Abstract
Purpose
The purpose of this study was to determine whether HDAC inhibitors (HDACIs) such as vorinostat or entinostat (SNDX-275) could increase the lethality of the dual Bcr/Abl-aurora kinase inhibitor KW-2449 in various Bcr/Abl+ human leukemia cells, including those resistant to imatinib mesylate (IM).
Experimental Design
Bcr/Abl+ CML and ALL cells, including those resistant to IM (T315I, E255K) were exposed to KW-2449 in the presence or absence of vorinostat or SNDX-275, after which apoptosis and effects on signaling pathways were examined. In vivo studies combining HDACIs and KW2449 were performed using a systemic IM-resistant ALL xenograft model.
Results
Co-administration of HDACIs synergistically increased KW-2449 lethality in vitro in multiple CML and Ph+ ALL cell types including human IM resistant cells (e.g. BV-173/E255K, Adult/T315I). Combined treatment resulted in inactivation of Bcr/Abl and downstream targets (e.g. STAT5 and CRKL), as well as increased ROS generation and DNA damage (γH2A.X). The latter events and cell death were significantly attenuated by free radical scavengers (TBAP). Increased lethality was also observed in primary CD34+ cells from patients with CML, but not in normal CD34+ cells. Finally, minimally active vorinostat or SNDX275 doses markedly increased KW2449 anti-tumor effects and significantly prolonged the survival of murine xenografts bearing IM-resistant ALL cells (BV173/E255K).
Conclusions
HDACIs increase KW-2449 lethality in Bcr/Abl+ cells in association with inhibition of Bcr/Abl, generation of ROS, and induction of DNA damage. This strategy preferentially targets primary Bcr/Abl+ hematopoietic cells and exhibits enhanced in vivo activity. Combining KW-2449 with HDACIs warrants attention in IM-resistant Bcr/Abl+ leukemias.
Introduction
Chronic myelogenous leukemia (CML) is characterized by a reciprocal translation t(9;22)(q34;q11.2) resulting in the Philadelphia chromosome (Ph), which produces a constitutively active tyrosine kinase, Bcr/Abl [1]. Philadelphia chromosome-positive ALL occurs in approximately 20–30% of patients with ALL and carries a relatively poor prognosis [2]. The treatment of CML, and to a lesser extent Ph+ ALL, has been revolutionized by the introduction of the Bcr/Abl kinase inhibitor imatinib mesylate; and subsequently, second-generation inhibitors such as nilotinib and the dual Src-Bcr/Abl inhibitors dasatinib and bosutinib [3, 4]. Unfortunately, resistance to imatinib or other kinase inhibitors generally develops, frequently reflecting the emergence of point mutations within various regions of the Bcr/Abl kinase although other mechanisms (e.g. increased Bcr/Abl expression, pharmacokinetic factors) may also be involved [5]. Mutations within the “gatekeeper”, or contact region (e.g., T315I) are particularly intractable and confer resistance to most Bcr/Abl kinase inhibitors, including imatinib, nilotinib, dasatinib, and bosutinib [6]. Consequently, novel and more effective therapeutic strategies are urgently needed in this setting.
Progress in this area was stimulated by the observation that certain aurora kinase inhibitors (e.g., XL228, VX-680/MK0457, and PHA-379358) targeted both wild-type and mutant Bcr/Abl [4]. Aurora kinases represent a family of serine-threonine kinases intimately involved in cell cycle regulation, particularly mitotic progression. Aurora A kinase is associated with centrosome maturation and separation, required for spindle assembly; whereas Aurora B kinase is a chromosome passenger protein required for spindle checkpoint activation and cytokinesis [7, 8]. Recently, an orally active multi-kinase inhibitor (KW-2449) has been developed which inhibits T315I Bcr/Abl in vitro and in vivo, as well as multiple other kinases, including aurora kinase, FLT3, and FGFR-1 [9]. Notably, KW-2449 displays minimal cardiotoxicity, a toxicity which halted the development of VX-680/MK-0457. Importantly, KW-2449, like VX-680, has shown activity in cells obtained from imatinib (IM)-resistant patients bearing the T315I mutation [9]. Of late, the clinical development of KW-2449 has focused primarily on patients with FLT3-mutated AML [10].
HDAC inhibitors (HDACIs) represent a class of agents that block histone acetylation, induce a more open chromatin configuration, and activate genes that promote cell differentiation or death [11]. HDACIs (e.g., vorinostat and romidepsin) have been approved for the treatment of cutaneous T-cell lymphoma (CTCL) [12]. These agents kill leukemia cells through diverse mechanisms, including induction of oxidative injury, up-regulation of death receptors, and acetylation of non-histone proteins, among others [13]. We and other groups have shown that HDACIs promote the lethality of IM and other kinase inhibitors in Bcr/Abl+ leukemias [14, 15].
Recently, our group and others reported that the pan-HDACI vorinostat synergistically potentiated the activity of VX-680 in Bcr/Abl+ leukemia cells, and was particularly effective against cells bearing various TKI resistance-conferring mutations (e.g., T315I) [16–18]. Unfortunately, VX-680-associated cardiotoxicity (QTc prolongation) rendered it unavailable for clinical use. However, the dual Bcr/Abl and aurora kinase inhibitor KW-2449, which has undergone clinical evaluation in Bcr/Abl+ and Bcr/Abl− leukemias, represents a plausible alternative. The purpose of this study was to assess the effects of HDACIs (e.g., vorinosat, SBHA, SNDX-275) on responses to the dual Bcr/Abl-aurora kinase inhibitor KW-2449 in various Bcr/Abl+ lymphoid and myeloid leukemia cells, with a particular emphasis on those bearing mutations conferring resistance to imatinib mesylate (i.e. T315I and E255K).
Materials and Methods
Cell lines
The BV173/E255K cell line was generated from parental BV173 by increasing concentration of imatinib. Ba/F3 cells expressing wild-type and mutant forms of Bcr/Abl provided by Dr Brian J. Druker (Oregon Health & Science University Cancer Institute, Portland, OR). All cells above were cultured in RPMI1640 medium containing 10% fetal bovine serum (FBS). Adult/T315I cells were established from a Ph+ acute lymphoblastic leukemia patient and co-cultured with irradiated OP9 cells in MEM alpha supplemented with 20% FBS.
Isolation of CD34+ cells
Bone marrow or peripheral blood was obtained with informed consent from CML patients. Mononuclear cells were isolated by Ficoll-Hypaque (Sigma-Aldrich, St Louis, MO) density gradient separation and enriched for CD34+ cells using a Miltenyi microbead separation system (Miltenyi BioTech, Auburn, CA) according to the manufacturer's protocol. Normal samples were obtained from cord blood with informed consent. Normal CD34+ cells were isolated from cord blood and processed as above. All studies have been approved by the VCU Investigational Review Board (VCU IRB #HM 12517).
Reagents
KW-2449 was provided by Kyowa Hakko Kirin Co Ltd. Vorinostat was supplied by Merck & Co., Inc, Whitehouse Station, NY. SNDX-275, entinostat, (formerly MS-275) was provided by Syndax, Waltham MA. All compounds were dissolved in dimethyl sulfoxide (DMSO) for in vitro study. Manganese (III) tetrakis (4-benzoic acid) porphyrin (Mn-TBAP) was purchased from Calbiochem. Z-VAD-fmk was purchased from MP Biomedicals (Irvine, CA). Suberic bishydroxamate (SBHA) and apicidin were purchased from Biomol International.
Assessment of apoptosis
The extent of apoptosis was evaluated by either annexin V-fluorescein isothiocyanate (FITC) staining (BD Pharmigen) or 7-aminoactinomycin D (7-AAD, Sigma–Aldrich) by flow cytometry as described previously [19, 20].
Measurement of ROS production
Cells were treated with either 20 µmol/L 5-6-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA, Invitrogen) or 5 µmol/L dehydroethidium (HE-Invitrogen) for 45 min at 37°C. Fluorescence was monitored by flow cytometry and analyzed with CellQuest software.
Immunoblot (Western blot)
Western blot analysis was performed as previously described [16]. The following were used as primary antibodies: cleaved caspase-3, cleaved PARP, p-STAT5 (Y694), p-CRKL (Y207), p-Aurora B from Cell Signaling Technology, Beverly, MA); antiphosphotyrosine (4G10), p-Histone H3, γ-H2A.X (Upstate, Lake Placid, NY); Abl, Cytochrome-c and apoptosis-inducing factor (AIF) (Santa Cruz, CA); and actin and tubulin (Sigma-Aldrich). Blots were stripped and reprobed with actin or tubulin antibodies to ensure equal loading and transfer of proteins. Analysis of cytosolic released proteins was performed as previously described [21].
Sequencing of the BCR/ABL ATP binding site
Total RNA was extracted using the RNeasy mini kit (QIAGEN). One microgram of RNA was subjected to RT-PCR (AccuScript™ High Fidelity 1st Strand cDNA Synthesis Kit (Stratagene). The cDNA was PCR amplified using primers as forward primer, CM10 (5′-GAAGCTTCTCCCTGACATCCGT-3′) and reverse primer, ABL-KD-R2 (5′-AACATTGTTTCAAAGGCTTGGT -3′). This reaction produced a 1.6-kb fragment that corresponds to the Bcr-Abl fusion junction and ABL kinase domain. This 1.6-kb fragment was purified by use of the QIAquick gel extraction kit (Qiagen) and then used as a template for a second PCR with the primers ABL-Fs ′(5-GCGCAACAAGCCCACTGTCTATGG-3′) and ABL-KD-R2. This reaction amplified the ABL kinase domain as a 0.8-kb fragment, which was purified and sequenced. Sequence data was aligned and analyzed with Bioedit software.
Methylcellulose colony formation assays
A total of 104 CML CD34+ cells were isolated and plated in Methocult GFH4434 (StemCell Technologies) in the presence or absence of drugs. Leukemic colony-forming units (L-CFU), consisting of groups of ≥ 20 cells, were scored at the end of 10–14 d incubation. Values for each condition were expressed as a percentage of untreated, control cell colony formation.
Statistical analysis
The significance of differences between experimental conditions was determined using the Student's t-test. PASW statistics 18 software was used to evaluate survival rate (Kaplan Meier). Characterization of synergistic and antagonistic interactions in cells exposed to a range of KW2449 and HDACI concentrations administered at a fixed ratio was performed using Median Dose Effect analysis in conjunction with a commercially available software program (CalcuSyn). Combination Index values <1.0 denote synergistic interactions.
Animal studies
All animal studies were performed in accordance with a protocol approved by the Virginia Commonwealth University’s institutional animal care. pGL4.51 (luc2/CML/Neo) was purchased from Promega and transfected into BV173/E255K by using an Amaxa nucleofector (Koeln, Germany). Stable clones were selected in the presence of G418 (500 µg/ml, Cellgro, Herndon, VA). Robust and stable expression of a luciferase clone, referred to as BV173/E255K/Luc cl4, was used for in vivo experiments.
Animal studies were performed in CBySmn.CB17-Prkds scid/J (BALB/C) mice (The Jackson Laboratory). A total of 2 × 106 BV173/E255K/Luc cl4 cells in 100uL phosphate-buffered saline (PBS) were injected into tail vein. Tumor infiltration was monitored by bioluminescence imaging one or twice a week. These animals were noninvasively imaged using the In Vivo Imaging System (IVIS-200; Xenogen, Hopkinton, MA) after injection with the luciferase substrate (D-Luciferin, Research Products International). For in vivo studies, KW2449 was dissolved in 0.5% methylcellulose 400 solution (Wako). SNDX-275 and vorinostat were first dissolved in DMSO and stored in –80°C in small aliquots. SNDX-275 was further diluted in sterile water before use. Vorinostat was further diluted in 1:1 poethylene glycol 400 (Fluka analytical) and sterile water to a final composition of 10% DMSO, 45% PEG400, 45% water before use. Both SNDX-275 and KW2449 was orally administered at 15mg/kg/day and 32mg/kg/day respectively. Vorinostat 70 mg/kg/day was administered intraperitoneally (IP). All drugs were given 5 days/week. The weight of each mouse was monitored once or twice a week.
Results
KW2449 interacts synergistically with HDACIs to induce apoptosis in Ph+ CML cells in a time- and concentration-dependent manner
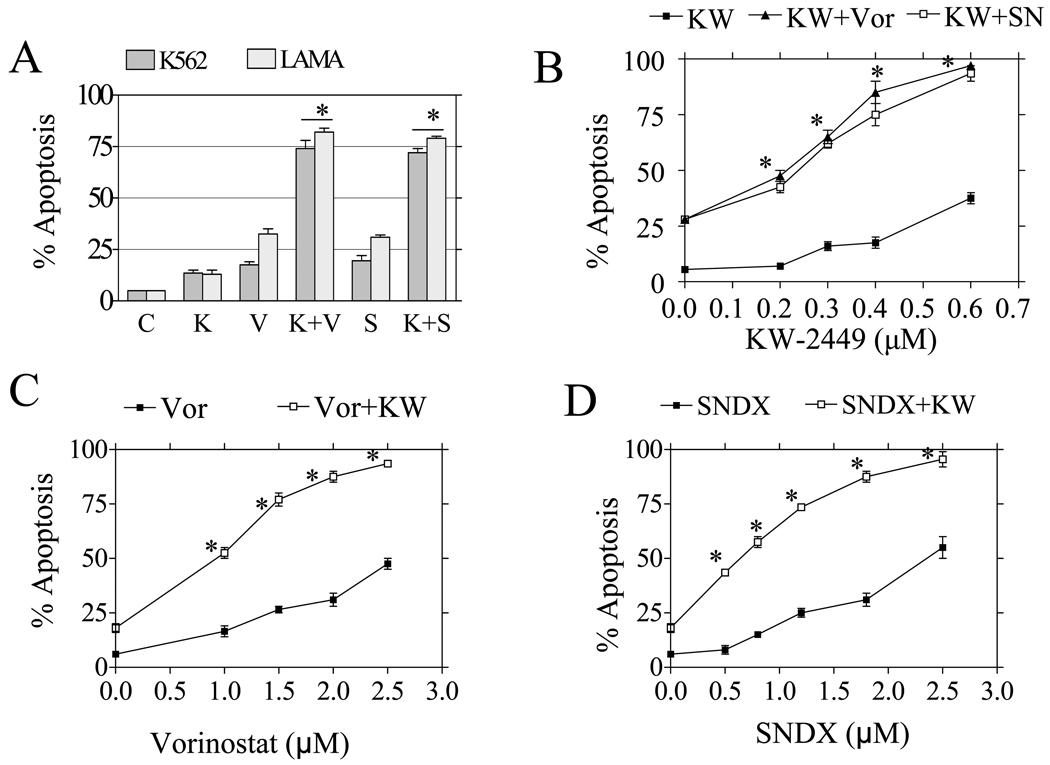
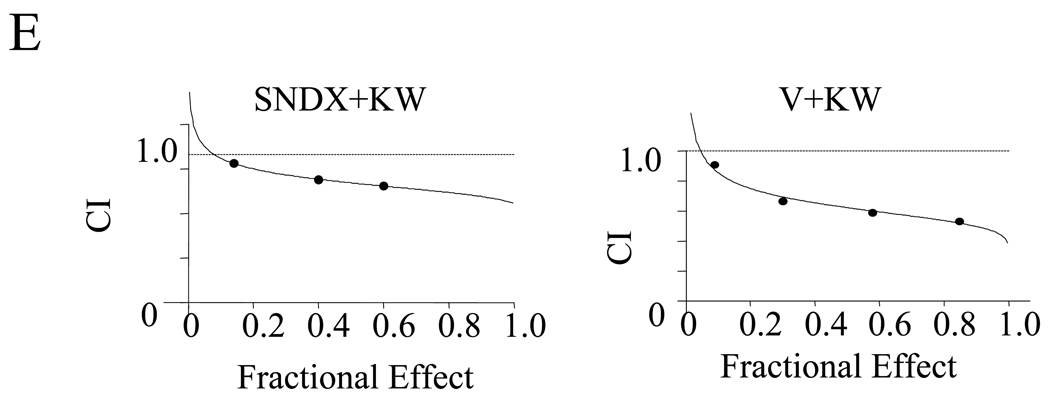
Interactions between KW2449 and HDACIs were first examined in Bcr/Abl+ human chronic leukemia cells. Individual exposure of K562 (72 h) or LAMA84 cells (48 h) to 0.4 µM KW2449 or HDACIs (i.e., 1.5 µM vorinostat, 1.3 µM SNDX-275) minimally induced cell death (less than 25 %). However, combined treatment with KW2249 and either vorinostat or SNDX275 triggered a pronounced increase in apoptosis (~75%) (P<0.01, Figure 1A). Dose-response studies revealed that KW2449 concentration as low as 0.2 µM potentiated the lethality of marginally toxic concentrations of vorinostat or SNDX-275 in K562 cells. Cell death increased substantially at higher KW2449 concentrations (Figure 1B). Conversely, vorinostat and SNDX-275 concentrations as low as 0.5 µM increased the lethality of a marginally toxic KW2449 concentration (0.4 µM), and to an even greater extent at higher concentrations (P<0.01, Figure 1C and 1D). Time-course analysis indicated that simultaneous exposure of K562 to 0.4 µM KW2449 or 1.5 µM vorinostat resulted in only a small increase in apoptosis by 30h hours and was very extensive at later intervals (Supplemental Figure 1A). Median dose effect analysis of apoptosis induction, in which K562 cells were exposed to a range of KW2449 and vorinostat or SNDX concentration alone and in combination, at a fixed concentration ratio, yielded combination index (CI) values less than 1.0, consistent with synergistic interactions (Figure 1E). Finally, similar synergistic interactions were observed when KW2449 was combined with other HDACIs, including apicidin or SBHA (Supplemental Figure 1B).
Figure 1. KW2449 synergistically enhances the lethality of vorinostat/SNDX275 in CML cells.
A) K562 (gray) and LAMA (dots) were exposed to KW2449 (K) 0.4 µM alone or in combination with 1.5 µM vorinostat (V) or 1.3 µM SNDX275 (S) for 72 h (K562) or for 48h (LAMA) after which the percentage of apoptosis were determined as 7-AAD positive by flow cytometry. B) K562 cells were incubated with increasing concentrations (0.2–0.6 µM) of KW2449 in the absence or presence of 1.5 µM vorinostat or 1.3 µM SNDX275 for 72 h after which the percentage of 7AAD+ cells was determined by flow cytometry. C) K562 cells were incubated with increasing concentration of vorinostat alone or in conjunction with 0.4µM KW2449 for 72 hr, after which the percentage of apoptotic cells was determined. D) Similarly, cells were incubated with increasing concentrations of SNDX275 alone or in conjunction with KW2449 0.4µM for 72 hr, after which the percentage of apoptotic cells was determined (* = P<0.01 for values obtained for single agents versus the combination). E) K562 were treated with a range of KW2449 and vorinostat at a fixed ratio 3.75:1 (left panel) or KW2449 and SNDX275 at a fixed ratio 3:1 for 72 h. At the end of this period, the percentage of 7AAD+ cells was determined by flow cytometry; then combination index (CI) values were determined in relation to the fractional effect using a commercially available software program as described in “Materials and Methods.” Combination index values less than 1.0 correspond to a synergistic interaction. For all studies, values represent the means ± S.D. for triplicate determinations.
HDACIs potentiate KW2449 lethality in Ph+ ALL cells, including new IM-resistant cell lines
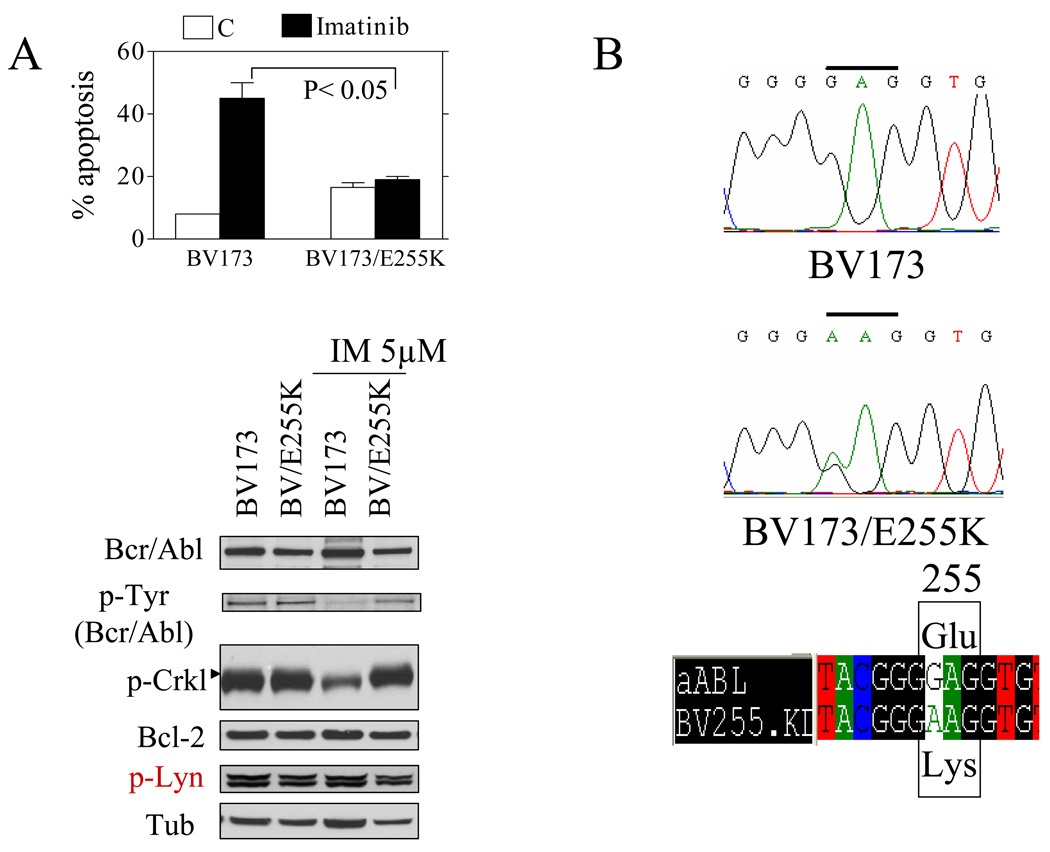
Studies were then undertaken to determine if similar interactions occurred in Ph+ ALL cells, particularly those resistant to IM. To generate human IM-resistant cells, Ph+ ALL cells (BV173) cells were cultured in the presence of increasing IM concentrations. After approximately 2–3 months, a cell line was generated that was capable of proliferating in the presence of 1 µM IM, and designated BV173/E255K. The cells displayed significant resistance to IM compared to parental BV173 cells (Figure 2A upper panel). Western blot analysis demonstrated that phosphorylation/activity of Bcr/Abl and its downstream target CRKL were completely suppressed by IM (5µM) in BV173 but not in BV173/E255K cells (Figure 2A, lower panel). Both BV173/E255K cells and parental BV173 cells expressed similar levels of Bcr/Abl, Bcl-2, p-Lyn (Figure 2A lower panel). Notably, sequencing the ATP binding site and kinase activation loop regions revealed a single substitution mutation at amino acid 255 of Abl (E255K; Figure 2B). Moreover, IM resistance as well as the presence of the E255K mutation remained stable in culture in both the presence and absence of IM for > 6 months.
Figure 2. Characterization of Ph+ALL imatinib-resistant cells and responses to HDACIs and KW2449.
A) BV173 and BV173/E255K cells were incubated with imatinib (IM) 1 µM for 48 h after which the percentage of apoptotic cells was determined (upper panel, * P<0.05). Alternatively, cells were incubated with IM 5 µM, 4h after which cells were lysed and Western blot performed as described in Methods (WB, lower panel). For this and subsequent studies, lanes were loaded with 25 µg of protein; blots were then stripped and re-probed with antibodies to tubulin to ensure equivalent loading and transfer. Two additional studies yielded equivalent results. B) A single point mutation was detected resulting in an amino acid change (E255K) in BV173/E255K cells. C) A single point mutation was detected at nucleotide 944 changing from a C to T resulting in the T315I mutation in Adult/T315I cells (left panel). K562 and Adult/T315I were cultured in imatinib (IM) 1 µM for 48 hr, after which the percentage of apoptotic cells was determined by 7AAD staining and flow cytometry. D) ALL TOM-1, SUP/B15, BV173, BV173/E255K and Adult/T315I cells were incubated with KW2449 (0.3–0.5 µM) alone or in combination with vorinostat (0.8–1.5 µM) / SNDX275 (0.6–1.2 µM) for 48 hr, after which the percentage of apoptotic cells was determined by 7AAD staining. For all studies, values represent the means ± S.D. for triplicate determinations, * P<0.01. E) Cells were treated with a range of KW2449 and vorinostat at a fixed ratio for 48 hr. At the end of this period, the percentage of apoptotic (7AAD+) cells was determined by flow cytometry, after which combination index (CI) values were determined in relation to fractional effects. CI values less than 1.0 correspond to a synergistic interaction.
Figure 3. Combined treatment with KW2449 and vorinostat or SNDX275 markedly increases mitochondrial injury and caspase activation, and diminishes expression/activation of Bcr/Abl and its downstream targets.
A) K562 cells were treated with KW 0.4 µM, vorinostat 1.5 µM or SNDX275 1.3 µM alone or in combination for 30 hours, after which mitochondria-free cytosolic fractions were obtained and subjected to Western blot to monitor cyt-c and AIF. B) Cells were exposed to agents alone or in combination (0.4 µM KW2449 was used in all cases; for K562, LAMA, Adult/T315I, BV173/E255K concentrations of 1.5, 1.5, 0.8, 0.7 µM vorinostat and 1.3, 1.3, 0.6, 0.5 µM SNDX275 were used respectively). After 24–30 h incubation, whole-cell lysates were harvested and subjected to Western blot analysis to monitor activation of caspases and PARP. C–D) Similarly, cells treated as above were prepared and subjected to Western blot using the indicated primary antibodies. For B–D, lanes were loaded with 25 µg of protein, after which blots were stripped and reprobed with anti-tubulin antibodies to ensure equal loading and transfer. Representative results are shown; two additional experiments yielded equivalent results.
The Adult/T315I cell line was established from an IM resistant Ph+ ALL patient. Sequencing of the ATP binding site of Adult/T315I cells revealed a nucleotide change from C-T at ABL resulting in the T315I substitution (Figure 2C left panel). As anticipated, Adult/T315I cells were highly resistant to imatinib and dasatinib whereas K562 cells were fully sensitive (Figure 2C).
To determine whether HDACIs increased KW2449 lethality in IM-sensitive and –resistant Ph+ ALL cells, parallel studies were performed employing two additional ALL cell lines (TOM1, SUP/B15), parental BV173 cells, as well as resistant BV173/E255K and Adult/T315I cells. Cells were exposed to 0.4 µM KW2449 in the presence or absence of 0.6–1.5 µM vorinostat or 0.5–1.2 µM SNDX for 48 hr, after which apoptosis was monitored. Exposure of Ph+ ALL to single agents minimally induced apoptosis; whereas combined exposure to KW2449 and either vorinostat or SNDX-275 dramatically increased apoptosis in all Ph+ ALL tested Figure 2D, including highly IM-resistant BV173/E255K and Adult/T315I. Notably, the sensitivity of the latter two cell lines was roughly equivalent to that of IM-sensitive cells. Finally, Median Dose Effect analysis of apoptosis induction, in which SUP/B15, BV173, BV173/E255K and Adult/T315I cells were exposed to a range of KW2449 and vorinostat concentrations alone or in combination at a fixed concentration ratio, yielded combination index (CI) values less than 1.0, consistent with synergistic interactions (Figure 2E).
HDACI/KW-2449 regimens are active against additional IM-resistant Bcr/Abl+ leukemia cells
Parallel studies were performed in other IM-resistant Bcr/Abl+ leukemia cells, including K562 cells displaying increased Bcr/Abl expression [22] (Supplemental Figure 2A, left panel) and BaF3 cells transfected with wild-type or mutant forms of Bcr/Abl. Whereas K562/R cells exhibited a significant reduction in apoptosis following exposure to 1 µM IM, they remained fully sensitive to KW2449/vorinostat or KW2449/SNDX-275 regimens (Supplemental Figure 2A, right panel). Similarly, BaF3/E255K and BaF3/T315I cells were as sensitive as their wild-type counterparts to KW2449/vorinostat or KW2449/SNDX-275 regimens (Supplemental Fig 2B, left panel). Furthermore, TUNEL-stained cells shows the marked increase in apoptosis in BaF3/T315I cells exposed to both KW2449 and the HDACI SBHA compared to those exposed to single agents (Supplemental Fig 2B, right panel).
HDACIs increase KW2449-mediated caspase activation and inhibition of Bcr/Abl-related signaling pathways in IM-sensitive and –resistant cells
Effects of KW2449 and HDACIs, alone and in combination, were then examined in relation to induction of the caspase cascade and activation of Bcr/Abl and related downstream targets. Studies were performed prior to extensive apoptosis (i.e., 30 h for K562; 20 h for LAMA, BV173/E255K, and Adult/T315I) to reduce the possibility that changes represented a consequence of the cell death process itself. Exposure of K562 cells to 0.4 µM KW2449, 1.5 µM vorinostat, and 1.3 µM SNDX individually for 30 h had little effect on AIF or cytochrome-c release, whereas the effects of combined exposure of KW with either vorinostat or SNDX were pronounced (Figure 3A). Consistent with these findings, co-treatment with KW2449 and vorinostat or SNDX-275 marked induced procaspase-3 activation and PARP cleavage in K562, LAMA, BV173/E255K and Adult/T315I cells, whereas the effects of individual agents were negligible or only modest (Figure 3B). Similar effects were observed in cells exposed to KW-2449 and SBHA (Supplemental Figure 3A).
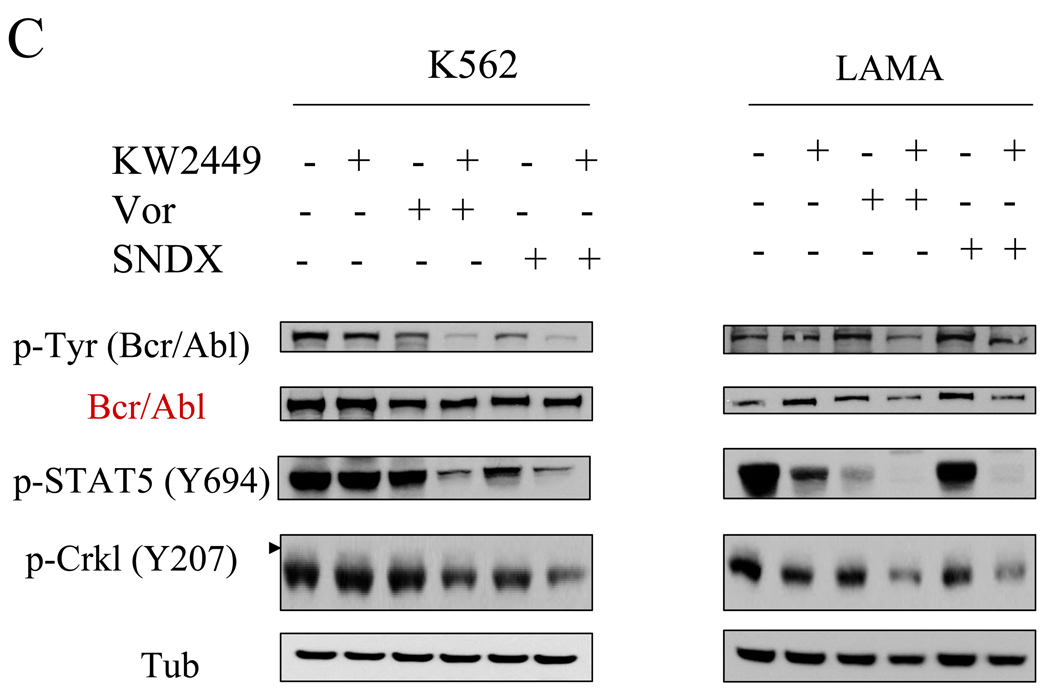
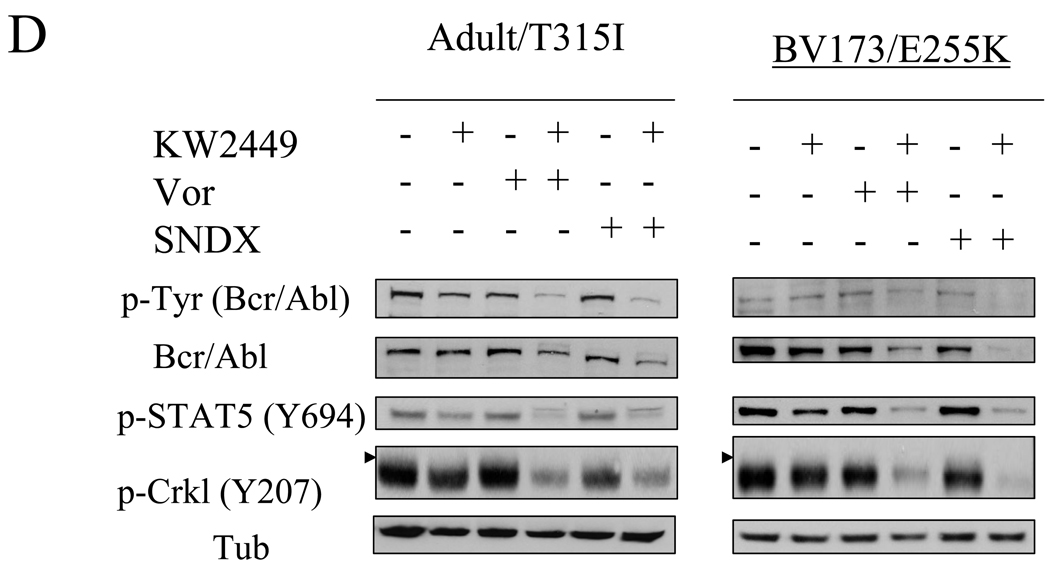
The effects of combined treatment on the status of various signaling pathways in Bcr/Abl+ leukemia cells were then investigated. First, in both K562 and LAMA84 cells, co-administration of vorinostat (1.5 µM) or SNDX (1.3 µM), which by themselves had minimal effects, sharply potentiated the inhibitory effects of KW2449 on Bcr/Abl activation, manifested by the pronounced dephosphorylation of Bcr/Abl and its downstream targets STAT5 (Y694) and CRKL (Y207; Figure 3C). Parallel results were obtained in K562 cells exposed to KW2449 and SBHA (Supplemental Fig 3A). Combined treatment with vorinostat or SNDX and KW2449 induced either slight declines or no changes in total Bcr/Abl protein expression in these cells.
In IM-resistant BV173/E255K and Adult/T315I cells, coadministration of KW2449 with either vorinostat or SNDX clearly inhibited Bcr/Abl activation and reduced total expression, whereas single drug treatment minimally affected Bcr/Abl levels or activation status. Consistent with these findings, expression of p-STAT5 and p-CRKL were substantially reduced by combined HDACI/KW2449 treatment compared to effects observed with agents administered individually (Figure 3D).
HDACIs promote KW2449-mediated ROS generation and DNA damage in IM-sensitive and –resistant Bcr/Abl+ leukemia cells
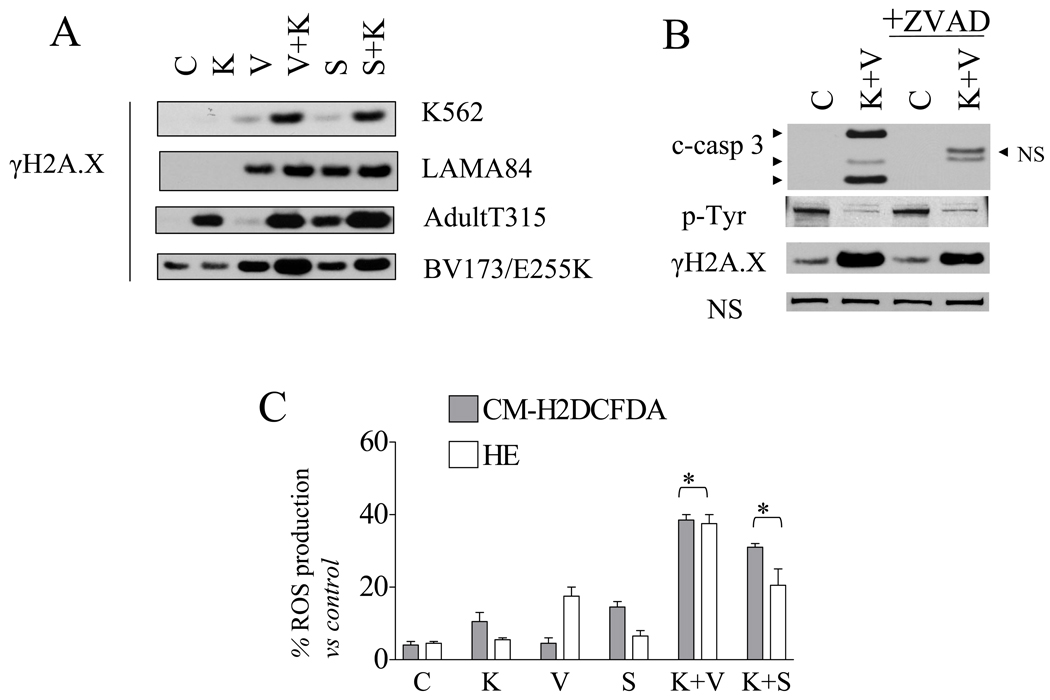
In view of evidence that Bcr/Abl kinase inhibitors can disrupt DNA repair mechanisms in Bcr/Abl+ cells [23], the effects of HDACIs on KW2449-induced DNA damage was examined. Expression of γH2A.X, a marker of double-stranded DNA breaks, was increased in K562, LAMA, BV173/E255K, and Adult/T315I cells exposed to KW24449/vorinostat or KW2449/SNDX compared to the effects of single drug exposure (Figure 4A). Moreover, induction of γH2A.X in cells exposed to KW2449/HDACi was detected as early as 6 hr after drug exposure and was extensive at 24 hr, as determined by confocal microscopy (data not shown). Notably, in contrast to effects on caspase-3 cleavage, which was completely blocked, the pan-caspase inhibitor fmk-ZVAD (20 µM) had little or no effect on KW2449/vorinostat-mediated inactivation of Bcr/Abl or γH2A.X formation (Figure 4B), arguing that these events do not simply represent consequences of caspase activation. Combined HDACI/KW2449 treatment induced a marked increase in G2M arrest in K562 (Supplemental Figure 3B) consistent with the pronounced induction of DNA damage. As previously reported [9], KW2449 moderately reduced phosphorylation of histone H3, an indicator of aurora B activity, in nocodozole-treated K562 cells (Supplemental Figure 3C). However, the degree of inhibition of histone H3 phosphorylation was not further enhanced by co-administration of vorinostat or SNDX-275. Similar results were obtained in other Bcr/Abl+ cells i.e. LAMA84, BV173/E255K, and Adult/T315I (data not shown).
Figure 4. HDACIs and KW2449 induce DNA damage and ROS in Bcr/Abl+ cells, events attenuated by the ROS scavenger Mn-TBAP.
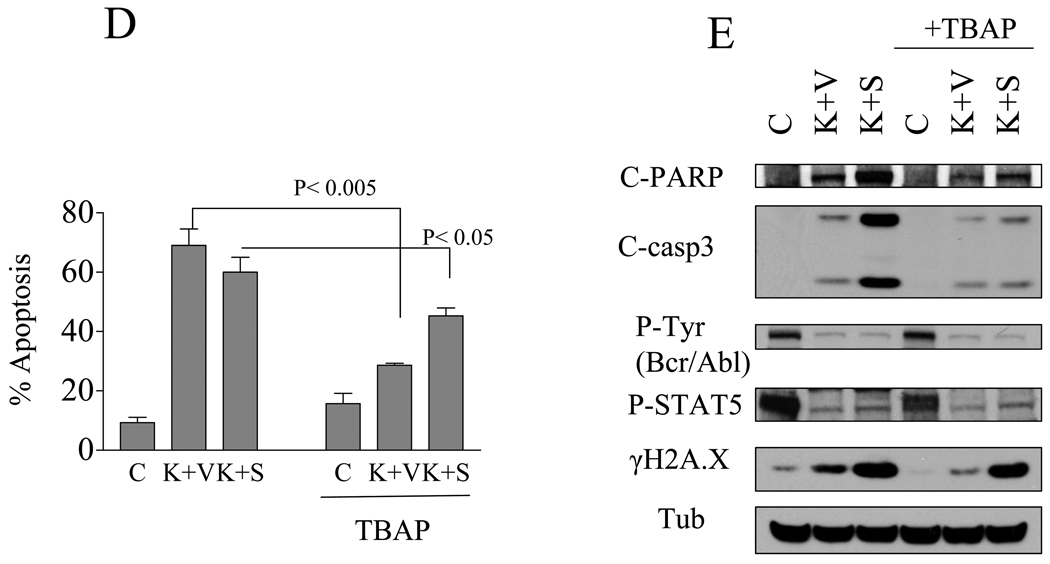
A) Following treatment as described in Figure 3, protein lysates were obtained and WB performed using γH2A.X antibodies. B) K562 cells were pretreated with 20 µM ZVAD-fmk for 2 hr followed by KW/vorinostat as described above for 30 hr. At the end of this period, the cells were lysed and subjected to WB (NS: non-specific). C) K562 cells were incubated with KW, vorinostat, SNDX alone or in combination for 24 hr, after which cells were labeled with CM-H2DCF-DA or HE for flow cytometric analysis. Values represent the means ± S.D. for triplicate determinations, (* P<0.05). D) K562 were exposed to either KW+vorinostat or KW+SNDX in the presence or absence of the free radical scavenger Mn-TBAP (300 µmol/L) for 72 h. The cells were collected and analyzed by flow cytometry to determine the percentage of 7AAD positive cells. E) Cells treated as in (D) were collected at 30 hr and Western blot analysis performed. Two additional experiments yielded equivalent results.
Reactive oxygen species (ROS) have been implicated in HDACI-mediated induced cell death [24, 25]. To explore the possible role of ROS generation in the lethality of HDACI/KW2449 regimens, ROS were monitored by flow cytometry using HE, a dye that is relatively specific for O2−, and CM-H2DCFDA, which can detect H2O2 and OH−. KW2449, vorinostat, or SNDX-275 administered alone had either no or only modest effects on ROS induction in K562, BV173/E255K, and Adult/T315 cells (Figure 4C and supplemental Figure 3D). In contrast, combined exposure to KW2449 and HDACIs resulted in significant increases in ROS production compared to single agent treatment (P < 0.05). To evaluate the functional significance of ROS generation in HDACI/KW2449 lethality, co-administration of Mn-TBAP, a ROS scavenger, substantially diminished ROS generation and significantly reduced apoptosis (Figure 4D; vor/KW2449 vs vor/KW2449+TBAP: 69±9.6% vs 28.6±1.5% respectively, P<0.005) or SNDX/KW2449 vs SNDX/KW2449+TBAP: 61±8% vs 45±4.5% with TBAP; P< 0.05). Consistent with the apoptosis data, TBAP partially reduced cleavage of caspase-3, PARP degradation, and γH2A.X formation (Figure 4E). In marked contrast, TBAP did not prevent dephosphorylation of Bcr/Abl or its downstream target, STAT5, suggesting the latter events are independent of ROS (Figure 4E). These findings argue that while ROS generation plays a significant functional role in HDACI/KW2449-mediated DNA damage and apoptosis in Bcr/Abl+ leukemia cells, additional factors may be involved in lethality.
HDACIs potentiate KW2449 lethality in primary CML cells
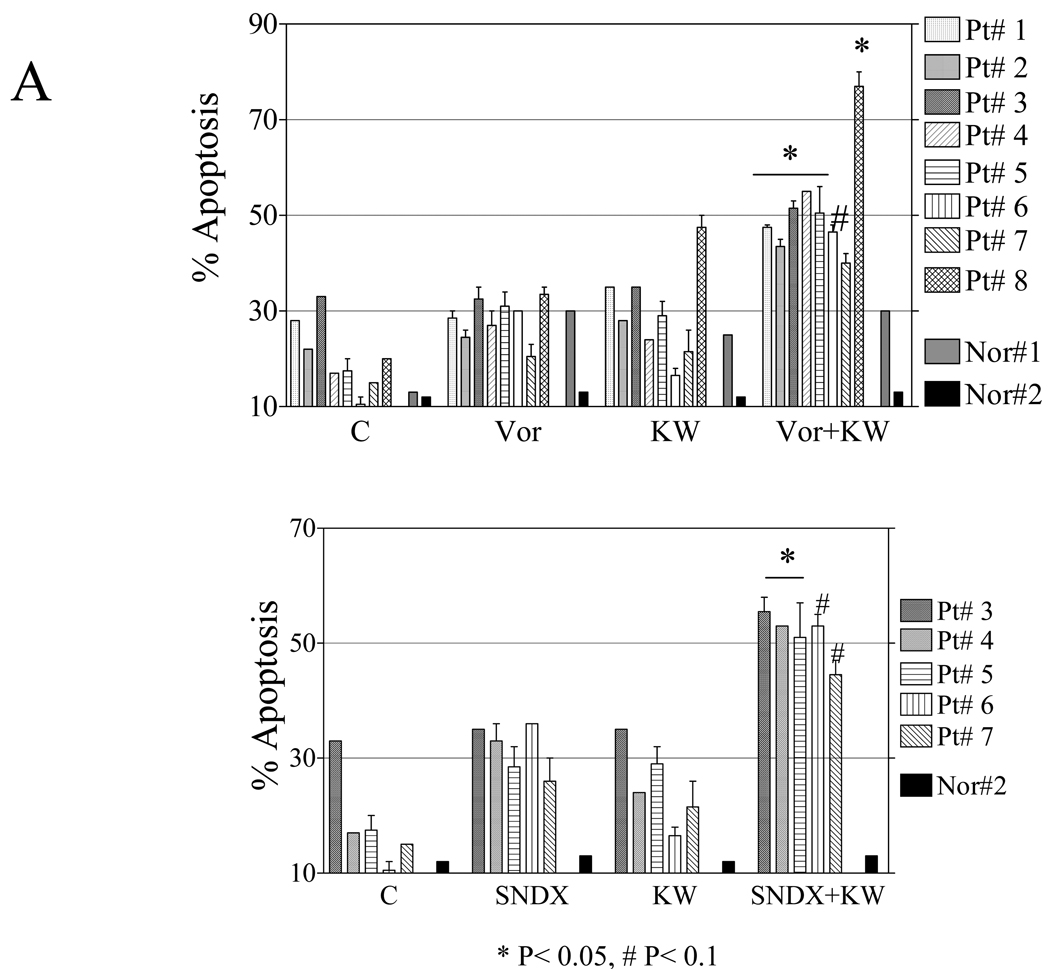
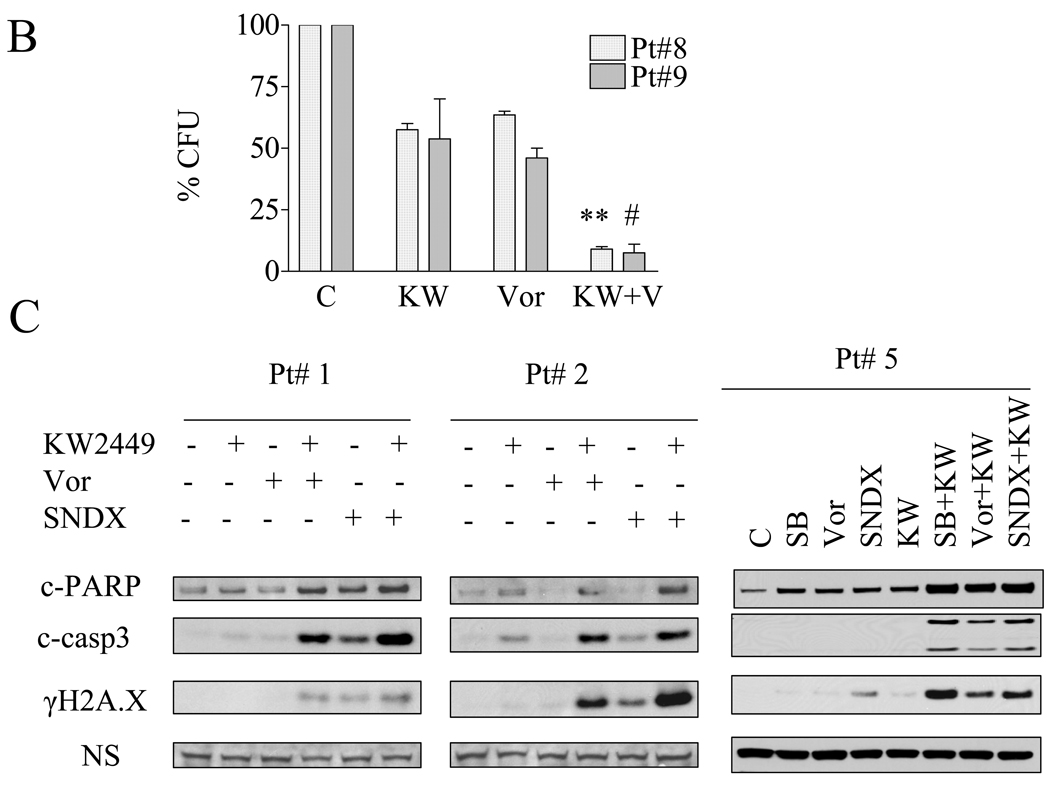
To determine whether the enhanced apoptosis following HDACI/KW2449 exposure is restricted to cell lines, parallel studies were performed employing bone marrow or peripheral blood isolated CD34+ cells from 8 patients with CML. Bcr/Abl sequence information was available for 4 patients and revealed no mutations in 3 patient samples (# 1, 3, 4) and I242T, Y257C mutations in one patient sample (# 6). Exposure (48 h) of CD34+ CML cells to 0.4–0.6 µM KW2449 or 1–1.5 µM vorinostat or 0.8–1.3 SNDX275 individually resulted in minimal toxicity; whereas combined treatment produced significant increases in cell death compared to single agent treatment for each of the patient samples, including one sample with the mutations (Figure 5A). Notably, the combination regimen exhibited only modest lethality toward normal CD34+ cells compared to untreated controls. Furthermore, KW2449/vorinostat resulted in a very pronounced reduction in colony formation of CD34+ CML cells for two tested samples (Figure 5B). Finally, CD34+ cells from three patients (Pt# 1, 2, 5) exhibited increases in expression of cleaved forms of caspase-3 and PARP, as well as γH2A.X, compared to cells exposed to single agents (Figure 5C).
Figure 5. Vorinostat and SNDX-275 potentiate KW2449 lethality in primary CML cells.
A) CD34+ cells were isolated from the bone marrow or peripheral blood samples obtained from 8 patients with CML. Mononuclear cells were exposed to 0.4–0.8 µM KW with or without 0.8–1.5µM vorinostat (upper panel) or 0.5–1.3µM SNDX-275 (lower panel) for 48 h, after which the percentage of Annexin V/PI or 7AAD positive cells was determined by flow cytometry (* P< 0.05, # P< 0.1). B) CD34+ cells isolated from patients # 8 and 9 were treated with KW2449 (0.4 µM) or vorinostat (0.8–1.2 µM) alone or in combination for 48 hr and then plated in methylcellulose for 14 days after which leukemic colony forming units (L-CFUs) were enumerated and expressed as a percentage relative to untreated cells, ** P<0.005, # P<0.1). C) CD34+ cells from patients #1, 2, and 5 were treated with KW, vorinostat, or SNDX-275 as above for 36 h, after which cell lysates were prepared and subjected to Western blot analysis as described above to monitor cleavage of caspase-3 and PARP. Results of a representative study are shown; duplicate experiments yielded equivalent results.
In vivo interactions between HDACIs and KW2449 against IM-resistant Ph+ ALL cells
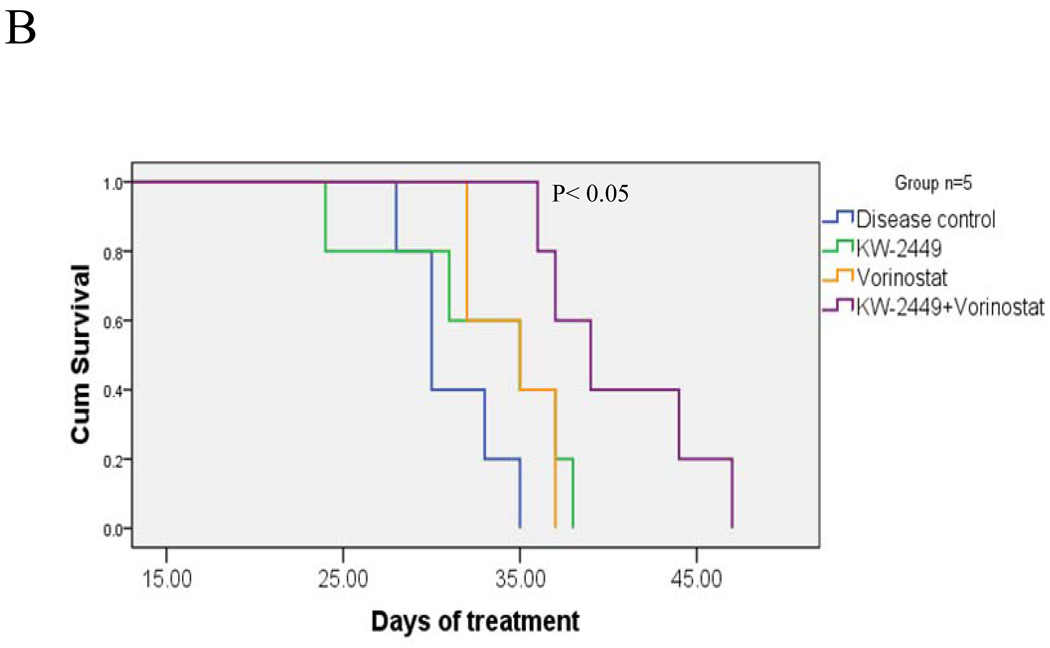
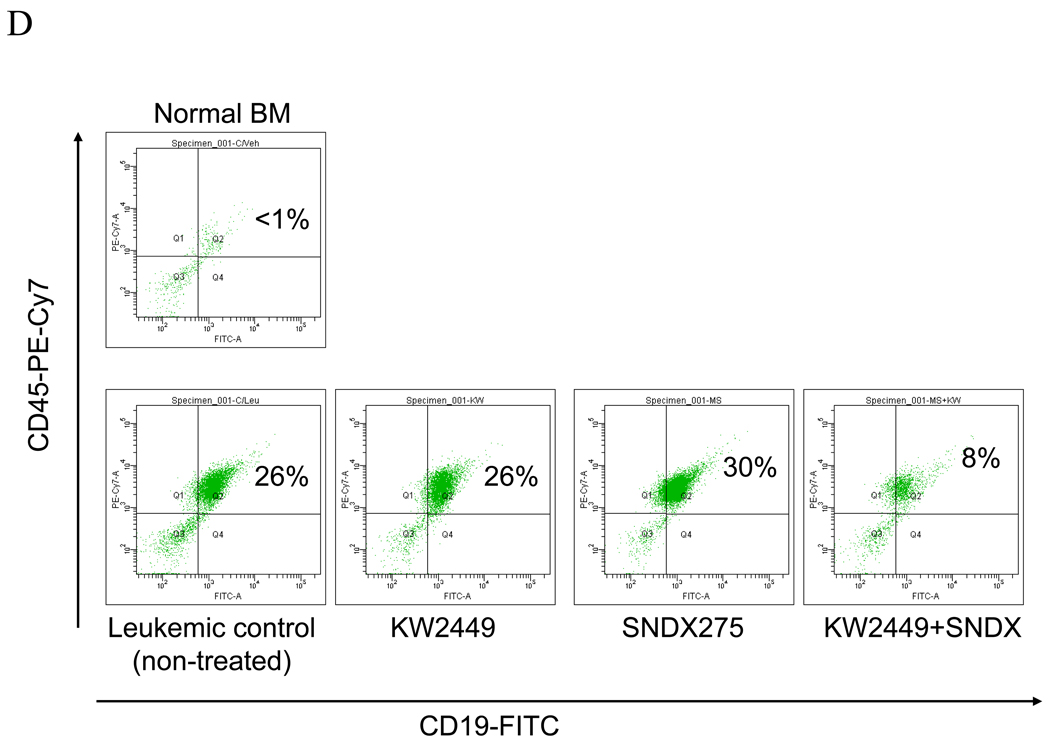
The in vivo activity of KW2449/HDACI against IM-resistant ALL cells was then investigated in BALB-C mice. To this end, 2.5 × 106 BV173/E255K/Luc cl4 were injected intravenously by tail vein. After 7–10 days, when signal intensity (indicating tumors) was visible by luminescence imaging, mice were treated with KW2449 32 mg/kg/day p.o. with or without vorinostat 70 mg/kg/day i.p. for 5 days/week. As shown in Figure 6A, mice treated with both KW2449 and vorinostat displayed a reduction in the leukemia signal relative to single agent treatment as early as two weeks after initiation of therapy. By 4 weeks the difference was very pronounced. In addition, mice treated with KW2449/vorinostat experienced an average survival of 40 ± 4 days which was significantly longer than that of controls (31.2 ± 2.8) or KW2449 (32.4 ± 5.9) or vorinostat (34.6 ± 2.5) (P< 0.05, Figure 6B). Treatment with agents alone or in combination did not result in significant weight loss compared to controls (Supplemental Figure 4, upper panel). Alternatively, in separate experiments, mice were treated with KW2449 as described above with or without SNDX275 15 mg/kg/day p.o 5 days/wk. As in the case of SNDX-275, survival of mice receiving both agents was significantly greater than that of either no or single agent treatment (P < 0.04). Notably, flow cytometric analysis of bone marrow cells obtained from mice after 3 weeks of single agent treatment did not reveal a decrease in the percentage of CD45+/CD19+ cells, reflecting leukemia cells of human origin. In contrast, a substantial reduction in CD45+/CD19+ cells (i.e., from 26% to 8%) in marrows obtained from animals receiving both KW2449 and SNDX-275 (Figure 6D) was observed.
Figure 6. HDACIs potentiate KW2449 activity against IM-resistant ALL in vivo.
A) BV173/E255K cells stably transfected with Luc/pcDNA3 (BV173/E255K/Luc cl4) were injected into BALB/SCID mice via tail vein (2.5 × 106 cells). The mice were then injected with firefly luciferin and imaged by Xenogen once or twice weekly. Empty boxes represent deceased mice. B) Mice were treated with KW2449 (32 mg/kg po once daily; green line), vorinostat (70 mg/kg ip daily (orange line) or the combination of vorinostat+KW2449 (purple line). Mice were also injected with sterile water as a control control (blue line). Drugs were administered 5 days per week. Survival was evaluated from the first day of treatment until death using Kaplan-Meier curves, (* P<0.05). C) Mice were treated with KW2449 (32 mg/kg po daily, SNDX275 (15 mg/kg po daily) or the combination of SNDX+KW2449 or sterile water as a control. Drugs were administered 5 days a week, (* P<0.05). D). Cells from a representative bone marrow sample obtained from one mouse from each group after 3 weeks of treatment were stained with fluorescently-labeled CD19 and CD45 antibodies, after which they were analyzed by flow cytometry.
DISCUSSION
Despite encouraging progress in the treatment of Bcr/Abl+ malignancies resulting from the development of Bcr/Abl kinase inhibitors such as IM, dasatinib, and nilotinib, numerous challenges persist [3]. First, the accelerated and blast crisis phases of CML are considerably less responsive to these agents than chronic phase disease. Furthermore, despite initial responses, patients with CML as well as Ph+ ALL develop resistance to IM and second-generation agents (niloditib, dasatinib) [5, 26]. This frequently involves mutations, often in the gatekeeper region (e.g. T315I, F317L), which disrupt contact between IM (or second-generation Bcr/Abl kinase inhibitors) and Bcr/Abl, conferring resistance on affected cells [4]. The fortuitous finding that certain aurora kinase inhibitors inhibited Bcr/Abl+ leukemia cells bearing such mutations provided a new therapeutic strategy in the setting of IM-resistant disease [27]. Although the multi-kinase inhibitor KW2449, which also inhibits Bcr/Abl and aurora kinases, has primarily been developed as a FLT3 inhibitor in AML [10], preclinical studies demonstrate that it effectively kills Bcr/Abl+ leukemias, including Ph+ ALL, bearing gatekeeper mutations [9]. The finding that HDACIs promoted the activity of first and second generation Bcr/Abl inhibitors [14, 15, 28, 29] prompted efforts to determine whether they could also potentiate the lethality of third generation agents. Indeed, the pan-HDACI vorinostat increased VX-680 activity against IM-sensitive and IM–resistant Bcr/Abl+ myeloid and lymphoid leukemia cells in preclinical studies [16–18]. However, discontinuation of VX-680 clinical development due to cardiotoxicity prevented further testing of this approach. The results of the present study suggest that combining KW2449 with HDACIs represents a plausible alternative strategy for IM-resistant Bcr/Abl+ leukemias.
In the present study, the concept of employing HDACIs to enhance KW2449 activity has been extended to Ph+ ALL and other IM-resistant leukemias. To this end, two new human IM-resistant ALL cell lines, BV173/E255K and Adult/T315I, have been established which bear two of the most common mutation responsible for IM resistance i.e. E255K and T315I [1]. Although in the past, murine cell lines (BaF3-Bcr/Abl) bearing these and other Bcr/Abl mutations have been of value in investigating the activity of Bcr/Abl kinase inhibitors, such cells may not precisely recapitulate the behavior of their human counterparts or patient-derived, primary leukemic cells. Consequently, the novel cell lines described here represent potentially valuable new tools for examination of in vitro and in vivo interactions between Bcr/Abl kinase inhibitors and other targeted agents.
Because HDACIs act through multiple mechanisms, the basis for HDACI/KW2449 interactions is likely to be complex. For example, certain HDACIs acetylate non-histone proteins, including chaperone proteins such as Hsp90, which are required for the maintenance and function of mutant oncoproteins, including Bcr/Abl [30]. Previous studies have implicated HDACI-mediated down-regulation of Bcr/Abl in synergistic antileukemic interactions involving Bcr/Abl kinase inhibitors [15, 17]. However, vorinostat and SNDX-275, when combined with KW2449, induced Bcr/Abl down-regulation principally in cells expressing mutant Bcr/Abl. Whether or not Bcr/Abl is down-regulated, the present results argue that potentiation of Bcr/Abl inhibition represents an important contributor to lethality. In support of this notion, combined treatment of both wild-type and mutant cells with KW2449 and vorinostat or SNDX-275 resulted in a marked reduction in Bcr/Abl phosphorylation compared to the effects of the agents administered alone. Significantly, these events were accompanied by diminished phosphorylation of two Bcr/Abl downstream targets, CRKL and STAT5, both of which have been implicated in the pathogenesis of Bcr/Abl+ leukemias [31, 32]. Importantly, similar events were observed in IM-resistant cells, consistent with a common mode of action. The mechanism(s) by which HDACIs potentiate the Bcr/Abl inhibitory activity of KW2449 independently of Bcr/Abl down-regulation remains to be determined.
To the best of our knowledge, the present findings are the first to demonstrate that HDACIs promote oxidative injury induced by KW2449, and that this event plays a significant functional role in enhanced lethality. Specifically, both vorinostat and SNDX-275 potentiated KW2449-induced ROS formation in both IM-sensitive and IM-resistant mutant cells, and the lethality of HDACI/KW2449 regimens, was significantly attenuated by the antioxidant TBAP. Among their diverse actions, HDACIs, including both vorinostat and SNDX-275, have been shown to trigger transformed cell death through ROS generation [24, 25]. The preferential induction of oxidative injury has also been implicated in HDACI selectivity toward transformed cells [33]. On the other hand, evidence that Bcr/Abl kinase inhibitors exert their lethal effects toward Bcr/Abl+ leukemias via oxidative injury is largely lacking. Interestingly, it has been reported that Bcr/Abl kinase inhibitors such as IM trigger genomic instability through ROS generation, leading to the development of resistance-conferring mutations in Bcr/Abl [34]. One possible explanation for these apparently paradoxical phenomena is that in cells exposed to kinase inhibitors alone, ROS generation may be sub-lethal but mutagenic; whereas in the presence of HDACIs, the levels of ROS exceed the threshold for cell death induction. The basis for the observed increases in ROS generation is unclear, but may be related to events stemming from Bcr/Abl kinase inhibition i.e., DNA damage induction, diminished DNA repair, and impaired DNA damage checkpoint regulation.
The finding that HDACIs potentiated DNA damage induced by KW2449 (or other Bcr/Abl kinase inhibitors) reflected by increased expression of λH2A.X, a marker for double-strand DNA breaks, has also not been previously described. Because such breaks can occur in cells undergoing apoptosis, it is possible that these phenomena represent secondary events. However, the finding that pan-caspase inhibitors, which blocked apoptosis, failed to diminish λH2A.X formation, argues against this possibility. The observation that antioxidant exposure reduced λH2A.X expression in HDACI/KW2449-treated cells, albeit partially, suggests that generation of ROS by the regimen plays a functional role in DNA damage induction. However, the finding that rescue from HDACI/KW-2449-mediated DNA damage and cell death by TBAP was incomplete suggests that additional factors very likely contribute to lethality. It may be relevant that Bcr/Abl kinase inhibitors such as IM have been shown to compromise genomic stability and DNA repair by inhibiting c-Abl [35], and to interfere with nucleotide excision repair in Bcr/Abl-expressing cells, including K562 and BV173 [23]. Analogously, HDACIs can disrupt DNA repair through multiple mechanisms i.e., acetylation of Ku70 [36] and down-regulation of DNA repair proteins such as RAD51 [37]. Interestingly, such phenomena have been reported to occur preferentially in transformed cells [38]. It is conceivable that these events cooperate to promote DNA damage in the face of increased ROS production. The possibility that disruption of DNA damage checkpoints contributes to these events also cannot be excluded.
Histone H3 phosphorylation is a hallmark of mitosis which is mediated by Aurora kinase B. In previous studies involving VX-680 and vorinostat, a clear reduction of phospho-histone H3 was observed with combined treatment [16]. Although in the present study, KW2449 alone diminished expression of phospho-histone H3, consistent with previous reports [39], potentiation of this effect by HDACIs was not observed. This may reflect the fact that VX680 primarily inhibits Aurora kinase B and A compared to Abl, with IC50 values of 0.18, 0.6 and 20 nM respectively; whereas KW2449 exhibits a stronger affinity for Abl than Aurora kinases (A and B), with IC50 values of 14 and 48nM respectively [40]. However, the possibility that aurora kinase-related effects of KW2449 on DNA damage checkpoints or DNA repair processes contribute to interactions with HDACIs cannot be excluded.
It is noteworthy that HDACIs increased KW-2449 lethality toward primary Bcr/Abl+ leukemia cells, but exhibited relatively little toxicity toward normal hematopoietic cells. HDACIs, administered alone, have been reported to induce oxidative injury [33] and DNA damage [38] preferentially in neoplastic versus normal cells. Furthermore, Bcr/Abl+ leukemia cells are known to be dependent upon Bcr/Abl function for their survival. These factors may cooperate to render Bcr/Abl+ leukemia cells more susceptible to HDACI/KW-2449 regimens. The observation that HDACI/KW-2449 regimens reduced tumor growth reinforces the notion that this strategy may also exert in vivo selectivity.
In summary, the present findings indicate that clinically relevant HDACIs promote the lethality of KW-2449 in multiple Bcr/Abl+ CML and ALL cell types, including those expressing highly IM-resistant variants, including phosphorylation loop (E255K) and gatekeeper (T315I). The basis for lethality is likely to be multi-factorial, including induction of oxidative injury and DNA damage, interruption of Bcr/Abl signaling pathways (e.g., CRKL, STAT-5), and possibly interference with DNA damage checkpoints and/or DNA repair events. Importantly, HDACI/KW-2449 regimens significantly increased the survival of mice bearing IM-resistant cells expressing the E255K mutation. One remaining question is whether this strategy would be active against quiescent CML stem cells, which are characteristically resistant to kinase inhibitors [41]. In this context, HDACIs have recently been shown to potentiate the activity of IM against such cells [28]. It would therefore be interesting to learn whether they might exert similar effects in combination with third-generation Bcr/Abl kinase inhibitors such as KW-2449. Accordingly, studies to test this possibility are underway.
Supplementary Material
Acknowledgments
This study is supported by award 6181-10 from the Leukemia and Lymphoma Society of America; Grants CA63753, CA93738, and CA100866 from the National Institutes of Health. Flow and image cytometry were supported in part by NIH Grant P30 CA16059.
Abbreviation
- CML
chronic myeloid leukemia
- ALL
Acute lymphoblastic leukemia
- NS
non-specific
- N/A
not applied
- ROS
reactive oxygen species.
Reference List
- 1.Druker BJ. Translation of the Philadelphia chromosome into therapy for CML. Blood. 2008;112:4808–4817. doi: 10.1182/blood-2008-07-077958. [DOI] [PubMed] [Google Scholar]
- 2.Carpiuc KT, Stephens JM, Botteman MF, Feng W, Hay JW. A review of the clinical and economic outcomes of imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia. Expert Opin Pharmacother. 2007;8:2775–2787. doi: 10.1517/14656566.8.16.2775. [DOI] [PubMed] [Google Scholar]
- 3.Quintas-Cardama A, Kantarjian H, Cortes J. Imatinib and beyond--exploring the full potential of targeted therapy for CML. Nat Rev Clin Oncol. 2009;6:535–543. doi: 10.1038/nrclinonc.2009.112. [DOI] [PubMed] [Google Scholar]
- 4.Quintas-Cardama A, Kantarjian H, Cortes J. Flying under the radar: the new wave of BCR-ABL inhibitors. Nat Rev Drug Discov. 2007;6:834–848. doi: 10.1038/nrd2324. [DOI] [PubMed] [Google Scholar]
- 5.La RP, Deininger MW. Resistance to imatinib: mutations and beyond. Semin Hematol. 2010;47:335–343. doi: 10.1053/j.seminhematol.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 6.O'Hare T, Eide CA, Deininger MW. Bcr-Abl kinase domain mutations, drug resistance and the road to a cure of chronic myeloid leukemia. Blood. 2007 doi: 10.1182/blood-2007-03-066936. [DOI] [PubMed] [Google Scholar]
- 7.Ruchaud S, Carmena M, Earnshaw WC. Chromosomal passengers: conducting cell division. Nat Rev Mol Cell Biol. 2007;8:798–812. doi: 10.1038/nrm2257. [DOI] [PubMed] [Google Scholar]
- 8.Marumoto T, Zhang D, Saya H. Aurora-A - a guardian of poles. Nat Rev Cancer. 2005;5:42–50. doi: 10.1038/nrc1526. [DOI] [PubMed] [Google Scholar]
- 9.Shiotsu Y, Kiyoi H, Ishikawa Y, et al. KW-2449, a novel multikinase inhibitor, suppresses the growth of leukemia cells with FLT3 mutations or T315I-mutated BCR/ABL translocation. Blood. 2009;114:1607–1617. doi: 10.1182/blood-2009-01-199307. [DOI] [PubMed] [Google Scholar]
- 10.Pratz KW, Cortes J, Roboz GJ, et al. A pharmacodynamic study of the FLT3 inhibitor KW-2449 yields insight into the basis for clinical response. Blood. 2008 doi: 10.1182/blood-2008-09-177030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 12.Grant S, Easley C, Kirkpatrick P. Vorinostat. Nature Review Drug Discovery. 2007;6:1–2. doi: 10.1038/nrd2227. [DOI] [PubMed] [Google Scholar]
- 13.Bhalla KN. Epigenetic and chromatin modifiers as targeted therapy of hematologic malignancies. J Clin Oncol. 2005;23:3971–3993. doi: 10.1200/JCO.2005.16.600. [DOI] [PubMed] [Google Scholar]
- 14.Yu C, Rahmani M, Almenara J, et al. Histone deacetylase inhibitors promote STI571-mediated apoptosis in STI571-sensitive and -resistant Bcr/Abl+ human myeloid leukemia cells. Cancer Res. 2003;63:2118–2126. [PubMed] [Google Scholar]
- 15.Fiskus W, Pranpat M, Balasis M, et al. Cotreatment with vorinostat (suberoylanilide hydroxamic acid) enhances activity of dasatinib (BMS-354825) against imatinib mesylate-sensitive or imatinib mesylate-resistant chronic myelogenous leukemia cells. Clin Cancer Res. 2006;12:5869–5878. doi: 10.1158/1078-0432.CCR-06-0980. [DOI] [PubMed] [Google Scholar]
- 16.Dai Y, Chen S, Venditti CA, et al. Vorinostat synergistically potentiates MK-0457 lethality in chronic myelogenous leukemia cells sensitive and resistant to imatinib mesylate. Blood. 2008 doi: 10.1182/blood-2007-10-116376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiskus W, Wang Y, Joshi R, et al. Cotreatment with vorinostat enhances activity of MK-0457 (VX-680) against acute and chronic myelogenous leukemia cells. Clin Cancer Res. 2008;14:6106–6115. doi: 10.1158/1078-0432.CCR-08-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okabe S, Tauchi T, Ohyashiki K. Efficacy of MK-0457 and in combination with vorinostat against Philadelphia chromosome positive acute lymphoblastic leukemia cells. Ann Hematol. 2010;89:1081–1087. doi: 10.1007/s00277-010-0998-x. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen TK, Rahmani M, Gao N, et al. Synergistic interactions between DMAG and mitogen-activated protein kinase kinase 1/2 inhibitors in Bcr/abl+ leukemia cells sensitive and resistant to imatinib mesylate. Clin Cancer Res. 2006;12:2239–2247. doi: 10.1158/1078-0432.CCR-05-2282. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen TK, Rahmani M, Harada H, Dent P, Grant S. MEK1/2 inhibitors sensitize Bcr/Abl+ human leukemia cells to the dual Abl/Src inhibitor BMS-354/825. Blood. 2007;109:4006–4015. doi: 10.1182/blood-2006-09-045039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao N, Kramer L, Rahmani M, Dent P, Grant S. The three-substituted indolinone cyclin-dependent kinase 2 inhibitor 3-[1-(3H-imidazol-4-yl)-meth-(Z)-ylidene]-5-methoxy-1,3-dihydro-indol-2-on e (SU9516) kills human leukemia cells via down-regulation of Mcl-1 through a transcriptional mechanism. Mol Pharmacol. 2006;70:645–655. doi: 10.1124/mol.106.024505. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen TK, Rahmani M, Harada H, Dent P, Grant S. MEK1/2 inhibitors sensitize Bcr/Abl+ human leukemia cells to the dual Abl/Src inhibitor BMS-354/825. Blood. 2007;109:4006–4015. doi: 10.1182/blood-2006-09-045039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sliwinski T, Czechowska A, Szemraj J, Morawiec Z, Skorski T, Blasiak J. STI571 reduces NER activity in BCR/ABL-expressing cells. Mutat Res. 2008;654:162–167. doi: 10.1016/j.mrgentox.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Rosato RR, Almenara JA, Grant S. The histone deacetylase inhibitor MS-275 promotes differentiation or apoptosis in human leukemia cells through a process regulated by generation of reactive oxygen species and induction of p21CIP1/WAF1 1. Cancer Res. 2003;63:3637–3645. [PubMed] [Google Scholar]
- 25.Ruefli AA, Ausserlechner MJ, Bernhard D, et al. The histone deacetylase inhibitor and chemotherapeutic agent suberoylanilide hydroxamic acid (SAHA) induces a cell-death pathway characterized by cleavage of Bid and production of reactive oxygen species. Proc Natl Acad Sci U S A. 2001;98:10833–10838. doi: 10.1073/pnas.191208598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee HJ, Thompson JE, Wang ES, Wetzler M. Philadelphia chromosome-positive acute lymphoblastic leukemia: current treatment and future perspectives. Cancer. 2010 doi: 10.1002/cncr.25690. [DOI] [PubMed] [Google Scholar]
- 27.Weisberg E, Manley PW, Cowan-Jacob SW, Hochhaus A, Griffin JD. Second generation inhibitors of BCR-ABL for the treatment of imatinib-resistant chronic myeloid leukaemia. Nat Rev Cancer. 2007;7:345–356. doi: 10.1038/nrc2126. [DOI] [PubMed] [Google Scholar]
- 28.Zhang B, Strauss AC, Chu S, et al. Effective targeting of quiescent chronic myelogenous leukemia stem cells by histone deacetylase inhibitors in combination with imatinib mesylate. Cancer Cell. 2010;17:427–442. doi: 10.1016/j.ccr.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiskus W, Pranpat M, Bali P, et al. Combined effects of novel tyrosine kinase inhibitor AMN107 and histone deacetylase inhibitor LBH589 against Bcr-Abl-expressing human leukemia cells. Blood. 2006;108:645–652. doi: 10.1182/blood-2005-11-4639. [DOI] [PubMed] [Google Scholar]
- 30.Bali P, Pranpat M, Bradner J, et al. Inhibiition of histone deacetylase 6 acetylates and disrupts the chaperone function of heat shock protein 90: A novel basis of antileukemia activity of histone deacetylase inhibitors. J Biol Chem. 2005 doi: 10.1074/jbc.C500186200. [DOI] [PubMed] [Google Scholar]
- 31.Seo JH, Wood LJ, Agarwal A, et al. A specific need for CRKL in p210BCR-ABL-induced transformation of mouse hematopoietic progenitors. Cancer Res. 2010;70:7325–7335. doi: 10.1158/0008-5472.CAN-10-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shuai K, Halpern J, ten HJ, Rao X, Sawyers CL. Constitutive activation of STAT5 by the BCR-ABL oncogene in chronic myelogenous leukemia. Oncogene. 1996;13:247–254. [PubMed] [Google Scholar]
- 33.Ungerstedt JS, Sowa Y, Xu WS, et al. Role of thioredoxin in the response of normal and transformed cells to histone deacetylase inhibitors. Proc Natl Acad Sci U S A. 2005;102:673–678. doi: 10.1073/pnas.0408732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koptyra M, Falinski R, Nowicki MO, et al. BCR/ABL kinase induces self-mutagenesis via reactive oxygen species to encode imatinib resistance. Blood. 2006;108:319–327. doi: 10.1182/blood-2005-07-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fanta S, Sonnenberg M, Skorta I, et al. Pharmacological inhibition of c-Abl compromises genetic stability and DNA repair in Bcr-Abl-negative cells. Oncogene. 2008;27:4380–4384. doi: 10.1038/onc.2008.68. [DOI] [PubMed] [Google Scholar]
- 36.Subramanian C, Opipari AW, Jr, Bian X, Castle VP, Kwok RP. Ku70 acetylation mediates neuroblastoma cell death induced by histone deacetylase inhibitors. Proc Natl Acad Sci U S A. 2005;102:4842–4847. doi: 10.1073/pnas.0408351102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosato RR, Almenara JA, Maggio SC, et al. Role of histone deacetylase inhibitor-induced reactive oxygen species and DNA damage in LAQ-824/fludarabine antileukemic interactions. Mol Cancer Ther. 2008;7:3285–3297. doi: 10.1158/1535-7163.MCT-08-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee JH, Choy ML, Ngo L, Foster SS, Marks PA. Histone deacetylase inhibitor induces DNA damage, which normal but not transformed cells can repair. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1008522107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oke A, Pearce D, Wilkinson RW, et al. AZD1152 rapidly and negatively affects the growth and survival of human acute myeloid leukemia cells in vitro and in vivo. Cancer Res. 2009;69:4150–4158. doi: 10.1158/0008-5472.CAN-08-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore AS, Blagg J, Linardopoulos S, Pearson AD. Aurora kinase inhibitors: novel small molecules with promising activity in acute myeloid and Philadelphia-positive leukemias. Leukemia. 2010;24:671–678. doi: 10.1038/leu.2010.15. [DOI] [PubMed] [Google Scholar]
- 41.Copland M, Hamilton A, Elrick LJ, et al. Dasatinib (BMS-354825) targets an earlier progenitor population than imatinib in primary CML but does not eliminate the quiescent fraction. Blood. 2006;107:4532–4539. doi: 10.1182/blood-2005-07-2947. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.