Abstract
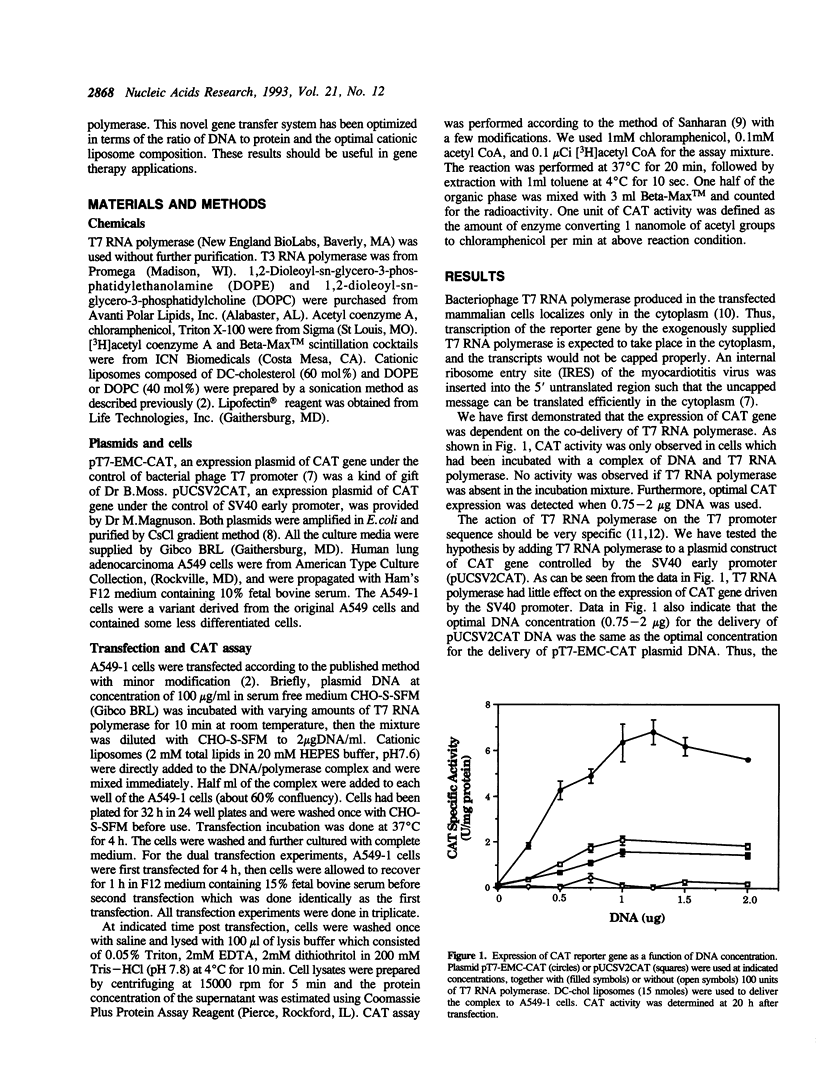
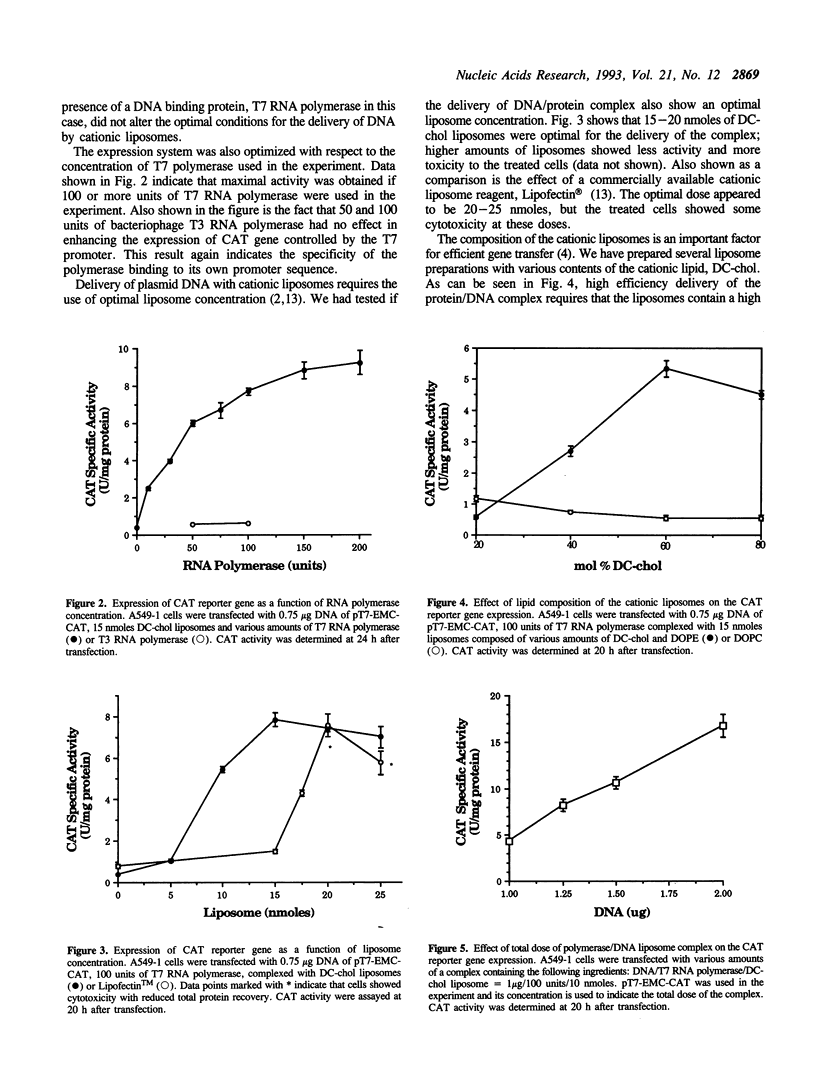
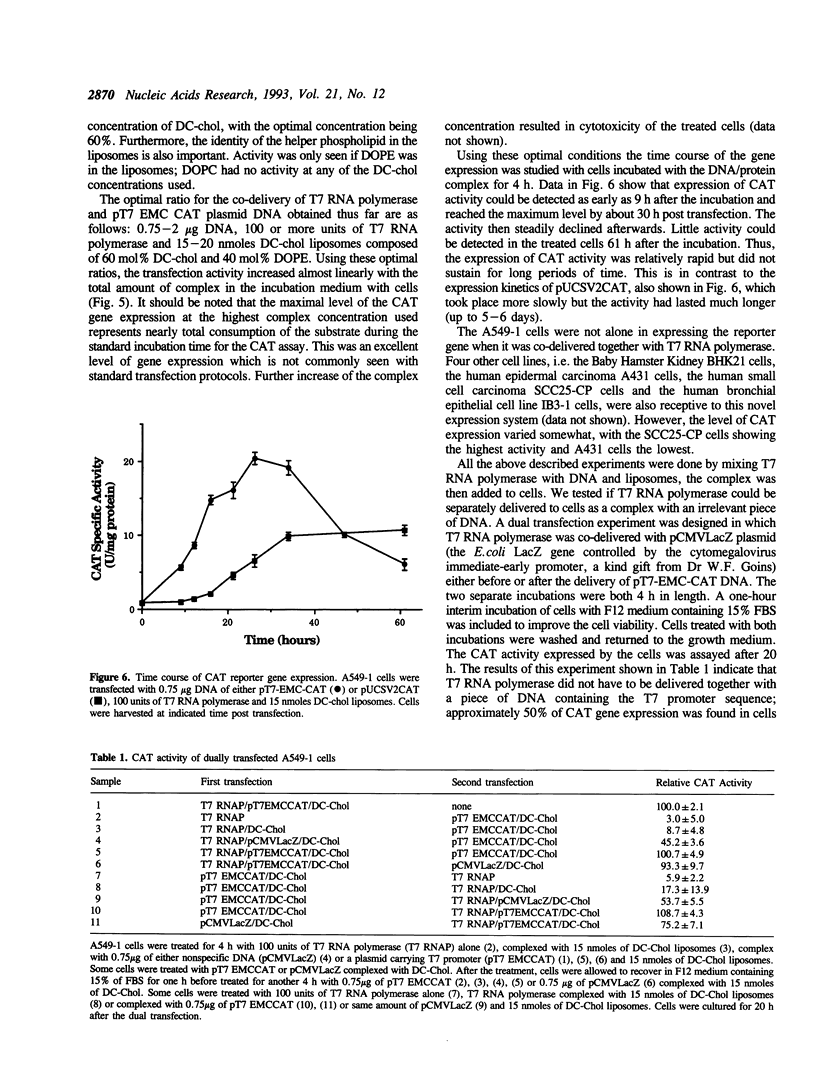
Expression of bacteriophage T7 RNA polymerase in mammalian cells can efficiently drive the transcription of a foreign gene controlled by the T7 promoter (Elroy-Stein et al., Proc. Natl. Acad. Sci. USA. 86, 6126-6130, 1989). We have tested the hypothesis that purified T7 RNA polymerase can be co-delivered into mammalian cells together with a reporter gene (chloramphenicol acetyltransferase, CAT) controlled by the T7 promoter (pT7-EMC-CAT) using DC-chol cationic liposomes. Indeed, significant level of CAT activity was observed in human lung adenocarcinoma (A549-1) cells which had been incubated with a complex of T7 RNA polymerase, pT7-EMC-CAT DNA and DC-chol cationic liposomes. The expression was specific in that T3 RNA polymerase could not replace the T7 RNA polymerase, and that co-delivered T7 RNA polymerase did not enhance the expression of a CAT gene controlled by the SV40 early promoter. The system was optimized in terms of enzyme, DNA and liposome concentrations. Time course experiment indicated that the expression of the T7 system was about 8-10 hours sooner than the SV40 system, consistent with the notion that T7 RNA polymerase does not enter into the nucleus and the transcription takes place in the cytoplasm of the transfected cells. The expression of the T7 system was transient; it declined after 30 hours post transfection, probably due to turnover of the phage enzyme in the mammalian cells. The expression system described here should be useful for gene transfer experiments which require a fast but transient expression of a foreign gene. We have also compared our delivery system with a commercial reagent, Lipofectin, which has been used to deliver T3 or T7 RNA polymerase with a reporter plasmid encoding the T3 or T7 promoter.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander W. A., Moss B., Fuerst T. R. Regulated expression of foreign genes in vaccinia virus under the control of bacteriophage T7 RNA polymerase and the Escherichia coli lac repressor. J Virol. 1992 May;66(5):2934–2942. doi: 10.1128/jvi.66.5.2934-2942.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi M. R. High efficiency transformation by direct microinjection of DNA into cultured mammalian cells. Cell. 1980 Nov;22(2 Pt 2):479–488. doi: 10.1016/0092-8674(80)90358-x. [DOI] [PubMed] [Google Scholar]
- Deng H., Wang C., Acsadi G., Wolff J. A. High-efficiency protein synthesis from T7 RNA polymerase transcripts in 3T3 fibroblasts. Gene. 1991 Dec 30;109(2):193–201. doi: 10.1016/0378-1119(91)90609-f. [DOI] [PubMed] [Google Scholar]
- Dubendorff J. W., Studier F. W. Creation of a T7 autogene. Cloning and expression of the gene for bacteriophage T7 RNA polymerase under control of its cognate promoter. J Mol Biol. 1991 May 5;219(1):61–68. doi: 10.1016/0022-2836(91)90857-3. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Krippl B., Bernstein K. E., Westphal H., Studier F. W. Targeting bacteriophage T7 RNA polymerase to the mammalian cell nucleus. Gene. 1988 Sep 7;68(2):259–266. doi: 10.1016/0378-1119(88)90028-5. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Complete nucleotide sequence of bacteriophage T7 DNA and the locations of T7 genetic elements. J Mol Biol. 1983 Jun 5;166(4):477–535. doi: 10.1016/s0022-2836(83)80282-4. [DOI] [PubMed] [Google Scholar]
- Elroy-Stein O., Fuerst T. R., Moss B. Cap-independent translation of mRNA conferred by encephalomyocarditis virus 5' sequence improves the performance of the vaccinia virus/bacteriophage T7 hybrid expression system. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6126–6130. doi: 10.1073/pnas.86.16.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhood H., Bottega R., Epand R. M., Huang L. Effect of cationic cholesterol derivatives on gene transfer and protein kinase C activity. Biochim Biophys Acta. 1992 Nov 9;1111(2):239–246. doi: 10.1016/0005-2736(92)90316-e. [DOI] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst T. R., Niles E. G., Studier F. W., Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Huang L. A novel cationic liposome reagent for efficient transfection of mammalian cells. Biochem Biophys Res Commun. 1991 Aug 30;179(1):280–285. doi: 10.1016/0006-291x(91)91366-k. [DOI] [PubMed] [Google Scholar]
- Hoeben R. C., Valerio D., van der Eb A. J., van Ormondt H. Gene therapy for human inherited disorders: techniques and status. Crit Rev Oncol Hematol. 1992 Jul;13(1):33–54. doi: 10.1016/1040-8428(92)90015-i. [DOI] [PubMed] [Google Scholar]
- Immunotherapy of malignancy by in vivo gene transfer into tumors. Hum Gene Ther. 1992 Aug;3(4):399–410. doi: 10.1089/hum.1992.3.4-399. [DOI] [PubMed] [Google Scholar]
- Iost I., Guillerez J., Dreyfus M. Bacteriophage T7 RNA polymerase travels far ahead of ribosomes in vivo. J Bacteriol. 1992 Jan;174(2):619–622. doi: 10.1128/jb.174.2.619-622.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klement J. F., Moorefield M. B., Jorgensen E., Brown J. E., Risman S., McAllister W. T. Discrimination between bacteriophage T3 and T7 promoters by the T3 and T7 RNA polymerases depends primarily upon a three base-pair region located 10 to 12 base-pairs upstream from the start site. J Mol Biol. 1990 Sep 5;215(1):21–29. doi: 10.1016/s0022-2836(05)80091-9. [DOI] [PubMed] [Google Scholar]
- Moffatt B. A., Dunn J. J., Studier F. W. Nucleotide sequence of the gene for bacteriophage T7 RNA polymerase. J Mol Biol. 1984 Feb 25;173(2):265–269. doi: 10.1016/0022-2836(84)90194-3. [DOI] [PubMed] [Google Scholar]
- Palmer T. D., Rosman G. J., Osborne W. R., Miller A. D. Genetically modified skin fibroblasts persist long after transplantation but gradually inactivate introduced genes. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1330–1334. doi: 10.1073/pnas.88.4.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaran L. A simple quantitative assay for chloramphenicol acetyltransferase by direct extraction of the labeled product into scintillation cocktail. Anal Biochem. 1992 Jan;200(1):180–186. doi: 10.1016/0003-2697(92)90296-j. [DOI] [PubMed] [Google Scholar]
- Tunitskaya V. L., Akbarov A. Kh, Luchin S. V., Memelova L. V., Rechinsky V. O., Kochetkov S. N. Inactivation of bacteriophage T7 DNA-dependent RNA polymerase by 5'-p-fluorosulfonylbenzoyladenosine. Identification of the modification site and the effect of the modification on enzyme action. Eur J Biochem. 1990 Jul 20;191(1):99–103. doi: 10.1111/j.1432-1033.1990.tb19098.x. [DOI] [PubMed] [Google Scholar]
- Zhou X. H., Klibanov A. L., Huang L. Lipophilic polylysines mediate efficient DNA transfection in mammalian cells. Biochim Biophys Acta. 1991 May 31;1065(1):8–14. doi: 10.1016/0005-2736(91)90003-q. [DOI] [PubMed] [Google Scholar]



