Abstract
The present study examined how haloperidol (typical) and clozapine (atypical) treatment to mother rats affected their pups’ ultrasonic vocalization (USV) response to maternal separation and re-separation (termed “maternal potentiation”). Clozapine (10 mg/kg, sc) but not haloperidol (0.2 mg/kg, sc) significantly enhanced the maternal potentiation of 40 kHz USVs in pups that were briefly reunited with their dams. This novel paradigm provides an indirect way of assessing the impact of antipsychotic treatment on the quality of maternal care. It may also be useful in examining the impact of antipsychotic treatment on social bonding between infants and mothers.
Keywords: pup ultrasonic vocalization, maternal potentiation of pup vocalization, maternal separation, maternal behavior, rat
Rat maternal behavior is a highly motivated social behavior. A variety of antipsychotics, from typical (e.g., haloperidol) to atypical (e.g., clozapine, risperidone, olanzapine and quetiapine), to even more recent novel antipsychotics (e.g., aripiprazole and amisulpride), possess a common disruptive effect on active maternal responsiveness (e.g., pup retrieval, pup licking, nest building) in postpartum rats [1–3].
So far, all work has been focused on the direct effect of antipsychotic treatment on the behaviors of mothers. How the treatment indirectly affects the offspring’s emotional states and behaviors has not been assessed. Rat pups often emit ultrasonic vocalizations (USV centered around 40 kHz) as a response to various stressors (e.g. isolation, shock, temperature change, etc), and as an early communicative behavior between pup and mother [4]. An isolated pup would increase its emission of USV when it is briefly reunited with its mother and separated again. This potentiated effect is termed “maternal potentiation” of pup USV and is believed to reflect a filial bond that has been formed by the pup during the first weeks of life [4]. Many researchers have used pup USVs to study the emotional states of the infant rodents (e.g. “distress”) [5], their communication with mothers [6], and drug effects [7, 8]. The present study examined the effect of antipsychotic treatment to mother rats on their offspring’s USV as an indirect way of assessing the impact of antipsychotic treatment on the quality of maternal care. We hypothesized that pups of antipsychotic-treated mother rats would show an increase in USV as a compensatory response to their lack of adequate maternal care.
This study consisted of two experiments. Experiment 1 examined the treatment effect of clozapine on isolation-induced pup USV and “maternal potentiation” of pup vocalization [4], while experiment 2 examined that of haloperidol. The basic procedure consisted of three phases: 1st USV testing phase (3 minutes), reunion/isolation phase (4.5 minutes), and 2nd USV testing phase (3 minutes) [9]. In experiment 1, 11 litters from 11 Sprague-Dawley pregnant female rats purchased from Charles River Inc. (Portage, MI) that arrived on gestation days 13–15 were used. On the postnatal day 1 (PND 1) (the morning when pups were found), each litter was culled to 8 pups (4 males and 4 females with the most visible milk bands. In 3 cases, the litter sizes were 5, 6 and 7. No culling was performed). The 11 litters were divided into two groups: a clozapine group (n=6) and a control group (n=5). On postnatal days 10, 12 and 14, mother rats were injected subcutaneously with either clozapine (10.0 mg/kg) or vehicle (sterile water). After the injection, the mothers were returned to their pups. One hour later, the pups from the same litter were taken away from their dam and placed in two plastic bowls (4/bowl: 14.5 and 9.0 cm wide at the top and bottom, 7.5 cm high) on a heating pad (~35°C) in a 48.3 cm × 26.7 cm × 20.3 cm transparent polycarbonate cage. Twenty minutes later, the pups were placed individually in separate bowls, each situated in a chamber (64 cm W×30 cm H×24 cm D) housed in a ventilated, sound-insulated isolation cubicle (96.52 cm W×35.56 cm D ×63.5 cm H, Med Associates, St. Albans, VT) for 3 minutes (the 1st test). Pup USV was recorded by an ultrasonic vocalization microphone (P48 Avisoft Bioacoustics / Emkay Microphone, Avisoft Bioacoustics, Berlin, Germany) mounted on the ceiling of the chamber. Acoustic data were displayed in real time by the Avisoft RECORDER, a multi-channel triggering hard-disk recording software (version 3.4; Avisoft Bioacoustics), and were recorded at a sampling rate of 192 kHz in 16 bit format and analyzed by Avisoft SASLab Pro (version 4.51; Avisoft Bioacoustics) with the lower cut-off frequency set at 23 kHz. After the 1st test, half of the littermates were returned to the dam for 4.5 minutes (termed “maternal”), whereas another half were returned to the bowls with the heating pad (termed “control”) underneath. At the end of the 4.5 minutes, the pups were tested again for USV for 3 minutes (the 2nd test), after which, all pups were returned to their mothers. The 11 litters (82 pups) were predetermined to be assigned into four groups: clozapine-maternal (n=22), clozapine-control (n=21), vehicle-maternal (n=20), and vehicle-control (n=19) on the basis of the drug treatment (i.e. clozapine vs. sterile water) and maternal reunion condition (i.e. reunited with dams or not). Experiment 2 used 6 litters (48 pups) with 3 litters being assigned to haloperidol (0.2 mg/kg, sc, n=24) and 3 being assigned to vehicle (sterile water, sc, n=24). Half of the pups from each litter were returned to their dams during the reunion/separation period (n=24), and half remained isolated (n=24). All animal procedures were conducted in accordance to the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and approved by the University of Nebraska Institutional Animal Care and Use Committee.
For acoustical analysis, recordings were transferred to Avisoft SASLab Pro (Version 4.51) and a fast Fourier transformation (FFT) was conducted. Spectrograms were generated with an FFT-length of 256 points and a time window overlap of 50% (100% Frame, FlatTop window). The spectrogram was produced at a frequency resolution of 750 Hz and a time resolution of 0.6667 ms. Call detection was provided by an automatic dual threshold-based algorithm and a hold-time mechanism (hold time: 0.02 s). The primary threshold was defined at −40 dB, while the start/end threshold was defined at −20 dB. Pup USV were classified into three types by a custom-built computer program. “Long” USVs were defined as waveforms that were greater than 50 ms long, with a frequency deviation of less than 3 kHz. “Short” USVs were any waveforms that were less than 50 ms long. “Frequency-modulated” USVs were greater than 50 ms long and had a frequency deviation of greater than 3 kHz (see examples in Figure 1). A comparison between this automatic quantification method and human scoring on four randomly selected tests revealed a high degree of agreement, with a Pearson’s product moment correlation ranging from 0.979 to 0.998.
Figure 1.
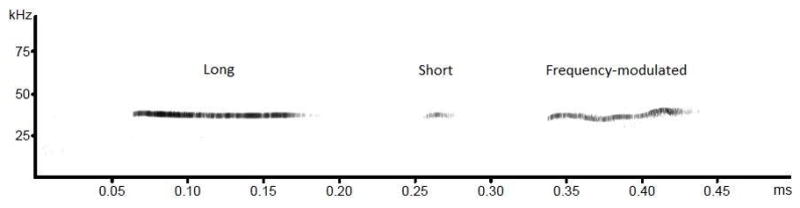
Exemplary spectrograms of the three types of pup ultrasonic vocalizations (from left to right): Long, short and Frequency-modulated. The “x” axis represents time (duration in milliseconds), and the “y” axis represents acoustic frequency (kHz).
Data from each testing day were analyzed separately using repeated measures analysis of variance (ANOVA) (SPSS package, v17) with drug treatment (e.g. clozapine vs. vehicle) and reunion/separation condition (i.e. maternal vs. control) as between-subjects factors and test session (i.e. 1st vs. 2nd test) as a within-subjects factor. Because no sex difference was found in any of these USV measures, data from male and female pups were combined. Each type of USV was also analyzed separately. Group differences at specific testing points for each type of USV were assessed using one-way ANOVA followed by Tukey post hoc test.
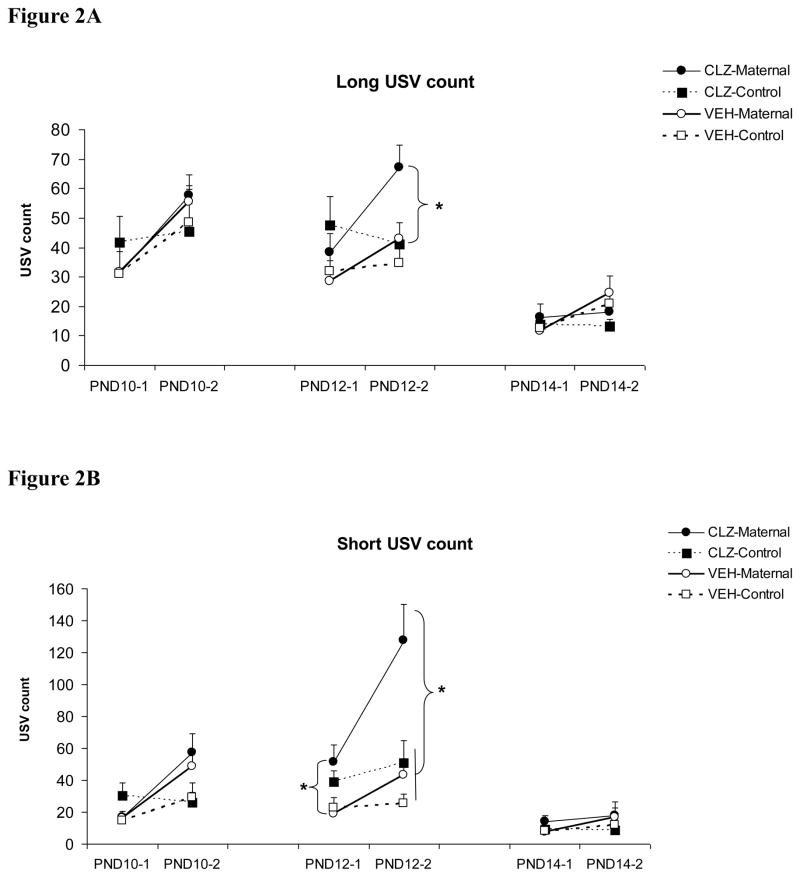
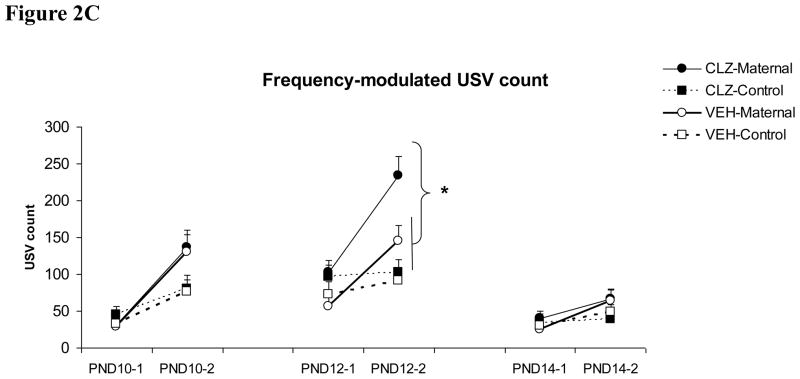
As can be seen in Figure 2, initial maternal separation (1st test) induced pup USVs and re-separation (2nd test in the “maternal” condition) further enhanced the number of pup USVs (a typical maternal potentiation effect). Clozapine treatment tended to increase the number of pup USVs, and this effect appeared to be more pronounced on “maternal potentiation” of pup USVs during the 2nd test and differed across the three test days. On PND 10, repeated measures ANOVA showed a significant interaction between the reunion/separation condition and the test session on the “Short” and “Frequency-modulated” USV (F(1, 78) = 11.590 and 11.871, ps <0.001, respectively), indicating a maternal potentiation effect. The main drug effects on the three types of USVs were not significant (F(1, 78) = 0.084–0.774, ps = 0.382–0.772), suggesting that clozapine did not significantly change pup USVs on this day. On PND 12, pups from the clozapine-treated mothers, especially those who were reunited with their mothers, made more USVs during the 2nd test. There was a main effect of clozapine treatment on all three types of pup USV: “Long” (F(1, 78) = 5.198, p = 0.025), “Short” (F(1, 78) = 15.514, p < 0.001), and “Frequency-modulated” USV (F(1, 78) = 6.648, p = 0.012). Once again, there was a maternal potentiation effect on all three pup USVs, as the interactions between the reunion/separation condition and the test session on all USVs were significant (ps < 0.008). One-way ANOVA and Tukey post hoc tests showed that during the 1st test, the clozapine-maternal pups made significantly more “Short” USV than the vehicle-maternal pups (p = 0.019). During the 2nd test, the clozapine-maternal group emitted significantly more USVs than the other three groups on all three USVs except for the “Long” USV, where it was marginally different from the vehicle-maternal group (p = 0.073). On PND 14, the overall number of pup USV was lower in comparison to the first two testing days. There was no main effect of clozapine treatment on any types of USV (F(1, 78) = 0.039–0.412, p = 0.523–0.843). No significant group difference was found.
Figure 2.
Effects of clozapine treatment (10.0 mg/kg, sc) on the “Long” (A), “Short” (B) and “Frequency-modulated” USV (C) in pups that were either reunited with their mothers (i.e. “CLZ-maternal” and “VEH-Maternal”) or remained isolated (i.e. “CLZ-Control” and “VEH-Control”). Pup USVs were recorded for three minutes in two tests on postnatal day 10, 12 and 14. * P < 0.05 significant group difference between CLZ-Maternal and other groups.
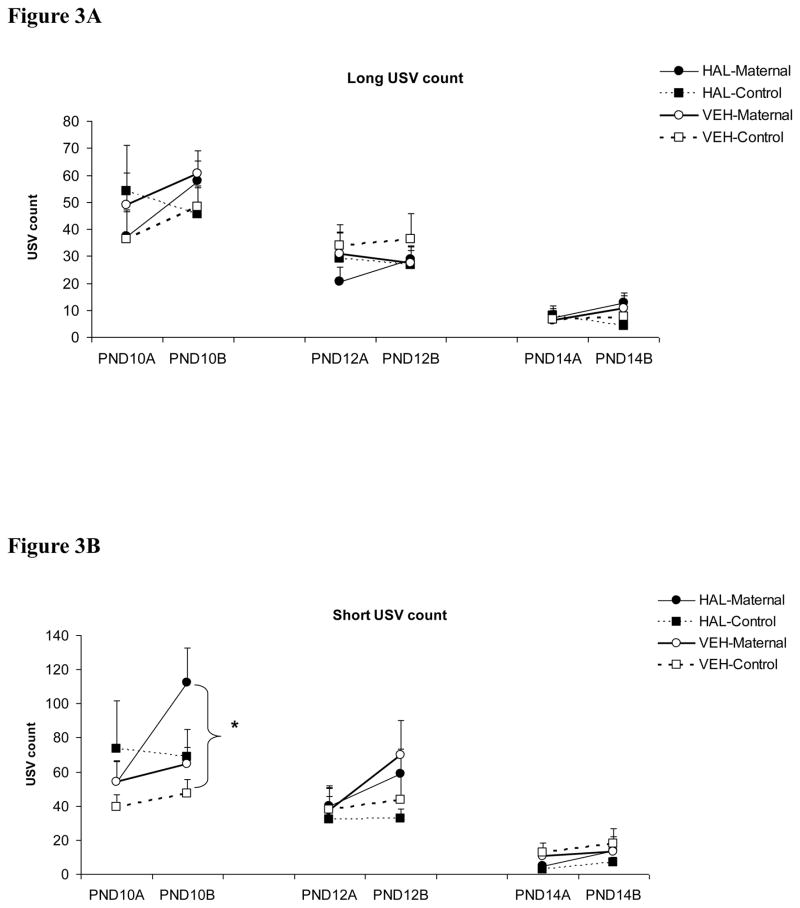
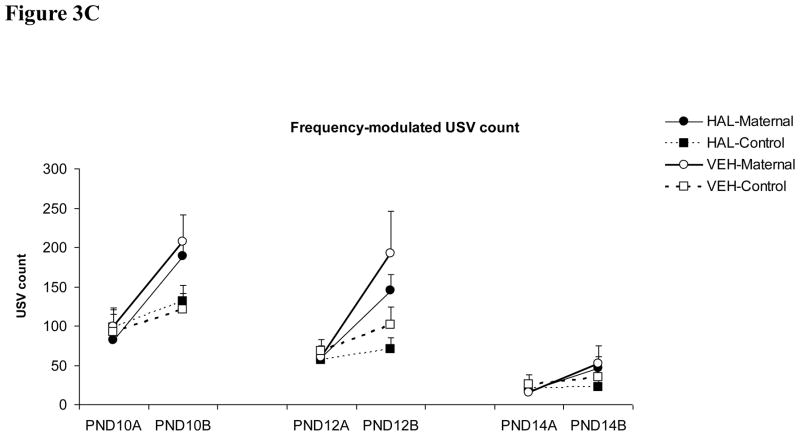
In contrast to the effect of clozapine, haloperidol (0.2 mg/kg) did not seem to have a great impact on pup USV (Figure 3). Across the three test days, the only significant group difference was between the haloperidol-maternal pups and the vehicle-control pups on the “Short” USV made on the second test on day 10 (one-way ANOVA followed by post hoc Tukey test, p = 0.013). No other significant group difference was found.
Figure 3.
Effects of haloperidol treatment (0.2 mg/kg, sc) on the “Long” (A), “Short” (B) and “Frequency-modulated” USV (C) in pups that were either reunited with their mothers (i.e. “HAL-maternal” and “VEH-Maternal”) or remained isolated (i.e. “HAL-Control” and “VEH-Control”). Pup USVs were recorded for three minutes in two tests on postnatal day 10, 12 and 14. * P < 0.05 significant group difference between HAL-Maternal and VEH-Control.
Antipsychotic drugs impair maternal behavior in rats [1–3, 10–12]. Our present study described an innovative approach to study the behavioral effects of antipsychotic medication on rat maternal behavior. Instead of focusing on how antipsychotic treatment affects maternal behavior itself, as we have done in the past [1–3, 10–12], we examined how typical (haloperidol) and atypical (clozapine) antipsychotic treatment to dams affected their offspring’s emotional responses, as measured by pup USV. We took advantage of the facts that rat pups often increase emission of USV in response to separation and re-separation from dams, and that the potentiation of pup USV after a brief contact with dams presumably reflects different maternal experience [4]. Therefore, changes in pup USV may reveal the subtle negative impact of antipsychotic treatment on maternal behavior of mother rats. We found that clozapine 10 mg/kg significantly enhanced the maternal potentiation of pup USVs: clozapine increased pup USVs in pups that were briefly reunited with their dams between the two tests, while it had little effect in pups that were not reunited with their dams. This finding suggests that it was the altered maternal behavior exhibited by clozapine-treated dams during the 4.5-minute reunion period that led to an increase in the number of pup USVs. Our previous study shows that clozapine tends to decrease dams’ licking and nursing towards pups, thus the impaired pup licking and nursing may be contributing factors for the increased pup USVs. However, haloperidol also decreases pup licking but did not significantly affect “maternal potentiation” of pup USVs (Figure 3), therefore, the disruptive effect of clozapine on pup licking may not be the major cause. Haloperidol does differ from clozapine on pup nursing (i.e. haloperidol increases pup nursing, whereas clozapine decreases it), suggesting that the most likely cause of the potentiating effect of clozapine on pup USV is its disruptive effect on pup nursing. Neurochemically, haloperidol has a strong antagonism against D2 receptors, whereas clozapine has a much more potent antagonism on the 5-HT2 receptor in addition to relatively weak antagonism on D2 receptor [13]. Clozapine also antagonizes histamine H1 receptors and/or adrenergic receptors and causes a severe sedation [14], so we speculate that the observed effect of clozapine on pup USVs could be attributed to drug-induced sedation and sedation-induced alteration of maternal behavior.
Previous work on maternal potentiation of pup USVs has not made an attempt to classify types of pup USVs [4]. Studies on adult animals suggest that different types of ultrasonic vocalizations may have different behavioral functions and respond to various drug treatments differently [15, 16]. For example, “Frequency-modulated” 50 kHz USV, but not other types, is proposed to reflect a positive emotional state in rats [17, 18]. In this study, we classified pup USVs into the “Long”, “Short” and “Frequency-modulated” types. Previous work suggests that the “Frequency-modulated” USVs can be more easily detected and localized by mothers than the other types [19], and reunion with mother rats seems to have the largest effect on this type of USV, it can be suggested that the “Frequency-modulated” USVs may reflect emotional state of the pups more closely than other types and provide a better mean for pup-mother communication. More detailed analysis of behavioral correlates of each type of pup USV may allow us to determine their behavioral functions.
It is important to note several limitations with this study. First, we only tested one dose of haloperidol and clozapine. Although these doses are known to disrupt maternal behavior [1], other doses may alter separation-induced pup USV and its maternal potentiation differently. Second, the number of litters tested in the haloperidol experiment was small. A large sample is needed to confirm the effects of haloperidol. Third, we did not directly observe how mother rats under haloperidol and clozapine responded to their pups differently from vehicle-treated rats during the 4.5 minutes of reunion. Thus, the behavioral mechanisms underlying the clozapine effect on maternal potentiation should be considered speculative. In addition, pups were repeatedly tested six times over the five-day period. How this testing schedule altered maternal behavior and its impact on pup USVs is unclear. Finally, since we did not measure plasma drug levels in pups, it was impossible to determine whether clozapine passed into pups and whether it might have altered pup USV through this direct effect. Future work needs to incorporate behavioral observation and neurochemical measurements to gain a better understanding of how antipsychotic treatment to mother rats affects maternal potentiation of pup USV.
Maternal care has a pronounced impact on the cognitive, emotional, behavioral, and social development of offspring and their vulnerability to physical and mental illnesses [20, 21]. The present study is not only important for understanding the impact of antipsychotic treatment on behaviors of mother rats, but is also useful in providing a novel paradigm for examining the impact of antipsychotic treatment on the behavioral and emotional development of the young and their quality of social bonding with mothers. This paradigm may also be valuable for examining the effects of other psychoactive drugs on the developmental trajectory of the young and allows us to investigate the neurobiological processes underlying the mother-infant interactions.
Acknowledgments
This research was supported by a grant from the National Institute of Mental Health (5R03MH080822-02) to Dr. Ming Li. We would like to thank the Undergraduate Creative Activities and Research Experience program for funding support for Katherine Heupel.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Li M, Davidson P, Budin R, Kapur S, Fleming AS. Effects of typical and atypical antipsychotic drugs on maternal behavior in postpartum female rats. Schizophr Res. 2004;70:69–80. doi: 10.1016/j.schres.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Li M, Budin R, Fleming AS, Kapur S. Effects of chronic typical and atypical antipsychotic drug treatment on maternal behavior in rats. Schizophr Res. 2005;75:325–36. doi: 10.1016/j.schres.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 3.Li M, Budin R, Fleming AS, Kapur S. Effects of novel antipsychotics, amisulpiride and aripiprazole, on maternal behavior in rats. Psychopharmacology (Berl) 2005;181:600–10. doi: 10.1007/s00213-005-0091-7. [DOI] [PubMed] [Google Scholar]
- 4.Shair HN. Acquisition and expression of a socially mediated separation response. Behav Brain Res. 2007;182:180–92. doi: 10.1016/j.bbr.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hofer MA, Shair H. Ultrasonic vocalization during social interaction and isolation in 2-weeek-old rats. Dev Psychobiol. 1978;11:495–504. doi: 10.1002/dev.420110513. [DOI] [PubMed] [Google Scholar]
- 6.Hofer MA, Shair H. Sensory processes in the control of isolation-induced ultrasonic vocalization by 2-week-old rats. J Comp Physiol Psychol. 1980;94:271–9. doi: 10.1037/h0077665. [DOI] [PubMed] [Google Scholar]
- 7.Zimmerberg B, Brunelli SA, Hofer MA. Reduction of rat pup ultrasonic vocalizations by the neuroactive steroid allopregnanolone. Pharmacol Biochem Behav. 1994;47:735–8. doi: 10.1016/0091-3057(94)90181-3. [DOI] [PubMed] [Google Scholar]
- 8.Gardner CR. Distress vocalization in rat pups. A simple screening method for anxiolytic drugs. J Pharmacol Methods. 1985;14:181–7. doi: 10.1016/0160-5402(85)90031-2. [DOI] [PubMed] [Google Scholar]
- 9.Hofer MA, Masmela JR, Brunelli SA, Shair HN. Behavioral mechanisms for active maternal potentiation of isolation calling in rat pups. Behav Neurosci. 1999;113:51–61. doi: 10.1037//0735-7044.113.1.51. [DOI] [PubMed] [Google Scholar]
- 10.Zhao C, Li M. C-Fos identification of neuroanatomical sites associated with haloperidol and clozapine disruption of maternal behavior in the rat. Neuroscience. 2010;166:1043–55. doi: 10.1016/j.neuroscience.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao C, Li M. The receptor mechanisms underlying the disruptive effects of haloperidol and clozapine on rat maternal behavior: A double dissociation between dopamine D(2) and 5-HT(2A/2C) receptors. Pharmacol Biochem Behav. 2009 doi: 10.1016/j.pbb.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao C, Li M. Sedation and disruption of maternal motivation underlie the disruptive effects of antipsychotic treatment on rat maternal behavior. Pharmacol Biochem Behav. 2009;92:147–56. doi: 10.1016/j.pbb.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meltzer HY, Li Z, Kaneda Y, Ichikawa J. Serotonin receptors: their key role in drugs to treat schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1159–72. doi: 10.1016/j.pnpbp.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Fleischhacker WW, Meise U, Günther V, Kurz M. Compliance with antipsychotic drug treatment: influence of side effects. Acta Psychiatr Scand Suppl. 1994;382:11–5. [PubMed] [Google Scholar]
- 15.Portfors CV. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J Am Assoc Lab Anim Sci. 2007;46:28–34. [PubMed] [Google Scholar]
- 16.Vivian JA, Miczek KA. Diazepam and gepirone selectively attenuate either 20–32 or 32–64 kHz ultrasonic vocalizations during aggressive encounters. Psychopharmacology (Berl) 1993;112:66–73. doi: 10.1007/BF02247364. [DOI] [PubMed] [Google Scholar]
- 17.Wöhr M, Houx B, Schwarting RK, Spruijt B. Effects of experience and context on 50-kHz vocalizations in rats. Physiol Behav. 2008;93:766–76. doi: 10.1016/j.physbeh.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 18.Burgdorf J, Kroes RA, Moskal JR, Pfaus JG, Brudzynski SM, Panksepp J. Ultrasonic vocalizations of rats (Rattus norvegicus) during mating, play, and aggression: Behavioral concomitants, relationship to reward, and self-administration of playback. J Comp Psychol. 2008;122:357–67. doi: 10.1037/a0012889. [DOI] [PubMed] [Google Scholar]
- 19.Brudzynski SM, Kehoe P, Callahan M. Sonographic structure of isolation-induced ultrasonic calls of rat pups. Dev Psychobiol. 1999;34:195–204. doi: 10.1002/(sici)1098-2302(199904)34:3<195::aid-dev4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 20.Bifulco A, Brown GW, Adler Z. Early sexual abuse and clinical depression in adult life. Br J Psychiatry. 1991;159:115–22. doi: 10.1192/bjp.159.1.115. [DOI] [PubMed] [Google Scholar]
- 21.Mackinnon A, Henderson AS, Andrews G. Parental ‘affectionless control’ as an antecedent to adult depression: a risk factor refined. Psychol Med. 1993;23:135–41. doi: 10.1017/s0033291700038927. [DOI] [PubMed] [Google Scholar]