Abstract
Although the role of macromolecular interactions in cell function has attracted considerable attention, important questions about the organization of cells remain. To help clarify this situation, we used a simple protocol that measures macromolecule release after gentle permeabilization for the examination of the status of endogenous macromolecules. Treatment of Chinese hamster ovary cells with saponin under carefully controlled conditions allowed entry of molecules of at least 800 kDa; however, there were minimal effects on internal cellular architecture and protein synthesis remained at levels comparable to those seen with intact cells. Most importantly, total cellular protein and RNA were released from these cells extremely slowly. The release of actin-binding proteins and a variety of individual cytoplasmic proteins mirrored that of total protein, while marker proteins from subcellular compartments were not released. In contrast, glycolytic enzymes leaked rapidly, indicating that cells contain at least two distinct populations of cytoplasmic proteins. Addition of microfilament-disrupting agents led to rapid and extensive release of cytoplasmic macromolecules and a dramatic reduction in protein synthesis. These observations support the conclusion that mammalian cells behave as highly organized, macromolecular assemblies (dependent on the actin cytoskeleton) in which endogenous macromolecules normally are not free to diffuse over large distances.
Tremendous progress has been made in our understanding of cell function. For the most part, this has been accomplished through the use of a traditional reductionist approach in which individual cellular components are identified and isolated and their cellular roles are reconstructed on the basis of their functions in vitro. While such an approach has proven to be highly successful, especially for determining the “players” in cell metabolism, it falls short in explaining how these components actually function within the cell. In fact, in many cases, particularly those involving complex cellular processes, it often has not been possible to recreate the efficiency of cellular reactions in vitro. Understanding what accounts for such differences in efficiency is essential if we are to explain cellular function in its entirety.
In recent years, considerable attention has focused on the importance of macromolecular interactions in cell function (see, e.g., reference 10) and on the fact that enzymes contributing to complex processes often are bound to each other and that intermediates in the process may be channeled (see, e.g., references 6 and 16 and the review in reference 19). As a consequence of such organization, processes within cells may be able to proceed much more efficiently than those carried out by the same enzymes dispersed in solution in vitro. Thus, important questions that remain to be answered are (i) how extensive is cellular organization, (ii) what cellular components are responsible for maintaining it, and (iii) are macromolecular interactions confined to individual functional units or are they a global property of the cell?
A variety of approaches have been employed to examine the organization of macromolecules in cells. Early experiments by Kempner and Miller (15) showed that cellular contents become stratified upon centrifugation of intact Euglena cells and that the zone thought to be the cytoplasm is devoid of proteins, implying that these molecules are not free. Other experiments, employing high-voltage electron microscopy and cell extraction procedures, demonstrated the presence of an organized network in cells (22, 23) which might act as a scaffold for cell organization (20). Subsequent work revealed that some glycolytic enzymes (5) and some detergent-extractable proteins (2) are not freely diffusible in vivo, suggesting that at least some cellular components might be present in highly organized structures (reviewed in reference 26).
With the advent of new techniques to study protein-protein interactions (see, e.g., references 8, 11, 13, and 31), thousands of interactions among cellular macromolecules have been identified. However, these types of studies often lead to a high number of false-positive results, raising uncertainties about the actual extent of in vivo organization. In contrast to the aforementioned studies, another body of work (reviewed in reference 32) supports a different conclusion. The results of these studies indicate that extensive macromolecule diffusion can occur intracellularly, implying the absence of organization, but that movement is hindered by crowding and transient binding. Thus, questions about structural and functional organization, and how this might be maintained in vivo, persist.
In the present work, we have used a simple, straightforward approach that directly examines the status of endogenous macromolecules in an attempt to clarify this situation. To do this, we employed procedures that gently permeabilize a cell's plasma membrane under conditions that appear to have minimal effects on internal cellular architecture and have used such a system to examine the release from cells of various classes of macromolecules. Our data suggest that the entire mammalian cell behaves as an organized, macromolecular assembly. We show, in addition, that macromolecular organization is essential for the high efficiency of a complex cellular process, namely, protein synthesis. Finally, we demonstrate that cellular organization is largely dependent on an intact actin cytoskeleton. These observations support the conclusion that endogenous macromolecules in mammalian cells are highly organized and are not free to diffuse over large volumes. The data provide important insights into our understanding of cell structure and function.
MATERIALS AND METHODS
A mixture of five 3H-labeled amino acids (leucine, lysine, phenylalanine, proline, and tyrosine) was purchased from Amersham. Unlabeled amino acids, ATP, GTP, creatine phosphokinase, phosphocreatine, saponin, trypsin inhibitor, and trypan blue all were obtained from Sigma. Latrunculin B was purchased from Calbiochem. Rabbit liver tRNA was prepared as described previously (27). Fluorescent immunoglobulins were purchased from Jackson ImmunoResearch Laboratories. Rabbit anti-protein kinase Bα (PKBα/Akt 1) Immunoglobulin G (IgG) fraction of antiserum, polyclonal affinity isolated anti-actin, monoclonal anti-ezrin, and anti-heat shock protein 70 (hsp70) antibodies were from Sigma, and monoclonal anti-EF1α antibody was from Upstate Biotechnology. Anti-mouse and anti-rabbit IgG (horseradish peroxidase conjugated) were purchased from Promega. All other chemicals were reagent grade.
Cell culture.
Chinese hamster ovary cells (ATCC no. CRL-1781) were grown as monolayer cultures at 37°C in alpha minimal essential medium containing ribonucleosides, deoxyribonucleosides, 2 mM glutamine, and 10% fetal bovine serum in air containing 5% CO2. Cells were transferred every 2 to 3 days and were harvested for experiments 1 day after reaching confluence.
Permeabilization with saponin.
Cells were permeabilized as previously described (28) with the following modifications: all buffers were at room temperature and cells were not cold shocked and recovered. Cells were treated with 75 μg of saponin/ml in S buffer (130 mM sucrose, 50 mM KCl, 50 mM potassium acetate, 20 mM HEPES, pH 7.4) for 7 min at 37°C. The cell concentration was adjusted to be 1 × 107 to 2 × 107 cells per ml for each experiment. After permeabilization, cells were pelleted by centrifugation at 2,500 × g for 30 s at room temperature. In all experiments, cells were between 97 and 99% permeable (as determined by trypan blue entry). When present during the permeabilization procedure, latrunculin B (in dimethyl sulfoxide [DMSO]) and colchicine were each used at 100 μg/ml. The final concentration of DMSO was 3.75%.
Intact and permeabilized cells were subjected to ultrastructural examination to determine the effects of saponin and latrunculin treatment. After permeabilization, cell pellets were fixed overnight in 2% paraformaldehyde-2.5% glutaraldehyde in Millonic's phosphate buffer, washed in Millonic's phosphate buffer, fixed in 1% osmium tetroxide for 1 h, dehydrated, and embedded in Spurr's reagent. Thin sections were stained with uranyl acetate and lead citrate and viewed on a JEOL CX100 transmission electron microscope.
To measure the size of holes created by saponin, permeabilized and intact cells were incubated for 15 min at room temperature with fluorescent Igs (IgG, IgA, and IgM). Cells were subsequently pelleted for 30 s, resuspended in S buffer, repelleted for 30 s, and resuspended in phosphate-buffered saline (PBS)-4% formaldehyde. Cells were fixed for 30 min at room temperature, pelleted, and resuspended in SlowFade Antifade mounting medium (Molecular Probes). The suspension was transferred to a glass slide, covered with a coverslip, and sealed with nail polish. Slides were then examined on a Zeiss LSM-510 confocal laser scanning microscope.
Leakage studies.
A portion of cells was counted in a hemocytometer prior to permeabilization. After the saponin treatment, cells were pelleted as indicated above and the supernatant fraction was removed and saved. Cells were resuspended in the original volume of S buffer and pelleted under the same conditions, and the supernatant fraction was saved. The final cell pellet was resuspended in the original volume of S buffer, and a portion was counted to determine cell recovery. During processing, some cells are invariably lost or lysed. The amount of leakage is presented as the percentage of a particular cell component present in the permeabilization supernatant plus the wash supernatant fractions corrected for that amount accounted for by the loss of cells. The 100% value was determined from an assay of an equivalent amount of intact cells.
Protein synthesis.
Translation in intact cells was carried out in PBS medium supplemented with 25 mM glucose, 2 mM CaC12, 1 mM MgC12, and 250 μM of each of the amino acids plus a mixture of 3H-labeled leucine, lysine, phenylalanine, proline, and tyrosine. Protein synthesis in permeabilized cells was carried out in PSW buffer (130 mM sucrose, 50 mM KCl, 50 mM potassium acetate, 20 mM HEPES [pH 7.4], 5 mM ATP, 13 mM phosphocreatine, 6.1 mM MgCl2, 2.6 mM CaCl2, 5.3 mM EGTA, 5 mM glucose) supplemented with 0.1 mM GTP, 30 μg of creatine phosphokinase/ml, and 250 μM (each) amino acid plus a mixture of 3H-labeled leucine, lysine, phenylalanine, proline, and tyrosine. All protein synthesis assays were performed at 28°C. Portions were removed at the times indicated, and protein synthesis was determined as to hot-acid-precipitable radioactivity (16).
Enzyme assays.
The activity of glucose 6-phosphatase was determined by measuring the release of orthophosphate (4, 30). DNA polymerase activity was determined (using denatured calf thymus DNA as the template) by measuring the rate of incorporation of [32P]dATP (21). Citrate synthase was assayed as described by Jarreta et al. (14). The activities of lactate dehydrogenase, glucose 6-phosphate dehydrogenase, and glyceraldehyde 3-phosphate dehydrogenase were determined as described in the Worthington Biochemical Corp. manual. Aminoacyl-tRNA synthetase assays were performed at 37°C in reaction mixtures containing the following ingredients: 250 mM Tris-HCl (pH 7.5), 5 mM MgC12, 0.2 mM EDTA, 0.2 mg of bovine serum albumin/ml, 1.5 mg of rabbit liver tRNA/ml, 0.1 mM 3H- or 14C-labeled amino acid (∼20 to 100 cpm/pmol), and sufficient cell extract or supernatant fraction to measure synthetase activity. Reactions were stopped by the addition of 10% trichloroacetic acid containing 0.5% Casamino Acids (Difco). Aminoacyl-tRNA precipitates were collected and counted as described previously (1). Release of protein and RNA from prelabeled cells was performed as previously described (18).
Western blotting.
After electrophoresis on 8% polyacrylamide gels, proteins were transferred to a polyvinylidene difluoride membrane in Tris-glycine buffer (0.375 M Tris, 0.192 M glycine, 20% methanol). Blocking of the membrane was performed with a 3% solution of nonfat milk in PBS buffer for 2 h at room temperature with shaking. The membrane was then treated with the antibodies mentioned above in PBS buffer supplemented with 3% nonfat milk (2 to 5 μg of antibody/10 ml of buffer) overnight at 4°C with shaking. Visualization of the protein bands followed the ECL Western blotting protocol using anti-mouse IgG (for anti-ezrin, anti-hsp70, and anti-EF1α antibodies) or anti-rabbit IgG (for anti-actin and anti-AKT antibodies) conjugated with horseradish peroxidase as the secondary antibody.
RESULTS
In previous work from our laboratory (18), we described a saponin-permeabilized CHO cell system that retained the ability to synthesize protein at rates comparable to those seen with intact cells. Although it was not studied in detail at that time, we also observed that the permeabilized cells retained a high degree of structural integrity, releasing only small amounts of protein and RNA while apparently allowing entry of molecules as large as 23S rRNA (1.2 × 106 Da). This system seemed ideally suited for a more extensive examination of the organization of the entire cell through simple measurement of which components might be released from such cells under a variety of conditions. With time, macromolecules which are free to diffuse within the cell would be expected to be able to leak out through the holes created in the plasma membrane by saponin. In contrast, those molecules which are bound to cell structure would remain with the cell unless the components responsible for their organization were to be destroyed. Thus, by examination of the release of appropriate marker molecules, by measurement of the time course of release, and by evaluating the effects of agents that affect cell structure, it should be possible to obtain considerable information about the internal organization of the cell. The studies detailed here demonstrate the feasibility of this approach and support the conclusion that the cell is highly organized.
Structural characterization of permeabilized CHO cells.
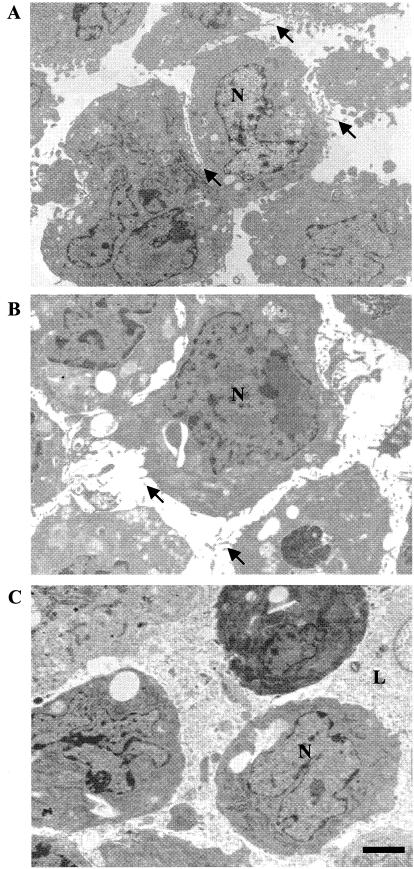
To carefully evaluate the effects of saponin treatment on cell structure, permeabilized CHO cells were examined by light and electron microscopy and compared to untreated cells. The extent of cell permeabilization was monitored by trypan blue dye entry and amounted to 97 to 99% in all experiments presented. Ultrastructural examination revealed no gross differences between intact and permeabilized cells (Fig. 1A and B, respectively). Moreover, in both cases, the cell populations were homogeneous. Less than 1% of the cells were lysed, and many microvilli were present on the cell surface in each case. In contrast, latrunculin-treated, permeabilized cells were quite heterogeneous. About 15% of these cells were lysed and about one-third were more electron dense (Fig. 1C). In addition, few microvilli were present. Analysis at higher magnification showed no damage to either the cytoplasm or the internal membranes of the permeabilized cells (data not shown). This is consistent with the known differential sensitivity of the plasma membrane to saponin (33). Mitochondria in the permeabilized cells appeared to be slightly distended but were otherwise intact. The endoplasmic reticulum was not disrupted. Several indistinct areas of the plasma membrane were seen. However, it has not been determined whether these areas represent holes in the membrane or just regions of the membrane oblique to the plane of the thin sections. The relative intactness of the permeabilized cells prepared by the methods described here is in contrast to that found with other permeabilization procedures (3, 5), which appeared more destructive.
FIG. 1.
Ultrastructural characterization of intact and permeabilized cells. Intact cells (A), permeabilized cells (B), and latrunculin-treated permeabilized cells (C) were examined by electron microscopy. Arrows indicate microvilli. N, nucleus; L, lysed cell. Bar, 2 μm.
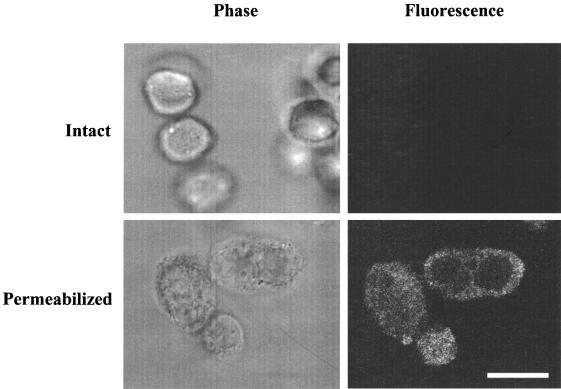
Dye-labeled immunoglobulin molecules were used to estimate the size of the holes in the plasma membrane that were generated by saponin. Intact and permeabilized cells were each incubated with solutions containing fluorescent immunoglobulin molecules of different sizes. As determined by fluorescence microscopy, fluorescein-IgG (160 kDa), rhodamine-IgA (480 kDa), and fluorescein IgM (800 kDa) all were able to diffuse into permeabilized cells but no fluorescence was observed in intact cells (data not shown). To confirm that the IgM had actually entered the permeabilized cells, confocal images also were obtained (Fig. 2). These showed that within 15 min, fluorescein-IgM was distributed throughout the cytoplasm of permeabilized cells but that it did not enter intact cells. These observations indicate that the plasma membrane of permeabilized cells contains holes large enough to allow passage of proteins of at least 800 kDa and that an exogenous molecule of this size can diffuse throughout the permeabilized cell cytoplasm.
FIG. 2.
Entry of fluorescent molecules into permeabilized CHO cells. Intact and permeabilized cells were treated with various fluorescent immunoglobulins as described in Materials and Methods. In the experiment whose results are shown, cells were incubated with fluorescent IgM and examined by phase contrast and fluorescence confocal microscopy. The exposure times for the fluorescence photographs were the same for both intact and permeabilized cells. The fluorescent confocal image is an optical section through the cell at the level of the nucleus. Bar, 10 microns.
Functional analysis of permeabilized CHO cells.
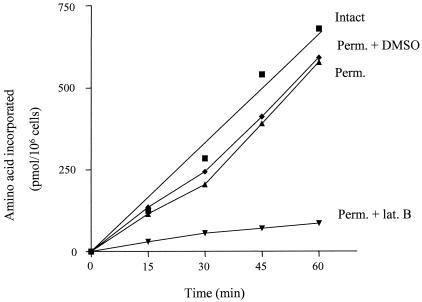
As one measure of the functional intactness of permeabilized cells, they were compared to untreated cells with regard to their ability to carry out protein synthesis. As shown in Fig. 3, permeable cells incorporate amino acids into protein at a rate comparable to that of intact cells for a period of at least 60 min. In view of the complexity of protein synthesis and previous work demonstrating how easily this process can be disrupted by loss of cellular organization (18, 29), these data provide strong evidence that permeabilized cells, prepared as described here, retain much of the organization of the intact cell and do so even during incubation for 60 min at 28°C.
FIG. 3.
Protein synthesis in intact cells and in permeabilized (Perm.) cells in the presence and absence of latrunculin B (lat. B). Cells were harvested, permeabilized, and incubated for protein synthesis as described in Materials and Methods. The time course of amino acid incorporation into protein over 60 min was determined with a mixture of five 3H-labeled amino acids. Latrunculin B, when present, was at 100 μg/ml.
As previously observed for intact cells (29), protein synthesis in permeabilized cells is dramatically inhibited by the presence of latrunculin B (100 μg/ml) (Fig. 3), an agent known to promote disruption of actin microfilaments (the presence of DMSO to which the latrunculin B was added did not affect the process). In contrast, treatment of the permeabilized cells with either colchicine (100 μg/ml) or nocodazole (62.5 μg/ml) had no significant effect on protein synthesis (data not shown), suggesting that intact microtubules are not required. These data indicate that even in permeabilized cells the high efficiency of protein synthesis is dependent on an intact microfilament system and focus attention on this part of the cytoskeleton as an important mediator of cell organization.
Release of macromolecules.
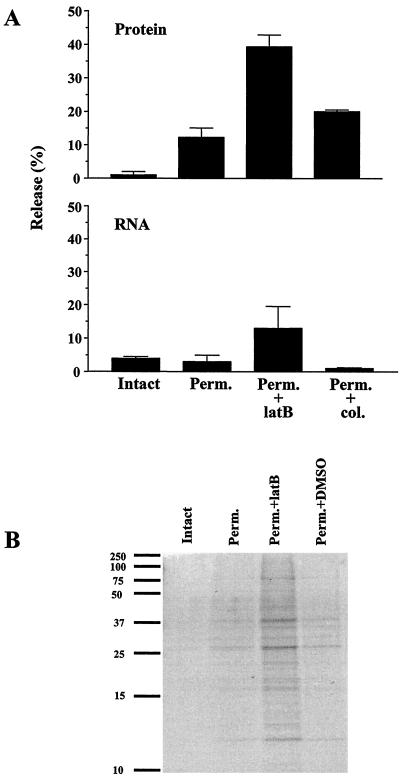
To determine the degree of organization of proteins in CHO cells, the cells were prelabeled with [3H]amino acids and then subjected to the usual saponin permeabilization procedure followed by centrifugation to separate those proteins that were released from those that remained with the cell. As shown in Fig. 4A, only ∼12% of total cellular protein was released from the permeabilized cells, indicating that the vast majority of proteins were in some way sequestered. This situation changed dramatically upon the addition of the microfilament-disrupting agent, latrunculin B. Under these conditions, total protein leakage increased to 40%. It should be noted that proteins do not leak from intact cells (Fig. 4A), and this was not altered by the addition of latrunculin B (data not shown). These data suggest that a large percentage of cellular proteins are sequestered by direct or indirect association with the actin cytoskeleton. In contrast, the addition of colchicine, a microtubule-disrupting agent, led to only a small increase of protein leakage. Thus, microtubules appear to play a less important role in macromolecule organization.
FIG. 4.
(A) Release of protein and RNA from permeabilized cells. Cells were labeled, harvested, and permeabilized as described in Materials and Methods. Acid-precipitable radioactivity present in supernatant and pellet fractions was measured in a scintillation counter. The activity present in the supernatant fraction divided by the total activity in the intact cell is indicated as percent leakage. The upper panel shows the results for the release of 3H-labeled protein, and the lower panel shows the results for the release of 3H-labeled RNA. The values shown represent the averages of two experiments. (B) SDS-PAGE analysis of protein release. Supernatant fractions from intact cells, permeabilized cells (Perm.), permeabilized cells treated with latrunculin B (Perm.+latB), and permeabilized cells treated with DMSO (Perm.+DMSO) were analyzed. A 1/20 volume of each supernatant fraction was fractionated on a 14% polyacrylamide gel. Bands were visualized with Coomassie blue. The positions of prestained broad range protein markers (Bio-Rad) are shown on the left side of the panel.
The population of proteins released from permeabilized cells was evaluated by separation on sodium dodecyl sulfate (SDS)-8% polyacrylamide gelsfollowed by staining with Coomassie blue (Fig. 4B). While little protein was present in the supernatant fraction from permeabilized cells, proteins of all sizes were observed. The addition of latrunculin B led to an increased amount of protein release; however, the size distribution of these proteins was unchanged. On the basis of this information, it appears that proteins of all sizes are equally able to be released from permeabilized cells, suggesting that neither the saponin-generated holes in the plasma membrane nor internal architecture would act as a barrier to the release of proteins if they were free to diffuse to the site of release.
Similar studies were carried out using cells prelabeled with [3H]uridine to assess leakage of RNA (Fig. 4A). Very little RNA leaked from permeabilized cells or from permeabilized cells treated with colchicine. In contrast, upon the addition of latrunculin B, RNA leakage increased to ∼15% (the presence of DMSO to which the latrunculin was added had no effect on leakage). Analysis by polyacrylamide gel electrophoresis (PAGE) of the RNA that is released under these conditions revealed that it was primarily tRNA (data not shown). Thus, these data indicate that tRNA also is normally sequestered in cells and that its retention is dependent on an intact actin cytoskeleton. The data do not allow any conclusions regarding ribosomes. They also may be sequestered or they may be too large to pass through the holes generated by saponin.
Release of specific proteins.
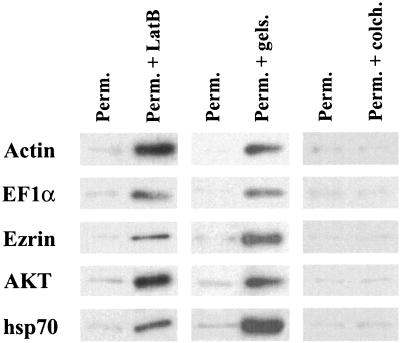
We next addressed the issue of whether the total protein release presented in Fig. 4 was representative of all proteins or whether proteins of different pathways or cellular locations might behave differently. Accordingly, proteins from different cellular compartments and several different cytoplasmic proteins were each examined for release in the absence or presence of agents that affect the cytoskeleton. We turned our attention first to actin and to the actin-binding proteins EFlα and ezrin. As shown in Fig. 5, the results of Western blot analysis indicated that only relatively small amounts of these proteins were released from permeabilized cells. Likewise, very little actin was released from intact cells or permeabilized cells treated with DMSO (data not shown). In contrast, upon treatment with latrunculin B, which leads to actin filament depolymerization, or gelsolin, which severs the filaments, there was a dramatic increase in the release of the three proteins. In contrast, the presence of colchicine, which disrupts microtubules, had essentially no effect on release of any of these proteins (Fig. 5). Essentially identical results were obtained with AKT (protein kinase Bα) and hsp70 (Fig. 5). Given the low level of actin release from permeabilized cells, it is likely that even the populations of monomeric actin that are known to be present at discrete sites in cells complexed with other proteins (7) remain bound in some manner.
FIG. 5.
Release of cytoplasmic proteins from permeabilized cells. A 1/20 volume of each indicated supernatant fraction was fractionated on SDS-PAGE, transferred to a PVDF membrane, and probed with antibodies against actin, EF1α, ezrin, AKT and hsp70. Horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG was used as a secondary antibody, and bands were visualized by enhanced chemiluminescence (see Materials and Methods). Abbreviations: Perm., permeabilized cells; Perm. + LatB, permeabilized cells treated with latrunculin B; Perm. + gels., permeabilized cells treated with gelsolin; Perm. + colch., permeabilized cells treated with colchicine.
A number of marker enzymes (specific for distinct organellar compartments) were also examined. These included citrate synthase (specific for mitochondria), glucose 6-phosphatase (endoplasmic reticulum), and DNA polymerase (nuclei) (Table 1). In each case, essentially no release from permeabilized cells was observed in either the absence or the presence of latrunculin B. These data confirm, first, that gentle saponin treatment permeabilizes the plasma membrane but does not significantly disrupt internal membranes and, second, that depolymerization of microfilaments by latrunculin does not appear to affect organellar integrity.
TABLE 1.
Release of specific proteins from permeabilized cellsa
| Protein | Leakage (%)
|
|
|---|---|---|
| Permeabilized cells | Permeabilzed cells plus latrunculin | |
| DNA polymerase | <1 | 5 |
| Citrate synthase | 4 | 10 |
| Glucose 6-phosphatase | <1 | 5 |
| Leu-tRNA synthetase | 1 | 75 |
| Gly-tRNA synthetase | 10 | 81 |
| Lys-tRNA synthetase | 1 | 85 |
| Glucose 6-P dehydrogenase | 8 | 43 |
| Lactate dehydrogenase | 40 | 78 |
| Glyceraldehyde 3-P dehydrogenase | 34 | 80 |
To determine release, enzyme assays were carried out on supernatant fractions and on intact cells as described in Materials and Methods. Percent leakage is defined as the amount of activity in the supernatant fraction (corrected for cell loss) divided by the activity in an equivalent amount of intact cells. The data represent the averages of at least two experiments.
The effects on enzymes of the cytoplasmic compartment were quite different. One group of cytoplasmic enzymes, the aminoacyl-tRNA synthetases, did not leak from permeabilized cells (Table 1). However, upon treatment of these cells with latrunculin B, massive amounts of the three synthetases tested were released. Thus, under normal circumstances, the aminoacyl-tRNA synthetases are immobilized (due in some manner to the actin cytoskeleton). An additional cytoplasmic protein, glucose 6-phosphate dehydrogenase, behaved similarly, although the release after latrunculin treatment was somewhat reduced (Table 1). In contrast, a second group of proteins, the glycolytic enzymes (glyceraldehyde 3-phosphate dehydrogenase and lactate dehydrogenase), behaved quite differently. They were released from permeabilized cells in significant amounts (Table 1), and treatment with latrunculin B resulted in additional leakage of both enzymes. Thus, it appears that at any given time only a portion of the population of these proteins is sequestered. The data suggest that at least two populations of proteins are present in the cytoplasm of mammalian cells. One group is tightly associated, directly or indirectly, with the actin cytoskeleton and is therefore not released from permeabilized cells. The second group may be only partially bound or associates only transiently, allowing for significant release from the permeabilized cell.
Kinetics of cytoplasmic protein leakage.
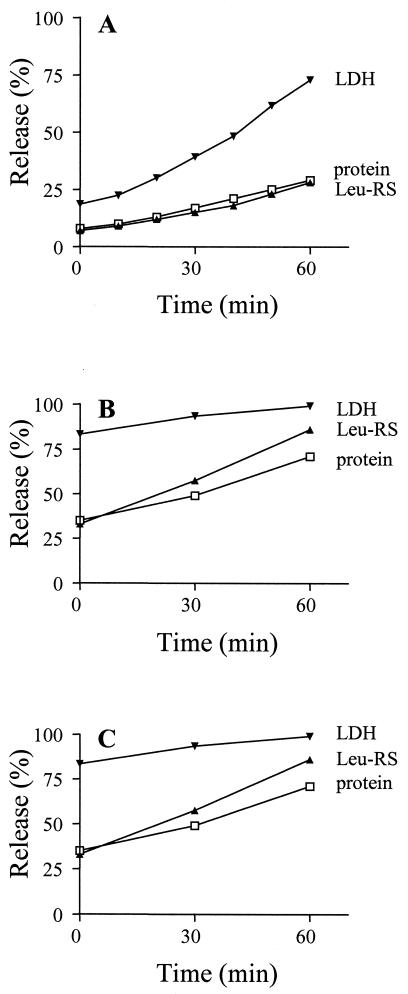
To examine in more detail the two putative populations of cytoplasmic proteins, we followed the time course of their release from permeabilized cells (Fig. 6A). The leakage of total protein was also measured for comparison. Over the course of 60 min, the amount of total protein released increased from ∼10 to ∼25%. This increase of 15% represents the maximum amount of additional protein leakage, because some of it may have been due to cell lysis during the extended incubation. Nevertheless, these data indicate that protein release remained relatively low, even during 1 h of incubation. Leucyl-tRNA synthetase was released in concert with that of total protein, in keeping with the single time point measurement (Table 1). Lactate dehydrogenase, on the other hand, was released at a much more rapid rate during the 60-min time period, reaching a level of ∼75% at 60 min and confirming the existence of at least two populations of cytoplasmic proteins. The data suggest that lactate dehydrogenase, and presumably other proteins of this group, is transiently sequestered but can leak from permeabilized cells during the period in which it is not associated with cellular structures. Moreover, it appears (as determined by total protein release) that the majority of cellular proteins are more stably sequestered.
FIG. 6.
Time course of protein release. Samples of supernatant fractions were taken at 10-min intervals and assayed for total protein, leucyl-tRNA synthetase (Leu-RS) activity, and lactate dehydrogenase (LDH) activity as described in Materials and Methods. Values represent the averages of two experiments. The percentages of release are relative to those in an equal amount of intact cells. (A) Permeabilized cells; (B) permeabilized cells treated with latrunculin B; (C) permeabilized cells treated with both latrunculin B and colchicine.
To obtain information about the maximum possible leakage of cellular constituents, similar time course experiments were carried out in the presence of latrunculin B (Fig. 6B) and latrunculin B plus colchicine (Fig. 6C). Over the course of a 60-min incubation, total protein release in the presence of latrunculin increased to a level of ∼70%; however, the additional presence of colchicine did not change the rate or total amount (within 5%) of protein leakage (compare Fig. 6C to 6B). These data suggest that sequestration of proteins is largely due to the actin cytoskeleton. Under these conditions, ∼90% of leucyl-tRNA synthetase and ∼95% of lactate dehydrogenase were released in 60 min and, again, the presence of colchicine had no additional effect. The high level of release of the two cytoplasmic enzymes suggests that cytoplasmic proteins are able to leak essentially to completion once the actin cytoskeleton has been disrupted. In contrast, since ∼30% of total protein remained with the cell it is likely that organellar proteins were not released, even under these extreme conditions.
DISCUSSION
The studies described here support the conclusion that endogenous macromolecules are highly organized within the cytoplasm of mammalian cells. The findings, which rely on the conceptually simple approach of measuring release of macromolecules from a permeabilized cell, suggest that the idea of a mammalian cell as a “bag of enzymes” with an aqueous cytosol containing dissolved macromolecules is no longer tenable. If that were the case, it would be expected that the dissolved macromolecules would be rapidly released from permeabilized cells, but this did not occur. The inability of most macromolecules to be released does not appear to be limited by the size of the holes in the plasma membrane generated by saponin, because molecules at least as large as 800 kDa can pass through these holes and enter the permeabilized cell. Likewise, the lack of appreciable leakage is apparently not due to extremely slow diffusion of the macromolecules. Fluorescent IgM (800 kDa) diffused throughout the cytoplasm in at most a few minutes, whereas the vast majority of endogenous macromolecules were retained in the cells for at least 1 h. In fact, it has been estimated that it should take only 7 seconds for a freely diffusible 500-kDa molecule to traverse the cytoplasm of a 10-μm-diameter cell (24). Second, the fact that there was no differential release of small proteins compared to that seen with large ones also suggests that intracellular barriers to diffusion are not playing a role, especially considering the much longer time frame of our measurements compared to those routinely used for measurement of intracellular diffusion (32). Rather, our data are most consistent with the conclusion that most macromolecules are not released from permeabilized cells because they are sequestered as part of an organized cell structure.
The data also suggest that the cytoskeleton, particularly the actin microfilament network, plays an important role in maintaining this organization. On the basis of an examination of protein synthesis (the results of which are presented here and elsewhere) (18, 29), both supramolecular organization and the actin cytoskeleton are necessary for maintaining the high efficiency of this process in vivo compared to the same reactions in vitro. Thus, components of the translation apparatus such as tRNA, aminoacyl-tRNA synthetases, and EFlα are normally tightly sequestered in permeabilized cells. However, upon disruption of microfilaments there is a dramatic reduction of protein synthesis accompanied by release of these translation components from the cells. The fact that protein synthesis in these permeabilized cells remains at such a high level, despite the complexity of the process, also suggests that this system has retained much of the original structural integrity of intact cells.
Permeabilized cells have been used by other investigators to examine cell architecture (5, 22). Compared to other permeabilizing agents, however, saponin, the permeabilizing agent used here, has the advantage when carefully titrated of causing minimal damage to internal membranes and leading to a very uniform population (>97%) of permeabilized cells (17). Although earlier work was limited largely to some glycolytic enzymes, those studies also supported the conclusion that extensive enzyme organization was a probable feature of animal cells (5, 22). In the present work, the analysis has been extended to total protein, to enzymes of multiple organelles, to structural proteins, to cytoplasmic enzymes of several metabolic pathways, and to RNA. Thus, these studies have revealed that organization extends to many components of the cell. Moreover, it has led to the finding that at least two populations of cytoplasmic proteins exist, those that are stably associated with cellular structure and those that appear to associate transiently, which results in their partial release from permeabilized cells. Differential release of glycolytic enzymes and the idea of stable and transient association with cell structure have been suggested earlier (12). Most significantly, these studies have also revealed the importance of the cytoskeleton in maintaining the organization of cytoplasmic macromolecules. While many macromolecules are known to bind directly to cytoskeletal elements, it remains to be determined how much of the organization is due to direct binding and how much is due to secondary binding to already bound molecules.
The extensive organization of cellular macromolecules that we propose does not mean that they are unable to move in cells. It is well known, for example, that upon microinjection many macromolecules can diffuse to specific cellular sites. However, our data suggest that diffusion is not the primary means by which endogenous macromolecules are transported. Rather, we would propose that macromolecules and macromolecular complexes normally are actively transported by molecular motors along the cytoskeleton which might be considered the “railroad tracks” of the cell (for reviews, see references 9 and 25). The increasing number and diversity of known molecular motors underscores the importance of their function in the cell and the large number and types of cargo that need to be transported.
How then does one reconcile our observations that endogenous macromolecules do not freely diffuse throughout cells with the large body of work (32) indicating that such molecules are able to move? There are several possibilities. First, measurements of macromolecule diffusion in cells generally use techniques (such as fluorescence recovery after photobleaching) that examine movement over relatively short distances whereas, in our experiments, long-range movement is necessary for molecules to be released from cells. One would expect that even if macromolecules were part of an organized structure, they would dissociate and rebind allowing for movements within small volumes. The extent of movement would depend on the rate of rebinding compared to the rate of diffusion. In fact, this is what we propose for the more rapid rate of leakage of the glycolytic enzymes. The second consideration is that measurements of diffusion generally employ microinjected or modified macromolecules that may exceed the available binding sites or may bind more weakly, either of which could result in movement that does not occur with the endogenous molecules. Further work will be needed to clarify these differences.
The data and conclusions presented here supporting the idea that the entire cell is a macromolecular assembly fits well with observations in other closely related areas. Thus, the increasing evidence that intermediates in many metabolic pathways are channeled demands associations among components of the pathway, at least transiently. Extensive organization among cellular components would also be in keeping with the tenets of metabolic control analysis and the behavior of metabolic networks such that perturbations in one part of a system can have profound effects on components elsewhere in the system. Such functional interrelationships are best understood in terms of structural organization. Finally, the thousands of interactions among cellular proteins that are now being reported in a variety of systems are exactly what would be expected from cells organized as we propose. It is becoming clear that if we wish to understand how cells function in vivo, we will have to use more integrative approaches and study the biochemistry of organized systems (10, 20).
Acknowledgments
We thank Dora Vega-Salas for helpful discussions, Beata Frydel and the University of Miami Analytical Imaging Core for help with the confocal microscopy, Susan Decker for electron microscopy, and David Fisher for technical assistance.
This work was supported by grant GM16317 from the National Institutes of Health.
REFERENCES
- 1.Bandyopadhyay, A. K., and M. P. Deutscher. 1971. Complex of aminoacyl-transfer RNA synthetases. J. Mol. Biol. 60:113-122. [DOI] [PubMed] [Google Scholar]
- 2.Cameron, I. L., K. E. Hunter, N. K. Smith, C. F. Hazelwood, A. Ludany, and M. Kellermayer. 1988. Role of plasma membrane and of cytomatrix in maintenance of intracellular to extracellular ion gradients in chicken erythrocytes. J. Cell. Physiol. 137:299-304. [DOI] [PubMed] [Google Scholar]
- 3.Cande, W. Z., K. McDonald, and R. L. Meeusen. 1981. A permeabilized cell model for studying cell division: a comparison of anaphase chromosome movement and cleavage furrow constriction in lysed Ptk1 cells. J. Cell Biol. 88:618-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, P. S., T. Y. Toribara, and H. Warner. 1956. Microdetermination of phosphorus. Anal. Chem. 28:1756-1758. [Google Scholar]
- 5.Clegg, J. S., and S. A. Jackson. 1988. Glycolysis in permeabilized L-929 cells. Biochem. J. 255:335-344. [PMC free article] [PubMed] [Google Scholar]
- 6.Clegg, J. S., and S. A. Jackson. 1990. Glucose metabolism and the channeling of glycolytic intermediates in permeabilized L-929 cells. Arch. Biochem. Biophys. 278:452-460. [DOI] [PubMed] [Google Scholar]
- 7.Fechheimer, M., and S. H. Zigmond. 1993. Focusing on unpolymerized actin. J. Cell Biol. 123:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fields, S., and O. Song. 1989. A novel genetic system to detect protein-protein interactions. Nature 340:245-246. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein, L. S. 2001. Kinesin molecular motors: transport pathways, receptors, and human disease. Proc. Natl. Acad. Sci. USA 98:6999-7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartwell, L. H., J. J. Hopfield, S. Leibler, and A. W. Murray. 1999. From molecular to modular cell biology. Nature 402(Suppl.):C47-C52. [DOI] [PubMed] [Google Scholar]
- 11.Ho, Y., et al. 2002. Systematic identification of protein complexes in Saccharomyces cerevisiae by mass spectrometry. Nature 415:180-183. [DOI] [PubMed] [Google Scholar]
- 12.Humphreys, L., and C. Masters. 1986. On the differential release of glycolytic enzymes from cellular structure. Biochem. Int. 13:71-77. [PubMed] [Google Scholar]
- 13.Ito, T., T. Chiba, R. Ozawa, M. Yoshida, M. Hattori, and Y. Sakaki. 2001. A comprehensive two-hybrid analysis to explore the yeast protein interactome. Proc. Natl. Acad. Sci. USA 98:4569-4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaretta, D., J. Orus, A. Barrientos, O. Miro, E. Roig, M. Heras, C. T. Moraes, F. Cardellach, and J. Casademont. 2000. Mitochondrial function in heart muscle from patients with idiopathic dilated cardiomyopathy. Cardiovasc. Res. 45:860-865. [DOI] [PubMed] [Google Scholar]
- 15.Kempner, E. S., and J. H. Miller. 1968. The molecular biology of Euglena gracilis. IV. Cellular stratification by centrifuging. Exp. Cell Res. 51:141-149. [DOI] [PubMed] [Google Scholar]
- 16.Negrutskii, B. S., and M. P. Deutscher. 1991. Channeling of aminoacyl-tRNA for protein synthesis in vivo. Proc. Natl. Acad. Sci. USA 88:4991-4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Negrutskii, B. S., and M. P. Deutscher. 1992. A sequestered pool of aminoacyl-tRNA in mammalian cells. Proc. Natl. Acad. Sci. USA 89:3601-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Negrutskii, B. S., R. Stapulionis, and M. P. Deutscher. 1994. Supramolecular organization of the mammalian translation system. Proc. Natl. Acad. Sci. USA 91:964-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ovadi, J., and P. A. Srere. 2000. Macromolecular compartmentation and channeling. Int. Rev. Cytol. 192:255-280. [DOI] [PubMed] [Google Scholar]
- 20.Penman, S. 1995. Rethinking cell structure. Proc. Natl. Acad. Sci. USA 92:5251-5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sambrook, J., E. F. Fritsch, and T. Maniatis (ed.). 1989. Molecular cloning: a laboratory manual, 2nd ed., p. 5.44-5.47. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 22.Schliwa, M., U. Euteneuer, and K. R. Porter. 1987. Release of enzymes of intermediary metabolism from permeabilized cells: further evidence in support of a structural organization of the cytoplasmic matrix. Eur. J. Cell Biol. 44:214-218. [PubMed] [Google Scholar]
- 23.Schliwa, M., J. van Blerkom, and K. R. Porter. 1981. Stabilization and the cytoplasmic ground substance in detergent-opened cells and a structural and biochemical analysis of its composition. Proc. Natl. Acad. Sci. USA 78:4329-4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seksek, O., J. Biwersi, and A. S. Verkman. 1997. Translational diffusion of macromolecule-sized solutes in cytoplasm and nucleus. J. Cell Biol. 138:131-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spudich, J. A. 2001. The myosin swinging cross-bridge model. Nat. Rev. Mol. Cell Biol. 2:387-392. [DOI] [PubMed] [Google Scholar]
- 26.Srere, P. A. 2000. Macromolecular interactions: tracing the roots. Trends Biochem. Sci. 25:150-153. [DOI] [PubMed] [Google Scholar]
- 27.Stapulionis, R., and M. P. Deutscher. 1995. A channeled tRNA cycle during mammalian protein synthesis. Proc. Natl. Acad. Sci. USA 92:7158-7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stapulionis, R., and M. P. Deutscher. 1998. Permeabilized mammalian cells as a system for protein synthesis. Methods Mol. Biol. 77:23-31. [DOI] [PubMed] [Google Scholar]
- 29.Stapulionis, R., S. Kolli, and M. P. Deutscher. 1997. Efficient mammalian protein synthesis requires an intact F-actin system. J. Biol. Chem. 272:24980-24986. [DOI] [PubMed] [Google Scholar]
- 30.Swanson, M. A. 1955. Glucose-6-phosphatase from liver. Methods Enzymol. 2:541-543. [Google Scholar]
- 31.Uetz, P., L. Giot, G. Cagney, T. A. Mansfield, R. S. Judson, J. R. Knight, D. Lockshon, V. Narayan, M. Srinivasan, P. Poschart, A. Qureshi-Emili, Y. Li, B. Godwin, D. Conover, T. Kalbfleisch, G. Vijayadamodar, M. Yang, M. Johnston, S. Fields, and J. M. Rothberg. 2000. A comprehensive analysis of protein-protein interactions in Saccharomyces cerevisiae. Nature 403:623-627. [DOI] [PubMed] [Google Scholar]
- 32.Verkman, A. S. 2002. Solute and macromolecule diffusion in cellular aqueous compartments. Trends Biochem. Sci. 127:27-33. [DOI] [PubMed] [Google Scholar]
- 33.Wassler, M., I. Jonasson, R. Persson, and E. Fries. 1987. Differential permeabilization of membranes by saponin treatment of isolated rat hepatocytes. Release of secretory proteins. Biochem. J. 247:407-415. [DOI] [PMC free article] [PubMed] [Google Scholar]