Abstract
Chromosomal instability (CIN) is associated with poor prognosis in human cancer. However, in certain animal tumour models elevated CIN negatively impacts upon organism fitness, and is poorly tolerated by cancer cells. To better understand this seemingly contradictory relationship between CIN and cancer cell biological fitness and its relationship with clinical outcome, we applied the CIN70 expression signature, which correlates with DNA-based measures of structural chromosomal complexity and numerical chromosomal instability in vivo, to gene expression profiles of 2125 breast tumours from 13 published cohorts. Tumours with extreme CIN, defined as the highest quartile CIN70 score, were predominantly of the estrogen receptor (ER) negative, basal-like phenotype and displayed the highest chromosomal structural complexity and chromosomal numerical instability. We found that the extreme CIN/ER-negative tumours were associated with improved prognosis relative to tumours with intermediate CIN70 scores in the third quartile. We also observed this paradoxical relationship between CIN and prognosis in ovarian, gastric and non-small cell lung cancer, with poorest outcome in tumours with intermediate, rather than extreme, CIN70 scores. These results suggest a non-monotonic relationship between gene signature expression and hazard ratio for survival outcome, which may explain the difficulties encountered in the identification of prognostic expression signatures in ER negative breast cancer. Furthermore, the data are consistent with the intolerance of excessive CIN in carcinomas and provide a plausible strategy to define distinct prognostic patient cohorts with ER-negative breast cancer. Inclusion of a surrogate measurement of CIN may improve cancer risk stratification and future therapeutic approaches.
Introduction
Chromosomal instability (CIN) results in numerical and structural chromosomal complexity and is associated with poor prognosis in solid tumours (1, 2), and the acquisition of phenotypic variation promoting drug resistance in yeast models (3). In contrast, in mammalian cells and yeast models, aneuploidy may have a negative impact upon organism fitness and proliferation (4, 5). Indeed, CIN may confer both a tumour promoting and tumour suppressive function in animal model systems (6, 7). Excessive CIN can be introduced in model systems by inactivation of mitotic spindle checkpoint components; this results in gross aneuploidy and cell death (8). Accordingly, elevation of the frequency of chromosome missegregation has been proposed as a strategy to kill tumour cells (8, 9).
Such an opposing relationship suggests that there may be an optimal level of CIN for tumour progression, beyond which further instability provides no growth advantage that may even be deleterious for cancer cell survival (10) through an evolutionary scenario analogous to “mutational meltdown” in bacteria (11) or “error catastrophe” in viruses (12). However, it is not known whether CIN, over a certain threshold, may impact negatively on human tumour growth, or whether very high levels of CIN in human tumours might be associated with improved patient prognosis relative to intermediate levels. If such a relationship exists, it may have important implications for risk stratification and for future therapeutic approaches directed against CIN tumours.
The CIN70 expression signature was derived from a surrogate measure of chromosomal instability and is defined as the average expression of 70 genes that correlate with “total functional aneuploidy” in solid tumours (1). Here we demonstrate that the CIN70 expression signature correlates with both structural chromosomal complexity and numerical chromosomal instability, and we use this signature to address the relationship between CIN and outcome in cancer.
Materials and Methods
Gene expression datasets
We obtained raw microarray expression data for 13 publicly available breast cancer cohorts (13–23) and GSE2109 and GSE16446, representing 2125 individual patients. Additionally, we obtained gene expression data from 3 ovarian cancer cohorts (24–26), 2 squamous NSCLC cohorts (25, 27) and one gastric cancer cohort (28).
Data analysis
Duplicate patients were present in some cohorts, but were removed from the analysis. Censored recurrence-free or metastasis-free survival data was available for 1168 breast cancer patients from seven cohorts. ER and ERBB2 status was inferred by k-medoids clustering of the expression levels of the ESR1 and ERBB2 genes, an approach that correlates well with histological assessment (29). CIN70 scores were calculated as the mean expression of the 70 probe sets matching the 70 genes of the CIN70 signature as described (1). For analysis of combined cohorts, CIN70 scores were first normalized within each cohort by centering the values, and then dividing by the standard deviation. All breast cancer samples in the combined cohorts were stratified into CIN70 quartiles according to the normalized CIN70 scores. These groups were fixed, and were not re-defined for analysis of specific subtypes. Thus, in analysis of specific subtypes, each quartile does not necessarily contain a quarter of the samples. Similarly, for non-breast cancer cohorts, CIN70 quartiles were defined based on all tumours of the given cancer type.
SNP based measurements of chromosomal instability
Publicly available SNP data based on the Affymetrix 100k platform(30), representing 281 breast tumor specimens with paired expression data (23), was acquired from GEO. We determined the Genome Integrity Index (GII) as described (31). To determine the total number of DNA breakpoints, we counted the number of DNA segments with an inferred log2 ratio of greater than 0.3 or less than −0.3. The total number of LOH regions was inferred from regions of allelic imbalance as described (32). To determine a combined GII/copy number/LOH score, we linearly transformed each set of scores such that its values ranged between 0 and 1, with no aberrations/GII being assigned to 0, and the highest number of aberrations/highest GII score in our cohort assigned a value of 1. We defined the combined aberration score as the mean of the three transformed scores.
Statistical analysis
For the breast cancer cohorts and the gastric cancer cohort, survival analysis was performed with time to relapse or, if not available, time to distant metastasis as outcome variable. For the ovarian and squamous lung cohorts, the outcome variable was overall survival. Survival curves were calculated using the Kaplan-Meier method. For univariate Cox regression, significance was estimated with a log rank test. For multivariate regression, significance was estimated using a chi-squared test. In the meta-analyses, the summary estimate was calculated as a weighted average of the individual estimates, where summary weights were calculated as the reciprocal of the variance defined as the squared standard errors. All data analysis and statistics was performed in the R statistical environment version 2.11. All P values are two-sided. Full methods are available in Supplementary Methods.
Results
CIN70 signature is a surrogate measure of structural chromosomal complexity and numerical chromosomal instability in vivo
To assess structural chromosomal complexity, we used a published cohort of 281 primary breast cancer patients whose tumours were profiled with paired SNP and mRNA expression arrays (23, 30). From the mRNA data we calculated the CIN70 score. From the SNP data we calculated three summary measures of structural chromosomal complexity: 1) The Genome Integrity Index (GII), a measure of the proportion of the cancer genome subject to copy number aberrations (31). 2) The total number of DNA copy number changes and 3) The total number of genomic regions with allelic imbalance (AI) (32), a measure correlated with loss of heterozygosity (LOH). Each of the three measures was positively and significantly correlated with the CIN70 score (Supplementary Figure 1, a–c). We combined these three DNA-based measures into a single “structural chromosomal complexity” measurement (Supplementary Methods), which was more strongly correlated with the CIN70 score than any of the individual measures (Supplementary Figure 1d). We then stratified patients into CIN70 score quartiles and found that the tumours with the highest CIN70 score quartile displayed the highest mean structural chromosomal complexity (Figure 1a), both in all tumors (P = 1.8 × 10−6, t-test) and in ER-/ERBB2-tumors only (P = 0.0002).
Fig. 1. CIN70 score and structural chromosomal complexity.
(A) The structural chromosomal complexity score based on SNP array data is shown for each CIN70 quartile in 271 breast cancer samples. (B) Nuclear DNA content versus CIN70 quartile in 44 breast cancer samples. (C) Frequency of diploid, genomically stable (dGS); aneuploid, genomically stable (aGS); and aneuploid, genomically unstable (aGU) in each CIN70 quartile.
To address the relationship between the CIN70 score and numerical CIN, we analysed a cohort of 44 breast cancers with paired DNA image cytometry and gene expression measurements (33) and stratified patients into CIN70 score quartiles. DNA image cytometry provides two relevant readouts: DNA index as a proxy for DNA ploidy; and stemline scatter index, discriminating genomically stable from genomically unstable tumours, providing a measure of numerical chromosomal instability (33). We found only a borderline significant enrichment of high DNA index in the highest CIN70 quartile (P = 0.08, t-test; Figure 1b). In contrast, we found a strong enrichment for genomically unstable tumours in the highest CIN70 quartile (OR = 15.4, P = 0.0006, Fisher's exact test; Figure 1c), indicating that the CIN70 score more closely reflects numerical chromosomal instability than ploidy status. Together, these results demonstrate that the CIN70 score reflects both structural chromosomal complexity and numerical chromosomal instability. Furthermore, the greatest chromosomal instability was observed in the highest CIN70 score quartile, which we defined as CINextreme.
CIN70 distribution in breast tumour subtypes
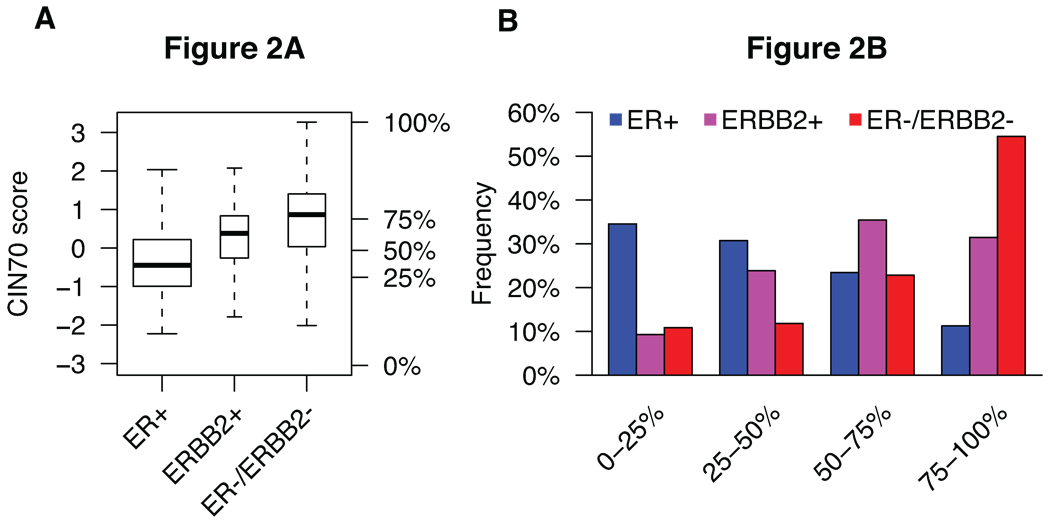
To assess whether higher-risk breast cancer subtypes exhibit increased levels of tumour chromosomal instability, we investigated the distribution of the CIN70 score in ER positive, ER-/ERBB2- and ERBB2 positive breast cancer subtypes in a pooled analysis of 2125 patients from 13 publicly available microarray expression datasets. As ER and ERBB2 status were not available for all samples, we inferred ER and ERBB2 subtype from gene expression data using published methods (29). In general, ER positive tumours displayed the lowest average CIN70 score, whereas ER-/ERBB2- tumours displayed the highest average and the broadest distribution of CIN70 scores (Figure 2a). The majority of ER-/ERBB2- tumours occur in the CINextreme, highest CIN70 score quartile, whereas ER positive breast cancers encompass the lower CIN70 score quartiles (Figure 2b). We also analyzed CIN70 scores in tumours stratified by intrinsic subtype and noted higher scores in basal-like tumours (P < 10−16, Supplementary Figure 2a,b) (34).
Fig. 2. Distribution of CIN70 scores across breast cancer subtypes.
(A) Overall distribution of CIN70 scores across 2125 breast cancer patients stratified by ER and ERBB2 receptor subtype. Percentages on Y-axis denote quartile thresholds. (B) Distribution of each breast cancer subtype within each of the four CIN70 score quartiles.
Non-monotonic relationship between CIN70 score and prognosis in ER-/ERBB2- breast cancer
As we previously reported (1), a CIN70 score higher than the median was associated with significantly poorer outcome when considering all patients (Supplementary Figure 3). However, we hypothesised that if animal and eukaryotic cell models describing intolerance of high levels of CIN were relevant to human breast cancer (4, 5, 8), then excessive CIN, determined by the CIN70 expression signature in the CINextreme quartile, might be associated with improved clinical outcome in breast cancer patient cohorts. Such an effect would manifest as a non-monotonic relationship, showing first increasing then decreasing risk of relapse as a function of increasing CIN70 score. ER-/ERBB2- breast cancer is considered a high-risk subtype and displays both the greatest range of CIN70 scores and the highest frequency of CINextreme tumours, providing a relevant patient cohort to address whether CINextreme might be associated with improved prognosis relative to tumours with intermediate CIN.
In the combined ER-/ERBB2- cohort of 265 patients, we observed better clinical outcome for patients with CINextreme tumours (highest CIN70 score quartile) compared to patients with tumours in the 3rd CIN70 score quartile (Figure 3a, HR = 0.55, P = 0.021, log-rank test). To confirm that this finding was not an artefact of the combined cohort, we also performed a meta-analysis of the individual datasets, with a similar result (Supplementary Figure 4, HR = 0.51, P = 0.017). When the cohorts were separated into those patients treated with and without adjuvant therapy, the same relationship was observed, indicating that improved outcome in CINextreme tumours appears to be independent of treatment (data not shown). In a multivariate analysis of ER-/ERBB2- patients including age, grade, nodal status, size, and CINextreme versus 3rd CIN70 score quartiles, CINextreme was a significant predictor of improved recurrence-free survival (HR = 0.40, P = 0.03, Supplementary Table 1). This difference in predicted relapse-free survival between tumours in the CINextreme cohort compared to tumours in the 3rd CIN70 score quartile was not detected with Adjuvant!Online (Supplementary Figure 5, P = 0.60, t-test), indicating that standard histopathological approaches do not distinguish these two groups.
Fig. 3. Survival of cancer patients stratified by CIN70 score quartile.
Kaplan-Meier survival curves are presented for (A) 265 ER-/ERBB2- breast cancer patients with recurrence-free or distant metastasis-free survival, (B) 652 patients with ovarian cancer, (C) 183 patients with squamous NSCLC, (D) 197 patients with gastric cancer.
CINextreme is associated with improved prognosis in ovarian cancer, squamous non-small cell lung cancer and gastric adenocarcinoma
To investigate whether the improved survival observed for patients with CINextreme tumours was specific for ER-/ERBB2- breast cancers or was a more general phenomenon in epithelial carcinomas, we acquired data from three ovarian carcinoma cohorts, two squamous non-small cell lung carcinoma (NSCLC) cohorts, and a gastric cancer cohort (Supplementary Methods). In the ovarian carcinoma meta-dataset, CINextreme tumours were associated with improved recurrence-free survival relative to tumours in the 3rd CIN70 score quartile (Figure 3b), as observed in the breast carcinoma cohorts. A similar relationship was observed in squamous NSCLC, although CINextreme was associated with improved prognosis relative to the two middle CIN70 score quartiles (Figure 3c). In the gastric cancer cohort, CINextreme was associated with similar prognosis to the two lowest CIN quartiles, and the 3rd CIN quartile was associated with the poorest prognosis (Figure 3d).
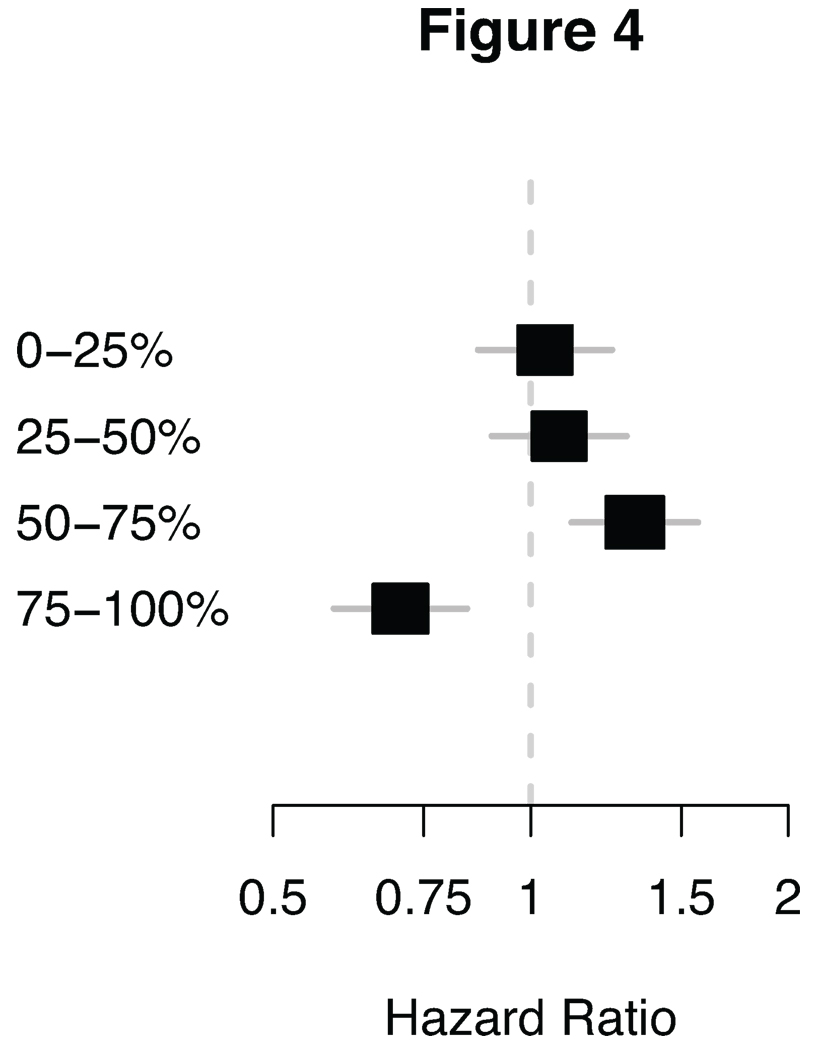
When we specifically assessed the hazard ratio for relapse for each of the four CIN quartiles relative to patients in the remaining three quartiles in a meta-analysis of the 1297 patients across the four epithelial carcinoma subtypes (ER-/ERBB2- breast, ovarian, squamous NSCLC, and gastric cancer), the CINextreme quartile was associated with a significantly improved hazard ratio (HR = 0.70, P = 0.0001), in contrast to the 3rd CIN70 score quartile carcinomas which were associated with a significantly worse hazard ratio for relapse (HR = 1.32, P = 0.001, Figure 4, Supplementary Figure 6a–d). This result is consistent with a non-monotonic relationship between CIN and outcome, with carcinomas in the CINextreme score quartile having the best prognosis, whilst carcinomas in the intermediate 3rd CIN70 score quartile are associated with the poorest clinical outcome.
Fig. 4. Quartile hazard ratio.
Forest plots showing the hazard ratio of the quartiles. The hazard ratio of each quartile is based on a summary estimate of ER-/ERBB2-breast tumours, ovarian tumours, squamous NSCLC and gastric tumours.
Discussion
Our results based on the CIN70 signature support the hypothesis that whilst genomic instability improves cancer cell biological fitness and may impact adversely on prognosis, excessive genomic instability may surpass a threshold compatible with cell viability (10). This suggestion is supported by evidence that aneuploidy in eukaryotic cell systems negatively impacts upon cell biological fitness (4, 5) and excessive CIN may induce cell autonomous lethality (8, 9). Given the non-monotonic nature of the relationship between CIN expression and prognosis, therapeutic strategies to modulate genomic instability may provide a rational approach for future drug development (8, 9). We have presented evidence that the CIN70 signature correlates with both structural chromosome complexity, an expected finding due to the derivation of this signature from a surrogate measure of CIN known as “total functional aneuploidy” (1), and numerical CIN measured by DNA image cytotmetry, and thus may serve as a stratification tool for genome instability within clinical cohorts.
Development of robust methods to predict patient outcome in ER-negative breast cancer is of paramount importance to enhance personalised treatment stratification approaches in this disease. Prognostic expression signatures in ER positive breast cancer such as those underlying the Mammaprint and Oncotype DX tests have been shown to predict tumour genome instability status (33) but have limited prognostic value in ER negative breast cancer (35). Our results derived from a plausible biological hypothesis, demonstrating a non-monotonic relationship between CIN70 expression and clinical outcome, begin to define an ER negative patient cohort with extreme CIN associated with good prognosis and a cohort with intermediate CIN associated with the worst prognosis, independent of standard histopathological criteria. This non-monotonic relationship between expression and survival provides a basis for the difficulties encountered in applying “prognostic” gene expression signature sets, which also predict genome instability status, to ER negative breast cancer outcome (35).
In a parallel paper by Roylance et al., we confirm these findings in ER negative breast cancer, where we directly quantify CIN using centromeric FISH in a cohort of 246 primary breast cancers with survival outcome data. Here we confirm that there is over-representation of ER-negative breast cancers within the most extreme-CIN cohort relative to ER-positive breast cancer and demonstrate that primary ER-negative breast cancers with extreme-CIN are associated with the best prognosis. Furthermore, concordant with the work presented here, extreme-CIN is a predictor of favourable outcome in a multivariate analysis in ER negative breast cancer.
In summary, the classification of tumour CIN status might be considered for incorporation into new prognostic models in cancer to validate this approach and form the basis for novel treatment strategies in order to improve patient prognosis and clinical outcome directed against this pattern of genome instability.
Supplementary Material
Acknowledgements
CS is a senior Medical Research Council (MRC) clinical research fellow. This work was funded by CR-UK, MRC, NIH (grants NCI SPORE P50 CA 89393, R21LM008823-01A1) and by the Breast Cancer Research Foundation. NJB was funded by the Danish Council for Independent Research-Medical Sciences (FSS). Some results are in whole or part based upon data generated by The Cancer Genome Atlas Pilot Project established by the NCI and NHGRI (24).
References
- 1.Carter SL, Eklund AC, Kohane IS, Harris LN, Szallasi Z. A signature of chromosomal instability inferred from gene expression profiles predicts clinical outcome in multiple human cancers. Nat Genet. 2006;38:1043–1048. doi: 10.1038/ng1861. [DOI] [PubMed] [Google Scholar]
- 2.Walther A, Houlston R, Tomlinson I. Association between chromosomal instability and prognosis in colorectal cancer: a meta-analysis. Gut. 2008;57:941–950. doi: 10.1136/gut.2007.135004. [DOI] [PubMed] [Google Scholar]
- 3.Pavelka N, Rancati G, Zhu J, Bradford WD, Saraf A, Florens L, et al. Aneuploidy confers quantitative proteome changes and phenotypic variation in budding yeast. Nature. 2010;468:321–325. doi: 10.1038/nature09529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torres EM, Sokolsky T, Tucker CM, Chan LY, Boselli M, Dunham MJ, et al. Effects of aneuploidy on cellular physiology and cell division in haploid yeast. Science. 2007;317:916–924. doi: 10.1126/science.1142210. [DOI] [PubMed] [Google Scholar]
- 5.Williams BR, Prabhu VR, Hunter KE, Glazier CM, Whittaker CA, Housman DE, et al. Aneuploidy affects proliferation and spontaneous immortalization in mammalian cells. Science. 2008;322:703–709. doi: 10.1126/science.1160058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schvartzman JM, Sotillo R, Benezra R. Mitotic chromosomal instability and cancer: mouse modelling of the human disease. Nat Rev Cancer. 2010;10:102–115. doi: 10.1038/nrc2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker DJ, Jin F, Jeganathan KB, van Deursen JM. Whole chromosome instability caused by Bub1 insufficiency drives tumorigenesis through tumor suppressor gene loss of heterozygosity. Cancer Cell. 2009;16:475–486. doi: 10.1016/j.ccr.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kops GJ, Foltz DR, Cleveland DW. Lethality to human cancer cells through massive chromosome loss by inhibition of the mitotic checkpoint. Proc Natl Acad Sci U S A. 2004;101:8699–8704. doi: 10.1073/pnas.0401142101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janssen A, Kops GJ, Medema RH. Elevating the frequency of chromosome mis-segregation as a strategy to kill tumor cells. Proc Natl Acad Sci U S A. 2009;106:19108–19113. doi: 10.1073/pnas.0904343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cahill DP, Kinzler KW, Vogelstein B, Lengauer C. Genetic instability and darwinian selection in tumours. Trends in cell biology. 1999;9:M57–M60. [PubMed] [Google Scholar]
- 11.Lynch M, Burger R, Butcher D, Gabriel W. The mutational meltdown in asexual populations. The Journal of heredity. 1993;84:339–344. doi: 10.1093/oxfordjournals.jhered.a111354. [DOI] [PubMed] [Google Scholar]
- 12.Eigen M. Error catastrophe and antiviral strategy. Proc Natl Acad Sci U S A. 2002;99:13374–13376. doi: 10.1073/pnas.212514799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andre F, Job B, Dessen P, Tordai A, Michiels S, Liedtke C, et al. Molecular characterization of breast cancer with high-resolution oligonucleotide comparative genomic hybridization array. Clin Cancer Res. 2009;15:441–451. doi: 10.1158/1078-0432.CCR-08-1791. [DOI] [PubMed] [Google Scholar]
- 14.Chin K, DeVries S, Fridlyand J, Spellman PT, Roydasgupta R, Kuo WL, et al. Genomic and transcriptional aberrations linked to breast cancer pathophysiologies. Cancer Cell. 2006;10:529–541. doi: 10.1016/j.ccr.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Desmedt C, Haibe-Kains B, Wirapati P, Buyse M, Larsimont D, Bontempi G, et al. Biological processes associated with breast cancer clinical outcome depend on the molecular subtypes. Clin Cancer Res. 2008;14:5158–5165. doi: 10.1158/1078-0432.CCR-07-4756. [DOI] [PubMed] [Google Scholar]
- 16.Hess KR, Anderson K, Symmans WF, Valero V, Ibrahim N, Mejia JA, et al. Pharmacogenomic predictor of sensitivity to preoperative chemotherapy with paclitaxel and fluorouracil, doxorubicin, and cyclophosphamide in breast cancer. J Clin Oncol. 2006;24:4236–4244. doi: 10.1200/JCO.2006.05.6861. [DOI] [PubMed] [Google Scholar]
- 17.Lu X, Wang ZC, Iglehart JD, Zhang X, Richardson AL. Predicting features of breast cancer with gene expression patterns. Breast Cancer Res Treat. 2008;108:191–201. doi: 10.1007/s10549-007-9596-6. [DOI] [PubMed] [Google Scholar]
- 18.Miller LD, Smeds J, George J, Vega VB, Vergara L, Ploner A, et al. An expression signature for p53 status in human breast cancer predicts mutation status, transcriptional effects, and patient survival. Proc Natl Acad Sci U S A. 2005;102:13550–13555. doi: 10.1073/pnas.0506230102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–524. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pawitan Y, Bjohle J, Amler L, Borg AL, Egyhazi S, Hall P, et al. Gene expression profiling spares early breast cancer patients from adjuvant therapy: derived and validated in two population-based cohorts. Breast Cancer Res. 2005;7:R953–R964. doi: 10.1186/bcr1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silver DP, Richardson AL, Eklund AC, Wang ZC, Szallasi Z, Li Q, et al. Efficacy of neoadjuvant Cisplatin in triple-negative breast cancer. J Clin Oncol. 2010;28:1145–1153. doi: 10.1200/JCO.2009.22.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sotiriou C, Wirapati P, Loi S, Harris A, Fox S, Smeds J, et al. Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst. 2006;98:262–272. doi: 10.1093/jnci/djj052. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F, et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005;365:671–679. doi: 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
- 24.NCI. The Cancer Genome Atlas (TGCA) http://cancergenome.nih.gov/
- 25.Bild AH, Yao G, Chang JT, Wang Q, Potti A, Chasse D, et al. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature. 2006;439:353–357. doi: 10.1038/nature04296. [DOI] [PubMed] [Google Scholar]
- 26.Dressman HK, Berchuck A, Chan G, Zhai J, Bild A, Sayer R, et al. An integrated genomic-based approach to individualized treatment of patients with advanced-stage ovarian cancer. J Clin Oncol. 2007;25:517–525. doi: 10.1200/JCO.2006.06.3743. [DOI] [PubMed] [Google Scholar]
- 27.Raponi M, Zhang Y, Yu J, Chen G, Lee G, Taylor JM, et al. Gene expression signatures for predicting prognosis of squamous cell and adenocarcinomas of the lung. Cancer Res. 2006;66:7466–7472. doi: 10.1158/0008-5472.CAN-06-1191. [DOI] [PubMed] [Google Scholar]
- 28.Ooi CH, Ivanova T, Wu J, Lee M, Tan IB, Tao J, et al. Oncogenic pathway combinations predict clinical prognosis in gastric cancer. PLoS Genet. 2009;5 doi: 10.1371/journal.pgen.1000676. e1000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gong Y, Yan K, Lin F, Anderson K, Sotiriou C, Andre F, et al. Determination of oestrogen-receptor status and ERBB2 status of breast carcinoma: a gene-expression profiling study. The lancet oncology. 2007;8:203–211. doi: 10.1016/S1470-2045(07)70042-6. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Martens JW, Yu JX, Jiang J, Sieuwerts AM, Smid M, et al. Copy number alterations that predict metastatic capability of human breast cancer. Cancer Res. 2009;69:3795–3801. doi: 10.1158/0008-5472.CAN-08-4596. [DOI] [PubMed] [Google Scholar]
- 31.Chin SF, Teschendorff AE, Marioni JC, Wang Y, Barbosa-Morais NL, Thorne NP, et al. High-resolution aCGH and expression profiling identifies a novel genomic subtype of ER negative breast cancer. Genome Biol. 2007;8:R215. doi: 10.1186/gb-2007-8-10-r215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li C, Beroukhim R, Weir BA, Winckler W, Garraway LA, Sellers WR, et al. Major copy proportion analysis of tumor samples using SNP arrays. BMC bioinformatics. 2008;9:204. doi: 10.1186/1471-2105-9-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Habermann JK, Doering J, Hautaniemi S, Roblick UJ, Bundgen NK, Nicorici D, et al. The gene expression signature of genomic instability in breast cancer is an independent predictor of clinical outcome. Int J Cancer. 2009;124:1552–1564. doi: 10.1002/ijc.24017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 35.Teschendorff AE, Miremadi A, Pinder SE, Ellis IO, Caldas C. An immune response gene expression module identifies a good prognosis subtype in estrogen receptor negative breast cancer. Genome Biol. 2007;8:R157. doi: 10.1186/gb-2007-8-8-r157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.