Abstract
Venous stenting has been shown to effectively treat iliofemoral venous obstruction with good short- and mid-term results. The aim of this study was to investigate long-term clinical outcome and stent patency. Twenty patients were treated with venous stenting for benign disease at our institution between 1987 and 2005. Fifteen of 20 patients (15 female, mean age at time of stent implantation 38 years [range 18–66]) returned for a clinical visit, a plain X-ray of the stent, and a Duplex ultrasound. Four patients were lost to follow-up, and one patient died 277 months after stent placement although a good clinical result was documented 267 months after stent placement. Mean follow-up after stent placement was 167.8 months (13.9 years) (range 71 (6 years) to 267 months [22 years]). No patient needed an additional venous intervention after stent implantation. No significant difference between the circumference of the thigh on the stented side (mean 55.1 cm [range 47.0–70.0]) compared with the contralateral thigh (mean 54.9 cm [range 47.0–70.0]) (p = 0.684) was seen. There was a nonsignificant trend toward higher flow velocities within the stent (mean 30.8 cm/s [range 10.0–48.0]) and the corresponding vein segment on the contralateral side (mean 25.2 cm/s [range 12.0–47.0]) (p = 0.065). Stent integrity was confirmed in 14 of 15 cases. Only one stent showed a fracture, as documented on x-ray, without any impairment of flow. Venous stenting using Wallstents showed excellent long-term clinical outcome and primary patency rate.
Keywords: Iliofemoral venous stent, Benign venous stenosis, Long-term result
Introduction
Benign iliofemoral venous occlusion is often difficult to treat. The main goal is to improve patient quality of life and prevent chronic venous insufficiency (CVI), which may lead to an inability to work [1].
In the past, different surgical techniques involving venous bypass were used in cases of iliocaval venous obstruction [2–4]. The main problem with bypass surgery is its invasiveness and associated complications. The reported outcomes are rather poor, with patency rates between 54 and 84%. Therefore, this therapy option is rarely used today [5].
In 1985, the first use of a self-expanding stent was described in the venous system of a dog [6]. Soon afterward the first studies in humans were published [7–9]. Good short- and mid-term patency rates were reported in these publications; however, the question of long-term clinical outcome and stent patency remained unanswered. The aim of the present study was to investigate clinical outcome as well as stent patency and integrity after follow-up >5 years.
Materials and Methods
The study was approved by our Institutional Review Board. Written informed consent was obtained from all patients.
Patients
From January 1987 to October 2005, 20 patients were admitted to our department for endovenous treatment of benign iliofemoral stenosis or occlusion. Five patients were excluded from further analysis. Four of these five patients were lost to follow-up. One patient died 277 months (23 years) after common iliac vein stent placement from a cause unrelated to the venous stent. For this patient, the last clinical visit 267 months (22 years) after stent placement showed a good clinical outcome after iliac vein stenting with no leg swelling. Fifteen patients (all female; mean age at time of stent implantation 38 years [range 18–66]) were included in the study. The etiology of obstruction was iatrogenic stenosis of an iliofemoral vein in four cases (patients no. 1, 2, 6, and 11) and stenosis of a venous interposition graft of the superficial femoral (patient no. 12) and the common femoral veins (patient 14). Six patients were treated with stents after thrombectomy for pelvic vein thrombosis caused by May–Thurner syndrome (patients no. 3, 7, 8, 9, 10, and 15). Two patients were stented after recanalization of a chronic postthrombotic obstruction of the common and/or external iliac veins (patient no. 5 and 13). One patient was treated with common iliac vein stent during treatment of an acute four-level thrombosis (patient no. 4). Details concerning patient data are listed in Table 1.
Table 1.
Summary of patient data
| Patient no. | Age at time of stent placement (y) | Follow-up (mo) | Indication for stent insertion | Stent localisation | Wallstent size (mm) | Stent integrity | Velocity within stent and corresponding contralateral segment (cm/s) | Diameter of the thigh and the calf of the leg with the stent compared with the contralateral side (cm) | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 66 | 219 | Iatrogenic stenosis after crossectomy for varicose veins | Common femoral vein left | 14/50 | Yes | 10 within stent, 20 contralateral side | Stent extremity: | Nonstent extremity: |
| Thigh: 53 | Thigh: 53 | ||||||||
| Lower part: 34 | Lower part: 33 | ||||||||
| 2 | 46 | 196 | Iatrogenic stenosis due to a hematoma after coronary angiography | External iliac vein right | 14/50 | Yes | 18 within stent, 15 contralateral side | Stent extremity: | Nonstent extremity: |
| Thigh: 49 | Thigh: 50 | ||||||||
| Lower part: 33 | Lower part: 34 | ||||||||
| 3 | 47 | 171 | May-Thurner syndrome with acute thrombosis. | Common iliac vein left | 16/60 | Yes | 30 within stent, 20 contralateral side | Stent extremity: | Nonstent extremity: |
| Thigh: 55 | Thigh: 54 | ||||||||
| Lower part: 40 | Lower part: 41 | ||||||||
| 4 | 18 | 111 | Factor V Leiden mutation and acute 4-level thrombosis of the left leg | Common iliac vein left | 16/90 | Yes | 20 within stent; 47 contralateral side | Stent extremity: | Nonstent extremity: |
| Thigh: 70 | Thigh: 70 | ||||||||
| Lower part:4 4 | Lower part: 44 | ||||||||
| 5 | 54 | 180 | Chronic thrombosis of the iliac vein; massive signs of CVI despite operative Palma shunt with persistent AV shun. | Common iliac vein + external iliac vein left | 12/89; 12/66; 14/96; 16/6.1 | Yes | 41 within stent, 13 contralateral side | Stent extremity: | Nonstent extremity: |
| Thigh: 57 | Thigh: 57 | ||||||||
| Lower part: 42 | Lower part: 41 | ||||||||
| 6 | 45 | 205 | Iatrogenic thrombosis of the common iliac vein left after crossectomy | Common iliac vein left | 16/90 | Yes | 43 within stent; 30 contralateral side | Stent extremity | Nonstent extremity: |
| Thigh: 65 | Thigh: 59 | ||||||||
| Lower part: 36 | Lower part: 31 | ||||||||
| 7 | 40 | 186 | Chronic left pelvic vein thrombosis in the case of May-Thurner syndrome and persistent pressure gradient despite a Palma shunt | Common and external iliac vein left | 10/60; 12/60; 14/60 | Yes | 44 within stent; 33 contralateral side | Stent extremity: | Nonstent extremity: |
| Thigh: 53 | Thigh: 58 | ||||||||
| Lower part: 40 | Lower part: 37 | ||||||||
| 8 | 50 | 168 | May-Thurner syndrome with acute thrombosis | Common iliac vein left | 16/90 | Yes | 15 within stent; 20 contralateral side | Stent extremity: | Nonstent extremity: |
| Thigh: 54 | Thigh: 54 | ||||||||
| Lower part: 37 | Lower part: 37 | ||||||||
| 9 | 18 | 71 | May-Thurner syndrome with acute thrombosis | Common iliac vein left | 14/70 | Yes | 24 within stent; 19 contralateral side | Stent extremity: | Nonstent extremity: |
| Thigh: 61 | Thigh: 60 | ||||||||
| Lower part: 41 | Lower part: 40 | ||||||||
| 10 | 23 | 148 | May-Thurner syndrome with acute thrombosis | Common iliac vein left | 12/80 | Yes | 43 within stent; 33 contralateral side | Stent extremity: | Nonstent extremity: |
| Thigh: 47 | Thigh: 47 | ||||||||
| Lower part: 35 | Lower part: 35 | ||||||||
| 11 | 41 | 135 | Iatrogenic stenosis after venous stripping | Common femoral vein right | 12/80 | Yes | 21 cm/seconds within the stent 12 cm/seconds contralateral side | Stent extremity: | Nonstent extremity: |
| Thigh: 51 | Thigh:52 | ||||||||
| Lower part:35 | Lower part:35 | ||||||||
| 12 | 34 | 222 months | Stenosis after jugular vein interponate in the common femoral vein after iatrogenic transection | Common femoral vein right | 2 × 12/75 | Yes | 48 within stent; 40 contralateral side | Stent extremity: | Nonstent extremity: |
| Thigh: 47 | Thigh: 48 | ||||||||
| Lower part: 30 | Lower part: 33 | ||||||||
| 13 | 31 | 83 | Chronic thrombosis after hip surgery and factor V Leiden mutation | Common femoral vein left | 12/90; 14/90 | Yes | 30 within stent; 28 contralateral side | Stent extremity: | Nonstent extremity: |
| Thigh: 49 | Thigh:48 | ||||||||
| Lower part:34 | Lower part:33 | ||||||||
| 14 | 33 | 267 | Stab wound injury; stenosis of the jugular vein interponate | Superficial femoral vein right | 2 × 14/70 | Stent fracture | 30 within stent; 28 contralateral side | Stent extremity: | Nonstent extremity: |
| Thigh: 61 | Thigh: 60 | ||||||||
| Lower part: 49 | Lower part: 48 | ||||||||
| 15 | 24 | 155 | May-Thurner syndrome with acute thrombosis | Common iliac vein left | 14/80 | Yes | 45 within stent; 20 contralateral side | Stent extremity: | Nonstent extremity: |
| Thigh: 55 | Thigh: 54 | ||||||||
| Lower part: 36 | Lower part: 34 | ||||||||
Venous Intervention
The procedure was performed in the interventional suite using either an ipsilateral retrograde percutaneous femoral vein access in cases of a common or external iliac vein obstruction or a crossover technique in cases of a common femoral vein stenosis. In one patient with a long-standing occlusion of the common and external iliac veins, a transjugular approach had to be used (patient no. 5). In all patients, self-expanding Wallstents (Boston Scientific, Natick, MA) were used (details of stent dimensions are listed in Table 1). We did not use other stent types. In the cases of iatrogenic or postoperative stenoses as well as chronic obstruction, a probatory test balloon angioplasty was performed to evaluate adequate stent size. The Wallstent was oversized by at least 1 mm. In cases of insufficient spontaneous self-expansion after placement, the stent was dilated to the estimated diameter of the treated vein. During the procedure, 5000 U heparin were administrated intravenously. After stent placement all patients were treated routinely with warfarin for 6 months. Patients no. 4 and 13 (Table 1) had a factor V Leiden mutation, which was treated with long-term therapy during the entire observation period.
Follow-Up
Follow-up was conducted from October 2009 to November 2009. Follow-up included a clinical examination with measurement of leg circumferences, Duplex ultrasound, and x-ray of the stent. Fourteen of 15 follow-up examinations were performed at the institution where the stents were placed. In one case (patient no. 14), the follow-up examination was performed in another hospital overseas.
Clinical Investigation
The patients were questioned for any intervention or surgery concerning their veins after stent placement. Signs of venous insufficiency, including varicosities and ulcers were recorded. Maximum circumferences of the thighs and calves were measured.
Duplex Ultrasound
The patients were examined using a state-of-the-art ultrasound scanner (Acuson Sequoia 512; Siemens, Munich, Germany). 4- and 8-MHz probes were used. The presence or absence of venous flow through the stent was investigated. In addition, maximum velocities within the stent and the contralateral untreated vein were recorded. Furthermore, it was documented if there was venous reflux under Valsalva maneuver.
X-Ray
Plain X-ray of the stent area was performed in anteroposterior view. In 14 patients, a film of the pelvis was obtained, and a film of the thigh was taken in one patient (patient no. 14). X-rays were analyzed for stent fractures on a PACS system (Impax 4.2, Agfa) that allowed magnified and inverted views.
Statistical Analysis
Statistical analyses were performed using the statistical program SPSS version 17 (SPSS version 17.0.1; SPSS, Chicago, IL). The data were analyzed, and a mean of the circumferences of the treated and nontreated legs, as well as flow measurements within the stent, were evaluated. Wilcoxon signed-rank test was used for comparisons within paired samples.
Results
Clinical Outcome
Mean follow-up after stent placement was 167.8 months (13.9 years) (range 71–267 [6 to 22 years]). No patient needed any additional intervention after stent implantation.
Two patients showed mild varicosis (patients no. 3 and 5), which had existed before venous stenting. In one patient, severe venous insufficiency (patient no. 6) with chronic venous ulcers was detected. This patient had severe varicosity documented before the intervention.
No significant difference (p = 0.684) between the circumference of the thigh on the stented side (mean 55.1 cm [range 47.0–70.0]) and the contralateral thigh (mean 54.9 cm [range 47.0–70.0]) was found. There was a nonsignificant tendency toward a greater circumference of the calf on the treated side (mean 37.7 cm [range 30.0–49.0]) compared with the nontreated side (mean 37.1 cm [range 31.0–48.0]) (p = 0.191).
Duplex Ultrasound
All stents were patent and had respiratory variation of flow. No stenosis or occlusion within the stent was seen. There was a nonsignificant trend of higher flow velocities within the stent compared with the corresponding segment of the contralateral side (p = 0.065). The median maximal velocity within the stent was 30.0 cm/s (range 10–48), and the median maximal velocity of the contralataral side was 20.0 cm/s (range 12–47). No patient showed venous reflux under the Valsalva maneuver within the stented segment.
X-Ray
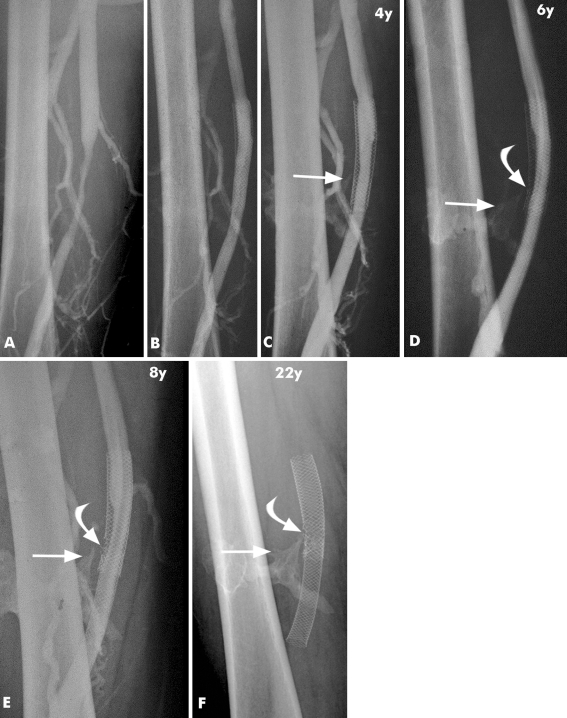
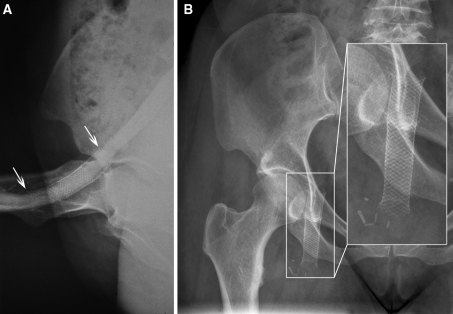
Stent integrity, with normal stent morphology, was documented in 14 of 15 cases (Fig. 1). One stent showed a single stent fracture (patient no. 14). We guess that the reason for this fracture was a calcified hematoma adjacent to the stent, which resulted in chronic mechanical stress (Fig. 2A–F). It is remarkable that even stents placed in the common femoral vein directly over the hip joint (patients no. 1 and 11 through 13) showed no fracture after 83 to 222 months later (Fig. 3A, B).
Fig. 1.
Shows X-ray of the pelvis, including a magnified view demonstrating the integrity of the stent in the left common iliac vein 13 years after placement for acute thrombosis due to a May–Thurner syndrome
Fig. 2.
Serial venograms during a follow-up period of 22 years (patient no. 14) after a stab wound injury of the right superficial femoral vein, which was treated with a venous interposition graft, resulting in stenosis of the femoral vein. A Venogram shows severe stenosis corresponding to the venous interposition graft. B Venogram taken after placement of two 14 × 70-mm Wallstents in the superficial femoral vein. C Venogram taken 4 years later shows new soft-tissue calcification adjacent to the patent stent (arrow), which was interpreted as a calcified hematoma or myositis ossificans. The stent is intact at follow-up of 6, 8, and 22 years. D, F A stent fracture can be noticed (curved arrow), which remained mostly unchanged over the years, with no signs of flow impairment or clinical signs of leg swelling. The likely cause of the stent fracture can be identified as the pointed part of the calcification (arrow) interfering with the stent
Fig. 3.
A X-ray of the right hip in a bent position after placement of two 12 × 75-mm Wallstents in the common femoral vein (patient no. 12) shows good adaption of the Wallstent to the curving course of the common femoral vein, with no signs of kinking or damage to the stent The upper and lower edges of the stent are marked with arrows. B X-ray of the hip of the same patient 18.5 years after placement shows that the Wallstent remained intact
Discussion
Our data suggest that stenting of benign iliofemoral venous obstruction has a good long-term clinical outcome with excellent stent patency. After a mean follow-up of 167.8 months (13.9 years), no additional venous intervention was needed in any patient. There was no significant leg swelling of the stented extremity compared with the contralateral side.
Since its introduction in the mid-1980 s, venous stenting has become a widely accepted treatment option [10–14]. Venous stenting has replaced surgical techniques, such as the Palma operation or the axial bypass technique for venous obstruction [15]. Not only is surgery more invasive than stenting, it also shows low patency rates between 44% and 85% [16].
Few reports about the mid- and long-term results after stenting of benign iliocaval obstructions [17–22] have shown patency rates approaching 100% without any stent fractures or other stent-related complications.
Our study focused on long-term outcomes >5 years. The results confirm excellent long-term result, with a patency rate of 100%, which corresponds to the good clinical outcome as measured by the lack of leg swelling.
Stent integrity has generally not been assessed in the venous system in the published literature [17–22] compared with the arterial system, in which stent fractures are of concern, particularly in the lower extremity [23, 24]. We encountered only one stent fracture (6.7%) in a special situation, i.e., the stent was placed in the superficial femoral vein (patient no. 14). During follow-up, soft-tissue calcifications developed lateral to the stent, which led to focal stent fracture after 6 years. The stent remained patent without any impairment of flow, despite the fracture, during a follow-up of 22 years.
Interestingly, no fractures were seen in the four stents placed in the common femoral vein across the hip joint. One patient (patient no. 15) with a common iliac stent had a normal pregnancy without any damage to the stent.
In the present study, patients were treated with warfarin for 6 months. Thereafter, no further anticoagulation was given routinely. Because no thrombosis occurred during our study, long-term anticoagulation did not seem necessary. The ability to stop anticoagulation is helpful because long-term anticoagulation during a period of years has its own risks [25].
Our study has several limitations. First, we only include a small study group, especially in the age group <50 years. Therefore, the long-term results should be interpreted with caution. The second limitation is the use a single device type (Wallstent), and the third limitation is the variety of underlying causes for venous obstruction.
In conclusion, venous stenting with Wallstents showed an excellent patency rate with good clinical outcomes between 71 months (6 years) and 267 months (22 years).
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
A. Gutzeit, Email: andreas.gutzeit@ksw.ch
Ch. L. Zollikofer, Email: Christoph.Zollikofer@ksb.ch
M. Dettling-Pizzolato, Email: Mira.Dettling@ksw.ch
N. Graf, Email: nicole.graf@usz.ch
J. Largiadèr, Email: jon.largiader@gefaesschirurgie-zuerich.ch
C. A. Binkert, Email: christoph.binkert@ksw.ch
References
- 1.Meissner MH, Eklof B, Smith PC, et al. Secondary chronic venous disorders. J Vasc Surg. 2007;46(Suppl S):68S–83S. doi: 10.1016/j.jvs.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 2.Alimi YS, DiMauro P, Fabre D, Juhan C. Iliac vein reconstructions to treat acute and chronic venous occlusive disease. J Vasc Surg. 1997;25:673–681. doi: 10.1016/S0741-5214(97)70294-5. [DOI] [PubMed] [Google Scholar]
- 3.Jost CJ, Gloviczki P, Cherry KJ, Jr, et al. Surgical reconstruction of iliofemoral veins and the inferior vena cava for nonmalignant occlusive disease. J Vasc Surg. 2001;33:320–327. doi: 10.1067/mva.2001.112805. [DOI] [PubMed] [Google Scholar]
- 4.Alimi YS, Juhan C. New trends in the surgical and endovascular reconstructions of large veins for nonmalignant chronic venous occlusive disease. Curr Opin Cardiol. 1998;13:375–383. doi: 10.1097/00001573-199809000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Halliday P, Harris J, May J. Femoro-femoral crossover grafts (Palma operation): a long-term follow up study. In: Bergan KK, Yao JST, editors. Surgery of the veins. Orlando: Grune & Stratton; 1985. pp. 241–254. [Google Scholar]
- 6.Wright KC, Wallace S, Charnsangavej C, Carrasco CH, Gianturco C. Percutaneous endovascular stents: an experimental evaluation. Radiology. 1985;156:69–72. doi: 10.1148/radiology.156.1.4001423. [DOI] [PubMed] [Google Scholar]
- 7.Zollikofer CL, Largiader I, Bruhlmann WF, Uhlschmid GK, Marty AH. Endovascular stenting of veins and grafts: Preliminary clinical experience. Radiology. 1988;167:707–712. doi: 10.1148/radiology.167.3.2966417. [DOI] [PubMed] [Google Scholar]
- 8.Antonucci F, Salomonowitz E, Stuckmann G, Stiefel M, Largiadèr J, Zollikofer CL. Placement of venous stents: clinical experience with a self-expanding prosthesis. Radiology. 1992;183:493–497. doi: 10.1148/radiology.183.2.1561356. [DOI] [PubMed] [Google Scholar]
- 9.Zollikofer CL, Antonucci F, Stuckmann G, Mattias P, Brühlmann WF, Salomonowitz EK. Use of the Wallstent in the venous system including hemodialysis-related stenoses. Cardiovasc Intervent Radiol. 1992;15:334–341. doi: 10.1007/BF02733959. [DOI] [PubMed] [Google Scholar]
- 10.Oguzkurt L, Tercan F, Ozkan U, Gulcan O. Iliac vein compression syndrome: outcome of endovascular treatment with long-term follow-up. Eur J Radiol. 2008;68:487–492. doi: 10.1016/j.ejrad.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 11.Schwarzbach MH, Schumacher H, Böckler D, et al. Surgical thrombectomy followed by intraoperative endovascular reconstruction for symptomatic ilio-femoral venous thrombosis. Eur J Vasc Endovasc Surg. 2005;29:58–66. doi: 10.1016/j.ejvs.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 12.Neglén P, Hollis KC, Olivier J, Raju S. Stenting of the venous outflow in chronic venous disease: Long-term stent-related outcome, clinical, and hemodynamic result. J Vasc Surg. 2007;46:979–990. doi: 10.1016/j.jvs.2007.06.046. [DOI] [PubMed] [Google Scholar]
- 13.Carlson JW, Nazarian GK, Hartenbach E, et al. Management of pelvic venous stenosis with intravascular stainless steel stents. Gynecol Oncol. 1995;56:362–369. doi: 10.1006/gyno.1995.1064. [DOI] [PubMed] [Google Scholar]
- 14.Ley EJ, Hood DB, Leke MA, Rao RK, Rowe VL, Weaver FA. Endovascular management of iliac vein occlusive disease. Ann Vasc Surg. 2004;18:228–233. doi: 10.1007/s10016-003-0096-9. [DOI] [PubMed] [Google Scholar]
- 15.Palma EC, Esperon R. Treatment of the post-thrombophlebitic syndrome by means of internal saphenous transplants. Bol Soc Cir Urug. 1959;30:115–125. [PubMed] [Google Scholar]
- 16.Nicolaides AN, Allegra C, Bergan J, et al. Management of chronic venous disorders of the lower limbs: guidelines according to scientific evidence. Int Angiol. 2008;27:1–59. [PubMed] [Google Scholar]
- 17.Kölbel T, Lindh M, Akesson M, Wassèlius J, Gottsäter A, Ivancev K. Chronic iliac vein occlusion: midterm results of endovascular recanalization. J Endovasc Ther. 2009;16:483–491. doi: 10.1583/09-2719.1. [DOI] [PubMed] [Google Scholar]
- 18.Hartung O, Loundou AD, Barthelemy P, Arnoux D, Boufi M, Alimi YS. Endovascular management of chronic disabling ilio-caval obstructive lesions: Long-term results. Eur J Vasc Endovasc Surg. 2009;38:118–124. doi: 10.1016/j.ejvs.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Hartung O, Otero A, Boufi M, et al. Mid-term results of endovascular treatment for symptomatic chronic nonmalignant iliocaval venous occlusive disease. J Vasc Surg. 2005;42:1138–1144. doi: 10.1016/j.jvs.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Hartung O, Benmiloud F, Barthelemy P, Dubuc M, Boufi M, Alimi YS. Late results of surgical venous thrombectomy with iliocaval stenting. J Vasc Surg. 2008;47:381–387. doi: 10.1016/j.jvs.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Wohlgemuth WA, Weber H, Loeprecht H, Tietze W, Bohndorf K. PTA and stenting of benign venous stenoses in the pelvis: Long-term results. Cardiovasc Intervent Radiol. 2000;23:9–16. doi: 10.1007/s002709910002. [DOI] [PubMed] [Google Scholar]
- 22.Nazir SA, Ganeshan A, Nazir S, Uberoi R. Endovascular treatment options in the management of lower limb deep venous thrombosis. Cardiovasc Intervent Radiol. 2009;32:861–876. doi: 10.1007/s00270-009-9662-z. [DOI] [PubMed] [Google Scholar]
- 23.Boehm G, Gschwendtner M, Schillinger M. Carotid stent fracture: diagnosis and management. Catheter Cardiovasc Interv. 2009;74:273–277. doi: 10.1002/ccd.22051. [DOI] [PubMed] [Google Scholar]
- 24.Schlager O, Dick P, Sabeti S, et al. Long-segment SFA stenting—the dark sides: in-stent restenosis, clinical deterioration, and stent fractures. J Endovasc Ther. 2005;12:676–684. doi: 10.1583/05-1672.1. [DOI] [PubMed] [Google Scholar]
- 25.Wells PS, Forgie MA, Simms M (2003) The outpatient bleeding risk index: validation of a tool for predicting bleeding rates in patients treated for deep venous thrombosis and pulmonary embolism. Arch Intern Med 163:917–920 [DOI] [PubMed]