Abstract
Posttranscriptional regulation is a critical control point for the expression of genes that promote or retard tumor growth. We previously found that the mRNA binding protein, ELAV 1 (HuR), is upregulated in primary brain tumors and stabilizes growth factor mRNAs such as VEGF and IL-8. To better understand the role of HuR in brain tumor growth, we altered levels of HuR in glioma cells by shRNA or ectopic expression and measured tumor cell phenotype using in vitro and in vivo models. In HuR-silenced cells, we found a significant decrease in anchorage-independent growth and cell proliferation with a concomitant induction of apoptosis. Using an intracranial tumor model with primary glioblastoma cells, HuR silencing produced a significant decrease in tumor volume. In contrast, overexpression of HuR produced in vitro chemoresistance to standard glioma therapies. Since bcl-2 is abundantly expressed in glioma and associated with tumor growth and survival, we determined the impact of HuR on its regulation as a molecular validation to the cellular and animal studies. Using UV crosslinking and RNA immunoprecipitation, we show that HuR bound to the 3' untranslated region of all bcl-2 family members. Silencing of HuR led to transcript destabilization and reduced protein expression. Polysome profiling indicated loss of HuR from the translational apparatus. In summary, these findings reveal a HuR-dependent mechanism for cancer cell survival and sensitivity to chemotherapeutic drugs suggesting that HuR should be considered as a new therapeutic target.
Introduction
Post-transcriptional regulation of RNA by RNA-binding proteins (RBP) and miRNA involves interactions with untranslated regions (UTR) of the mRNA, particularly the 3' UTR (1–3). This level of gene regulation is essential for normal development but also is active in disease conditions such as cancer. The impact of RBPs and miRNAs on mRNA range from effects on stability, subcellular location, and/or efficiency of translation (4). Hu antigen R (HuR), is a member of the ELAV family that binds to adenine- and uridine-rich elements (ARE) located in the 3' UTR (5). We have previously characterized expression patterns in cancers of the nervous system and identified several functional classes of targets important in glioma progression (6, 7). An expanding list of mRNAs regulated by HuR has been identified and include regulators of numerous cellular processes including inflammation (8, 9), cell cycle (10), angiogenesis (11), survival (12, 13), and apoptosis (14). Expression of HuR has also been characterized in cancers of the breast (15), ovaries (16), colon (17), and pancreas (18) and may be a biomarker for disease activity (19–24). In vivo studies indicate that elav1 knockout is embryonic lethal (25) and transgenic overexpression in specific tissue compartments alters the expression of ARE containing transcripts (26).
Glioblastoma (GBM) remains one of the most aggressive cancers with tremendous morbidity and mortality. The limited effectiveness of traditional cytotoxic therapies is most likely multifactorial; however, GBM is characterized by marked overexpression of the anti-apoptotic bcl-2 family, which is associated with poor prognosis (27) and treatment resistance (28). In this work, we show that the conditioned expression of HuR with either inducible or silencing constructs alters cellular growth and sensitivity to apoptosis. Molecular analysis reveals that HuR binds to members of the bcl-2 family and promotes mRNA stability and protein expression. In addition, we show for the first time a significant impact of HuR on glioma tumor growth using an in vivo animal model. These observations support the viability of HuR as a novel molecular target in cancer.
Materials and Methods
Cell Culture and Expression of HuR
The U251 Tet-on cells were a gift from Dr. Erwin Van Meir. For stable transfections, pTRE2 plasmids were transfected into U251 Tet-On cells and the clones were selected with hygromycin. The maintenance, propagation, and transfection of U251 Tet-On cells are described elsewhere (7). Clones were selected with blasticidin and verified for transgene expression by Western blot using an anti-Flag antibody. The primary glioblastoma lines utilized for the in vivo experiments included the D456 glioma xenograft, a gift of Darell D. Bigner (Duke University) and human lines GBM2, GBM10, and GBM12 were provided by David James and Jann Sarkaria (Mayo Clinic).
RNA Interference
We used the SureSilencing shRNA plasmid (SABiosciences, Frederic, MD) for human ELAVL1 (UniGene# Hs.184492) with the insert sequence 5'-TGCCGTCACCAATGTGAAAGT-3' to generate stable clones. A random sequence 5'-GGAATCTCATTCGATGCATAC-3' was used for the control clones. Transfection of U251 was performed with Mirus transfection reagent (Mirus Bio LLC, Madison, WI) followed by selection with neomycin.
RNA Analysis
Clones were treated with actinomycin D, and RNA was collected at different intervals over a 6-hour time period. Levels of mRNA were determined with qRT-PCR at each interval and expressed as a percentage of target mRNA present at time 0. Total RNA was extracted, purified, and quantified as previously described (7).
Western Blotting, Immunoprecipitation, and RNA Immunopreciptiation
Cytosolic or whole cell lysates from cultured cells were prepared in the presence of protease inhibitors and sodium orthovanadate using the M-PER kit (Pierce Endogen). One hundred micrograms of cell extract were incubated with 1 μl of antibody overnight at 4 °C. The following antibodies were used HuR (Santa Cruz Biotechnology) at 1:1000, anti-HuC/HuD monoclonal 16A11 (Invitrogen, Eugene, OR), and FLAG (Sigma) 1:1000 and equivalent amounts of control IgG (mouse IgG (Santa Cruz Biotechnology)). One-fifth of the supernatant served as a loading control. Protein G beads were added, and the antibody-antigen complex was then precipitated, washed, eluted in 1× Laemmli sample buffer, and subjected to SDS-PAGE electrophoresis followed by Western blot analysis. After IP, RNA was eluted from protein G beads using the RNeasy kit and analyzed by qRT-PCR mRNA. Standard real-time PCR amplification curves were generated (r2 > 0.98) for bcl-2, mcl-1, and bcl-xL mRNA and S9 or GAPDH controls using the threshold cycle (Ct) method. GAPDH primers and probe were obtained from Assays on Demand (Applied Biosystems). All qRT-PCR analyses were performed on an ABI 7900 PCR instrument (Applied Biosystems). Western blot band density was determined with NIH Image J.
UV Crosslinking
Nuclear extracts were prepared from U251 MG cells using the Nu-Per kit (Pierce Endogen, Rockford, IL) and the protein concentration was determined with the BCA protein assay kit. The UV crosslinking was performed as previously described (7). The samples were electrophoresed on a 4–15% tris gradient gel (BioRad, Hercules, CA), dried and exposed on a phosphorimager. For immunoprecipitation, anti-HuR IgG or control IgG was added to the UV-crosslinked sample in immunoprecipitation buffer as previously described (7).
Polyribosome Isolation
Cells were grown to 80–90% confluency and treated for 15 mins at 37°C and 5% CO2 with 100 mg/ml of cyclohexamide in complete media (1× DMEM/F12K 50/50, 7% fetal bovine serum). Cells were washed with PBS with cycloheximide (100mg/ml) and trypsinized with 0.25%Trypsin/2.21mM EDTA and washed with ice-cold 1× PBS containing cycloheximide (100mg/ml) and pelleted. Protein collection, fractionation by sucrose gradient, RNA isolation and analysis are as previously described (29).
Proliferation and Soft agar assays
To measure cell proliferation, 5000 cells were plated in 96-well plates and viable cell numbers were quantified using the ViaLIght kit (Lonza, Rockland, ME). Colony formation was performed in SeaPlaque agarose (0.9%) with 8% fetal calf serum in 6-well plates. A cell density of 500 per well was used. After 4-weeks, 250 uL of p-iodonitroterazolium violet solution was added per well and incubated overnight. The following day the plate was photographed and colony number quantified with NIH Image J software.
Chemosensitivity Assays
The chemosensitivity assays were performed by plating cells into 96-well plates. Prior to plating, cells were incubated for 24 hours in media containing doxycycline at 0.5 ug/mL for transgene induction. Chemotherapeutic agents were added to fresh culture media 24 hours after plating into 96-well plates. Cell viability was determined after 48 hours with the ViaLight Cytotoxicity assay. Three independent assays were performed each in triplicate. For each construct, two independent clones were evaluated and averaged for each experiment. Data were analyzed with Prism software and fit to a nonlinear regression equation to describe the relationship between the log of the chemotherapy concentration and the response (viable tumor cells): Y=100/(1+10^((LogIC50−X)*HillSlope)).
Generation of shHuR lentivirus
To generate the pLVTHM-shHUR vector shHuR primers were annealed and cloned into the Mlu1 and Cla1 sites of the pLVTHM vector. The control pLVTHM plasmid was obtained from Addgene, plasmid # 12247. Viral particles were packaged by as previously described (29). A total of 106 U251 cells were infected with 2 ml of viral supernatant in the final volume of 4 mL. At 48 h postinfection, cells were washed with dMEM-F12 media. Protein extracts and RNA from control and shHuR cells were obtained and analyzed after 4 weeks of cells culture expansion.
In vivo animal studies
All animal studies were carried out in accordance with the policies set by the UAB IACUC. Each mouse was placed in a stereotaxic head holder with coordinates set to target the subcortical right frontal region. A linear incision (~5mm) was made in the scalp. A dental drill with a 0.45 mm non-cutting bit was used to make a small burr hole and expose the dura. Using a 250 μl Hamilton syringe fitted with a 30-gauge needle, 0.5×10^6 or 10^6 GBM cells were injected. The burr hole was then filled with bone wax and the incision closed with glue..
Tumor analysis
Following euthanasia, the animals were decapitated. The brains were fixed in 10% zinc formalin overnight and stored. Coronal 30–50 μm brain slices (from front to back) were prepared using a Microm HM 355S (Alabama Neuroscience Blueprint Core C) and stored in antifreeze solution. The fluorescence spectrum from slices was obtained and analyzed on a model N-msi-500-FL multispectral imaging system (Leica Microsystems, Welzlar GmbH, type 307-072.55, Germany), GFP filter, and Nuance software (Cambrige Research Instrumentation, MA) in the UAB Small Animals Imaging Facility. To quantify tumor size in each brain section, a background coronal image was subtracted from a fluorescent image leaving the tumor whose area was calculated by using the NIH Image J program. Tumor size was determined on every sixth 40 μm slice for each brain. Nineteen slices (from olfactory bulb through cerebellum) were analyzed for each animal, and tumor size was estimated by totaling the tumor area for all sections.
Statistics
A paired Student's t test was applied for statistical analysis using GraphPad Prism v.4 (San Diego, CA).
Results
HuR is required for anchorage-independent growth, survival, and chemoresistance
We investigated the role of HuR in promoting the transformed phenotype of glioma cells. Cells from shControl or shHuR were plated and assessed for proliferation over four days. We observed a 50% reduction in cell number, as determined by ATP bioluminescence, in shHuR cells relative to control (Fig. 1A). This difference was significant (p=0.03). We next assessed colony formation in soft agar. Colonies formed by shHuR clones (n=2) were strikingly sparse and small compared to shControl (n=2) (Fig. 1C, left panel, lower versus upper row). Colony counts from three independent experiments showed a significant 10-fold reduction of colonies in shHuR clones compared to control (p =0.0016). Together, these data support a role for HuR in proliferation and growth of malignant glioma.
Figure 1. Silencing of HuR alters glioma growth and sensitizes cells to chemotherapeutic induction of apoptosis.
(A.) The growth of U251 is constrained compared to controls over a four day time course. (B.) HuR silencing in U251 induces activation of the intrinsic pathway of apoptosis with cleavage of caspase 3 and PARP. The time course is after the addition of the siRNA oligos. (C.) Colony formation in soft agar is markedly reduced for HuR knockdown compared to control. Quantitation of colony number in soft agar experiment is represented by the bar graph; *(p=0.0016). (D.) The graph depicts a sensitization of glioma cell line to cytotoxic agents with HuR knockdown for common types of chemotherapy.
To determine if growth inhibitory effects of HuR knockdown were due, in part to induction of apoptosis or cell cycle arrest, we examined regulators of apoptosis (Fig. 1 B). Knockdown resulted in a time-dependent appearance of cleaved caspase-3 suggesting that HuR silencing induced cell death through a caspase-3 dependent process. The presence of cleaved PARP further supports activation of the caspase cascade. Analysis of cell cycle distributions in control and HuR silenced clones did not reveal a difference (data not shown). These results suggest that apoptotic cell death, induced by loss of HuR, contributes to growth inhibition. The effect of standard chemotherapy on glioma viability was analyzed in the setting of HuR knockdown. Inhibitory Concentration 50 (IC50) curves were generated for shHuR, shControl and wild type U251 cells (Fig. 1 D). Compared to control cells, there was a 2–3 fold reduction of IC50 values for all the three agents. Wild-type U251 had similar IC50 values as shControls (data not shown).
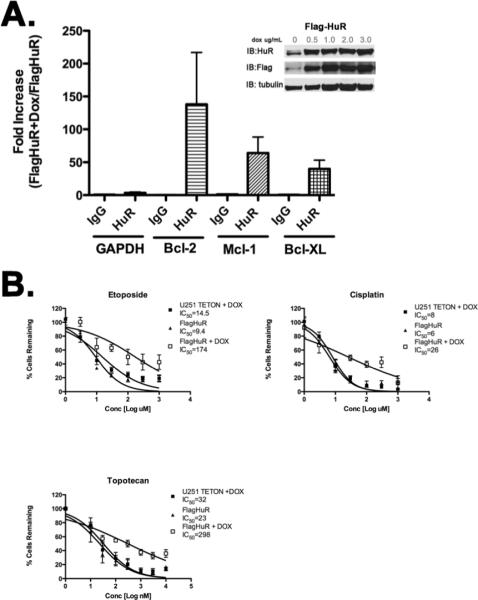
The ability of HuR to influence glioma chemoresistance was evaluated by examining HuR overexpression. We used U251 clones that over express Flag-tagged HuR following doxycycline induction (7). To determine whether ectopically expressed Flag-HuR increased overall binding to bcl-2 mRNA, we performed RNA immunoprecipitation (RIP) in Flag-HuR cells following doxycycline induction (Figure 2A). We quantified the amount of bcl-2 mRNA precipitated and expressed it as fold-increase over non-induced cells. With HuR induction, we found a significant increase in bcl-2 mRNA recovered by IP in the induced clones (140-fold for bcl-2, 64-fold for mcl-1, 40-fold for bcl-XL), but not GAPDH. The RIP assay also confirms the functionality of the induced Flag-tagged HuR. We treated the cells with etoposide, cisplatin, and topotecan (Fig. 2B). In the bottom panel, the IC50 curves are shifted to the right following induction of Flag-HuR with doxcycyline, indicating a resistance to chemotherapy cytotoxicity. Controls included the parent clone, U251 Teton, and uninduced Flag-HuR clones. The increase in IC50 values of ½ to 1 log difference is biologically and clinically significance. In summary, the cellular experiments indicate an important role forHuR in glioma proliferation and chemotherapy resistance.
Figure 2. Inducible overexpression of HuR enhances Bcl-2 expression and chemoresistance.
(A.) The Flag-HuR inducible clones (n=2) demonstrate a specific and marked increase in bcl-2 family interaction with HuR following induction (dox treated is normalized by the untreated) and immunoprecipitation with a control IgG or HuR antibody (Experiment done in triplicate). The error bars represent standard deviation. An inset reveals robust expression of the Flag tagged HuR with dox. (B.) The IC50 curves with standard cytotoxic agents in a cell viability assay demonstrate chemoresistance after the induction of HuR, a shift to the right is seen for all agents.
HuR Binds to the 3'UTR of the Bcl-2 Family and Regulates Expression
The bcl-2 family of anti-apoptotic genes have large AREs in their 3' UTRs that could serve as targets for HuR binding. An interaction between the bcl-2 family RNA and HuR would implicate the process of post-transcriptional regulation in the promotion of glioma growth and resistance to cytotoxic agents. To determine whether HuR interacts with bcl-2 3'UTR in glioma cells, we performed UV crosslinking of U251 glioma extract with radiolabeled 3' UTR probes from all three members of the bcl-2 family (Figure 3 and Supplemental Figure 1). In Fig. 3A, the pattern of the UV crosslinking is seen in lane 1 for each riboprobe. The multiple bands are similar across the various 3'UTR riboprobes suggesting interactions with similar groups of proteins. To determine a specific interaction with HuR, we performed an immunoprecipitation (IP) of the crosslinked extract with an anti-HuR antibody. We detected radiolabeled bands representative of HuR for all three bcl-2 family members (Fig. 3A, lanes 2). No bands were detected with IgG control (lanes 3). Using the mcl-1 3'UTR, we mapped the binding to areas generally rich in AREs. The right panel of Fig. 3A is a UV crosslinking experiment with four fragments of the mcl-1 3'UTR. Following IP with HuR antibodies, the most intense bands were detected in fragments 2 and 3, which have the highest AU content. There was no interaction with the 3'UTR riboprobe mcl-1 #1, which has the lowest AU content. A faint band may be present for mcl-1 #4, the distal most fragment of the 3'UTR.
Figure 3. A specific molecular interaction exist between HuR and the bcl-2 family RNA.

(A.) - Lane 1 shows the UVX pattern of glioma protein extract and 32P labeled riboprobes for the indicated bcl-2 member 3'UTR, lane 2 is an IP of the UVX with a HuR Ab to define the HuR band, and lane 3 is a control IP with normal mouse IgG. In panel A right, UVX using 4 riboprobes representing various regions of the mcl-1 3'UTR (Supplement Table 1 for definition of regions). The UVX lanes are overexposed to better visualize the HuR IP in lane 2. (B.) - qRT-PCR for the bcl-2 family from total RNA isolated by HuR IP confirms an interaction. The bcl-2 family can be quantified from HuR IP compared to the control IP with IgG. (C.) – A schematic illustrating the location of riboprobes (P) relative to bcl-2 family 3' UTR and the open reading frame (ORF).
To quantify the interaction between HuR and the bcl-2 family, we immunoprecipitated HuR from U251 extracts, purified the bound mRNA, and quantified the mRNA targets. Bound target mRNA was expressed as a percentage of total target mRNA for the specific target in the supernatant (Fig. 3B). An isotype specific mouse IgG was included as a control. The bar graph illustrates increased amounts of bound bcl-2 family members with the HuR antibody compared to control IgG. GAPDH does not have an ARE and showed no binding difference between HuR and control IgG. Taken together, these results support a direct interaction between HuR and the 3' UTRs of the bcl-2 family.
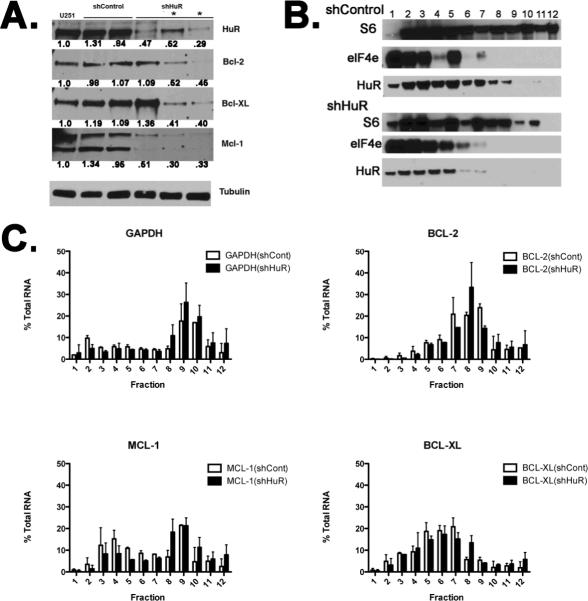
The impact of HuR binding to bcl-2 mRNA was determined by HuR knockdown. We generated two stable U251 clones expressing short-hairpin (sh) RNA directed to HuR (shHuR). A scrambled sequence was utilized to generate control (shControl) clones. Effective and specific knockdown of HuR was observed at the mRNA and protein levels (Supplemental Fig. 1). The shHuR clones showed an 80% reduction in HuR mRNA compared to shControl clones. Western blot confirmed a specific reduction in HuR protein without affecting the highly homologus ELAV family member, HuC.
The functional consequence of the HuR interaction with the bcl-2 families' 3'UTR was examined by determining the mRNA half-life (t1/2). The t1/2 of all members was reduced in shHuR cells by more than half that of control shRNA (from 4–6 h to less than 2 h, Fig. 4B). The data represent an average of two independent clones for shControl and shHuR. Although there was a significant reduction in mRNA half-life for each member, the overall mRNA levels were not significantly reduced in shHuR cells (Fig. 4A). The change in RNA degradation had a significant impact on protein expression of bcl-2, mcl-1, and bcl-XL. Western blot analysis of protein extracts from shControl and shHuR clones is shown in figure 5A. After immunoblotting for bcl-2, mcl-1 or bcl-XL, markedly diminished bands are present in the shHuR clones but normal levels are present in the shControls when compared to wild-type U251. We identified two independent HuR silenced clones with significant reductions in all three members of the bcl-2 family (noted with *) and were the ones used for phenotypic studies. Since HuR plays an integral role in mRNA localization to the polysome, we next examined the distribution of RNA in the translational machinery. We isolated polysome fractions by sucrose gradient and then measured mRNA targets in those fractions by qRT-PCR. The polyribosome profiles were assessed by UV spectrophotometry with fractions 1–2 represent nonribosomal components, fractions 3–6 the ribosomal components (subunits 18 S and 28 S), and fractions 7–12 polyribosomes (Fig. 5B). We analyzed the fractions by Western blot and found a decrease in HuR from shHuR clones, particularly in the polyribosome fractions. The distribution of ribosomal protein S6 and elF4E is provided as controls for the integrity of fractionation. The distribution profiles differ slightly for the bcl-2 family members with a general shift toward more RNA within the polyribosome fractions (7–12) in the clones with HuR silenced compared to the control clones (Fig. 5C). The pattern was similar to GAPDH RNA profile, a species which lacks an AU rich 3'UTR. These data support a functional role of HuR in the post-transcriptional regulation of the bcl-2 family, with the impact primarily on the mRNA destabilization and serve as a molecular validation of a HuR silencing effect.
Figure 4. The knockdown of HuR primarily effects the degradation kinetics of the bcl-2 family.
(A) A significant difference was not noted in RNA expression levels for the bcl-2 family following knockdown with shHuR or shControl. (Replicates of 3 for 2 shHuR clones and 6 for single shControl clone). (B) The degradation kinetics of the bcl-2 family was markedly reduced after silencing for HuR. (Replicates of 3)
Figure 5. Impact of HuR knockdown on bcl-2 family RNA distribution within the polyribosome and protein expression.
(A.) The silencing of HuR in multiple clones with the concurrent reduction in protein expression of all bcl-2 family members in select clones (*) which were used for phenotypic studies. Band density was normalized to tubulin and then compared to U251 for to generate percent expression (shown below the blot). (B.) Western blot analysis from protein samples of 12 fractions collected following polyribosome isolation. S6 and elf4E are ribosomal components and used for control. HuR is reduced in the polysome fractions of shHuR cells compared to controls. (C.) The quantitation of RNA from each fraction for GAPDH and the bcl-2 family. Overall, the patterns differ depending on the target with a slight increase in distribution to later fractions (8–12) following HuR knockdown. The data is presented so that each bar represents the percent RNA from that fraction divided by the total for all 12 fractions.
In vivo Effect of HuR Silencing on Primary GBM Xenografts
To evaluate the effect of HuR protein knockdown on in vivo tumor growth, we cloned different shHuR sequences (V1–V3) into the lentiviral vector pLVTHM (Fig. 6A). This vector also contains the coding sequence for green fluorescent protein (GFP) to monitor infectivity. Initial characterization of three shHuR lentiviral constructs (V1–3) was performed in U251 cells (Fig 6A) and showed robust HuR knockdown at the RNA and protein levels (Fig. 6B). Control lentivirus (V-GFP) had no effect on HuR expression. In conjunction with this knockdown, we observed a reduction in Bcl-2 protein (Fig. 6B). For in vivo characterization of HuR knockdown, we chose primary human GBM cell lines passaged subcutaneously in mice to maintain a tumor phenotype more reflective of human disease than established glioma cell lines (30, 31). A total of four primary GBM lines (D456, GBM 12, GBM 6, and GBM 10) were infected with V-GFP or V2shHuR lentivirus illustrated in figure 6 C and D. HuR silencing in primary GBM lines infected with V2shHuR was 70–80 % compared to V-GFP control. Figure 6 E documents a western blot for the primary GBM line, D456 with knockdown of HuR with V2shHuR.
Figure 6. Lentivirus mediated HuR silencing in established and primary GBM lines.
(A.) Fluorescent microscopy showing robust infectivity of control (V-GFP) and three shHuR GFP-lentiviral constructs (V1–3-shHuR) in U251. (B.) The lentivirus constructs robustly knockdown HuR. Panel B quantifies HuR RNA levels by TAQMAN and a following Western blot shows effective knockdown of HuR with each of the shHuR lentiviruses (V1–3) but not with control (V-GFP). The most consistent and robust was V2, which was carried forward in animal experiments. (C.) The four primary GBM lines available are shown in phase contrast (top) and after transduction with the GFP lentivirus (bottom). (D.) All lines were uniformly infected with 100% cellular transduction by 20 viral units per cell. (E.) Western blotting of protein extracts from D456 confirms knockdown of HuR in V2shHuR cells compared to shControl. Similar results were seen for the other primary GBM lines.
Infected primary glioma cells were injected intracranially into the right frontal subcortex of mice. The in vivo experiments were completed on 3 sets of animals using the primary GBM lines D456, GBM6, and GBM12. Each set of 10 mice received a different primary GBM line with 5 animals receiving cells infected with the V2-shHuR construct and 5 animals the control (V-GFP). Mice were sacrificed 3–4 weeks after tumor cell injection and whole brains were imaged for GFP fluorescence. Figs. 7 and 8 represent results from the D456 primary GBM line. We observed a marked increase in tumor initiation and dissemination in sacrifice shows tumor initiation and dissemination in the control V-GFP control animals compared to those with HuR knockdown (V2-shHuR) suggesting a growth disadvantage for HuR silenced tumors (Fig. 7A). There was significant attenuation of tumor growth and invasiveness in the shHuR tumors compared to control. Tumors were analyzed in detail on 40 μm thick coronal brain sections (rostral to caudal) using a stereo fluorescence microscope for GFP detection (Fig. 7B). The control and shHuR mice groups for D456 and GBM12 had similar patterns of tumor distribution within the area of injection; however, control animals had tumor cell migration from right to the left hemispheres, dissemination into and through the lateral ventricles, the striatum, hippocampus, and the third ventricle. GBM 6 produced multifocal disease in control mice (defined as tumor populations completely separated from the primary injection site and not in continuity with other areas of tumor). There was a greater tumor size in V-GFP control mice (shControl) compared to V2-shHuR mice for all 3 lines (D456, GBM6, and GBM12). In figure 7 C, we provide a table of the data as the absolute total tumor area in shControl and shHuR. There was a difference in tumor size development across the 3 cell lines with consistent trends in control animals (wild-type HuR) having larger tumors than HuR silenced animals (shHuR). This variation may reflect difference in behavior of the primary GBM lines with some growing faster than others. The time from implantation to animal sacrifice was the same for all at 3 weeks. The difference was statistically significant for D456 and GBM6 and approached for GBM12. GBM12 had a greater SEM (standard error of the mean) suggesting greater variability in tumor behavior in the model. This could be technical or biologically driven by this particular primary line. The distance of saggital (rostral/caudal) migration of tumor cells was greater for control group compared to shHuR group. Tumor cells were detected from olfactory bulb to cerebellum in 4 of 5 control mice compared to only one of five mice in the shHuR group for both D456 and GBM12. GBM6 had a very different phenotype compared to the others. It appeared to disseminate more through the brain with numerous tumor foci. The difference in the number of discrete tumor foci was statistically significant with few following HuR silencing compared to the control (shControl). The number of tumor foci were counted and reported in figure 7 C. For this primary line, the effect of HuR silencing appears to impact both growth and migration. Overall, the primary GBM xenografts displayed more variability than would be seen with established lines but were clearly rendered less proliferative and invasive following HuR silencing.
Figure 7. The GFP marker in the lentivirus facilitates in vivo assessment of GBM growth.
(A.) Whole brain dissection after sacrfice showing extensive and bilateral green fluorescence in the shControl mouse versus limited signal in the shHuR mouse. (B.) A background coronal section is shown at the top followed by a GFP image and then a subtraction. The subtraction allows for quantification of tumor volume and extent of infiltration and confirms the extensive growth and infiltration of the shControl-infected cells. (C.) A table present data for the 3 sets of animal experiments with each primary GBM line. When tumor size is the primary endpoint, a consistent size difference is seen following silencing of HuR (shHuR). GBM6 demonstrated a very migratory behavior and a difference in tumor foci was quantified as a second primary endpoint.
Figure 8. HuR knockdown reduces GBM tumor growth in-vivo.
(A) Coronal sections of mouse brains injected with shControl or V2-shHuR- infected D456 GBM cells are shown. V2shHuR cells produced a smaller and less infiltrative tumor compared to shControl (arrows) (B) Representative tumor sections immunostained for HuR (red) and counterstained with DAPI (blue) showing reduced expression of HuR in the V2shHuR-infected GBM cells.
We next analyzed immunohistochemical features of the tumors. shControl tumors were more invasive in appearance compared to shHuR animals (Fig. 8A). Staining of tumor sections confirmed a reduction in HuR in the shHuR animals by both immunohistochemistry and immunofluorescence (Fig. 8B). The impact of HuR silencing on bcl-2 protein extended to the in vivo setting with a reduction in bcl-2 immunofluorescence in shHuR animals versus the shControls as shown in supplemental Fig. 2. Thus, our data suggest that HuR knockdown significantly decreases tumor proliferation and migration in an orthotopic in vivo mouse model with primary GBM xenograft lines.
DISCUSSION
Aberrant regulation of gene expression in cancer is typically attributed to transcriptional control. The molecular events occurring after production of the mature mRNA molecule (post-transcriptional regulation), however,, have a substantial impact on genes that fuel tumor growth (3). Our work illustrates this impact for the first time using an animal model of primary malignant glioma. We also expand the mRNA targets for HuR in cancer to include the bcl-2 family.
Expression of HuR in cancer was first described by our group in primary brain tumors but has been extensively expanded by others to include multiple tumor types (6). In normal growth and development, HuR is primarily located in the nucleus; however, in malignant tumors a cytoplasmic shift has been reported that correlates with a worse prognosis(20). Alterations in expression and subcellular localization of HuR suggest a role for post-transcriptional regulation in multiple cancer-associated pathways such as cell cycle, inflammation, invasion and metastasis, angiogenesis, survival, and therapeutic resistance. The number of potential HuR-regulated mRNAs active in these disease pathways is potentially large as up to 8% of the transcribed genome may contain an AU-rich 3'UTR (32). Here, we have focused on the bcl-2 family as a molecular target of HuR. While other mRNA targets may be affected by HuR and contribute to the tumor phenotype, the bcl-2 family is implicated as an important control point in glioblastoma survival and treatment resistance, and it serves as a molecular validation for our tumor models. Utilizing established glioma cell lines and primary GBM lines, we have extended previous reports and identified the entire bcl-2 family as a candidate for regulation by HuR (14). All family members (bcl-2, mcl-1, and bcl-xl) contain a 3' UTR rich in AREs which serve as binding targets for HuR. This interaction promotes expression of the protein product by reducing RNA degradation. In addition, we observed a loss of HuR in the polysomes following silencing with substantial decrease in bcl-2 family protein but without a change in bcl-2 family member RNA levels in the polysomes suggesting HuR may stimulate translation of this family as described by other for XIAP (X chromosome-linked inhibitor of apoptosis) (33).
Our data support a role for HuR in cell survival more than proliferation. Thus, we saw significant induction of apoptosis and loss of anchorage-dependent growth with HuR knockdown, but no impact on cell cycle distribution. Likewise, overexpression of HuR in glioma cell lines resulted in significantly greater resistance to cytotoxic chemotherapy agents (Fig. 2). These results suggest that inactivation of HuR, as a molecular target in glioma tumors, could provide therapeutic synergy with traditional chemotherapy. With knockdown of HuR, the enhanced cytotoxicity at lower concentrations would ameliorate two clinically relevant limitations for chemotherapy: systemic toxicities and poor penetration into the brain.
The transition of molecular and cellular studies to animal models has been undergoing a rapid evolution in the field of glioma research and other cancers. The traditional intracranial model using established glioma cell lines does not recapitulate the pathological or clinical behavior of human disease. To address this deficiency, we used patient-derived primary GBM xenograft lines. This model demonstrates rapid tumor progression with extensive CNS dissemination and mimics human disease (30, 31). By use of a lentiviral vector, we were able to knockdown HuR in a panel of primary GBM lines prior to implantation. The resultant effect was twofold: (i) a significant diminution of tumor growth and (ii) significantly less invasion. The behavior of the patient-derived tumors did vary, as with human disease, with some more aggressive and highly invasive and others that that were smaller but multi-focal. With all cell lines, however, the tumors were smaller, less invasive, or more unifocal with HuR knockdown. These results support our hypothesis that HuR is important in glioma growth and the viability of this RNA-binding protein as a therapeutic target in malignant glioma.
Supplementary Material
The bar graph quantifies RNA expression of HuR compared to the neural specific family members, HuD and HuC after silencing with HuR specific constructs in the U251 glioma line. The western blot confirms a specific reduction of HuR protein and sparing of the more neural restricted family members. Lanes 1 and 2 are two clones with shHuR constructs and lanes 3 and 4 are transfected with the shControl construct.
The nuclear stain, DAPI is illustrated in panel (A) to provide a reference for the nucleus and cell density. Immunostaining for bcl-2 is provided in panel B. The bright fluorescent areas represent artifact and background from tumor vascular structures. In panel (C), the DAPI and bcl-2 staining is overlaid.
ACKNOWLEDGEMENTS
Supported by National Cancer Institute R01 CA112397 (L.B.N) and VA Merit Review (PHK).
Supported by UAB Small Animal Imaging Shared Facility (P30CA013148).
REFERENCES
- 1.Fan XC, Myer VE, Steitz JA. AU-rich elements target small nuclear RNAs as well as mRNAs for rapid degradation. Genes Dev. 1997 Oct 1;11(19):2557–68. doi: 10.1101/gad.11.19.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pullmann R, Jr., Kim HH, Abdelmohsen K, Lal A, Martindale JL, Yang X, et al. Analysis of turnover and translation regulatory RNA-binding protein expression through binding to cognate mRNAs. Mol Cell Biol. 2007 Sep;27(18):6265–78. doi: 10.1128/MCB.00500-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mazan-Mamczarz K, Hagner PR, Corl S, Srikantan S, Wood WH, Becker KG, et al. Post-transcriptional gene regulation by HuR promotes a more tumorigenic phenotype. Oncogene. 2008 Oct 16;27(47):6151–63. doi: 10.1038/onc.2008.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keene JD. RNA regulons: coordination of post-transcriptional events. Nat Rev Genet. 2007 Jul;8(7):533–43. doi: 10.1038/nrg2111. [DOI] [PubMed] [Google Scholar]
- 5.Brennan CM, Steitz JA. HuR and mRNA stability. Cell Mol Life Sci. 2001 Feb;58(2):266–77. doi: 10.1007/PL00000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nabors LB, Gillespie GY, Harkins L, King PH. HuR, a RNA stability factor, is expressed in malignant brain tumors and binds to adenine- and uridine-rich elements within the 3' untranslated regions of cytokine and angiogenic factor mRNAs. Cancer Res. 2001 Mar 1;61(5):2154–61. [PubMed] [Google Scholar]
- 7.Nabors LB, Suswam E, Huang Y, Yang X, Johnson MJ, King PH. Tumor necrosis factor alpha induces angiogenic factor up-regulation in malignant glioma cells: a role for RNA stabilization and HuR. Cancer Res. 2003 Jul 15;63(14):4181–7. [PubMed] [Google Scholar]
- 8.Casolaro V, Fang X, Tancowny B, Fan J, Wu F, Srikantan S, et al. Posttranscriptional regulation of IL-13 in T cells: role of the RNA-binding protein HuR. J Allergy Clin Immunol. 2008 Apr;121(4):853–9. e4. doi: 10.1016/j.jaci.2007.12.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dean JL, Wait R, Mahtani KR, Sully G, Clark AR, Saklatvala J. The 3' untranslated region of tumor necrosis factor alpha mRNA is a target of the mRNA-stabilizing factor HuR. Mol Cell Biol. 2001 Feb;21(3):721–30. doi: 10.1128/MCB.21.3.721-730.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang W, Caldwell MC, Lin S, Furneaux H, Gorospe M. HuR regulates cyclin A and cyclin B1 mRNA stability during cell proliferation. Embo J. 2000 May 15;19(10):2340–50. doi: 10.1093/emboj/19.10.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang K, Breen EC, Wagner PD. Hu protein R-mediated posttranscriptional regulation of VEGF expression in rat gastrocnemius muscle. Am J Physiol Heart Circ Physiol. 2002 Oct;283(4):H1497–504. doi: 10.1152/ajpheart.00813.2001. [DOI] [PubMed] [Google Scholar]
- 12.Mazan-Mamczarz K, Galban S, Lopez de Silanes I, Martindale JL, Atasoy U, Keene JD, et al. RNA-binding protein HuR enhances p53 translation in response to ultraviolet light irradiation. Proc Natl Acad Sci U S A. 2003 Jul 8;100(14):8354–9. doi: 10.1073/pnas.1432104100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yi J, Chang N, Liu X, Guo G, Xue L, Tong T, et al. Reduced nuclear export of HuR mRNA by HuR is linked to the loss of HuR in replicative senescence. Nucleic Acids Res. 2009 Dec 8; doi: 10.1093/nar/gkp1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdelmohsen K, Lal A, Kim HH, Gorospe M. Posttranscriptional orchestration of an anti-apoptotic program by HuR. Cell Cycle. 2007 Jun 1;6(11):1288–92. doi: 10.4161/cc.6.11.4299. [DOI] [PubMed] [Google Scholar]
- 15.Heinonen M, Fagerholm R, Aaltonen K, Kilpivaara O, Aittomaki K, Blomqvist C, et al. Prognostic role of HuR in hereditary breast cancer. Clin Cancer Res. 2007 Dec 1;13(23):6959–63. doi: 10.1158/1078-0432.CCR-07-1432. [DOI] [PubMed] [Google Scholar]
- 16.Erkinheimo TL, Lassus H, Sivula A, Sengupta S, Furneaux H, Hla T, et al. Cytoplasmic HuR expression correlates with poor outcome and with cyclooxygenase 2 expression in serous ovarian carcinoma. Cancer Res. 2003 Nov 15;63(22):7591–4. [PubMed] [Google Scholar]
- 17.Denkert C, Koch I, von Keyserlingk N, Noske A, Niesporek S, Dietel M, et al. Expression of the ELAV-like protein HuR in human colon cancer: association with tumor stage and cyclooxygenase-2. Mod Pathol. 2006 Sep;19(9):1261–9. doi: 10.1038/modpathol.3800645. [DOI] [PubMed] [Google Scholar]
- 18.Costantino CL, Witkiewicz AK, Kuwano Y, Cozzitorto JA, Kennedy EP, Dasgupta A, et al. The role of HuR in gemcitabine efficacy in pancreatic cancer: HuR Up-regulates the expression of the gemcitabine metabolizing enzyme deoxycytidine kinase. Cancer Res. 2009 Jun 1;69(11):4567–72. doi: 10.1158/0008-5472.CAN-09-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez de Silanes I, Fan J, Yang X, Zonderman AB, Potapova O, Pizer ES, et al. Role of the RNA-binding protein HuR in colon carcinogenesis. Oncogene. 2003 Oct 16;22(46):7146–54. doi: 10.1038/sj.onc.1206862. [DOI] [PubMed] [Google Scholar]
- 20.Heinonen M, Bono P, Narko K, Chang SH, Lundin J, Joensuu H, et al. Cytoplasmic HuR expression is a prognostic factor in invasive ductal breast carcinoma. Cancer Res. 2005 Mar 15;65(6):2157–61. doi: 10.1158/0008-5472.CAN-04-3765. [DOI] [PubMed] [Google Scholar]
- 21.Cho NP, Han HS, Soh Y, Lee KY, Son HJ. Cytoplasmic HuR over-expression is associated with increased cyclooxygenase-2 expression in laryngeal squamous cell carcinomas. Pathology. 2007 Dec;39(6):545–50. doi: 10.1080/00313020701684391. [DOI] [PubMed] [Google Scholar]
- 22.Lim SJ, Kim HJ, Kim JY, Park K, Lee CM. Expression of HuR is associated with increased cyclooxygenase-2 expression in uterine cervical carcinoma. Int J Gynecol Pathol. 2007 Jul;26(3):229–34. doi: 10.1097/01.pgp.0000236946.82334.07. [DOI] [PubMed] [Google Scholar]
- 23.Ido K, Nakagawa T, Sakuma T, Takeuchi H, Sato K, Kubota T. Expression of vascular endothelial growth factor-A and mRNA stability factor HuR in human astrocytic tumors. Neuropathology. 2008 Dec;28(6):604–11. doi: 10.1111/j.1440-1789.2008.00926.x. [DOI] [PubMed] [Google Scholar]
- 24.Ortega AD, Sala S, Espinosa E, Gonzalez-Baron M, Cuezva JM. HuR and the bioenergetic signature of breast cancer: a low tumor expression of the RNA-binding protein predicts a higher risk of disease recurrence. Carcinogenesis. 2008 Nov;29(11):2053–61. doi: 10.1093/carcin/bgn185. [DOI] [PubMed] [Google Scholar]
- 25.Katsanou V, Milatos S, Yiakouvaki A, Sgantzis N, Kotsoni A, Alexiou M, et al. The RNA-binding protein Elavl1/HuR is essential for placental branching morphogenesis and embryonic development. Mol Cell Biol. 2009 May;29(10):2762–76. doi: 10.1128/MCB.01393-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katsanou V, Papadaki O, Milatos S, Blackshear PJ, Anderson P, Kollias G, et al. HuR as a negative posttranscriptional modulator in inflammation. Mol Cell. 2005 Sep 16;19(6):777–89. doi: 10.1016/j.molcel.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 27.Korshunov A, Golanov A, Sycheva R, Pronin I. Prognostic value of tumour associated antigen immunoreactivity and apoptosis in cerebral glioblastomas: an analysis of 168 cases. J Clin Pathol. 1999 Aug;52(8):574–80. doi: 10.1136/jcp.52.8.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Streffer JR, Rimner A, Rieger J, Naumann U, Rodemann HP, Weller M. BCL-2 family proteins modulate radiosensitivity in human malignant glioma cells. J Neurooncol. 2002 Jan;56(1):43–9. doi: 10.1023/a:1014448721327. [DOI] [PubMed] [Google Scholar]
- 29.Lu L, Wang S, Zheng L, Li X, Suswam EA, Zhang X, et al. Amyotrophic lateral sclerosis-linked mutant SOD1 sequesters Hu antigen R (HuR) and TIA-1-related protein (TIAR): implications for impaired post-transcriptional regulation of vascular endothelial growth factor. J Biol Chem. 2009 Dec 4;284(49):33989–98. doi: 10.1074/jbc.M109.067918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarkaria JN, Carlson BL, Schroeder MA, Grogan P, Brown PD, Giannini C, et al. Use of an orthotopic xenograft model for assessing the effect of epidermal growth factor receptor amplification on glioblastoma radiation response. Clin Cancer Res. 2006 Apr 1;12(7 Pt 1):2264–71. doi: 10.1158/1078-0432.CCR-05-2510. [DOI] [PubMed] [Google Scholar]
- 31.Giannini C, Sarkaria JN, Saito A, Uhm JH, Galanis E, Carlson BL, et al. Patient tumor EGFR and PDGFRA gene amplifications retained in an invasive intracranial xenograft model of glioblastoma multiforme. Neuro Oncol. 2005 Apr;7(2):164–76. doi: 10.1215/S1152851704000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bakheet T, Williams BR, Khabar KS. ARED 3.0: the large and diverse AU-rich transcriptome. Nucleic Acids Res. 2006 Jan 1;34(Database issue):D111–4. doi: 10.1093/nar/gkj052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Durie D, Lewis SM, Liwak U, Kisilewicz M, Gorospe M, Holcik M. RNA-binding protein HuR mediates cytoprotection through stimulation of XIAP translation. Oncogene. Nov 22; doi: 10.1038/onc.2010.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The bar graph quantifies RNA expression of HuR compared to the neural specific family members, HuD and HuC after silencing with HuR specific constructs in the U251 glioma line. The western blot confirms a specific reduction of HuR protein and sparing of the more neural restricted family members. Lanes 1 and 2 are two clones with shHuR constructs and lanes 3 and 4 are transfected with the shControl construct.
The nuclear stain, DAPI is illustrated in panel (A) to provide a reference for the nucleus and cell density. Immunostaining for bcl-2 is provided in panel B. The bright fluorescent areas represent artifact and background from tumor vascular structures. In panel (C), the DAPI and bcl-2 staining is overlaid.