Abstract
Purpose
Rhabdomyosarcoma (RMS) is a common pediatric soft-tissue tumor. In this study, we evaluated the efficacy and selectivity of drozitumab, a death receptor DR5-targeted therapeutic antibody, in RMS preclinical models.
Experimental design
A panel of 11 RMS cell lines was used for in vitro studies. The molecular marker predictive of response to drozitumab was interrogated. Selected RMS cell lines were injected into the gastrocnemius muscle of mice for in vivo assessment of the potency and selectivity of drozitumab.
Results
We report that DR5, but not DR4, persisted at high levels and on the surface of all RMS cell lines. DR5 antibody drozitumab was effective in vitro against the majority of RMS cell lines. There was a strong correlation between caspase-8 expression and the sensitivity to drozitumab, which induced the rapid assembly of the death-induced signaling complex (DISC) and the cleavage of caspase-8 only in sensitive cells. More importantly, caspase-8 catalytic activity was both necessary and sufficient for mediating the sensitivity to drozitumab. Furthermore, drozitumab had potent anti-tumor activity against established RMS xenografts with a specificity predicted from the in vitro analysis and with tumor-free status in half of the treated mice.
Conclusion
Our study provides the first preclinical evaluation of the potency and selectivity of a death receptor antibody in rhabdomyosarcoma. Drozitumab is effective, in vitro, against the majority of RMS cell lines that express caspase-8 and, in vivo, may provide long-term control of RMS.
Introduction
Rhabdomyosarcoma (RMS) is the most common pediatric soft-tissue tumor. Despite aggressive management including surgery, radiation and chemotherapy, the outcome for children with metastatic disease is dismal, and this prognosis has remained unchanged for decades (1–2). The cure rate for advanced RMS is not expected to improve significantly until effective targeted and tumor-specific agents are developed (3). Recent advances in targeted therapies provide fresh alternatives for therapeutic development against RMS. Many novel investigational agents are in various stages of clinical development, including those targeting IGF1R, mTOR, PDGFR and c-Kit (3).
We recently showed that a therapeutic antibody against IGF1R effectively induced cell death via intrinsic apoptosis in selected RMS cell lines, which express high levels of IGF1R and minimal levels of Bcl-2 (4). This antibody demonstrated only modest growth inhibitory activity, however, against the majority of RMS cell lines in vitro. Thus, there is a need to further explore other agents capable of inducing apoptosis in a greater percentage of RMS cells. Interestingly, a previous report showed that a significant number of RMS cell lines were susceptible to TNF-related apoptosis inducing ligand (TRAIL)-induced apoptosis but resistant to FAS-induced cell death in vitro (5). However, many issues remain to be resolved, including the identification of the receptors mediating the activity of TRAIL, the detection of biomarkers predictive of tumor sensitivity, and the demonstration of in vivo anti-tumor activity. Indeed, little is known about the anti-tumor activity of agonistic antibodies to TRAIL receptors in RMS, and in vivo preclinical evaluation of a therapeutic composition targeting TRAIL receptors is needed.
Apoptosis or programmed cell death is a naturally occurring process for removing unwanted cells in the body. Defects in apoptotic pathways have been implicated in disease conditions, such as cancer, which are characterized by uncontrolled cell growth. Apoptosis can be achieved by the activation of the intrinsic, mitochondria-dependent pathway or the extrinsic, death receptor-mediated pathway. The frequent inactivation of p53 enables cancer cells not only to bypass the intrinsic apoptotic response to their genomic aberrations, but also to escape apoptosis initiation in response to DNA damage induced by various conventional cancer therapies (6). Therefore, targeting the extrinsic, death receptor-mediated pathway provides a fresh alternative to current cancer therapies (7).
The extrinsic pathway depends on ligand-mediated activation of cell-surface receptors, including CD95 (Fas), tumor necrosis factor (TNF) receptor, and TRAIL receptors (8). Binding of TRAIL to death receptors DR4 and/or DR5 results in the assembly of the death-induced signaling complex (DISC) involving FADD and caspase-8 or -10 (9–10). Due to the selectivity of TRAIL towards cancer cells, there has been a significant interest in developing agents targeting TRAIL receptors for the treatment of various cancers (7, 11). Recent analysis reveals that sensitivity to the ligand appears to be controlled mainly by apical events including DISC assembly and caspase-8 activation (12).
Multiple factors have been suggested to affect TRAIL-induced apoptosis, including decoy receptors DcR1, DcR2 and OPG that bind to TRAIL without mediating death signaling (13) and c-FLIP that may compete with the recruitment of caspases-8 and -10 at the DISC (14). It was also suggested that the mitochondria-dependent apoptotic pathway may augment TRAIL-induced cell death (7). Recently, both the post-translational modifications of the DR4 and DR5 receptors, including O-glycosylation (15) and endocytosis (16), as well as the ubiquitination of caspase-8 (17) were implicated as mechanisms for affecting TRAIL-induced cell death. These important studies may facilitate the identification and implementation of predictive biomarkers for the clinical development of TRAIL-based therapeutics for cancer.
Recent clinical trial results showed that treatment with the recombinant human rhApo2L/TRAIL was associated with responses in several sarcoma patients in a phase I study (18). Various agonist therapeutic antibodies against DR4 and DR5 also exhibited anti-tumor activities in pre-clinical models (19–22) and are in clinical development (11). The antibodies for death receptors have unique characteristics including different pharmacokinetic properties (much longer half-life), greater receptor selectivity, and reduced sensitivity to the effects of decoy receptors or receptor post-translational modification. One of the human DR5 antibodies, drozitumab (Apomab), displayed encouraging anti-tumor activity in a mouse xenograft model (20). Phase I study of drozitumab showed that the agent was safe and well-tolerated with a mean plasma half-life between 1 to 3 weeks (23). Yet, to date, there is little information on the determinants of cancer cell susceptibility to antibody-based agents and on predicting cancer cell responses. Uncovering these determinants would be very important for promoting the clinical development of these antibody-based therapies.
To further explore the potential of targeting death receptors against RMS, we performed a preclinical investigation of DR5 therapeutic antibody drozitumab on RMS. The results illustrate that RMS is a rational target for drozitumab and other DR5-targeted agents. Our data further demonstrate that caspase-8 expression and activity is crucial for predicting the therapeutic efficacy of antibodies against death receptors.
Materials and Methods
Cell lines and reagents
The RMS cell lines Rh4, Rh18, Rh28, Rh30, Rh36 and Rh41 were from Dr. Peter Houghton at Nationwide Children’s Hospital, and CTR was from Dr. Lee Helman at US National Cancer Institute (NCI). RMS cell lines RD, A204, HS729, RMS13 and Ewing’s cell line A673 (EWS) were acquired from ATCC. The authenticity of the cell lines were verified with immunoblots using a specific antibody against the PAX3-FKHR fusion protein (24). All cell lines were maintained in RPMI-1640 with 10% fetal bovine serum and antibiotics. Human skeletal muscle derived cells (SkMDC) were obtained from Cook Myosite (Pittsburgh, PA) and cultured per manufacturer’s instructions. Recombinant human TRAIL, mouse antibodies against human DR4 (MAB347) and DR5 (MAB631) for cell-based assays were purchased from R&D Systems (Minneapolis, MN). The human DR5 antibody drozitumab and placebo antibody were kindly provided by Genentech Inc. (San Francisco, CA). The goat anti-Fc antibody was obtained from Jackson Lab (Bar Harbor, ME). Caspase-8 specific inhibitor Z-I-E(OMe)-T-D(OMe)-FMK was obtained from R&D Systems. The expression plasmids with CASP8 and enzyme defective CASP8mt (C360S) were provided by Drs. Michael Lenardo and Lixin Zheng at the US National Institutes of Health. Transfection was performed with Lipofectamine per manufacturer’s instructions (Invitrogen, Carlsbad, CA).
Cell viability and clonogenic assays
Cell proliferation assays were performed with ATPLite reagent as previously described (25). All experiments were performed in triplicate at least three times for each result. Clonogenic assays were performed with 0.5% crystal violet (26). All clonogenic experiments were reproducible.
DR5 immunoprecipitation
Rh18 or Rh41 cells were stimulated with 1 μg/ml drozitumab+anti-Fc antibody or placebo antibody+anti-Fc for 10 min at 37°C. Stimulation was stopped by washing twice with 25 ml of ice-cold PBS. Cells were lysed in IP lysis buffer (30 mM Tris-HCl, 150 mM NaCl, 10% glycerol, 2 mM EDTA, containing 0.5% triton, and 1x PMSF and protease inhibitor cocktail). After centrifugation, the supernatant was pre-cleared with agarose resin. The pre-cleared lysate was immunoprecipitated via DR5 with drozitumab (for cells stimulated with placebo+anti-Fc, 0.5 μg drozitumab was also added) for 3 hrs at 4°C with protein G beads. The protein G beads were washed with IP lysis buffer and the precipitated proteins were resuspended in 100 μl of 1x sample buffer. The protein concentrations of the pre-IP total cell lysates were measured and normalized. The IP samples and pre-IP total lysates were analyzed by immunoblotting.
Immunoblot analysis
Both sensitive and resistant RMS cells were treated with 1 μg/ml drozitumab+anti-Fc antibody or placebo antibody for the indicated time. Cell and tissue lysates were prepared as previously described (25). The DR4 and DR5 antibodies were from Imgenex (San Diego, CA), the FADD antibody was from BD Pharmingen (San Diego, CA), and the FLIP, PARP, caspase-3, caspase-8, caspase-9, α-tubulin, GAPDH, and actin antibodies were from Cell Signaling Technology (Danvers, MA).
Flow cytometric analysis of surface DR4 and DR5 expression
Fluorescence-activated cell sorting analysis of DR4 and DR5 cell surface expression was performed using PE-conjugated antibodies (R&D Systems, Minneapolis, MN) as per manufacturer’s suggestion and previously described (16). In brief, cells were blocked with 1% goat serum, incubated with 10 μg/ml anti-DR4-PE or anti-DR5-PE (mouse IgG1-PE and IgG2b-PE as respective controls), and analyzed by FACS.
Human tissues and animal studies
Anonymous human RMS tumor and control skeletal muscle tissues were obtained from patients at NCI. IRB exemption for using human specimens was obtained from the National Institutes of Health. The animal protocol was approved by the NCI Animal Care and Use Committee. Female 4 to 6-week-old CB17.B6-Prkdcscid mice were purchased from Charles River Laboratories (Wilmington, MA). The animal study was done as previously described (25) except that drozitumab treatment was initiated once tumors reached about 1–2 mm in maximal length (subtracted that of the un-inoculated leg) to determine its ability to induce tumor regression. The drug or placebo was given at 10 mg/kg i.p. once weekly for a total of ten weeks. Tumors were measured with calipers. The tissues were obtained four days after drozitumab or placebo treatment, fixed with 10% buffered formalin and paraffin embedded. The H&E staining and TUNEL assay were performed by the pathology facility within the Advance Technology Program at NCI Frederick. Images were acquired with a ScanScope (Aperio, Vista, CA) at 20x resolution.
Immunohistochemistry
RMS tissue arrays were acquired from Biomax (Rockville, MD). Control slides were obtained from mouse xenografts generated with Rh18 and Rh4 cells. IHC staining was performed as previously described (27). In brief, the slides were incubated with caspase-8 polyclonal antibody (1:100 dilution) (NeoMarkers, Fremont, CA) or DR5 polyclonal antibody (1:250 dilution) (ProSci, Inc., Poway, CA) overnight at 4°C, following the DAKO Visualization System instructions. Both the percentage of positive staining in tumor cells and the intensity of staining were scored. The intensity of IHC staining was measured by using a numerical scale (0 = no expression, 1 = weak expression, 2 = moderate expression, 3 = strong expression). The staining data were finally quantified as the weighted index (WI) (WI = % positive stain in tumor × intensity score) as previously described (27). The final values were the average of readings from two replicate cores.
Statistical analysis
Statistical analyses were performed with Prism (GraphPad, La Jolla, CA). All cell-based studies were done in triplicate. Data were presented as mean + SEM. Statistical comparisons were determined by Two-way ANOVA test using Prism. Statistical significance was defined as P < 0.05. Kaplan-Meier survival analysis was performed with Prism.
Results
TRAIL ligand effectively induces RMS cell death mediated through DR5 receptor
To determine the in vitro susceptibility of RMS cell lines to TRAIL receptor targeted agents, drug sensitivity analysis was performed with a panel of 11 RMS cell lines and 1 Ewing’s sarcoma line (A673), with various concentrations of TRAIL ligand. MDA-231 cells were selected as a highly sensitive cell line since our previous study showed that it was the most sensitive breast cancer cell line to TRAIL or DR4 and DR5 antibodies in vitro (16). As a negative control, primary human skeletal muscle derived cells (SkMDC) were used. The SkMDC were entirely insensitive to treatment with TRAIL, or the DR4 and DR5 antibodies in vitro (Table 1). There was a significant difference between RMS cell lines, however, in terms of their response to TRAIL (Fig. 1A). Rh18 and Rh30 showed very high sensitivity to TRAIL, comparable to that of MDA-231 cells; whereas Rh4 and Rh41 had minimal to no response to TRAIL. The detailed dose studies of these RMS cell lines revealed four additional cell lines that were highly sensitive to TRAIL, Rh28, Rh36, RD, and CTR (Table 1), with more than 50% reduction of viability at 0.1μg/ml TRAIL. Thus, approximately half of the RMS cell lines examined are sensitive to TRAIL.
Table 1.
Drug sensitivity of RMS cell lines to TRAIL, mouse anti-DR4 and anti-DR5, drozitumab (DRO), and DRO+anti-Fc antibodies. Dose studies of the agents on the panel of RMS cells as described in Figures 1 and 2. The percent viability was defined as the ratio between viable cells at the highest ligand or antibody dose and in the absence of ligand or antibody.
| RMS lines |
Sub- type |
TRAIL Ligand | DR4 Mouse Mab | DR5 Mouse Mab | DRO | DRO + Anti Fc | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50 (μg/ml) |
Viability (%) at 0.1μg/ml |
IC50 (μg/ml) |
Viability (%) at 1μg/ml |
IC50 (μg/ml) |
Viability (%) at 1μg/ml |
IC50 (μg/ml) |
Viability (%) at 1μg/ml |
IC50 (μg/ml) |
Viability (%) at 1μg/ml |
||
| RH4* | ARMS | >0.100 | 100 | >1 | 100 | >1 | 100 | >1 | 98 | >1 | 80 |
| RH18 | ERMS | 0.004 | 4 | >1 | 85 | 0.014 | 4 | >1 | 56 | 0.014 | 5 |
| RH28 | ARMS | 0.001 | 14 | >1 | 100 | 0.093 | 15 | 0.659 | 70 | 0.018 | 17 |
| RH30 | ARMS | 0.001 | 7 | >1 | 90 | 0.039 | 7 | >1 | 78 | 0.079 | 15 |
| RH36 | ERMS | 0.030 | 45 | 0.398 | 50 | 0.136 | 21 | 0.986 | 48 | 0.156 | 15 |
| RH41* | ARMS | >0.100 | 85 | >1 | 90 | >1 | 90 | >1 | 98 | >1 | 80 |
| RD | ERMS | 0.003 | 29 | >1 | 95 | 0.398 | 32 | >1 | 78 | 0.108 | 34 |
| A673 | EWS | 0.001 | 3 | 0.076 | 21 | 0.112 | 4 | 0.681 | 39 | 0.006 | 12 |
| CTR | ERMS | 0.011 | 12 | >1 | 85 | 0.088 | 24 | 0.600 | 42 | 0.006 | 16 |
| A204* | ERMS | 0.070 | 50 | >1 | 100 | >1 | 65 | >1 | 98 | 0.608 | 45 |
| HS729* | ERMS | >0.100 | 80 | >1 | 100 | >1 | 80 | >1 | 95 | >1 | 85 |
| RMS13* | ARMS | >0.100 | 68 | >1 | 45 | >1 | 52 | >1 | 92 | >1 | 53 |
| MDA231 | BrCa | 0.001 | 9 | 0.026 | 42 | 0.026 | 18 | >1 | 69 | 0.115 | 19 |
| SkMDC | Normal | >0.100 | 95 | >1 | 100 | >1 | 95 | >1 | 100 | >1 | 100 |
RMS cell lines resistant to TRAIL ligand and drozitumab+anti-FC. MDA231, TRAIL sensitive breast cancer cell line; A673, EWS cell line, SkMDC, primary human skeletal muscle derived cells.
Figure 1.
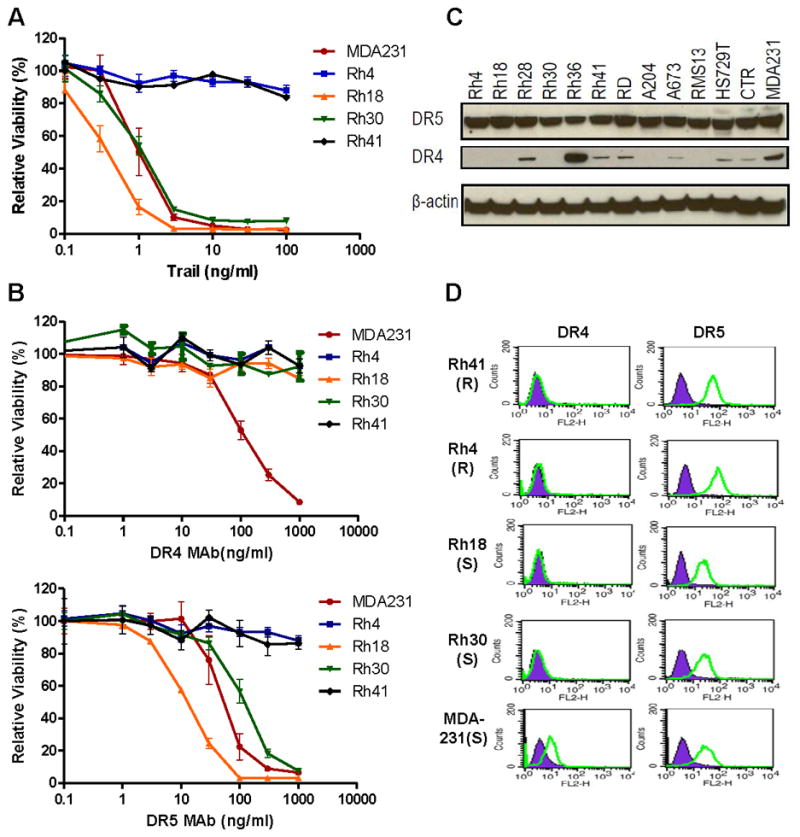
TRAIL reduces RMS cell viability mediated via the DR5 receptor. A. Cell viability analysis of RMS cell lines treated with TRAIL for 72 hrs, followed by ATPLite assay. A highly sensitive breast cancer cell line, MDA-231, was used as a reference. B. Sensitivity of RMS cell lines to mouse monoclonal antibody against DR4 (upper) or DR5 (lower). The cells were treated with the indicated concentrations of these antibodies for 72 hrs prior to the viability assay. C. The expression of DR4 and DR5 in the panel of RMS cell lines was determined by immunoblotting. D. The cell surface expression of DR4 and DR5 was analyzed by FACS in selected TRAIL and DR5 resistant (R) or sensitive (S) cells. The purple signal was from the control non-specific antibody while the green signal was from either the DR4 or DR5 specific antibody. Increased intensity of the green signal over the purple reflects a specific expression of the analyte.
To determine the specific receptor that mediated the cytotoxic activity of TRAIL, the RMS cell lines were treated with mouse agonist monoclonal antibodies against DR4 and DR5. The results showed that the six RMS cell lines sensitive to TRAIL were also selectively sensitive to the DR5 antibody (Fig. 1B, Table 1). In contrast, they were mostly resistant to the DR4 antibody (Fig. 1B, Table 1). In comparison, MDA-231 cells were equally sensitive to both DR4 and DR5 antibodies. To understand the selective sensitivity of RMS to the DR5 antibody, the expression of DR4 and DR5 receptors were analyzed by immunoblotting. The data showed that DR5 was widely expressed in RMS, whereas DR4 was generally expressed at much lower levels (Fig. 1C). Further analysis of the receptor expression on the cell surface by flow cytometry indicated ubiquitous cell surface expression of DR5 in all RMS cell lines (Fig. 1D, and data not shown). In contrast, only A673 has significant surface DR4 expression (Fig. 1D, and data not shown). In comparison, both DR4 and DR5 were detected on the surface of MDA-231 cells. Thus, our results suggest that TRAIL treatment reduces RMS cell viability mainly through the DR5 receptor. It is worth noting that DR5 was expressed and present on the surface of all RMS cell lines examined, including those cell lines that failed to respond to TRAIL or the agonist DR5 antibody (Fig. 1). Our data suggest that other factor(s) may be involved in determining the cellular sensitivity to DR5 targeted agents in RMS.
Drozitumab, a human agonist antibody against DR5, is effective against RMS in vitro
The fact that a large percentage of RMS cell lines was sensitive to a DR5 antibody allowed us to explore the therapeutic potential of a human DR5 antibody, drozitumab, for RMS. Previous studies showed that receptor aggregation enhances the pro-apoptotic signal of drozitumab (20, 28). Our results showed that the RMS cell lines were minimally sensitive to drozitumab alone (Fig. 2A, Table 1). However, they were susceptible to drozitumab cross-linked with an anti-Fc antibody (Fig. 2A, Table 2), a result that was consistent with these reports (20, 28). This result was in contrast to that of the mouse DR5 monoclonal antibody described above, which did not require cross-linking. All subsequent in vitro studies were performed with drozitumab and an equal amount of the anti-Fc antibody.
Figure 2.
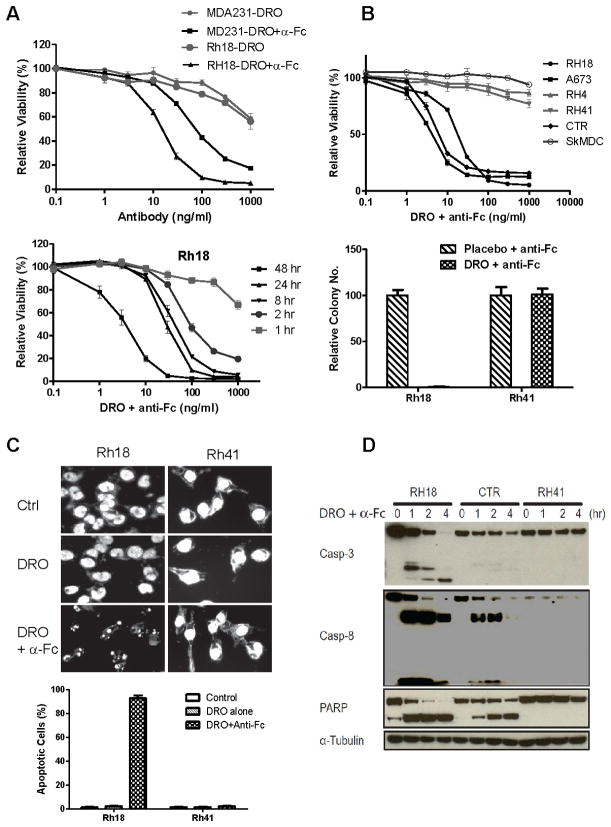
Drozitumab (DRO) with anti-Fc antibody treatment induces apoptosis in the sensitive RMS cells. A. The sensitive Rh18 cells were treated with drozitumab or a 1:1 ratio of drozitumab+anti-Fc antibodies at the indicated doses for 72 hrs prior to the viability assay (upper). Also, Rh18 cells were treated with various doses of drozitumab+anti-Fc for the indicated time points and analyzed immediately for cell viability (lower). B. Selected sensitive and resistant RMS cell lines and primary skeletal muscle derived cells (SkMDC) were treated with drozitumab+anti-Fc or placebo+anti-Fc for 72 hr, followed by cell viability assay (upper); or clonogenic assay (lower). C. Both sensitive Rh18 and resistant Rh41 cells were treated with indicated antibodies for 4 hrs, fixed, stained with DAPI, and analyzed for fragmented nuclei as a marker for apoptotic cells. D. RMS cells were treated with drozitumab+anti-Fc for the indicated time points and analyzed for the cleavage of apoptotic markers, caspases-3 and -8 and PARP.
Our study showed that the loss of cell viability was rapid with a substantially decreased viability after only two hours of antibody treatment (Fig. 2A). RMS cell lines had the same sensitivity profile to drozitumab as they did to TRAIL and the mouse DR5 antibody. The cell lines that were most resistant to TRAIL (marked *) were also most resistant to the mouse DR5 antibody and to drozitumab (Fig. 2B, Table 1). All together six RMS cell lines, including Rh18, Rh28, Rh30, Rh36, RD and CTR, were highly sensitive to drozitumab+anti-Fc treatment, with sensitivities comparable to that of MDA-231 (Table 1). In contrast, SkMDC were entirely resistant to drozitumab+anti-Fc (Fig. 2B, Table 1). The cellular sensitivity to drozitumab using the short-term proliferation assay was also confirmed with a long-term clonogenic assay (Fig. 2B). Thus, drozitumab+anti-Fc induced rapid RMS cell death with the same selectivity as TRAIL and the mouse DR5 antibody.
As expected, drozitumab-mediated cell death was due to a rapid onset of apoptosis. After 4 hrs of drozitumab+anti-Fc treatment, greater than 90% of the sensitive Rh18 cells displayed fragmented nuclei (Fig. 2C). Immunoblot analysis showed that drozitumab+anti-Fc treatment induced the cleavage of caspase-3, 8 and PARP in these sensitive cells with the maximum induction seen 1 hr following the antibody treatment (Fig. 2D). The resistant Rh41 cells, even though they expressed DR5 on their cell surface (Fig. 1D), failed to respond to drozitumab+anti-Fc treatment (Fig. 2C, 2D). Therefore, we sought to understand the determinants of sensitivity to drozitumab in RMS cells.
Caspase-8 is associated with the selective activity of drozitumab in RMS
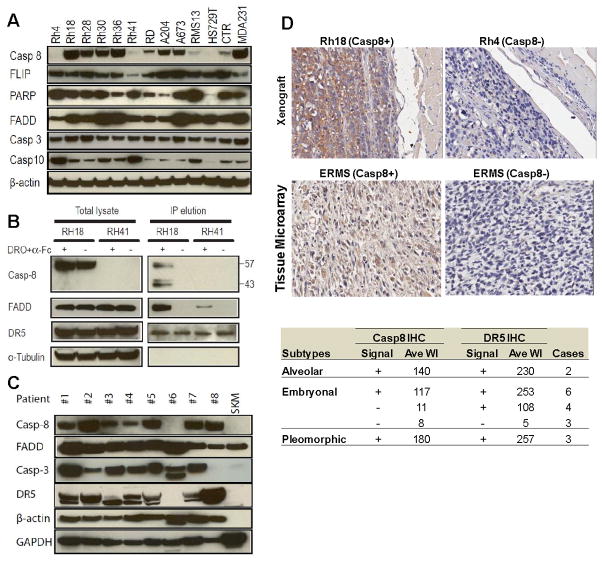
To explore the potential link between drozitumab resistance and the expression of components of ligand-induced apoptosis, immunoblots were performed with antibodies against caspases-3, 8 and 10, FADD, and FLIP. Of the RMS cell lines (Rh4, Rh41, HS729, and RMS13) that were resistant to drozitumab+anti-Fc with an IC50 > 1 μg/ml, a level that was achieved for drozitumab in phase I clinical study (23), the most remarkable correlation was the absence of caspase-8 protein in all four (Fig. 3A). No correlation was seen with FADD, FLIP or caspase-3. Despite the homology between caspase-8 and caspase-10 and the roles of caspase-10 in mediating ligand-dependent apoptosis, there was no association between drozitumab sensitivity and reduced caspase-10 expression. On the contrary, three of the four drozitumab resistant cell lines (Rh4, Rh41, and RMS13) had the highest expressed levels of caspase-10. Thus, our results point to a specific correlation of reduced caspase-8 expression with RMS cell sensitivity to drozitumab+anti-Fc treatment.
Figure 3.
Caspase-8 expression is associated with drozitumab (DRO) sensitivity in RMS cells. A. Immunoblot analysis of RMS cell lines for the expression of proteins relevant to DISC and its effectors. B. Both sensitive Rh18 and resistant Rh41 cells were treated with drozitumab+anti-Fc for 10 min. Following immunoprecipitation via DR5, both the total cell lysate and the precipitated DISC complex were analyzed for FADD and caspase-8 by immunoblot. C. Analysis of the expression of proteins relevant to DR5-DISC and its effectors in a panel of RMS tumor samples. Patients #1 and 3 represent embryonal RMS (ERMS); Patients #4, 5, 6 and 8 represent alveolar RMS; Patients #2 and 7are RMS of unknown subtype; SKM is normal skeletal muscle. D. Caspase-8 staining (brown) of control RMS xenografts, Rh18 and Rh4, and 2 representative ERMS tumors from a RMS tissue array. The staining results for caspase-8 and DR5 of 18 RMS cases are summarized in the table: 11 were DR5+/casp-8+, 4 were DR5+/casp-8−, 3 were DR5−/casp-8−. Further details can be found in Supplemental Table 1. WI, weight index (see Methods).
As the assembly of DISC is the critical initiating step in TRAIL ligand-mediated apoptosis, we examined the assembly of antibody-induced DISC formation and its subsequent activation in both drozitumab-sensitive Rh18 and -resistant Rh41 cells via immunoprecipitation (IP) through DR5. The precipitates were analyzed by immunoblotting for components of the DISC complex, FADD and caspase-8. The results showed that the incubation with drozitumab+anti-Fc for 10 min induced the formation of the DISC complex, which associated with FADD and caspase-8 in the sensitive Rh18 cells (Fig. 3B). Significant cleavage of caspase-8 was observed on the DISC complex, which was not apparent in the total cell lysate, suggesting that caspase-8 was being cleaved within the complex in Rh18 cells. In contrast, there was reduced FADD in the DR5 IP from the resistant Rh41 cells and caspase-8 was clearly absent from the complex (Fig. 3B). This is not surprising since the resistant Rh41 cell lysate had no detectable caspase-8, though comparable levels of DR5 and FADD were detected. The results suggest that the absence of caspase-8 likely plays a critical role in determining the resistance of Rh41 cells to drozitumab.
To determine the expression of caspase-8 and DR5 in human RMS tumors, immunoblots were performed on RMS tumors from eight patients (Fig. 3C). Seven of them were positive for caspase-8 expression, which associated with DR5 expression. One RMS tumor, however, was negative for both caspase-8 and DR5. It was also apparent that both caspase-8 and DR5 were absent in a control normal skeletal muscle tissue. Additional immunostaining against RMS tissue arrays showed that 11 of 18 RMS cases were positive for both caspase-8 and DR5 (Fig. 3D, Supplemental Table 1). As the majority of the cases (13) in the array were embryonal RMS (ERMS), the IHC showed that 7 of 13 ERMSsamples were negative for caspase-8, three of which were negative for both caspase-8 and DR5. The results demonstrated the specific expression of both DR5 and caspase-8 in a significant percentage of RMS which may make them susceptible to the effects of DR5 targeted agents.
Caspase-8 is both necessary and sufficient to confer drozitumab-induced apoptosis
To extend our finding beyond the association between caspase-8 and drozitumab sensitivity, we addressed the issue of whether caspase-8 activity was necessary or sufficient to confer drozitumab sensitivity in RMS. By using a blocking peptide specific for caspase-8, we showed that this peptide was capable of inhibiting drozitumab+anti-Fc-mediated loss of cell viability (Fig. 4A) and induction of caspase-8 cleavage (Fig. 4B), indicating that the activity of caspase-8 was crucial for transmitting the death signal from the activated DR5-dependent DISC complex.
Figure 4.
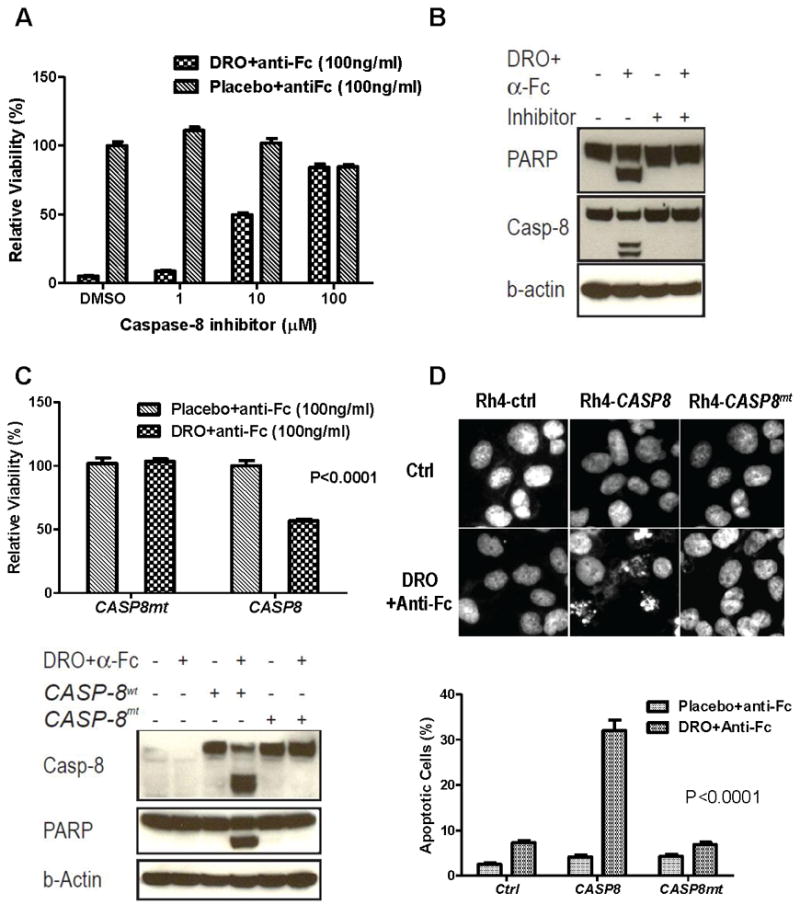
Caspase-8 is both necessary and sufficient for mediating drozitumab (DRO)+anti-Fc induced apoptosis of RMS cells. A. Sensitive Rh18 cells were treated with drozitumab+anti-Fc in the presence of a caspase-8 specific peptide blocker for 72 hrs followed by cell viability measurement. B. Sensitive Rh18 cells were treated with drozitumab+anti-Fc for 3 hrs, with or without the caspase-8 specific inhibitor. Cell lysates were analyzed by immunoblotting for activated caspase-8 and PARP. C. Resistant Rh4 cells were transfected with CASP8 and caspase-defective CASP8mt (C360S) for 2 days. The cells were then treated with drozitumab+anti-Fc for 3 days and analyzed for cell viability (upper). The cells were also analyzed for caspase-8 and PARP cleavage after 5 hrs of treatment with drozitumab+anti-Fc (lower). D. Resistant Rh4 cells were transfected with CASP8 and CASP8mt (C360S) for 2 days. Following drozitumab+anti-Fc treatment for 4 hrs, the cells were fixed and analyzed for nuclear fragmentation via DAPI (upper). The percentage of apoptotic cells is shown (lower). Statistical significance (P value) between drozitumab+anti-Fc and CASP8 was determined using two-way ANOVA.
Equally important, we asked if the introduction of an active caspase-8 was sufficient to convert an insensitive RMS line to a sensitive one. For the gene transfer experiment, we transfected resistant Rh4 cells with a wild-type CASP8-GFP (green fluorescence protein) and a protease-defective CASP8mt-GFP with a site-specific C360S mutation at the active-site cysteine of the caspase domain (29). After transfection with CASP8-GFP, it was apparent that the GFP positive cells were selectively killed by drozitumab+anti-Fc when examined under a microscope (data not shown). DAPI staining results showed that CASP8 but not CASP8mt transiently transfected into the resistant Rh4 cells led to significantly reduced cell viability following drozitumab+anti-Fc treatment (Fig. 4C). Immunoblots showed that although both wide-type and mutant caspase-8 were expressed at comparable levels, drozitumab+anti-Fc treatment only induced the cleavage of caspase-8 and PARP in CASP8, but not CASP8mt, transfected cells (Fig. 4C). Further, the introduction of CASP8, but not CASP8mt, led to significantly increased nuclear fragmentation upon drozitumab+anti-Fc treatment (Fig. 4D). Thus, our results indicate that caspase-8 is both necessary and sufficient for mediating drozitumab+anti-Fc induced apoptosis in RMS cells.
Drozitumab is effective against RMS in vivo with a selectivity predicted from in vitro studies
The anti-tumor activity of drozitumab, without the help of the anti-Fc antibody, was evaluated in a xenograft mouse in which RMS cells were inoculated intramuscularly. After about 4–5 weeks, when tumors became just visible (1–2mm), the mice were randomized and treated with either control antibody (Placebo) or drozitumab alone, once weekly, for up to 10 weeks. While drozitumab treatment had minimal effects on the resistant Rh41 cell line, it exhibited remarkable anti-tumor activity against the sensitive Rh18 cells (Fig. 5A). Pathological analysis and TUNEL assay of tumors obtained four days after drozitumab treatment showed a significant number of apoptotic cells following drozitumab treatment (Fig. 5B). Kaplan-Meier analysis showed improved survival only with the sensitive Rh18 cells (Fig. 5C). Four months after the termination of drozitumab administration, half of the mice inoculated with Rh18 cells remained free from visible tumors (Fig. 5C). Thus, the results indicate a significant anti-tumor activity of drozitumab with a selectivity predicted from in vitro cell-based assays, and this activity is associated with drug-induced apoptosis of tumor cells.
Figure 5.
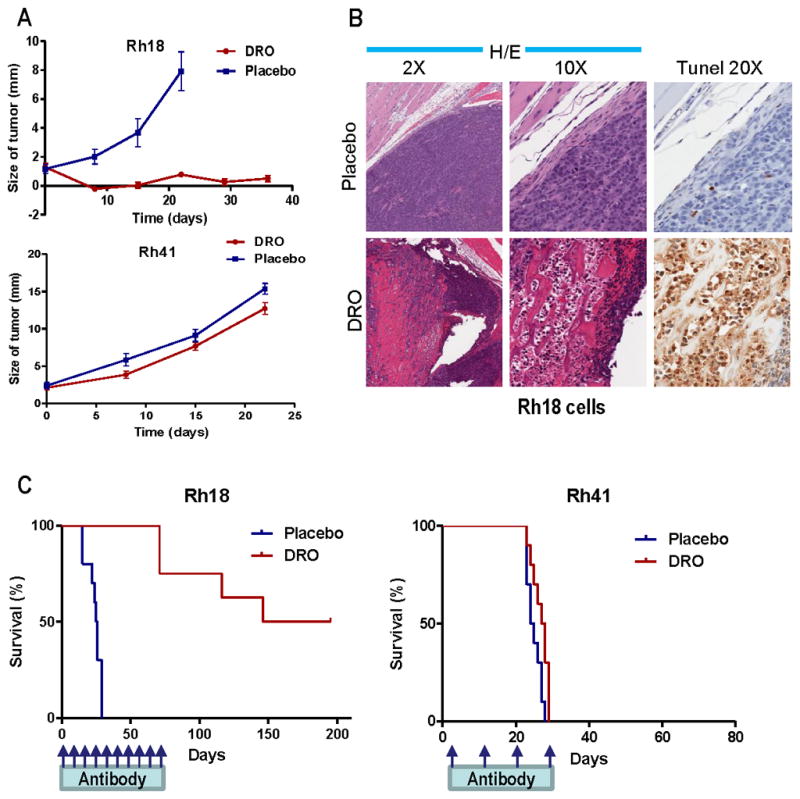
Drozitumab (DRO) has potent anti-tumor activity and a specificity predicted by our in vitro results. A. Both sensitive Rh18 and resistant Rh41 were inoculated intramuscularly into SCID mice. After 4–5 weeks, when tumors just became palpable, mice were treated with 10 mg/kg i.p. drozitumab or placebo antibody alone once weekly. The size of the tumors was measured and shown (n=10). B. Mice with sensitive Rh18 tumors were treated with drozitumab or placebo for 4 days. Tumors were resected, and stained with H&E and TUNEL for apoptotic cells. C. Kaplan-Meier survival analysis of the Rh18- or Rh41-tumor bearing mice treated with drozitumab or placebo. The administration of drozitumab for Rh18 was stopped after 10 weeks and the mice were followed for another 18 weeks without treatment until all mice that developed visible tumors died.
Discussion
To achieve long-term control and a potential cure for metastatic RMS, a disease with consistently poor patient outcome, requires the clinical evaluation of novel targeted therapies. Our study demonstrates that TRAIL receptor DR5 targeted agents, such as drozitumab, may have significant therapeutic potential against RMS. Drozitumab induces rapid apoptosis in vitro via DR5-dependent DISC assembly to activate caspase-8. It is very interesting that drozitumab+anti-Fc is effective against more than half of the RMS cell lines tested, with a potency comparable to that of the most sensitive breast cancer cell line MDA-231 (16, 28), yet with no detectable activity against primary human skeletal muscle cells. Our results show that the determinants of a response to drozitumab, the expression of DR5 and caspase-8, are commonly present in RMS tumors, but not in normal skeletal muscle. This result suggests that normal cells will be able to escape the cytotoxic effect of drozitumab, thereby lowering the potential for side effects in vivo.
We extended our cell culture studies using an in vivo intramuscular xenograft mouse model. The once weekly administration of drozitumab results in the regression of established tumors due to RMS cell apoptosis and is associated with the long-term survival and potential cure of half of the treated animals. In the breast cancer MDA-231 mammary fat pad xenograft model described by Zinonos et al, single agent drozitumab also achieved long-term control and cure of freshly inoculated tumor cells (28). It is important to point out that a recent clinical trial with TRAIL ligand in patients with advanced cancers showed documentable anti-tumor activity in sarcoma patients, without evidence of activity in patients with carcinomas (18). Until now, drozitumab has not undergone clinical studies in pediatric patients, but, our study provides a comprehensive preclinical evaluation of a DR5 targeted therapeutic agent for pediatric RMS.
In addition to determining the specific types of sensitive cancers, the identification of predictive biomarkers is a critical component in the development of targeted therapies. Since the antibodies targeting the various death receptors have different specificities to TRAIL ligand, and may not be sensitive to decoy receptors or to receptor glycosylation, it is important to identify biomarkers representative of a response to those antibodies. Our results provide the first analysis of a predictive biomarker for a death-receptor-targeted therapeutic antibody in RMS. The sensitivity of RMS to drozitumab is strongly associated with the expression of caspase-8; whereas there is no correlation with its sister protein, caspase-10. Drozitumab induces the rapid assembly of DISC and the associated cleavage of caspase-8 in sensitive RMS cells. The activity of caspase-8 is essential for mediating drozitumab-induced RMS cell death. In fact, the expression of a wild type caspase-8, but not a protease-defective mutant, converts a resistant RMS cell line to a susceptible one. A lack of caspase-8 expression was found in some primary tumor samples, and previous studies have showed that CASP8 is frequently methylated in RMS, (30) which may be related to its silencing. Our study suggests that caspase-8 deficiency could be used as a biomarker to exclude patients with RMS from DR5 targeted antibody therapy.
It was evident to us that cross-linking drozitumab with an anti-Fc antibody was essential for restoring its activity in vitro since the single-agent drozitumab was ineffective. A similar requirement for cross-linking was previously demonstrated with both drozitumab (20) and conatumumab (22). It was also clear that an independent monoclonal antibody can be as effective without cross-linking, as in the case of the mouse anti-DR5 antibody (Table 1). While these human DR5 antibodies were effective against tumors in previous in vivo studies (20, 22) and in ours, other components might be necessary for activity, i.e., Fcγ receptor-dependent in vivo cross-linking of drozitumab (A. Ashkenazi, personal communication). Therefore, the penetration of Fc-receptor positive macrophages and neutrophils into a tumor and the affinity of a patient’s Fc-receptor to these engineered human antibodies may significantly affect the effectiveness of the DR5 antibodies. There may be a need to obtain alternative human antibodies that do not need Fc-receptor for its activity, as in the case of the mouse monoclonal antibody used in this study. Alternatively, in vitro cross-linking of these human antibodies to form dimers (31) may significantly enhance their activity.
In summary, we report that the high degree of RMS sensitivity to TRAIL is specifically mediated though the DR5 receptor. The DR4 receptor is expressed at a lower level and is generally absent from the surface of most RMS cell lines. These results also provide a rationale for the use of an anti-DR5, but not an anti-DR4, antibody for the treatment of these malignancies. The therapeutic antibody against DR5, drozitumab, is selective and is effective in vitro, in the presence of an anti-Fc antibody, against the majority of RMS cell lines with an activity profile similar to TRAIL or the mouse DR5 antibody. There is a strong correlation between caspase-8 expression and sensitivity to drozitumab treatment in RMS. Drozitumab induces rapid assembly of the DISC and cleavage of caspase-8 in RMS cells that express this caspase. Caspase-10 is not involved in TRAIL or drozitumab sensitivity in RMS. In addition, our results show that caspase-8 is both necessary and sufficient for mediating the sensitivity to drozitumab, which is dependent on its protease activity. Moreover, both DR5 and caspase-8 are frequently expressed in RMS tumors. Furthermore, drozitumab is effective in vivo against RMS with specificity predicted from the in vitro analysis, and may offer value in providing long-term control or a cure for RMS. Thus, our study provides the basis for the clinical evaluation of DR5 targeted agents against RMS and presents a biomarker for correlative studies.
Supplementary Material
Acknowledgments
We are very grateful to Genentech, Inc. and Avi Ashkenazi for providing drozitumab and placebo antibody, Drs. Michael Lenardo and Lixin Zheng for CASP8 and CASP8mt (C360S) expression plasmids, Lee Helman for RMS tumors, Linnia Mayeenuddin for reading the manuscript, and Arnulfo Mendoza and Danielle O’Mard for excellent technical assistance. This research was supported by the Intramural Research Program of the US National Cancer Institute (NCI). This project was also funded in part with federal funds from the NCI, NIH, under contract N01-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Conflict of interest
All authors declare no conflict of interest.
Translational Relevance Despite aggressive management including surgery, radiation and chemotherapy, the outcome for children with metastatic rhabdomyosarcoma (RMS) is dismal, and this prognosis has remained unchanged for decades. This study shows that the susceptibility of RMS cell lines to TNF-related apoptosis inducing ligand (TRAIL)-induced apoptosis is entirely mediated through the death receptor DR5. Drozitumab, a human anti-DR5 antibody, is both selective and effective against RMS cells in vitro. Further analysis reveals that the sensitivity of RMS to drozitumab is correlated with the expression of caspase-8 and that its catalytic activity is both necessary and sufficient to confer this sensitivity. In vivo, drozitumab is both selective and highly effective against RMS and, four months after drug administration, is capable of achieving long-term control of established tumors in half of the treated animals. Thus, our study provides the basis for the clinical evaluation of DR5 targeted agents against RMS and indicates a biomarker for correlative studies.
References
- 1.Dagher R, Helman L. Rhabdomyosarcoma: an overview. Oncologist. 1999;4:34–44. [PubMed] [Google Scholar]
- 2.Paulino AC, Okcu MF. Rhabdomyosarcoma. Curr Probl Cancer. 2008;32:7–34. doi: 10.1016/j.currproblcancer.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Wachtel M, Schafer BW. Targets for cancer therapy in childhood sarcomas. Cancer Treat Rev. 2010;36:318–27. doi: 10.1016/j.ctrv.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Mayeenuddin LH, Yu Y, Kang Z, Helman LJ, Cao L. Insulin-like growth factor 1 receptor antibody induces rhabdomyosarcoma cell death via a process involving AKT and Bcl-x(L) Oncogene. 2010 doi: 10.1038/onc.2010.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petak I, Douglas L, Tillman DM, Vernes R, Houghton JA. Pediatric rhabdomyosarcoma cell lines are resistant to Fas-induced apoptosis and highly sensitive to TRAIL-induced apoptosis. Clin Cancer Res. 2000;6:4119–27. [PubMed] [Google Scholar]
- 6.Lee JM, Bernstein A. Apoptosis, cancer and the p53 tumour suppressor gene. Cancer Metastasis Rev. 1995;14:149–61. doi: 10.1007/BF00665797. [DOI] [PubMed] [Google Scholar]
- 7.Ashkenazi A. Directing cancer cells to self-destruct with pro-apoptotic receptor agonists. Nat Rev Drug Discov. 2008;7:1001–12. doi: 10.1038/nrd2637. [DOI] [PubMed] [Google Scholar]
- 8.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 9.Kischkel FC, Lawrence DA, Chuntharapai A, Schow P, Kim KJ, Ashkenazi A. Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5. Immunity. 2000;12:611–20. doi: 10.1016/s1074-7613(00)80212-5. [DOI] [PubMed] [Google Scholar]
- 10.Kischkel FC, Lawrence DA, Tinel A, LeBlanc H, Virmani A, Schow P, et al. Death receptor recruitment of endogenous caspase-10 and apoptosis initiation in the absence of caspase-8. J Biol Chem. 2001;276:46639–46. doi: 10.1074/jbc.M105102200. [DOI] [PubMed] [Google Scholar]
- 11.Ashkenazi A, Herbst RS. To kill a tumor cell: the potential of proapoptotic receptor agonists. J Clin Invest. 2008;118:1979–90. doi: 10.1172/JCI34359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes MA, Harper N, Butterworth M, Cain K, Cohen GM, MacFarlane M. Reconstitution of the death-inducing signaling complex reveals a substrate switch that determines CD95-mediated death or survival. Mol Cell. 2009;35:265–79. doi: 10.1016/j.molcel.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalvez F, Ashkenazi A. New insights into apoptosis signaling by Apo2L/TRAIL. Oncogene. 2010;29:4752–65. doi: 10.1038/onc.2010.221. [DOI] [PubMed] [Google Scholar]
- 14.Safa AR, Day TW, Wu CH. Cellular FLICE-like inhibitory protein (C-FLIP): a novel target for cancer therapy. Curr Cancer Drug Targets. 2008;8:37–46. doi: 10.2174/156800908783497087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagner KW, Punnoose EA, Januario T, Lawrence DA, Pitti RM, Lancaster K, et al. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat Med. 2007;13:1070–7. doi: 10.1038/nm1627. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Zhang B. TRAIL resistance of breast cancer cells is associated with constitutive endocytosis of death receptors 4 and 5. Mol Cancer Res. 2008;6:1861–71. doi: 10.1158/1541-7786.MCR-08-0313. [DOI] [PubMed] [Google Scholar]
- 17.Jin Z, Li Y, Pitti R, Lawrence D, Pham VC, Lill JR, et al. Cullin3-based polyubiquitination and p62-dependent aggregation of caspase-8 mediate extrinsic apoptosis signaling. Cell. 2009;137:721–35. doi: 10.1016/j.cell.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 18.Herbst RS, Eckhardt SG, Kurzrock R, Ebbinghaus S, O’Dwyer PJ, Gordon MS, et al. Phase I dose-escalation study of recombinant human Apo2L/TRAIL, a dual proapoptotic receptor agonist, in patients with advanced cancer. J Clin Oncol. 2010;28:2839–46. doi: 10.1200/JCO.2009.25.1991. [DOI] [PubMed] [Google Scholar]
- 19.Luster TA, Carrell JA, McCormick K, Sun D, Humphreys R. Mapatumumab and lexatumumab induce apoptosis in TRAIL-R1 and TRAIL-R2 antibody-resistant NSCLC cell lines when treated in combination with bortezomib. Mol Cancer Ther. 2009;8:292–302. doi: 10.1158/1535-7163.MCT-08-0918. [DOI] [PubMed] [Google Scholar]
- 20.Adams C, Totpal K, Lawrence D, Marsters S, Pitti R, Yee S, et al. Structural and functional analysis of the interaction between the agonistic monoclonal antibody Apomab and the proapoptotic receptor DR5. Cell Death Differ. 2008;15:751–61. doi: 10.1038/sj.cdd.4402306. [DOI] [PubMed] [Google Scholar]
- 21.Jin H, Yang R, Ross J, Fong S, Carano R, Totpal K, et al. Cooperation of the agonistic DR5 antibody apomab with chemotherapy to inhibit orthotopic lung tumor growth and improve survival. Clin Cancer Res. 2008;14:7733–40. doi: 10.1158/1078-0432.CCR-08-0670. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan-Lefko PJ, Graves JD, Zoog SJ, Pan Y, Wall J, Branstetter DG, et al. Conatumumab, a fully human agonist antibody to death receptor 5, induces apoptosis via caspase activation in multiple tumor types. Cancer Biol Ther. 2010:9. doi: 10.4161/cbt.9.8.11264. [DOI] [PubMed] [Google Scholar]
- 23.Camidge DR, Herbst RS, Gordon MS, Eckhardt SG, Kurzrock R, Durbin B, et al. A phase I safety and pharmacokinetic study of the death receptor 5 agonistic antibody PRO95780 in patients with advanced malignancies. Clin Cancer Res. 2010;16:1256–63. doi: 10.1158/1078-0432.CCR-09-1267. [DOI] [PubMed] [Google Scholar]
- 24.Cao L, Yu Y, Bilke S, Walker RL, Mayeenuddin LH, Azorsa DO, et al. Genome-wide identification of PAX3-FKHR binding sites in rhabdomyosarcoma reveals candidate target genes important for development and cancer. Cancer Res. 2010;70:6497–508. doi: 10.1158/0008-5472.CAN-10-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao L, Yu Y, Darko I, Currier D, Mayeenuddin LH, Wan X, et al. Addiction to elevated insulin-like growth factor I receptor and initial modulation of the AKT pathway define the responsiveness of rhabdomyosarcoma to the targeting antibody. Cancer Res. 2008;68:8039–48. doi: 10.1158/0008-5472.CAN-08-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nature protocols. 2006;1:2315–9. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 27.Elrod HA, Fan S, Muller S, Chen GZ, Pan L, Tighiouart M, et al. Analysis of death receptor 5 and caspase-8 expression in primary and metastatic head and neck squamous cell carcinoma and their prognostic impact. PLoS One. 2010;5:e12178. doi: 10.1371/journal.pone.0012178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zinonos I, Labrinidis A, Lee M, Liapis V, Hay S, Ponomarev V, et al. Apomab, a fully human agonistic antibody to DR5, exhibits potent antitumor activity against primary and metastatic breast cancer. Mol Cancer Ther. 2009;8:2969–80. doi: 10.1158/1535-7163.MCT-09-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siegel RM, Martin DA, Zheng L, Ng SY, Bertin J, Cohen J, et al. Death-effector filaments: novel cytoplasmic structures that recruit caspases and trigger apoptosis. J Cell Biol. 1998;141:1243–53. doi: 10.1083/jcb.141.5.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harada K, Toyooka S, Shivapurkar N, Maitra A, Reddy JL, Matta H, et al. Deregulation of caspase 8 and 10 expression in pediatric tumors and cell lines. Cancer Res. 2002;62:5897–901. [PubMed] [Google Scholar]
- 31.Ghetie MA, Podar EM, Ilgen A, Gordon BE, Uhr JW, Vitetta ES. Homodimerization of tumor-reactive monoclonal antibodies markedly increases their ability to induce growth arrest or apoptosis of tumor cells. Proc Natl Acad Sci U S A. 1997;94:7509–14. doi: 10.1073/pnas.94.14.7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.