Abstract
Pulmonary metastasis of breast cancer requires recruitment and expansion of T regulatory cells (Tregs) that promote escape from host protective immune cells. However, it remains unclear precisely how tumors recruit Tregs to support metastatic growth. Here we report the mechanistic involvement of a unique and previously undescribed subset of regulatory B cells. These cells, designated tumor-evoked Bregs (tBregs), phenotypically resemble activated but poorly proliferative mature B2 cells (CD19+ CD25High CD69High) that express constitutively active Stat3 and B7-H1High CD81High CD86High CD62LLowIgMInt. Our studies with the mouse 4T1 model of breast cancer indicate that the primary role of tBregs in lung metastases is to induce TGFβ-dependent conversion of FoxP3+ Tregs from resting CD4+ T cells. In the absence of tBregs, 4T1 tumors cannot metastasize into the lungs efficiently due to poor Treg conversion. Our findings have important clinical implications, since they suggest that tBregs must be controlled to interrupt the initiation of a key cancer-induced immunosuppressive event that is critical to support cancer metastasis.
Keywords: Breast cancer, regulatory B cells, Bregs, Tregs, lung metastasis
Introduction
Cancer escape is an active process that regulates immune responses by employing at least two types of suppressive cells, myeloid suppressive cells (MSCs) and regulatory T cells (Tregs) (1–3). For example, in the mouse mammary adenocarcinoma 4T1 cancer model, which represents a highly aggressive model of human breast carcinoma (4), cancer-produced GM-CSF, IL-1β and TGFβ promote the generation of MSCs to impair antitumor immune responses and promote metastases either through direct action or indirectly by activating Tregs (5). Tregs are key subsets of CD4+ T cells that control peripheral tolerance to self- and allo- antigens (6), and the majority of them phenotypically can be identified by the expression of CD25 (IL-2Rα) and a fork-head box P3 (FoxP3) gene product (7). They suppress T cell responses by acting directly or through the inhibition of APCs (7–9) involving cell contact-, FasL/Fas - and PD1/B7-H1-dependent processes (10–13), or secreted factors such as IL-10, TGF-β, IL-27 and IL-35 (14–17). As a result, Tregs are considered to play a key role in the escape of cancer cells from anti-tumor effector T cells (20–23), although the role of other immune cells remains poorly understood. Although a handful of reports indicate that B cells can disable CD4+ T cell help for CTL mediated tumor immunity in mice (18), or promote epithelial carcinogenesis and castration-resistant prostate cancer (19, 20), the role or existence of cancer-associated regulatory B cells (Bregs) remains unknown. However, suppressive B cells and Bregs were shown to exist and participate in the protective immune responses against autoimmune diseases (21–24). For example, T cell –dependent autoimmunity in mice is associated with the lack of a small subset (1–2% of B220+ cells) of IL-10 –producing CD1dHigh CD5+ B cells (so called B10 cells) (25). In addition, B1b (CD5−CD1dHigh B220 Low CD11b+ IgM+) suppressive cells in mice and CD19+CD24HighCD38High B cells in humans are linked with protection from murine chronic colitis (26, 27) and systemic lupus erythematosus, respectively (28). Interestingly, although the suppressive activity of B cells can be IL-10 –independent, for example, in protection from EAE (29), the majority of them appear to utilize IL-10 (23–25, 28). In fact, LPS activation of naïve B cells alone can induce IL-10 production and suppress T cell responses inducing H-Y antigen tolerance (30).
Recently, we have reported that lung metastasis of mammary adenocarcinoma 4T1 implanted in the mammary gland of immune competent BALB/C mice requires an active participation of Tregs to inactivate antitumor defenses of NK cells (4). The process was also associated with the expansion of Tregs that appeared to be induced without involvement of MSCs (4). Here, we demonstrate that FoxP3+Treg conversion from non-regulatory CD4+ T cells (non-Tregs) is mediated by a unique subset of regulatory B cells, designated tumor-evoked Bregs (tBregs), utilizing TGFβ, a pathway commonly used for the generation iTregs (31). To our knowledge, this is the first report on the existence and function of Bregs in cancers. Our data suggest that as long as cancer persists, it will generate tBregs to thereby convert Tregs to initiate a chain of suppressive events leading to successful lung metastasis.
MATERIALS AND METHODS
Reagents, cells and mice
The majority of reagents were described in our recent report (3). In brief, female BALB/C, C57Bl/6, non-obese diabetic severe combined immunodeficient NOD/SCID (NOD.CB17-Prkdcscid/J; H-2d) mice and mice with mature B cell deficiency (B6.129P2-Igh-Jtm1Cgn/J) were from the Jackson Laboratory (Bar Harbor, ME). 4T1 cells, B16F10 melanoma, MCF-7 and MDA-MB-231, OVCAR3, B-2008, BG-1 UCI101, SW480 cells were purchased from American Type Culture Collection. 4T1.2 cells, a subset of 4T1 cells, were a gift from Dr. Robin L. Anderson (Peter McCallum Cancer Center, Australia). 4T1-PE cells were generated from 4T1 by using TARC-PE38 chemotoxin (3). Splenocytes from FoxP3-GFP mice were the gift from Dr. Rachel Caspi (NEI/NIH) and Dr. Vijay Kochroo (Harward Mediacl School). Primary untransformed murine embryonic fibroblasts CD1 and DR4 were used at passage 3–6 and were the gift of Dr. Lioudmila Sharova (NIA/NIH).
In vitro tBreg and Treg generation and T cell suppression assay
Murine splenic B cells were isolated by negative selection using the RoboSep magnetic purification system (StemCell Technologies, Vancouver, Canada). For tBreg generation, B cells were incubated with 50% cancer CM for two days in cRPMI. Control B cells were treated with PBS, or 100 ng/ml BAFF, or 5 μg/ml LPS. Cancer CM was collected from confluent cancer cells, and for some experiments, to generate serum-free CM, cRMPI was replaced and the cells cultured for 72 hours in serum-free media (Life Sciences). Mouse CD3+ T cells were isolated from naïve mouse spleen using T cell enrichment columns (R&D Systems, Minneapolis, MN). To generate non-Tregs (purity >99.5 %), CD4+ T cells were isolated by mouse T cell CD4 Subset Column Kit and separated from CD25+ cells using CD25 Microbead kit (Miltenyi Biotec, Auburn, CA). The CD25+CD4+ cells were used as Tregs. To test the suppressive effects of B cells, B cells were cultured together with carboxyfluorescein succinimidyl ester (CFSE, Invitrogen, Carlsbad, CA) -labeled non-Tregs or Tregs (1×105) in the presence of anti-CD3/CD28-coupled beads and IL-2 (500 U/ml) for 5 days. Decrease in CFSE expression of T cells correlates with the proportion of cells that underwent divisions. B cells were also isolated from spleens of tumor-bearing mice using anti-CD19 Ab beads after three rounds of depletion of MSCs using anti-Gr1 Ab beads (Miltenyi Biotec).
To generate Tregs, tBregs were incubated with non-Treg cells (CD25−CD4+ from BALB/C mice or GFP−CD4+ cells from FoxP3-GFP mice, purity >99.5%) at a 1:1 ratio and cultured for 5 days in the presence of bead-conjugated anti-CD3/CD28 Abs and 500 U/ml IL-2 in the absence or presence of DMSO or SB431542 (Tocris bioscience, Ellisville, MO). To test their activity, Tregs were re-isolated by two rounds of B cell depletion with FITC-CD19 and PE-B220 Abs (BD Pharmingen) and anti-FITC and anti-PE beads (Miltenyi Biotec) and mixed with naïve CFSE-labeled CD8+ T cells in the presence of bead-conjugated anti-CD3/CD28 Abs and 500 U/ml IL-2 for 5 days. TGFβ1 secretion was quantified by ELISA following manufacturer’s protocol (R&D systems, Inc., Minneapolis, MN).
In vivo manipulations
Animal care was provided in accordance with the procedures outlined in the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 86-23, 1985). The experiments were performed using 4–8 weeks old female mice in a pathogen-free environment at the National Institute on Aging Animal Facility, Baltimore, MD. To deplete Tregs and tBregs, mice were i.p. injected with Abs to mouse CD25 (500 μg; PC-61, BioXcell, West Lebanon, NH) and B220 (400 μg; RA3.3A1, BioXcell) or control IgG at days 3, 10 and 18 post tumor challenge. To restore metastasis, NOD/SCID mice were i.v. injected with 1×107 tBregs, generated from naïve BALB/C B cells two days treated with CM-4T1PE, together with equal amounts of BALB/C non-Tregs at days −1, 3 and 7 after tumor challenge. Control NOD/SCID mice were injected with 1×107 BALB/C non-Tregs. Congeneic mice were challenged s.c 4T1.2 tumor cells (in 4th mammary gland with 1×104 cells) or 1×105 B16 melanoma F10 cells; tumor growth was measured every other day. Mice were culled after 28 days of tumor challenge and lungs were analyzed for metastasis as previously described (3). Tumor burden in the lungs was quantified by counting nodules.
RESULTS
Lung metastases require the participation of a unique subset of B cells
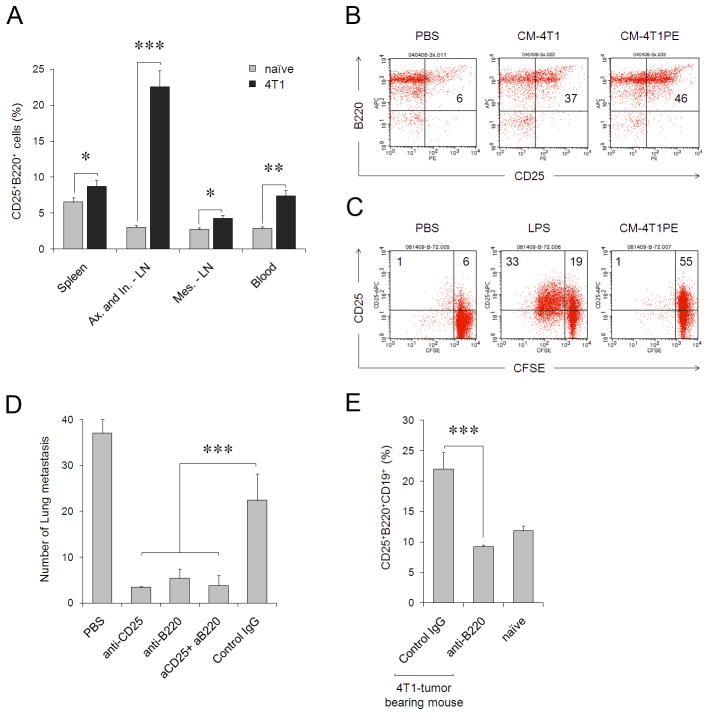
We recently reported that murine 4T1 breast cancer promotes expansion of Tregs to utilize them in lung metastases (3). Interestingly, although this expansion can be induced by MSCs, which are also expanded in tumor-bearing mice, MSCs appear not to be involved in the de novo conversion of Tregs (3). The search for other Treg-converting APCs led us to find an enhanced population of CD25-expressing B220+CD19+ B cells in the peripheral blood and secondary lymphoid organs of tumor-bearing mice (Fig. 1A). Similar cells were also readily generated in naïve mice s.c. injected with conditioned media (CM) of 4T1 cells (CM-4T1, and, specifically, by their non-metastatic 4T1 cells, CM-4T1PE, Fig. 1B, and CM-4T1R4, Suppl. Fig. 1); or in vitro from purified naïve mouse B cells treated with cancer CM (Fig. 1C). CD25 expression can also be induced in B cells treated with mitogens like LPS (Fig. 1C). However, unlike LPS, tumor CM-treated B cells only up-regulated CD25 without proliferation (Fig. 1C). It appears that this was the 4T1 cancer CM-associated process, as CM from untransformed murine fibroblasts or murine A20 B cell lymphoma did not activate B cells either in vitro or in vivo (data not shown and also see Suppl. Fig. 2). To test whether tumor-induced CD25+B220+ B cells are involved in lung metastases, 4T1 tumor-bearing mice were treated with antibody (Ab) targeting B220 alone (to deplete B cells) or in combination with CD25 (PC61 Ab, routinely used for Treg depletion). While control Ab-treated mice succumbed to massive lung metastases, both anti-B220 and anti-CD25 Abs, which depleted splenic B220+ and CD25+ cells (Suppl. Fig. 3a), almost completely abrogated lung metastases (Fig. 1D). Importantly, treatment with anti-B220 Ab also reversed the enhanced proportion of CD25-expressing B220+CD19+ cells in tumor-bearing mice to the levels of naïve mice (Fig. 1E and Suppl. Fig. 3b), further indicating the importance of CD25+B cells in lung metastases.
FIGURE 1.
(A) 4T1 cancer -bearing mice (black bars) have a higher proportion of CD25+B220+ cells (% ± SEM of three mice per group) in peripheral blood and secondary lymphoid organs (Ax, axillary; In, inguinal; and Mes, mesenteric) compared with naïve BALB/C mice (grey bars). (B) CM from non-metastatic 4T1-PE cells (CM-4T1PE) had a greater ability to generate CD25+CD19+B220+ cells in vivo, than CM from metastatic 4T1 cells (CM-4T1). Shown is proportion of cells within CD19+ cells. Naïve BALB/C mice were i.p. injected with 0.5 ml CMs or control medium (Mock) once a day four times and splenocytes were stained for CD25 and B220 cells five days after last treatment. (C) Poorly proliferative CD25+B220+ B cells are generated in vitro from naïve mouse B cells after treatment with CM-4T1PE for two days. Control B cells were treated with LPS (B-LPS), or PBS (B-PBS). Histograms show percentage of proliferated (CFSE-diluted) B cells. Numbers are for % of cells in corresponding quadrants. The results in A–C were repeated at least three times. (D, E) B220+CD25+ tBregs are required for lung metastasis. (D) Mean lung metastatic foci ± SEM of four mice per group experiments reproduced three times. 4T1.2 tumor-bearing BALB/C mice were depleted of B220+ and CD25+ cells by i.p. injecting anti-B220 and anti-CD25 Abs alone or together (aCD25+aB220), respectively. Control mice were treated with isotype-matched antibody (IgG). (E) Y-axis shows % ± SEM of CD25+B220+ cells (within CD19+ cells) in spleens of four tumor-bearing and naive mice per group experiments treated with anti-B220 Ab or IgG. From here on, *P<0.05, **P<0.01; *** P<0.001.
Cancer induces the generation of regulatory B cells
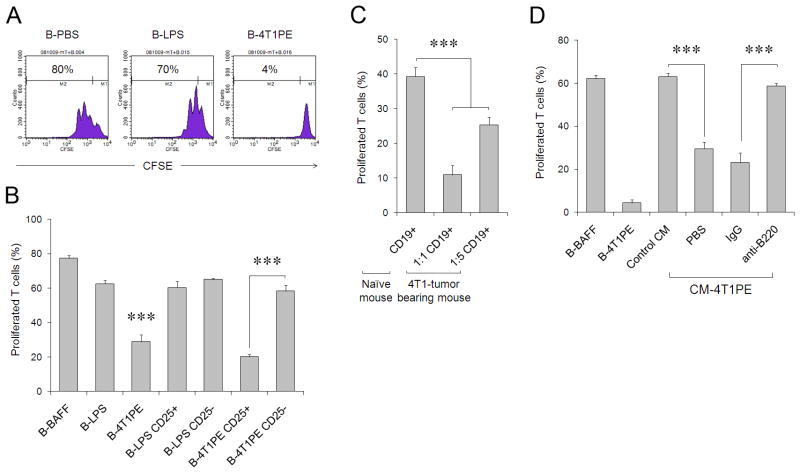
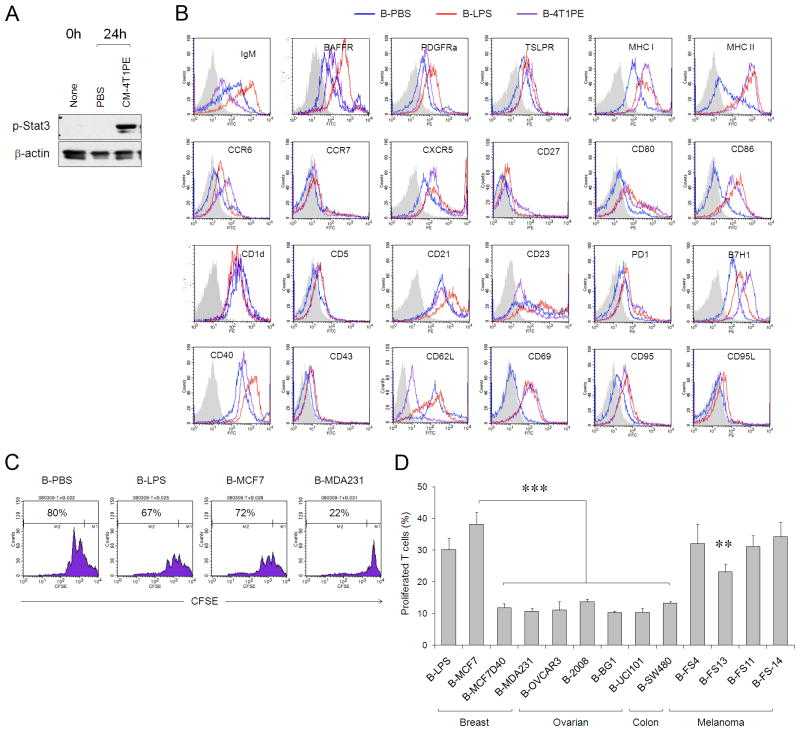
Next, to explain the metastasis-promoting role of B cells, we hypothesized that CD25+ B cells could have regulatory activity. To test this idea, three types of B cells were generated in vitro by treating purified naive B cells with control CM, LPS, or cancer cell-derived CM (from here on, only the data from the use of CM-4T1PE will be shown, as it consistently activated B cells more strongly (Fig. 1B,C)). The cells were then washed and mixed with CFSE-labeled CD3+ T cells in the presence of anti-CD3/CD28 Ab to activate TCR-mediated proliferation. Unlike B cells treated with control CM (B-PBS) or LPS (B-LPS, Fig. 2A), the cancer CM-treated B cells almost completely prevented T cell proliferation (B-4T1PE, Fig. 2A) in both CD4+ and CD8+ cells (Suppl. Fig. 4 and data not shown). The inhibitory activity was only retained in the CD25+, but not CD25−, subset of cancer CM-treated B cells (Fig. 2B). In contrast, control B cells that were treated with CM from untransformed fibroblasts (Fig. Suppl.2) or LPS (Fig. 2B) did not inhibit T cell proliferation regardless of CD25 expression. Moreover, confirming a positive association between CD25+CD19+B220+ B cells and lung metastases (Fig. 1A,D,E), CD19+ B cells isolated from 4T1 tumor-bearing mice (Fig. 2C) or cancer CM-injected mice (CM-4T1PE, Fig. 2D) efficiently inhibited T cell proliferation ex vivo. Control B cells from naïve or control CM-treated mice failed to affect T cell activity (Fig. 2C,D). Importantly, the inhibition was completely prevented if B220+ B cells were removed from the suppression assay (anti-B220, CM-4T1PE, Fig. 2D). Taken together, 4T1 cancer cells directly induce the generation of a unique and suppressive subset of CD25+B220+CD19+ B cells. These cells (from here on designated tumor-evoked Bregs, tBregs) inhibit proliferation of resting and pre-activated T cells equally well in a mouse strain-independent way (Suppl. Fig. 4A–D) even when used at a 1:16 ratio of B cells to T cells (Suppl. Fig. 5). Unlike resting B cells, tBregs retained high viability (at least up to 96 hours, Suppl.6) and expressed constitutively active Stat3 (Fig. 3A). Phenotypically the cells resembled activated mature B2 cells (IgDHigh and CD21Int/low, CD23Int/low CD43− and IgMInt, Fig. 3B), as they reduced expression of CD62L and up-regulated CD69, B7-H1 and CD81 (Fig. 3B).
FIGURE 2.
(A) CM-4T1PE, but not mock (B-PBS) or LPS (B-LPS), treated B cells (B-4T1PE) inhibit proliferation of T cells stimulated with anti-CD3/CD28 Abs. B cells and CFSE-labeled T cells (responder) cells were cultured at a 1:1 ratio for four days in the presence of 50 U/ml IL-2. (B) The suppressive activity of B-4T1PE is retained in CD25+ subset. CD25+ and CD25− subsets of B-LPS and B-4T1PE cells were purified using anti-CD25 Ab and tested as in (A). (C) B cells isolated from 4T1 tumor-bearing mice also suppress T cell activity. Splenic CD19+ were isolated from 4T1 tumor-bearing mice and tested as in (A) after mixing with CFSE-labeled T cells in 1:1 and 1:5 B and T cell ratio, while control naïve mouse B cells were used at 1:1 ratio. (D) Splenic CD19+ B cells were isolated from naïve BALB/C mice i.p. injected with control CM or cancer CM (CM-4T1PE, see Fig. 1B) and tested in vitro for the ability to inhibit T cell proliferation as in (A). Purified B cells from CM-4T1PE-treated mice were also depleted using anti-B220 Ab or control IgG prior to mixing with T cells. Controls were B cells in vitro cultured with BAFF (to maintain viability of control cells) or CM-4T1PE (B-BAFF and B-4T1PE, respectively). Shown is % ± SEM of proliferated T cells of triplicates repeated at least three times.
FIGURE 3.
(A) The CM-4T1PE –treated B cells (tBregs), freshly isolated (None) and PBS-treated B cells (PBS) were lysed and tested for phospho-Stat3 and β-actin using corresponding Abs by western blotting. (B) Surface marker expression (FACS analysis) of purified murine B cells treated with tumor CM (tBregs, pink line), or LPS (B-LPS, red line), or PBS (B-PBS, blue line) after staining with Abs to corresponding surface markers (indicated), or isotype-matched control Ab (grey filled area). (C) Human B cells treated with CM of MDA-MB-231 cells (B-MDA231), but not MCF-7 cells (B-MCF7), or LPS (B-LPS), or PBS (B-PBS), suppress proliferation of T cells. Histograms (B,C) and graphs (D) show % (± SEM of triplicates) of CD3+ T cells that diluted CFSC (proliferated) when mixed with B cells at a 1:1 ratio as in Fig. 2A. (D) Similarly with B-MDA231, B cells treated with CM of other human cancers (depicted on X-axis) also inhibit T cell proliferation. All data were repeated at least three times.
This does not appear to be an isolated mouse phenomenon, as healthy donor peripheral blood CD19+ B cells treated with CM from a number of human cancer cells, including ovarian and colon, up regulated CD25 (Suppl. Fig. 7A) and suppressed proliferation of human CD3+ T cells stimulated with anti-CD3/CD28 Abs and IL-2 (Fig. 3C,D). As with mice, B cells treated with LPS did not suppress T cell activity (Fig. 3C,D). Thus, human cancers also induce the generation of tBregs.
tBregs induce the generation of FoxP3+ Tregs
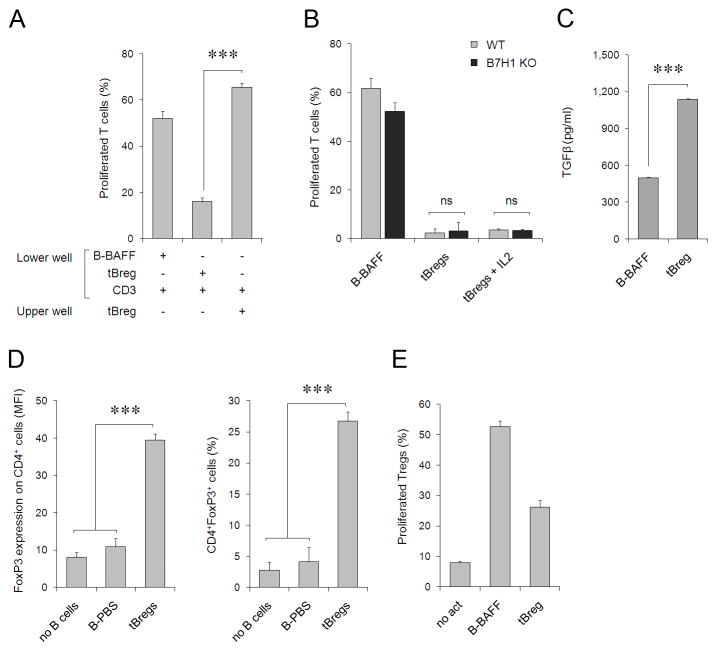
Next, to test the mechanism of Breg-mediated suppression, we separated tBregs from T cells by a porous membrane, and found that this disabled the suppressive activity of tBregs (Fig. 4A). Thus, the process required cell-contact to exert T cell suppression. However, all our attempts to link the suppression with known mechanisms failed. For example, tBregs did not affect viability of T cells and did not utilize common regulatory pathways, such as the use of B7-H1 (Fig. 4B) and Fas (Suppl. Fig. 7B,C), or soluble cytokines IL-10, IL-27 and IL-35 (the factors abundantly expressed in tBregs, Suppl. Fig. 7D–F). Lastly, the IL-2 competition theory can be also ruled out, as the suppression was not affected by the presence of high doses of IL-2 (Fig. 4B and Suppl. Fig. 7D).
FIGURE 4.
(A) The suppressive activity of tBregs requires cell contact, as they cannot suppress proliferation of T cells if the cells are physically separated (placed in the upper and lower chambers of trans-well plate, respectively). Controls were B-BAFF and T cells activated with anti-CD3/CD28 Abs (αCD3/CD28). (B) Despite high levels of B7-H1 (Fig. 3B), both w.t. tBregs (grey bars) and B7-H1 deficient tBregs (black bars) can suppress T cells regardless of the presence of exogenously added IL-2 (tBregs+IL2). (C) tBregs secrete high levels of TGFβ1 (pg/ml), as tested by ELISA assay after two days of incubation as in Fig. 1C. (D) tBregs promote Treg conversion in vitro when co-cultured with purified non-Treg CD4+ T cells for five days in the presence anti-CD3/CD28 Abs and 500 U/ml IL-2. Y-axis shows mean fluorescence intensity (MFI, left panel) and percentage (right panel) of FoxP3+ within CD4+ T cells. (E) Purified and CFSE-labeled Tregs (CD25+CD4+) were stimulated with anti-CD3/CD28 Abs and IL-2 (500 U/ml) in the presence of tBregs or control B cells. Y-axis shows % of proliferated (CFSE diluted) cells ± SEM of triplicate experiments. The experiments were reproduced at least three times.
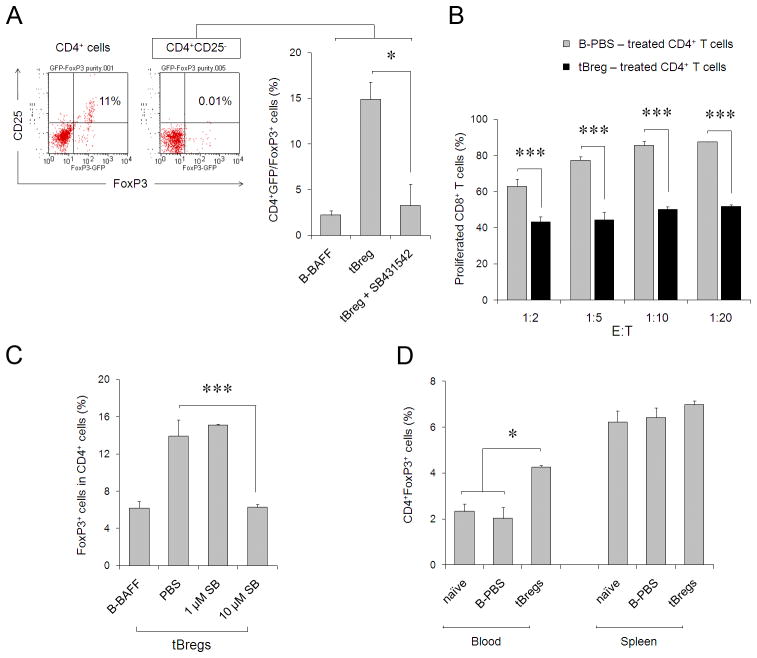
On the other hand, tBregs expressed high levels of CD40, CD80, CD86 and MHC class I and II molecules (Fig. 3B) and high levels of TGFβ (Fig. 4C), suggesting that tBregs could be promoting Treg conversion. To test this idea, purified non-regulatory CD4+ T cells (CD25− FoxP3− CD4+, non-Tregs) were stimulated with anti-CD3/CD28 Abs and high doses of IL-2 in the presence of tBregs or mock-treated B cells. Unlike T cells incubated with control B cells (B-PBS), a significant number of non-Tregs co-cultured with tBregs expressed FoxP3 (tBregs, Fig. 4D), a key marker of Tregs (7). This is not a result of the expansion of contaminating pre-existing Tregs, as their proportion in our non-Treg preparations was below 0.01% (see also Fig. 5C) and, compared with control B cells, tBregs did not enhance (if not reduced) proliferation of purified FoxP3+CD25+CD4+ cells stimulated with anti-CD3/CD28 Abs in the presence of high doses of IL-2 (Fig. 4E). Importantly, highly purified green fluorescent protein-negative (GFP−) CD4+ T cells from FoxP3-GFP mice (32) were also induced to express GFP/FoxP3 when only incubated with tBregs, but not control B cells (Fig. 5A). To confirm that these newly converted FoxP3+ T cells also acquired regulatory activity, the CD4+ T cells (non-Tregs co-cultured with control B cells or tBregs) were re-isolated by two rounds of B cell depletion (using anti-CD19 and -B220 Abs which resulted in at least 98% pure T cells) and tested for their ability to inhibit proliferation of naïve CD8+ T cells. Indeed, the tBreg-generated FoxP3+ T cells, but not with T cells incubated with control B cells, inhibited proliferation of CD8+ T cells (Fig. 5B). The process required TGFβ, as the ability to convert Tregs was completely blocked in the presence of ALK5 inhibitor SB431542, a selective inhibitor of the TGFβ type I receptor activity (Fig. 5A,C). Moreover, the fact that naïve BALB/C mice transferred with tBregs, but not mock-treated B cells, had significantly enhanced numbers of FoxP3+ Tregs in peripheral blood (and at lesser degrees in spleens, although not statistically significant) (Fig. 5D), suggest that tBregs also promote Treg conversion in vivo as in cancer-bearing mice.
FIGURE 5.
(A) Purified GFP−CD4+ T cells from FoxP3-GFP were depleted of CD25+ cells (< 0.01%, right dot blot, inlet) and stimulated with anti-CD3/CD28 Abs and IL-2 (500 U/ml) in the presence of tBregs or control B cells. Y-axis shows % ± SEM of GFP+(FoxP3+)Tregs after 5 days of culture of a triplicate assay. Left dot blot (inlet) shows proportion of GFP+CD25+ of purified CD4+ T cells before CD25 depletion. (B) The converted FoxP3+ T cells (as in Fig. 4D) are Tregs, as they (after two rounds of depletion of B cells, >98% pure CD4+ cells) suppressed proliferation of CFSE-labeled CD8+ T cells stimulated with anti-CD3/CD28 Abs and 500 U/ml IL-2 at the indicated E:T ratio. Y-axis shows percentage of proliferated CFSE-labeled CD8+ cells of a triplicate experiment repeated twice. P values are for comparisons between tBreg- (black bars) and normal B cell- (grey bars) treated CD4+ T cells. tBreg-mediated Treg conversion from non-Tregs (% of GFP+CD4+ cells in A or FoxP3+CD4+ cells in C, Y-axis) requires TGFβ signaling, as it was blocked with 10 μM SB431542. (D) Unlike B-PBS, tBregs expand FoxP3+CD4+ T cells in vivo. Three naïve BALB/C mice per group were i.p. injected with 107 B cells and the proportion of FoxP3+CD4+ T cells (% ± SEM, Y-axis) was evaluated after 5 days in the blood and spleens by FACS. All data shown were reproduced at least three times.
tBreg-induced Tregs promote lung metastasis
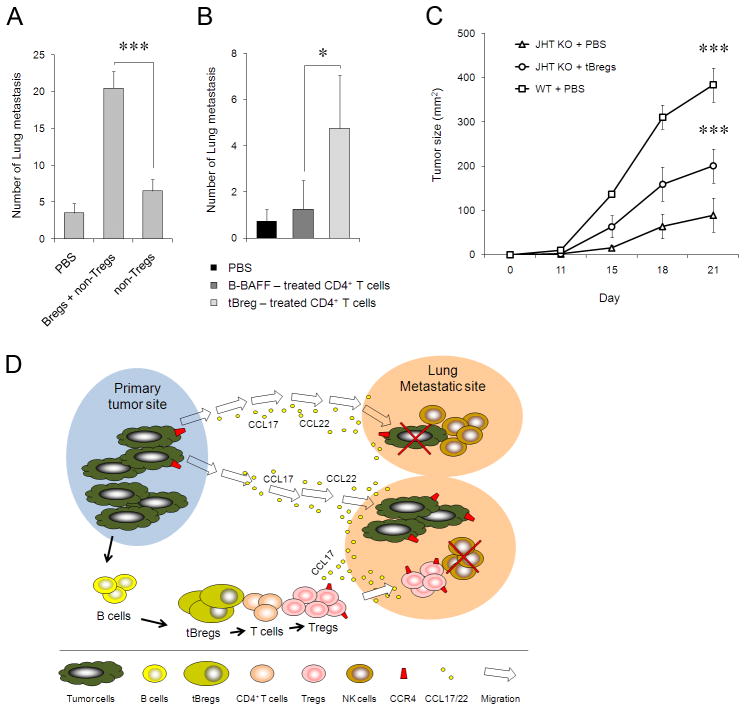
Since lung metastasis of 4T1 cancer cells is a Treg-dependent process that is also associated with the expansion of Tregs (3), the role of tBregs in lung metastasis (see Fig. 1D,E) is to promote conversion of Tregs. To demonstrate this, we utilized T and B cell deficient H-2d –matched NOD/SCID mice that do not support metastasis of 4T1 cancer cells unless adoptively transferred with Tregs (3). While control mice transferred with either tBregs alone (data not shown) or non-Tregs were almost free of lung metastases (Fig. 6A), the mice that received ex vivo-generated tBregs together with non-Tregs from BALB/C mice succumbed to massive lung metastasis (Fig. 6A), suggesting that the process utilized tBreg-induced Tregs. Indeed, consistent with our report on the importance of Tregs in lung metastases (3), the transfer of newly in vitro converted Tregs (cultured with tBregs and then depleted of tBregs, >98%, see Fig. 5A), but not normal B cell-treated T cells, also restored the ability of 4T1 cells to metastasize in NOD/SCID mice (Fig. 6B). Thus, taken together with the inability of 4T1 cancer cells to metastasize in immune competent BALB/C mice depleted of B220+ B cells (Fig. 1D), our data suggest that the role of tBregs in lung metastases is to induce Treg conversion. This is not a 4T1 cancer-associated phenomenon, as B16 melanoma could not efficiently progress in mice that did not have mature B cells (33), unless they were adoptively transferred with congenic tBregs generated by treating them with cancer CM (JHT KO + tBregs, Fig. 6C).
FIGURE 6.
(A–B) tBregs support lung metastases via Treg generation. The inability of 4T1.2 cells to metastasize in T and B cell deficient NOD/SCID mice is reversed by transfer of tBregs together with non-Tregs (A) or newly tBreg-generated Tregs (B, depleted of B cells as in Fig. 4D). Control mice received CD25−CD4+ T cells (non-Tregs, A) alone, or tBregs alone (not shown), or T cells cultured with mock-treated B cells (B). (C) Poor growth of s.c. challenged B16 melanoma in mice deficient in mature B cells (JHT KO) can be reversed by adoptive transfer of congeneic tBregs (splenic B cells from naïve C57BL/6 mice treated with CM-4T1PE as in Fig. 1C). Shown, mean lung metastatic foci (A,B) or tumor size (C) ± SEM of four-five mice per group experiments reproduced three times. (D) Summary schema which expands our recent report on the importance of Tregs in lung metastases (3) by adding a “missing link”, tBregs. Our data indicate that as long as cancer persists, it induces the generation of tBregs from resting B cells by producing yet to be identified soluble factors. As a result, tBregs induce TGFβ-dependent FoxP3+ Treg conversion of non-Treg T cells and thereby promote lung metastasis by presumably utilizing the same mechanism we previously reported, such as by migrating into CCL17/CCL22-producing lungs to directly kill antitumor NK cells (3). In the absence of tBregs, cancer cannot metastasize into the lungs due to a poor Treg conversion.
DISCUSSION
We previously reported that murine breast cancer induces expansion of Tregs to utilize them in lung metastasis (3). The search for the cells responsible for this expansion has led us to find a new subset of regulatory B cells, tBregs, which are actively generated from normal B cells in response to the direct effects of cancer-produced factors. tBregs also play an essential role in lung metastases, as they at least induce conversion of metastasis-supporting FoxP3+ Tregs from non-Treg CD4+ T cells. Although tBregs did not affect expansion of pre-existing (purified) Tregs in vitro, their role on Tregs in vivo cannot be ruled out. However, using highly purified CD4+ T cell (which contained less than 0.01% contaminating Tregs) from spleens of naïve BALB/C and FoxP3-GFP mice we demonstrated that tBregs mediate de novo Treg conversion utilizing TGFβ–dependent process, suggesting that they may represent iTregs (31). Moreover, we also demonstrate that tBregs generate Tregs from non-Tregs in vivo and thereby to support lung metastasis of 4T1 cancer cells. For example, 4T1 cancer cells only grow at the primary site (mammary gland) but cannot metastasize in T and B cell deficient NOD/SCID mice, unless the mice receive a transfer of Tregs (but not non-Tregs) (3). However, we were able to restore the ability to metastasize if the mice were also adoptively transferred with either ex vivo converted Tregs alone or tBregs together with non-Tregs. The transfer of non-Tregs or tBregs alone did not promote metastasis. It should be noted that MSCs were expanded in NOD/SCID mice as in BALB/C mice, yet the fact that the transfer of non-Tregs did promote metastasis further supports the importance of the tBreg/Treg axis in this process (data not shown and see Ref. (3)). Interestingly, we detected a positive association between Treg and MSC proportions in tumor-bearing mice. For example, the transfer of Tregs into tumor-bearing NOD/SCID mice (Suppl. Fig. 8A) or the depletion of Tregs in tumor-bearing BALB/C mice (Suppl. Fig. 8B) enhanced or reduced MSCs numbers, respectively. Thus, it is tempting to speculate that Tregs and tBregs may cross-talk with MSCs, which can also induce Tregs and supported metastasis (1, 2, 5, 34), by providing or receiving survival benefits to further enhance immune suppression.
Although we do not know how cancers induce the generation of tBregs, a subject of a separate study being conducted in the authors’ laboratory, this is (to our knowledge) the first report on the existence of tBregs. It remains to be seen whether tBregs may represent a population of B cells shown to have tumor-promoting properties (18–20). Interestingly, unlike regulatory B cells (B10 cells and B1b cells in mice (26, 27) and CD19+CD24HighCD38High B cells in humans (28)) shown in the autoimmune field, tBregs represent a functionally and phenotypically unique subset of B cells. Phenotypically, tBregs resemble mature B2 cells (IgDHigh) that constitutively express Stat3, but poorly proliferate and do not express CD27 and CD5 or up regulate CD1d. In the absence of a unique marker, tBregs can be also defined as CD19+ B cells that are CD25High B7-H1High CD81High CD86HighCCR6High and CD62LLowIgMInt/Low. Interestingly, B cells treated with S. aureus Cowan 1 antigen also up regulate CD25 but induce anergy of activated T cells competing for IL-2 (35), suggesting that they may represent the same type of cells. However, our tBregs efficiently inhibit both resting and activated T cells, including CD4+ and CD8+ T cells, without induction of cell death or use of IL-2. tBregs also differ functionally from other Bregs which often utilize IL-10 –dependent suppression. Unlike other Bregs (23, 25, 27) and LPS- or BCR -activated B cells (30, 36), the suppressive activity of tBregs did not require IL-10 or other known suppressive pathways, such as B7-H1-PD1, Fas-FasL, and IL27/IL35. Instead, the primary role of tBregs in lung metastasis is to promote the conversion of Tregs by utilizing TGFβ and its TGFβ type I receptor signaling axis. However, it is conceivable that cancer-induced B cells and, specifically tBregs, may also exert additional tumorigenic functions as B cells that promote epithelial carcinogenesis and castration-resistant prostate cancer (19, 20).
Collectively, as summarized in Fig. 6D, our findings indicate that tumor-evoked regulatory B cells are crucial for lung metastasis, acting to convert resting T cells to regulatory T cells that promote immune escape in the target tissue. However, the tBregs by themselves are actively generated from normal B cells in response to cancer cell-produced factors utilizing yet unknown mechanisms, and this appears to be quite a widespread phenomenon, as a number of human cancer lines (breast, ovarian and colon carcinomas) also induced the generation of tBregs. Thus, the clinical implication of our finding is that, as long as cancer persists, it will induce the generation of tBregs and thereby initiate the chain of suppressive events leading to metastasis. Hence, tBregs need to be controlled to efficiently combat cancers, as in the absence of tBregs, cancer cannot metastasize into the lungs due to a poor Treg conversion. For example, clinically available antibodies such as the pan B cell antibody, anti-CD20 antibody (rituximab) or the anti-IL2Rα antibody (daclizumab) could bypass the tBreg-mediated blockade of the immune response to some cancers.
Supplementary Material
Acknowledgments
We are grateful to Drs. Edward Goetzl (UCSF) and Dan Longo (NIA/NIH) and Ana Lustig (NIA/NIH) for helpful comments and suggestions; Drs. Ashani Weeraratna and Dr. Lioudmila Sharova (NIA/NIH) for the gift of melanoma cell lines and murine embryonic fibroblasts, respectively; Karen Madara (NIA/NIH) for providing human blood samples; Dr. Cornelia Bergmann, (Lerner Research Institute) for the gift of B7-H1 KO splenocytes; Dr. Rachel Caspi (NEI/NIH) and Dr. Vijay Kochroo (Harvard Medical School) for the gift of FoxP3-GFP splenocytes. This research was supported by the Intramural Research Program of the National Institute on Aging, NIH.
Footnotes
Conflict-of-interest disclosure: The authors work for the US government and declare no competing financial interests.
References
- 1.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu VC, Wong LY, Jang T, Shah AH, Park I, Yang X, et al. Tumor evasion of the immune system by converting CD4+ J Immunol. 2007;178:2883–92. doi: 10.4049/jimmunol.178.5.2883. [DOI] [PubMed] [Google Scholar]
- 3.Olkhanud PB, Baatar D, Bodogai M, Hakim F, Gress R, Anderson RL, et al. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer Res. 2009;69:5996–6004. doi: 10.1158/0008-5472.CAN-08-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lelekakis M, Moseley JM, Martin TJ, Hards D, Williams E, Ho P, et al. A novel orthotopic model of breast cancer metastasis to bone. Clin Exp Metastasis. 1999;17:163–70. doi: 10.1023/a:1006689719505. [DOI] [PubMed] [Google Scholar]
- 5.DuPre SA, Redelman D, Hunter KW., Jr The mouse mammary carcinoma 4T1: characterization of the cellular landscape of primary tumours and metastatic tumour foci. Int J Exp Pathol. 2007;88:351–60. doi: 10.1111/j.1365-2613.2007.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 7.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 8.Serafini P, De SC, Marigo I, Cingarlini S, Dolcetti L, Gallina G, et al. Derangement of immune responses by myeloid suppressor cells. Cancer Immunol Immunother. 2004;53:64–72. doi: 10.1007/s00262-003-0443-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–31. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 10.Okudaira K, Hokari R, Tsuzuki Y, Okada Y, Komoto S, Watanabe C, et al. Blockade of B7-H1 or B7-DC induces an anti-tumor effect in a mouse pancreatic cancer model. Int J Oncol. 2009;35:741–9. doi: 10.3892/ijo_00000387. [DOI] [PubMed] [Google Scholar]
- 11.Phares TW, Ramakrishna C, Parra GI, Epstein A, Chen L, Atkinson R, et al. Target-dependent B7-H1 regulation contributes to clearance of central nervous system infection and dampens morbidity. J Immunol. 2009;182:5430–8. doi: 10.4049/jimmunol.0803557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Probst HC, McCoy K, Okazaki T, Honjo T, van den BM. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nat Immunol. 2005;6:280–6. doi: 10.1038/ni1165. [DOI] [PubMed] [Google Scholar]
- 13.Baatar D, Olkhanud P, Sumitomo K, Taub D, Gress R, Biragyn A. Human Peripheral Blood T Regulatory Cells (Tregs), Functionally Primed CCR4+ Tregs and Unprimed CCR4-Tregs, Regulate Effector T Cells Using FasL. J Immunol. 2007;178:4891–900. doi: 10.4049/jimmunol.178.8.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 15.Annacker O, Pimenta-Araujo R, Burlen-Defranoux O, Barbosa TC, Cumano A, Bandeira A. CD25+ CD4+ T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. J Immunol. 2001;166:3008–18. doi: 10.4049/jimmunol.166.5.3008. [DOI] [PubMed] [Google Scholar]
- 16.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–9. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 17.Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–9. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 18.Qin Z, Richter G, Schuler T, Ibe S, Cao X, Blankenstein T. B cells inhibit induction of T cell-dependent tumor immunity. Nat Med. 1998;4:627–30. doi: 10.1038/nm0598-627. [DOI] [PubMed] [Google Scholar]
- 19.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–23. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 20.Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature. 2010;464:302–5. doi: 10.1038/nature08782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rafei M, Hsieh J, Zehntner S, Li M, Forner K, Birman E, et al. A granulocyte-macrophage colony-stimulating factor and interleukin-15 fusokine induces a regulatory B cell population with immune suppressive properties. Nat Med. 2009;15:1038–45. doi: 10.1038/nm.2003. [DOI] [PubMed] [Google Scholar]
- 22.Ito T, Ishikawa S, Sato T, Akadegawa K, Yurino H, Kitabatake M, et al. Defective B1 cell homing to the peritoneal cavity and preferential recruitment of B1 cells in the target organs in a murine model for systemic lupus erythematosus. J Immunol. 2004;172:3628–34. doi: 10.4049/jimmunol.172.6.3628. [DOI] [PubMed] [Google Scholar]
- 23.Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420–30. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Byrne SN, Halliday GM. B cells activated in lymph nodes in response to ultraviolet irradiation or by interleukin-10 inhibit dendritic cell induction of immunity. J Invest Dermatol. 2005;124:570–8. doi: 10.1111/j.0022-202X.2005.23615.x. [DOI] [PubMed] [Google Scholar]
- 25.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–50. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 26.Shimomura Y, Mizoguchi E, Sugimoto K, Kibe R, Benno Y, Mizoguchi A, et al. Regulatory role of B-1 B cells in chronic colitis. Int Immunol. 2008;20:729–37. doi: 10.1093/intimm/dxn031. [DOI] [PubMed] [Google Scholar]
- 27.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–30. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 28.Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32:129–40. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Frommer F, Heinen TJ, Wunderlich FT, Yogev N, Buch T, Roers A, et al. Tolerance without clonal expansion: self-antigen-expressing B cells program self-reactive T cells for future deletion. J Immunol. 2008;181:5748–59. doi: 10.4049/jimmunol.181.8.5748. [DOI] [PubMed] [Google Scholar]
- 30.Fuchs EJ, Matzinger P. B cells turn off virgin but not memory T cells. Science. 1992;258:1156–9. doi: 10.1126/science.1439825. [DOI] [PubMed] [Google Scholar]
- 31.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-beta to convert naive CD4+CD25− cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol. 2007;178:2018–27. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 32.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 33.Gu H, Zou YR, Rajewsky K. Independent control of immunoglobulin switch recombination at individual switch regions evidenced through Cre-loxP-mediated gene targeting. Cell. 1993;73:1155–64. doi: 10.1016/0092-8674(93)90644-6. [DOI] [PubMed] [Google Scholar]
- 34.Nagaraj S, Gabrilovich DI. Tumor escape mechanism governed by myeloid-derived suppressor cells. Cancer Res. 2008;68:2561–3. doi: 10.1158/0008-5472.CAN-07-6229. [DOI] [PubMed] [Google Scholar]
- 35.Tretter T, Venigalla RK, Eckstein V, Saffrich R, Sertel S, Ho AD, et al. Induction of CD4+ T-cell anergy and apoptosis by activated human B cells. Blood. 2008;112:4555–64. doi: 10.1182/blood-2008-02-140087. [DOI] [PubMed] [Google Scholar]
- 36.Hussain S, Delovitch TL. Intravenous transfusion of BCR-activated B cells protects NOD mice from type 1 diabetes in an IL-10-dependent manner. J Immunol. 2007;179:7225–32. doi: 10.4049/jimmunol.179.11.7225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.