Abstract
The neuropeptides substance P (SP) and calcitonin gene-related peptide are believed to be involved in the axon reflex-mediated component of cutaneous thermal hyperaemia, but no studies have specifically addressed this issue. The purpose of this study was to determine whether neurokinin-1 (NK1) receptors, which preferentially bind SP, contribute to the axon-reflex component of cutaneous thermal hyperaemia. Nine subjects were equipped with four microdialysis fibres and received one of four treatments: 1) lactated Ringer’s (control); 2) 10mM L-NAME to inhibit NO synthase; 3) 10μM SP; and 4) 10μM SP + 10mM L-NAME. Skin blood flow was monitored via laser-Doppler flowmetry (LDF) and local skin temperature was controlled using local heating devices. Sites 3 and 4 were perfused with 10μM SP for 15 minutes at a rate of 4μl min−1 and the ensuing vasodilatation was allowed to return to baseline. Following SP-induced vasodilatation, all skin sites were locally heated from a baseline temperature of 33°C to 42°C at a rate of 0.5°C every 5 seconds. Cutaneous vascular conductance (CVC) was calculated as LDF/MAP and normalized to maximal (%CVCmax) via 28mM nitroprusside and local heating to 43°C. Initial peak did not differ between control (79±3%CVCmax) and SP only sites (79±2%CVCmax). Initial peak at L-NAME (43±3%CVCmax) and SP + L-NAME (53±3%CVCmax) sites were significantly reduced compared to both control and SP only sites (p<0.001 for both) and L-NAME sites were attenuated compared to SP + L-NAME sites (p<0.01). There was no observable nadir response at sites pretreated with SP. Compared to control sites (57±4%CVCmax), nadir at L-NAME (14±2%CVCmax) and SP + L-NAME (31±5%CVCmax) sites were significantly reduced (p<0.01 for all conditions). L-NAME significantly reduced the nadir compared to SP + L-NAME (p<0.01). Plateau CVC values did not differ between control (86±3%CVCmax) and SP sites (91±1%CVCmax). At L-NAME (36±4%CVCmax) and SP + L-NAME (56±6%CVCmax) sites, plateau CVC was significantly reduced compared to control and SP only sites (p<0.01 for all conditions). The plateau at L-NAME sites was significantly reduced compared to SP + L-NAME sites (p<0.01). These data suggest NK1 receptors contribute to both the axon reflex component and secondary plateau phase of cutaneous thermal hyperaemia.
Keywords: Skin blood flow, microdialysis, nitric oxide, local heating, laser-Doppler
INTRODUCTION
An increase in local temperature of human skin results in a robust and reproducible biphasic increase in skin blood flow (Kellogg et al., 1999; Minson et al., 2001). The first phase consists of an initial peak and nadir, which are thought to be mediated, in part, by an axon reflex mechanism (Magerl and Treede, 1996; Minson et al., 2001). Following the initial peak and nadir there is a prolonged secondary plateau mediated predominantly by nitric oxide (NO) (Kellogg et al., 1999; Minson et al., 2001). Under conditions in which the local heating stimulus results in a sensation of even brief periods of pain, the initial peak and nadir response become indistinguishable and the NO-dependent plateau is rendered insensitive to NO synthase inhibition (Kellogg et al., 1999). Data suggests cutaneous thermal hyperaemia is dependent on cutaneous sensory nerves and production of local vasodilators and also on intact adrenergic vasoconstrictor nerves and noradrenaline (Hodges et al., 2008, 2009b; Houghton et al., 2006). Cutaneous vasodilatation to local heating relying on adrenergic vasoconstrictor nerves and substances is counterintuitive and, taken together, the aforementioned data suggest a complex interaction between neurally mediated vasodilatation, local production of vasodilator substances, and adrenergic vasoconstrictor nerves. As such, mechanism(s) underlying cutaneous thermal hyperaemia are not fully understood.
Data from Minson et al. (2001) suggest the initial peak and nadir are primarily dependent on cutaneous axon reflexes and recent data from Wong and Fieger (2010) further suggest functional transient receptor potential vanilloid-1 (TRPV-1) channels are required for full expression of the initial peak and nadir components of thermal hyperaemia. The data from Wong and Fieger (2010) suggest activation of TRPV-1 channels by heat may constitute one mechanism by which cutaneous sensory nerves are deploarised and ultimately leads to axon reflex-mediated vasodilatation (Minson et al., 2001); however, the question as to which neuro-peptides/transmitters are ultimately released from cutaneous sensory nerve terminals remains unresolved. The neuropeptides substance P and calcitonin gene-related peptide (CGRP) have long been been postulated to be released from heat-sensitive C-fibre afferent nerves upon application of heat to the skin and mediate the initial peak and nadir response of thermal hyperaemia; however, the involvement of substance P and CGRP in cutaneous thermal hyperaemia has not yet been directly investigated.
Both substance P and CGRP have been shown to be co-localized in nerve terminals in human skin (Brain, 1996; Wallengren, 1997) and both peptides have been implicated in axon reflex-mediated vasodilatation in human skin (Brain, 1996; Sann and Pireau, 1998; Wallengren, 1997; Wallengren & Hakanson, 1987). Substance P and, to a lesser extent, CGRP have been shown to be attenuated in the presence of an NO synthase inhibitor (Klede et al., 2003; Wong et al., 2005) and both peptides have been shown to cause the release of NO from cutaneous endothelial cells (Bull et al., 1996) and release of CGRP has been shown to be an NO-dependent process (Hughes & Brain, 1994). In the context of cutaneous thermal hyperaemia, the initial peak and nadir has been shown to be attenuated in the presence of an NO synthase inhibitor and are further reduced with topical application of EMLA cream, which blocks the axon reflexes in human skin, suggesting the initial peak and nadir are predominantly mediated by axon reflexes but are also partially dependent on NO (Kellogg et al., 1999; Minson et al., 2001).
The characteristics of substance P- and CGRP-induced vasodilatation in human skin differ. Substance P-induced vasodilatation is robust but short-lived (Klede et al., 2003; Weidner et al., 2000; Wong et al., 2005), where the transient nature of substance P-mediated vasodilatation is believed to be due to internalization, or desensitisation, of the neurokinin-1 (NK1) receptor upon binding of substance P (Klede et al., 2003; Quartara & Maggi, 1997; Weidner et al., 2000; Wong et al., 2005). In contrast, CGRP-induced vasodilatation results in a prolonged cutaneous vasodilatation and this pattern of vasodilatation is qualitatively similar to the cutaneous vasodilatation to a painful local heating stimulus (Brain et al., 1986; Brain & Williams, 1988; Weidner et al., 2000). Using intradermal injections, it has been shown the prolonged vasodilator response to CGRP can be attenuated when substance P is co-injected with CGRP (Brain & Williams, 1988; Wallengren & Wang, 1993) and this pattern of cutaneous vasodilatation when substance P and CGRP are co-injected is similar to the initial peak and nadir response to a rapid, non-painful local heating stimulus. This regulatory role of substance P on CGRP-induced vasodilatation is believed to be due to the release of proteases from cutaneous mast cells initiated by substance P binding to NK1 receptors on cutaneous mast cells (Brain & Williams, 1988; Wallengren, 1997; Wallengren & Wang, 1993). The aforementioned studies suggest a role for both substance P and CGRP to a rapid, non-painful local heating stimulus; however, to date, no study has provided evidence to support this hypothesis. We have recently demonstrated a desensitisation of NK1 receptors to two consecutive microdialysis infusions of substance P (Wong et al., 2005). We sought to exploit these previous findings to investigate a possible role for NK1 receptors and, indirectly, substance P, in cutaneous thermal hyperaemia. We tested the hypothesis that pretreatment of the skin with substance P prior to local heating would modulate the initial peak and nadir response of thermal hyperaemia but would have no effect on the secondary (NO-dependent) plateau. The rationale was that infusion of substance P would render the NK1 receptors desensitised and, as such, substance P would not be able to modulate the prolonged action of CGRP. The initial peak and nadir would be indistinguishable and the vasodilator response to local heating would be mediated by CGRP and would resemble the pattern of cutaneous vasodilatation to a painful local heating stimulus. We further hypothesised that in sites pretreated with substance P and an NO synthase inhibitor the initial peak and nadir would be abolished and the NO-dependent plateau would be attenuated compared to both control and substance P only sites.
METHODS
Ethical Approval and Subjects
Seven men (24 ± 1 year) and two women (22 ± 1 year) participated in this study. The Institutional Review Board of the University of Oregon approved all methods and all subjects gave written informed consent prior to participation in this study. All subjects were healthy, normotensive, and non-smokers. All protocols in this study conformed to the guidelines as set forth in the Declaration of Helsinki. Subjects were not taking any medications with the exception of the two female subjects who were taking oral contraceptives. Phase of oral contraceptive usage was noted, but not controlled for, in these studies. As only two females participated in this study, separate statistics could not be used for the female subjects; therefore, data from both men and women were grouped and used for subsequent analysis.
Instrumentation
Subjects were equipped with an electrocardiogram and blood pressure was measured via automated brachial auscultation (CardioCap, Datex-Ohmeda, Tewksbury, MA, USA). All studies were performed in a thermoneutral laboratory with the subjects in the supine position and the experimental arm at heart level.
Each subject was instrumented with four microdialysis fibres (MD2000, Bioanalytical Systems, West Lafayette, IN, USA) on the ventral surface of the forearm. The membrane of the microdialysis fibres was 10mm in length and had a 20kDa molecular mass cut-off. To place the fibres, a 25-gauge needle was inserted into the dermal layer of the skin in the absence of anesthaesia; however, ice was used to numb the skin (Hodges et al., 2009). The microdialysis fibre was then threaded through the lumen of the needle, the needle and microdialysis fibre were pulled through the skin, leaving the membrane in place and completely removing the needle. Placement of the microdialysis fibres results in a minor trauma hyperaemia, which was allowed to subside (~45–90 minutes) prior to beginning the experimental protocol. During the trauma resolution period, lactated Ringer’s solution was perufsed through each fibre at a rate of 2μl min−1 via a microinfusion pump (CMA/102, CMA Microdialysis, Stockholm, Sweden).
Red blood cell (RBC) flux was used as an index of skin blood flow and was monitored via laser-Doppler flowmetry (Moor LAB, Moor Instruments, Devon, UK). To control local skin temperature, local skin heating devices (SH02 Skin Heaters, Moor Instruments, Devon, UK) were placed directly over each microdialysis membrane. Integrated laser-Doppler probes designed to fit in the center of the local heating device were used to measure RBC flux directly over each microdialysis membrane.
Drugs
Substance P (Calbiochem, San Diego, CA, USA) was dissolved in sterile lactated Ringer’s solution to a final concentration of 10μM. We have previously shown that consecutive microdialysis infusions of 10μM substance P desensitise the NK1 receptors (Wong et al. 2005). We have further shown the skin blood flow response to whole body heating is significantly attenuated following pretreatment with 10μM substance P (Wong & Minson, 2006). To inhibit NO synthase, 10mM of the L-arginine analog, NG-nitro-L-arginine-methyl ester (L-NAME; Calbiochem, San Diego, CA, USA), dissolved in sterile lactated Ringer’s solution was used. This concentration of L-NAME has been shown previously to adequately inhibit NO synthase in human skin (Minson et al., 2001). Skin blood flow was normalized to maximal vasodilatation via 28mM infusion of sodium nitroprusside (SNP; Nitropress, Abbott Laboratories, Chicago, IL, USA). This concentration of SNP has been shown to elicit maximal vasodilatation in human skin (Kellogg et al., 1999; Minson et al., 2001).
Protocol
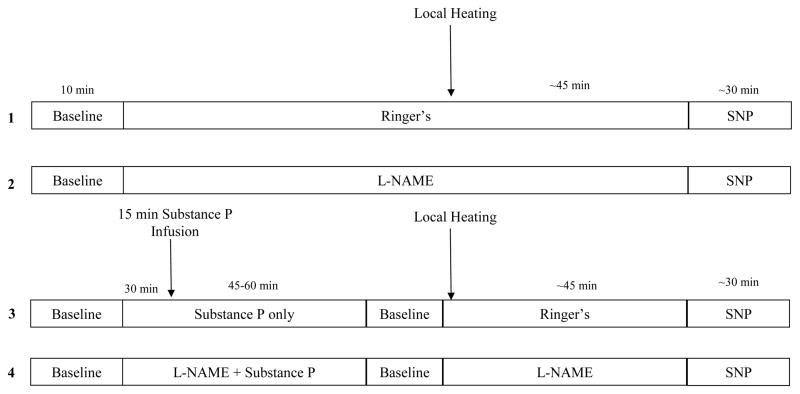
Microdialysis sites were randomly assigned to receive one of four treatments: 1) Ringer’s solution to serve as a control; 2) 10mM L-NAME to inhibit NO synthase; 3) 10μM substance P; or 4) substance P combined with L-NAME (final concentrations were 10μM and 10mM, respectively). Site 3 was used to investigate the contribution of NK1 receptor activation to the thermal hyperaemic response and site 4 was used to investigate the contribution of NO and NK1 receptor activation to the thermal hyperaemic response. Figure 1 provides a schematic illustration of the protocol and microdialysis sites.
Figure 1. Schematic Drawing of the General Experimental Protocol.
Numbers on left refer to microdialysis sites. Site 1 served as a control. Site 2 served as an L-NAME “control” and was used to determine the independent contribution of NO to cutaneous thermal hyperaemia. Site 3 was used to determine the independent contribution of substance P in cutaneous thermal hyperaemia. Site 4 was used to determine any interaction between substance P and NO in cutaneous thermal hyperaemia.
Following the trauma resolution period, baseline data was collected for 10 minutes with local skin heating devices set to 33°C. Following the baseline collection period, sites 2 and 4 were perfused with 10mM L-NAME for 30–45 minutes at a rate of 2μl min−1. Sites 3 and 4 were then perfused with 10μM substance P and 10μM substance P combined with 10mM L-NAME, respectively. Substance P and substance P plus L-NAME (sites 3 and 4, respectively) were perfused for 15 minutes at a rate of 4μl min−1. We have shown previously this infusion time and rate results in a reproducible and significant increase in skin blood flow (Wong et al., 2005; Wong & Minson, 2006). The ensuing substance P-induced vasodilatation was then allowed to return to baseline (~45–60 minutes).
Once a stable 5–10 minute period of baseline was achieved, local skin heaters were increased in all sites from a baseline temperature of 33°C to 42°C at a rate of 0.5°C every 5 seconds. This rate of local skin heating has been shown previously to elicit a reproducible increase in skin blood flow in the absence of pain (Kellogg et al., 1999; Minson et al., 2001). During the local heating protocol, subjects did not report any sensation of pain. The local skin heaters were held at 42°C until skin blood flow reached a stable 20–30 minute plateau. Following the plateau in skin blood flow at 42°C, each site was perfused with 28mM SNP at a rate of 4μl min−1 and locally heated to 43°C to maximally vasodilate the skin.
Data Collection and Statistical Analyses
Data were digitized and stored at 20Hz on a personal computer and were analyzed offline using signal-processing software (Windaq, Dataq Instruments, Akron, OH, USA). Cutaneous vascular conductance was calculated as RBC flux ÷ mean arterial pressure and is expressed as a percentage of maximal vasodilatation (%CVCmax).
Initial peak and nadir responses of cutaneous thermal hyperaemia were analyzed by averaging a stable 30–60 second period of skin blood flow. For the secondary plateau phase, a stable 3–5 minute period of skin blood flow used for subsequent analysis.
Each phase of the thermal hyperaemic response (initial peak, nadir, and secondary plateau) across treatments was analyzed using a one-way ANOVA with repeated measures. Similarly, each phase of thermal hyperaemia within a treatment was analyzed using a one-way ANOVA with repeated measures. Raw CVC values between treatment sites for each phase of thermal hyperaemia were compared using a one-way ANOVA with repeated measures. For all ANOVAs, a Tukey post hoc analysis was used to determine where significant differences occurred. P-values <0.05 were considered statistically significant and all data are presented as mean ± SEM.
RESULTS
Group cardiovascular variables, including systolic blood pressure, diastolic blood pressure, mean arterial pressure, and heart rate, at baseline and at the end of the local heating protocol (i.e., during the local heating plateau) are shown in Table 1. There were no statistical differences in any of the cardiovascular variables at baseline versus the end of the local heating protocol.
TABLE 1.
Subject Hemodynamic Data
| Baseline | Local Heating Plateau | |
|---|---|---|
| Systolic Blood Pressure (mmHg) | 112 ± 4 | 111 ± 3 |
| Diastolic Blood Pressure (mmHg) | 62 ± 2 | 68 ± 3 |
| Mean Arterial Pressure (mmHg) | 79 ± 3 | 82 ± 2 |
| Heart Rate (beats/min) | 67 ± 5 | 71 ± 6 |
Values are means ± SEM; n = 9
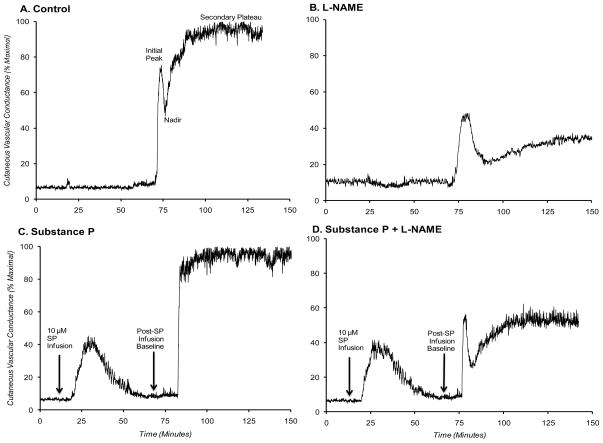
Figure 2A–D is a representative tracing of the cutaneous thermal hyperaemic response from one subject in (A) control, (B) L-NAME, (C) pretreatment with substance P, and (D) combined substance P plus L-NAME sites. Depicted are the baseline, initial peak, nadir, and secondary plateau phases of the thermal hyperaemic response for each treatment site. In panels C and D, initial baseline, period of substance P infusion and ensuing vasodilatation, and post-substance P infusion baseline are depicted.
Figure 2. Representative tracing of cutaneous thermal hyperaemic in the four treatment sites.
Data are from one representative subject. A) Control; B) L-NAME; C) pretreatment with substance P; and D) substance P plus L-NAME sites. Depicted are the initial peak, nadir, and secondary (NO-dependent) plateau phases. In panels (C) and (D), initial baseline, substance P-induced vasodilatation, and post-infusion baseline are shown.
Comparison of Thermal Hyperaemic Response across Treatment Sites
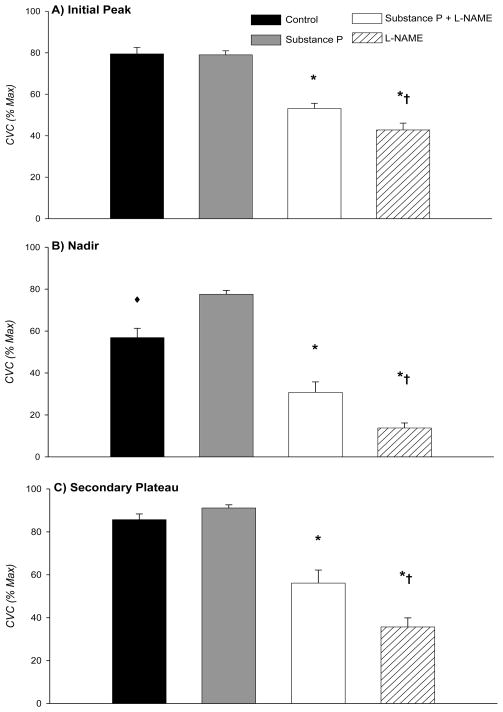
The group data for the initial peak in the four sites are summarized in Figure 3A. The initial peak CVC averaged 79±3 %CVCmax at control sites and 79±2 %CVCmax at sites pretreated with substance P. There was no statistical difference between these two sites (P >0.05). The L-NAME sites (43±3 %CVCmax) and substance P plus L-NAME sites (53±3 %CVCmax) were significantly reduced compared to both control and substance P sites (P <0.001). Additionally, L-NAME sites were significantly reduced compared to substance P plus L-NAME sites (P <0.01).
Figure 3. A–C. Group mean data comparing each phase of thermal hyperaemia across treatment sites.
Shown are: A) Initial Peak; B) Nadir; and C) Secondary Plateau. *, significantly reduced compared to control and substance P sites; †, significantly reduced compared to substance P plus L-NAME sites; ◆, significantly reduced compared to substance P sites.
The group data for the nadir are shown in Figure 3B. At control sites, the nadir averaged 57±4 %CVCmax, which was significantly reduced compared to the nadir at sites pretreated with substance P (78±2 %CVCmax; P <0.001). The nadir at L-NAME sites averaged 14±2 %CVCmax and at substance P plus L-NAME sites the nadir averaged 31±5 %CVCmax. The nadir at L-NAME sites was significantly reduced compared to control, substance P, and substance P plus L-NAME sites (P <0.01 for all conditions). The nadir at substance P plus L-NAME sites was significantly reduced compared to control and substance P sites (P <0.001 for all conditions).
Summary data for the secondary plateau are shown in Figure 3C. There was no statistical difference between control (86±3 %CVCmax) and substance P (91±1 %CVCmax) sites (P >0.05). However, L-NAME sites (36±4 %CVCmax) and substance P plus L-NAME sites (56±6 %CVCmax) were significantly reduced compared to both control and substance P sites (P <0.001 for all conditions). Furthermore, L-NAME sites were significantly reduced compared to substance P plus L-NAME sites (P <0.01).
Comparison of Thermal Hyperaemic Response within Treatment Sites (Data not shown)
At control sites, the initial peak and nadir were significantly reduced compared to the secondary plateau (P <0.05 for both conditions) and the nadir was significantly reduced compared to the initial peak (P <0.001). There was no observable or statistical difference between the initial peak and the nadir at sites pretreated with substance P (P >0.05). However, the initial peak/nadir was significantly reduced compared to the secondary plateau (P <0.01). The initial peak and secondary plateau were not significantly different from each other at L-NAME sites (P >0.05). However, the nadir was significantly reduced compared to the initial peak and the secondary plateau (P <0.001). There was no significant difference between the initial peak and secondary plateau at sites pretreated with substance P plus L-NAME (P >0.05). However, the nadir was significantly reduced compared to the initial peak and the secondary plateau (P <0.001 for both conditions).
Raw CVC Data
Raw CVC values for the initial peak, nadir, secondary plateau, and maximal vasodilatation are shown in Table 2. Units for raw CVC data are volts · mmHg−1.
TABLE 2.
Raw CVC Values
| Initial Peak CVC | Nadir CVC | Plateau CVC | Maximal CVC | |
|---|---|---|---|---|
| Control | 2.23 ± 0.10 | 1.64 ± 0.15 | 2.53 ± 0.21 | 2.94 ± 0.20 |
| L-NAME | 1.3 ± 0.07*#† | 0.45 ± 0.10*#† | 1.09 ± 0.09*#† | 3.07 ± 0.23 |
| Substance P | 2.31 ± 0.11 | 2.25 ± 0.10* | 2.66 ± 0.21 | 2.90 ± 0.20 |
| Substance P + L-NAME | 1.59 ± 0.08*# | 0.92 ± 0.09*# | 1.65 ± 0.10*# | 3.00 ± 0.25 |
Values are mean ± SEM. CVC, cutaneous vascular conductance. Units are volts/mmHg.
p < 0.05 vs. Control;
p < 0.05 vs. substance P;
p < 0.05 vs. substance P + L-NAME
DISCUSSION
The major finding of this study is that pretreatment of human skin with substance P, either alone or in the presence of an NO synthase inhibitor, significantly alters the cutaneous thermal hyperaemic response to rapid, non-painful local heating. Previous data from our laboratory has demonstrated a desensitisation of NK1 receptors to two consecutive microdialysis infusions of substance P (Wong et al., 2005). Therefore, we sought to exploit these previous findings in order to investigate the role of NK1 receptors and, indirectly, substance P, in cutaneous thermal hyperaemia. In our previous study (Wong et al., 2005), we found the cutaneous vasodilation to a second infusion of substance P was significantly attenuated, and often times, abolished. Similarly, we have previously reported (Wong & Minson, 2006) that reflex cutaneous vasodilation is significantly attenuated following a single microdialysis infusion of 10 μM substance P. Therefore, the experimental paradigm in the present investigation utilised a single 10 μM microdialysis infusion of substance P as a means to desenstise the NK1 receptors. The data from the present investigation provide evidence to suggest NK1 receptors are involved in cutaneous thermal hyperaemia. This was evidenced by the lack of a distinct initial peak and nadir response in sites pretreated with substance P compared to control sites and the partial restoration of the secondary plateau in sites pretreated with substance P plus an NO synthase inhibitor compared to NO synthase inhibition only sites (Figures 3 and 4). These data suggest substance P is involved in cutaneous thermal hyperaemia, based on the known location of substance P in the skin and its preferential binding to NK1 receptors.
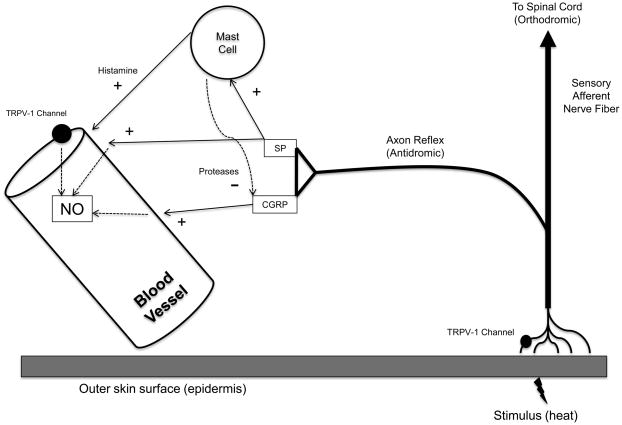
Figure 4. Schematic illustration of proposed working model.
Based on available data, our model suggests heat activates cutaneous sensory afferent nerves via TRPV-1 channels. The axon reflex releases substance P and CGRP from nerve terminals to directly mediate a portion of thermal hyperaemia and possibly via NO. Substance P also liberates histamine and proteases from cutaneous mast cells where the proteases have an inhibitory (−) effect on CGRP-mediated vasodilatation. Activation of endothelial TRPV-1 channels may constitute another mechanism by which NO increases. SP, substance P; NO, nitric oxide
There are four key lines of evidence to support the hypothesis that sensory nerves and axon reflexes mediate the initial, rapid increase in skin blood flow in response to local heating. First, Magerl and Treede (1996) observed an increase in skin blood flow in response to direct heat at sites distal from the heat source. Second, Minson and colleagues (2001) demonstrated the initial peak and nadir response of thermal hyperaemia are significantly reduced in the presence of an NO synthase inhibitor and are further reduced in skin treated with EMLA cream, which blocks the axon reflexes. Third, Wong and Fieger (2010) reported TRPV-1 channels, which are putative channels located on sensory nerves and are activated by heat, are required for full expression of the axon reflex component of thermal hyperaemia. The data from Wong and Fieger (2010) further suggest activation of TRPV-1 channels may serve as a functional sensory link between application of heat to the skin and sensory nerve depolarization. Fourth, Hodges (2008, 2009b) and Houghton (2006) found that the axon reflex is either abolished or significantly attenuated when adrenergic vasoconstrictor nerves are blocked or the receptors for noradrenaline and neuropeptide Y are inhibited. Although these studies all implicate cutaneous sensory nerves, axon reflexes, and adrenergic vasoconstrictor nerves in mediating the initial peak and nadir components of thermal hyperaemia, it remains unclear which vasoactive substances are released from sensory nerve terminals and ultimately cause cutaneous vasodilatation.
While substance P has been shown to induce a robust, but transient, increase in skin blood flow (Klede et al., 2003; Weidner et al., 2000; Wong et al., 2005) CGRP has been shown to elicit a sustained increase in skin blood flow (Brain 1996; Brain et al., 1986; Brain and Williams, 1988; Weidner et al., 2000). The prolonged vasodilator action of CGRP can be attenuated when substance P is co-injected with CGRP (Brain and Williams, 1988; Wallengren, 1997; Wallengren & Wang, 1993) and this pattern of vasodilatation qualitatively resembles the axon reflex-mediated initial peak and nadir response of cutaneous thermal hyperaemia. Data from the present investigation suggests a regulatory role for NK1 receptors in cutaneous thermal hyperaemia, as evidenced by the differing responses in control versus substance P-treated sites (Figures 3 and 4), and are consistent with the hypothesis that substance P regulates the vasodilator action of CGRP, although this was not directly investigated.
Based on the available evidence from this and previous studies, we have constructed a working model of cutaneous thermal hyperaemia (Figure 4). Under control conditions, the initial peak and nadir response are mediated, in part, by NO and an axon reflex mechanism (Magerl & Treede, 1996; Minson et al., 2001; Wong & Fieger, 2010), where heat activates TRPV-1 channels on cutaneous sensory nerves and substance P and CGRP are co-released and are the neuropeptides that mediate axon reflex-mediated vasodilatation. The role of substance P is to modulate the prolonged vasodilator activity of CGRP via release of proteases from cutaneous mast cells. Substance P may further serve to increase the local production and availability of NO, which mediates the prolonged secondary plateau phase (Kellogg et al., 1999; Minson et al., 2001) while CGRP may act directly on vascular smooth muscle to mediate a portion of the vasodilatation.
In sites pretreated with substance P, the initial peak and nadir response become indistinguishable from each other due to desensitisation of NK1 receptors prior to local heating. Under these conditions, substance P and CGRP are co-released during local heating but mast cells become unresponsive to substance P. Thus, proteases are not released from mast cells and the prolonged CGRP-induced vasodilatation is left unregulated, resulting in elimination of the initial peak and nadir response. A limitation to this mechanism is the NK1 receptor isoform has not been specifically identified on human cutaneous mast cells. Rather, it has been suggested that non-specific receptors that bind a host of substances, including substance P are located on cutaneous mast cells (Church et al., 1991). The possibility remains these receptors, similar to NK1 receptors, undergo a process of endocytosis and/or desensitisation upon binding of a ligand.
The possible mechanism(s) underlying cutaneous thermal hyperaemia under conditions in which the skin is pretreated with substance P plus L-NAME are not as clear. As the skin was pretreated with substance P, we hypothesised the initial peak and nadir would be modulated such that these two phases of thermal hyperaemia would be indistinguishable from each other, similar to what was observed in substance P only sites (figure 2B). In contrast to our hypothesis, we observed a distinct, but reduced, initial peak and nadir response and an attenuated secondary plateau compared to control and substance P only sites; however, all three phases of cutaneous thermal hyperaemia exhibited a partial restoration when compared to L-NAME only sites.
It is possible the partial restoration of the thermal hyperaemic response observed in sites treated with substance P + L-NAME is due to an unmasking of either an unknown vasodilator(s) or unmasking of one or more of the currently known contributors to cutaneous thermal hyperaemia. While the data from the present study do not allow for us to directly address the identity of the potential vasodilators, several possible candidates exist, including vasoactive prostanoids, histamine receptor activation, and A1/A2 adenosine receptor activation. Vasoactive prostanoids and histamine receptor activation do not appear to be involved in cutaneous thermal hyperaemia. Prostaglandins seem unlikely as both Golay et al. (2004) and McCord et al. (2006) found no direct role for vasoactive prostanoids in thermal hyperaemia and McCord et al. (2006) were unable to unmask any further role for prostaglandins when both the cyclooxygenase pathway and NO synthase pathway were blocked simultaneously. Inasmuch as our laboratory has recently demonstrated only a modest role for H1 receptor activation to the nadir component and no role for H2 receptors to any phase of thermal hyperaemia (Wong et al. 2006), it appears histamine receptor activation is not involved.
Recent data from Fieger and Wong (2010) have demonstrated a role for A1/A2 adenosine receptor activation in cutaneous thermal hyperaemia. Inhibition of A1/A2 adenosine receptors modestly attenuated all components of thermal hyperaemia and when A1/A2 adenosine receptor inhibition was combined with NO synthase inhibition there was a greater attenuation of the plateau compared to the independent A1/A2 inhibition or NO synthase inhibition. It is possible a larger role for A1/A2 adenosine receptor activation may be unmasked under conditions when NK1 receptors are desensitised and NO synthase is inhibited.
Limitations
A limitation to the interpretation of the data from the present study is that we did not use a specific NK1 antagonist. Preliminary work in our laboratory using several different classical NK1 receptor antagonists was problematic in that low doses of the antagonists were without effect on substance P-mediated vasodilatation whereas higher doses of antagonists resulted in non-specific vasodilation, which was often near maximal. As such, we took advantage of previous findings that exposure of NK1 to substance P renders these receptors unresponsive to a subsequent exposure to substance P. Because we did not use a classical NK1 receptor antagonist we cannot be certain that the data and observations from the present study are entirely due to NK1 receptors. Further, based on the present data and experimental paradigm we cannot speak to whether substance P per se is involved in the cutaneous thermal hyperaemic response.
Specific to human skin, we have previously demonstrated the aforementioned phenomenon in two separate publications investigating the mechanisms of substance P-mediated vasodilation in human skin (Wong et al., 2005) as well as the effect of NK1 receptor desensitisation on the skin blood flow response to whole body heat stress (Wong & Minson, 2006). During whole body heat stress we found desensitisation of NK1 receptors plus inhibition of NO synthase accounts for ~70% of the increase in skin blood flow. Conversely, in the present study we found combined NK1 receptor desensitisation combined with inhibition of NO synthase partially restores the cutaneous thermal hyperaemic response. Although we areunable to directly address the contribution of substance P and NK1 receptors to cutaneous thermal hyperaemia, we believe the data from the present study provide convincing evidence of a potential contribution of NK1 receptor activation and, indirectly, substance P to cutaneous thermal hyperaemia. Based on our previously published data demonstrating an attenuated, and often times abolished, cutaneous vasodilation to repeated infusions of substance P, as well as the stark contrast in cutaneous vascular responses between local and whole body heating at sites pretreated with substance P plus L-NAME, we are also confident in our experimental paradigm used in the current study.
In conclusion, this is the first study to provide evidence of a regulatory role for NK1 receptors in cutaneous thermal hyperaemia. We found that pretreatment of the skin with substance P prior to local heating modulates the initial peak and nadir response but does not affect the secondary plateau phase. Furthermore, in skin sites pretreated with substance P plus L-NAME we observed a partial restoration of all phases of thermal hyperaemia when compared to L-NAME only sites. Although these data suggest a regulatory role for NK1 receptors and are consistent with a role for substance P in the axon reflex-mediated component of thermal hyperaemia, more research is needed to fully elucidate the mechanisms underlying cutaneous thermal hyperaemia.
Acknowledgments
The authors would like to extend their appreciation to all of the subjects who participated in this series of studies.
FUNDING SOURCES
This study was funded by National Institutes of Health Grant HL-70928.
Footnotes
DISCLOSURES
The authors have no conflict of interest or other disclosure to report.
References
- 1.Brain SD. Sensory neuropeptides in the skin. In: Geppetti P, Holzer P, editors. Neurogenic Inflammation. Boca Raton, FL: CRC Press; 1996. [Google Scholar]
- 2.Brain SD, Tippins JR, Morris HR, MacIntyre I, Williams TJ. Potent vasodilator activity of calcitonin gene-related peptide in human skin. J Invest Dermatol. 1986;87:533–536. doi: 10.1111/1523-1747.ep12455620. [DOI] [PubMed] [Google Scholar]
- 3.Brain SD, Williams TJ. Substance P regulates the vasodilator activity of calcitonin gene-related peptide. Nature. 1988;335:73–75. doi: 10.1038/335073a0. [DOI] [PubMed] [Google Scholar]
- 4.Bull HA, Hothersall J, Chowdhury N, Cohen J, Dowd PM. Neuropeptided induce release of nitric oxide from human dermal microvascular endothelial cells. J Invest Dermatol. 1996;106:655–660. doi: 10.1111/1523-1747.ep12345471. [DOI] [PubMed] [Google Scholar]
- 5.Church MK, Suhad E-L, Caulfield JP. Neuropeptide-induced secretion from human skin mast cells. Int Arch Allergy Appl Immunol. 1991;94:310–318. doi: 10.1159/000235393. [DOI] [PubMed] [Google Scholar]
- 6.Fieger SM, Wong BJ. Adenosine receptor inhibition with theophylline attenuates the skin blood flow response to local heating in humans. Exp Physiol. 2010a;95:946–954. doi: 10.1113/expphysiol.2010.053538. [DOI] [PubMed] [Google Scholar]
- 7.Golay S, Haeberli C, Delachaux A, Liaude L, Kucera P, Waeber B, Feihl F. Local heating of human skin causes hyperaemia without mediation by muscarinic cholinergic receptors or prostanoids. J Appl Physiol. 2004;97:1781–1786. doi: 10.1152/japplphysiol.00814.2003. [DOI] [PubMed] [Google Scholar]
- 8.Hodges GJ, Chiu C, Kosiba WA, Zhao K, Johnson JM. The effect of microdialysis needle trauma on cutaneous vascular responses in humans. J Appl Physiol. 2009a;106:1112–1118. doi: 10.1152/japplphysiol.91508.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodges GJ, Kosiba WA, Zhao K, Johnson JM. The involvement of norepinephrine, neuropeptide Y, and nitric oxide in the cutaneous vasodilator response to local heating in humans. J Appl Physiol. 2008;105:233–240. doi: 10.1152/japplphysiol.90412.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodges GJ, Kosiba WA, Zhao K, Johnson JM. The involvement of heating rate and vasoconstrictor nerves in the cutaneous vasodilator response to skin warming. Am J Physiol Heart Circ Physiol. 2009b;296:H51–H56. doi: 10.1152/ajpheart.00919.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Houghton BL, Meendering JR, Wong BJ, Minson CT. Nitric oxide and noradrenaline contribute to the temperature threshold of the axon reflex response to gradual local heating in human skin. J Physiol. 2006;572:811–820. doi: 10.1113/jphysiol.2005.104067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hughes SR, Brain SD. Nitric oxide-dependent release of vasodilator quantities of calcitonin gene-related peptide from capsaicin-sensitive nerves in rabbit skin. Br J Pharmacol. 1994;111:425–430. doi: 10.1111/j.1476-5381.1994.tb14752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huttunen M, Harvima IT, Ackermann L, Harvima RJ, Naukkarinen A, Horsmanheimo M. Neuropeptide- and capsaicin-induced histamine release in skin monitored with the microdialysis technique. Acta Derm Venereol (Stockh) 1996;76:205–209. doi: 10.2340/0001555576205209. [DOI] [PubMed] [Google Scholar]
- 14.Irani AA, Schechter NM, Craig S, DeBlois G, Schwartz LB. Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci USA. 1986;83:4464–4468. doi: 10.1073/pnas.83.12.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kellogg DL, Jr, Liu Y, Kosiba IF, O’Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol. 1999;86:1185–1190. doi: 10.1152/jappl.1999.86.4.1185. [DOI] [PubMed] [Google Scholar]
- 16.Klede M, Clough G, Lischetzki G, Schmelz M. The effect of the nitric oxide synthase inhibitor N-nitro-L-arginine-methyl ester on neuropeptide-induced vasodilation and protein extravasation in human skin. J Vasc Res. 2003;40:105–114. doi: 10.1159/000070707. [DOI] [PubMed] [Google Scholar]
- 17.Lowman MA, Rees PH, Benyon RC, Church MK. Human mast cell heterogeneity: histamine release from mast cells disperse from skin, lung, adenoids, tonsils and intestinal mucosa in response to IgE-dependent and non-immunological stimuli. J Allergy Clin Immunol. 1988;81:590–597. [PubMed] [Google Scholar]
- 18.Magerl W, Treede R-D. Heat-evoked vasodilatation in human hairy skin: axon reflexes due to low-level activity of nociceptive afferents. J Physiol. 1996;497:837–848. doi: 10.1113/jphysiol.1996.sp021814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCord GR, Cracowski J-L, Minson CT. Prostaglandins contribute to cutaneous active vasodilatation in humans. Am J Physiol Heart Circ Physiol. 2006 doi: 10.1152/ajpregu.00710.2005. In Press. [DOI] [PubMed] [Google Scholar]
- 20.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol. 2001;91:1619–1626. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- 21.Petersen LJ, Poulsen LK, Søndergarrd J, Skov PS. The use of cutaneous microdialysis to measure substance P-induced histamine release in intact human skin in vivo. J Allergy Clin Immunol. 1994;94:773–783. doi: 10.1016/0091-6749(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 22.Petersen LJ, Winge K, Brodin E, Skov PS. No release of histamine an substance P in capsaicin-induced neurogenic inflammation in intact human skin in vivo: a microdialysis study. Clin Exp Allergy. 1997;27:957–965. [PubMed] [Google Scholar]
- 23.Quartara L, Maggi CA. The tachykinin NK1 receptor. Part I: ligands and mechanisms of cellular activation. Neuropeptides. 1997;31:537–563. doi: 10.1016/s0143-4179(97)90001-9. [DOI] [PubMed] [Google Scholar]
- 24.Ralevic V, Khalil Z, Dusting GJ, Helme RD. Nitric oxide and sensory nerves are involved in the vasodilator response to acetylcholine but not calcitonin gene-related peptide in rat skin microvasculature. Br J Pharmacol. 1992;106:650–655. doi: 10.1111/j.1476-5381.1992.tb14390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sann H, Pierau FK. Efferent functions of C-fibre nociceptors. Z Rheumatol. 1998;57:8–13. doi: 10.1007/s003930050226. [DOI] [PubMed] [Google Scholar]
- 26.Wallengren J. Vasoactive peptides in the skin. J Invest Dermatol Symposium Proceed. 1997;2:49–55. doi: 10.1038/jidsymp.1997.11. [DOI] [PubMed] [Google Scholar]
- 27.Wallengren J, Hakanson R. Effects of substance P, neurokinin A and calcitonin gene-related peptide in human skin and their involvement in sensory nerve-mediated responses. Eur J Pharmacol. 1987;143:267–73. doi: 10.1016/0014-2999(87)90542-5. [DOI] [PubMed] [Google Scholar]
- 28.Wallengren J, Wang Z-Y. Interaction between tachykinins and CGRP in human skin. Acta Derm Venereol (Stockh) 1993;73:259–261. doi: 10.2340/0001555573259261. [DOI] [PubMed] [Google Scholar]
- 29.Weidner C, Klede M, Rukwied R, Lischetzki G, Neisius U, Skov PS, Petersen LJ, Schmelz M. Acute effects of substance P and calcitonin gene-related peptide in human skin—a microdialysis study. J Invest Dermatol. 2000;115:1015–1020. doi: 10.1046/j.1523-1747.2000.00142.x. [DOI] [PubMed] [Google Scholar]
- 30.Wong BJ, Fieger SM. Transient receptor potential vanilloid type-1 (TRPV-1) channels contribute to cutaneous thermal hyperaemia in humans. J Physiol. 2010;588:4317–4326. doi: 10.1113/jphysiol.2010.195511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong BJ, Minson CT. Neurokinin-1 receptor desensitisation attenuates cutaneous active vasodilatation in humans. J Physiol. 2006;577:1043–1051. doi: 10.1113/jphysiol.2006.112508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong BJ, Tublitz NJ, Minson CT. Neurokinin-1 receptor desensitisation to consecutive microdialysis infusions of substance P in human skin. J Physiol. 2005;568:1047–1056. doi: 10.1113/jphysiol.2005.095372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong BJ, Williams SJ, Minson CT. Minimal role for H1 and H2 histamine receptors in cutaneous thermal hyperaemia to local heating in humans. J Appl Physiol. 2006;100:535–540. doi: 10.1152/japplphysiol.00902.2005. [DOI] [PubMed] [Google Scholar]