Abstract
The expression of autism spectrum disorder (ASD) is highly heterogeneous, owing to the complex interactions between genes, the brain, and behavior throughout development. Here we present a model of ASD that implicates an early and initial failure to develop the specialized functions of one or more of the set of neuroanatomical structures involved in social information processing (i.e., the “social brain”). From this early and primary disruption, abnormal brain development is canalized because the individual with an ASD must develop in a highly social world without the specialized neural systems that would ordinarily allow him or her to partake in the fabric of social life, which is woven from the thread of opportunities for social reciprocity and the tools of social engagement. This brain canalization gives rise to other characteristic behavioral deficits in ASD including deficits in communication, restricted interests, and repetitive behaviors. We propose that focused efforts to explore the brain mechanisms underlying the core, pathognomic deficits in the development of mechanisms for social engagement in ASD will greatly elucidate our understanding and treatment of this complex, devastating family of neurodevelopmental disorders. In particular, developmental studies (i.e., longitudinal studies of young children with and without ASD, as well as infants at increased risk for being identified with ASD) of the neural circuitry supporting key aspects of social information processing are likely to provide important insights into the underlying components of the full-syndrome of ASD. These studies could also contribute to the identification of developmental brain endophenotypes to facilitate genetic studies. The potential for this kind of approach is illustrated via examples of functional neuroimaging research from our own laboratory implicating the posterior superior temporal sulcus (STS) as a key player in the set of neural structures giving rise to ASD.
Defining autism spectrum disorders
The considerable heterogeneity in the expression and severity of the core and associated symptoms is a challenge that has hindered progress towards understanding autism spectrum disorder (ASD). To illustrate, within autistic disorder, variability in the social domain ranges from a near absence of interest in interacting with others to more subtle difficulties managing complex social interactions that require an understanding of other people's goals and intentions and other cues of social context. Similarly, stereotyped and repetitive behaviors range from simple motor stereotypies and/or a preference for sameness to much more complex and elaborate rituals, accompanied by emotional dysregulation or “meltdowns” when these rituals are interrupted. Some individuals with ASD lack basic speech abilities, while others can have language deficits that are mild and limited to language pragmatics. While a majority of individuals with ASD exhibit some level of intellectual impairment, intelligence quotients vary from the severe and profoundly impaired range to well above average.
Mirroring the phenotypic heterogeneity, there is also great genetic variability in ASD (Abrahams & Geschwind, 2008; Geschwind & Levitt, 2007; Gupta & State, 2007). For instance, many susceptibility loci have been identified, yet each is thought to account for only a small number (1-2%) of overall cases (e.g., Weiss et al., 2008). Various defined mutations, genetic syndromes, and de novo copy number variation account for as many as 10-20% of cases (Abrahams & Geschwind, 2008). Given the large number of potential genetic mechanisms, it is likely that no single molecular pathophysiological explanation will be sufficient to explain the majority of cases (Abrahams & Geschwind, 2008). The pace of discovery is accelerating toward the identification of a myriad of genetic mechanisms, all leading through a smaller but still substantial number of molecular pathophysiological mechanisms, to a set of neurodevelopment disorders that comprise ASD.
Constraining heterogeneity
Reflecting upon the genetic and phenotypic heterogeneity, Geschwind and Levitt (2006) articulated an influential three-part hypothesis stating that: (1) there are many “autisms”; (2) a common brain-systems-level feature across the autisms is the concept of “developmental disconnection”; (3) developmental disconnection manifests as a failure to develop normal connections between higher-order association areas of the temporal and parietal cortices and regions of the frontal cortices. This developmental disconnection or “underconnectivity” component of the hypothesis builds from and complements other similar viewpoints (e.g., Brock, Brown, Boucher, & Rippon, 2002; Courchesne & Pierce, 2005; Hughes, 2007; Just, Cherkassky, Keller, & Minshew, 2004).
Functional neuroimaging studies of adolescents and adults with ASD have provided considerable evidence in support of underconnectivity in ASD (e.g., Castelli, Frith, Happé , & Frith, 2000; Just et al., 2004; Kana, Keller, Cherkassky, Minshew, & Just, 2006; Kana et al., 2007; Kleinhans et al., 2008; Koshino et al., 2008; Koshino, Carpenter, Minshew, Cherkassky, Keller, Just, 2005; Mason et al., 2008; Monk et al., 2009; Noonan, Haist, & Müller, 2009; Villalobos, Mizuno, Dahl, Kemmotsu, & Müller, 2005; Wicker et al., 2008). Similarly, structural neuroimaging studies of older children, adolescents, and adults with ASD support the underconnectivity hypothesis (e.g., Barnea-Goraly et al., 2004; Sivaswamy et al., 2010; Fletcher et al., 2010; Cheng et al., 2010; Sahyoun et al., 2010; Kumar et al., 2010, Cheung et al., 2009; Keller, Kana, & Just, 2007). Underconnectivity appears to be present in the brains of older children, adolescents, and adults with ASD. The appeal of the underconnectivity notion lies partly in the fact that it appears to offer a systems-level model of brain dysfunction that purports to account for the specific symptoms of ASD as well as the heterogeneity of etiology, behaviors and cognition (Geschwind & Levitt, 2006). However, it is not yet clear how the underconnectivity perspective accounts for the specific patterns of dysfunction in individuals with ASD. That is, how might this perspective explain what is common among individuals with ASD and what separates ASD from other neurodevelopmental disorders that also feature underconnectivity?
We believe that it is premature to conclude that underconnectivity reflects a primary, unifying neural-systems-level mechanism in ASD. There are several reasons for our caution. First, only a handful of fMRI studies have tested children with ASD (e.g., Lee et al., 2008; Brito et al., 2009). The results of these studies have been somewhat mixed, if not tending to run counter to key aspects of the disconnection hypothesis. For instance, using functional magnetic resonance imaging (fMRI), Lee and colleagues (2008) examined functional connectivity in data collected during a Go/No-go task in samples of 8- to 12-year-old children with and without an ASD. They focused on the connectivity values between the left and right inferior frontal cortices (IFC; BA 47) and regions of the respective frontal, striatal, and parietal cortices. The two groups of children did not differ in their functional connectivity. Intriguingly, in the ASD group, there was a significant negative correlation between age and two long-range IFC correlation pairs: the right IFC ↔ bilateral presupplementary motor area (BA 6) and right IFC ↔ right caudate. These findings indicate normal prefrontal cortical functional connectivity in school-age children with ASD, but also suggest that some functional connections may abnormally decrease with age in the children with ASD. In other words, the brains of these children become more “disconnected” over time instead of starting that way.
Second, the specificity of reduced functional connectivity for ASD relative to other neurodevelopmental and neuropsychiatric disorders has not been demonstrated. Reduced functional connectivity, including reductions in the long-range frontal ↔ temporal cortical and frontal ↔ parietal cortical connections, have been reported in a variety of disparate neurological, neuropsychiatric, and neurodevelopmental disorders and conditions including: Alzheimer's disease (e.g., Supekar et al, 2008), schizophrenia (e.g., Esslinger et al., 2009; Friston & Frith, 1995), adolescent depression (Cullen et al., 2009), chronic heroin use (Liu et al., 2009), post-traumatic stress disorder (Shaw et al., 2009), and dyslexia (e.g., Richards & Berninger, 2008).
Germane to the point of specificity, one study of Romanian orphans reported prominent effects on brain connectivity from the experience of profound, early, and severe socioemotional deprivation. Eluvathingal and colleagues (2006) examined, using diffusion tensor imaging (DTI) tractography, the integrity of white matter tracts that connect limbic and paralimbic structures, including the orbital frontal gyrus, infralimbic prefrontal cortex, hippocampus / amygdala, lateral temporal cortex, and the brainstem. These regions were selected a priori on the basis of a prior positron emission tomography (PET) study that identified glucose hypometabolism in each of these neuroanatomical structures in children with ASD (Chugani et al., 2001). The children exhibited relatively mild specific cognitive impairment and impulsivity, but they did not have an ASD. Fractional anisotropy values in the left uncinate fasciculus (which connects the gyri of the frontal lobe with the anterior end of the temporal lobe) were decreased significantly in the early deprivation group compared with typically developing (TD) comparison children. This finding highlights the possibility that the observed findings of reduced long-range functional connectivity in adults with ASD are actually the result of the ASD. If individuals with an ASD lack the necessary, early developing mechanisms for social engagement that ensure normative social development, then opportunities for social interaction are inherently reduced, particularly experiences sought out by the individual.
Our field has also overemphasized the heterogeneity in ASD, at the expense of recognizing the homogeneity of core disruptions in social information processing. While diverse with regard to phenotypic expression and genetic etiology, the ASDs share the common, pathognomic feature of dysfunctional reciprocal social interaction. In this review we present recent evidence from our program of research of inherent and lifelong impairments in the structure and function of neuroanatomical systems that are related to the universal and pathognomic ASD deficits in reciprocal social interaction. We recognize that these disruptions in brain structure and function may arise from a number of different genetic and molecular etiologies and are also further transformed across development by the experiences and activity of the individual in the world (Kandel, 1998). We argue that the reciprocal relationship between brain disruption and atypical social development drives homogeneity in the syndrome's presentation even in the presence of enormous phenotypic and genotypic heterogeneity.
Our contention is that despite the etiological heterogeneity and despite such high phenotypic variability, it might be the case that the various factors contributing to the now well-characterized triad of impairments in ASD exert their effect through a circumscribed set of neural structures and their interconnections that both give rise to and are shaped by social development. That is, it is possible that the simplest and potentially most powerful marker of ASD will be found at the level of brain systems, particularly those regions devoted to social information processing, and their development (i.e., the dynamic interactions among genes, brain, and behavior over time).
We propose that ASD begins with a failure in the emergence of the specialized functions of one or more of the set of neuroanatomical structures involved in social information processing. This failure happens early in ontogeny, within the first nine months to one year of life, if not earlier. In turn, because the affected regions do not generate the normal stream of both intrinsic and stimulus driven signals, the normal developmental pattern of connections among these brain regions is greatly altered. Abnormal brain development is canalized because the individual with an ASD must develop in a highly social world without the specialized neural systems that would ordinarily allow him or her to partake in the fabric of social life, which is woven from opportunities for social reciprocity. These opportunities provide the basis for normal cognitive development in typically developing children, but are greatly decreased in ASD.
Our proposal borrows from and builds upon contributions beginning even before neuroscientists recognized the existence of a “social brain”. In the late 1980s, researchers began to discuss an early form of what we now call the social motivation hypothesis (e.g., Sahley & Panksepp, 1987, Journal of Autism and Developmental Disorders). In 1991, Fotheringham put forward an early form of the amygdala hypothesis of ASD, followed by Simon Baron-Cohen's groundbreaking exposition on this hypothesis in 1999. More broadly, Baron-Cohen (1995) formulated a theory of social brain dysfunction in Mindblindness. At approximately the same time, Uta and Chris Frith integrated the available neuroscientific evidence to put forward a social brain hypothesis to explain theory of mind deficits in ASD (Frith & Frith, 1999). Indeed, one might argue that Leo Kanner captured the essence of our argument, without empirical data on the central nervous system, in his original descriptions of ASD, wherein he states: “We must, then, assume that these children have come into the world with innate inability to form the usual, biologically-provided affective contact with other people, just as other children come into the world with innate physical or intellectual handicaps” (p. 250). These are just a few of the ideas predating our own conceptualization, which have served to shape our ideas of abnormal social brain development in ASD.
The social brain and social perception
Social perception refers to the initial stages of evaluating the intentions and psychological dispositions of others by using their gaze direction, body movements, hand gestures, facial expressions, and other biological-motion cues (Allison, Puce, & McCarthy, 2000). Neuroscientists became deeply interested in social perception when it was discovered that neurons within the temporal cortex and amygdaloidal complex of monkeys were sensitive to and selective for social objects (e.g., faces and hands) and complex social stimuli (actions in a social context and direction of gaze) (Brothers & Ring, 1993; Brothers, Ring, & Kling, 1990; Desimone, 1991; Desimone, Albright, Gross, & Bruce, 1984; Perrett, Rolls, & Caan, 1979, 1982; Perrett, Smith, Potter, Mistlin, Head, Milner, & Jeeves, 1984, 1985; Rolls, 1981, 1995). On the basis of these pioneering findings, the field began to think seriously about the possibility of a network of brain regions dedicated to processing social information. The label “the social brain” was coined by Leslie Brothers (1990), and it served to elegantly capture the core, emerging idea. The social brain is defined as the complex network of areas that enable us to recognize other individuals and to evaluate their mental states (e.g., intentions, dispositions, desires, and beliefs). The key idea is that human beings, in response to the unique computational demands of their highly social environments, have evolved cognitive mechanisms and an associated, dedicated neural system that support such abilities as recognizing other agents and their actions, individuating others, perceiving the emotional states of others, analyzing the intentions and dispositions of others, sharing attention with one another, and representing another person's perceptions and beliefs.
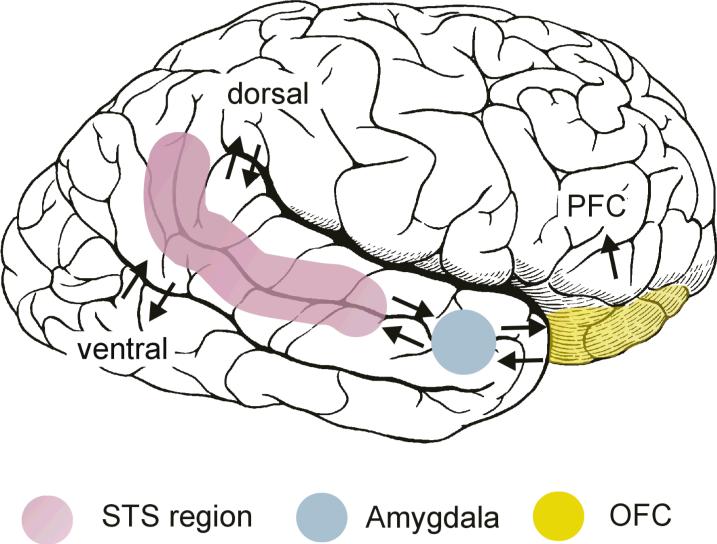
In an early and influential model of the social brain, Brothers (1990) emphasized the contributions of the superior temporal sulcus (STS), amygdala, orbital frontal cortex (OFC), and fusiform gyrus (FFG) to social perception. These four neuroanatomical structures are illustrated in Figure 1. In humans, the STS region, particularly the posterior STS in the right hemisphere, analyzes biological motion cues, including eye, hand, and other body movements, to interpret and predict the actions and intentions of others (e.g., Bonda, Petrides, Ostryi, & Evans, 1996; Pelphrey, Morris, Michelich, Allison, & McCarthy, 2005). The FFG, located in the ventral occipitotemporal cortex, contains a region termed the fusiform face area (FFA), which has been implicated in face detection (identifying a face as a face) and face recognition (identifying one's friend versus a stranger) (e.g., Kanwisher, McDermott, & Chun, 1997; Puce, Allison, Asgari, Gore, & McCarthy, 1996). The OFC has been strongly implicated in social reinforcement and reward processes more broadly (e.g., Rolls, 2000, 2009). Finally, the amygdala, a complex structure that is highly interconnected with cortical and other subcortical brain structures, has been implicated in helping to recognize the emotional states of others through analysis of facial expressions (e.g., Morris et al., 1996), as well as in multiple aspects of the experience and regulation of emotion (e.g., Davis & Whalen, 2001; Kluver & Bucy, 1997; LeDoux, 2000).
Figure 1.
Pictured are the components of the social brain as originally described by Leslie Brothers (1990) as well as their interconnections. The figure is borrowed with permission from Allison, Puce, and McCarthy (2000) Trends in Cognitive Science.
To understand social brain function, we must be as attentive to the interconnections of neuroanatomical structures as we are to their individual contributions. Currently, in humans, much is known about the roles played by the individual brain regions, but very little is known about the ways in which they are interconnected, and thus even less is known about how they interact functionally. However, we can take some initial guidance from the monkey brain; where it is known that the STS region has reciprocal connections to the amygdala (Amaral et al., 1992). The amygdala, in turn, is connected to the OFC (Amaral et al., 1992), and the STS is also connected with the OFC (Barbas, 1988). The OFC is connected to prefrontal cortex (Pandya & Yeterian, 1996), which is connected to motor cortex and the basal ganglia, thus completing what Allison and colleagues (2000) described as a pathway from perception to action.
The role of the STS region in social perception and its dysfunction in ASD
Representing actions
In an initial fMRI study of typically developing young adults, we compared the response from the posterior STS to four different types of motion conveyed via animated virtual-reality characters (Pelphrey, Mitchell, et al., 2003). Participants viewed walking, a biological motion conveyed by a robot or a human. They also viewed a nonmeaningful but complex nonbiological motion in the form of a disjointed mechanical figure as well as a complex, meaningful, and nameable nonbiological motion involving the movements of a grandfather clock. We reasoned that a region selectively responsive to biological motion should respond strongly to both the man and the robot walking, but not respond to the mechanical figure or the grandfather clock.
We found strong and equivalent activity in the right hemisphere posterior STS to the human and robot walking and very little activity to the moving clock and the mechanical figure. This pattern of results was very different from that observed in the nearby motion-responsive visual area called MT or V5 (Zeki et al., 1991), which responded robustly to all four of our stimulus conditions. We concluded that the posterior STS selectively processes biological motion. This finding led us to begin to view the posterior STS as one component of the neural system supporting social perception, via its identification and representation of observed human actions as compared to the movements of other objects.
Perceiving intentions
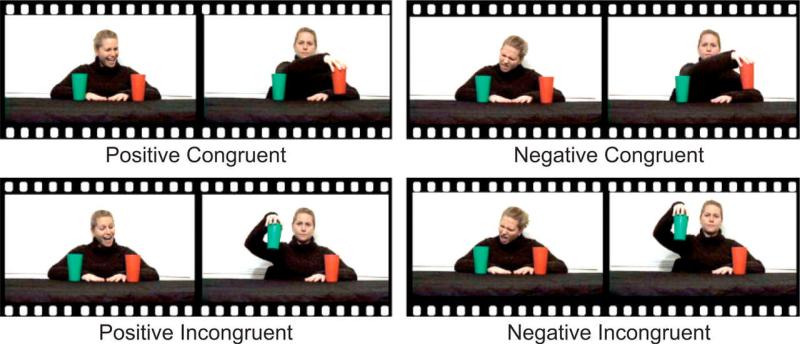
Having established a “baseline” role for the posterior STS region in social perception, we went on to examine whether this region simply serves as a biological motion detector or if it is involved more broadly in aspects of social perception through the evaluation of the intentions and dispositions that are conveyed by biological motions. For example, we asked if this region might be involved in representing another person's intentions with respect to the objects in his or her visual field (Vander Wyk et al., 2009). This kind of intention understanding involves integrating actions with the social and physical context and it is possible that the STS may show regional sensitivity to environmental or cognitive sources of information that inform this context. An especially salient component of social context is the perceived emotions of other individuals, particularly when a perceived emotion indicates another person's like or dislike of an object or event. We examined whether the posterior STS exhibits differences in activity depending on the previous emotional context related to understanding another's preferences, which would thus inform the viewer about that person's underlying intentions. This experiment used a paradigm adapted from a study of young children by Phillips et al. (2002), in which the participants observed an actress on video express positive or negative regard towards one of two cups. She then reached and picked up that same cup or the other one. Viewing the actress’ emotional expression allowed the participant to attribute an intention to her: positive expressions toward an object warranted the attribution of the intent to pick up the object, while negative expressions warranted the opposite attribution. The subsequent reaching gesture was then interpreted as being either congruent or incongruent with the intention. As illustrated in Figure 2, by crossing emotional expression and congruency of the action, we created four experimental conditions: Positive-Incongruent, Negative-Incongruent, Positive-Congruent, and Negative-Congruent.
Figure 2.
Schematic illustration of the four conditions in the experiment. In the positive conditions, the actress expressed positive regard toward one of the cups, and in the negative conditions, she expressed negative regard toward one of the cups. In the congruent conditions, the actress's reach was expected, given her previous expression. In the incongruent conditions, her reach was unexpected, given her previous expression. The figure is borrowed with permission from Vander Wyk et al., (2009) Psychological Science.
Our results indicated that the right posterior STS exhibited significantly more activity for the incongruent trials than for the congruent trials. This showed that the activity of the right posterior STS to a given biological motion is sensitive to prior emotional context. Specifically, the posterior STS showed a greater response when participants viewed a reach that was incongruent with a prior emotional expression (i.e., when the actress reached for the object not targeted by a prior positive expression or when she reached for the object targeted by a prior negative expression) than when they viewed a reach congruent with the actress’ expression. In both cases in which positive and negative emotions set up these expectations about the actress's future behavior towards an object, the response in the posterior STS was greater upon viewing unanticipated actions.
We concluded that the STS does not merely represent the surface features of biological motion, but also participates in integrating biological motion, or actions, with the social and/or physical context, and thereby plays a role in analyzing and interpreting the intentions underlying observed biological motions. This view of the functional role of the posterior STS is further bolstered by additional findings regarding a role for the posterior STS in analyzing the intentions and dispositions of others as conveyed by biological motion (e.g., Brass, Schmitt, Spengler, & Gergely, 2007; Castelli, Happé, Frith, & Frith, 2000; Pelphrey, Singerman, et al., 2003; Saxe, Xiao, Kovacs, Perrett, & Kanwisher, 2004).
Prior to our study by Vander Wyk and colleagues (2009), potential limitations in the experimental designs employed by our group and others left the findings open to an alternative interpretation. Corbetta, Patel, and Shulman (2008) argued that the prior results could be more parsimoniously explained by uncontrolled stimulus differences in demands for shifting attention. After all, the posterior STS region is known to be involved in the allocation of spatial attention in response to visual cues (e.g., Hopfinger, Buonocore, & Mangun, 2000). When we scrutinized our own study designs (e.g., Pelphrey, Singerman, et al., 2003) we recognized that our conditions could plausibly differ in the number of attention shifts evoked. However, in the Vander Wyk et al. (2009) study described above, by using both positive and negative emotions directed toward an object, we ensured that the number of attention shifts was fully balanced between congruent and incongruent trials. Our results supported an intention understanding as opposed to an attention-shifting hypothesis. That is, the pattern of findings we observed in this study fully contradicted the argument that increased activity in the posterior STS region while viewing unexpected actions in earlier findings, was related to additional shifts in attention required when observed movements differ from expectations (Corbetta, Patel, & Shulman, 2008). By our estimation, the attention reorienting account would predict greater activity for the Positive-Incongruent than for the Negative-Incongruent condition, because the former requires the participant to reorient attention twice and the latter only once. Likewise, the Positive-Congruent condition would be expected to involve only one shift in attention, while the Negative-Congruent condition should demand two shifts in attention (i.e., shifting attention to the cup at which the actress directed negative regard and then shifting attention to the cup picked up by the actress). This attention account would therefore predict equivalent activation in the Positive-Incongruent and Negative-Congruent conditions and equivalent activity in the Positive-Congruent and Negative-Incongruent conditions. We observed a very different pattern of effects: within the right posterior STS, the incongruent condition was greater than the congruent condition, regardless of the affect, a pattern of effects that an attention reorienting account cannot explain.
Failing to read intentions: A role for STS dysfunction in ASD
A large body of research demonstrates that individuals with ASD exhibit characteristic and early appearing deficits in the use of gaze information to understand the intentions and mental states of others, as well as to coordinate joint attention (Baron-Cohen et al., 1999; Baron-Cohen, Wheelwright, Hill, Raste, & Plumb, 2001; Dawson, Meltzoff, Osterling, Rinaldi, & Brown, 1998; Frith & Frith, 1999; Leekam, Hunnisett, & Moore, 1998; Leekam, Lopez, & Moore, 2000; Loveland & Landry, 1986; Mundy, Sigman, Ungerer, & Sherman, 1986). This failure to understand the mentalistic significance of eye gaze and the focus of another person's attention motivated a recent study of posterior STS function in individuals with ASD.
Adolescent and young adult participants with (N = 12) and without (N = 14) high-functioning ASD viewed actions that were congruent or incongruent with expectations given positive or negative emotional content. The two participant groups were matched on IQ, age, and gender. The individuals with ASD (not PDD-NOS or Asperger's Syndrome) were evaluated by an expert clinician and met gold-standard research criteria using the Autism Diagnostic Observation Schedule (ADOS, Lord et al., 2000) and the Autism Diagnostic Interview-Revised (ADI-R, Lord et al., 1994). Neurotypical participants scored below the threshold for ASD using the Autism Screening Questionnaire (ASQ, Berument et al., 1999) or the ADOS. This experiment made use of the identical reach-to-cups paradigm as our prior study (Vander Wyk, et al., 2009). To recap, as illustrated in Figure 2, participants were instructed to watch videos in which an actress was seated in front of a red cup and a green cup. At the start of a trial, the actress shifted her head and gaze to show preference to a cup by either smiling (Positive regard) or frowning (Negative regard), followed by a return to a neutral expression and eye contact with the camera. She maintained this pose while reaching towards, lifting, and setting down either cup to end the trial. Thus, given the initial preference and whether the actress chose to reach toward and pick up the ignored or attended cup, four conditions were created: (1) a Positive-Congruent condition where the actress expressed a positive emotion towards an object and then reached for that object; (2) a Positive-Incongruent condition, in which the actress reached for the ignored object; (3) a Negative-Congruent conditions where the actress expressed a negative emotion and reach to the ignored object; and (4) a Negative-Incongruent condition, in which the actress expressed a negative emotion to an object, but then reached to that object.
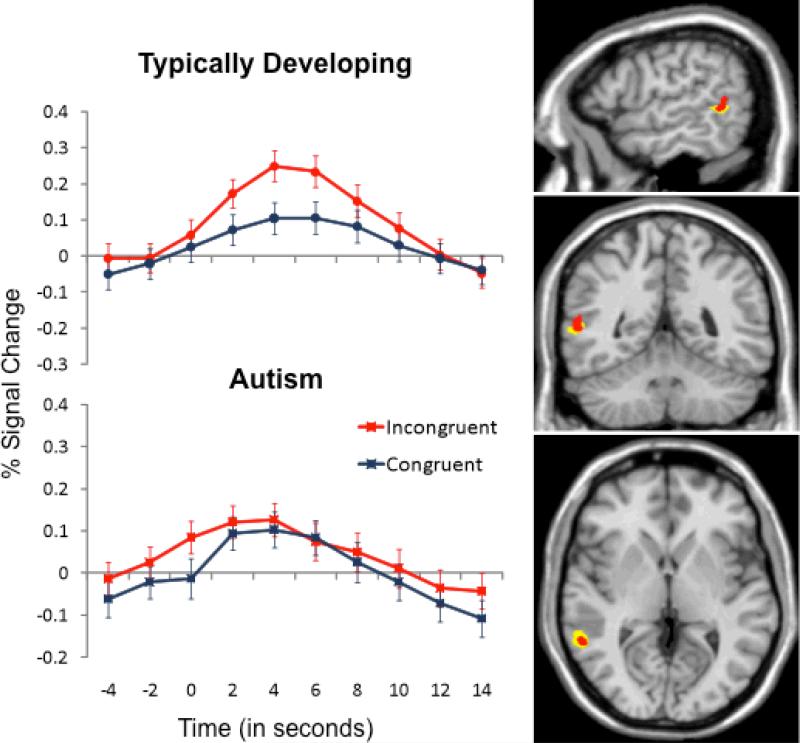
Based on our prior work, we hypothesized that the right posterior STS would not differentiate incongruent and congruent actions in ASD (Pelphrey, Morris, et al., 2005). As illustrated in the right panel of Figure 3, the new sample of typically developing participants demonstrated a strong effect of congruency (Incongruent > Congruent) within the right posterior STS. This area of activation nicely overlaps with the previously published reference region, clearly replicating our prior findings (Vander Wyk et al., 2009). In sharp contrast, individuals with ASD failed to exhibit Incongruent > Congruent activation in the posterior STS region. As shown in the left panel of Figure 3, participants with ASD responded with significant activation to the two stimulus categories; however, the activation levels did not differ as a function of congruency.
Figure 3.
Right panel, The new sample of typically developing participants (yellow color map) demonstrated a strong effect of congruency (Incongruent > Congruent) within the right posterior STS. This area of activation generally overlapped with the previously reported (Vander Wyk et al., 2009) reference region of the posterior STS (red color map). These activation maps are threshold at a value of q < .001 and k > 12. Left panel, participants with ASD (bottom graph) responded with significant activation to the two stimulus categories; however, the activation levels did not differ as a function of congruency as they did in typically developing participants (top graph). Error bars represent standard errors of the mean at each time point.
Consistent with our findings, other neuroimaging studies have revealed dysfunction of the STS in ASD during tasks involving eye movement perception (Pelphrey et al., 2005), the attribution of intentions to moving geometric figures (Castelli et al., 2002), and human speech perception (Boddaert et al., 2003; Gervais et al., 2004). Bilateral hypoperfusion of temporal lobe areas at rest has been observed in children with ASD (Ohnishi et al., 2000; Zilbovicius et al., 2000). A positron emission tomography (PET) study of speech perception reported abnormal laterality of responses and hypoactivation of the left superior temporal gyrus (Boddaert et al., 2003), and an fMRI study observed abnormal responses in the STS to human voices (Gervais et al., 2004). Finally, a study comparing cortical sulcal maps in individuals with and without ASD found anterior and superior displacements of the STS (Levitt et al., 2003), and Boddaert and colleagues (2004) reported abnormal STS volumes in ASD.
Amygdala and fusiform gyrus contributions to disrupted face perception in ASD
In comparison to typically developing participants, hypoactivation of the amygdala (e.g. Baron-Cohen et al., 1999; Ogai et al., 2003; Pelphrey et al., 2007) and the FFG (e.g. Critchley et al., 2000; Pelphrey et al., 2007; Schultz et al., 2000) have often been observed in people with ASD relative to typically developing participants. However, the question of whether or not hypoactivation in these regions is an aspect of the brain phenotype in ASD remains open and widely debated. Indeed, critical questions have been raised regarding the mechanisms underlying the amygdala. In particular, to what degree is the hypoactivation accounted for by known differences in the visual scanpaths exhibited by individuals with and without autism in response to faces?
In a recent study (Perlman et al., 2010), we experimentally manipulated activity in the face processing system of individuals with ASD by compelling them to look at the eyes of a face to varying degrees. This allowed us to determine whether hypoactivation in the amygdala and FFG can be accounted for by known differences in the visual scanpaths exhibited by individuals with and without autism in response to faces. We directly manipulated visual scanpaths across four experimental conditions including free viewing, central fixation, low, medium, or high amounts of fixating on the eyes in a group of high-functioning adults with autistic disorder and a matched (on IQ, age, and sex) group of typically developing participants during fMRI.
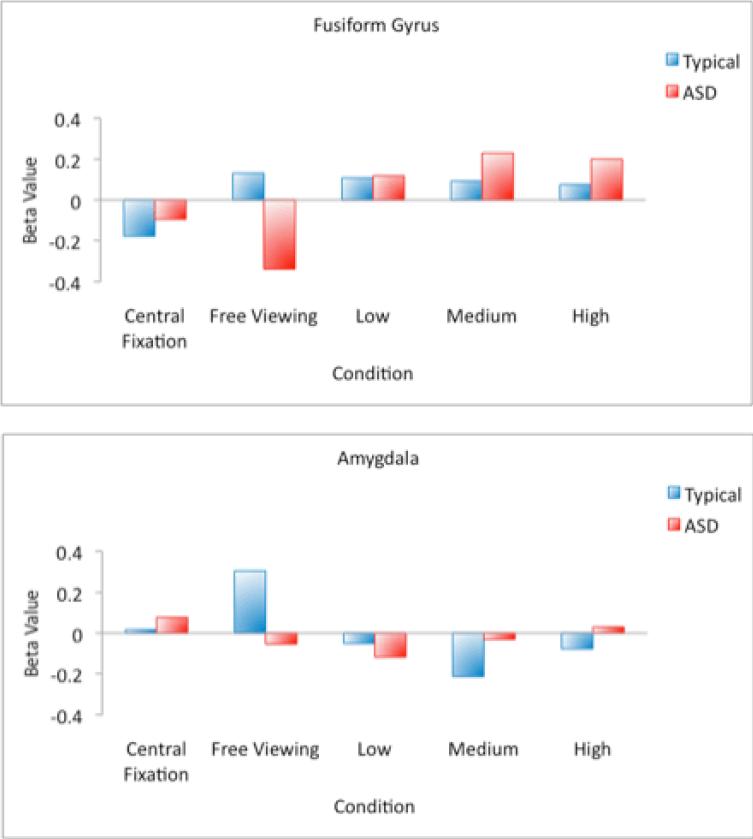
Comparison of activation during free viewing revealed hypoactivation of the right FFG and bilateral amygdala in the ASD group relative to typically developing participants. As illustrated in the top panel of Figure 4, activity in the right FFG of individuals with ASD increased from a slightly negative response during central fixation to a robustly positive response for each of the eye fixation conditions. In contrast, central fixation reduced activity in typically developing participants to below-zero levels, suggesting an inhibition of activity in the FFG when typically developing individuals have their scanpaths artificially constrained. Within the amygdala, there was no effect of the scanpath manipulation for individuals with autism and the scanpath manipulation reduced amygdala activation in typically developing participants (see Figure 4, bottom panel) relative to the free viewing condition. Our findings concerning increases in FFG activity when participants with ASD are compelled to look at the eyes were generally consistent with the two prior studies that, using varying methodology, constrained fixation to a central crosshair on a face (Hadjikhani et al., 2004; Pierce et al., 2004). However, we demonstrated that activity in the right FFG of individuals with ASD increases from a below-zero level during the central fixation condition to robustly positive levels during execution of a scanpath involving eye contact. By simply manipulating visual attention to the eyes, we were able to “normalize” activity in one component of the face processing system in individuals with ASD. Furthermore, our findings from the free viewing condition (Figure 4, top panel) reveal that the effect is not attributable to decreases in activity in the FFG of our typically develop participants as a function of artificially constraining their visual scanpaths during the low, medium, and high eye fixation conditions. It is noteworthy that when the fixation patterns were constrained to the center of the face during our Central Fixation condition, as they were constrained to the center of the eyes in the study by Hadjikhani et al. (2004) and to the center of the screen by Pierce et al. (2004), we found reduced activity to the point of significant deactivation in TD participants. In contrast to the FFG, our manipulation of eye contact had no effect on amygdala activation in our group of participants with ASD. In typically developing individuals, constraining eye movements to the center of the screen or directing eye movements to the eyes of the stimulus face served to significantly decrease amygdala activity (bottom panel of Figure 4). This observation is consistent with previous research conceptualizing the role of the amygdala as directing attention towards salient environmental stimuli such as the eyes of faces (e.g., Adolphs et al., 2005). It is possible that we do not observe amygdala activity when we constrain the eye movements of typically developing participants because we are circumventing the need for the amygdala to direct eye movements in response to the presentation of an emotionally expressive face. In the case of ASD, the lack of amygdala activity during free viewing, combined with the observation that this hypoactivation cannot be reversed via the manipulation of eye movements, suggests that disruption in this brain mechanism for directing eye movements to the key, most socially relevant features of a face is a core feature of the brain phenotype in autism.
Figure 4.
Graphs representing average beta value (across all voxels of a region of activation) in the right FFG (top panel) and the bilateral amygdala (bottom panel) for Free Viewing, Central Fixation, Low, Medium, and High Eyes conditions.
Comparing ASD and schizophrenia: Overlapping and distinct social brain dysfunction
We suggested earlier the necessity of demonstrating specificity for ASD relative to other neurodevelopmental disorders in construing theories of brain system dysfunction. To this end, Pinkham and her colleagues (2008) conducted a study that directly compared age- and IQ-matched adults with schizophrenia (paranoid and non-paranoid), adults with ASD, and typically developing adults. ASD and schizophrenia were compared because individuals with either of these very distinct neurodevelopmental disorders present with some overlapping deficits in aspects of social cognition and social behavior. A trustworthiness task, developed by Adolphs and his colleagues (1998), was used. The task required participants to look at a series of gray-scale frontal images of faces and to perform a forced-choice rating as trustworthy or untrustworthy. As a control task, participants viewed the same kind of faces, but judged age, classifying faces as “30 years of age or younger” or “over 30 years of age”.1 The design was partially modeled after an fMRI study by Winston and colleagues (2002) that found explicit trustworthiness judgments to robustly activate key components of the social brain including the right posterior STS, bilateral VLPFC/AI, amygdala, and lateral FFG.
The behavioral results indicated that the groups did not differ on the trustworthiness or age judgments in terms of the percentages rated as trustworthy / over thirty years of age, nor did they differ in reaction times. Each group exhibited activation of key, a priori selected regions of the social brain, including the bilateral amygdala, lateral FFG, and VLPFC/AI. The ASD group and the paranoid schizophrenia group exhibited significantly reduced activity in the right amygdala, FFG, and left VLPFC/AI as compared to the typically developing adults, and in the left VLPFC/AI as compared to non-paranoid schizophrenics. Strikingly, activity in the right posterior STS distinguished the participants with ASD from the three other participant groups. That is, during trustworthiness judgments, activation of the right posterior STS was observed for the typically developing participants, the non-paranoid schizophrenic group, and the paranoid schizophrenic group, but not in the group of participants with ASD. This suggests that dysfunction in this region might represent a “neural signature” of ASD.
On the one hand, the finding of overlapping areas of dysfunction in three components of the social brain (the amygdala, the VLPFC/AI, and the lateral FFG) across two very different neurodevelopmental disorders suggests that social cognition deficits are the result of specific neurofunctional impairments in the social brain. These deficits appear to be deficit-specific as opposed to disorder-specific (Pinkham et al., 2008). On the other hand, the finding that activity in the right posterior STS differentiates individuals with ASD from individuals with schizophrenia suggests that this aspect of social brain dysfunction is disorder specific. Note, too, that there was no reduced activity in any brain area in any of the clinical groups relative to the typically developing participants during age judgments, demonstrating that the reduced activity observed for social judgments cannot be attributed to overall reductions in activation, a failure to view stimuli, or a failure to engage in the task.
An emerging developmental perspective on the social brain
The work reviewed above indicates that the posterior STS in typically developing young adults is highly specialized for detecting biological motion and interpreting the actions and intentions of others. This region is not specialized for these functions in young adults with ASD. A fundamental question remains to be addressed: How does the posterior STS become specialized across development for processing biological motion and intentional actions, and when does this process go astray in ASD? Answering this question would constrain distinct, but not mutually exclusive, interpretations of findings of posterior STS dysfunction in young adults with ASD. One interpretation is that very early disruptions in the posterior STS may drive the deficits in social perception, characteristic of ASD. Alternatively, posterior STS dysfunction in ASD may emerge as a consequence of the failure to follow the course of normative social development. From early on, children with ASD demonstrate deficits in basic mechanisms that predispose the allocation of attention to socially relevant signals that facilitate engagement with others (Jones &Klin, 2009). For example, while infants as young as two days old preferentially attend to point-light displays of biological motion (Simion et al., 2008), two-year-olds with ASD fail to do so (Klin et al., 2009). Derailment of these basic social mechanisms early in life likely has cascading effects on subsequent development, causing increasing divergence in the processes impacting brain development (Jones &Klin, 2009). Given what is known from the behavioral literature regarding the early development of key aspects of social perception, determining precisely how the specialization of the posterior STS emerges will require longitudinal studies of infants in the first two years of life and methodological approaches that allow for the consideration and measurement of the impact of genes and experience (and their interaction) on the developing social brain.
At present, our knowledge of early typical and atypical development of social brain structures such as the posterior STS remains limited, in part due to technological and practical limitations. Imaging data is difficult to acquire for infants and young children who lack the ability to remain still while awake. Despite these limitations, studies have begun to elucidate the emergence of the specialization of the posterior STS for detecting agents and evaluating intentions.
Detecting agents
Carter and Pelphrey (2006) investigated the development of social brain regions involved in the perception of biological motion in 7- to 10-year-old typically developing children using fMRI. A key aim of the study was to test the specificity of the response to biological motion in the posterior STS by presenting participants with four sets of stimuli described earlier: a walking human, a walking robot, nonmeaningful but complex nonbiological motion (a disjointed mechanical figure), and meaningful, complex, and nameable nonbiological motion (a grandfather clock). As in adults (Pelphrey, Mitchell, et al., 2003), BOLD activity in the posterior STS of this sample of school age children clearly differentiated biological from non-biological motion. However, individual differences in the magnitude of this differentiation were apparent. A significant positive correlation was found between the magnitude of differentiation between biological and non-biological motion in the right posterior STS and age. A recent functional near-infrared spectroscopy study examined whether 5-month-old infants show a differential hemodynamic response over the posterior temporal lobe (a region encompassing the posterior STS) in response do dynamic biological motion stimuli (an actress moving her hands, eyes and mouth) compared with dynamic non-biological motion (moving machinery) (Lloyd-Fox et al., 2009). Significant hemodynamic changes were observed in response to the dynamic biological motion stimuli in both left and right posterior sensors. Additionally, more activation was observed in response to biological compared with non-biological motion, suggesting that in 5-month old infants there are already areas of the temporal lobe, located roughly in an area corresponding to the adult posterior STS, dedicated to the perception of biological motion. Together these studies suggest early specialization of a cortical network for the perception of biological motion, which becomes increasingly fine-tuned throughout development.
Reading intentions
Mosconi and colleagues (2005) used fMRI to examine the neural circuitry underlying eye-gaze processing and intention understanding in 7- to 10-year-old typically developing children. Children viewed an animated actor who shifted her eyes toward a target object (a congruent gaze shift) or towards empty space (an incongruent gaze shift). Consistent with prior adult studies, the posterior STS was sensitive to the intentions underlying the actor's eye movements, suggesting that by 7 years of age the neural circuitry underlying the detection of intentions through eye-gaze shifts may already be specialized for this particular social perception task. A similar study, using the same paradigm, was conducted in both adults and 9-month-old infants using event-related potential (ERP) measurements (Senju et al., 2006). In adults, incongruent gaze shifts elicited larger amplitudes at occipitotemporal sites (N330). A similar posterior component (N290) was observed in infants in response to incongruent gaze shifts. An additional frontal component (N200), showing higher amplitude in response to congruent gaze shifts was observed in infants but not in adults. While these results suggest that infants and adults recruit a similar cortical network to encode intentions of others, the more widespread activation in infants may imply less specialization early in development.
These studies represent initial advances in developmental social neuroscience. However, much work needs to be done in order to advance our understanding of the emerging functional specialization of the posterior STS and other areas of the social brain network. First, although infant studies demonstrate neural activity in response to biological motion versus mechanical motion, the exact degree of specialization of this response remains unclear. Second, while we have some understanding of brain development at the level of specific cortical regions (e.g. the posterior STS), few infant studies have examined how networks of brain regions become specialized for supporting social perception. Finally, studies have not yet addressed the disruption of neural systems for social perception in ASD. Because ASD are typically diagnosed after the second year of life, data on infants with ASD younger than two years of age are rare. However, research programs investigating infants at a high genetic risk for ASD may provide the opportunity for longitudinal studies of the development of cortical circuitry supporting social perception, an important step for developing optimal treatments for ASD early in life.
Conclusions
Given the nature of neurodevelopmental disorders, the expression of ASD is expected to be heterogeneous, particularly due to the pivotal interactions between gene, brain, and behavior throughout development (Cicchetti & Cohen, 2006). Focused efforts to explore the genetic and neural mechanisms underlying the core, homogeneous components of ASD and the deficits in tools for social engagement, are necessary to elucidate this complex, devastating family of neurodevelopmental disorders. Future work in this area should focus on identifying the earliest departures from the typical development of the social brain and follow the complex interactions among genes, brain, and behavior that drive and constrain the atypical development of the social brain in ASD. Such work holds remarkable potential for informing the development of rational approaches to intervention and treatment of ASD.
Acknowledgements
The research reviewed herein was supported by grants from the Simons Foundation, the National Institute of Mental Health, the National Institute of Child Health and Development, the John Merck Scholars Fund, the U.S. Veterans Affairs Administration, and Autism Speaks. Kevin Pelphrey was supported by a Career Development Award from the National Institutes of Health (NIMH Grant MH071284). We gratefully acknowledge our collaborators, especially Gregory McCarthy, James Morris, and Truett Allison. We are deeply grateful to the families, adults, and children who participate in our research. We confirm that all of the research from our laboratory presented in this review meets the ethical guidelines, including adherence to the legal requirements, of the USA. Informed consent was obtained from each of the study participants or their parents.
Footnotes
Participants were young adults, and thus it was unlikely that they would be familiar with Jack Weinberger's suggestion: “Don't trust anyone over thirty.”
References
- Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nature Reviews Genetics. 2008;9(5):341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393:470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- Allison T, Puce A, McCarthy G. Social perception from visual cues: Role of the STS region. Trends in Cognitive Sciences. 2000;4:267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biological Psychiatry. 2004;55(3):323–326. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. Mindblindness: An Essay on Autism and Theory of Mind. MIT Press; 1995. [Google Scholar]
- Baron-Cohen S, Ring HA, Wheelright S, Bullmore ET, Brammer MJ, Simmons A, et al. Social Intelligence in the Normal and Autistic Brain: An fMRI Study. European Journal of Neuroscience. 1999;11:1891–1898. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The reading the mind in the eyes test revised version: A study with normal adults, and adults with Asperger syndrome or high-functioning autism. Journal of Child Psychology and Psychiatry. 2001;42:241–251. [PubMed] [Google Scholar]
- Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism screening questionnaire: diagnostic validity. British Journal of Psychiatry. 1999;175:444–451. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- Boddaert N, Belin P, Chabane N, Poline JB, Barthélémy C, Mouren-Simeoni MC, Brunelle F, Samson Y, Zilbovicius M. Perception of complex sounds: abnormal pattern of cortical activation in autism. American Journal of Psychiatry. 2003;160(11):2057–2060. doi: 10.1176/appi.ajp.160.11.2057. [DOI] [PubMed] [Google Scholar]
- Boddaert N, Chabane N, Gervais H, Good CD, Bourgeois M, Plumet MH, Barthélémy C, Mouren MC, Artiges E, Samson Y, Brunelle F, Frackowiak RS, Zilbovicius M. Superior temporal sulcus anatomical abnormalities in childhood autism: a voxel-based morphometry MRI study. NeuroImage. 2004;23(1):364–369. doi: 10.1016/j.neuroimage.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Bonda E, Petrides M, Ostry D, Evans A. Specific involvement of human parietal systems and the amygdala in the perception of biological motion. Journal of Neuroscience. 1996;16:3737–3744. doi: 10.1523/JNEUROSCI.16-11-03737.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass M, Schmitt RM, Spengler S, Gergely G. Investigating action understanding: inferential processes versus action simulation. Current Biology. 2007;17:2117–2121. doi: 10.1016/j.cub.2007.11.057. [DOI] [PubMed] [Google Scholar]
- Brock J, Brown C, Boucher J, Rippon G. The temporal binding deficit hypothesis of autism. Developmental Psychopathology. 2002;142:209–224. doi: 10.1017/s0954579402002018. [DOI] [PubMed] [Google Scholar]
- Brito AR, Vasconcelos MM, Domingues RC, Hygino da Cruz LC, Jr., Rodrigues Lde S, Gasparetto EL, Calçada CA. Diffusion tensor imaging findings in school-aged autistic children. Journal of Neuroimaging. 2009;19(4):337–343. doi: 10.1111/j.1552-6569.2009.00366.x. [DOI] [PubMed] [Google Scholar]
- Carter EJ, Pelphrey KA. School-aged children exhibit domain-specific responses to biological motion. Social Neuroscience. 2006;1:396–411. doi: 10.1080/17470910601041382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli F, Happé F, Frith U, Frith C. Movement and mind: a functional imaging study of perception and interpretation of complex intentional movement patterns. NeuroImage. 2000;12:314–325. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- Cheung C, Chua SE, Cheung V, Khong PL, Tai KS, Wong TK, Ho TP, McAlonan GM. White matter fractional anisotrophy differences and correlates of diagnostic symptoms in autism. Journal of Child Psychology and Psychiatry. 2009;50(9):1102–1112. doi: 10.1111/j.1469-7610.2009.02086.x. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Behen ME, Muzik O, Juhasz C, Nagy F, Chugani DC. Local brain functional activity following early deprivation: A study of postinstitutionalized Romanian orphans. NeuroImage. 2003;14(6):1290–1301. doi: 10.1006/nimg.2001.0917. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Cohen D, editors. Developmental psychopathology: Theory and method. 2nd Edition Vol. 1. Wiley; New York: 2006. [Google Scholar]
- Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: Local over-connectivity but long-distance disconnection. Brain Research: Cognitive Brain Research. 2005;23(2-3):221–234. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Cullen KR, Gee DG, Klimes-Dougan B, Gabbay V, Hulvershorn L, Mueller BA, et al. A preliminary study of functional connectivity in comorbid adolescent depression. Neuroscience Letters. 2009;460(3):227–231. doi: 10.1016/j.neulet.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: Vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Dawson G, Meltzoff AN, Osterling J, Rinaldi J, Brown E. Children with autism fail to orient to naturally occurring social stimuli. Journal of Autism and Developmental Disorders. 1998;28(6):479–485. doi: 10.1023/a:1026043926488. [DOI] [PubMed] [Google Scholar]
- Eluvathingal TJ, Chugani HT, Behen ME, Juhász C, Muzik O, Maqbool M, et al. Abnormal brain connectivity in children after early severe socioemotional deprivation: A diffusion tensor imaging study. Pediatrics. 2006;117(6):2093. doi: 10.1542/peds.2005-1727. [DOI] [PubMed] [Google Scholar]
- Esslinger C, Walter H, Kirsch P, Erk S, Schnell K, Arnold C, Haddad L, Mier D, Opitz von Boberfeld C, Raab K, Witt SH, Rietschel M, Cichon S, Meyer-Lindenberg A. Neural mechanisms of a genome-wide supported psychosis variant. Science. 2009;324(5927):605. doi: 10.1126/science.1167768. [DOI] [PubMed] [Google Scholar]
- Fecteau S, Lepage J-F, Theoret H. Autism spectrum disorder: Seeing is not understanding. Current Biology. 2006;16(4):R131–R133. doi: 10.1016/j.cub.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Fletcher PT, Whitaker RT, Tao R, DuBray MB, Froehlich A, Ravichandran C, Alexander AL, Bigler ED, Lange N, Lainhart JE. Microstructural connectivity of the arcuate fasciculus in adolescents with high-functioning autism. Neuroimage. 2010;51(3):1117–1125. doi: 10.1016/j.neuroimage.2010.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proceedings of the National Academy of Sciences. 2006;103(26):10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox R, McDaniel C. The perception of biological motion by human infants. Science. 1982;218(4571):486–487. doi: 10.1126/science.7123249. [DOI] [PubMed] [Google Scholar]
- Fotheringham JB. Autism: its primary psychological and neurological deficit. Canadian Journal of Psychiatry. 1991;36(9):686–692. doi: 10.1177/070674379103600913. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clinical Neuroscience. 1995;3(2):89–97. [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds--a biological basis. Science. 1999;286(5445):1692–1705. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Gervais H, Belin P, Boddaert N, Leboyer M, Coez A, Sfaello I, Barthélémy C, Brunelle F, Samson Y, Zilbovicius M. Abnormal cortical voice processing in autism. Nature Neuroscience. 2004;7(8):801–802. doi: 10.1038/nn1291. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Levitt P. Autism spectrum disorders: Developmental disconnection syndromes. Current Opinion in Neurobiology. 2007;17(1):103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Gupta AR, State MW. Recent advances in the genetics of autism. Biological Psychiatry. 2007;61(4):429–437. doi: 10.1016/j.biopsych.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Autism: the first firm finding = underconnectivity? Epilepsy & Behavior. 2007;11(1):20–24. doi: 10.1016/j.yebeh.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Johansson G. Visual perception of biological motion and a model for its analysis. Perception & Psychophysics. 1973;14:201–211. [Google Scholar]
- Jones W, Klin A. Heterogeneity and homogeneity across the autism spectrum: The role of development. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(5):471–473. doi: 10.1097/CHI.0b013e31819f6c0d. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: Evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Sentence comprehension in autism: Thinking in pictures with decreased functional connectivity. Brain. 2006;129:2484–2493. doi: 10.1093/brain/awl164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Minshew NJ, Just MA. Inhibitory control in high functioning autism: Decreased activation and underconnectivity in inhibition networks. Biological Psychiatry. 2007;62:198–206. doi: 10.1016/j.biopsych.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER. A new intellectual framework for psychiatry. American Journal of Psychiatry. 1998;155:457–469. doi: 10.1176/ajp.155.4.457. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The Fusiform Face Area: A module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller TA, Kana RK, Just MA. A developmental study of the structural integrity of white matter in autism. NeuroReport. 2007;18:23–27. doi: 10.1097/01.wnr.0000239965.21685.99. [DOI] [PubMed] [Google Scholar]
- Kumar A, Sundaram SK, Sivaswamy L, Behen ME, Makki MI, Ager J, Janisse J, Chugani HT, Chugani DC. Alterations in frontal lobe tracts and corpus callosum in young children with autism spectrum disorder. Cerebral Cortex. 2010;20(9):2103–2113. doi: 10.1093/cercor/bhp278. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Sterling L, Stegbauer KC, Mahurin R, Johnson CL, Greenson J, Dawson G, Aylward E. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131(4):1000–1012. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz RT, Volkmar FR, Cohen DJ. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Archives of General Psychiatry. 2002;59:809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- Klin A, Lin DJ, Gorrindo P, Ramsay G, Jones W. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459(7244):257–261. doi: 10.1038/nature07868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klüver H, Bucy PC. Preliminary analysis of functions of the temporal lobes in monkeys. Journal of Neuropsychiatry Clinical Neuroscience. 1997;9:606–620. doi: 10.1176/jnp.9.4.606. [DOI] [PubMed] [Google Scholar]
- Koshino H, Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. fMRI investigation of working memory for faces in autism: Visual coding and underconnectivity with frontal areas. Cerebral Cortex. 2008;18:289–300. doi: 10.1093/cercor/bhm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshino H, Carpenter PA, Minshew NJ, Cherkassky VL, Keller TA, Just MA. Functional connectivity in an fMRI working memory task in high-functioning autism. NeuroImage. 2005;24:810–821. doi: 10.1016/j.neuroimage.2004.09.028. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion Circuits in the Brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee PS, Yerys BE, Rosa AD, Foss-Feig J, Barnes KA, James JD, VanMeter J, Vaidya CJ, Gaillard WD, Kenworthy LE. Functional connectivity of the inferior frontal cortex changes with age in children with autism spectrum disorders: A fcMRI study of response inhibition. Cerebral Cortex. 2009;19:1787–1794. doi: 10.1093/cercor/bhn209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leekam SR, Hunnisett E, Moore C. Targets and cues: gaze-following in children with autism. Journal of Child Psychology and Psychiatry. 1998;39(7):951–962. [PubMed] [Google Scholar]
- Leekam SR, López B, Moore C. Attention and joint attention in preschool children with autism. Developmental Psychology. 2000;36(2):261–273. doi: 10.1037//0012-1649.36.2.261. [DOI] [PubMed] [Google Scholar]
- Levitt JG, Blanton RE, Smalley S, Thompson PM, Guthrie D, McCracken JT, Sadoun T, Heinichen L, Toga AW. Cortical sulcal maps in autism. Cerebral Cortex. 2003;13(7):728–735. doi: 10.1093/cercor/13.7.728. [DOI] [PubMed] [Google Scholar]
- Liu J, Liang J, Qin W, Tian J, Yuan K, Bai L, et al. Dysfunctional connectivity patterns in chronic heroin users: An fMRI study. Neuroscience Letters. 2009;460(1):72–77. doi: 10.1016/j.neulet.2009.05.038. [DOI] [PubMed] [Google Scholar]
- Lloyd-Fox S, Blasi A, Volein A, Everdell N, Elwell CE, Johnson MH. Social perception in infancy: A near infrared spectroscopy study. Child Development. 2009;80(4):986–999. doi: 10.1111/j.1467-8624.2009.01312.x. [DOI] [PubMed] [Google Scholar]
- Lord C, Pickles A, McLennan J, Rutter M, Bregman J, Folstein S, Fombonne E, Leboyer M, Minshew N. Diagnosing autism: Analyses of data from the Autism Diagnostic Interview. Journal of Autism and Developmental Disorders. 1997;27(5):501–517. doi: 10.1023/a:1025873925661. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Loveland KA, Landry SH. Joint attention and language in autism and developmental language delay. Journal of Autism and Developmental Disorders. 1986;16(3):335–349. doi: 10.1007/BF01531663. [DOI] [PubMed] [Google Scholar]
- Mason RA, Williams DL, Kana RK, Minshew NJ, Just MA. Theory of mind disruption and recruitment of the right hemisphere during narrative comprehension in autism. Neuropsychologia. 2008;46:269–280. doi: 10.1016/j.neuropsychologia.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Peltier SJ, Wiggins JL, Weng S-J, Carrasco M, Risi S, Lord C. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. NeuroImage. 2009;47(2):764–772. doi: 10.1016/j.neuroimage.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Mosconi MW, Mack PB, McCarthy G, Pelphrey KA. Taking an “intentional stance” on eye-gaze shifts: A functional neuroimaging study of social perception in children. NeuroImage. 2005;27:247–252. doi: 10.1016/j.neuroimage.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Mundy P, Sigman M, Ungerer J, Sherman T. Defining the social deficits of autism: The contribution of non-verbal communication measures. Journal of Child Psychology and Psychiatry. 1986;27(5):657–669. doi: 10.1111/j.1469-7610.1986.tb00190.x. [DOI] [PubMed] [Google Scholar]
- Noonan SK, Haist F, Muller R-A. Aberrant functional connectivity in autism: Evidence from low-frequency BOLD signal fluctuations. Brain Research. 2009;1262:48–63. doi: 10.1016/j.brainres.2008.12.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi T, Matsuda H, Hashimoto T, Kunihiro T, Nishikawa M, Uema T, Sasaki M. Abnormal regional cerebral blood flow in childhood autism. Brain. 2000;123:1838–1844. doi: 10.1093/brain/123.9.1838. [DOI] [PubMed] [Google Scholar]
- Omori E, Watanabe S. Discrimination of Johansson's stimuli in pigeons. International Journal of Comparative Psychology. 1996;9:92. [Google Scholar]
- Oram MW, Perrett DI. Integration of form and motion in the anterior superior temporal polysensory area (STPa) of the macaque monkey. Journal of Neurophysiology. 1996;76(1):109–129. doi: 10.1152/jn.1996.76.1.109. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Carter EJ. Brain mechanisms for social perception: Lessons from autism and typical development. Annals of the New York Academy of Science. 2008;1145:283–299. doi: 10.1196/annals.1416.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Mitchell TV, McKeown MJ, Goldstein J, Allison T, McCarthy G. Brain activity evoked by the perception of human walking: Controlling for meaningful coherent motion. Journal of Neuroscience. 2003;23:6819–6825. doi: 10.1523/JNEUROSCI.23-17-06819.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP. Brain mechanisms for interpreting the actions of others from biological-motion cues. Current Directions in Psychological Science. 2006;15:136–140. doi: 10.1111/j.0963-7214.2006.00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G. Grasping the intentions of others: The perceived intentionality of an action influences activity in the superior temporal sulcus during social perception. Journal of Cognitive Neuroscience. 2004;16:1706–1716. doi: 10.1162/0898929042947900. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Sasson NJ, Reznick JS, Paul G, Goldman BD, Piven J. Visual scanning of faces in autism. Journal of Autism and Developmental Disorders. 2002;32:249–261. doi: 10.1023/a:1016374617369. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Singerman JD, Allison T, McCarthy G. Brain activation evoked by perception of gaze shifts: The influence of context. Neuropsychologia. 2003;41:156–170. doi: 10.1016/s0028-3932(02)00146-x. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Viola RJ, McCarthy G. When strangers pass: Processing of mutual and averted social gaze in the superior temporal sulcus. Psychological Science. 2004;15:598–603. doi: 10.1111/j.0956-7976.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- Perlman SB, Hudac CM, Pegors T, Minshew NJ, Pelphrey KA. Experimental manipulation of face-evoked activity in the fusiform gyrus of individuals with autism. Social Neuroscience. 2010 2010 May 27; doi: 10.1080/17470911003683185. electronic publication ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AT, Wellman HM, Spelke ES. Infants’ ability to connect gaze and emotional expression to intentional action. Cognition. 2002;85:53–78. doi: 10.1016/s0010-0277(02)00073-2. [DOI] [PubMed] [Google Scholar]
- Pinkham AE, Hopfinger JB, Pelphrey KA, Piven J, Penn DL. Neural bases for impaired social cognition in schizophrenia and autism spectrum disorders. Schizophrenia Research. 2008;99:164–175. doi: 10.1016/j.schres.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puce A, Allison T, Asgari M, Gore JC, McCarthy G. Differential sensitivity of human visual cortex to faces, letterstrings, and textures: A functional MRI study. The Journal of Neuroscience. 1996;16:5205–5215. doi: 10.1523/JNEUROSCI.16-16-05205.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards TL, Berninger VW. Abnormal fMRI connectivity in children with dyslexia during a phoneme task: Before but not after treatment. Journal of Neurolinguistics. 2008;21(4):294–304. doi: 10.1016/j.jneuroling.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahley TL, Panksepp J. Brain opioids and autism: An updated analysis of possible linkages. Journal of Autism and Developmental Disorders. 1987;17(2):201–216. doi: 10.1007/BF01495056. [DOI] [PubMed] [Google Scholar]
- Sahyoun CP, Belliveau JW, Soulières I, Schwartz S, Mody M. Neuroimaging of the functional and structural networks underlying visuospatial vs. linguistic reasoning in high-functioning autism. Neuropsychologia. 2010;48(1):86–95. doi: 10.1016/j.neuropsychologia.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R, Xiao D-K, Kovacs G, Perrett DI, Kanwisher N. A region of right posterior superior temporal sulcus responds to observed intentional actions. Neuropsychologia. 2004;42:1435–1446. doi: 10.1016/j.neuropsychologia.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senju A, Johnson MH, Csibra G. The development and neural basis of referential gaze perception. Social Neuroscience. 2006;1(3-4):220–234. doi: 10.1080/17470910600989797. [DOI] [PubMed] [Google Scholar]
- Shaw ME, Strother SC, McFarlane AC, Morris PLP, Anderson J, Egan GF, Clark RC. Abnormal functional connectivity in posttraumatic stress disorder. NeuroImage. 2001;15:661–674. doi: 10.1006/nimg.2001.1024. [DOI] [PubMed] [Google Scholar]
- Sivaswamy L, Kumar A, Rajan D, Behen M, Muzik O, Chugani D, Chugani H. A diffusion tensor imaging study of the cerebellar pathways in children with autism spectrum disorder. Journal of Child Neurology. 2010 2010 February 22; doi: 10.1177/0883073809358765. electronic publication ahead of print. [DOI] [PubMed] [Google Scholar]
- Simion F, Regolin L, Bulf H. A predisposition for biological motion in the newborn baby. Proceedings of the National Academy of Sciences. 2008;105:809–813. doi: 10.1073/pnas.0707021105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Menon V, Rubin D, Musen M, Greicius MD. Network analysis of intrinsic functional brain connectivity in Alzheimer's disease. PLoS Computational Biology. 2008;4(6):e1000100. doi: 10.1371/journal.pcbi.1000100. doi:10.1371/journal.pcbi.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences. 2008;105(34):12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Menon V. The anterior insula in autism: Under-connected and under-examined. Neuroscience and Biobehavioral Reviews. 2009 doi: 10.1016/j.neubiorev.2009.06.002. doi:10.1016/j.neubiorev.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Wyk BC, Hudac CM, Carter EJ, Sobel DM, Pelphrey KA. Action understanding in the superior temporal sulcus region. Psychological Science. 2009;20:771–777. doi: 10.1111/j.1467-9280.2009.02359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos MM, Brito AR, Domingues RC, da Cruz LC, Jr., Gasparetto EL, Werner J, Jr., Gonçalves JP. Proton magnetic resonance spectroscopy in school-aged autistic children. Journal of Neuroimaging. 2008;18(3):288–95. doi: 10.1111/j.1552-6569.2007.00200.x. [DOI] [PubMed] [Google Scholar]
- Weiss LA, Shen Y, Korn JM, Arking DE, Miller DT, Fossdal R, Saemundsen E, Stefansson H, Ferreira MA, Green T, Platt OS, Ruderfer DM, Walsh CA, Altshuler D, Chakravarti A, Tanzi RE, Stefansson K, Santangelo SL, Gusella JF, Sklar P, Wu BL, Daly MJ, Autism Consortium Association between microdeletion and microduplication at 16p11.2 and autism. New England Journal of Medicine. 2008;358(7):667–675. doi: 10.1056/NEJMoa075974. [DOI] [PubMed] [Google Scholar]
- Wicker B, Fonlupt P, Hubert B, Tardif C, Gepner B, Deruelle C. Abnormal cerebral effective connectivity during explicit emotional processing in adults with autism spectrum disorder. Social Cognitive and Affective Neuroscience. 2008;3:135–143. doi: 10.1093/scan/nsn007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki S, Watson JD, Lueck CJ, Friston KJ, Kennard C, Frackowiak RS. A direct demonstration of functional specialization in human visual cortex. Journal of Neuroscience. 1991;11:641–649. doi: 10.1523/JNEUROSCI.11-03-00641.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilbovicius M, Boddaert N, Belin P, Poline JB, Remy P, Mangin JF, Thivard L, Barthélémy C, Samson Y. Temporal lobe dysfunction in childhood autism: A PET study. American Journal of Psychiatry. 2000;157(12):1988–1993. doi: 10.1176/appi.ajp.157.12.1988. [DOI] [PubMed] [Google Scholar]