Abstract
Disturbed cortical γ-aminobutyric acid (GABA) neurotransmission in schizophrenia is evident from lamina- and cell type- specific alterations in presynaptic markers. In the dorsolateral prefrontal cortex (DLPFC), these alterations include lower transcript expression of glutamic acid decarboxylase (GAD67) and somatostatin (SST), a neuropeptide expressed in the Martinotti subpopulation of GABA neurons whose axons innervate the distal apical dendrites of pyramidal neurons. However, whether the alterations in SST-containing interneurons are associated with changes in post-synaptic receptors for SST has not been examined. Thus, we used in situ hybridization to quantify the mRNA expression levels of SST receptors subtype 1 (SSTR1) and subtype 2 (SSTR2) in DLPFC area 9 from 23 matched pairs of subjects with schizophrenia and normal comparison subjects. We also assessed the effects of potential confounding variables within the human subjects and in brain specimens from macaque monkeys with long term exposure to antipsychotic drugs. SSTR1 mRNA levels did not differ between subject groups. In contrast, mean cortical SSTR2 mRNA levels were significantly 19% lower in the subjects with schizophrenia. Laminar and cellular level analyses revealed that lower SSTR2 mRNA levels were localized to pyramidal cells in cortical layers 5-6. Expression of SSTR2 mRNA did not differ between monkeys exposed chronically to high doses of haloperidol or olanzapine and control animals, or between subjects with schizophrenia on or off antipsychotic medications at the time of death. However, levels of SSTR2 mRNA were significantly 37.6% lower in monkeys exposed chronically to low dose haloperidol, suggesting that the lower levels of SSTR2 mRNA selectively in pyramidal neurons in DLPFC layers 5-6 in schizophrenia should be interpreted with caution. In concert with prior findings of lower SST mRNA expression in the same subjects, the results of this study suggest the convergence of pre- and post-synaptic mechanisms to reduce inhibitory inputs to pyramidal neurons in the infragranular layers of the DLPFC.
Keywords: GABA, interneurons, inhibition, dendrite, prefrontal cortex, postmortem
1. Introduction
Dysfunction of the dorsolateral prefrontal cortex (DLPFC) in schizophrenia is associated with abnormalities in cortical γ-aminobutyric acid (GABA) neurotransmission. These abnormalities include lower levels of the mRNA for the key enzyme regulating GABA synthesis, the 67 kDa isoform of glutamic acid decarboxylase (GAD67). Several lines of evidence suggest that these alterations are not present in the ~50% of GABA neurons that express calretinin (Hashimoto et al, 2008; Sakai et al, 2008; Woo et al., 1998), but are prominent in a subset (~25-35%) of interneurons that express either the calcium-binding protein parvalbumin (Hashimoto et al, 2008) or the neuropeptide somatostatin (Morris et al, 2008). For example, a reduction in the expression of SST mRNA in DLPFC, initially described by microarray analysis (Hashimoto et al, 2008), has been confirmed by real-time qPCR and in situ hybridization (Hashimoto et al., 2008; Morris et al, 2008). However, whether the alterations in presynaptic markers of inhibitory neurotransmission in SST-containing interneurons are associated with alterations in post-synaptic receptors for SST has not been yet examined.
The effects of SST are mediated by five known G-protein coupled receptor subtypes (SSTR1-5) (Moller et al., 2003); stimulation of these receptors appears to have an inhibitory effect on neuronal excitability (Baraban and Tallent, 2004; Vezzani and Hoyer, 1999). SSTR1 and SSTR2 subtypes are the most abundant subtypes in the cerebral cortex (Videau et al, 2003), and although both are present in pyramidal and nonpyramidal neurons (Hervieu and Emson, 1998a, b; Schindler et al, 1997), these receptor subtypes appear exhibit a high degree of specialization with regard to their cellular expression (Kumar, 2005) and subcellular targeting (Schulz et al., 2000). For example, SSTR2 immunolabeling is present mainly in pyramidal neurons, suggesting a major postsynaptic role in these neurons, whereas SSTR1 shows comparable expression in both pyramidal cells and interneurons (Kumar, 2005). In addition, SSTR1 immunoreactivity is found on varicose axons, suggesting a presynaptic role for this receptor (Schulz et al, 2000).
Consequently, in this study we used in situ hybridization to examine the lamina-and cell-specific expression levels of SSTR1 and SSTR2 transcripts in the DLPFC from schizophrenia and matched normal comparison subjects in which we had previously measured SST mRNA expression using an identical approach (Morris et al., 2008).
2. Material and methods
2.1 Human subjects
Brain specimens from 46 subjects were obtained after consent from the next-of-kin during autopsies conducted at the Allegheny County Medical Examiner's Office (Pittsburgh, PA). All procedures were approved by the University of Pittsburgh's Committee for the Oversight of Research Involving the Dead and Institutional Review Board for Biomedical Research.
In order to control for experimental variance and to reduce biological variance between groups, each subject in the schizophrenia group (n = 23), which included 8 subjects diagnosed with schizoaffective disorder, was matched for sex and, as closely as possible, for age, with one normal comparison subject (for demographic details, see Table 1). Subject groups did not differ in mean age, postmortem interval (PMI), RNA integrity number (RIN), brain pH, or tissue storage time at −80 °C (Table 1).
TABLE 1.
| Parameter | Comparison | Schizophrenia* |
|---|---|---|
| Sex | 17 M, 6 F | 17 M, 6 F |
| Race | 18 W, 5 B | 15 W, 8 B |
| Age (years) | 48.0 (15.5) | 47.9 (14.1) |
| PMI (hours) | 18.0 (5.5) | 17.8 (9.3) |
| Brain pH | 6.9 (0.2) | 6.8 (0.3) |
| RIN | 8.7 (0.4) | 8.4 (0.7) |
| Storage time (months at −80 °C) |
113.6 (23.5) | 117.8 (23.5) |
Note: Abbreviations: PMI, postmortem interval; RIN, RNA integrity number.
Values are mean (± SD).
This group includes 8 individuals with diagnosed with schizoaffective disorder.
2.2. Tissue preparation
For each brain specimen, coronal blocks from the right frontal cortex were frozen and stored at −80°C. Serial cryostat sections (20 μm) containing the superior frontal gyrus were thaw-mounted onto glass slides and stored at −80°C until processed. Adjacent sections were collected into tubes containing Trizol (Invitrogen Corp, Carlsbad, CA) in order to obtain RNA for RIN measures using the Agilent Bioanalyzer 2100 (Agilent Technologies, Inc., Santa Clara, CA) according to the manufacturer's protocol as previously described (Hashimoto et al., 2008). The location of DLPFC area 9 was identified by cytoarchitectonic criteria in Nissl-stained sections as previously described (Glantz et al., 2000; Volk et al, 2001; Volk et al., 2000b). Three sets of adjacent sections per subject, separated at rostro-caudal intervals of approximately 300 μm, were matched within subject pairs and used to assess SSTR1 and SSTR2 mRNA expression.
2.3. In situ hybridization
Templates for the synthesis of the antisense and sense riboprobes for human SSTR1 and SSTR2 mRNAs were first generated by polymerase chain reaction (PCR). The specific primers amplified 484 and 247 base pair fragments of human SSTR1 and SSTR2, respectively. These fragments corresponded to bases 886-1370 of the human SSTR1 (GenBank NM_001049) and 1018-1264 of the human SSTR2 (GenBank NM_001050) genes. Nucleotide sequencing confirmed 100% homology of the amplified fragment to the previously reported sequences. The fragments were then subcloned into a plasmid (pSTBlue-1, Novagen, Madison, WI). The antisense and sense riboprobes were transcribed in the presence of 35S-CTP (Amersham Biosciences, Piscataway, NJ) using T7 and SP6 RNA polymerase, respectively. DNase I was used to digest the DNA template. The riboprobes were purified using RNeasy mini-spin columns (Qiagen, Valencia, CA).
One section from each subject pair was processed side-by-side in three separate runs for each gene of interest. Prior to the hybridization reaction, tissue sections were fixed with 4% paraformaldehyde in PBS solution, acetylated with 0.25% acetic anhydrate in 0.1 M triethanolamine/0.9% NaCl for 10 minutes, dehydrated with a graded alcohol series, and then defatted in chloroform for 10 minutes. The sections were then hybridized with 35S-labeled riboprobes (1.0 × 10 cpm/μl) in hybridization buffer at 56°C for 16 hours. The hybridization buffer contained 50% formamide, 0.75 M NaCl, 20 mM 1,4-piperazine diethane sulfonic acid, pH 6.8, 10 mM EDTA, 10% dextran sulfate, 5X Denhardt's solution (0.2 mg/ml Ficoll, 0.2 mg/ml polyvinylpyrrolidone, 0.2 mg/ml BSA), 50 mM dithiothreitol, 0.2% SDS, and 100 μg/ml yeast tRNA. Following the hybridization reaction, sections were washed in a solution of 0.3 M NaCl, 20 mM Tris-HCl, pH 8.0, 1 mM EDTA, pH 8.0, and 50% formamide at 63°C, treated with RNase A (20 μg/ml) at 37°C, washed in 0.1 X SSC (1.5 mM NaCl, 150 μM sodium citrate) at 67°C, dehydrated through a graded ethanol series, and air dried. Sections from both subjects in a pair were exposed on the same BioMax MR film (Kodak, Rochester, NY) for 7 days, and then coated with undiluted NTB2 emulsion (Kodak) for SSTR2 and NTB2 emulsion diluted 2:1 with water for SSTR1. Utilizing DLPFC sections from normal comparison subjects, different emulsion exposure times were systematically evaluated in order to achieve an optimal signal to noise ratio. The emulsion was exposed for 77 and 71 days at a constant temperature of 4°C for SSTR1 and SSTR2, respectively. The slides were developed with D-19 (Kodak) and counterstained with Cresyl-violet.
2.4. Quantification of mRNA expression levels
Each section was randomly coded, so that subject number and diagnosis were unknown to a single rater. Trans-illuminated autoradiographic film images were captured by a video camera under precisely controlled conditions, digitized, and quantified using a Microcomputer Imaging Device (MCID) system (Imaging Research Inc., London, ON, Canada). Quantification was performed without knowledge of subject diagnosis by random coding of the sections. Images of Nissl-stained sections were also captured and superimposed onto the autoradiographic images to draw contours of the full thickness of the cortex exclusively in the zones where the cortex was cut perpendicular to the pial surface. Optical density (OD) was measured within the contours and expressed as nCi/g of tissue by reference to radioactive 14C standards (ARC Inc., St Louis, MO, USA) exposed on the same autoradiographic film. The mean (SD) total area of gray matter sampled in each subject was 360 (178) and 344 (117) mm2 for SSTR1, and 358 (185) mm2 and 304 (91) mm2 for SSTR2, in comparison subjects and subjects with schizophrenia, respectively.
2.5. Laminar analysis of mRNA expression
SSTR1 and SSTR2 mRNA expression as a function of cortical layer was determined in a series of cortical traverses (1-2 mm in width) extending from the pial surface to the white matter. Three cortical traverses were sampled for each section (9 traverses per subject). Each traverse was divided into 50 equal bins parallel to the pial surface and the OD was determined for each bin. These bins were then combined into six zones that approximated the laminar boundaries in the DLPFC based on previous studies (Akbarian et al, 1995; Pierri et al, 1999). These zones (i.e., bins 1–5, 6–10, 11–25, 26–30, 31–40, and 41–50) corresponded to cortical layers 1-6, respectively. The mean OD was calculated for each zone. Background measures in each section were sampled from layer 1, where no specific expression of mRNA for either receptor subtype was observed. All sampled areas for both total gray and laminar analyses were corrected by subtracting the corresponding background measure from the same slide.
2.6. Cellular analysis of mRNA expression
Evaluation of mRNA expression at the cellular level was performed for SSTR2 transcript in a subset of 13 subject pairs where the total cortical expression level was decreased by >15% in the subject with schizophrenia relative to the matched comparison subject. Silver grain accumulation on emulsion-dipped, Nissl-counterstained sections was conducted as previously described (Beneyto and Meador-Woodruff, 2006, 2008; Hashimoto et al, 2003; Morris et al, 2008). Briefly, using the MCID imaging software and a Zeiss microscope with a motorized stage, four 1 mm-wide cortical traverses extending from the pial surface to the white matter were placed on each tissue section in locations where area 9 was cut perpendicular to the cortical surface. In each of the cortical traverses, four sampling frames (120 × 170 μm) were systemically and randomly placed in deep layer 5-superficial layer 6 (defined as 70%-90% of the distance from the pial surface to the white matter border), corresponding to the laminar distribution of the major change in mRNA expression observed for SSTR2. The edges of the frames were equidistant from the border of each traverse and the edge of the next sampling frame.
Because RNase-A treatment during the in situ hybridization procedure degrades Nissl-stainable substances within the cytoplasm, it is not possible to draw contours around neuronal soma. Thus, the number of grains/cell was counted in each frame by placing circles over nuclei of cells in a bright field image. As previously described, grain clusters confined within a 22 μm diameter circle were considered to be interneurons and those within a 30 μm diameter circle were considered to be pyramidal cells (Benes et al, 1986; Beneyto and Meador-Woodruff, 2006, 2008; Hashimoto et al., 2003; Morris et al, 2008; Rajkowska et al, 1998). In the corresponding dark-field image, the software determined the number of grains in each circle. Background grain density was measured in each slide by counting the grains in a sampling frame placed over layer 1. The smaller size and intense Nissl staining of glial nuclei distinguished them from the larger, more faintly stained neuronal nuclei. A total number of 2201 large cells (1151 from comparison subjects vs. 1050 from schizophrenia subjects) and 1105 small cells (571 from comparison subjects vs. 534 from schizophrenia subjects) were sampled. For both subject groups, frequency histograms of the number of grains from all sampled neurons, normalized by the background for each slide, showed unimodal and normal distributions for both large and small cells in both subject groups.
2.7. Antipsychotic-exposed monkeys
Two sets of experimentally naive, macaque monkeys (Macaca facsicularis) were used to assess the possible effects of antipsychotic medications on SSTR2 mRNA expression. In the first set, male animals 4 – 6 years of age were exposed to twice daily oral doses of haloperidol, olanzapine or placebo (n = 6 monkeys per group), for 17–27 months (Dorph-Petersen et al, 2005). Stable trough drug plasma levels were within the range associated with clinical efficacy in humans (~1.5 ng/ml for haloperidol and ~15 ng/ml for olanzapine) (Dorph-Petersen et al, 2005). In the second set, 4 other pairs of young adult male macaque monkeys matched for age and weight were chronically exposed to haloperidol decanoate administered every 4 weeks (trough plasma level, 4.3 ± 1.1 ng/mL) and benztropine mesylate (1 mg twice daily) to treat extrapyramidal symptoms, or placebo for 9-12 months, as previously described (Pierri et al, 1999). Animals within each triad or pair were euthanized on the same day. Brains were rapidly removed, and the right frontal lobe was cut into coronal blocks, frozen in isopentane on dry ice and stored at −80°C. Serial coronal sections (16 μm) were cut from the slabs containing the anterior one-third of the principal sulcus and mounted on glass slides. Two sections evenly spaced at 224 μm were hybridized with an antisense RNA probe against human SSTR2 mRNA as described above in the human study. The optical density for SSTR2 mRNA was measured in the gray matter of the DLPFC bordered by the cingulate and principal sulci and corrected by subtracting the layer 1 density measures. Density values of two sections were averaged before statistical analysis. All studies were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
2.8. Statistical analyses
Analyses were performed on SPSS (SPSS Inc., Chicago, IL). The data were averaged across the three sections for each subject before statistical analysis. Two analyses of covariance (ANCOVA) models were used to test differences in SSTR1 and SSTR2 mRNA expression between control subjects and subjects with schizophrenia. The first ANCOVA model used diagnostic group as the main effect, pair as a blocking effect, and storage time and RIN as covariates. The pair effect reflects the matching of individual subject pairs for sex, age, and PMI. RIN was included as a covariate because it reflects mRNA integrity (Stan et al, 2006). Subject pairing may be considered an attempt to balance the two diagnostic groups with regard to the experimental factors instead of a true statistical paired design. Thus, to validate the first model, a second ANCOVA model was performed with a main effect of diagnostic group and covariates of sex, age, PMI, RIN, and storage time. Storage time as a covariate was not significant in either model and was excluded in the reported analyses. Both models produced comparable results for diagnostic group effect; however, because the effect of age on SSTR1 and SSTR2 mRNA expression was significant, the results of the second model are reported.
For the laminar analysis, multiple comparisons were controlled by adjusting for simultaneous inference of significance levels using the Bonferroni-Holm method (Volk et al., 2000a) in which p values are ordered from the smallest (i=1) to the largest (i=N) among multiple comparisons; the significance level for each comparison is defined as α=0.05/([N+1]-i).
The potential influence of sex, diagnosis of schizoaffective disorder, history of substance abuse/dependence, medications at time of death, or death by suicide on the within-pair percentage differences in mRNA expression was assessed by two-sample t-test analyses. Correlations were assessed by Pearson's correlation analyses.
For the antipsychotic-treated monkey data analysis, the effects of drug exposure on mRNA expression levels were evaluated by paired t-test analysis for the olanzapine exposure and by analysis of covariance (ANCOVA), with route of administration as covariate, for the exposure to haloperidol.
3. Results
3.1. Specificity of SSTR1 and SSTR2 hybridization signal
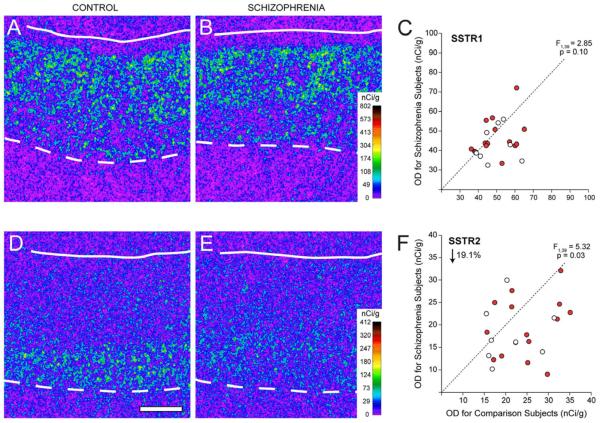
Several lines of evidence confirmed the specificity of the riboprobes for SSTR1 and SSTR2 mRNAs used in this study. First, the laminar distribution of the SSTR1 signal matched that previously reported for SSTR1 mRNA in the human frontal cortex (Thoss et al, 1996). Specifically, the density of SSTR1 mRNA was high in the superficial and middle layers and lower in the deep layers (Figs. 1A, B and 2A, B). Similarly, SSTR2 mRNA was primarily expressed in layers 5-6 (Figs. 1D, E and 2C, D), similar to prior reports for SSTR2 mRNA in human frontal cortex and for SSTR2 immuno-positive cell bodies in human temporal cortex (Schindler et al, 1997; Thoss et al., 1996). Second, emulsion-coated sections demonstrated silver grain clusters over large, faintly Nissl-stained neuronal nuclei, whereas the smaller and more intensely stained glial nuclei lacked silver grains (Fig. 3A). Third, sense riboprobes for SSTR1 and SSTR2 mRNA showed an absence of signal above background (data not shown).
Figure 1.
Representative autoradiograms illustrating expression of SSTR1 (A,B) and SSTR2 (D,E) in DLPFC area 9 of a comparison subject (A,D) and a matched subject with schizophrenia (B,E). The density of hybridization signal for each transcript is represented in pseudocolor according to the calibration scale (nCi/g) from the 14C standards. Solid and broken lines denote the pial surface and the gray matter-white matter border, respectively. Scale bar (1 mm) applies to all panels. Comparison of film autoradiogram optical density (OD) measures for SSTR1 (C) and SSTR2 (F) in total gray matter of DLPFC in matched pairs of normal comparison subjects and subjects with schizophrenia (red circles) or schizoaffective disorder (open circles). Circles below the dashed unity line indicate pairs for which the subject with schizophrenia or schizoaffective disorder had a lower mean expression level than the matched comparison subject.
Figure 2.
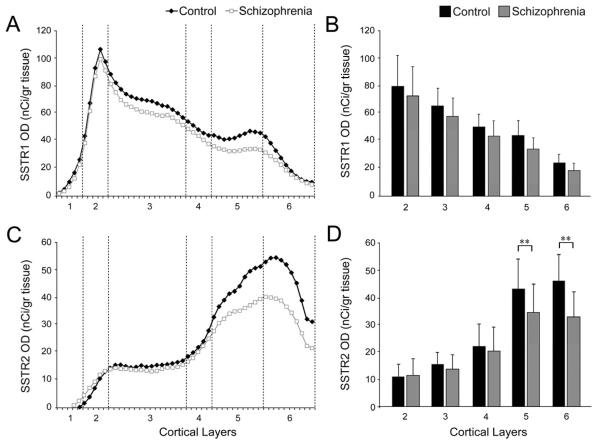
Laminar expression of the mRNAs for SSTR1(A,B) and SSTR2(C,D). Left panels: Mean mRNA OD across cortical layers from the pial surface to the white matter border in schizophrenia (gray) and comparison groups (black). Right panels: Mean (SD) film OD for mRNA expression in each cortical layer between comparison and schizophrenia groups. **P<.005.
Figure 3.
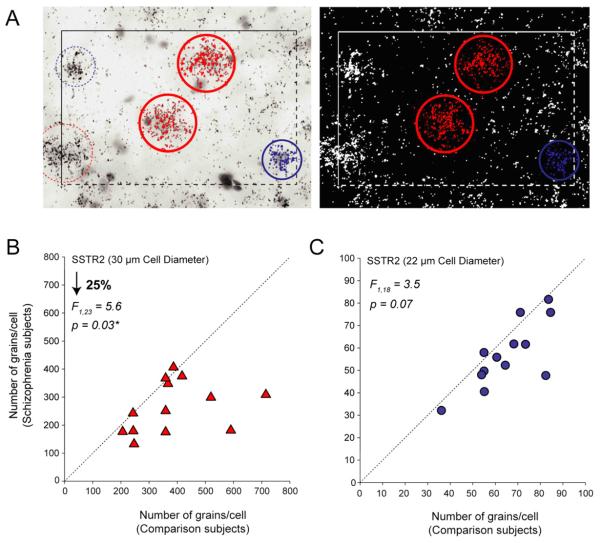
Cellular level analysis of the difference in SSTR2 mRNA expression in deep layers of the DLPFC. A: Photomicrographs illustrating expression of SSTR2 mRNA expression in Nissl-stained emulsion-dipped sections of DLPFC. The left panel shows the bright-field image in which the cell nuclei were selected, and the right shows the corresponding dark-field image were the grains were quantified. Comparison of number of grains per cell measures for SSTR2 in large (triangles)(B) and small cells (circles)(C) in matched pairs of normal comparison subjects and subjects with schizophrenia, showed significant reduction only in grains per large (putative pyramidal) cell. Markers below the dashed unity line indicate pairs for which the subject with schizophrenia or schizoaffective disorder had a lower mean expression level than the matched comparison subject.
3.2. Expression of SSTR1 and SSTR2 in DLPFC
Total gray matter expression levels of SSTR1 mRNA were not significantly (F1,39 = 2.85; p = 0.10) different between subjects with schizophrenia (45.4 ± 9.2 nCi/g) and matched comparison subjects (49.6 ± 8.9 nCi/g) (Fig. 1C). In contrast, mean (±SD) SSTR2 mRNA levels were significantly (F1,39 = 5.32; p = 0.03) 19.1% lower the subjects with schizophrenia (19.0 ± 6.4 nCi/g) relative to comparison subjects (23.5 ± 6.4 nCi/g) (Fig. 1F).
Laminar analyses confirmed the absence of differences in SSTR1 mRNA expression between subject groups (Fig. 2A, B) and demonstrated that the difference in SSTR2 mRNA expression was due to lower transcript levels in layers 5 (20.4%; F1,39 = 7.33, p = 0.01) and layer 6 (28.7%; F1,39 = 22.75, p < 0.0001) of the subjects with schizophrenia (Fig. 2C, D).
3.3. Cellular mRNA expression levels of SSTR2
Although SSTR2 protein is mainly present in pyramidal cells, it is also expressed in nonpyramidal cells in human neocortex (Kumar, 2005); therefore, we sought to determine if SSTR2 expression was preferentially lower in putative pyramidal cells or interneurons in layers deep 5 and superficial 6 in the subjects with schizophrenia. Quantification of silver grains revealed that the number of grains per putative pyramidal neuron (i.e., 30 μm diameter sampling circle) was significantly (F1,23 = 5.6, P = 0.03) 25% lower in the subjects with schizophrenia (246.6 ± 102.6) than in the comparison subjects (331.6 ± 157.9) (Fig. 3A, B). In contrast, the mean number of grains per putative interneuron (i.e., 22 μm diameter sampling circle) in the subjects with schizophrenia (46.51 ± 17.5) was only 6 % lower relative to comparison subjects (49.5 ± 18.8), a difference that showed a only trend level of significance (F1,18 = 3.5, P = 0.07) (Fig. 3A, C).
3.4. Analysis of potential confounding factors
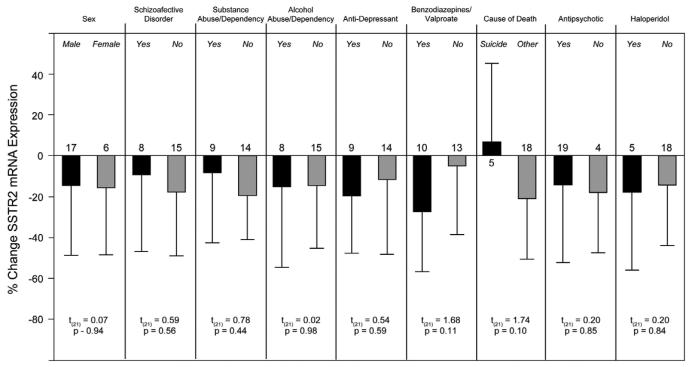
In the second ANCOVA model, age was a significant (F1,39 = 4.72; p < 0.036) determinant of SSTR2 mRNA expression. Further analyses demonstrated that OD measures were negatively correlated with age in comparison subjects (r = −0.42; p < 0.044), but showed only a trend level in subjects with schizophrenia (r = −0.39; p = 0.067). The within-subject pair percent differences in SSTR2 mRNA expression did not differ as a function of sex, diagnosis of schizoaffective disorder, diagnosis of substance or alcohol abuse at time of death (ATOD), use of antidepressant medication ATOD, use of benzodiazepine/valproate ATOD, death by suicide, or antipsychotic medication use ATOD (all t(21) < 1.74, all p > 0.10) (Fig. 4).
Figure 4.
Effects of potential confounding factors on the expression differences in SSTR2 mRNA in subjects with schizophrenia. The bars represent the mean (SD) percent differences from control subjects for SSTR2 mRNA within subject pairs; numbers above each bar indicate the number of subject pairs. Sex, diagnosis of schizoaffective disorder, diagnosis of substance or alcohol abuse or dependency at time of death (ATOD), use of antidepressant medications ATOD, use of benzodiazepines/valproate ATOD, death by suicide, use of antipsychotic medication ATOD, or use of haloperidol ATOD did not significantly affect the expression changes in SSTR2 mRNA.
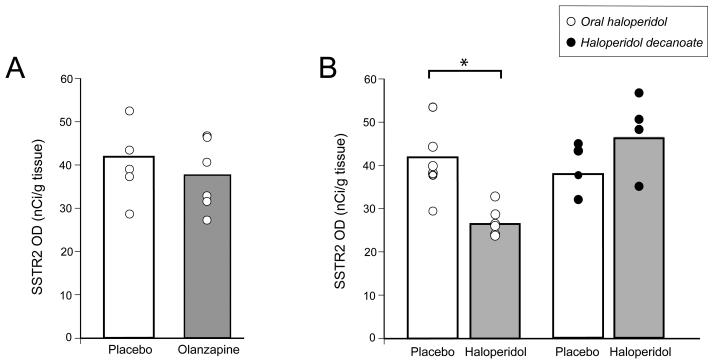
To further evaluate the potential effect of long-term exposure to typical and atypical antipsychotic medications, we examined the expression of SSTR2 mRNA in the DLPFC of monkeys chronically exposed to haloperidol (oral and decanoate) vs. placebo or olanzapine vs. placebo (Fig. 5). The laminar distribution of SSTR2 mRNA in all groups matched the pattern observed in the human DLPFC. Expression of SSTR2 mRNA in the total grey matter did not differ between monkeys chronically exposed to olanzapine or placebo (t5 = 1.49, p = 0.19) (Fig. 5A). Likewise, mean (±SD) SSTR2 mRNA expression did not differ (F1,7 = 2.3; p = 0.18) between all haloperidol-exposed monkeys (38.8 ± 5.7 nCi/g) and their sex-, age-, and weight-matched controls (46.8 ± 9.0 nCi/g); however, break down of the haloperidol group by oral vs. haloperidol decanoate administration revealed a significant (F1,12 = 15.6; p = 0.003) 37.6% lower levels of SSTR2 mRNA levels in the oral haloperidol animals (26.4 ± 3.3 nCi/g) relative to placebo-exposed animals (42.3 ± 9.3 nCi/g) (Fig. 5B). Analysis of the effect of haloperidol or any typical antipsychotic medication use in the subjects with schizophrenia revealed no within-subject pair differences in SSTR2 mRNA expression (Fig. 4). Furthermore, when we removed all subject pairs in which the subject with schizophrenia was taking a typical antipsychotic medication ATOD, a significant reduction (F1,15 = 4.81; p = 0.04) of 19.9% in SSTR2 mRNA expression was maintained in the subjects with schizophrenia relative to comparison subjects.
Figure 5.
The effect of chronic exposure to antipsychotic medications on the expression of SSTR2 mRNA in the monkey PFC. Graphs showing the mean (±SD) expression levels of SSTR2 mRNA in olanzapine (A) and oral haloperidol (open circles) or haloperidol decanoate (black circles) (B) treated monkeys (grey bars) relative to the corresponding group of placebo-exposed animals (open bars). *F1,12 = 15.6; p = 0.003.
4. Discussion
Our results suggest that SST neurotransmission in the DLPFC of subjects with schizophrenia might be altered at the postsynaptic level in a receptor subtype-, layer-and cell type-specific manner. That is, in individuals with schizophrenia or schizoaffective disorder the expression of SSTR2, but not SSTR1, mRNA is preferentially lower in layers 5-6, and in larger, putative pyramidal neurons in those layers. These findings do not appear to be attributable to a number of potential confounds, although not all of our findings exclude the possible influence of antipsychotic medications. Specifically, although SSTR2 levels were similarly lower in subjects with schizophrenia who were on or off antipsychotic medication at time of death, and were not lower in monkeys exposed chronically to olanzapine or to haloperidol decanoate, SSTR2 mRNA levels were significantly reduced in monkeys exposed to oral haloperidol. The apparent difference in the effects of oral haloperidol versus haloperidol decanoate is surprising, as the latter was associated with higher trough serum levels and marked extrapyramidal symptoms that required anticholinergic treatment. Previous studies also reported that long-term haloperidol exposure in rats did not produce changes in SST receptor binding levels in the frontal cortex after one week of withdrawal (Perez-Oso et al., 1990). Although the lower levels of SSTR2 mRNA associated with oral haloperidol exposure in monkeys observed in our study suggest caution in interpreting the findings in schizophrenia subjects, the bulk of the existing data suggest that haloperidol exposure does not alter SSTR2 mRNA expression.
Our results on the laminar and cellular expression of SSTR2 transcripts in pyramidal neurons in layers 5-6 are consistent with previous reports of the distribution of SSTR2 immuno-labeled somata in the deep layers of the human neocortex (Kumar, 2005; Schindler et al, 1997; Thoss et al, 1996) and with previous reports in rodents (Schindler et al, 1997) and humans (Kumar, 2005) that SSTR2 is primarily concentrated in the ascending dendrites of deep layer pyramidal neurons. In addition, in the rat and monkey neocortex SST-containing interneurons predominately innervate the dendritic shafts of pyramidal neurons (de Lima and Morrison, 1989; Hendry et al., 1984; Kawaguchi and Kubota, 1996; Melchitzky and Lewis, 2008), suggesting that SSTR2 is primarily expressed on the apical dendritic shaft of pyramidal neurons in layers 5 and 6, postsynaptic to inputs from SST-containing inhibitory interneurons.
Consistent with this idea, the within-pair percentage differences in SSTR2 mRNA expression were significantly correlated (r = 0.77, p < 0.0001) with those found for SST mRNA expression in the same subjects, suggesting that the down regulation of SSTR2 might be related to alterations in the SST interneuron population (Morris et al., 2008). In contrast, within subject pair differences in SSTR1 and SST mRNA levels were not correlated (r = 0.17, p = 0.43).
Based on existing data, lower levels of SSTR2 in schizophrenia might have the following effects. At the cellular level, SST has inhibitory effects on neurons by direct inhibition of excitatory neurotransmission (Boehm and Betz, 1997; Tallent and Siggins, 1997), or by augmentation of two distinct K+ current, the voltage-sensitive M-current (IM) (Moore et al., 1988) and a voltage-insensitive leak current (Schweitzer et al, 1998). A recent investigation of the coupling to IM of different SSTR subtypes in receptor knockout mice and by subtype-selective ligands demonstrated that SST mediated increase in IM is intact in SSTR2 knock-outs, indicating SSTR2 does not couple to this channel (Qiu et al., 2008). Although the mechanism through which SSTR2 acts is unknown, the SSTR2-selective ligands, seglitide and octreotide, mediate inhibition of excitatory postsynaptic currents (EPSCs) by SST in rat hippocampal neurons,(Boehm and Betz, 1997). Furthermore, data on SST action in dentate granule cells (Baratta et al., 2002) suggests that SSTR2 activation contributes to the modulation of dendritic depolarization during seizure events by inhibiting Ca2+ channels (Traub and Wong, 1983; Vezzani and Hoyer, 1999). Consequently, and independently of the mechanism secondary to the activation of the receptor, the reduced SSTR2 expression in schizophrenia suggests decreased inhibition of EPSCs in the pyramidal cells of DLPFC layers 5-6, representing another source of altered DLPFC inhibitory neurotransmission (Lewis et al, 2005).
Recent studies have identified the presence of GABAA receptors containing α5 subunits on pyramidal cell apical dendrites postsynaptic to GABA inputs from SST-containing Martinotti cells (Ali and Thomson, 2008). Interestingly, GABAA α5 mRNA expression was also found to be lower in the deep layers of DLPFC of the same schizophrenia subjects used in this study (Beneyto et al, 2010). These findings suggest that schizophrenia is associated with a correlated alteration of GABA and SST inhibitory neurotransmission to the apical dendrites of pyramidal neurons whose cell bodies are present in the deep layers of the DLPFC. Consistent with this interpretation, we detected positive correlations between the within-subject expression of SSTR2 (present study) and GABAA α5 subunit levels (Beneyto et al, 2010) (r=0.305, P=0.039) and between their within-pair percentage differences (r=0.415, P=0.04) in DLPFC layers 5-6.
The synaptic inhibition provided by GABA inputs from the affected SST cells in schizophrenia contributes to the synchronization of pyramidal cell firing at specific frequencies. For example, the GABA inputs from SST-containing Martinotti cells mediated by the slower kinetics of α5 containing GABAa receptors (Ali and Thomson, 2008; Zarnowska et al, 2009) appear to modulate theta oscillations (4-7 Hz) (Fanselow et al., 2008). Interestingly, gamma oscillations, known to be altered in schizophrenia (Cho et al, 2006; Gonzalez-Burgos and Lewis, 2008), are thought to be embedded in theta frequency oscillations in the context of certain cognitive tasks, such as working memory (Lisman and Idiart, 1995), raising the possibility that the working memory disturbances in schizophrenia reflect the behavioral outcome of convergent disturbances in different GABA circuits in the DLPFC.
In conclusion, our findings suggest that lower SST mRNA expression in schizophrenia is accompanied by a selective reduction of SSTR2 mRNA expression levels in pyramidal neurons of deep layers in the DLPFC. Together, these findings suggest converging pre- and post-synaptic mechanisms to reduce inhibitory neurotransmission to pyramidal neurons in the DLPFC, which could result in alterations in the synchronization of low frequency oscillations and consequent disturbances in working memory performance in subjects with schizophrenia.
Acknowledgments
The authors thank Mary Brady, BS for her assistance in editing the graphics, Holly Bazmi, MS, for her assistance in the in situ hybridization procedures, and Jim Kosakowski for developing the Matlab program for laminar analysis. We thank the members of the Clinical Services and Diagnostics Core of the Conte Center for the Neuroscience of Mental Disorders (MH084053) for their assistance in diagnostic assessments.
Funding
This work was supported by the National Institutes of Health grants MH043784 and MH084053.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure
David A. Lewis currently receives investigator-initiated research support from the BMS Foundation, Bristol-Myers Squibb, Curridium Ltd and Pfizer and in 2007-2009 served as a consultant in the areas of target identification and validation and new compound development to AstraZeneca, BioLine RX, Bristol-Myers Squibb, Hoffman-Roche, Lilly, Merck, Neurogen and SK Life Science.
REFERENCES
- Akbarian S, Huntsman MM, Kim JJ, Tafazzoli A, Potkin SG, Bunney WE, Jr., Jones EG. GABAA receptor subunit gene expression in human prefrontal cortex: comparison of schizophrenics and controls. Cereb Cortex. 1995;5:550–560. doi: 10.1093/cercor/5.6.550. [DOI] [PubMed] [Google Scholar]
- Ali AB, Thomson AM. Synaptic alpha 5 subunit-containing GABAA receptors mediate IPSPs elicited by dendrite-preferring cells in rat neocortex. Cereb Cortex. 2008;18:1260–1271. doi: 10.1093/cercor/bhm160. [DOI] [PubMed] [Google Scholar]
- Baraban SC, Tallent MK. Interneuron Diversity series: Interneuronal neuropeptides--endogenous regulators of neuronal excitability. Trends Neurosci. 2004;27:135–142. doi: 10.1016/j.tins.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Baratta MV, Lamp T, Tallent MK. Somatostatin depresses long-term potentiation and Ca2+ signaling in mouse dentate gyrus. J Neurophysiol. 2002;88:3078–3086. doi: 10.1152/jn.00398.2002. [DOI] [PubMed] [Google Scholar]
- Benes FM, Davidson J, Bird ED. Quantitative cytoarchitectural studies of the cerebral cortex of schizophrenics. Arch Gen Psychiatry. 1986;43:31–35. doi: 10.1001/archpsyc.1986.01800010033004. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Meador-Woodruff JH. Lamina-specific abnormalities of AMPA receptor trafficking and signaling molecule transcripts in the prefrontal cortex in schizophrenia. Synapse. 2006;60:585–598. doi: 10.1002/syn.20329. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Meador-Woodruff JH. Lamina-specific abnormalities of NMDA receptor-associated postsynaptic protein transcripts in the prefrontal cortex in schizophrenia and bipolar disorder. Neuropsychopharmacology. 2008;33:2175–2186. doi: 10.1038/sj.npp.1301604. [DOI] [PubMed] [Google Scholar]
- Beneyto M, Abbott A, Hashimoto T, Lewis DA. Lamina-specific alterations in cortical GABAa receptor subunit expression in schizophrenia. Cerebral Cortex. 2010 doi: 10.1093/cercor/bhq169. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm S, Betz H. Somatostatin inhibits excitatory transmission at rat hippocampal synapses via presynaptic receptors. J Neurosci. 1997;17:4066–4075. doi: 10.1523/JNEUROSCI.17-11-04066.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci U S A. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lima AD, Morrison JH. Ultrastructural analysis of somatostatin-immunoreactive neurons and synapses in the temporal and occipital cortex of the macaque monkey. J Comp Neurol. 1989;283:212–227. doi: 10.1002/cne.902830205. [DOI] [PubMed] [Google Scholar]
- Dorph-Petersen KA, Pierri JN, Perel JM, Sun Z, Sampson AR, Lewis DA. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology. 2005;30:1649–1661. doi: 10.1038/sj.npp.1300710. [DOI] [PubMed] [Google Scholar]
- Fanselow EE, Richardson KA, Connors BW. Selective, state-dependent activation of somatostatin-expressing inhibitory interneurons in mouse neocortex. J Neurophysiol. 2008;100:2640–2652. doi: 10.1152/jn.90691.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz LA, Austin MC, Lewis DA. Normal cellular levels of synaptophysin mRNA expression in the prefrontal cortex of subjects with schizophrenia. Biol Psychiatry. 2000;48:389–397. doi: 10.1016/s0006-3223(00)00923-9. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull. 2008;34:944–961. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Arion D, Unger T, Maldonado-Aviles JG, Morris HM, Volk DW, Mirnics K, Lewis DA. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Volk DW, Eggan SM, Mrnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry SH, Jones EG, Emson PC. Morphology, distribution, and synaptic relations of somatostatin- and neuropeptide Y-immunoreactive neurons in rat and monkey neocortex. J Neurosci. 1984;4:2497–2517. doi: 10.1523/JNEUROSCI.04-10-02497.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervieu G, Emson PC. The localization of somatostatin receptor 1 (sst1) immunoreactivity in the rat brain using an N-terminal specific antibody. Neuroscience. 1998a;85:1263–1284. doi: 10.1016/s0306-4522(98)00024-4. [DOI] [PubMed] [Google Scholar]
- Hervieu G, Emson PC. Visualisation of non-glycosylated somatostatin receptor two (ngsst2) immunoreactivity in the rat central nervous system. Brain Res Mol Brain Res. 1998b;58:138–155. doi: 10.1016/s0169-328x(98)00120-x. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. Physiological and morphological identification of somatostatin- or vasoactive intestinal polypeptide-containing cells among GABAergic cell subtypes in rat frontal cortex. J Neurosci. 1996;16:2701–2715. doi: 10.1523/JNEUROSCI.16-08-02701.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar U. Expression of somatostatin receptor subtypes (SSTR1-5) in Alzheimer's disease brain: an immunohistochemical analysis. Neuroscience. 2005;134:525–538. doi: 10.1016/j.neuroscience.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Idiart MA. Storage of 7 +/− 2 short-term memories in oscillatory subcycles. Science. 1995;267:1512–1515. doi: 10.1126/science.7878473. [DOI] [PubMed] [Google Scholar]
- Melchitzky DS, Lewis DA. Dendritic-targeting GABA neurons in monkey prefrontal cortex: comparison of somatostatin- and calretinin-immunoreactive axon terminals. Synapse. 2008;62:456–465. doi: 10.1002/syn.20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller LN, Stidsen CE, Hartmann B, Holst JJ. Somatostatin receptors. Biochim Biophys Acta. 2003;1616:1–84. doi: 10.1016/s0005-2736(03)00235-9. [DOI] [PubMed] [Google Scholar]
- Moore SD, Madamba SG, Joels M, Siggins GR. Somatostatin augments the M-current in hippocampal neurons. Science. 1988;239:278–280. doi: 10.1126/science.2892268. [DOI] [PubMed] [Google Scholar]
- Morris HM, Hashimoto T, Lewis DA. Alterations in somatostatin mRNA expression in the dorsolateral prefrontal cortex of subjects with schizophrenia or schizoaffective disorder. Cereb Cortex. 2008;18:1575–1587. doi: 10.1093/cercor/bhm186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Oso E, Lopez-Ruiz MP, Arilla E. Effect of haloperidol withdrawal on somatostatin level and binding in rat brain. Biosci Rep. 1990;10:15–22. doi: 10.1007/BF01116846. [DOI] [PubMed] [Google Scholar]
- Pierri JN, Chaudry AS, Woo TU, Lewis DA. Alterations in chandelier neuron axon terminals in the prefrontal cortex of schizophrenic subjects. Am J Psychiatry. 1999;156:1709–1719. doi: 10.1176/ajp.156.11.1709. [DOI] [PubMed] [Google Scholar]
- Qiu C, Zeyda T, Johnson B, Hochgeschwender U, de Lecea L, Tallent MK. Somatostatin receptor subtype 4 couples to the M-current to regulate seizures. J Neurosci. 2008;28:3567–3576. doi: 10.1523/JNEUROSCI.4679-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Selemon LD, Goldman-Rakic PS. Neuronal and glial somal size in the prefrontal cortex: a postmortem morphometric study of schizophrenia and Huntington disease. Arch Gen Psychiatry. 1998;55:215–224. doi: 10.1001/archpsyc.55.3.215. [DOI] [PubMed] [Google Scholar]
- Sakai T, Oshima A, Nozaki Y, Ida I, Haga C, Akiyama H, Nakazato Y, Mikuni M. Changes in density of calcium-binding-protein-immunoreactive GABAergic neurons in prefrontal cortex in schizophrenia and bipolar disorder. Neuropathology. 2008;28:143–150. doi: 10.1111/j.1440-1789.2007.00867.x. [DOI] [PubMed] [Google Scholar]
- Schindler M, Sellers LA, Humphrey PP, Emson PC. Immunohistochemical localization of the somatostatin SST2(A) receptor in the rat brain and spinal cord. Neuroscience. 1997;76:225–240. doi: 10.1016/s0306-4522(96)00388-0. [DOI] [PubMed] [Google Scholar]
- Schulz S, Handel M, Schreff M, Schmidt H, Hollt V. Localization of five somatostatin receptors in the rat central nervous system using subtype-specific antibodies. J Physiol Paris. 2000;94:259–264. doi: 10.1016/s0928-4257(00)00212-6. [DOI] [PubMed] [Google Scholar]
- Schweitzer P, Madamba SG, Siggins GR. Somatostatin increases a voltage-insensitive K+ conductance in rat CA1 hippocampal neurons. J Neurophysiol. 1998;79:1230–1238. doi: 10.1152/jn.1998.79.3.1230. [DOI] [PubMed] [Google Scholar]
- Stan AD, Ghose S, Gao XM, Roberts RC, Lewis-Amezcua K, Hatanpaa KJ, Tamminga CA. Human postmortem tissue: what quality markers matter? Brain Res. 2006;1123:1–11. doi: 10.1016/j.brainres.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallent MK, Siggins GR. Somatostatin depresses excitatory but not inhibitory neurotransmission in rat CA1 hippocampus. J Neurophysiol. 1997;78:3008–3018. doi: 10.1152/jn.1997.78.6.3008. [DOI] [PubMed] [Google Scholar]
- Thoss VS, Perez J, Probst A, Hoyer D. Expression of five somatostatin receptor mRNAs in the human brain and pituitary. Naunyn Schmiedebergs Arch Pharmacol. 1996;354:411–419. doi: 10.1007/BF00168430. [DOI] [PubMed] [Google Scholar]
- Traub RD, Wong RK. Synchronized burst discharge in disinhibited hippocampal slice. II. Model of cellular mechanism. J Neurophysiol. 1983;49:459–471. doi: 10.1152/jn.1983.49.2.459. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Hoyer D. Brain somatostatin: a candidate inhibitory role in seizures and epileptogenesis. Eur J Neurosci. 1999;11:3767–3776. doi: 10.1046/j.1460-9568.1999.00838.x. [DOI] [PubMed] [Google Scholar]
- Videau C, Hochgeschwender U, Kreienkamp HJ, Brennan MB, Viollet C, Richter D, Epelbaum J. Characterisation of [125I]-Tyr0DTrp8-somatostatin binding in sst1- to sst4- and SRIF-gene-invalidated mouse brain. Naunyn Schmiedebergs Arch Pharmacol. 2003;367:562–571. doi: 10.1007/s00210-003-0758-8. [DOI] [PubMed] [Google Scholar]
- Volk D, Austin M, Pierri J, Sampson A, Lewis D. GAB A transporter-1 mRNA in the prefrontal cortex in schizophrenia: decreased expression in a subset of neurons. Am J Psychiatry. 2001;158:256–265. doi: 10.1176/appi.ajp.158.2.256. [DOI] [PubMed] [Google Scholar]
- Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000a;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- Volk M, Strotzer M, Holzknecht N, Manke C, Lenhart M, Gmeinwieser J, Link J, Reiser M, Feuerbach S. Digital radiography of the skeleton using a large-area detector based on amorphous silicon technology: image quality and potential for dose reduction in comparison with screen-film radiography. Clin Radiol. 2000b;55:615–621. doi: 10.1053/crad.2000.0493. [DOI] [PubMed] [Google Scholar]
- Woo TU, Whitehead RE, Melchitzky DS, Lewis DA. A subclass of prefrontal gamma-aminobutyric acid axon terminals are selectively altered in schizophrenia. Proc Natl Acad Sci U S A. 1998;95:5341–5346. doi: 10.1073/pnas.95.9.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarnowska ED, Keist R, Rudolph U, Pearce RA. GABAA receptor alpha5 subunits contribute to GABAA,slow synaptic inhibition in mouse hippocampus. J Neurophysiol. 2009;101:1179–1191. doi: 10.1152/jn.91203.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]