Abstract
An inflammatory response is a pathological hallmark of amyotrophic lateral sclerosis (ALS), a relentless and devastating degenerative disease of motoneurons. This response is not simply a late consequence of motoneuron degeneration, but actively contributes to the balance between neuroprotection and neurotoxicity; initially infiltrating lymphocytes and microglia slow disease progression, while later, they contribute to the acceleration of disease. Since motor weakness begins in the hindlimbs of ALS mice and only later involves the forelimbs, we determined whether differential protective versus injurious inflammatory responses in the cervical and lumbar spinal cords explained the temporally distinct clinical disease courses between the limbs of these mice. Densitometric evaluation of immunohistochemical sections and quantitative RT-PCR (qRT-PCR) demonstrated that CD68 and CD11c were differentially increased in their spinals cords. qRT-PCR revealed that protective and anti-inflammatory factors, including BDNF, GDNF, and IL-4, were increased in the cervical region compared with the lumbar region. In contrast, the toxic markers TNF-α, IL-1β and NOX2 were not different between ALS mice cervical and lumbar regions. T lymphocytes were observed infiltrating lumbar spinal cords of ALS mice prior to the cervical region; mRNA levels of the transcription factor gata-3 (Th2 response) were differentially elevated in the cervical cord of ALS mice whereas t-bet (Th1 response) was increased in the lumbar cord. These results reinforce the important balance between specific protective/injurious inflammatory immune responses in modulating clinical outcomes and suggest that the delayed forelimb motor weakness in ALS mice is partially explained by augmented protective responses in the cervical spinal cords.
Keywords: ALS, IL-4, microglia, inflammation, protective microglia, toxic microglia, spinal cord
1. Introduction
The central nervous system (CNS) has traditionally been considered immunologically privileged, but there has been a re-evaluation of this tenet and neuroinflammation is now recognized to be a prominent feature of many classic neurodegenerative disorders including amyotrophic lateral sclerosis (ALS) (Carson et al., 2006; Barbeito et al., 2010). ALS is an adult-onset, relentlessly progressive neurodegenerative disorder and although numerous pathological processes appear to contribute to motoneuron injury in this inexorable disease, there is a neuroinflammatory response, highlighted by the presence of activated microglia and infiltrating lymphocytes at sites of motoneuron injury, that also contributes to this pathogenic process (Engelhardt et al. 1993; McGeer and McGeer, 2002; Henkel et al. 2004; Boillée et al. 2006; Kassa et al. 2009). Recent evidence suggests that this response is not simply a late consequence of motoneuron degeneration, but actively contributes to the balance between neuroprotection and neurotoxicity; initially activated microglia and infiltrating lymphocytes slow disease progression, while later, they can contribute to the acceleration of disease (Beers et al. 2006; Beers et al. 2008).
As a component of the innate immune system, microglia are of hematopoietic origin and colonize the CNS during early development; microglia are resident macrophages that sample the extracellular space through continuous extension, retraction, and remodeling of their cellular processes (McKercher et al. 1996; Beers et al. 2006; Ransohoff and Perry 2009). As primary intrinsic immune effector cells, microglia are involved in virtually all pathological processes of the CNS including inflammatory, neurodegenerative, traumatic, neoplastic, and vascular diseases, and as identified more recently, neuroprotection (Carson et al., 2006; Appel et al., 2010). Cumulative data suggest that when activated, microglia can produce and release an array of pro-and anti-inflammatory molecules, and may exert either a toxic or protective effect on neurons depending on the physiologic conditions; earlier reports proposed that microglial activation is detrimental to the CNS, but other evidence suggests that inhibition of this activation does not improve neurological outcomes (Arvin et al., 2002; Tsuji et al., 2004; Henkel et al., 2009; Appel et al., 2010; Polazzi and Monti 2010). In response to injury, microglia undergo rapid morphological and functional activation which includes phagocytosis, antigen presentation, as well as the production and secretion of ROS, cytokines and growth factors (Kreutzberg 1996; Zhao et al. 2004; Hanisch and Kettenmann 2007; Ransohoff and Perry 2009). More recently, several studies have documented the neuroprotective attributes of microglia that may enhance neuronal survival through the release of the trophic factors BDNF and IGF-1, and anti-inflammatory factors IL-4 and IL-10 (Zhao et al., 2006; Lalancette-Hébert et al., 2007; Simard and Rivest, 2007). Although there is considerable morphological and neurochemical evidence for the proliferation and activation of microglia in ALS, it had remained unclear whether these innate immune cells protect motoneurons or contribute to neuronal injury (Moisse and Strong 2006). The most direct evidence that both outcomes are possible is the temporal association between neuroinflammation and the progression of motoneuron disease observed in animal models of ALS (Beers et al., 2006; Beers et al., 2008).
In addition to microglial involvement in the ALS pathogenic process, recent studies have shown that the presence of T lymphocytes, while they have little influence on disease onset, played a fundamental role in the rate of disease progression. CD4+ T lymphocytes infiltrating ALS mice CNS slowed disease progression, prolonged survival, and enhanced the levels of anti-inflammatory cytokines and trophic factors; T lymphocytes in ALS mice play an endogenous neuroprotective function by augmenting the protective potentials of microglia and attenuating their toxic responses (Beers et al., 2008; Chiu et al., 2008). Another study demonstrated that passively transferred ex vivo activated T lymphocytes delayed motoneuron loss, improved neurological function, and increased life expectancy of ALS mice (Banerjee et al., 2008). These results suggest that, in a model of chronic neurodegeneration, T lymphocytes may play an endogenous neuroprotective role by modulating beneficial microglial responses and supports the concept of a well-orchestrated and complex dialogue among microglia, T cells, and neurons (Appel et al. 2010).
In ALS mice, motor weakness begins in the hindlimbs, culminating in hindlimb paralysis, and slowly progresses to include forelimb weakness by end-stage (ES) disease. In this report, we determined whether the temporal functional discrepancies between the onset of disease and levels of disease burden in the limbs was associated with differences in protective versus injurious inflammatory responses within the cervical and lumbar spinal cord regions. We demonstrate that after disease onset and detectable pathology, there is a toxic response in both the lumbar and cervical spinal cord regions of ALS mice, but the cervical region had an augmented and sustained protective inflammatory response as disease progresses.
2. Methods
2.1. Mice
ALS mice, on a C57Bl/6 genetic background and overexpressing the G93A mutation in the Cu2+/Zn2+ superoxide dismutase gene (mSOD1), a transgenic animal model of inherited human ALS (Gurney et al., 1994), were bred and maintained in The Methodist Hospital Research Institute’s animal facility. All animals were housed in microisolator cages with access to food and water ad libitum. All experimental procedures involving animals were approved by The Methodist Hospital Research Institute’s Institutional Animal Care and Use Committee in compliance with National Institutes of Health guidelines. ALS mice were identified and mSOD1 copy number verified by quantitative PCR (qRT-PCR) as previously described (Beers et al., 2008). Disease symptoms and course were assessed using the BASH scoring system (supplementary materials, table 1; (Beers et al., 2006; Beers et al., 2008). Specific cytokines/chemokines/neurotrophic factor mRNA levels were assessed at disease onset (11 weeks), the stable disease phase (14 and 16 weeks), the point at which disease progression rapidly accelerates (18 weeks), the rapidly progressing phase (20 weeks), and end stage (ES) disease; age-matched wild-type (WT) mice served as controls.
2.2. Immunohistochemistry
The mice were lethally anesthetized and perfused with phosphate buffered saline (PBS: pH: 7.4) followed by 3% neutral paraformaldehyde. Thirty-micron thick sections were cut from the spinal cord and washed three times in PBS. The sections were blocked for endogenous peroxidase activity (0.3% H2O2 in distilled water, 30 min.). The sections were pretreated with normal serum for 1 hour at room temperature (RT) to block the non-specific IgG binding sites. The CD68, CD11c, CD3, and CD4 primary antibodies were diluted in PBS containing normal serum and incubated overnight at 4°C. As a negative control the primary antibodies were omitted during the reaction. After rinsing in PBS, the sections were incubated with a biotinylated labeled secondary IgG for 2 hours at RT. The immunostained sections were examined using a Zeiss Imager-Z1m microscope equipped with a Zeiss AxioCam MRc5 color camera and Zeiss digital image analysis system (Karl Zeiss, Oberkochen, Germany).
2.3. Image analysis
CD68 and CD11c immunoreactivities in the cervical and lumbar spinal cords were analyzed by densitometry. Photomicrographed immune-stained spinal cord sections (n = 3 sections at each time point) were imported into Photoshop 6.0 (Adobe Systems Inc., San Jose, CA) and assessed using quantitative densitometric analyses adapted from Lindley et al. (2008). Briefly, a constant ventral horn size in each section was captured and the total number of pixels within that area was isolated, and the number and the average gray level of pixels darker than background was determined for each section. The same constant ventral horn size was captured and analyzed from WT mice spinal cords. The number of pixels darker than WT background was divided by the total number of pixels within the isolated image, to provide a relative signal.
2.3. qRT-PCR
RNA was isolated from homogenized frozen spinal cord tissue using Trizol (Gibco) and purified using RNeasy (Qiagen) according to the manufacturers’ recommendations. The RNA concentrations were determined spectrophotometrically (NanoDrop Spectrophotometer ND-1000). qRT-PCR was performed on 10 ng of mRNA as previously described (Beers et al., 2008; Henkel et al., 2006). Primer efficiency was assessed by analyzing a dilution series of mRNA. The relative expression level of each mRNA was calculated using the ΔΔCt method normalizing to β-actin and relative to the control samples. The presence of one product of the correct size was verified by gel electrophoresis and melting curve analyses.
2.4. Statistical analyses
Data were analyzed using two-tailed Student's t-test using Excel software. Data are expressed as mean ± standard error of the mean (SEM); p < 0.05 was considered statistically significant. Differences between groups were analyzed using a two-way ANOVA (SigmaStat, Richmond CA).
3. Results
3.1. Morphological evaluation of microglia in the cervical and lumbar spinal cord regions of ALS mice
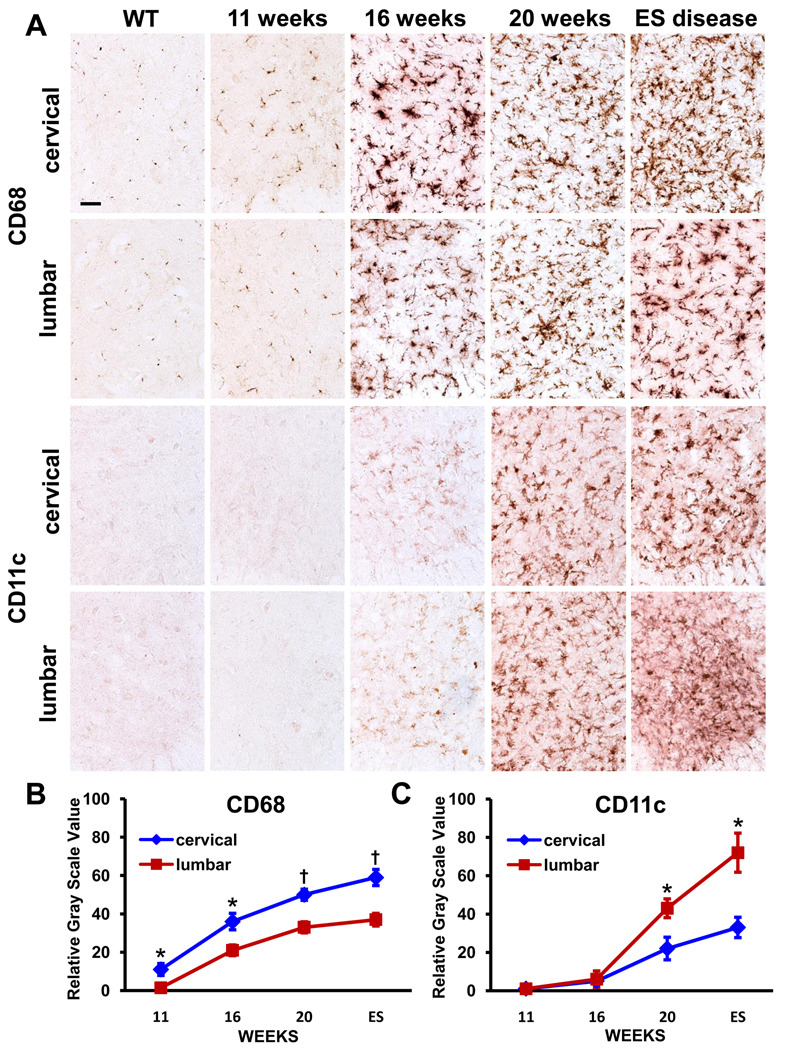
Because motor weakness begins in the hindlimbs of ALS mice and slowly progresses to include the forelimbs, we immunohistochemically assayed the cervical and lumbar spinal cord regions from these mice to detect any observable differences in microglial morphological activation states. The cervical and lumbar spinal cord sections of ALS mice were stained at disease onset (11 weeks), the stable disease phase (16 weeks), and the rapidly progressing phase (20 weeks), and at near ES disease, and compared with age-matched WT mice. Using CD68 antibodies, a marker of phagocytic microglia, there appeared to be relatively more signal in the cervical regions of ALS mice compared with their lumbar regions; the signal increased in both regions of ALS mice spinal cords as disease progressed (Fig. 1A). In contrast, when using antibodies to CD11c, a marker for immature dendritic cell (DC), there appeared to be relatively more signal in the lumbar regions of ALS mice compared with their cervical cords; there was no detectable CD11c signal in WT mice and ALS mice at 11 weeks (Fig. 1A).
Fig. 1.
Immunohistochemical evaluations of CD68 and CD11c in the cervical and lumbar spinal cords of ALS mice. (A) CD68 is relatively increased in the cervical region whereas CD11c is relatively increased in lumbar region of these mice. (B) and (C) Densitometric analyses confirm the relative difference of CD68 and CD11c immuno-signals. * ≤ 0.05, ALS mice cervical region compared with their lumbar region; † ≤ 0.01, ALS mice cervical region compared with their lumbar region. Scale bar: 50 µm.
To quantitatively assess whether the observed immuno-signals in the cervical and lumbar spinal cords of ALS were different, sections from each region were subjected to densitometric analyses. The CD68 signal in immune-stained sections was increased in the cervical cords of ALS mice at all assessed time points compared with their lumbar regions (Fig. 1B). In contrast, the CD11c signal was similar between cervical and lumbar regions at 11 and 16 weeks of age, but increased in the lumbar spinal cords of ALS mice at 20 weeks of age compared with their cervical regions, and remained elevated in this region through ES disease (Fig. 1C).
3.2. Expression levels for phagocytic and dendritic phenotypes are differentially elevated in ALS mice
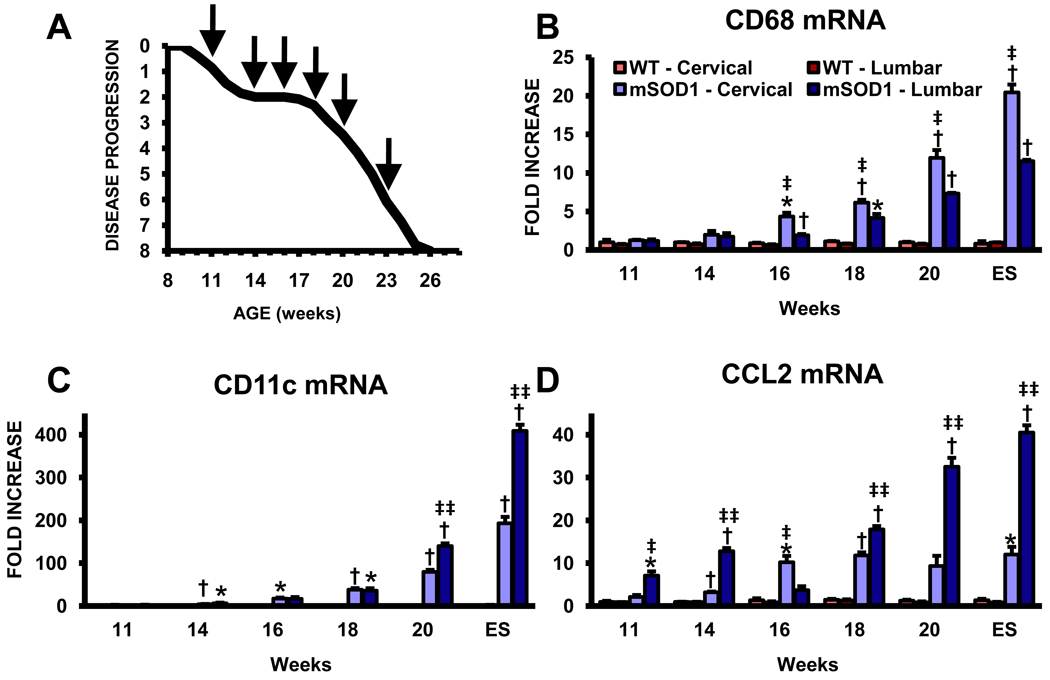
Since there were observable morphological differences in the activation states of microglia from the cervical spinal cords of ALS mice compared with their lumbar regions, we used qRT-PCR to accurately assess the levels of phagocytic and DC phenotypes in the cervical and lumbar spinal cords of these mice at disease onset (11 weeks), the stable disease phase (14 and 16 weeks), the point at which disease progression rapidly accelerates (18 weeks), the rapidly progressing phase (20 weeks), and end stage (ES) disease (Fig. 2A). The mRNA levels for CD68 were increased at 16 weeks in the cervical spinal cords of ALS mice compared with their levels in the lumbar spinal cords (Fig. 2B). Although CD68 levels increased in both regions as disease progressed, they increase at a more rapid rate in the cervical cords than in the lumbar cords of these mice. On the contrary, CD11c mRNA levels were differentially increased at 20 weeks in the lumbar spinal cords of these mice compared with the cervical region of their spinal cords, at a time when disease rapidly accelerates in these mice (Fig. 2C). There was a greater discrepancy, approximately 2 fold, between the two regions at ES disease; although not observably detectable by immunohistochemistry, CD11c expression was detected in the spinal cords of WT and 11 week ALS mice by qRT-PCR. CCL2 mRNA, a cytokine involved in the recruitment of peripheral immune cells, was also differentially increased in the lumbar spinal cords of ALS mice compared with the cervical regions (Fig. 2D). The CCL2 mRNA expression levels were approximately 3.5 fold greater in the lumbar region at 20 weeks and ES disease than the levels in the cervical region. We have previously demonstrated that increased expression of DC markers was associated with a more rapidly progressing disease in ALS patients, and that CCL2 was elevated in ALS patient spinal cords, thus the current data support the involvement of immune/inflammatory responses in amplifying motoneuron degeneration (Henkel et al., 2004).
Fig. 2.
Quantitative RT-PCR analyses reveal differentially elevated markers for phagocytic and dendritic cell phenotypes in ALS mice. (A) Graph of the disease progression curve and time points (arrows) when qRT-PCR assays were performed on ALS mice; based upon the BASH score. Each time point represents the mean of n = 3 mice. Notice the stable disease phase from 14 to 18 weeks and the rapidly progressing phase subsequent to 18 weeks. (B) mRNA levels for CD68, a LAMP protein expressed on phagocytic cells CD68. (C) CD11c mRNA levels. Notice the scale of the ordinate in this graph. (D) CCL2 mRNA levels. * ≤ 0.05, ALS mice spinal cord regions compared with the same regions from WT mice; † ≤ 0.01, ALS mice spinal cord regions compared with the same regions from WT mice; ‡ ≤ 0.05, ALS mice cervical spinal cords compared with ALS mice lumbar spinal cords, ‡‡ ≤ 0.01, ALS mice cervical spinal cords compared with ALS mice lumbar spinal cords.
3.3. Protective inflammatory and neurotrophic factors are increased in the cervical cords of ALS mice
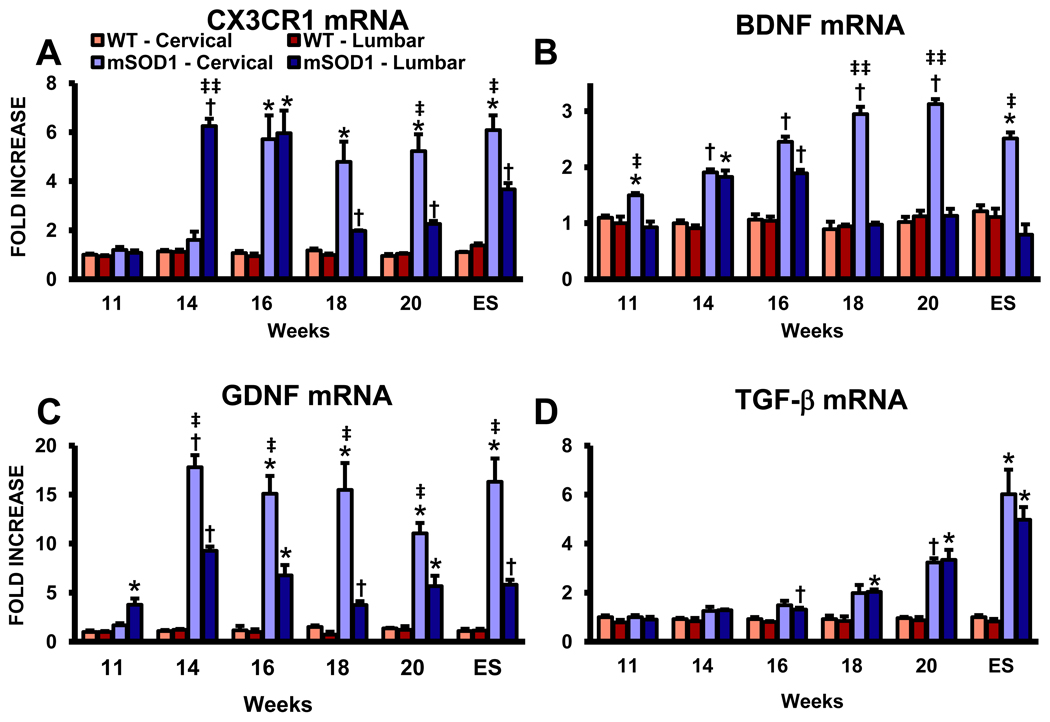
Because morphological and qRT-PCR of microglial and DC markers were differentially expressed between the cervical and lumbar regions of ALS mice spinal cords, and since we previously demonstrated that morphological markers of microglial “activation” do not accurately predict functional indicators of “activation,” we evaluated these discrete anatomical areas for the differential expression of a neuroprotective microglial and cytokine/neurotrophic markers (Cardona et al., 2006; Beers et al., 2008; Appel et al., 2010). Since microglia are the only cells in the CNS to express CX3CR1, and since signaling through CX3CR1 may ameliorate microglial neurotoxicity, we assessed the mRNA expression levels of CX3CR1 in the cervical and lumbar spinal cords of ALS mice; the replacement of microglia by circulating monocytes/macrophages does not take place in ALS mice without first subjected to γ-irradiation, suggesting that maintenance and local expansion of microglia in these models are solely dependent on the self-renewal of resident CNS cells (Cardona et al., 2006; Ajami et al., 2007). CX3CR1 mRNA levels were increased at 14 weeks in the lumbar spinal cords of ALS mice compared with the levels in the cervical region, but by 16 weeks, the levels in the two regions were equivalent (Fig. 3A). Although the increased CX3CR1 levels were sustained over the remaining course of disease in the cervical spinal cords in these mice, their levels precipitously dropped at 18 weeks in the lumbar regions at a time when disease rapidly accelerates.
Fig. 3.
Anti-inflammatory and neurotrophic factors are increased in the cervical cords of ALS mice. (A) CX3CR1 (fractalkine receptor) mRNA levels. (B) mRNA levels for BDNF. (C) GDNF mRNA levels. (D) The mRNA levels of TGF-β. Each time point represents the mean of n = 3 mice. * ≤ 0.05, ALS mice spinal cord regions compared with the same regions from WT mice; † ≤ 0.01, ALS mice spinal cord regions compared with the same regions from WT mice; ‡ ≤ 0.05, ALS mice cervical spinal cords compared with ALS mice lumbar spinal cords, ‡‡ ≤ 0.01, ALS mice cervical spinal cords compared with ALS mice lumbar spinal cords.
Recently, Liang et al. (2010) demonstrated that glutamate induced the release of neurotrophic factors, such as BDNF, GDNF and NGF from microglia; Serpe et al. (2005) demonstrated that CD4+ T lymphocyte derived BDNF protected facial motoneurons after corresponding nerve axotomy. Furthermore, BDNF released by microglia has been shown to modulate the expression of proteins involved in neural plasticity following focal brain ischemia (Madinier et al., 2009). In our study, BDNF was initially elevated only in the cervical spinal cords of ALS mice at 11 weeks, but by 14 weeks, the increased levels of BDNF mRNA were equivalent between the cervical and lumbar regions in these mice (Fig. 3B). At 16 weeks, the mRNA level of BDNF in the lumbar region began to wane and dropped to WT levels at 18 weeks and remained at this level until ES disease. However, in the cervical spinal cords of ALS mice, the increased mRNA levels of BDNF were sustained until ES disease. Although there was a trend for GDNF to be increased in the lumbar spinal cords of ALS mice at 11 weeks compared with the cervical region, by 14 weeks and through the remaining course of disease, GDNF was increased in the cervical region of these mice compared with the lumbar region; GDNF was increased throughout disease in the lumbar spinal cords of ALS mice compared with the same region in WT mice (Fig. 3C). Interestingly, TGF-β was only increased in the lumbar region of ALS mice at 18 weeks compared with WT mice and increased in both cervical and lumbar spinal cords of these mice at 20 weeks and ES disease compared with age-matched WT mice; there were no difference between the two regions in ALS mice (Fig. 3D).
3.4. Messages for injurious inflammatory markers were similar between the cervical and lumbar regions in ALS mice
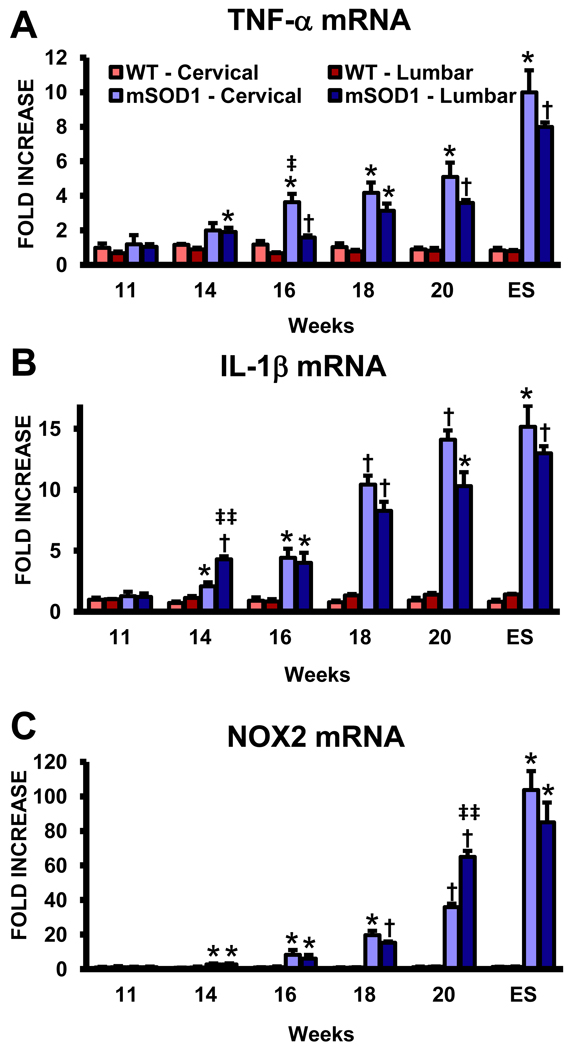
Toxic microglia secrete increased pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL)-1β, and release the potent reactive oxygen species (ROS) superoxide radicals (O2•−) and nitric oxide (NO) (Benoit et al., 2008; Appel et al., 2010). TNF-α mRNA was increased at 14 weeks in lumbar spinal cords of ALS mice compared with age-matched WT mice, and increased to a similar extent in both regions over the course of disease (Fig. 4A). IL-1β mRNA mirrored the expression profile for TNF-α; at 14 weeks, IL-1β was increased in both anatomical regions of the spinal cords of ALS mice and continued to increase as disease progressed (Fig. 4B). The mRNA for NOX2, the subunit of NADPH oxidase found in microglia producing O2•−, also increased rapidly in both the cervical and lumbar regions of ALS mice beginning at 14 weeks and was markedly elevated in both regions at ES disease; both regions had similar temporal NOX2 expression levels (Fig. 4C).
Fig. 4.
An equivalent up-regulation of mRNA for toxic factors in the cervical and lumbar spinal cords of ALS mice. (A) The mRNA for TNF-α. (B) IL-1β mRNA levels. (C) NOX2 mRNA levels. Notice the scale of the ordinate in this graph. Each time point represents the mean of n = 3 mice. * ≤ 0.05, ALS mice spinal cord regions compared with the same regions from WT mice; † ≤ 0.01, ALS mice spinal cord regions compared with the same regions from WT mice; ‡ ≤ 0.05, ALS mice cervical spinal cords compared with ALS mice lumbar spinal cords, ‡‡ ≤ 0.01, ALS mice cervical spinal cords compared with ALS mice lumbar spinal cords.
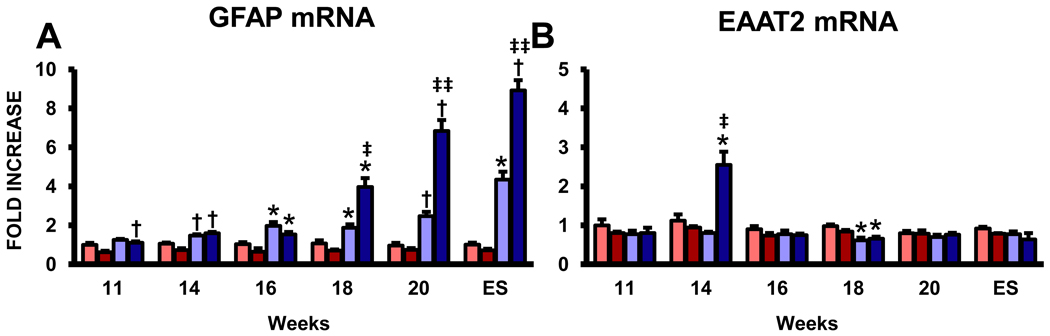
3.5. Astrocyte mRNA factors are elevated in the lumbar spinal cords of ALS mice
Glutamate-induced excitotoxicity may contribute to the pathogenesis of ALS; evidence of abnormal glutamate handling in ALS arose from the detection of large increases in the levels of glutamate in the cerebrospinal fluid of ALS patients (Cleveland and Rothstein, 2001). The astroglial glutamate transporter EAAT2 that is responsible for ~90% of the glutamate clearance for motoneurons. Although GFAP mRNA levels, a marker of astrocytes and astrocytosis, was increased in both the cervical and lumbar spinal cords of ALS mice beginning at 14 weeks and increasing thereafter, EAAT2 was increased in the lumbar spinal cord of these mice at 14 weeks, returning to WT levels at 16 weeks, then again at WT levels at 20 weeks and ES disease; EAAT2 was not increased in the cervical region of these mice at any age assayed (Fig. 5A and B). EAAT2 expression was reduced in the cervical and lumbar spinal cords of ALS mice at 18 weeks (Lin et al., 1998; Howland et al., 2002). GFAP levels increased in the lumbar region of ALS mice at 16 weeks compared with the cervical region and remained elevated in this region until ES disease.
Fig. 5.
Expression of astrocyte mRNAs in the cervical and lumbar spinal cords of ALS mice. (A) The mRNA for GFAP, a marker of astrocytes and astrocytosis. (B) The expression of EAAT2, the astroglial glutamate transporter responsible for the removal of glutamate from the synaptic cleft. Each time point represents the mean of n = 3 mice. * ≤ 0.05, ALS mice spinal cord regions compared with the same regions from WT mice; † ≤ 0.01, ALS mice spinal cord regions compared with the same regions from WT mice; ‡ ≤ 0.05, ALS mice cervical spinal cords compared with ALS mice lumbar spinal cords, ‡‡ ≤ 0.01, ALS mice cervical spinal cords compared with ALS mice lumbar spinal cords.
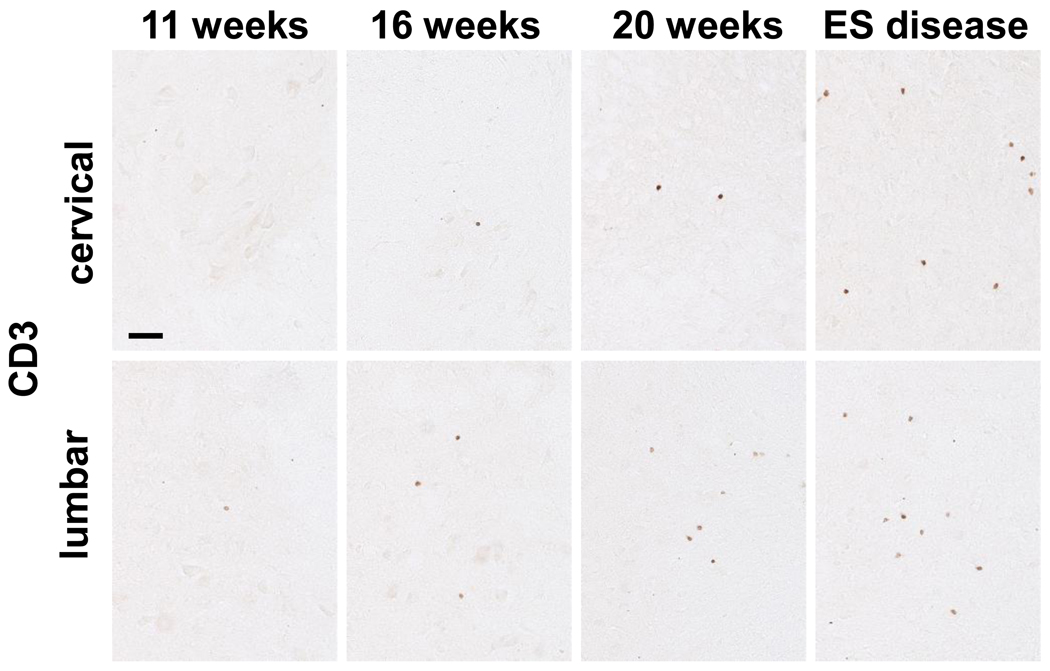
3.6. Lymphocytes are differentially increased in the cervical and lumbar regions of ALS mice
To assess whether infiltrating T lymphocytes were differentially increased between the cervical and lumbar spinal cord regions of ALS mice, sections from these respective regions were immuno-stained for CD3, a pan T lymphocyte marker. At 11 weeks of age, at the initiation of disease onset, CD3+ T lymphocytes were present in the lumbar region (4.5 ± 0.5 T lymphocytes/section) of ALS mice but were conspicuously rare in their cervical spinal cord region (Fig. 6). At 16 weeks, during the stable disease phase, CD3+ T lymphocytes were present in both the cervical and lumbar regions of ALS mice, but were increased in the lumbar region compared with the cervical region of ALS mice (10.2 ± 1.1 and 4.3 ± 0.5 T lymphocytes/section, respectively). At 20 weeks, T lymphocytes were similarly increased in the lumbar region of ALS mice (15.1 ± 0.6 T lymphocytes/section) compared with their cervical region (6.8 ± 0.1 T lymphocytes/section) of the same mice. At ES disease, there were fewer CD3+ T lymphocytes in the cervical region of ALS mice (10.6 ± 1.5 T lymphocytes/section) compared with their lumbar region (14.5 ± 0.4 T lymphocytes/section); the number of CD3+ T lymphocytes/section in the lumbar region of the ALS mice was comparable the numbers previously reported (Beers et al., 2008).
Fig. 6.
Immunohistochemical evaluations of CD3 in the cervical and lumbar spinal cords of ALS mice. Scale bar: 50 µm.
3.7. Differential mRNA levels of preferentially expressed T lymphocyte transcription factors and cytokines in ALS mice spinal cords
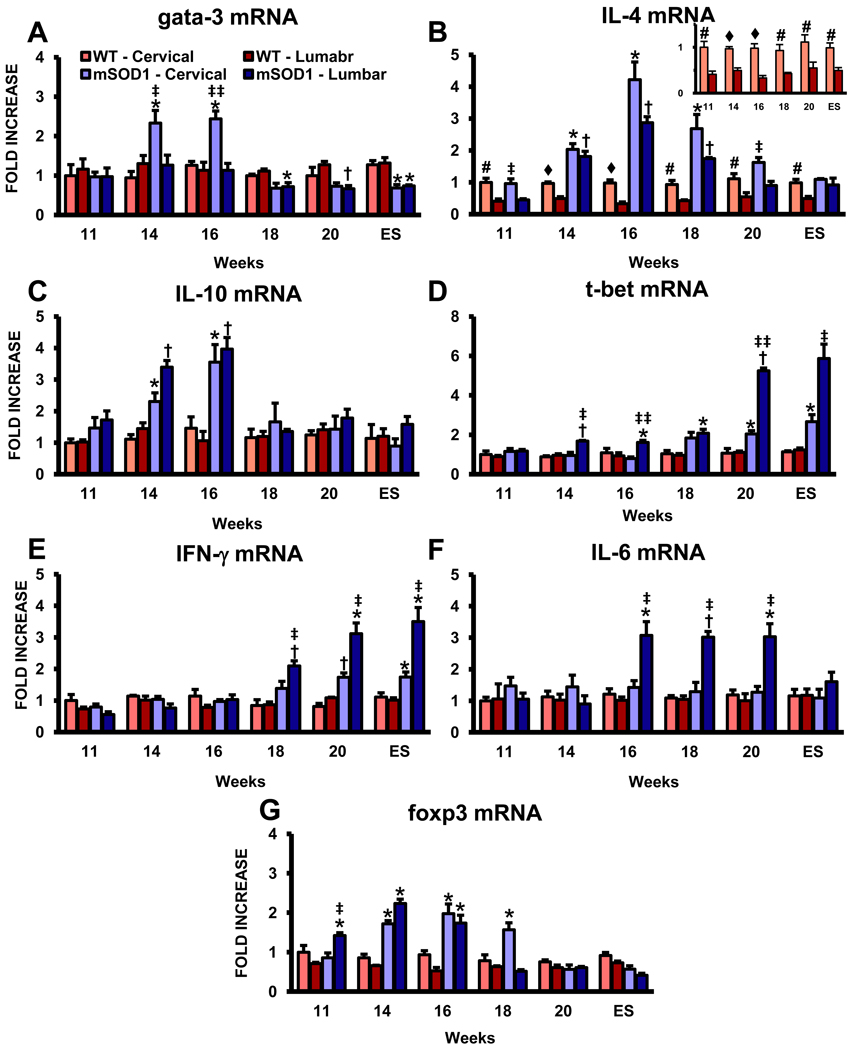
Since T lymphocytes were observed to infiltrate the spinal cords of ALS mice, we determine whether T lymphocyte transcription factors were expressed in a temporally and regional distinct manner. mRNA levels of gata-3, a master transcription factor preferentially expressed by Th2 lymphocytes, were increased at 14 and 16 weeks in the cervical region of ALS mice compared with the cervical region of WT mice or the lumbar region of ALS mice; gata-3 expression was not elevated in the lumbar region of ALS mice at any time point examined (Fig. 7A) (Zhu and Paul, 2010). Furthermore, gata-3 mRNA levels were suppressed in both the cervical and lumbar regions of ALS mice beginning at 18 weeks, and extending through ES disease, compared to the respective regions in WT mice.
Fig. 7.
Expression of lymphocyte mRNAs is elevated in the lumbar spinal cords of ALS mice. (A) mRNA for gata-3, a master transcription factor preferentially expressed in Th2 lymphocytes. (B) IL-4 mRNA levels, the prototypic anti-inflammatory cytokine released by Th2 lymphocytes. There was 2–2.5 fold more in the cervical cords of WT mice compared with their lumbar regions at all time points assayed (insert); IL-4 was the only factor assayed that was different in the two spinal cord regions of WT mice. (C) IL-10 mRNA expression levels. (D) Expression levels of t-bet mRNA, a master transcription factor for Th1 lymphocytes. (E) The mRNA for IFN-γ, the prototypic pro-inflammatory cytokine released by Th1 lymphocytes. (F) The expression level for IL-6, another Th1 induced cytokine. (G) mRNA levels for foxp3, a transcription factor currently accepted as a reliable marker of Tregs. Each time point represents the mean of n = 3 mice. * ≤ 0.05, ALS mice spinal cord regions compared with the same regions from WT mice; † ≤ 0.01, ALS mice spinal cord regions compared with the same regions from WT mice; ‡ ≤ 0.05, ALS mice cervical spinal cords compared with ALS mice lumbar spinal cords, ‡‡ ≤ 0.01, ALS mice cervical spinal cords compared with ALS mice lumbar spinal cords; # ≤ 0.05, WT mice cervical spinal cords compared with WT mice lumbar spinal cords; ♦ ≤ 0.01, WT mice cervical spinal cords compared with WT mice lumbar spinal cords.
We have previously demonstrated that T lymphocytes modulate microglial phenotypes and slow disease progression in ALS mice, however, specific T lymphocyte factors were not assessed (Beers et al., 2008). The prototypic cytokine secreted by Th2 lymphocytes is IL-4; however, a recent study demonstrated by qRT-PCR that IL-4 mRNA can be expressed by microglia in the CNS of WT mice and that its production is essential for controlling autoimmune inflammation by inducing a protective microglial phenotype (Ponomarev et al., 2007). In this study, IL-4 levels were elevated in the cervical cords of WT mice compared with their lumbar regions; IL-4 was expressed 2–2.5 fold more in WT cervical cords than lumbar cords at all ages assayed (Fig. 7B). At 11 weeks of age, the IL-4 message level was increased in the cervical region of ALS mice compared with their lumbar cords, but was equivalently increased in both cervical and lumbar spinal cord region of these mice at 14 of weeks, and continued to increase until 16 weeks. IL-4 levels dropped in both regions at 18 weeks, but were still elevated in the cervical cords of ALS mice at 20 weeks compared with their lumbar regions. Another Th2 lymphocyte secreted cytokine is IL-10 and its mRNA levels were increased in the two regions of ALS mice at 14 and 16 weeks compared with their two WT regions, but returned to WT levels as diseased progressed; IL-10 levels were not elevated in the cervical cords of WT mice compared with their lumbar regions at any age assayed (Fig. 7C).
In contrast, t-bet, also known as T-box 21 (Tbx21) and a master transcription factor preferentially expressed in Th1 lymphocytes, was first increased at 14 weeks in the lumbar region of ALS mice compared with the respective region in WT mice or the cervical region of ALS mice (Fig. 7D) (Zhu and Paul, 2010). The expression of t-bet was first elevated in the cervical region of ALS mice at 18 weeks compared with their WT mice counterparts; t-bet mRNA levels in the cervical region of ALS mice never increased to the same extent as in the lumbar region of these mice. The mRNA for IFN-γ, the prototypic pro-inflammatory cytokine released by Th1 lymphocytes inducing a Th1 response, was increased in the lumbar spinal cords of ALS mice at 18 weeks and thereafter; IFN-γ was not increased in the cervical spinal cords of these mice compared with age-matched WT mice over the entire course of disease (Fig. 7E). In addition, the relative expression levels of t-bet were similar to that of IFN-γ in the cervical and lumbar regions of ALS mice (Fig. 7B). Th1 lymphocytes also release IL-6 and its levels were also increased only in the lumbar spinal cords of ALS mice beginning at 16 weeks compared with levels in the cervical region of these mice and WT mice; IL-6 expression in ALS mice returned to WT levels at ES disease (Fig 7F). In contrast to IFN-γ, IL-6 was not increased in the cervical regions of ALS or WT mice at any time point assayed.
We next assayed for foxp3 mRNA levels, a transcription factor currently accepted as a reliable marker of regulatory T (Tregs) lymphocytes, in the cervical and lumbar spinal cords of WT and ALS mice; the suppressive effects of Tregs on the adaptive and innate immune systems have been previously documented (Sakaguchi 2005; Tiemessen et al., 2007; Sakaguchi et al., 2008). foxp3 was elevated in the cervical region of ALS mice at 14, 16, and 18 weeks compared with WT mice; the levels returned to WT levels at 20 weeks and ES disease (Fig. 7G). In contrast, foxp3 levels increased in the lumbar region of ALS mice at 11 through 16 weeks compared with WT mice, but returned to WT levels at 18 weeks through ES disease.
4. Discussion
Early studies of ALS mice overexpressing mutations in the SOD1 gene reported that the mice first developed hindlimb tremors, then progressive hindlimb weakness with rapidly deteriorating gates, which eventually culminated in paralysis of one or both hindlimbs (Gurney et al., 1994; Wong et al., 1995; Bruijn et al., 1997). Forelimb weakness occurred later if at all in disease; in some ALS mice, disease progressed so rapidly that they were sacrificed before any signs of forelimb involvement were observed (Bruijn et al., 1997). We also reported a similar set of clinical signs in our mutant SOD1G93A ALS mice; limb onset and involvement is more variable in the transgenic rat model of ALS (Howland et al., 2002; Beers et al., 2006; Beers et al., 2008). Therefore, because motor weakness begins and is more severe in the hindlimbs of ALS mice and only later involves the forelimbs, we asked whether cervical spinal cord pathology with regard to protective versus injurious inflammatory responses differed from their lumbar regions over the course of disease. The current study demonstrates that the levels of BDNF, GDNF, and IL-4, specific neuroprotective factors expressed by glia and lymphocytes, were either augmented and/or sustained in the cervical spinal cords of these mice as disease progressed (Madinier et al., 2009; Liang et al. 2010). Furthermore, T lymphocytes were observed first infiltrating the lumbar region of these mice, and only later infiltrated the cervical region. The elevated levels of gata-3, a master transcription factor preferentially expressed by Th2 lymphocytes, suggest that Th2 lymphocytes infiltrate the cervical region of ALS mice. Thus, a potential additional source of IL-4, aside from that produced by microglia, may include infiltrating Th2 lymphocytes in the cervical spinal cords of ALS mice; IL-4, the critical cytokine driving Th2 lymphocyte differentiation, especially in vitro, and suppresses the development of Th1 lymphocytes (Zhu and Paul, 2010). Then, as disease progresses, there is an elevation in both IFN-γ and t-bet, a master transcription factor preferentially expressed in Th1 lymphocytes, suggesting that Th1 lymphocytes infiltrate the cervical region and augment a toxic inflammatory response; with the increased expression of IFN-γ, which is important for inducing Th1 lymphocytes and also inhibiting Th2 lymphocyte differentiation, there is a concomitant decrease in mRNA levels for IL-4. However, as recently reported by Hegazy et al. (2010), fully differentiated Th2 lymphocytes can be reprogrammed into gata-3+/t-bet+ lymphocytes capable of producing both IL-4 and IFN-γ. Thus, caution must be exercised in attributing specific cytokine or chemokine signaling to any single cell type, including subtypes of T lymphocytes as well as microglia or astrocytes.
Cardona et al., (2006) showed using three different in vivo models that CX3CR1 deficiency dysregulates the response of microglia, resulting in neurotoxicity and suggested that augmenting CX3CR1 signaling may protect neurons against toxicity. Our data support this concept, but that regionally specific microglia can be more efficient in upregulating CX3CR1 and suppressing the effects of the concurrent toxic microglial responses. Toxic microglia phenotypes are present in the cervical and lumbar spinal cords of ALS mice, and are capable of expressing mRNAs for neurotoxic substances such as O2•− and producing the injurious cytokines TNF-α and IL-1β. Interestingly, the message levels for CD11c, a marker of DC, and CCL2, a chemokine that attracts peripheral immune cells, were augmented in the lumbar region of these mice at a time when disease rapidly accelerates; they remained relatively stable in the cervical region of ALS mice. These results suggest that after disease onset and subsequently during disease progression, the cervical region may contain a heterogeneous population of protective and injurious microglia, whereas the lumbar region possesses a population of microglia that is predominantly of a toxic phenotype.
IL-4 is a well known immune regulatory cytokine that is able to suppress inflammation; CNS-derived IL-4 ameliorates the resulting inflammation and clinical signs of experimental autoimmune encephalomyelitis in mice, a model of multiple sclerosis, and induces a M2 microglial phenotype (Ponomarev et al., 2007). Thus, in addition to Th2 lymphocytes, another possible cellular source of the CNS-derived IL-4 was microglia. We have previously shown that exogenously applied IL-4 suppressed microglial release of O2•− and NO, and lessened the lipopolysaccharide-induced microglia-mediated motoneuron injury (Zhao et al., 2006). In the current study, the constitutive level of IL-4 expression was more in the cervical spinal cords of WT mice compared with their lumbar spinal cord regions; IL-4 was the only factor assayed that was elevated in the WT cervical cord compared with their lumbar region. The cellular source of the IL-4 was again probably microglia; few T lymphocytes are found in the CNS under normal physiologic conditions. This result suggests that cervical region of the mouse spinal cord is possibly predisposed to maintain a protective microglial response and thus better able to modulate toxic inflammatory insults. Furthermore, IL-4 was recently shown to induce BDNF mRNA expression in astrocytes and BDNF has been demonstrated to attenuate facial motoneuron loss after axotomy, a peripheral nerve insult that initiates a cascade of events that resemble the peripheral nerve “dying back” process detected in ALS mice (Fisher et al., 2004; Serpe et al., 2005; Derecki et al., 2010).
Because we and others have demonstrated in different mouse models of CNS injury that responses from specific subsets T lymphocytes are neuroprotective and elevate levels of the anti-inflammatory cytokines IL-4 and IL1–10, and the neurotrophic factor BDNF, T lymphocytes may act as an addition cellular reservoir for these cytokines and factor; these responses do not occur in physiologically intact mice (Kipnis et al., 2004; Serpe et al., 2005; Banerjee et al., 2008: Beers et al., 2008, Chiu et al., 2008). In ALS mice, the infiltration of CD4+ T lymphocytes into their spinal cords contributes to the stable phase of disease progression (Fig. 2A) (Beers et al., 2008). We determined that CD4+ T lymphocytes enhanced the stable disease phase and prolonged survival and that they modulated the functional attributes of the resident microglia at sites of motoneuron injury; the T lymphocytes were associated with the induction of protective microglia and enhanced IGF-1 secretion. We further demonstrated that while microglia were functionally more activated without T lymphocytes, they appeared morphologically less activated, indicating that microglial function cannot be determined by traditional morphological markers of microglial activation; morphological (CD68 and CD11c) markers of microglial “activation” do not accurately predict the functional (TNF-α and NOX2) markers of “activation” (Beers et al., 2008). Therefore, during the stable disease phase, T lymphocytes, through the release of protective inflammatory cytokines and neurotrophic factors, augmented a protective microglial response in the cervical cord and promoted their development in the lumbar cord of ALS mice.
We have previously demonstrated that the state of microglia activation can directly influence the extent of motoneuron injury and the rate of disease progression in ALS mice (Beers et al, 2006; Zhao et al., 2006; Xiao et al., 2007; Beers et al., 2008). Microglia display functional plasticity during activation, which involves changes in cell number, morphology, surface receptor expression, and production of growth factors and cytokines (Ransohoff and Perry, 2009). The changes reflect altered activation states induced by signals that arise from injured neurons and surrounding glia, and possibly from peripheral targets. As with macrophages, microglia exhibit a classically activated M1 phenotype or an alternatively activated M2 phenotype (Greissmann et al., 2008; Martinez et al., 2008). Classically activated M1 microglia secrete increased pro-inflammatory cytokines and potent ROS, and reduced neurotrophic factors. The alternatively activated M2 phenotypes, as identified based upon their gene expression profile and cover a continuum of functional states, enhance neurotrophic factor release, reduce pro-inflammatory cytokines production, and assist in inflammation resolution (Benoit et al., 2008). Classical microglial activation, alternative activation and acquired deactivation are each found in the brain during chronic neuroinflammatory diseases and may demonstrate regional differences in expression levels (Colton and Wilcock, 2010).
The differences between the activation states of the microglia in these two spinal cord regions of ALS mice after the initiation of disease may partially be due to the signals they receive from the corresponding skeletal muscle targets of the respective motoneurons and their axons. Denervation at the neuromuscular junction is observed prior to motoneuron loss in the spinal cord, which suggests that motoneuron pathology begins at the distal axon and proceeds in, as previously mentioned, a “dying back” process; we have recently demonstrated that hindlimb denervation occurs prior to loss of neuromuscular junction integrity of the forelimbs (Fisher et al., 2004; Kano et al., 2010). After peripheral nerve injury, neurons are damaged outside the CNS and hence, are the only injured cells, and while the blood–brain barrier remains essentially intact, this injury triggers a cascade of microglia morphologic and metabolic processes within the affected areas; initial signals for the activation of microglia surrounding the injured neurons therefore originate from these neurons (Blinzinger and Kreutzberg, 1968; Kreutzberg, 1996; Raivich et al., 1998). Flügel et al. (2001) demonstrated that the recruitment of peripheral immune cells was in response to the rapid up-regulation of neuronal CCL2 after nerve injury; CCR2, the constitutively expressed receptor for CCL2, did not change after axotomy. We also demonstrated that CCL2 mRNA and immunoreactivity were upregulated in the neurons and glial cells of ALS mice early in disease followed by the upregulation of CD68 expression. Although it is not clear if these responses were protective or injurious, the early increased CCL2 levels and CD68+ cells suggest an early preexisting injury. In addition, following facial nerve axotomy, BDNF, which can be released by microglia and T lymphocytes under pathological conditions, protected the corresponding facial motoneurons from injury (Serpe et al., 2005; Liang et al., 2010).
Regional differences in microglial reactivity have been reported in animal models studying the neuropathologies underlying disease-, trauma- and chemically-induced neurodegeneration. A recent study concluded that there are marked differences in the regional density, distribution and/or activity of microglia in a MPTP-induced neurodegenerative mouse model of Parkinson’s disease and that microglial-derived factors influenced the region-specific role for this cell type (Sriram et al., 2006). Furthermore, in the rat spinal cord, microglial population represents 7% of the white and 11% of the grey matter, and after pathological intrathecal stimulation with IFN-γ, the highest numbers of microglia were detected in the lumbar spinal cord, suggesting a region-specific microglial regulation within the spinal cord (Ling, 1976; Vass and Lassmann, 1990). In this report, the detected microglial heterogeneity may have been dictated by the local motoneuron/astrocytic/lymphocyte microenvironments that the microglia encountered in the two anatomically distinct regions of their spinal cord, which in turn induced a specific set of different mRNA expression profiles in the respective resident microglia in these two regions; the distinct phenotypes of activated microglia might result from this heterogeneity (Sawada, 2009).
Regionally specific cellular phenotype differences are not restricted to microglia. Astrocytes also are a major component of the resident non-neuronal glial cell population of the CNS. Mounting data suggests that astrocytes do not simply support neuronal activity but directly contribute to it and that these glial cells are not a homogeneous population of cells; current evidence supports the concept that astrocytes are remarkably heterogeneous and astrocytic populations within functionally defined regions of the CNS also exhibit phenotypic heterogeneity (Hewett, 2009). Astrocytes in various anatomically distinct regions of the normal CNS possess unique phenotypic characteristics that may directly influence the particular neuronal activities that define these regions (Bachoo et al., 2004). Many phenotypic astrocytic traits are responsive to local microenvironmental cues, suggesting that plasticity contributes to this diversity.
We have hypothesized that the default activation state of microglia is a protective phenotype and is responsible for the maintenance of CNS homeostasis and prevention of neuronal damage (Appel et al., 2010). The data presented in this report suggest that under pathological stress there are regional and temporal differences in the population of microglia and lymphocyte phenotypes in the spinal cord of mice. Although protective microglia are present in the lumbar spinal cords of ALS mice at early stages of disease, there is a rapid transition to microglia exhibiting a toxic response. However, in the cervical region of these mice, there are anti-inflammatory microglia and infiltrating lymphocytes contributing to an enhancement of a protective response which assists in maintaining this response for a longer period of time. These results reinforce the importance of a balance between specific protective and/or injurious inflammatory immune responses in modulating clinical outcomes.
Supplementary Material
Acknowledgments
We thank Xiaoli Wang for her technical assistance. This study was supported by grants from the NIH (NS067153 and NS048950), the Muscular Dystrophy Association, the Texas Methodist Foundation, and The Methodist Hospital Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
All authors declare that there are no conflicts of interest.
References
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FMV. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- Appel SH, Beers DR, Henkel JS. T cell-microglial dialogue in Parkinson's disease and amyotrophic lateral sclerosis: are we listening? Trends Immunol. 2010;31:7–17. doi: 10.1016/j.it.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvin KL, Han BH, Du Y, Lin SZ, Paul SM, Holtzman DM. Minocycline markedly protects the neonatal brain against hypoxic-ischemic injury. Ann Neurol. 2002;52:54–61. doi: 10.1002/ana.10242. [DOI] [PubMed] [Google Scholar]
- Bachoo RM, Kim RS, Ligon KL, Maher EA, Brennan C, Billings N, Chan S, Li C, Rowitch DH, Wong WH, DePinho RA. Molecular diversity of astrocytes with implications for neurological disorders. Proc. Natl. Acad. Sci. USA. 2004;101:8384–8389. doi: 10.1073/pnas.0402140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee R, Mosley RL, Reynolds AD, Dhar A, Jackson-Lewis V, Gordon PH, Przedborski S, Gendelman HE. Adaptive immune neuroprotection in G93A-SOD1 amyotrophic lateral sclerosis mice. PLoS ONE. 2008;3:e2740. doi: 10.1371/journal.pone.0002740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers DR, Henkel JS, Xiao Q, Zhao W, Wang J, Yen AA, Siklós L, McKercher SR, Appel SH. Wild-type microglia extends survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. USA. 2006;103:16021–16026. doi: 10.1073/pnas.0607423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers DR, Henkel JS, Zhao W, Wang J, Appel SH. CD4+ T-cells support glial neuroprotection, slow disease progression, and modify glial morphology in an animal model of inherited ALS. Proc. Natl. Acad. Sci. USA. 2008;105:15558–15563. doi: 10.1073/pnas.0807419105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J. Immunol. 2008;181:3733–3739. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- Blinzinger K, Kreutzberg GW. Displacement of synaptic terminals from regenerating motoneurons by microglial cells. Z. Zellforsch. 1968;85:145–157. doi: 10.1007/BF00325030. [DOI] [PubMed] [Google Scholar]
- Boillée S, Vande Velde C, Cleveland DW. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Bruijn LI, Becher MW, Lee MK, Anderson KL, Jenkins NA, Copeland NG, Sisodia SS, Rothstein JD, Borchelt DR, Price DL, Cleveland DW. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18:327–338. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- Carson MJ, Doose JM, Melchior B, Schmid CD, Ploix CC. CNS immune privilege: hiding in plain sight. Immunol. Rev. 2006;213:48–65. doi: 10.1111/j.1600-065X.2006.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu IM, Chen A, Zheng Y, Kosaras B, Tsiftsoglou SA, Vartanian TK, Brown RH, Jr, Carroll MC. T lymphocytes potentiate endogenous neuroprotective inflammation in a mouse model of ALS. Proc. Natl. Acad. Sci. USA. 2008;105:17913–17918. doi: 10.1073/pnas.0804610105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland DW, Rothstein JD. From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat. Rev. Neurosci. 2001;2:806–819. doi: 10.1038/35097565. [DOI] [PubMed] [Google Scholar]
- Colton CA, Wilcock DM. Assessing activation states in microglia. CNS Neurol. Disord. Drug Targets. 2010;9:174–191. doi: 10.2174/187152710791012053. [DOI] [PubMed] [Google Scholar]
- Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, Kipnis J. Regulation of learning and memory by meningeal immunity: a key role for IL-4. J. Exp. Med. 2010;207:1067–1080. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt JI, Tajti J, Appel SH. Lymphocytic infiltrates in the spinal cord in amyotrophic lateral sclerosis. Arch. Neurol. 1993;50:30–36. doi: 10.1001/archneur.1993.00540010026013. [DOI] [PubMed] [Google Scholar]
- Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, Khan J, Polak MA, Glass JD. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp. Neurol. 2004;185:232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Flügel A, Hager G, Horvat A, Spitzer C, Singer GM, Graeber MB, Kreutzberg GW, Schwaiger FW. Neuronal MCP-1 expression in response to remote nerve injury. J. Cereb. Blood Flow Metab. 2001;21:69–76. doi: 10.1097/00004647-200101000-00009. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Auffray C, Palframan R, Wirrig C, Ciocca A, Campisi L, Narni-Mancinelli E, Lauvau G. Blood monocytes: distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of T cell responses. Immunol. Cell Biol. 2008;86:398–408. doi: 10.1038/icb.2008.19. [DOI] [PubMed] [Google Scholar]
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, Chen W, Zhai P, Sufit RL, Siddique T. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Hegazy AN, Peine M, Helmstetter C, Panse I, Fröhlich A, Bergthaler A, Flatz L, Pinschewer DD, Radbruch A, Löhning M. Interferons direct Th2 cell reprogramming to generate a stable GATA-3(+)T-bet(+) cell subset with combined Th2 and Th1 cell functions. Immunity. 2010;32:116–128. doi: 10.1016/j.immuni.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Henkel JS, Engelhardt JI, Siklós L, Simpson EP, Kim SH, Pan T, Goodman JC, Siddique T, Beers DR, Appel SH. Presence of dendritic cells, MCP-1, and activated microglia/macrophages in amyotrophic lateral sclerosis spinal cord tissue. Ann. Neurol. 2004;55:221–235. doi: 10.1002/ana.10805. [DOI] [PubMed] [Google Scholar]
- Henkel JS, Beers DR, Siklos L, Appel SH. The chemokine MCP-1 and the dendritic and myeloid cells it attracts are increased in the mSOD1 mouse model of ALS. Mol. Cell. Neurosci. 2006;31:427–437. doi: 10.1016/j.mcn.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Henkel JS, Beers DR, Zhao W, Appel SH. Microglia in ALS: The good, the bad, and the resting. J. Neuroimmune Pharmacol. 2009;4:389–398. doi: 10.1007/s11481-009-9171-5. [DOI] [PubMed] [Google Scholar]
- Hewett JA. Determinants of regional and local diversity within the astroglial lineage of the normal central nervous system. J. Neurochem. 2009;110:1717–1736. doi: 10.1111/j.1471-4159.2009.06288.x. [DOI] [PubMed] [Google Scholar]
- Howland DS, Liu J, She Y, Goad B, Maragakis NJ, Kim B, Erickson J, Kulik J, DeVito L, Psaltis G, DeGennaro LJ, Cleveland DW, Rothstein JD. Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant-mediated amyotrophic lateral sclerosis (ALS) Proc. Natl. Acad. Sci. USA. 2002;99:1604–1609. doi: 10.1073/pnas.032539299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano O, Beers DR, Henkel JS, Zhao W, Liao B, Appel SH. Peripheral nerve inflammation and muscle denervation in mSOD1 transgenic mice: Which comes first?; 62nd American Academy of Neurology Meeting; Toronto Canada: 2010. [April 10–17]. p. A437. [Google Scholar]
- Kassa RM, Mariotti R, Bonaconsa M, Bertini G, Bentivoglio M. Gene, cell, and axon changes in the familial amyotrophic lateral sclerosis mouse sensorimotor cortex. J. Neuropathol. Exp. Neurol. 2009;68:59–72. doi: 10.1097/NEN.0b013e3181922572. [DOI] [PubMed] [Google Scholar]
- Kipnis J, Avidan H, Caspi RR, Schwartz M. Dual effect of CD4+CD25+ regulatory T cells in neurodegeneration: a dialogue with microglia. Proc. Natl. Acad. Sci. USA. 2004;101 Suppl 2:14663–14669. doi: 10.1073/pnas.0404842101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Lalancette-Hébert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J. Neurosci. 2007;27:2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Takeuchi H, Jin S, Noda M, Li H, Doi Y, Kawanokuchi J, Sonobe Y, Mizuno T, Suzumura A. Glutamate induces neurotrophic factor production from microglia via protein kinase C pathway. Brain Res. 2010;1322:8–23. doi: 10.1016/j.brainres.2010.01.083. [DOI] [PubMed] [Google Scholar]
- Lin CL, Bristol LA, Jin L, Dykes-Hoberg M, Crawford T, Clawson L, Rothstein JD. Aberrant RNA processing in a neurodegenerative disease: the cause for absent EAAT2, a glutamate transporter, in amyotrophic lateral sclerosis. Neuron. 1998;20:589–602. doi: 10.1016/s0896-6273(00)80997-6. [DOI] [PubMed] [Google Scholar]
- Lindley J, Deurveilhera S, Rusakb B, Semba K. Transforming growth factor-α and glial fibrillary acidic protein in the hamster circadian system: Daily profile and cellular localization. Brain Res. 2008;1197:94–105. doi: 10.1016/j.brainres.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Ling EA. Study in the changes of the proportions and numbers of the various glial cell types in the spinal cord of neonatal and young adult rats. Acta Anat. 1976;96:188–195. doi: 10.1159/000144672. [DOI] [PubMed] [Google Scholar]
- Madinier A, Bertrand N, Mossiat C, Prigent-Tessier A, Beley A, Marie C, Garnier P. Microglial involvement in neuroplastic changes following focal brain ischemia in rats. PLoS ONE. 2009;4:e8101. doi: 10.1371/journal.pone.0008101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front. Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Inflammatory processes in amyotrophic lateral sclerosis. Muscle Nerve. 2002;26:459–470. doi: 10.1002/mus.10191. [DOI] [PubMed] [Google Scholar]
- McKercher SR, Torbett BE, Anderson KL, Henkel GW, Vestal DJ, Baribault H, Klemsz M, Feeney AJ, Wu GE, Paige CJ, Maki RA. Targeted disruption of the PU.1 gene results in multiple hematopoietic abnormalities. EMBO J. 1996;15:5647–5658. [PMC free article] [PubMed] [Google Scholar]
- Moisse K, Strong MJ. Innate immunity in amyotrophic lateral sclerosis. Biochimica et Biophysica Acta. 2006;1762:1083–1093. doi: 10.1016/j.bbadis.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Polazzi E, Monti B. Microglia and neuroprotection: From in vitro studies to therapeutic applications. Prog. Neurobiol. Epub ahead of print. 2010 doi: 10.1016/j.pneurobio.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Ponomarev ED, Maresz K, Tan Y, Dittel BN. CNS-derived interleukin-4 is essential for the regulation of autoimmune inflammation and induces a state of alternative activation in microglial cells. J. Neurosci. 2007;27:10714–10721. doi: 10.1523/JNEUROSCI.1922-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu. Rev. Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- Raivich G, Jones LL, Kloss CU, Werner A, Neumann H, Kreutzberg GW. Immune surveillance in the injured nervous system: T-lymphocytes invade the axotomized mouse facial motor nucleus and aggregate around sites of neuronal degeneration. J. Neurosci. 1998;18:5804–5816. doi: 10.1523/JNEUROSCI.18-15-05804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat. Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Sawada M. Neuroprotective and toxic changes in microglia in neurodegenerative disease. Parkinsonism Relat. Disord. 2009;15 Suppl 1:S39–S41. doi: 10.1016/S1353-8020(09)70011-2. [DOI] [PubMed] [Google Scholar]
- Serpe CJ, Byram SC, Sanders VM, Jones KJ. Brain-derived neurotrophic factor supports facial motoneuron survival after facial nerve transaction in immunodeficient mice. Brain Behav. Immun. 2005;19:173–180. doi: 10.1016/j.bbi.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Simard AR, Rivest S. Neuroprotective effects of resident microglia following acute brain injury. J. Comp. Neurol. 2007;504:716–729. doi: 10.1002/cne.21469. [DOI] [PubMed] [Google Scholar]
- Sriram K, Matheson JM, Benkovic SA, Miller DB, Luster MI, O'Callaghan JP. Deficiency of TNF receptors suppresses microglial activation and alters the susceptibility of brain regions to MPTP-induced neurotoxicity: role of TNF-alpha. FASEB J. 2006;20:670–682. doi: 10.1096/fj.05-5106com. [DOI] [PubMed] [Google Scholar]
- Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS. CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc. Natl. Acad. Sci. USA. 2007;104:19446–19451. doi: 10.1073/pnas.0706832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vass K, Lassmann H. Intrathecal application of interferon gamma. Progressive appearance of MHC antigens within the rat nervous system. Am. J. Pathol. 1990;137:789–800. [PMC free article] [PubMed] [Google Scholar]
- Wong PC, Pardo CA, Borchelt DR, Lee MK, Copeland NG, Jenkins NA, Sisodia SS, Cleveland DW, Price DL. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron. 1995;14:1105–1116. doi: 10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]
- Xiao Q, Zhao W, Beers DR, Yen AA, Xie W, Henkel JS, Appel SH. Mutant SOD1(G93A) microglia are more neurotoxic relative to wild-type microglia. J. Neurochem. 2007;102:2008–2019. doi: 10.1111/j.1471-4159.2007.04677.x. [DOI] [PubMed] [Google Scholar]
- Zhao W, Xie W, Le W, Beers DR, He Y, Henkel JS, Simpson EP, Yen AA, Xiao Q, Appel SH. Activated microglia initiate motor neuron injury by a nitrite oxide and glutamate-mediated mechanism. J. Neuropathol. Exp. Neurol. 2004;63:964–977. doi: 10.1093/jnen/63.9.964. [DOI] [PubMed] [Google Scholar]
- Zhao W, Xie W, Xiao Q, Beers DR, Appel SH. Protective effects of an anti-inflammatory cytokine, interleukin-4, on motoneuron toxicity induced by activated microglia. J. Neurochem. 2006;99:1176–1187. doi: 10.1111/j.1471-4159.2006.04172.x. [DOI] [PubMed] [Google Scholar]
- Zhu J, Paul WE. CD4+ T Cell Plasticity—Th2 Cells Join the Crowd. Immunity. 2010;32:11–13. doi: 10.1016/j.immuni.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.