Abstract
Excessive free radical production due to various bacterial components released during bacterial infection has been linked to cell death and tissue injury. Peroxynitrite is a highly reactive oxidant produced by the combination of NO and superoxide anion, which has been implicated in cell death and tissue injury in various forms of critical illness. Pharmacological decomposition of peroxynitrite may represent a potential therapeutic approach in diseases associated with the overproduction of NO and superoxide. In the present study we tested the effect of the peroxynitrite decomposition catalyst in murine models of endotoxemia and sepsis. Mice were injected i.p. with LPS 40 mg/kg with or without FP15 (0.1, 0.3, 1, 3 or 10 mg/kg/h). Mice were sacrificed 12 hours later, followed by the harvesting of samples from the lung, liver and gut for MDA and MPO measurements. In other subsets of animals, blood samples were obtained by cardiac puncture at 1.5, 4, and 8 hours after LPS administration for cytokine (TNF-α, IL-1β and IL-10), nitrite/nitrate, alanine aminotransferase (ALT) and blood urea nitrogen (BUN) measurements. Endotoxemic animals showed an increase in survival from 25% to 80% at the FP15 doses of 0.3 and 1 mg/kg/h. The same dose of FP15 had no effect on plasma levels of nitrite/nitrate. There was a reduction in liver and lung MDA in the endotoxemic animals pre-treated with FP15, as well as in hepatic MPO and biochemical markers of liver and kidney damage (ALT and BUN). In a bacterial model of sepsis induced by CLP, FP15 treatment (0.3 mg/kg/day) significantly protected against mortality. The current data support the view that peroxynitrite is a critical factor mediating liver, gut and lung injury in endotoxemia and septic shock: its pharmacological neutralization may be of therapeutic benefit.
Introduction
Sepsis is the leading cause of death in intensive care units (1-3). Pathophysiological features of sepsis include the dysregulation of the immune response and excessive oxidant and free radical production due to various bacterial components released during bacterial infection. Superoxide, hydrogen peroxide, singlet oxygen, nitric oxide, peroxynitrite and many other oxygen- and nitrogen-derived reactive species have been shown to be generated; these species have been proposed to play both an anti-microbial role, as well as a deleterious role in damaging the host organism itself (4). Lipopolysaccharide (LPS), a cell wall component of gram-negative bacteria, is a highly potent activator of the innate immune system, which induces many features of septic shock via activation of the Toll-like receptor 4 (1-4). The interaction of LPS with lymphocytes and macrophages leads to the formation and release of a host of inflammatory mediators, which are critical to the adaptive antibacterial defense (1-6). In the initial process of bacterial infection, bacteria are rapidly eliminated by this aggressive inflammatory response (5-7).
Severe hypotension and multiple organ failure are frequent causes of death among patients who succumb to endotoxic shock (1-6). To date, inhibitors of the synthesis, release or actions of cytokines, eicosanoids, and NO have been implicated in the pathogenesis of circulatory shock in preclinical models; however, pharmacological approaches targeting the same pathways have not been shown to have significant benefit on mortality in randomized controlled clinical trials (1-10). Some of these discrepancies may be due to the fact that many mediators produced during circulatory shock possess both pro-inflammatory and anti-inflammatory properties; each playing important homeostatic functions in the complex host response to the septic challenge.
Peroxynitrite is a highly reactive oxidant produced spontaneously by the combination of NO and superoxide anion at rates approaching the diffusion limit (11,12). The formation of peroxynitrite in circulatory shock and sepsis has been demonstrated by several methods; the sources of NO for the generation of peroxynitrite include both the constitutive and inducible isoforms of NO synthase (11-14). Peroxynitrite is capable of oxidizing lipid membranes and sulfhydryl moieties, as well as hydroxylating and nitrating aromatics rings (11,12). Peroxynitrite formation has been proposed as a terminal event contributing to cell death and tissue injury in human sepsis (15,16). Peroxynitrite may be ultimately responsible for some of the pathophysiological responses in shock previously attributed to NO. On the other hand, NO may mediate numerous protective effects in cells and tissues and its inhibition can be more dangerous than beneficial (11,12). Thus, by interfering with the reactivity of peroxynitrite, one may selectively influence some of the deleterious events in the pathophysiology of various diseases that are associated with the overproduction of NO and superoxide.
We have previously reported that the water-soluble metalloporphyrin FP15 is an active peroxynitrite decomposition catalyst through peroxynitrite isomerization to nitrate (17). The compound increases the rate of peroxynitrite isomerization, reducing the formation of oxidizing radical species and generating the harmless nitrate anion. Beneficial effects of FP15, as well as other peroxynitrite decomposition catalysts, have been previously reported in a number of diseases associated with the overproduction of including ischemia-reperfusion, vascular injury, diabetes and other diseases (18). The aim of the current study was to characterize the in vivo effects of FP15 in a murine endotoxic shock induced by LPS and in a polymicrobial sepsis model induced by cecal ligature and puncture (CLP).
MATERIALS AND METHODS
All studies were performed in accordance with National Institutes of Health guidelines and with the approval of the local institutional animal care and use committee.
Effect of FP15 in the LPS model of circulatory shock
Eight weeks old Balb/c mice were used in this study. In order to induce circulatory shock, mice were injected i.p. with E. coli LPS at 40 mg/kg as previously described (19). FP15 treatment (0.1, 0.3, 1, 3 or 10 mg/kg/h or vehicle) was given in Alzet osmotic minipumps. For the implantation of the minipumps, mice were anesthetized with a mixture of ketamine (80 mg/kg) and xylazine (10 mg/kg), given i.p. Under aseptic conditions, a 1-cm midline laparotomy was performed to allow the introduction of osmotic minipump then the laparotomy was closed with 4.0 silk sutures. The animals were returned to their cages with free access to food and water. The effect of FP15 was studied on survival rate in n=20 mice in each group. An additional group of mice (n=20) received FP15 alone (10 mg/kg/h) without LPS treatment. The survival study utilized n=160 mice altogether. In a subsequent series of experiments, utilizing an identical experimental design, but only utilizing n=10 mice per group (n=80 mice in total), measurement of plasma levels of nitrite/nitrate, stable breakdown products of nitric oxide was performed at 12 hours after the administration of LPS. In an additional series of experiments, the most effective dose of FP15 on survival (0.3 mg/kg/h) was tested on a number of additional parameters. Animals were treated with vehicle (n=30 mice) or FP15 (0.3 mg/kg/h) (n=30 mice) and were sacrificed at baseline (0 h) and at 1.5, 4 and 8 hours for measurements of plasma cytokines, or at 12 hours for biochemical measurements (measurement of plasma markers of organ injury, or for harvesting of lung, liver and ileum for measurements of myeloperoxidase activity and malondialdehyde formation).
Measurement of plasma nitrate and nitrite
Plasma nitrate and nitrite were measured as an index of nitric oxide production, as previously described (19,20). First, nitrate in the plasma was reduced to nitrite by incubation with nitrate reductase (610 mU/ml) and NADPH (170 mM) at room temperature for 3h. After 3h, nitrite concentration in the samples was measured by the Griess reaction, by adding 100 μl of Griess reagent (0.1% naphthalethylenediamine dihydrochloride in H2O and 1% sulphanilamide in 5% concentrated H3PO4; vol. 1:1). The optical density at 550 nm (OD 550, corrected for absorbance at 650 nm) was measured in a Spectramax microplate reader. Nitrite concentrations were calculated by comparison of OD 550 of standard solutions of sodium nitrite prepared in phosphate buffered saline.
Malondialdehyde assay (MDA)
Malondialdehyde formation was utilized as previously described (19,20) to quantify the lipid peroxidation in tissues and measured as thiobarbituric acid-reactive material. Tissues were homogenized (100 mg/ml) in 1.15% potassium chloride buffer. 200 μl of the homogenates were then added to a reaction mixture consisting of 1.5 ml 0.8% thiobarbituric acid, 200 μl 8.1% sodium dodecyl sulfate, 1.5 ml 20% acetic acid (pH 3.5) and 600μl distilled water. The mixture was then heated at 90° C for 45 minutes. After cooling to room temperature, the samples were cleared by centrifugation (10000g, 10 minutes) and their absorbance measured at 532 nm, using 1,1,3,3-tetramethoxypropane as an external standard. The level of lipid peroxides was expressed as nmol MDA /mg protein (Bradford assay).
Myeloperoxidase assay (MPO)
Measurement of MPO, an enzyme contained in polymorphonuclear leukocytes was used to quantify the extent of neutrophil infiltration into various tissues as previously described (19,20). Tissues were homogenized (50 mg/ml) in 0.5 % hexadecyltrimethylammonium bromide in 10 mM 3-N-morpholinopropanesulfonic acid and centrifuged at 15,000g for 40 min. The suspension was then sonicated 3 times for 30 seconds. An aliquot of supernatant was mixed with a solution of 1.6 mM tetra-methyl-benzidine and 1mM hydrogen peroxide. Activity was measured spectrophotometrically as the change in absorbance at 650 nm at 37°C, using a Spectramax microplate reader (Molecular Devices, Sunnyvale, CA). Results are expressed as milliunits of MPO activity per mg protein, which were determined with the Bradford assay.
Measurement of plasma levels of alanine aminotransferase (ALT) and blood urea nitrogen (BUN)
Plasma concentrations of ALT and BUN were determined enzymatically using an automated VetScan chemistry analyzer (Abaxis, Union City, CA).
Measurement of plasma levels of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and interleukin-10 (IL-10)
Plasma concentration of immuno-reactive murine TNF-α, IL-1β, and IL-10 at various time points after LPS was determined by using commercially available enzyme-linked immuno-absorbent assays, according to manufacturer's protocol (R&D Systems, Minneapolis, MN).
Effect of FP15 in the CLP model of polymicrobial sepsis
Mice were anesthetized with a mixture of ketamine (80 mg/kg) and xylazine (10 mg/kg), given i.p. CLP was induced as previously described (20). Under aseptic conditions, a 2-cm midline laparotomy was performed to allow exposure of the cecum with adjoining intestine. The cecum was tightly ligated with a 3.0 silk suture at its base, below the ileo-cecal valve, and was perforated twice with an 18-gauge needle (top and bottom). Then the cecum was gently squeezed to extrude a small amount of feces from the perforation sites. The cecum was returned to the peritoneal cavity and an osmotic minipump (releasing either vehicle [n=25] of FP15 at 0.3 mg/kg/h [n=25] was placed in the peritoneal cavity. This dose of the peroxynitrite decomposition catalyst is an optimal dose selected from the results of the LPS shock studies. The laparotomy was closed with 4.0 silk sutures. The animals were returned to their cages with free access to food and water. Animals were monitored thereafter and survival rates were determined.
Reagents
All chemicals were purchased from Sigma Chemicals (St-Louis, MO), except when mentioned otherwise. Fe(III) tetrakis-2-(N-triethylene glycol monomethyl ether)pyridyl porphyrin (FP15) was synthesized as previously described (17).
Statistical analysis
Data are expressed as mean ± standard error of the mean (SEM) in all figures. For the biochemical measurements in tissues (MPO, MDA) and plasma studies, the means from groups were compared by ANOVA followed for Bonferroni test. In the survival experiments, the survival curves of the 2 groups were compared using the log-rank test. Statistical significance was assigned to p < 0.05.
RESULTS
The peroxynitrite decomposition catalyst FP15 decreases mortality rate and does not affect plasma nitrite/nitrate concentrations in a circulatory shock model induced by LPS
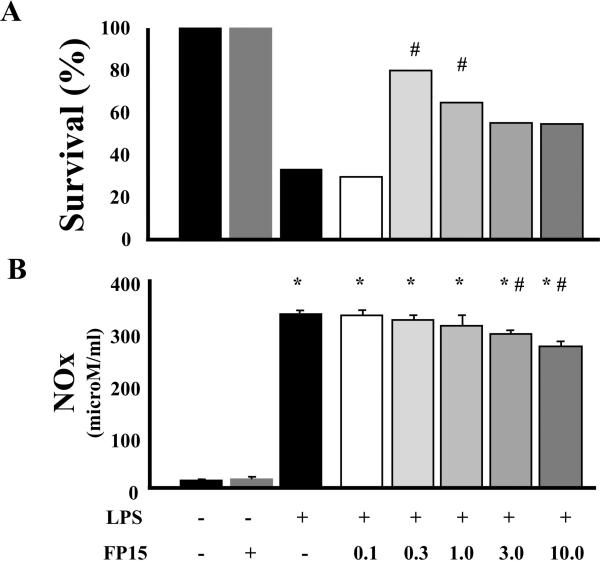
We have conducted a dose-response study with FP15, in order to define the most effective dose on the survival of the endotoxemic animals. Figure 1A shows that the lowest dose tested (0.1 mg/kg/h) was ineffective, while 0.3 mg/kg markedly improved survival (to approximately 80%, p<0.05). At doses higher than 0.3 mg/kg/h, the efficacy of FP15 gradually diminished; the survival benefit remained significant at 1 mg/kg/h, but not at the doses above.
Figure 1. Effect of FP15 on (A) survival and (B) circulating levels of nitrite/nitrate in endotoxemic mice.
(A): Endotoxemia was induced in mice with LPS (40 mg/kg i.p.) (n = 20 in each group). The animals were observed for 2 days, and mortality was recorded every 8 hours. FP15 was effective in reducing LPS-induced mortality at 0.3 and 1 mg/kg/h. #P < 0.05 indicates a significant improvement in mortality by FP15 treatment, as determined by Log-Rank test. (B): Using an identical experimental design, plasma levels of nitrite/nitrate were measured in a subgroup of mice, sacrificed 12 h after LPS administration. There was a marked increase in NO production after LPS. FP15 did not affect nitrite/nitrate plasma levels with the doses of 0.1 up to 1.0 mg/kg. The doses of 3 and 10 mg/kg/h produced a reduction in nitrite/nitrate plasma levels. Data shown represent mean ± SEM of n=10 animals per group. *P<0.05 indicates a significant increase in nitrite/nitrate levels when compared with baseline; #P<0.05 indicates a significant decrease in nitrite/nitrate levels after FP15 treatment. The FP15 alone (second bar from the left, in gray color) group represents the group of animals treated with the highest dose of FP15 used (10 mg/kg/h) alone, in the absence of LPS. We did not observe any adverse effects in this group (i.e. 100% survival) and this dose of FP15 not affect baseline nitrite/nitrate levels.
Next, we conducted experiments to test the effect of FP15 on breakdown products of circulating nitric oxide. Animals that received LPS exhibited a marked increase in nitric oxide breakdown product levels in the plasma (Figure 1B). In endotoxemic animals treated with FP15 there were no change in circulating levels of NO at the doses of 0.1, 0.3 and 1 mg/kg/h (Figure 1B). However, with the two highest doses of FP15 there was a slight, but statistically significant reduction in nitrite/nitrate concentrations. However, this decrease in circulating NO levels could not have accounted for the survival benefit of FP15, as the compound had no effect on nitrite/nitrate levels at the doses (0.3 and 1 mg/kg/h) where it improved survival.
The peroxynitrite decomposition catalyst FP15 reduces lipid peroxidation and neutrophil infiltration without affecting the production of inflammatory cytokines
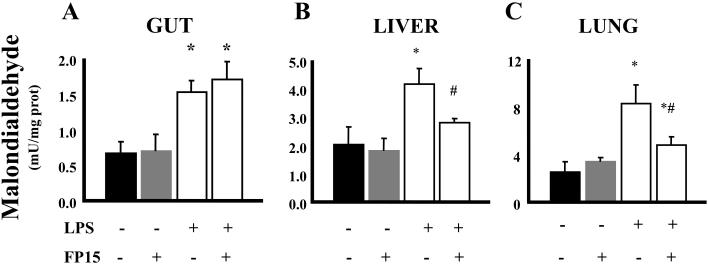
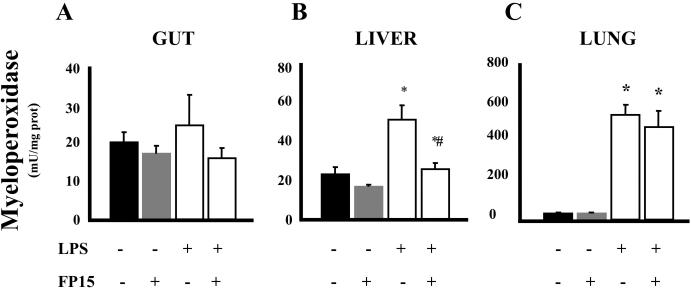
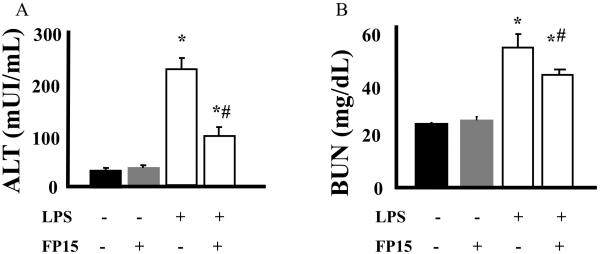
Endoxemia is associated with an increase of lipid peroxidation in various organs. In addition, there is a marked degree of neutrophil infiltration into various organs, which has been attributed to the subsequent organ damage. These features of endotoxin shock were all present in the lungs, livers and guts of mice exposed to LPS (Figures 2 and 3). Treatment with FP15 showed a marked reduction in MDA levels in liver and lung. On the other hand, no protective effect was found in the gut. The reduced amount of plasma ALT, a marker of liver injury after FP15 treatment is consistent with the reduced MDA and MPO levels in the same organ (Figure 4A). There was a tendency for reduction of MPO levels in the gut and lung of FP15-treated animals, but these changes were not statistically significant. Renal function was also evaluated by the measurement of blood urea nitrogen (BUN); a statistically significant protection was seen in FP15-treated group.
Figure 2. MDA in the gut, lung, and liver after LPS: effect of the peroxynitrite decomposition catalyst FP15.
Endotoxemia was induced in mice with LPS (40 mg/kg i.p). Samples from different organs (gut, liver and, lung) were harvested after 12 h. LPS induced a significant increase in MDA content in the tissues. FP15 treatment (0.3 mg/kg/h) reduced the tissue MDA levels in the liver and lung of LPS-treated animals. Data shown represent mean ± SEM of n=6 animals per group. *P<0.05 indicates a significant increase in tissue MDA levels when compared with baseline; #P<0.05 indicates a significant decrease in tissue MDA levels after FP15 treatment.
Figure 3. MPO activity in gut, lung, and liver after LPS: effect of the peroxynitrite decomposition catalyst FP15.
Endotoxemia was induced in mice with LPS (40 mg/kg i.p.). Samples from different organs (gut, liver and, lung) were harvested after 12 h. LPS induced a significant increase in MPO activity in all tissues. FP15 treatment (0.3 mg/kg/h) reduced MPO levels in the liver of LPS-treated animals. Data shown represent mean ± SEM of n=6 animals per group. *P<0.05 indicates a significant increase in tissue MPO levels when compared with baseline; #P<0.05 indicates a significant decrease in tissue MPO levels after FP15 treatment.
Figure 4. Biochemical markers of organ injury after LPS: effect of the peroxynitrite decomposition catalyst FP15.
Endotoxemia was induced in mice with LPS (40 mg/kg i.p.). Biochemical markers of liver injury (alanine aminotransferase, ALT) and renal damage (BUN, blood urea nitrogen) were determined after 12 h. LPS induced significant liver injury and renal damage. FP15 treatment (0.3 mg/kg/h) attenuated the LPS-induced increase in the various biochemical markers of organ injury. Data shown represent mean ± SEM of n=6 animals per group. *P<0.05 indicates a significant increase in ALT or BUN levels when compared with baseline; #P<0.05 indicates a significant decrease in ALT or BUN levels after FP15 treatment. UI=international units.
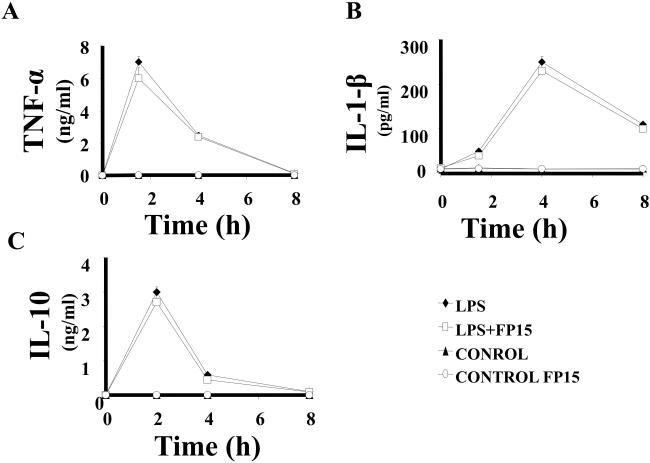
We have also measured the effect of FP15 on plasma levels of various pro-inflammatory cytokines as well as the anti-inflammatory cytokine IL-10 at various time points after the induction of endotoxemia. FP15 did not significantly affect TNF-α, IL-1β or IL-10 plasma levels (Figure 5).
Figure 5. Plasma levels of TNF-α, IL-1β, and IL-10 in after LPS: effect of the peroxynitrite decomposition catalyst FP15.
Endotoxemia was induced in mice with LPS (40 mg/kg i.p.). Plasma levels of selected pro- and anti-inflammatory cytokines were measured at 1.5, 3, 6 and 12 hours. Animals that received LPS showed a marked increase in cytokines levels. FP15 treatment (0.3 mg/kg/h) did not affect circulating levels of TNF-α, IL-1β or IL-10 at any of the time points. Data shown represent mean ± SEM of n=6 animals per group. Whenever error bars are not apparent on the figure, they are contained within the symbols. *P<0.05 indicates a significant increase in cytokine levels when compared with baseline.
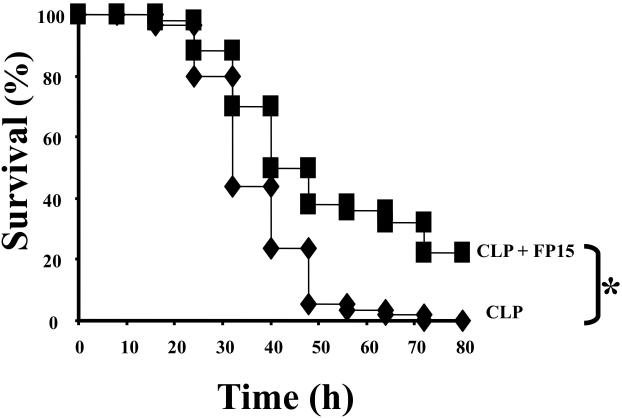
The peroxynitrite decomposition catalyst FP15 improves survival in polymicrobial sepsis
Using the most effective dose of FP15 on survival rate in the endotoxemia study, we have next evaluated the effect of FP15 in a polymicrobial model of sepsis. CLP resulted in a 100% mortality by the end of Day 3; FP15, at the dose of 0.3 mg/kg/h, provided a significant reduction in mortality (p<0.05) (Figure 6).
Figure 6. Effect of FP15 on survival of mice subjected to cecal ligation and puncture (CLP).
CLP was induced as described in the Materials and Methods section. Vehicle or FP15 (0.3 mg/kg/h) treatment was performed in the two groups of mice. Data represent percent survival at the indicated time points after CLP; n=25 animals per group. *P<0.05 represents a significant reduction in mortality in the FP15 treated group subjected to CLP compared to the vehicle-treated CLP group.
DISCUSSION
Septic patients develop progressive organ damage and dysfunction; the available therapeutic interventions are only supportive. Specific therapeutic interventions that can prevent or reverse organ damage are not available and are urgently needed (1-10). In this study, we used a metallporphyrinic compound, FP15, which has been synthesized and optimized for its redox properties to act as a selective peroxynitrite decomposition catalyst (17). The compound rapidly increases the rate of peroxynitrite isomerization, reducing the formation of oxidizing radical species to generate an inactive metabolite, nitrate anion (17). The results showed that animals treated with FP15 were protected from mortality and numerous hallmarks of end-organ injury (liver, kidney) in endotoxemic and in the septic rodent models of circulatory shock.
Nitric oxide and superoxide rapidly react to form the toxic reaction product, peroxynitrite anion (12-13). Pharmacological studies demonstrate that peroxynitrite is more cytotoxic than NO or superoxide in a variety of experimental systems and can induce both necrosis and apoptosis and can induce multiple downstream pathways of cell and organ injury (12-13, 16, 21). In the present study, first we analyzed the effect of different FP15 doses on survival rate. Interestingly one of the lowest doses of the compound tested was the most effective in terms of survival benefit, while at the highest doses tested, the statistically significant therapeutic benefit disappeared. At these highest doses, the compound exerted a slight inhibitory effect on plasma nitric oxide concentrations. Since NO (as opposed to peroxynitrite) has several anti-inflammatory and cytoprotective effects, it is conceivable that diminishment of tissue levels of free NO may have contributed to the loss of protective effect of FP15 at the highest doses tested. It is noteworthy that in previous studies, bell-shaped dose-responses have been reported in a number of studies with superoxide dismutase mimics and other antioxidants (22-27); considering the multitude of free radical actions, it is conceivable that only a partial, but not a complete neutralization of these reactive species is necessary or beneficial in various forms of critical illness.
The reaction of peroxynitrite with lipids leads to peroxidation (malondialdehyde and conjugated diene formation) and formation of nitrito-, nitro-, nitrosoperoxo- and/or nitrated lipid oxidation adducts (12-13). In our study the animals that received LPS presented a 2-3-fold increase in MDA levels in the lung, liver and gut. Treatment with FP15 showed a clear reduction in MDA content of liver and lung, but not the gut. It is conceivable that the lipid peroxidation in the gut of LPS-treated animals may be due to oxidant species other than peroxynitrite. It is also possible that differences in the tissue levels and pharmacokinetics of FP15 in different organs may have resulted in this organ-specific difference.
There are several lines of studies demonstrating the crucial role of peroxynitrite in the development of endothelial dysfunction in various models of cardiovascular disease and critical illness. Peroxynitrite has been suggested to contribute to the pathogenesis of organ failure in circulatory shock in many different ways (12,13): (a) it was suggested to exacerbate local vasospasm, may increase local neutrophil adhesion and migration into inflamed tissues; (b) it was suggested to exacerbate platelet activation and aggregation and (c) it was suggested to lead to hypo-perfusion of certain parts of various organs. It is generally accepted that endothelial injury is an early and significant trigger of neutrophil adhesion and tissue infiltration (28). We have, therefore, expected that FP15 will reduce the tissue levels of MPO in the current model. Surprisingly, only a trend was observed in most organs, and it was only in the liver that a partial, but statistically significant inhibition was seen. Although we do not have a clear explanation for the preferential protection by FP15 against liver injury, we must emphasize that in the liver, high levels of reactive oxidant species are produced in endotoxemia, as a result of the activation of multiple cell types (hepatocytes, Kupffer cells, stellate cells, endothelial cells as well as infiltrating leukocytes). We speculate that the hepatic protection by FP15 may involve two mechanisms: a direct protection by removing peroxynitrite and an indirect mechanism involving reduced neutrophil infiltration and consequent prevention of the release of cytotoxic mediators by these cells.
Even though there are many reports (mainly based on in vitro studies) suggesting that peroxynitrite plays a role in the up-regulation of pro-inflammatory signal transduction pathways (e.g. nuclear factor κB) (29,30), in the current study FP15 did not change plasma levels of pro-inflammatory cytokines and only exerted a slight inhibitory effect on NO levels at the highest doses tested. It is well known that cytokine-mediated inter-cellular signaling plays an important role in regulating immune defense; the fact that FP15 did not affect these cytokines distinguishes the mode of action of this compound from the mode of action of many previously studied experimental therapies and may, on one hand, actually represent an added benefit of the compound. On the other hand, the elevated pro-inflammatory cytokines may have induced an activation of endothelial cells and may have played a role in the attraction of neutrophils into the various organs studied. In summary, FP15 may have reduced peroxidation and the inflammatory amplification cycles governed by it; but at the same time the action of the pro-inflammatory cytokines that were still released may have masked some of its beneficial effects on neutrophil migration into lung and gut.
We must keep in mind that models such as the ones used in the current study represent an over-simplification of the disease state, which has many stages (pro-inflammatory, counter-regulating anti-inflammatory; immune activator; immune suppressive, hyperdynamic, hypodynamic, etc). It is conceivable that peroxynitrite plays a pathogenetic role in some of these stages, but not in others; it may well be that pharmacological modulation of peroxynitrite's action needs to be harmonized and adjusted to the stage of the disease. Further studies are needed to determine how the therapeutic efficacy of FP15 changes if the compound is given in a post-treatment design (i.e. at various time points after the administration of LPS or the initiation of CLP). Although in the current model, FP15 has provided clear benefits, it is conceivable that neutralization of peroxynitrite, in a clinical scenario, may not be sufficient to provide therapeutic benefit, unless it is combined with interventions that target other, parallel pathways of the disease.
Renal dysfunction is another common feature of circulatory shock. Hypoxia and re-oxygenation produce a marked increase in cellular generation of reactive oxidant species and triggered a significant degree of lactate dehydrogenase release. Various antioxidants have been shown to be effective in reducing the renal dysfunction and injury associated with ischemia/reperfusion of the kidney in various models of endotoxemia, shock and multiple organ injury (31-33). In line with these observations, in the current study, FP15 was markedly effective in protect kidney function in our model; based on the current and previous data, protection of the kidney by peroxynitrite neutralization may represent an important therapeutic possibility in various forms of critical illness.
It has been established by many laboratories that the mechanism by which LPS induces the pro-inflammatory response in endotoxin shock involves the activate the nuclear factor-κB/Rel family of transcription factors, enabling the expression of several critical genes involved in the pathogenesis of septic shock: TNF-α, interleukins (IL-1β, IL-2, IL-6 and IL-8), adhesion molecules, cyclooxygenase-2 and inducible NO synthase (4,5). Although the importance of these mediators in animal models of endotoxemia is unquestionable, their blockade fails to provide benefit most bacterial models of sepsis, and yielded disappointing results in clinical trials as well (1-10). The typical interpretation of these findings is that these mediators exert detrimental effects when extreme levels are present; on the other hand, complete inhibition of these cytokines in sepsis may be detrimental, because an appropriate level of cytokines are necessary for fighting the invading bacteria. The adequate balance between pro-inflammatory and anti-inflammatory cytokines in sepsis may be important to reduce self-damage, while maintaining anti-bacterial defense responses. The improvement in survival rate both in the LPS and the CLP models of circulatory shock is consistent with the hypothesis that (1) direct inhibition of end-organ injury by interfering with the action of terminal oxidant mediators of injury (in our case, peroxynitrite) can be beneficial in both endotoxemic and septic models of circulatory shock, and that (2) significant therapeutic benefits can be achieved with appropriate pharmacological therapeutic approaches even if they do not affect the cytokine response.
Starting with some landmark observations in rats by Cuzzocrea and co-workers in 1999, using the first-generation peroxynitrite neutralizing agent 5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrinato iron III chloride (FeTTPs) (33), activation of peroxynitrite is increasingly recognized as a common pathway of tissue damage in conditions associated with the formation of oxidant species and free radicals in various forms of critical illness. These early studies - demonstrating that FeTPPs was able to reduce mortality, plasma levels of ALT, AST, bilirubin, creatinine, amylase, lipase and alkaline phosphatase and improved histological less signs of liver damage and nitrotyrosine formation in the liver -, were subsequently confirmed and extended by several other groups in various rodent and large animal models of critical illness (18,34-37) using the later-generation peroxynitrite decomposition catalysts, such as FP15 (used in the current study, as well as in a number of other studies, e.g. 17, 35,39-44), as well as additional groups of peroxynitrite catalysts, including the compound WW-85 (36-38). A novel aspect of the current study is the demonstration of the efficacy of a peroxynitrite decomposition catalyst in a polymicrobial sepsis model (which may be a better approximation of the clinical sepsis than the LPS models). An additional novel aspect of the current study is the demonstration that the protection may be bell-shaped. A further novel aspect of the study is the demonstration that in circulatory shock, peroxynitrite does not appear to upregulate pro-inflammatory cytokines or iNOS, suggesting that all of the protection seen in this model is downstream from these processes.
Limitations of the current study include that comparison between pre- and post-treatment with FP15 was not performed, and the fact that tyrosine nitration, a marker of peroxynitrite formation was not measured. Nevertheless, many other studies, using comparable doses of FP15, or the other peroxynitrite catalysts have already demonstrated this effect (17,35,39-44). An additional limitation of the current study is that it exclusively focused on biochemical and survival endpoints, and did not follow hemodynamic parameters or indices of tissue oxygenation. Generally, however, these indices can be better measured in large animal models: in fact, it was recently demonstrated that peroxynitrite decomposition catalysts improve a number of hemodynamic and functional parameters in various large animal models of critical illness (36,37).
Taken together, the results of the present study, are consistent with an existing body of literature suggesting that peroxynitrite is a significant effector of sepsis mediating organ injury and death. Pharmacological blockade of peroxynitrite, therefore, continues to emerge as a potentially valuable tool in the experimental therapy of septic shock and other forms of critical illness.
Acknowledgements
The work of C.S. is supported by a grant from the National Institutes of Health (R01GM060915) and by a grant from the Shriners Burns Hospitals (#8661).
Abbreviations
- ALT
alanine aminotransferase
- BUN
blood urea nitrogen
- CLP
cecal ligation and puncture
- FeTTPS
5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrinato iron III chloride
- IL-1β
interleukin 1β
- IL-10
interleukin 10
- LPS
bacterial lipopolysaccharide
- NO
nitric oxide
- MDA
malon dialdehyde
- MPO
myeloperoxidase
- TNF-α
tumor necrosis factor α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Winters BD, Eberlein M, Leung J, Needham DM, Pronovost PJ, Sevransky JE. Long-term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38:1276–83. doi: 10.1097/CCM.0b013e3181d8cc1d. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 3.Texereau J, Pene F, Chiche JD, Rousseau C, Mira JP. Importance of hemostatic gene polymorphisms for susceptibility to and outcome of severe sepsis. Crit Care Med. 2004;32(5 Suppl):S313–9. doi: 10.1097/01.ccm.0000126363.46191.dc. [DOI] [PubMed] [Google Scholar]
- 4.Cuzzocrea S, Riley DP, Caputi AP, Salvemini D. Antioxidant therapy: a new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol Rev. 53:135–59. [PubMed] [Google Scholar]
- 5.Wittebole X, Castanares-Zapatero D, Laterre PF. Toll-like receptor 4 modulation as a strategy to treat sepsis. Mediators Inflamm. 2010;2010:568396. doi: 10.1155/2010/568396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RA. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–8. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 7.Freeman BD, Natanson C. Anti-inflammatory therapies in sepsis and septic shock. Expert Opin Investig Drugs. 2000;9:1651–63. doi: 10.1517/13543784.9.7.1651. [DOI] [PubMed] [Google Scholar]
- 8.Vincent JL, Sun Q, Dubois MJ. Clinical trials of immunomodulatory therapies in severe sepsis and septic shock. Clin Infect Dis. 2001;34:1084–93. doi: 10.1086/339549. [DOI] [PubMed] [Google Scholar]
- 9.Ulloa L, Brunner M, Ramos L, Deitch EA. Scientific and clinical challenges in sepsis. Curr Pharm Des. 2009;15:1918–35. doi: 10.2174/138161209788453248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marshall JC, Dellinger RP, Levy M. The Surviving Sepsis Campaign: a history and a perspective. Surg Infect (Larchmt) 2010;11:275–81. doi: 10.1089/sur.2010.024. [DOI] [PubMed] [Google Scholar]
- 11.Szabo C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 12.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szabo C, Salzman AL, Ischiropoulos H. Peroxynitrite-mediated oxidation of dihydrorhodamine 123 occurs in early stages of endotoxic and hemorrhagic shock and ischemia-reperfusion injury. FEBS Lett. 1995;372:229–232. doi: 10.1016/0014-5793(95)00984-h. [DOI] [PubMed] [Google Scholar]
- 14.Lange M, Connelly R, Traber DL, Hamahata A, Nakano Y, Esechie A, Jonkam C, von Borzyskowski S, Traber LD, Schmalstieg FC, Herndon DN, Enkhbaatar P. Time course of nitric oxide synthases, nitrosative stress, and poly(ADP ribosylation) in an ovine sepsis model. Crit Care. 2010;14:R129. doi: 10.1186/cc9097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soriano FG, Nogueira AC, Caldini EG, Lins MH, Teixeira AC, Cappi SB, Lotufo PA, Bernik MM, Zsengellér Z, Chen M, Szabó C. Potential role of poly(adenosine 5'-diphosphate-ribose) polymerase activation in the pathogenesis of myocardial contractile dysfunction associated with human septic shock. Crit Care Med. 2006;34:1073–1079. doi: 10.1097/01.CCM.0000206470.47721.8D. [DOI] [PubMed] [Google Scholar]
- 16.Pacher P, Szabo C. Role of the peroxynitrite-poly(ADP-ribose) polymerase pathway in human disease. Am J Pathol. 2008;173:2–13. doi: 10.2353/ajpath.2008.080019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szabó C, Mabley JG, Moeller SM, Shimanovich R, Pacher P, Virag L, Soriano FG, Van Duzer JH, Williams W, Salzman AL, Groves J. Pathogenetic role of peroxynitrite in the development of diabetes and diabetic vascular complications: studies with FP15, a novel potent peroxynitrite decomposition catalyst. Mol Med. 2001;8:571–80. [PMC free article] [PubMed] [Google Scholar]
- 18.Szabó C, Módis K. Pathophysiological roles of peroxynitrite in circulatory shock. Shock. 2010;34(Suppl 1):4–14. doi: 10.1097/SHK.0b013e3181e7e9ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jagtap P, Soriano FG, Virág L, Liaudet L, Mabley J, Szabó E, Haskó G, Marton A, Lorigados CB, Gallyas F, Jr, Sümegi B, Hoyt DG, Baloglu E, VanDuzer J, Salzman AL, Southan GJ, Szabó C. Novel phenanthridinone inhibitors of poly (adenosine 5'-diphosphate-ribose) synthetase: potent cytoprotective and antishock agents. Crit Care Med. 2002;30:1071–82. doi: 10.1097/00003246-200205000-00019. [DOI] [PubMed] [Google Scholar]
- 20.Soriano FG, Liaudet L, Szabó E, Virág L, Mabley JG, Pacher P, Szabó C. Resistance to acute septic peritonitis in poly(ADP-ribose) polymerase-1-deficient mice. Shock. 2002;17:286–92. doi: 10.1097/00024382-200204000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Jagtap P, Szabó C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov. 2005;4:421–40. doi: 10.1038/nrd1718. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka K, Ishihara T, Azuma A, Kudoh S, Ebina M, Nukiwa T, Sugiyama Y, Tasaka Y, Namba T, Ishihara T, Sato K, Mizushima Y, Mizushima T. Therapeutic effect of lecithinized superoxide dismutase on bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2010;298:L348–60. doi: 10.1152/ajplung.00289.2009. [DOI] [PubMed] [Google Scholar]
- 23.McCord JM. Superoxide dismutase, lipid peroxidation, and bell-shaped dose response curves. Dose Response. 2008;6:223–38. doi: 10.2203/dose-response.08-012.McCord. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishihara T, Tanaka K, Tasaka Y, Namba T, Suzuki J, Ishihara T, Okamoto S, Hibi T, Takenaga M, Igarashi R, Sato K, Mizushima Y, Mizushima T. Therapeutic effect of lecithinized superoxide dismutase against colitis. J Pharmacol Exp Ther. 2009;328:152–64. doi: 10.1124/jpet.108.144451. [DOI] [PubMed] [Google Scholar]
- 25.Nelson SK, Bose SK, McCord JM. The toxicity of high-dose superoxide dismutase suggests that superoxide can both initiate and terminate lipid peroxidation in the reperfused heart. Free Radic Biol Med. 1994;16:195–200. doi: 10.1016/0891-5849(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 26.Galiñanes M, Ferrari R, Qiu Y, Cargnoni A, Ezrin A, Hearse DJ. PEG-SOD and myocardial antioxidant status during ischaemia and reperfusion: dose-response studies in the isolated blood perfused rabbit heart. J Mol Cell Cardiol. 1992;24:1021–30. doi: 10.1016/0022-2828(92)91868-6. [DOI] [PubMed] [Google Scholar]
- 27.Omar BA, McCord JM. The cardioprotective effect of Mn-superoxide dismutase is lost at high doses in the postischemic isolated rabbit heart. Free Radic Biol Med. 1990;9:473–8. doi: 10.1016/0891-5849(90)90124-2. [DOI] [PubMed] [Google Scholar]
- 28.Lefer AM, Lefer DJ. Pharmacology of the endothelium in ischemia-reperfusion and circulatory shock. Annu Rev Pharmacol Toxicol. 1993;33:71–90. doi: 10.1146/annurev.pa.33.040193.000443. [DOI] [PubMed] [Google Scholar]
- 29.Levrand S, Pesse B, Feihl F, Waeber B, Pacher P, Rolli J, Schaller MD, Liaudet L. Peroxynitrite is a potent inhibitor of NF-κB activation triggered by inflammatory stimuli in cardiac and endothelial cell lines. J Biol Chem. 2005;280:34878–87. doi: 10.1074/jbc.M501977200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loukili N, Rosenblatt-Velin N, Rolli J, Levrand S, Feihl F, Waeber B, Pacher P, Liaudet L. Oxidants positively or negatively regulate nuclear factor kappaB in a context-dependent manner. J Biol Chem. 2010;285:15746–52. doi: 10.1074/jbc.M110.103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zacharowski K, Olbrich A, Cuzzocrea S, Foster SJ, Thiemermann C. Membrane-permeable radical scavenger, tempol, reduces multiple organ injury in a rodent model of gram-positive shock. Crit Care Med. 2000;28:953–61. doi: 10.1097/00003246-200006000-00044. [DOI] [PubMed] [Google Scholar]
- 32.Chatterjee PK, Cuzzocrea S, Brown PA, Zacharowski K, Stewart KN, Mota-Filipe H, Thiemermann C. Tempol, a membrane-permeable radical scavenger, reduces oxidant stress-mediated renal dysfunction and injury in the rat. Kidney Int. 2000;58:658–673. doi: 10.1046/j.1523-1755.2000.00212.x. [DOI] [PubMed] [Google Scholar]
- 33.Salvemini D, Riley DP, Lennon PJ, Wang ZQ, Currie MG, Macarthur H, Misko TP. Protective effects of a superoxide dismutase mimetic and peroxynitrite decomposition catalysts in endotoxin-induced intestinal damage. Br J Pharmacol. 1999;127:685–92. doi: 10.1038/sj.bjp.0702604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cuzzocrea S, Mazzon E, Di Paola R, Esposito E, Macarthur H, Matuschak GM, Salvemini D. A role for nitric oxide-mediated peroxynitrite formation in a model of endotoxin-induced shock. J Pharmacol Exp Ther. 2006;319:73–81. doi: 10.1124/jpet.106.108100. [DOI] [PubMed] [Google Scholar]
- 35.Naidu BV, Fraga C, Salzman AL, Szabo C, Verrier ED, Mulligan MS. Critical role of reactive nitrogen species in lung ischemia-reperfusion injury. J Heart Lung Transplant. 2003;22:784–793. doi: 10.1016/s1053-2498(02)00556-9. [DOI] [PubMed] [Google Scholar]
- 36.Maybauer DM, Maybauer MO, Szabó C, Westphal M, Traber LD, Enkhbaatar P, Murthy KG, Nakano Y, Salzman AL, Herndon DN, Traber D. Lung-protective effects of the metalloporphyrinic peroxynitrite decomposition catalyst WW-85 in interleukin-2 induced toxicity. Biochem Biophys Res Commun. 2008;377:786–791. doi: 10.1016/j.bbrc.2008.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maybauer DM, Maybauer MO, Szabó C, Cox RA, Westphal M, Kiss L, Horvath EM, Traber LD, Hawkins HK, Salzman AL, Southan GJ, Herndon DN, Traber DL. The peroxynitrite catalyst WW-85 improves pulmonary function in ovine septic shock. Shock. 2010 Jun 22; doi: 10.1097/SHK.0b013e3181eb4556. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Genovese T, Mazzon E, Esposito E, Di Paola R, Murthy K, Neville L, Bramanti P, Cuzzocrea S. Effects of a metalloporphyrinic peroxynitrite decomposition catalyst, ww-85, in a mouse model of spinal cord injury. Free Radic Res. 2009;43:631–45. doi: 10.1080/10715760902954126. [DOI] [PubMed] [Google Scholar]
- 39.Mabley JG, Liaudet L, Pacher P, Southan GJ, Groves JT, Salzman AL, Szabó C. Beneficial effects of the peroxynitrite decomposition catalyst FP15 in murine models of arthritis and colitis. Mol Med. 2002;8:581–90. [PMC free article] [PubMed] [Google Scholar]
- 40.Bianchi C, Wakiyama H, Faro R, Khan T, McCully JD, Levitsky S, Szabó C, Sellke FW. A novel peroxynitrite decomposer catalyst (FP-15) reduces myocardial infarct size in an in vivo peroxynitrite decomposer and acute ischemia-reperfusion in pigs. Ann Thorac Surg. 2002;74:1201–7. doi: 10.1016/s0003-4975(02)03953-x. [DOI] [PubMed] [Google Scholar]
- 41.Pacher P, Liaudet L, Bai P, Mabley JG, Kaminski PM, Virág L, Deb A, Szabó E, Ungvári Z, Wolin MS, Groves JT, Szabó C. Potent metalloporphyrin peroxynitrite decomposition catalyst protects against the development of doxorubicin-induced cardiac dysfunction. Circulation. 2003;107:896–904. doi: 10.1161/01.cir.0000048192.52098.dd. [DOI] [PubMed] [Google Scholar]
- 42.Tauskela JS, Brunette E, Hewitt M, Mealing G, Morley P. Competing approaches to excitotoxic neuroprotection by inert and catalytic antioxidant porphyrins. Neurosci Lett. 2006;401:236–41. doi: 10.1016/j.neulet.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 43.Obrosova IG, Drel VR, Oltman CL, Mashtalir N, Tibrewala J, Groves JT, Yorek MA. Role of nitrosative stress in early neuropathy and vascular dysfunction in streptozotocin-diabetic rats. Am J Physiol Endocrinol Metab. 2007;293:E1645–55. doi: 10.1152/ajpendo.00479.2007. [DOI] [PubMed] [Google Scholar]
- 44.Radovits T, Seres L, Gero D, Lin LN, Beller CJ, Chen SH, Zotkina J, Berger I, Groves JT, Szabó C, Szabó G. The peroxynitrite decomposition catalyst FP15 improves ageing-associated cardiac and vascular dysfunction. Mech Ageing Dev. 2007;128:173–81. doi: 10.1016/j.mad.2006.09.005. [DOI] [PubMed] [Google Scholar]