostate hyperplasia-1 (BPH-1) that were infected with control virus (control) or shAIM2 virus (shATM) were either left untreated (lanes 1 and 3) or treated with IFN-γ (lanes 2 and 4) for 18 h. After the treatment, total cell extracts were analyzed by immunoblotting for the indicated proteins. C, Cultures of human embryonic kidney cells (HEK-293) were either transfected with pCMV-AIM2 plasmid (2 μg) allowing the expression of the FLAG-tagged human AIM2 protein or along with pCMV-IFI16 plasmid allowing the expression of IFI16 protein. 40 h after the transfections, total cell lysates were analyzed by immunoblotting using antibodies specific to the IFI16 protein or FLAG-tag (left panel). Total cell extracts were also subjected to immunoprecipitation using antibodies to FLAG-tag and immunoprecipitates were analyzed by immunoblotting (right panel).
Given that levels of AIM2 protein in HDFs are inversely correlated with IFI16 protein (Figs. 1 and 7) and AIM2 protein was reported to bind IFI16 protein (40), we also explored binding of AIM2 protein with IFI16 in immunoprecipitation-western assays (IP-western assays). As shown in Fig. 7C, we were able to immunoprecipitate IFI16 protein using antibodies to FLAG-tag that was present in the AIM2 protein in IP-western assays. This observation indicated that AIM2 and IFI16 proteins interact with each other and their physical interaction, depending upon their relative expression levels and sub-cellular localization (cytoplasmic versus nuclear), could affect the extent and the type of the innate immune response (IFN-β production versus the proinflammatory cytokine production) following the detection of cytosolic dsDNA.
The Expression of the IFI16 Protein is Required for the Constitutive and dsDNA-induced Activation of the IFN-signaling in HDFs
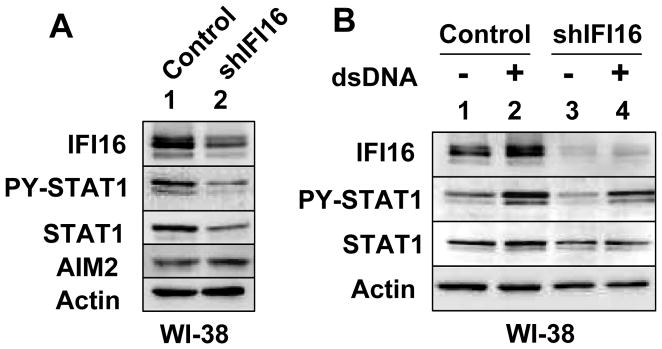
Our observations that nucleofection of dsDNA into WI-38 HDFs activated the expression IFNB gene (Fig. 6) prompted us to determine whether IFI16 expression is needed for the constitutive as well as dsDNA-induced activation of IFN-signaling in WI-38 HDFs. Therefore, we chose to knockdown the expression of IFI16 gene in WI-38 HDFs. As shown in Fig. 8A, the knockdown of IFI16 expression decreased basal levels of p-STAT1 and STAT1 proteins. Interestingly, the basal levels of the AIM2 protein increased moderately. Moreover, the knockdown also reduced basal and dsDNA-induced levels of p-STAT1 and STAT1 proteins after nucleofection of cells. Together, these observations demonstrated that the expression of IFI16 protein is needed for the basal and dsDNA-induced activation of the IFN-signaling in HDFs.
FIGURE 8.
Knockdown of IFI16 expression in HDFs reduces the constitutive and dsDNA-induced activation of IFN-signaling. A, Total cell extracts prepared from the young WI-38 HDFs that were infected with control virus (Vector) or shIFI16 virus (shIFI16) were analyzed by immunoblotting for the indicated proteins. B, Young WI-38 HDFs that were infected with control virus (Vector) or shIFI16 virus (shIFI16) were either nucleofected without (lanes 1 and 3) or with (lanes 2 and 4) dsDNA. 18 h after nucleofections, cells were harvested and total cell extracts were analyzed by immunoblotting for the indicated proteins.
Discussion
The loss of IFN-signaling in human cells that results in the lack of the expression of IFN-inducible genes is associated with the development of certain cancers (17–20). Consistent with the above observations, the loss of IFI16 expression in cells is associated with the development of cancers including the breast and prostate (23). Moreover, increased levels of IFI16 protein in human normal prostate epithelial cells (34) and HDFs (13) are associated with cellular senescence-associated cell growth arrest. Accordingly, reduced expression of IFI16 protein in Li–Fraumeni HDFs is associated with spontaneous immortalization (19) and the knockdown of IFI16 expression in WI-38 HDFs delays the onset of cellular senescence (13). Given that the recent studies identified IFN-inducible IFI16 and AIM2 proteins as sensors for cytosolic dsDNA, we investigated their roles in cellular senescence of HDFs. Our observations revealed that: (i) increased levels of IFI16, but not AIM2, protein in old (versus young or senescent) HDFs are associated with increased expression of the IFN-β and activation of IFN-signaling (Fig. 1); (ii) increased levels of the AIM2 protein in senescent (versus old or young) HDFs are associated with increased levels of IL-1β (Fig. 1); (iii) the knockdown of the type I IFN-receptor-α subunit expression in HDFs decreases IFI16, but not AIM2, protein levels and delays the onset of a senescence phenotype (Fig. 2); (iv) increased basal levels of IFI16 and AIM2 proteins in ataxia telangiectasia HDFs are associated with the activation of IFN-signaling and increased levels of IL-1β (Fig. 4); (v) a prolonged IFN-β treatment of young HDFs increases steady-state levels of IFI16, AIM2, and the proinflammatory cytokine IL-1β (Fig. 5); (vi) IFI16 protein is detected primarily in the cytoplasm of WI-38 HDFs and nucleofection of cells with dsDNA induces the IFN-β expression (Fig. 6); (vii) the knockdown of AIM2 expression in cells activates a DNA-damage response and the IFN-signaling, resulting in increases in IFI16 protein levels (Fig. 7); and (viii) the constitutive and dsDNA-induced IFN-signaling in HDFs depends on the expression of IFI16 (Fig. 8).
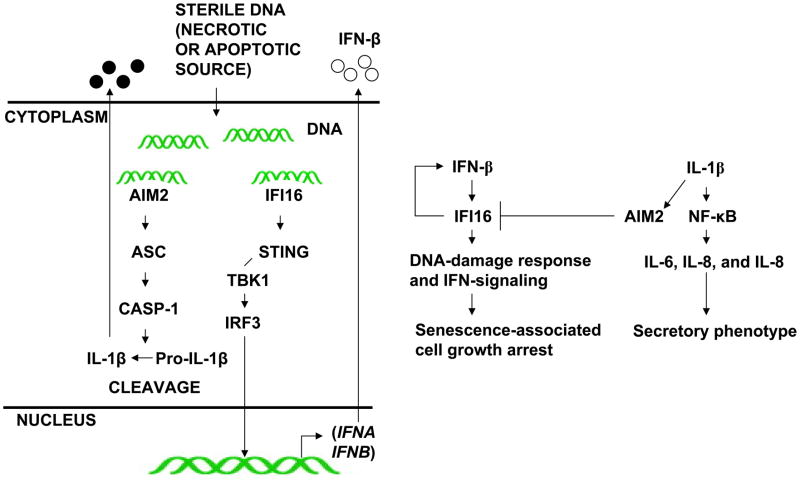
Given that: (i) the IFI16 and AIM2 proteins initiate different innate immune responses after sensing cytosolic DNA (the induction of IFN-β versus the production of the proinflammatory cytokines) (51); (ii) type I interferon inhibits interleukin-1 production and inflammasome activation (52); (iii) immune cells derived from the Aim2-deficient mice exhibit the constitutive activation of IFN-signaling (32), our observations that: (i) levels of the IFI16, but not AIM2, protein increase in old (versus young) HDFs (Fig. 1); (ii) the knockdown of type I IFN-receptor-α subunit expression in young HDFs decreases IFI16, but not AIM2, protein levels (Fig. 2); (iii) treatment of HDFs with bleomycin, which activates a DNA-damage response, increases IFI16, but not AIM2, protein levels (Fig. 3); (iv) the knockdown of AIM2 expression in HDFs activated a DNA-damage response and in BPH-1 cells activated an IFN-response (Fig. 7); and (v) the knockdown of IFI16 expression in HDFs inhibited the IFN-response and increased AIM2 protein levels (Fig. 8) are consistent with the idea that these two innate immune sensors play an opposite roles in the onset of cellular senescence-associated cell growth arrest and associated production of IL-1β (Fig. 9). Therefore, further work will be needed to elucidate their roles.
FIGURE 9.
Proposed roles of the IFI16 and AIM2 proteins in cellular senescence-associated cell growth arrest and secretory phenotype in HDFs.
HDFs from AT individuals, which express higher levels of IFN-inducible proteins, exhibit premature senescence (15). Therefore, our observations that AT HDFs express constitutively higher levels of IFI16 protein and exhibit the activation of IFN-signaling (Fig. 4), support the notion that increased levels of IFI16 protein in AT HDFs contribute to premature cellular senescence through the activation of the IFN-signaling.
Overexpression of IFI16 protein in prostate cancer cell lines induces a senescence-like phenotype, which is accompanied by the stimulation of p53-mediated transcription, an increase in the p21CIP1 protein levels (a transcriptional target of p53), and an inhibition of the E2F-mediated transcription of target genes (34). Furthermore, increased expression of IFI16 protein in HDFs decreases the hTERT expression by inhibiting c-Myc-mediated transcription (35). In contrast, the knockdown of IFI16 expression in HDFs increases c-Myc protein levels and decreases the p21CIP1 protein levels (35). Similarly, overexpression of the AIM2 protein in breast cancer cell lines inhibits cell proliferation in vitro (47). Together, these observations support the idea that increased levels of both IFI16 and AIM2 proteins contribute to inhibition of cell cycle progression whereas their reduced levels in cells provide the growth advantage.
Sub-cellular localization of IFI16 and other p200-family proteins (including the AIM2 protein) appear to depend on the cell type (23). In WI-38 HDFs, the bulk of the IFI16 protein was detected in the cytoplasm (Fig. 6). However, it was also detectable in the nuclear fraction. The nuclear localization of IFI16 protein is consistent with its proposed transcriptional regulatory functions (22). Given that the IFI16 and AIM2 proteins can form a heterodimers (Fig. 7), further work is required to determine whether the binding of IFI16 protein with AIM2 protein could regulate the sub-cellular localization of the AIM2 protein.
Most p200-family proteins share two protein domains: the PYD and HIN-200 domain (24). PYD of the AIM2 protein heterodimerizes with an adaptor protein ASC in response to cytoplasmic dsDNA and forms ASC speckles (25–27). However, the PYD of IFI16 protein does not form ASC speckles. This is consistent with the limited (only 29%) amino acid residue identities between the PYD of the AIM2 and IFI16 proteins. The HIN-200 domain consists of two oligonucleotide/oligosaccharide binding folds (OB-folds), which recognize nucleic acids (24). Consistent with this observation, the AIM2 protein requires this domain to sense cytosolic dsDNA and to assemble an inflammasome (24). Similarly, upon binding to dsDNA through the HIN-200 domain, the IFI16 protein recruits STING protein to stimulate the expression of IFN-β (6). Thus, supporting the idea that cytosolic IFI16 protein, upon sensing dsDNA that is taken up by the HDFs in cultures of old HDFs (5), stimulates the expression of IFN-β. In turn, the constitutively increased levels of the IFN-β in cultures of old HDFs stimulate the expression of IFI16 protein further, expression of other IFN-inducible proteins, activate a DNA-damage response, and potentiate senescence-associated cell growth arrest (Fig. 9).
Most p200-family proteins have the ability to homo- and heterodimerize (23, 24). Consistent with this observation, the human AIM2 protein was shown to heterodimerize with the IFI16 protein in vitro (40). Similarly, we noted that AIM2 protein can bind to IFI16 protein in immunoprecipitation-western assays (Fig. 7). These observations support the idea that the ability of AIM2 protein to heterodimerize with the IFI16 protein (and possibly other p200-proteins) may limit the ability of cells to produce IFN-β upon sensing cytosolic dsDNA. Accordingly, we noted that the knockdown of AIM2 expression in BPH-1 human prostate cell line, which express detectable levels of both AIM2 and IFI16 proteins, resulted in the activation of IFN-signaling and increases in IFI16 protein levels (Fig. 7).
Given that the IFN-β is produced during the innate immune responses that are induced after infections (48); that HDFs from individuals with AT express increased levels of IFN-inducible proteins (15); that the immune system of patients with autoimmune diseases, which are associated with increased levels of type I IFNs (IFN-α and β), shows signs of an accelerated aging (49); that IFN-β promotes atherosclerosis by stimulating macrophage recruitment to lesions (50), it is important to understand the regulation and role of IFN-β and the IFN-inducible proteins in cellular aging and aging-associated inflammatory diseases. Our observations that IFN-inducible IFI16 and AIM2 proteins differentially regulate the IFN-signaling in HDFs will serve basis to understand the role of IFN-inducible proteins in diseases that are associated with increased production of IFN-β and proinflammatory cytokines.
Table 1.
Differential regulation of the IFI16 and AIM2 proteins levels during cellular senescence of HDFs.
| HDFs | IFI16 levels | AIM2 levels | Cytokine/Signaling* |
|---|---|---|---|
| Old | Increase | No change | IFN-β, IS, and DDIS |
| Senescent | Decrease | Increase | IL-1β, IIS |
| IFN receptor knockdown | Decrease | No change | IS |
| DNA-damage response | Increase | No change | DDIS |
| Ataxia telangiectasia | Increase | Increase | IS, IIS |
| AIM2 knockdown | Increase | Decrease | IS, DDIS |
| IFI16 knockdown | Decrease | Increase | IS |
IS, IFN-signaling; DDIS, DNA-damage-induced signaling; IIS, IL-β-induced signaling
Acknowledgments
Grant Support
This work was supported by a grant (AG 025036) from the National Institutes of Health and a Merit Award from the Veterans Affairs (VA) to D.C.
Footnotes
Disclosure of Potential Conflicts of Interest
The authors have no financial conflicts of interest.
References
- 1.Campisi J. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 2001;11:S27– 31. doi: 10.1016/s0962-8924(01)02151-1. [DOI] [PubMed] [Google Scholar]
- 2.Campisi J. Cancer and ageing: rival demons? Nat Rev Cancer. 2003;3:339–49. doi: 10.1038/nrc1073. [DOI] [PubMed] [Google Scholar]
- 3.Young AR, Narita M. SASP reflects senescence. EMBO Rep. 2009;10:228–30. doi: 10.1038/embor.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mammone T, Gan D, Foyouzi-Youssefi R. Apoptotic cell death increases with senescence in normal human dermal fibroblast cultures. Cell Biol Int. 2006;30:903–9. doi: 10.1016/j.cellbi.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 5.Lehmann BD, Paine MS, Brooks AM, McCubrey JA, Renegar RH, et al. Senescence-associated exosome release from human prostate cancer cells. Cancer Res. 2008;68:7864–71. doi: 10.1158/0008-5472.CAN-07-6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, et al. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11:997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–5. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 8.Borden EC, Sen GC, Uze G, Silverman RH, Ransohoff RM, et al. Interferons at age 50: past, current and future impact on biomedicine. Nat Rev Drug Discov. 2007;6:975–90. doi: 10.1038/nrd2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stark GR. How cells respond to interferons revisited: from early history to current complexity. Cytokine Growth Factor Rev. 2007;18:419–23. doi: 10.1016/j.cytogfr.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taniguchi T, Takaoka A. A weak signal for strong responses: interferon-alpha/beta revisited. Nat Rev Mol Cell Biol. 2001;2:378–86. doi: 10.1038/35073080. [DOI] [PubMed] [Google Scholar]
- 11.Honda K, Yanai H, Takaoka A, Taniguchi T. Regulation of the type-I IFN induction: a current view. Int Immunol. 2005;17:1367–78. doi: 10.1093/intimm/dxh318. [DOI] [PubMed] [Google Scholar]
- 12.Tahara H, Kamada K, Sato E, Tsuyama N, Kim JK, et al. Increase in expression levels of interferon-inducible genes in senescent human diploid fibroblasts and in SV40-transformed human fibroblasts with extended lifespan. Oncogene. 1995;11:1125–32. [PubMed] [Google Scholar]
- 13.Xin H, Pereira-Smith OM, Choubey D. Role of IFI 16 in cellular senescence of human fibroblasts. Oncogene. 2004;23:6209–17. doi: 10.1038/sj.onc.1207836. [DOI] [PubMed] [Google Scholar]
- 14.Barzilai A, Rotman G, Shiloh Y. ATM deficiency and oxidative stress: a new dimension of defective response to DNA damage. DNA Repair. 2002;1:3–25. doi: 10.1016/s1568-7864(01)00007-6. [DOI] [PubMed] [Google Scholar]
- 15.Siddoo-Atwal C, Haas AL, Rosin MP. Elevation of interferon beta-inducible proteins in ataxia telangiectasia cells. Cancer Res. 1996;56:443–7. [PubMed] [Google Scholar]
- 16.Chen S, Wang G, Makrigiorgos GM, Price BD. Stable siRNA-mediated silencing of ATM alters the transcriptional profile of HeLa cells. Biochem Biophys Res Commun. 2004;317:1037–44. doi: 10.1016/j.bbrc.2004.03.149. [DOI] [PubMed] [Google Scholar]
- 17.Shou J, Soriano R, Hayward SW, Cunha GR, Williams PM, et al. Expression profiling of a human cell line model of prostatic cancer reveals a direct involvement of interferon signaling in prostate tumor progression. Proc Natl Acad Sci U S A. 2002;99:2830–5. doi: 10.1073/pnas.052705299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Untergasser G, Koch HB, Menssen A, Hermeking H. Characterization of epithelial senescence by serial analysis of gene expression: identification of genes potentially involved in prostate cancer. Cancer Res. 2002;62:6255–62. [PubMed] [Google Scholar]
- 19.Kulaeva OI, Draghici S, Tang L, Kraniak JM, Land SJ, et al. Epigenetic silencing of multiple interferon pathway genes after cellular immortalization. Oncogene. 2003;22:4118–27. doi: 10.1038/sj.onc.1206594. [DOI] [PubMed] [Google Scholar]
- 20.Fridman AL, Tainsky MA. Critical pathways in cellular senescence and immortalization revealed by gene expression profiling. Oncogene. 2008;27:5975–87. doi: 10.1038/onc.2008.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moiseeva O, Mallette FA, Mukhopadhyay UK, Moores A, Ferbeyre G. DNA damage signaling and p53-dependent senescence after prolonged β-interferon stimulation. Mol Biol Cell. 2006;17:1583–92. doi: 10.1091/mbc.E05-09-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnstone RW, Trapani JA. Transcription and growth regulatory functions of the HIN- 200 family of proteins. Mol Cell Biol. 1999;19:5833–38. doi: 10.1128/mcb.19.9.5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choubey D, Deka R, Ho SM. Interferon-inducible IFI16 protein in human cancers and autoimmune diseases. Front Biosci. 2008;13:598–608. doi: 10.2741/2705. [DOI] [PubMed] [Google Scholar]
- 24.Choubey D, Duan X, Dickerson E, Ponomareva L, Panchanathan R, et al. Interferon-inducible p200-family proteins as novel sensors of cytoplasmic DNA: role in inflammation and autoimmunity. J Interferon Cytkine Res. 2010;30:371–80. doi: 10.1089/jir.2009.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–18. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–13. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bürckstümmer T, Baumann C, Blüml S, Dixit E, Dürnberger G, et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10:266–72. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 28.Schroder K, Muruve DA, Tschopp J. Innate immunity: cytoplasmic DNA sensing by the AIM2 inflammasome. Curr Biol. 2009;19:R262–5. doi: 10.1016/j.cub.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 29.Rathinam VA, Jiang Z, Waggoner SN, Sharma S, Cole LE, et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11:395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernandes-Alnemri T, Yu JW, Juliana C, Solorzano L, Kang S, et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat Immunol. 2010;11:385–93. doi: 10.1038/ni.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones JW, Kayagaki N, Broz P, Henry T, Newton K, et al. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc Natl Acad Sci U S A. 2010;107:9771–6. doi: 10.1073/pnas.1003738107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panchanathan R, Duan X, Shen H, Rathinam VA, Erickson LD, et al. Aim2 Deficiency Stimulates the Expression of IFN-Inducible Ifi202, a Lupus Susceptibility Murine Gene within the Nba2 Autoimmune Susceptibility Locus. J Immunol. 2010;185:7385–93. doi: 10.4049/jimmunol.1002468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song LL, Alimirah F, Panchanathan R, Xin H, Choubey D. Expression of an IFN-inducible cellular senescence gene, IFI16, is up-regulated by p53. Mol Cancer Res. 2008;6:1732–41. doi: 10.1158/1541-7786.MCR-08-0208. [DOI] [PubMed] [Google Scholar]
- 34.Xin H, Curry J, Johnstone RW, Nickoloff BJ, et al. Role of IFI16, a member of the interferon-inducible p200-protein family, in prostate epithelial cellular senescence. Oncogene. 2003;22:4831–40. doi: 10.1038/sj.onc.1206754. [DOI] [PubMed] [Google Scholar]
- 35.Song LL, Ponomareva L, Shen H, Duan X, Alimirah F, et al. Interferon-inducible IFI16, a negative regulator of cell growth, down-regulates expression of human telomerase reverse transcriptase (hTERT) gene. PLoS One. 2010;5:e8569. doi: 10.1371/journal.pone.0008569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caposio P, Gugliesi F, Zannetti C, Sponza S, Mondini M, et al. A novel role of the interferon-inducible protein IFI16 as inducer of proinflammatory molecules in endothelial cells. J Biol Chem. 2007;282:33515–29. doi: 10.1074/jbc.M701846200. [DOI] [PubMed] [Google Scholar]
- 37.Baggetta R, De Andrea M, Gariano GR, Mondini M, Rittà M, et al. The interferon-inducible gene IFI16 secretome of endothelial cells drives the early steps of the inflammatory response. Eur J Immunol. 2010;40:2182–9. doi: 10.1002/eji.200939995. [DOI] [PubMed] [Google Scholar]
- 38.DeYoung KL, Ray ME, Su YA, Anzick SL, Johnstone RW, et al. Cloning a novel member of the human interferon-inducible gene family associated with control of tumorigenicity in a model of human melanoma. Oncogene. 1997;15:453–7. doi: 10.1038/sj.onc.1201206. [DOI] [PubMed] [Google Scholar]
- 39.Woerner SM, Kloor M, Schwitalle Y, Youmans H, Doeberitz MK, et al. The putative tumor suppressor AIM2 is frequently affected by different genetic alterations in microsatellite unstable colon cancers. Genes Chromosomes Cancer. 2007;46:1080–9. doi: 10.1002/gcc.20493. [DOI] [PubMed] [Google Scholar]
- 40.Cresswell KS, Clarke CJ, Jackson JT, Darcy PK, Trapani JA, et al. Biochemical and growth regulatory activities of the HIN-200 family member and putative tumor suppressor protein, AIM2. Biochem Biophys Res Commun. 2005;326:417–24. doi: 10.1016/j.bbrc.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 41.Hayward SW, Dahiya R, Cunha GR, Bartek J, Deshpande N, et al. Establishment and characterization of an immortalized but non-transformed human prostate epithelial cell line: BPH-1. In Vitro Cell Dev Biol Anim. 1995;31:14–24. doi: 10.1007/BF02631333. [DOI] [PubMed] [Google Scholar]
- 42.Choubey D, Lengyel P. Interferon action: cytoplasmic and nuclear localization of the interferon-inducible 52-kD protein that is encoded by the Ifi 200 gene from the gene 200 cluster. J Interferon Res. 1993;13:43–52. doi: 10.1089/jir.1993.13.43. [DOI] [PubMed] [Google Scholar]
- 43.Yazdi AS, Drexler SK, Tschopp J. The role of the inflammasome in nonmyeloid cells. J Clin Immunol. 2010;30:623–7. doi: 10.1007/s10875-010-9437-y. [DOI] [PubMed] [Google Scholar]
- 44.Cichowski K, Hahn WC. Unexpected pieces to the senescence puzzle. Cell. 2008;133:958–61. doi: 10.1016/j.cell.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodier F, Coppé JP, Patil CK, Hoeijmakers WA, Muñoz DP, et al. Persistent DNA damage signaling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–9. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Orjalo AV, Bhaumik D, Gengler BK, Scott GK, Campisi J. Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc Natl Acad Sci U S A. 2009;106:17031–6. doi: 10.1073/pnas.0905299106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen IF, Ou-Yang F, Hung JY, Liu JC, Wang H, et al. AIM2 suppresses human breast cancer cell proliferation in vitro and mammary tumor growth in a mouse model. Mol Cancer Ther. 2006;5:1–7. doi: 10.1158/1535-7163.MCT-05-0310. [DOI] [PubMed] [Google Scholar]
- 48.Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–36. doi: 10.1146/annurev.immunol.23.021704.115843. [DOI] [PubMed] [Google Scholar]
- 49.Thewissen M, Somers V, Venken K, Linsen L, van Paassen P, et al. Analyses of immunosenescent markers in patients with autoimmune disease. Clin Immunol. 2007;123:209–18. doi: 10.1016/j.clim.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 50.Goossens P, Gijbels MJ, Zernecke A, Eijgelaar W, Vergouwe MN, et al. Myeloid type I interferon signaling promotes atherosclerosis by stimulating macrophage recruitment to lesions. Cell Metab. 2010;12:142–53. doi: 10.1016/j.cmet.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 51.Goubau D, Rehwinkel J, Reis e Sousa C. PYHIN proteins: center stage in DNA sensing. Nat Immunol. 2010;11:984–6. doi: 10.1038/ni1110-984. [DOI] [PubMed] [Google Scholar]
- 52.Guarda G, Braun M, Staehli F, Tardivel A, Mattmann C, et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity. 2011;34:213–23. doi: 10.1016/j.immuni.2011.02.006. [DOI] [PubMed] [Google Scholar]