Abstract
The obligate intracellular parasite Toxoplasma gondii is exposed to a variety of physiological conditions while propagating in an infected organism. The mechanisms by which Toxoplasma overcomes these dramatic changes in its environment are not known. In yeast and plants, ion detoxification and osmotic regulation are controlled by vacuolar compartments. A novel compartment named the plant-like vacuole or vacuolar compartment (PLV/VAC) has recently been described in Toxoplasma gondii, which could potentially protect extracellular tachyzoites against salt and other ionic stresses. Here we report the molecular characterization of the vacuolar type Na+/H+ exchanger in T. gondii, TgNHE3, and its co-localization with the PLV/VAC proton-pyrophosphatase (TgVP1). We have created a TgNHE3 knockout strain, which is more sensitive to hyperosmotic shock and toxic levels of sodium, possesses a higher intracellular Ca2+ concentration [Ca2+]i, and exhibits a reduced host invasion efficiency. The defect in invasion correlates with a measurable reduction in the secretion of the adhesin TgMIC2. Overall, our results suggest that the PLV/VAC, has functions analogous to those of the vacuolar compartments of plants and yeasts, providing the parasite with a mechanism to resist ionic fluctuations, and potentially, regulate protein trafficking.
Keywords: Toxoplasma gondii, NHE, vacuole, invasion, secretion, VP1, processing
INTRODUCTION
The ability to adapt to diverse physiological conditions is one of the hallmarks of intracellular microorganisms. Among these, the protozoan Toxoplasma gondii is one of the most versatile and exhibits a remarkable aptness to withstand and adjust to a variety of biological contexts. T. gondii can infect virtually every warm blooded animal and is estimated to infect one third of the world's human population [1]. In the gut of felines, the only host in which the parasite is known to reproduce sexually, T. gondii gametocytes mate to form oocysts, which are shed in the cat's feces and can survive for months in soil or water. Ingestion or inhalation of these oocysts by a non-feline warm-blooded animal initiates the asexual stages of the parasite's life cycle, which include the fast propagating tachyzoite stage and the latent bradyzoite form. The tachyzoite form of T. gondii, which can invade and replicate in virtually any nucleated cell, is responsible for the pathogenicity of T. gondii because it lyses the host cell upon exit. Repeated cycles of entry, replication, and exit, in the tachyzoite stage, allow the parasite to disseminate throughout the host, and even to cross the blood-brain and placental barriers. Once the immune system responds to the presence of T. gondii, the tachyzoite converts to the encysted bradyzoite form. Thus, during this complex life cycle, T. gondii encounters several physiologically unique environments as it moves between host species, within different tissues, and from the inside to the outside of a cell.
The particular mechanisms and proteins that confer T. gondii with this remarkable adaptability are not known. Nonetheless, given that the concentrations of ions such as H+, Na+, and K+ are greatly varied in the different environments the parasite encounters, it is likely that ion transporters, exchangers and channels play an important role in the adaptability of the parasite. Of the various types of ion transporters present in T. gondii, Na+/H+ exchangers (NHEs) are likely candidates to influence survival since they are critical to the regulation of pH, cell volume, and ion detoxification [2].
NHEs are part of the CPA superfamily of monovalent cation proton antiporters and typically contain 10 to 12 transmembrane domains, within the first N-terminal half of the protein, involved in the antiport transport of sodium and protons. NHEs fulfill a wide array of cellular functions including ion homeostasis, cation movement, regulation of organellar and cytosolic pH, regulation of membrane movement, vesicle trafficking and biogenesis [2-9]. There are two distinct subgroups of NHEs, the plasma membrane localized NHEs, and the intracellular NHEs [10]. The plasma membrane localized exchangers have been implicated in the control of cytoplasmic pH, maintenance of cell volume, and sodium homeostasis [11]. Not much is known about the intracellular NHEs, although many of these have been recently identified, including the four human isoforms NHE6 to NHE9 [10]. By far, the best characterized of the intracellular NHEs is the Saccharomyces cerevisiae NHX1, ScNHX1, which localizes to the pre-vacuolar compartment. ScNHX1 is responsible for the sequestration of sodium in the yeast vacuole, thus providing yeast cells with a mechanism for tolerance to osmotic and ionic stresses [9].
ScNHX1 has also been shown to be involved in retrograde transport of vesicles from the Golgi to the plasma membrane, by association with ScGyp6, a GTPase-activating protein (of the Rab family of GTPases) [12]. Accordingly, disruption of ScNHX1 not only yields strains highly sensitive to hyperosmotic shock, but also causes deficient protein processing, trafficking, and sorting, with the concomitant accumulation of intermediates in vacuolar and pre-vacuolar compartments [11, 13]. A similar role has been ascribed to the NHEs localized in pre-vacuolar compartments, recycling endosomes, or exocytic compartments, such as the human HsNHE6 in recycling endosomes [6]. Interestingly, a novel organelle with similar composition and function to the plant and yeast vacuoles termed the plant-like vacuole or vacuolar compartment (PLV or VAC) [14, 15] has recently been described in T. gondii. This vacuolar compartment was shown to have Na+/H+ exchange activity [14] and an important role in processing of proteins destined to the micronemes [15].
Inspection of the T.gondii genome [16] revealed the presence of four proteins with strong homology to NHEs, out of which TgNHE1 and TgNHE2 have been characterized [17, 18]. TgNHE1 localizes to the plasma membrane [17] while TgNHE2 was shown to be localized at the rhoptries [18]. Here we present the molecular characterization of a third protein with homology to Na+/H+ exchangers in T. gondii, which we have named TgNHE3. This NHE represents a candidate to influence the environmental adaptability of T. gondii because it is most similar to NHEs usually found in plant and yeast vacuoles. We have found that TgNHE3 partly colocalizes with proteins known to be in the PLV/VAC, and we have determined that TgNHE3 is needed for normal resistance to osmotic stress and for microneme secretion, thus, providing direct evidence for the role of the PLV/VAC in osmoregulation and protein trafficking in T.gondii.
EXPERIMENTAL PROCEDURES
Parasite and cell culture
For the experiments described here we used parasites of the RH strain lacking a functional hpt gene, RHΔhpt [19]. Parasites were maintained by serial passage in human foreskin fibroblasts (HFFs) in a humidified incubator at 37°C with 5% CO2. The culture medium was Dulbecco's Modified Eagle Medium (DMEM, GIBCO), supplemented with 10% fetal bovine serum (Atlanta Biologicals), 2 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml of streptomycin. Stock solutions of 1 M NaCl (Sigma), 1.2 M KCl (Sigma), 2 M Sorbitol (Sigma), and 1 M Sodium Gluconate (Sigma) were prepared using Ringer Buffer (155 mM NaCl, 3 mM KCl, 2mM CaCl2, 1 mM MgCl2, 3 mM NaH2PO4-H20, 10 mM HEPES, 10 mM Glucose, pH 7.2) as solvent. Stock of 1 mM Calcimycin A23187 (Sigma) was prepared using 100% DMSO as solvent and Hygromycin (Sigma) solution was prepared in water to a final concentration of 50 mg/ml.
Cloning of TgNHE3
The RHΔhpt TgNHE3 gene was cloned and sequenced using a cDNA library to amplify overlapping fragments using primers against predicted exons. The 5’ and 3’ ends were obtained using 5’ and 3’ RACE respectively. For PCR amplification of the 5’ and 3’ region, the GeneRacer Kit (Invitrogen) and RNA isolated from an RHΔhpt strain were used. The 5’ end was reverse transcribed with the TgNHE3 specific primer, 5’-caagccacataatcgcgccg-3’ and the resulting cDNA was used in nested PCR reactions using the primers provided with the kit along with the gene specific primers 5’-gcgacaggcatctgcagc -3’ (for the primary reaction) and 5’-ctgctcagctcctcaagcga-3’ (for the nested reaction).
To identify the 3’ end of the transcript, RNA was reverse transcribed using the GeneRacer Oligo dT primer and the resulting cDNA was used as a template in nested PCR reactions using the GeneRacer 3’ primers in combination with 5’-catttccgaggagatgcgagacag-3’ (for the primary reaction) and 5’-tgaattgcagaatggatggcagcc -3’ (for the nested reaction).
Bioinformatic analysis
Protein-protein Blast (BlastP) search using the entire TgNHE3 was performed using the non-redundant protein database excluding proteins generated by models. Expected threshold was set at 10 and the scoring parameters matrix used was BLOSUM 62. Gap costs was set as existence: 11, extension: 1.
Alignment for phylogenetic analysis was done using the ClustalW program of the DNAStar/Megaling package. Gap penalty was set at 50 and Gap length penalty was 0.1. The BLOSUM matrix was used to weight mismatches. Because phylogeny estimation is sensitive to alignment errors (e.g., [20]), we used GBLOCKS v. 0.91b [21] to identify conserved regions where the alignment was conserved. We used the least stringent conditions, which will result in fewer sites excluded. The objective exclusion of ambiguously aligned regions using this approach has been shown to improve phylogeny estimation [22].
Conserved blocks of well-aligned regions were subjected to Bayesian phylogeny estimation with MRBayes (v. 3.2.1; [23]). We allowed the program to estimate the amino-acid sequence model (using the model jumping options: prset aamodelpr=mixed). We ran the two independent cold chains (each linked to three heated chains) for 106 generations and monitored the standard deviation of split frequencies between the two independent cold chains to assess convergence of the Markov chain Monte Carlo (MCMC) analysis and trees were sampled every 100 generations. The burn-in period was identified as the generations prior to the reduction standard deviation of split frequencies below a value of 0.01, and the two post-burn in distributions of trees were merged to generate the posterior distribution of topologies. A uniform topology prior and exponential prior on branch lengths were used.
Generation of TgNHE3 knockout strain
The knockout construct was generated using the pminiGFP.ht vector [17], which contains the HPT marker flanked by two multiple cloning sites. To design the knockout construct a PCR amplicon corresponding bases 4,322,126 to 4,324,133 of chromosome 9 (Fragment 1 in Fig. 5A, nucleotide numbers based on sequence of strain GT1) was digested with for NotI and XbaI, and ligated into pminiGFP.ht vector digested with the same enzymes. The resulting plasmid was digested with HindIII and KpnI and ligated to a PCR fragment that included bases 4316322 to 4319515 of chromosome 9 and the HindIII and KpnI restriction sites (Fragment 2 in Fig. 5A). The primers utilized to amplify the flanking fragments were 5’-ggggcgccggcgctacgtccaccctgtgt-3’ and 5’-ggggtctagactcgagacgtgagagaggac-3’ for fragment 1 and 5’-ggggaagcttcttccgcaactccagcgggt-3’ and 5’-ggggtacccgtgaagggtcttctacacc-3’ for fragment 2.
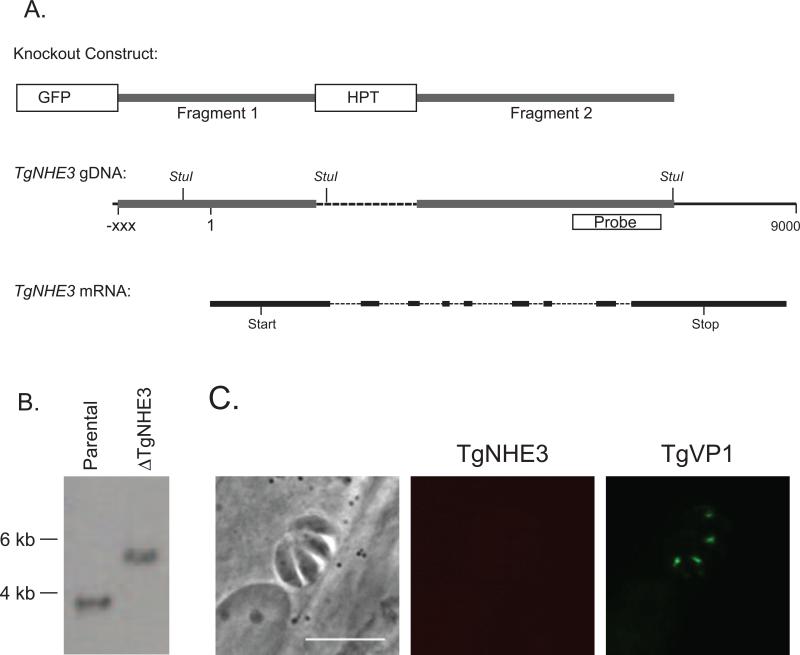
Figure 5.
Establishment of a TgNHE3 knockout strain. A. Schematic of the knockout construct (top diagram), the genomic region targeted by insertion of the construct (middle diagram) and the relative position of the introns and exons (bottom diagram). The knockout construct contains the HPT gene flanked by regions of the TgNHE3 genomic locus (fragments 1 and 2 in top and middle diagrams), and the GFP gene. When the knockout construct is incorporated into the NHE3 locus by double homologous recombination a 1.5 kb fragment of the gene (dotted lines in middle diagram) is replaced by the HPT selectable marker and the GFP marker is lost. The location of the probe and the StuI restriction sites used for Southern analysis are indicated in the genomic DNA schematic. B. Southern blot analysis of StuI digested DNA from the RHΔhpt and RHΔnhe3 parasite strains using a probe that recognizes a region of the NHE3 genomic locus (box in A). Insertion of pKONHE3 into the TgNHE3 locus deletes a StuI restriction site, which results in a larger restriction fragment. C. Immunofluorescence analysis of RHΔnhe3 using the TgNHE3 and VP1 antibodies. Scale bar = 10 μm
Fifty μg of the resulting vector, pKONHE3, were linearized with NotI, and transfected into RHΔhpt strain using standard methods [24]. Three independent transfection reactions were performed and stable transfectants were selected for with 50 μg/ml mycophenolic acid and 50 μg/ml xanthine and cloned by limiting dilution. Six clones were tested by PCR for the desired disruption using genomic DNA isolated with the DNeasy Blood and Tissue Kit (QIAGEN). The PCR reaction used a forward primer (5’-tgttggctcgtgtctctccg-3’) that anneals to a sequence in the TgNHE3 locus and a reverse primer (5’- gcatgtggagatgctgccgt-3’) that anneals to a sequence absent in the knockout strain (supplemental figure S4). Using this primer set, a 450 bp band is amplified from DNA of wild-type parasites, but not from parasites with a deletion in TgNHE3KO. As a control, PCR was also performed using the forward primer 5’-tgcatgcatccactgccacg-3’ and the reverse primer, 5’-tgcctgcgatcgaggtggag-3’, which yield an 800 bp band with DNA from both parental and knockout strains. Six clones were determined to be disrupted in the TgNHE3 locus by this PCR assay and were frozen for storage and analyzed further by Southern Blot analysis.
Complementation of the knockout strain was achieved using a vector containing the NHE3 cDNA under the control of the NHE3 promoter. This vector was generated using 3 fragments that included a) genomic DNA containing 2 kb upstream of the NHE3 start codon and part of the first intron (fragment 1), b) the open reading frame of TgNHE3 including an HA tag immediately before the stop codon (fragment 2) and c) the 3’UTR of TgNHE3 (fragment 3). These fragments were generated by PCR cDNA from genomic DNA (fragments 1 and 3) or (cDNA fragment 2) and cloned separately into Invitrogen's Topo cloning system vector pCR12.1. The primers used were 5’ggtaccgcactctccagctgcatgccgctcaacaacg3’ and 5’cctcagtgctagccgcgggagaacgcg3’ for fragment 1, 5’-atgtcgtttttgctgggacgaggctcg-3’ and 5’-ttaattaatcaCGCGTAGTCCGGGACGTCGTACGGGTAgagcacttgtctctttccaacg-3’ for fragment 2, and 5’-ttaattaaggaaggagtgcgaaagcgatg-3’ and 5’-gcggccgcccgcggagttaccgaattggc-3’for fragment 3. To assemble the TgNHE3 gene we first inserted fragment 1 into the EcoRV and NheI sites of the fragment 2 containing plasmid. This resulted in an in frame junction of the promoter and first intron with the rest of the TgNHE3 ORF. Next, fragment 3 was cloned into the PacI and HindIII sites of the pCR2.1NHE3prom vector containing the ORF. Finally, a copy of a pyrimethamine resistant allele of DHFR-TS digested from vector pDHFR-TSc3 [25] was cloned into the HindIII and SpeI sites of the vector containing the TgNHE3 gene. 30μg of the resulting vector, pNHE3RES, were linearized with EcoRV or HindIII, and transfected to RHΔhptΔnhe3 using standard methods [24]. Four independent transfections were performed and parasites were transferred to T25 culture flasks containing complete media. 24 hours following transfection, the media was replaced with culture medium containing 1μM pyrimethamine-sulfadiazine to select for stable transfectants and those parasites stably expressing the DHFR pyrimethamine resistant allele were cloned by limiting dilution. Six clones were tested by immunofluoresence assay for expression of TgNHE3.
Southern blot analysis
Southern blot analysis of the RHΔhptΔnhe3 clones was performed using established methods [26]. In brief 5 μg of genomic DNA from each of the clones and from the parental strain isolated by TELT DNA isolation protocol [27] were digested separately with StuI, and separated in a 0.7% agarose gel. Fragments were transferred overnight to a nylon membrane (Magna, Osmonics, Inc.) and DNA was cross-linked using UV light (UV Stratalinker 1800 -Stratagene). The DNA was hybridized to a [α32P-dCTP] labeled probe, which consisted of a 600 bp fragment of TgNHE3 genomic DNA (Fig. 7A). The probe was obtained by PCR using the primers 5’-tcagcgacgggtggggaagg-3’ and 5’-tcaaacctccgcgtcgcgga-3’.
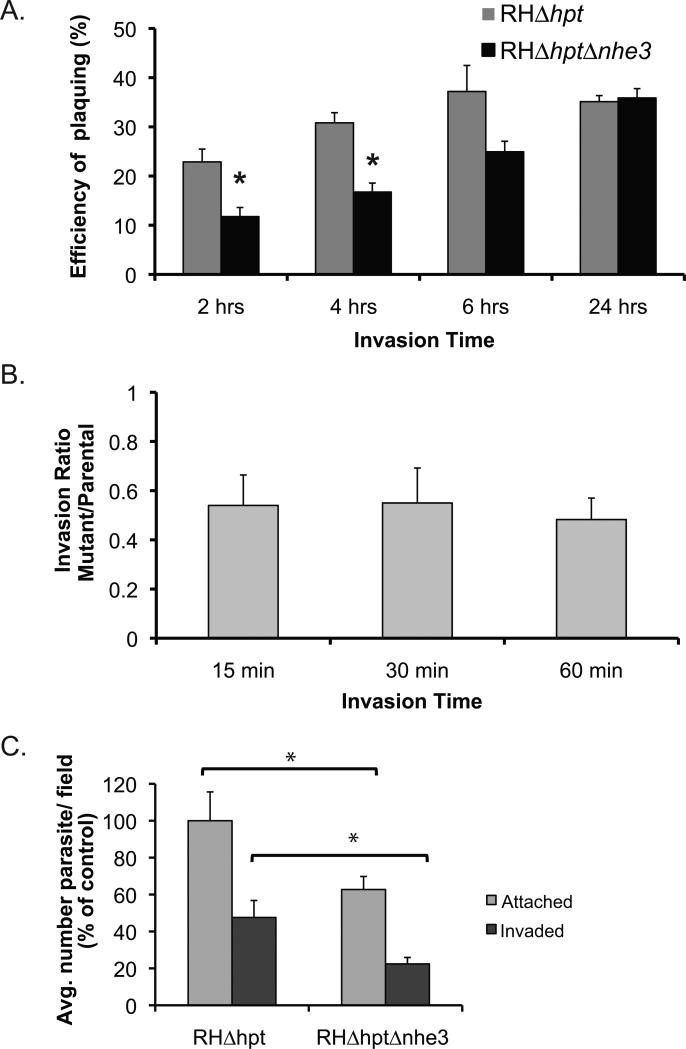
Figure 7.
Invasion efficiency of the Tgnhe3 knockout strain. A. Parasites of either the parental (RHΔhpt) or the Tgnhe3 knockout (RHΔhptΔnhe3) strains were allowed to invade cells for either 2, 4, 6, or 24 hours. After washing off extracellular parasites those that entered cells were allowed to develop plaques. Percent plaquing efficiency was calculated by dividing the number of plaques formed by the number of parasites initially added to the tissue. Data were compiled from 4 independent experiments. Error bars represent standard deviation and the asterisk depicts statistical significance as determined by a two-tailed t-test (p<0.05). B. Equal numbers of either RHΔhpt or RHΔhptΔnhe3 parasites were added to fibroblasts. After 15, 30, or 60 minutes the number of invaded parasite was determined. The data is expressed as a ratio of the knockout parasites that invaded over the number of parental strain parasites that entered a cell. Data were compiled from 4 independent experiments. Error bars represent standard deviation. C. Equal numbers of RHΔhpt or RHΔhptΔnhe3 parasites were incubated with HFFs for 30 minutes the number of invaded parasites (ones inside) and the number of attached parasites (one inside and outside) was counted for a total of 20 fields for each strain. The average of attached parasites per field for the parental strain was used as the standard to calculate the % of control. Data were compiled from 4 independent experiments (20 view fields per strain in each). Error bars represent standard deviation and the asterisk depicts statistical significance as determined by a two-tailed t-test (p<0.05).
Generation of TgNHE3 antibody
Antibody was raised against a 200 amino acids (AAs 742 to 941) non-transmembrane fragment of TgNHE3. This fragment was generated using bacterial expression, for which purpose a PCR fragment was obtained from cDNA using primers 5’-caccatgcgacgtcaaagtcgaga-3’ and 5’-ggagacctttcccgagggcctt-3’. This PCR fragment was cloned into the pBAD202/D-TOPO vector (Invitrogen) in frame with a N-terminal His-patch thioredoxin peptide and a C-terminal 6x His-tag. Protein expression was induced in E. coli with 0.2% arabinose, following the manufacturer's instructions and the protein fragment was purified using a Ni-NTA agarose (Qiagen). Recombinant protein and 100 μL MPL adjuvant (Invivogen) were injected every 3 weeks into a female BALB/C mouse (Simonsen Laboratories). For the guinea pig antibody a peptide [H]- CKKDVRVALPDALRASALLSDS -[NH2], which contains amino acids 1005 to 1023 of TgNHE3, was synthesized by Princeton BioMolecules. A guinea pig was injected with 100ug of KLH conjugated peptide in Freund's complete adjuvant, followed by four boosts with 50ug antigen in incomplete Freund's adjuvant. Each injection was performed subcutaneously in 4 sites. The antibody was affinity purified using the same peptide that was used for the immunization. Immunization and affinity purification for the guinea pig antibodies were performed by Open Biosystems.
Epitope tagging of TgNHE3
A construct for tagging the endogenous TgNHE3 locus was generated from the pTKO vector [28] (a gift from John Boothroyd), which has multiple restriction sites flanking sequences that contain the Gra2 3’UTR and the selectable marker HPT. Into the NotI and EcoRV sites upstream of the Gra2 3’UTR we cloned a 2.5 kb fragment corresponding to the genomic sequences that immediately precede the TgNHE3 stop codon. This fragment was generated by PCR using the primer set, 5-′-gcggccgcggaatgagatccaagtcgag-3′ and 5′-gatatctcacgcgtagtccgggacgtcgtacgggtagagcacttgtctctttccaa-3′. The second primer contains the bases right before the TgNHE3 stop codon followed by sequences encoding the HA epitope and a stop codon. We then introduced a 2 kb fragment corresponding to genomic DNA sequences directly downstream of the TgNHE3 stop codon into the ApaI and NheI sites downstream of the HPT gene in pTKO. This second fragment was generated using the forward primer, 5′-gctagcggaaggagtgcgaaagcgat-3′, which contains an NheI site, and the reverse primer, 5′-gggccctgcagggatgataggtttttgccca-3′, which contains an ApaI site in the amplified DNA. The resulting vector was linearized with ApaI, and 30 μg of the linearized DNA was transfected into RHΔhpt parasites. Following selection for the presence of the vector with mycophenolic acid, stable transformants were cloned and the integration of the construct into the TgNHE3 locus was confirmed by staining parasites with rabbit antibodies against the HA epitope (Rockland Immunochemicals). The established parasite line was named RHΔhpt+NHE3HA.
Immunofluorescence assays
Immunofluorescence analysis assays were performed on intracellular tachyzoites grown for 16 hours in HFFs on coverslips as described before [17]. For staining of extracellular parasites, freshly lysed 1 × 106 tachyzoites we allowed to precipitate onto glass coverslips by gravity and then stained by the same method as with intracellular parasites [17]. For primary antibodies we used either our mouse or guinea pigs anti-TgNHE3 at 1:500 or the Rabbit antiTgVP1 or the rabbit anti-HA antibodies (Rockland Immunochemicals) at 1:2000. The secondary antibodies, manufactured by Molecular Probes and conjugated to either Alexa Flour 594 or Alexa Flour 488, were used at a dilution of 1:2000. Stained parasites were observed by phase contrast and fluorescence using a 60x objective in a Nikon Eclipse E1000 microscope equipped with a C4742-95 camera.
For extracellular tachyzoite localization of TgNHE3 and TgVP1 by indirect immunofluorescence, endogenously NHE3-tagged parasites (HA epitope) were grown in htert mammalian cells. Upon egress parasites were collected and washed in Buffer A glucose (116 mM NaCl, 5.4 mM KCl, 0.8 mM MgSO4, 50 mM HEPES, pH 7.2, 5.5 mM glucose) with glucose and processed as previously described [29]. Primary antibodies were: TgNHE3 (guinea pig, 1:500 dilution) and TgVP1 (rabbit, 1:4000 dilution). Secondary antibodies (Molecular Probes) were Alexa 568nm (for TgNHE3) and Alexa 488nm (for TgVP1). Images were captured with an Olympus IX-71 inverted fluorescence microscope equipped with DIC optics and attached to a Photometrix CoolSnap CCD camera. All images were deconvolved using a DeltaVision Image Restoration System (Applied Precision, Seattle, WA).
Immunoelectron microscopy
For electron microscopy analysis, T. gondii of either the NHE3 HA-tagged strain or a TgVP1 over expressing line [14] were used. Extracellular tachyzoites were fixed in 4% paraformaldehyde/0.05% glutaraldehyde (Polysciences Inc., Warrington, PA) in 100mM PIPES/0.5mM MgCl2, pH 7.2 for 1 hr at 4°C. Samples were then embedded in 10% gelatin and infiltrated overnight with 2.3M sucrose/20% polyvinyl pyrrolidone in PIPES/MgCl2 at 4°C. Samples were trimmed, frozen in liquid nitrogen, and sectioned with a Leica Ultracut UCT cryo-ultramicrotome (Leica Microsystems Inc., Bannockburn, IL). 50 nm sections were blocked with 5% FBS/5% NGS for 30 min and subsequently incubated with primary antibodies at 1:2000 for rabbit anti-TgVP1, 1:50 for the guinea pig antibody and 1:250 for the rabbit anti HA antibody (Sigma). This was followed by incubation with the appropriate secondary antibody conjugated to 12 or 18 nm colloidal gold (Jackson ImmunoResearch Laboratories, Inc., West Grove PA). Sections were washed in PIPES buffer followed by a water rinse, and stained with 0.3% uranyl acetate/2% methyl cellulose. Samples were viewed with a JEOL 1200EX transmission electron microscope (JEOL USA Inc., Peabody, MA). All labeling experiments were conducted in parallel with controls omitting the primary antibody. These controls were consistently negative at the concentration of colloidal gold conjugated secondary antibodies used in these studies.
Quantification of osmotic sensitivity and plaquing efficiency
For all assays testing sensitivity to osmotic stresses, parasites were mechanically lysed from fibroblasts with a syringe and a 27.5 G needle. Cells were spun down 10 min at 2000 rpm, washed in 1 ml of Ringer buffer (155mM NaCl, 3mM KCl, 2mM CaCl2, 1mM MgCl2, 3mM NaH2PO4-H20, 10 mM HEPES, 10 mM Glucose, pH 7.2), and resuspended in 1 ml of Ringer buffer. Parasites were counted and diluted to a final concentration of 1.2 × 106 parasites/ml. Aliquots of 500 μl of parasites in Ringer buffer were combined with 500 μL of sorbitol to a final concentration of 0.5, 0.75 and 1 M, or with 500 μl NaCl to a final concentration of 0.5 or 1 M or with 500 μL KCl to a final concentration of 0.3 or 0.6 M, or with 500 μl sodium gluconate to a final concentration of 0.5 or 0.75 M. In all cases the salt or sugar was diluted in Ringer buffer. In addition, one aliquot of parasites was combined 500 μL of Ringer buffer alone to be used as a control. Parasites were then incubated at 37°C for 30 min, at which time, 5 μl of each aliquot, i.e. 600 parasites, were plated in triplicate in 24 well plates containing confluent HFFs. Parasites were allowed to invade for 30 min, at which time all wells were washed with PBS, the media was replaced with fresh culture media, and plates were incubated at 37°C for 4 days to allow the development of plaques. Cultures were then methanol-fixed, and stained with crystal violet to visualize plaques. Plaques formed were counted for each strain and condition, under a stereomicroscope. To determine the efficiency of plaquing under treatment we divided the number of plaques formed by treated parasites by the number of plaques formed by untreated ones. Data was compiled from 4 different experiments, each one done in triplicate. Statistical analyses were performed using a two-tailed t-student test. Differences were considered significant if p-values were < 0.05.
To monitor the propagation of the knockout strain as compared to that of the parental, monolayers of HFFs in 24 well plates were infected with 400 parasites per well, which were allowed to invade for 1, 2, 4, 6, and 24 hours at 37°C. Wells were then washed with PBS twice and the media replaced with normal culture medium. After 4 days the cultures were processed as described above to reveal the number of plaques. The data was calculated as the percentage of the parasites added to the cultured that formed a plaque (i.e. number of plaques over 400) for each strain and each invasion time.
Invasion assay
HFFs monolayers on cover slips were infected with 2 × 106 parasites/well, and after 15, 30, or 60 minutes incubation at 37°C, cultures were fixed with 4% formaldehyde. To differentiate those parasites that have entered cells from those that remained outside we utilized an established immunofluorescence-based assay [30]. The number of intracellular parasites in each coverslip was determined from 20 randomly chosen fields at 1000X total magnification in an Axiovert HBO 50 microscope. The data were expressed as a ratio of the total number of intracellular knockout parasites over the number of intracellular parental parasites.
Secretion assay and western blots
The secretion assay was performed as described by Huynh, et al., 2003 [31] with a few modifications. In all assays 1 × 108 parasites were resuspended in 100 μL of pre-warmed invasion medium. For induced secretion, parasites were incubated with 200 mM A23187 at 37°C for 4 minutes. For the constitutive secretion assays parasites were incubated at 37°C for 30 minutes in invasion media [31, 32]. After incubations parasites were spun down and both the supernatant and the precipitate were used in western blot analysis using standard methods [26]. For each of the assays, 66 μL of supernatant and 10 μL of cell lysate were separated in an 8% acrylamide gels, and transferred to a membrane. Antibody against MIC2 raised in mouse (a gift from Dr. Vern Carruthers) was used at a final concentration of 1:10000 and visualized with a peroxidase-conjugated Goat anti-mouse secondary antibody (Promega) and the Supersignal West Pico Chemiluminescent Substrate solution (Thermo Scientific).
Ca2+ measurements
Measurements of intracellular calcium concentrations in NHE3-knockout mutants were performed using Fura 2-AM (Invitrogen) as previously described [33].
RESULTS
TgNHE3 encodes a protein with homology to intracellular Na+/H+ exchangers
A Blastp search through the T. gondii genome [16] for homologs of human NHE1 revealed the presence of a putative uncharacterized NHE in chromosome IX (TGGT1_042320, aka 541.m01159). To determine whether the predicted gene is expressed and encodes an NHE homolog, we set out to clone and sequence the entire cDNA. Overlapping fragments of the internal section of the coding sequences were amplified from a cDNA library using primers designed against predicted exons. In addition, we determined the ends of the cDNA using 5’ and 3’ RACE. Using this approach we identified a 5,479 bp transcript encoded within a 9 kb genomic region containing 9 introns and 10 exons (Fig. 1A and supplemental figure S1, NCBI accession number GU230797.1). We consistently obtained the same results from different RNA samples suggesting that there are no alternative transcripts. We have named this gene TgNHE3 since it is the third characterized NHE homolog in T. gondii [17, 18].
Figure 1.
Molecular characterization of TgNHE3. A. TgNHE3 is within a 9 Kb region of chromosome 9 (base 1 above indicates site of transcription start). The encoded mRNA is 5,479 kb mRNA. The relative position of the 9 exons (dark lines) and 5 introns (broken lines) are indicated in the mRNA schematic. Conceptual translation of TgNHE3 reveals protein of 1,116 amino acids in length, which contains 12 predicted transmembrane domains (black boxes in protein schematic). B. Alignment of the 14 amino acids common to all eukaryotic sodium hydrogen exchangers and known to be important for ion exchange [10]. The corresponding fragments in TgNHE3, ScNHX1 from Saccharomyces cerevisiae, AtNHX1 and AtNHX6 from Arabidopsis thaliana, the rice OsNHX1 from Oryza sativa, and the human HsNHE6 from human were aligned using the Clustal W method within MegAlign (DNA Star). The numbers adjacent to the protein sequences denote the location of the first and last aligned residue within the corresponding protein. C. Unrooted consensus tree summarizing the posterior distribution of trees from the Bayesian MCMC analysis. The numbers associated with the internal branches are split frequencies. Shaded sequences represent intracellular sequences and the TgNE3 sequence (box) nests within this group with a very high probability. Complete lists of proteins and their accession numbers are in supplemental Figure S2.
Conceptual translation of TgNHE3 reveals a 1,116 amino acid protein with a molecular weight of 132 KDa. This protein contains 10 to 12 predicted transmembrane domains (TMpred [34] and TMHMM [35]), between residues 267 to 685 (Fig. 1A and supplemental figure S1). The distribution and amino acid composition of the transmembrane domains are consistent with members of the CPA1 family of transporters, which include the human plasma membrane NHE1, and yeast prevacuolar ScNHX1 [10]. Additionally, TgNHE3 contains a stretch of 14 amino acids that is conserved in all characterized members of the CPA1 family of Na+/H+ exchangers and is associated with function (Fig. 1B). However, in TgNHE3 this conserved domain is localized at the predicted second transmembrane domain, while in most orthologs it is localized at the fourth domain.
TgNHE3 shows limited identity to the previously characterized Toxoplasma's NHEs, TgNHE1 [17] and TgNHE2 [17, 18] and to the additional plasma membrane NHE, which we have recently characterized and refer to as TgNHE4 (unpublished data, NCBI accession number GU230798). Based on protein-protein Blast (blastp) searches through the non-redundant protein sequence (nr) database, TgNHE3 shows 7% identity and 18 % similarity to TgNHE, 4% identity and 6% similarity to TgNHE2, and 12% identity and 18% similarity to TgNHE4. This identity is solely within the strongly conserved transmembrane domain, which represent only 37% of the protein sequence. Instead of NHEs from T. gondii or other apicomplexan parasites, the proteins with most identity and similarity to TgNHE3, based on the blastp search, were putative NHE homologs from plant, algae and arthropods. An extensive phylogenetic analysis of 156 CPA1 Na+/H+ exchangers performed by Brett, Donowitz and Rao [10] resulted in the categorization of these proteins in four major clades: intracellular (IC-NHE), plasma membrane (PM-NHE), NhaP-SOS1, and NhaPII. Out of the 156 CPA1 Na+/H+ exchangers used by Brett and colleagues in their analysis [10], 10 (Homo sapiens NHE7 and NHE8, Drosophila melanogaster NHE1 and NHE3, Caenorhabditis elegans NHX8, Mus musculus NHE7 and NHE8, Lycopersicon esculentum NHX2 and Arabidopsis thaliana NHX5 and NHX6) are within the top 100 top matches (E value < 7e-50) obtained in a BlastP search using TgNHE3 as the query. Interestingly, all of these are within the IC-NHE category.
To confirm that TgNHE3 falls within the intracellular group of NHEs we performed phylogenetic analysis (see Experimental Procedures for details) with 18 proteins previously used to analyze the sequence characteristics of IC-NHE and PM-NHE proteins [10], along with TgNHE1, 2 and 3. The resulting phylogeny (Fig. 1C) indicates that TgNHE3 is nested within the intracellular group with a very high posterior probability. Furthermore, there are no trees in the posterior distribution in which the TgNHE3 sequence occurs outside the intracellular group. Thus, the phylogenetic analyses provide strong corroboration that the TgNHE3 sequence has likely evolved from an intracellular ancestral sequence.
TgNHE3 localizes to an intracellular compartment
Given the similarity of TgNHE3 to IC NHEs at the sequence level, we wanted to determine whether TgNHE3 localizes to an intracellular organelle. For this purpose we raised polyclonal antibodies to a bacterially expressed 200 amino acids fragment from the non-transmembrane C-terminal domain of TgNHE3 (residues 917 to 1116, supplemental figure S1) in mice and to a peptide (residues 1005 to 1023, supplemental figure S1) in guinea pig. Using these antibodies we performed immunofluorescence assays (IFA) of intracellular parasites (Fig. 2A and B respectively). In addition, to confirm the localization, we tagged the TgNHE3 endogenous locus with a haemagglutinin (HA) tag using a double homologous recombination strategy to generate the parasite line RHΔhpt+NHE3HA. Staining of these parasites with an anti-HA antibody and either of the anti-NHE3 antibodies shows coincidence between the signals (supplemental figure S3). With the mouse antibody we occasionally observe punctate staining outside of the region stain by the guinea pig or HA antibodies. This is likely background staining as it is observed in the pre-immune serum. Nonetheless, regardless of the antibody or parasite strain used, we observed the same intracellular staining pattern (Fig. 2). Consistent with our phylogenetic and sequence analysis, which suggests similarity to intracellular NHEs, TgNHE3 localizes intracellularly, within a defined domain near the nucleus toward the apical region of the parasite (Fig. 2). Co-staining experiments with antibodies against known markers of either the apicoplast, a non-photosynthetic plastid typically present near the nucleus, T. gondii's single mitochondrion, or the rhoptries, showed that TgNHE3 was not localized to any of these intracellular organelles (supplemental figure S4). Thus, TgNHE3 is not in any of the well-characterized parasite organelles.
Figure 2.
Localization of TgNHE3. A-B. Intracellular parasites of the RHΔhpt parental strain were stained with the anti-VP1 antibody (visualized in green) and either the mouse polyclonal antibody against TgNHE3 (A) or the guinea pig antibody against TgNHE3 (B) (both visualized in red). The last panel shows the overlay of the NHE3 and the VP1 stain. C. Intracellular parasites of the RHΔhpt+NHE3HA, in which the endogenous copy of TgNHE3 contains an HA epitope tag, were stained with a rabbit anti HA antibody (visualized in green) and antibodies raised against TgVP1 (visualized in red). The last panel shows overlay of both images. D. TgNHE3 (red) localizes to the PLV/VAC as represented by TgVP1 (green) in extracellular tachyzoites of T. gondii. Dapi staining of nuclear DNA is shown in blue. Scale bars = 10 μm in A to C, 5μm in D.
TgNHE3's sequence similarity to vacuolar NHXs led us to investigate whether its localization coincided with that of other T. gondii proteins with homology to vacuolar type proteins. A vacuolar type H+-translocating pyrophosphatase (TgVP1) has been described in T. gondii and shown to localize to both the acidocalcisomes [36, 37] and the newly described PLV/VAC [14, 15]. Co-staining with antibodies against TgNHE3 and TgVP1 [14] indicated that TgNHE3 co-localizes with TgVP1 (Fig. 2A, B and C). While the signals of both TgNHE3 and TgVP1 show significant overlap, the distribution is not identical. The TgNHE3 signal appears to occupy a smaller region than that of the TgVP1 stained region (Fig. 2A, B and C). We consistently see the TgNHE3 signal in the central portion of the TgVP1 stained area. In addition, when staining extracellular parasites for TgVP1 and TgNHE3 it became evident that TgVP1 is present in areas of the parasite in which TgNHE3 is not detected by neither anti-NHE3 antibodies nor the anti-HA, consistent with TgVP1 localizing to other organelles, such as the acidocalcisomes (Fig. 2D).
In order to better define the localization of TgNHE3 we performed immuno-gold electron microscopy experiments. We used the RHΔhpt+NHE3HA strain and anti-HA antibody to immuno-label cryo-sections of extracellular parasites. Consistent with the immunofluorescence assays, we detected positive labeling for TgNHE3 on the periphery of an apical membranous compartment, and closely associated vesicles (Fig. 3A). Consistently, all immuno-gold particles are observed next to or on the membrane of the compartment to which it localizes. To unequivocally show that this compartment is the PLV/VAC, we labeled extracellular parasites with anti-TgVP1 and anti-TgNHE3 antibodies. To ease detection of TVP1 we used the TgVP1 over-expressing strain previously utilized to define the PLV/VAC in immuno EM experiments [14]. Immuno-gold labeled sections of these parasites show significant coincidence between the signals for TgVP1 and TgNHE3 (Fig. 3B). These results further support the notion that TgNHE3 co-localizes with TgVP1 to the PLV/VAC.
Figure 3.
Electron microscopy of immunogold labeled parasites. A. Extracellular RHΔhpt+NHE3HA parasites were labeled with rabbit anti-HA antibody and 18 nm colloidal gold conjugated anti-rabbit secondary antibody. B. Parasites over expressing TgVP1 were stained with rabbit anti TgVP1 and guinea pig anti TgNHE3 antibodies. TgVP1 was visualized with 12 nm colloidal gold and TgNHE3 with 18 nm colloidal gold (black arrows). Small vacuoles (A, left) are probably acidocalcisomes. All images were taken at 40,000X, scale bar = 0.5μm.
TgVP1 has been shown to partly co-localize with a cathepsin L protease (TgCPL) to the PLV/VAC [14, 15]. Therefore, we assessed the localization of TgCPL in respect to TgNHE3, using an anti-CPL antibody (a gift from Vern Carruthers) for immunofluorescence experiments with the HA tagged TgNHE3 cell line (3A and B). TgNHE3 localization exhibits significant overlap with TgCPL in intracellular parasites (Fig. 4A). In extracellular parasites TgNHE3 shows a more dynamic relationship relative to TgCPL. While always in close proximity to TgCPL, the colocalization of TgNHE3 is only partial, both temporally and spatially (Fig. 4B shows partial colocalization in the left parasite, while no colocalization in the right parasite). This is consistent with the observation that the compartment containing these proteins might be highly dynamic [14, 15]. Thus, in general, TgNHE3 appears to have a localization pattern similar to that of VP1 and occupies the same region as TgCPL while the parasites are dividing.
Figure 4.
Epitope tagging of the endogenous TgNHE3. Intracellular (A) and extracellular (B) parasites of the RHΔhpt+NHE3HA strain were stained with an anti-HA antibody and the anti-CPL antibodies. Scale bars = 10 μm.
Generation of a TgNHE3 knockout T. gondii strain
In order to assess the function of TgNHE3 in the parasite, we generated a knock out strain of TgNHE3 and analyzed its phenotype. For this purpose we made a construct, pKONHE3, which consists of the selectable marker hypoxanthine-xanthineguanine-phosphoribosyl transferase (HPT), flanked by genomic regions of TgNHE3 such that when it integrates into the genome by double homologous recombination it replaces a 1543 bases fragment from the TgNHE3 locus with the HPT marker (Fig. 5A). The pKONHE3 plasmid also carries the green fluorescent protein (GFP) gene, which serves as a negative selectable marker to enrich for double recombination events (Fig. 5A). Following transfection of a wild type strain of T. gondii with pKONHE3 we established a stable GFP negative, HPT positive clone, which was confirmed to carry a disruption of the TgNHE3 locus by PCR (supplemental figure S5) and named RHΔhptΔnhe3. To unequivocally show that the established clone had the expected deletion, Southern blot analysis of StuI digested gDNA from the RHΔhptΔnhe3 and the parental strain, was performed using a 600 bp fragment of the TgNHE3 locus as a probe (Fig. 5B). Based on the known genomic sequence, this probe should detect a 3.1 kb fragment in StuI digested DNA from the parental strain and a 5.7 Kb fragment when pKONHE3 is integrated into the targeted locus. As seen in Fig. 5B, the Southern blot shows the expected band for each strain indicating that TgNHE3 has been disrupted as predicted in the knockout strain and that there is no other insertion of the construct in this clone.
To confirm that the genomic disruption of TgNHE3 affects gene expression we performed IFA of the knockout strain. Staining of the knockout strain with the anti-TgNHE3 and anti-VP1 antibodies shows that while the intracellular domain can still be detected by the VP1 antiserum, no detectable TgNHE3 signal can be detected anywhere in the parasite (Fig 5C). Thus, the RHΔhptΔnhe3, hereon referred to as Tgnhe3 mutant or knockout strain, represents an ideal tool to study the function of the protein and the compartment to where it localizes.
Lack of TgNHE3 affects tolerance to acute hyperosmotic stress
It has been shown extensively that both yeast and plant mutants lacking vacuolar and endosomal Na+/H+ exchangers exhibit defective osmotolerance following acute hyperosmotic shock [3, 4, 9, 38]. This is caused by the inability of mutant vacuoles to uptake the excess toxic ions in the cytoplasm [3, 4, 38]. Accordingly, we tested the ability of the RHΔhptΔnhe3 strain to adapt to various types of hyperosmotic stresses using osmolite concentrations of ions and sugars that parallel studies performed in yeast as to be able to compare our results to that of others [9, 11, 39]. The general assay consisted of exposing extracellular parasites of the parental and knockout strains to an osmotic stress, and assessing viability of the treated parasite by their ability to form plaques in tissue culture. In these assays, the survival rate was determined by dividing the total number of plaques formed by treated parasites over that of untreated parasites of the particular strain being tested. This method controls for any difference in viability between the parental and mutant strain since the survival rate is calculated for each strain independently. The first test consisted of exposure to high concentrations of sorbitol, a sugar derivative used to cause acute hyperosmotic shock in yeast at concentrations of 0.4 to 2 M [9]. We observed a significant difference in survivability between mutant and wild type parasites when exposed to all concentrations of sorbitol tested (Fig. 6A). While 88.9%, 30.9% and 20.2% of wild type parasites survived treatment with 0.5 M, 0.75 and 1.0 M respectively, only 45.4%, 12.9% and 9.2% of the knockout strain survived at those concentrations of sorbitol (Fig. 6A). Thus on average the knockout strain showed an approximately 50% reduction in resistance to high concentrations of sorbitol. These results indicate that lack of TgNHE3 causes increased sensitivity to acute osmotic stress. To confirm that this phenotype is due to the disruption of TgNHE3, we introduced a copy of the coding sequence under the control of its own promoter into the knockout strain. This strain shows normal expression of TgNHE3 and complements sensitivity to sorbitol phenotype seen in the knockout mutant (supplemental figure S6).
Figure 6.
Sensitivity of the Tgnhe3 knockout strain to osmotic stresses. Extracellular parasites of the parental strain, RHΔhpt, or the knockout RHΔhptΔnhe3 were allowed to infect tissue culture cells and form plaques after being treated with the indicated concentrations of Sorbitol (A), NaCl (B), Sodium glutamate (C) or KCl (D). Percent survival was calculated by dividing the number of plaques formed by the treated parasites over the number formed by untreated parasites of the same strain. Data were compiled from 4 independent experiments, each done in triplicate. Error bars represent standard deviation and * indicates statistically significant difference, as determined by a two-tailed t-test (p value < 0.05).
To further test the influence of TgNHE3 on sensitivity to general osmotic stress we exposed extracellular parasites to high concentrations of various salts. While we do not know the specific ions transported by TgNHE3 we focused our experiments on salts known to affect yeast strains lacking a vacuolar NHEs, which can transport sodium and potassium [11]. At both 0.5 and 1 M NaCl, we observed lower survival for the Tgnhe3 mutant strain as compared to the parental one (Fig. 6B). While 91% and 58% of the parental strain parasites survived the 0.5 M and 1 M NaCl treatment respectively, only 63% and 28% of the mutant parasites survived the same treatments. To confirm that the sensitivity of the Tgnhe3 knockout strain to hyperosmotic stress, we treated the parasites with sodium gluconate, a non-chloride containing sodium salt, at 0.5 and 0.75 M. We observed that, while 69% of the wild type parasites survived treatment with 0.75 M sodium gluconate, only 37% of the mutant parasites survived this ionic stress. Thus the mutant strain appears more sensitive to high salt levels (Fig. 6C).
Besides sodium, NHEs have been shown to transport other ions such as potassium [10]. Based on the homology of TgNHE3 to other NHEs, we tested the sensitivity of the mutant strain to extreme levels of KCl as performed to test the role of the yeast NHX1 in osmotolerance [11]. While the parental strain is able to survive hyperosmotic stress caused by KCl (91 and 82% survival at 0.3 and 0.6 M KCl respectively) the Tgnhe3 knockout strain exhibits an increased sensitivity to KCl (79% and 43 % survival at 0.3 and 0.6 M KCl respectively) (Fig. 6D). The over sensitivity to salt stress is reduced by addition of a wild type copy of TgNHE3 (supplemental figure S6). Taken together, these results suggest that TgNHE3 plays a role in the ability of T. gondii to overcome hyperosmotic stress, paralleling the function of NHXs in plants and yeast.
Lack of TgNHE3 affects invasion
During manipulation of the Tgnhe3 knockout strain we observed that the same number of mutant strain parasites, treated with the control buffer, occasionally formed a smaller number of plaques than the parental strain. Consequently, we suspected that the lack of TgNHE3 led to a disruption of the normal propagation of the parasite. To quantify this phenotype we allowed 1,000 parasites of either the knockout or parental strain to infect human fibroblasts for 2, 4, 6, or 24 hours and we counted the number of plaques formed 4 days later. We observed that when parasites were allowed to infect for 2 hours, 22.9% of the parental parasites formed plaques but only 11.7% of the knockout ones did (Fig. 7A). Similarly, after allowing the parasites to infect for 4 hours, we observed that the plaquing efficiency for the mutant was reduced 2-fold when compared to that of the parental strain (Fig. 7A). However, when allowed to invade for 6 hours the mutant strain showed a plaquing efficiency that was not significantly different from that of the parental, as determined by a two-tailed t-test. While efficiency of plaquing depends on various steps of the parasite's lytic cycle such as invasion, division, and egress, the fact that allowing the parasites to invade for longer time periods results in the mutant and parental to form similar number of plaques suggests that the knockout strain has a deficiency in either attachment and/or invasion and when allowed to invade for prolonged periods it catches up. Consistent with this hypothesis, if allowed to invade for prolonged periods of time such as 24 hours both strains formed the same number of plaques (Fig. 7A).
To corroborate that parasites of the Tgnhe3 knockout strain are defective in invasion we counted the number of parasites that invaded after short incubation times. To detect the intracellular parasites after such short incubation times we used a differentially labeling method with antibodies before permeabilization of HFFs (staining only extracellular parasites) and after permeabilization (staining both intra- and extracellular parasites) [31]. After determining the number of intracellular parasites for both parasite strains we expressed the result as the ratio of knockout invaded parasites over parental, so that a ratio of less than 1 would imply that the knockout strain has a defect in invasion as compared to its parental strain. Indeed, at the three invasion periods tested (15, 30 and 60 minutes) we found that the Tgnhe3 knockout strain is less invasive than the parental one (ratios of 0.54, 0.55 and 0.58 for 15, 30 and 60 min invasion periods respectively, Fig. 7B). As expected, this phenotype is significantly reduced by introducing a wild type copy of the gene into the mutant parasite (supplemental figure S6 C and d). In an independent assay we observed invasion ratios of 0.25 and 0.26 after 15 and 60 min. respectively for the mutant strain, while the ratios were .80 and .67 for the complemented strain at the same time points (supplemental figure S6 D).
Invasion by T. gondii depends on a sequence of events, which include motility and attachment to the host cell. To investigate events prior to the actual invasion were affected in the mutant we analyzed the number of parasites that attached to cells after 30 min incubation with fibroblasts. Number of attached parasites was determined by adding the number of parasites that remain attached to the outside of the cells to that of parasites inside cells (since parasites must have attached to the cell before entering). Fig. 7C shows the average number of parasites seen per field of view for both the parental and the mutant strain as a percentage of what is observed for the wild type control. This analysis indicates that the number of attached parasites is significantly reduced (62.7±7%) in the strain lacking TgNHE3. This analysis also confirmed that the number of parasites that invaded is reduced (Fig. 7C black bars). Thus, at least part of the defect in invasion is due to deficiency in events prior to the actual penetration into the cell.
Lack of NHE3 affects intracellular calcium concentration
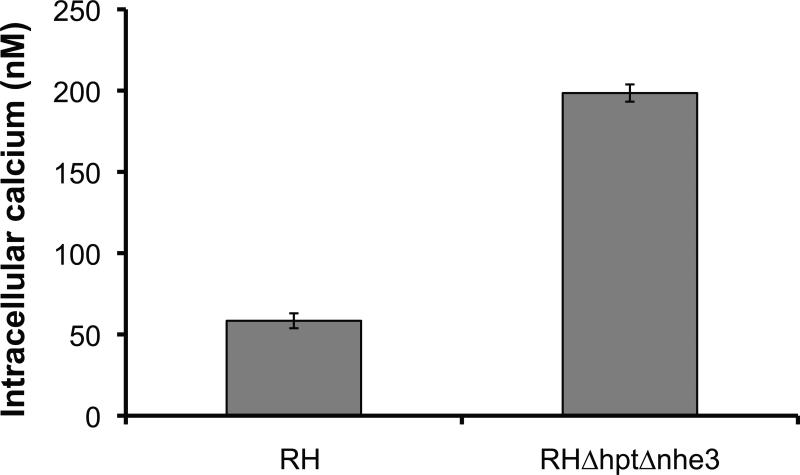
Many of the processes involved in invasion such as motility, secretion and cytoskeletal rearrangements are regulated by calcium fluxes within the parasite [40]. Interestingly, it has been shown that the TgVP-1-compartment, to which TgNHE3 localizes, acts as a calcium store [14]. Thus, to determine if ablation of TgNHE3 resulted in changes in calcium homeostasis, the cell permeable calcium indicator, fura2-AM, was used to measure cytosolic calcium concentration ([Ca2+]i) in extracellular tachyzoites. Measurements were made in the RH wild type strain and the Tgnhe3 knockout mutants. The average intracellular calcium concentration for four independent experiments is shown in Fig. 8. Tgnhe3 knockout mutants had a significantly higher concentration of intracellular calcium than the parental strain. The knockout mutants had an average cytosolic concentration of 198.5 nM (± 5.3 nM; SE), which was 3.6-fold greater than that of the parental strain (58.4 ± 4.86 nM). Although cytosolic calcium levels were higher in the knockout mutants, both groups had stable calcium levels (data not shown). As with other phenotypes of the knockout mutant, the increase in calcium is rescued in the complemented strain (supplemental figure S6 E).
Figure 8.
Cytosolic calcium concentration in the NHE3 mutant strain. Tachyzoites of either the wild type strain RH or the knockout strain RHΔhptΔnhe3 were loaded with fura2-AM, washed, and cytosolic calcium concentration was determined in a nominally calcium free extracellular buffer. Values are averages (n = 15; +/- SEM).
Lack of TgNHE3 affects microneme secretion
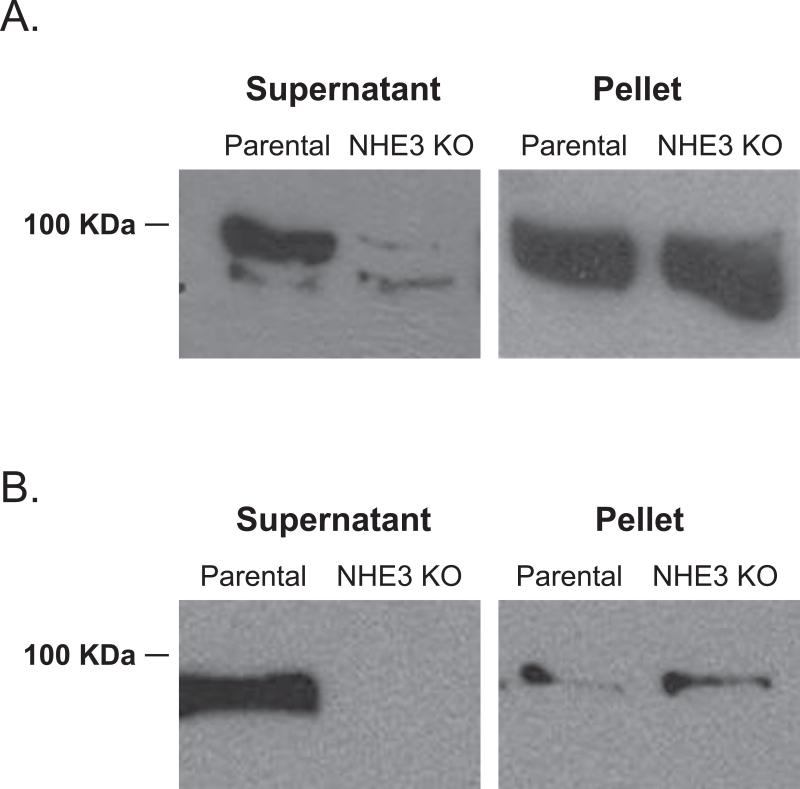
Another possible cause for reduction in invasion efficiency that would be consistent with vacuolar functions is secretion of adhesins and invasins. Various T. gondii proteins involved in invasion are secreted from specialized organelles in a calcium dependent manner. Furthermore, before secretion these proteins are required to be processed by numerous proteases including CPL [15]. Thus, it is plausible that lack of TgNHE3 causes a defect in invasion as a consequence of a direct or indirect disruption of protein trafficking, processing, or secretion. Consequently, we tested the secretion of the micronemal protein MIC2 in the knockout strain. Micronemal protein secretion normally occurs during parasite motility and during parasite attachment to the host cell and can be induced with the calcium ionophore A23187 [31, 41]. Our experiment consisted of incubating extracellular parasites of both the parental and the ΔTgnhe3 strain with 200 nM A23187 for 15 min. to induce secretion. After this treatment parasites were spun down. Both the precipitate, which contains the parasites, and the supernatant, which contains the secreted proteins, were used in Western blot analysis using a MIC2 antibody (Fig. 9A). While the levels of MIC2 are normal within the mutant parasites, as compared to the parental strain (Fig. 9A pellet), the levels of secreted MIC2 in the presence of A23187 are dramatically reduced in the mutant strain (Fig. 9A supernatant). This reduction in secreted MIC2 could be due to a defect in the secretion process itself, or in the response to calcium fluxes by the parasite. To explore this possibility we repeated the assay without the use of A23187 to look at basal level secretion (Fig. 9B). We observed that similar to induced secretion, basal secretion is affected in the knockout strain. This reduction in MIC2 secretion can account for the reduction in attachment and invasion efficiency seen in the mutant strain.
Figure 9.
Microneme secretion in the parental and Tgnhe3 knockout strain. A. Micronemal secretion was induced with 1 μM the calcium ionophore A23187 for 4 minutes. The microneme protein MIC2 was detected in western blot of both the supernatant (secreted material) and the pellet (intact parasites) using a rabbit anti-Mic2 polyclonal antibody. B. Constitutively secreted MIC2 was detected by Western blot analysis of both the supernatant and pellet of extracellular parasites incubated for 30 minutes at 37°C in invasion media.
DISCUSSION
Characterization of a vacuolar type pyrophosphatase, TgVP1, and a cathepsin L protease, TgCPL, led to the description of a novel compartment in Toxoplasma gondii [14, 15, 42]. In characterizing the TgVP1 compartment, Miranda and colleagues discovered that it contains other proteins typical of the vacuoles of plants. Consequently, they have named this compartment the plant-like vacuole or PLV. Simultaneously, Parussini et. al. described that TgCPL, which localizes to what they called the vacuolar compartment, or VAC, is involved in protein trafficking and processing, and colocalizes with TgVP1 for the most part. They hypothesized that this compartment is similar functionally to late endosomes. We have discovered that TgNHE3, which bears significant homology to well characterized vacuolar/endosomal NHEs, localizes to the PLV/VAC compartment. The presence of a vacuolar-type NHE in this eukaryote of distal origin underlines the evolutionary commonality of the intracellular NHEs. Furthermore, our results provide evidence that the PVL/VAC is involved in both detoxification and protein trafficking.
In extracellular parasites the TgNHE3 and TgVP1 localization are nearly identical. In intracellular parasites TgNHE3 seems to occupy a more internal domain of the PVL/VAC, which does not perfectly coincide with TgVP1's distribution. Interestingly, the vacuoles of yeast and plants occupied by TgNHE3 orthologs, are not void, homogenous compartments, but rather contain subdivisions and are heterogeneous, with formation of channels and accumulation of dense bodies. Similarly, the PLV/VAC has been shown to be multi-vesicular, and to have a dynamic structure that is developmentally regulated [14, 15, 36]. Thus, TgNHE3 might localize at more internal domains, and might be translocated to the outermost domains of this compartment only under certain conditions. Alternatively, TgNHE3 might serve its function at a more internal localization or the more diffuse staining pattern of TgVP1 might be due to the acidocalcisomes, which contain TgVP1 but not TgNHE3, fusing to the larger compartment. Nonetheless, VP1 and NHE3 appear to belong to the same compartment, and their co-localization may be dynamic.
Vacuolar and pre-vacuolar compartments have been implicated in a variety of functions including stress tolerance, osmotolerance, control of cytoplasmic pH, detoxification of xenobiotics, and storage of metal ions such as calcium. Yeast mutants impaired in vacuole biogenesis lose viability after 10 sec exposure to 1.5 M NaCl [43]. Similarly, the plant and yeast orthologs AtNHX1, OsNHX1, and ScNHX1, have been implicated in cation detoxification and lacking these vacuolar NHXs in their hosts renders these cells sensitive to excess salt [3, 9, 38]. Consistent with TgNHE3 being a functional paralog of yeast and plant NHXs, we observe a 50% increase in the sensitivity to sorbitol, as well as to high levels of NaCl and to KCl, in strains lacking TgNHE3. As mentioned above, T. gondii is a particularly versatile parasite in that it can survive not only in different hosts, but also in different tissue and cell types within the host, and also in the extracellular environment. Thus, it is not hard to envision the relevance of the ability to overcome osmotic stress in the life cycle of T. gondii. Resistance to ionic stress might be of particular significance in the extracellular environment in which the parasite encounters an abrupt increase in the concentration of both calcium and sodium, and a sudden drop in the concentration of potassium; the sodium concentration varies from 5-10 mM inside to 160 mM outside the cell, while the inverse situation exists for potassium.
One of the most significant defects of disrupting TgNHE3 is a reduction in the efficiency of invasion; likely directly caused by a decrease in the secretion of MIC2 [31, 32]. Surprisingly, the invasion deficiency we observed in the TgNHE3 knock out, did not translate into an appreciable reduction in the strain's virulence in mice (data not shown). Nonetheless, a slight or even a significant reduction in virulence might not be measureable with our mutant, since it was generated from a strain of which a single parasite is sufficient to kill a mouse.
Our results show that calcium regulation is significantly altered by the genetic ablation of TgNHE3. Calcium regulation by Na+/H+ exchangers has been documented for T.gondii [18] as well as in other protistan parasites such as Trypanosoma brucei [44]. The deregulation is likely the end result of several transport mechanisms that rely on the generation of a proton gradient via primary active transport utilizing ATP or PPi. Ablation of TgNHE3 led to higher cytosolic calcium, but with no demonstrable loss in overall stability. Miranda and colleagues determined that the PLV/VAC is acidic and contains a significant amount of releasable calcium [14]. It seems likely that the removal of TgNHE3 from the PLV results in the partial regulatory loss of calcium flux to and from the cytosol. This, in turn, establishes a new standing concentration of calcium in the cytosol. Even though, the defect in both invasion and micronemal secretion observed in the mutant might be due to the deregulation of calcium homeostasis, the fact that basal secretion of TgMIC2 (i.e. independent from calcium stimulated secretion) is also affected in the TgNHE3 mutant, suggests that a different phenomenon is likely affecting TgMIC2 secretion.
Another scenario connecting lack of TgNHE3 and a defect in secretion is that the compartment occupied by TgNHE3 acts as a site for sorting and or processing of proteins directed to the secretory organelles or other sites in the cell. Consistent with this idea, we have shown that TgNHE3 localization closely follows that of TgCPL's, a cathepsin-like protease, which has been proposed to carry out protein processing including that of TgM2AP [15, 45]. In terms of MIC2 secretion, the processing of TgM2AP is of importance; the correct processing of TgM2AP, has been shown to be critical for the stability and secretion of the TgMIC2/TgM2AP complex [31]. The particular region of the parasite where TgM2AP processing occurs is not known. Nevertheless, given that TgCPL, and TgNHE3 coincide in their localization and that, along many other places, we detect unprocessed TgM2AP (proTgM2AP) in the same spot as TgNHE3 (data not shown), it is plausible that the processing occurs in the PVL/VAC. If this is indeed the case, the disruption of TgNHE3 could change the compartment's pH, affecting TgCPL's activity, which has been shown to be pH-dependent.[45]. Alternatively, a change in the compartment's pH may disrupt the correct trafficking of the TgMIC2/TgM2AP towards the micronemes. While we have been able to determine that the cytosolic pH is not affected in the TgNHE3 knockout strain (data not shown) we have been unable to accurately determine the pH of the compartment in which TgNHE3 resides in the mutant strain.
CONCLUSIONS
Our identification and characterization of a novel vacuolar-like NHE sheds light onto the possible functions of the compartment in which it resides along with TgVP1 and TgCPL. Consistent with the functions of its vacuolar orthologs, we have shown that lack of TgNHE3 causes a mutant strain to be more sensitive to osmotic stress, as well as, to affect protein secretion. We hypothesize that the PLV/VAC provides the parasite with a mechanism to cope with the ionic fluctuations, potentially by mediating the sequestration of excess toxic ions in this compartment, thus, protecting ion-sensitive processes in the cytoplasm. An additional function of this compartment, consistent with vacuolar functions in plants and yeast, may be in protein sorting and processing as part of the endocytic pathway. This possibility is suggested by the microneme secretion defect in the mutant strain along with the co-localization with TgCPL1. Our studies pave the way for further determination of the mechanism of protection to osmotic stress and the mechanism by which the PLV/VAC provides the parasite with the ability to overcome osmotic stress. Finally, a more thorough understanding of the role of this compartment in T. gondii's secretory pathways will reveal novel drug targets by exploiting the divergence and absence of plant-vacuolar-like proteins in the mammalian counterparts.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to acknowledge Ana Pawlik and Kathryn Morris for their technical assistance in initiating the cloning of TgNHE3. We would like to thank Dr. Vern Carruthers for sharing reagents and their unpublished data with us. We are also grateful to Dr. Gus Zeisner and Dr. John Boothroyd for kindly providing the HA tagging vector. Technical assistance with cryoimmuno EM was provided by Wandy Beatty, Microbiology Imaging Facility (Washington University). This work was supported by an NIH grant from the NCRR Center of Biomedical Research Centers P20 RR15587 and an award from the American Cancer Society (RSG-08-19-01-MBC). The laboratory of G.A. is also funded by NIH grant R01 AI 89808-01. S.N.J.M. and D.A.P. were supported by NIH grant AI-079625. D.A.P. was also supported by NIH T-32 training grant AI-060546. J.S. is supported by NIH grant P20 RR016454.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 2.Putney LK, Denker SP, Barber DL. The changing face of the Na+/H+ exchanger, NHE1: structure, regulation, and cellular actions. Annu Rev Pharmacol Toxicol. 2002;42:527–552. doi: 10.1146/annurev.pharmtox.42.092001.143801. [DOI] [PubMed] [Google Scholar]
- 3.Gaxiola RA, Rao R, Sherman A, Grisafi P, Alper SL, Fink GR. The Arabidopsis thaliana proton transporters, AtNhx1 and Avp1, can function in cation detoxification in yeast. Proc Natl Acad Sci U S A. 1999;96:1480–1485. doi: 10.1073/pnas.96.4.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yokoi S, Quintero FJ, Cubero B, Ruiz MT, Bressan RA, Hasegawa PM, Pardo JM. Differential expression and function of Arabidopsis thaliana NHX Na+/H+ antiporters in the salt stress response. Plant J. 2002;30:529–539. doi: 10.1046/j.1365-313x.2002.01309.x. [DOI] [PubMed] [Google Scholar]
- 5.Wunsch S, Sanchez CP, Gekle M, Grosse-Wortmann L, Wiesner J, Lanzer M. Differential stimulation of the Na+/H+ exchanger determines chloroquine uptake in Plasmodium falciparum. J Cell Biol. 1998;140:335–345. doi: 10.1083/jcb.140.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brett CL, Wei Y, Donowitz M, Rao R. Human Na(+)/H(+) exchanger isoform 6 is found in recycling endosomes of cells, not in mitochondria. Am J Physiol Cell Physiol. 2002;282:C1031–1041. doi: 10.1152/ajpcell.00420.2001. [DOI] [PubMed] [Google Scholar]
- 7.Aronson PS. Kinetic properties of the plasma membrane Na+-H+ exchanger. Annu Rev Physiol. 1985;47:545–560. doi: 10.1146/annurev.ph.47.030185.002553. [DOI] [PubMed] [Google Scholar]
- 8.Vercesi AE, Rodrigues CO, Catisti R, Docampo R. Presence of a Na(+)/H(+) exchanger in acidocalcisomes of Leishmania donovani and their alkalization by anti-leishmanial drugs. FEBS Lett. 2000;473:203–206. doi: 10.1016/s0014-5793(00)01531-3. [DOI] [PubMed] [Google Scholar]
- 9.Nass R, Rao R. The yeast endosomal Na+/H+ exchanger, Nhx1, confers osmotolerance following acute hypertonic shock. Microbiology. 1999;145(Pt 11):3221–3228. doi: 10.1099/00221287-145-11-3221. [DOI] [PubMed] [Google Scholar]
- 10.Brett CL, Donowitz M, Rao R. Evolutionary origins of eukaryotic sodium/proton exchangers. Am J Physiol Cell Physiol. 2005;288:C223–239. doi: 10.1152/ajpcell.00360.2004. [DOI] [PubMed] [Google Scholar]
- 11.Brett CL, Tukaye DN, Mukherjee S, Rao R. The yeast endosomal Na+K+/H+ exchanger Nhx1 regulates cellular pH to control vesicle trafficking. Mol Biol Cell. 2005;16:1396–1405. doi: 10.1091/mbc.E04-11-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ali R, Brett CL, Mukherjee S, Rao R. Inhibition of sodium/proton exchange by a Rab-GTPase-activating protein regulates endosomal traffic in yeast. J Biol Chem. 2004;279:4498–4506. doi: 10.1074/jbc.M307446200. [DOI] [PubMed] [Google Scholar]
- 13.Bowers K, Levi BP, Patel FI, Stevens TH. The sodium/proton exchanger Nhx1p is required for endosomal protein trafficking in the yeast Saccharomyces cerevisiae. Mol Biol Cell. 2000;11:4277–4294. doi: 10.1091/mbc.11.12.4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miranda K, Pace DA, Cintron R, Rodrigues JC, Fang J, Smith A, Rohloff P, Coelho E, de Haas F, de Souza W, Coppens I, Sibley LD, Moreno SN. Characterization of a novel organelle in Toxoplasma gondii with similar composition and function to the plant vacuole. Mol Microbiol. 2010;76:1358–1375. doi: 10.1111/j.1365-2958.2010.07165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parussini F, Coppens I, Shah PP, Diamond SL, Carruthers VB. Cathepsin L occupies a vacuolar compartment and is a protein maturase within the endo/exocytic system of Toxoplasma gondii. Mol Microbiol. 2010;76:1340–1357. doi: 10.1111/j.1365-2958.2010.07181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gajria B, Bahl A, Brestelli J, Dommer J, Fischer S, Gao X, Heiges M, Iodice J, Kissinger JC, Mackey AJ, Pinney DF, Roos DS, Stoeckert CJ, Jr., Wang H, Brunk BP. ToxoDB: an integrated Toxoplasma gondii database resource. Nucleic Acids Res. 2008;36:D553–556. doi: 10.1093/nar/gkm981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arrizabalaga G, Ruiz F, Moreno S, Boothroyd JC. Ionophore-resistant mutant of Toxoplasma gondii reveals involvement of a sodium/hydrogen exchanger in calcium regulation. The Journal of cell biology. 2004;165:653–662. doi: 10.1083/jcb.200309097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karasov AO, Boothroyd JC, Arrizabalaga G. Identification and disruption of a rhoptry-localized homologue of sodium hydrogen exchangers in Toxoplasma gondii. Int J Parasitol. 2005;35:285–291. doi: 10.1016/j.ijpara.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Donald RG, Carter D, Ullman B, Roos DS. Insertional tagging, cloning, and expression of the Toxoplasma gondii hypoxanthine-xanthine-guanine phosphoribosyltransferase gene. Use as a selectable marker for stable transformation. J Biol Chem. 1996;271:14010–14019. doi: 10.1074/jbc.271.24.14010. [DOI] [PubMed] [Google Scholar]
- 20.Wang L, Leebens-Mack J, Wall P, Beckmann K, dePamphilis C, Warnow T. The Impact of Multiple Protein Sequence Alignment on Phylogenetic Estimation. IEEE/ACM Transactions on Computational Biology and Bioinformatics. 2009;PP:1–1. doi: 10.1109/TCBB.2009.68. [DOI] [PubMed] [Google Scholar]
- 21.Castresana J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol. 2000;17:540–552. doi: 10.1093/oxfordjournals.molbev.a026334. [DOI] [PubMed] [Google Scholar]
- 22.Talavera G, Castresana J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst Biol. 2007;56:564–577. doi: 10.1080/10635150701472164. [DOI] [PubMed] [Google Scholar]
- 23.Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 24.Soldati D, Boothroyd JC. Transient transfection and expression in the obligate intracellular parasite Toxoplasma gondii. Science. 1993;260:349–352. doi: 10.1126/science.8469986. [DOI] [PubMed] [Google Scholar]
- 25.Donald RG, Roos DS. Stable molecular transformation of Toxoplasma gondii: a selectable dihydrofolate reductase-thymidylate synthase marker based on drug-resistance mutations in malaria. Proc Natl Acad Sci U S A. 1993;90:11703–11707. doi: 10.1073/pnas.90.24.11703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sambrook J.a.D.W.R. Molecular cloning : a laboratory manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, N.Y: 2001. [Google Scholar]
- 27.Medina-Acosta E, Cross GA. Rapid isolation of DNA from trypanosomatid protozoa using a simple ‘mini-prep’ procedure. Mol Biochem Parasitol. 1993;59:327–329. doi: 10.1016/0166-6851(93)90231-l. [DOI] [PubMed] [Google Scholar]
- 28.Kafsack BF, Pena JD, Coppens I, Ravindran S, Boothroyd JC, Carruthers VB. Rapid membrane disruption by a perforin-like protein facilitates parasite exit from host cells. Science (New York, N.Y. 2009;323:530–533. doi: 10.1126/science.1165740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo S, Vieira M, Graves J, Zhong L, Moreno SN. A plasma membrane-type Ca(2+)-ATPase co-localizes with a vacuolar H(+)-pyrophosphatase to acidocalcisomes of Toxoplasma gondii. EMBO J. 2001;20:55–64. doi: 10.1093/emboj/20.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lavine MD, Knoll LJ, Rooney PJ, Arrizabalaga G. A Toxoplasma gondii mutant defective in responding to calcium fluxes shows reduced in vivo pathogenicity. Mol Biochem Parasitol. 2007;155:113–122. doi: 10.1016/j.molbiopara.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huynh MH, Rabenau KE, Harper JM, Beatty WL, Sibley LD, Carruthers VB. Rapid invasion of host cells by Toxoplasma requires secretion of the MIC2-M2AP adhesive protein complex. Embo J. 2003;22:2082–2090. doi: 10.1093/emboj/cdg217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huynh MH, Carruthers VB. Toxoplasma MIC2 is a major determinant of invasion and virulence. PLoS Pathog. 2006;2:e84. doi: 10.1371/journal.ppat.0020084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moreno SN, Zhong L. Acidocalcisomes in Toxoplasma gondii tachyzoites. Biochem J. 1996;313(Pt 2):655–659. doi: 10.1042/bj3130655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hofmann K, Stoffel W. TMbase - A database of membrane spanning proteins segments. Biol. Chem. Hoppe-Seyler. 1993;374:166. [Google Scholar]
- 35.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 36.Drozdowicz YM, Shaw M, Nishi M, Striepen B, Liwinski HA, Roos DS, Rea PA. Isolation and characterization of TgVP1, a type I vacuolar H+-translocating pyrophosphatase from Toxoplasma gondii. The dynamics of its subcellular localization and the cellular effects of a diphosphonate inhibitor. J Biol Chem. 2003;278:1075–1085. doi: 10.1074/jbc.M209436200. [DOI] [PubMed] [Google Scholar]
- 37.Luo S, Ruiz FA, Moreno SN. The acidocalcisome Ca2+-ATPase (TgA1) of Toxoplasma gondii is required for polyphosphate storage, intracellular calcium homeostasis and virulence. Mol Microbiol. 2005;55:1034–1045. doi: 10.1111/j.1365-2958.2004.04464.x. [DOI] [PubMed] [Google Scholar]
- 38.Fukuda A, Nakamura A, Tagiri A, Tanaka H, Miyao A, Hirochika H, Tanaka Y. Function, intracellular localization and the importance in salt tolerance of a vacuolar Na(+)/H(+) antiporter from rice. Plant & cell physiology. 2004;45:146–159. doi: 10.1093/pcp/pch014. [DOI] [PubMed] [Google Scholar]
- 39.Chowdhury S, Smith KW, Gustin MC. Osmotic stress and the yeast cytoskeleton: phenotype-specific suppression of an actin mutation. J Cell Biol. 1992;118:561–571. doi: 10.1083/jcb.118.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arrizabalaga G, Boothroyd JC. Role of calcium during Toxoplasma gondii invasion and egress. Int J Parasitol. 2004;34:361–368. doi: 10.1016/j.ijpara.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 41.Carruthers VB, Sibley LD. Mobilization of intracellular calcium stimulates microneme discharge in Toxoplasma gondii. Mol Microbiol. 1999;31:421–428. doi: 10.1046/j.1365-2958.1999.01174.x. [DOI] [PubMed] [Google Scholar]
- 42.van Dooren GG, Ralph SA. Novel vacuoles in Toxoplasma. Mol Microbiol. 76:1335–1339. doi: 10.1111/j.1365-2958.2010.07197.x. [DOI] [PubMed] [Google Scholar]
- 43.Latterich M, Watson MD. Evidence for a dual osmoregulatory mechanism in the yeast Saccharomyces cerevisiae. Biochemical and biophysical research communications. 1993;191:1111–1117. doi: 10.1006/bbrc.1993.1331. [DOI] [PubMed] [Google Scholar]
- 44.Vercesi AE, Grijalba MT, Docampo R. Inhibition of Ca2+ release from Trypanosoma brucei acidocalcisomes by 3,5-dibutyl-4-hydroxytoluene: role of the Na+/H+ exchanger. Biochem J. 1997;328(Pt 2):479–482. doi: 10.1042/bj3280479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harper JM, Huynh MH, Coppens I, Parussini F, Moreno S, Carruthers VB. A cleavable propeptide influences Toxoplasma infection by facilitating the trafficking and secretion of the TgMIC2-M2AP invasion complex. Mol Biol Cell. 2006;17:4551–4563. doi: 10.1091/mbc.E06-01-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.