Abstract
We have explored the functional implications of inducible α4 integrin deletion during adult hematopoiesis by generating a conditional-knockout mouse model, and we show that α4 integrin-deficient hematopoietic progenitor cells accumulate in the peripheral blood soon after interferon-induced gene deletion. Although their numbers gradually stabilize at a lower level, progenitor cell influx into the circulation continues at above-normal levels for more than 50 weeks. Concomitantly, a progressive accumulation of progenitors occurs within the spleen. In addition, the regeneration of erythroid and myeloid progenitor cells is delayed during stress hematopoiesis induced by phenylhydrazine or by 5-fluorouracil, suggesting impairment in early progenitor expansion in the absence of α4 integrin. Moreover, in transplantation studies, homing of α4−/− cells to the bone marrow, but not to the spleen, is selectively impaired, and short-term engraftment is critically delayed in the early weeks after transplantation. Thus, conditional deletion of α4 integrin in adult mice is accompanied by a novel hematopoietic phenotype during both homeostasis and recovery from stress, a phenotype that is distinct from the ones previously described in α4 integrin-null chimeras and β1 integrin-conditional knockouts.
Development of hematopoietic stem cells and their differentiated descendants in selective anatomic sites during fetal or adult life relies on favorable interactions with a specialized microenvironment that provides mechanical support and facilitates the enhancement of hematopoietic stem cells' proliferation and differentiation. The relationship between hematopoietic cells and cells within the microenvironment is a highly dynamic one, such that in response to stimuli, their coordinated responses can accommodate acute demands in cell expansion and migration in or out of the hematopoietic compartment to meet physiologic needs. As the molecular demands of stem cells residing in each anatomical site are likely to be different from those of differentiated cells, specialized niches are envisioned to accommodate these requirements (61). Members of the integrin family of cytoadhesive molecules are widely expressed in the hematopoietic system (56, 63) and exercise decisive roles in the interactions between hematopoietic cells and their microenvironment. This specialized function is dependent on the ability of integrins to serve not only as adhesion receptors but also as bona fide bidirectional signaling molecules that transduce signals to downstream effectors (20, 55). Integration of signaling networks is of particular importance in hematopoiesis, permitting cross talk with additional integrin molecules or with growth factor receptors, metalloproteinases, and chemokines in order to influence motility and other cellular functions (22, 26, 47). Moreover, the specificity in the actions of integrins in different anatomic locations may rely on the fact that signaling after ligation to immobilized ligands is both topographically constrained and cell type dependent.
To uncover integrin functions in the process of hematopoiesis, early studies used antibodies that not only defined their expression patterns but also uncovered their function and responses to stimuli in vitro and in vivo. These studies found that the β1 integrins are expressed by a wide variety of cell types, whereas the β2 integrins are found exclusively in hematopoietic cells (56, 63). Expression of β1 and β2 integrins is also differentiation dependent, with mature cells exhibiting diminished or inactive expression of α4β1 integrin (41, 56, 63) in contrast to stem and progenitor cells, which express α4 integrin in a constitutively active state (21, 38). Cells from different stages of development also display differential patterns of integrin expression. For example, fetal cells express more α2β1 integrin than do adult cells, a factor which dictates functional differences in their ability to adhere to collagen IV (52). Similarly, yolk sac-derived primitive erythroid cells display little α4 or α5 integrin expression in contrast to high levels expressed by fetal liver (FL) erythroid and adult bone marrow (BM) cells (40, 41). Perturbation of α4 integrin function in long-term BM cultures by an anti-α4 integrin antibody blocked the in vitro development of progenitors into mature erythroid, lymphoid, and myeloid cells (36, 70). Similarly, anti-α4 integrin antibodies injected into pregnant mice resulted in inhibition in FL erythroid development, but with little effect on lymphoid or myeloid development (15). In vivo studies have revealed additional roles for α4β1 integrin and its ligand, vascular cell adhesion molecule 1 (VCAM-1), in the homing to and short-term engraftment in the BM (42, 75). In vivo studies also stressed the role of α4 integrin in influencing migration of hematopoietic progenitors from the BM into circulation (9, 23, 43, 62, 71).
Experiments in mice deficient in β1 or α4 integrins have provided direct evidence of the involvement of integrins in hematopoiesis. Deletion of either the α4 or β1 integrin gene caused embryonic lethality from nonhematological defects (10, 58, 72); deletion of VCAM-1 resulted in a phenotype identical to that of the α4 integrin knockout (13, 28). Early lethality consequently precluded significant analysis of the roles of these adhesion molecules in hematopoietic development, although yolk sac hematopoiesis did remain intact in the α4 integrin knockout (3). The transition from α4 integrin independence to dependence at the liver colonization stage might explain the preservation of primitive erythropoiesis in α4 knockout animals. Studies with chimeric mice showed that α4 integrin-deficient hematopoiesis was compromised in the FL and was extinguished shortly after birth (3, 4). Although FL progenitor cells differentiated normally in vitro, their terminal differentiation in the FL and BM was impaired in the absence of α4 integrin, resulting in decreased proliferation and differentiation. Furthermore, β1 integrin-deficient cells were not able to colonize the FL, nor were they able to colonize the adult BM, spleen, or thymus of chimeras (18). However, in contrast to the significant defects in hematopoiesis in α4 chimeric mice and the migratory failure of β1-null cells during development, recent analysis of adult mice with conditional deletion of β1 integrin demonstrated no perturbations in myelopoiesis in the absence of all β1 integrins (6). Moreover, it is noteworthy that both of these genetic studies appear to diverge from studies obtained in vivo with the use of α4 or β1 antifunctional antibodies, although the interpretation of data with monoclonal antibodies has been questioned because of unwanted side effects either by steric hindrance of other interactions or by partial activation of target cells and/or binding to irrelevant cells (6).
Because of the difficulties in assigning specific roles to α4 integrin in hematopoiesis, especially between fetal and adult stages, we have generated conditional-knockout mice with α4 integrin alleles designed to be disrupted upon treatment of adult animals with interferon. This approach not only bypasses the embryonic lethality observed in α4 integrin knockouts but also causes a cell-intrinsic deficiency that cannot be restored by a normal BM microenvironment. By studying myelopoiesis before and after induction of stress, a novel hemopoietic phenotype was unveiled in these mice that has certain similarities to that of α4 integrin chimeras but displays significant differences from both these mice and the β1 integrin-conditional knockouts. The present studies expand our understanding of the role of α4 integrin in adult hematopoiesis at both the cellular and molecular levels.
MATERIALS AND METHODS
Gene targeting and generation of α4 integrin knockout mice.
A 13.9-kb clone containing the proximal end of the murine α4 integrin gene (α4) was isolated from a 129S4/SvJaeSor-derived genomic library (Stratagene, La Jolla, Calif.). The targeting vector was constructed from a 5.95-kb XbaI/SphI α4 restriction fragment that included the promoter and first two exons, a PGK-neo-p(A) cassette flanked by loxP elements, inserted at a KpnI site in the promoter, and an additional loxP, inserted at a HindIII site distal to the second exon. The diphtheria toxin A chain gene was added as a negative selection marker (68). AK7 embryonic stem (ES) cells were maintained on mitomycin C-inactivated SNL 76/7 feeder cells in medium containing 500 U of leukemia inhibitory factor (Gibco-BRL, New York, N.Y.) per ml. A total of 8 × 106 AK7 cells were electroporated at 240 V and 500 μF with 25 μg of linearized targeting vector by using a GenePulser electroporator (Bio-Rad Laboratories, Hercules, Calif.); the cells were then selected in 300 μg of G418. Clones with a floxed (that is, flanked by loxP) α4 allele (α4flox) resulting from homologous recombination were identified by amplification reactions with primers specific to the distal loxP and a region of intron II not included in the targeting vector (5′-TGAAGAGGAGTTTACCCAGC-3′ and 5′-CACCCTTAGCTCATCATCATCG-3′). Candidate clones were analyzed by Southern blot analyses using probes proximal and distal to the 5.95-kb XbaI/SphI restriction fragment. Targeted clones with a normal XY karyotype were injected into C57BL/6 blastocysts 3.5 days postcoitum and transferred into pseudopregnant females. The resulting high percentage of male chimeras, identified by the level of agouti coat color, were bred to C57BL/6 females. Offspring were genotyped by tail tipping and polymerase chain reactions with primers flanking the distal loxP sequence (5′-GTCCACTGTTGGGCAAGTCC-3′ and 5′-AAACTTGTCTCCTCTGCCGTC-3′), which were carried out with an annealing temperature of 61°C. Animals heterozygous for the floxed α4 allele (α4flox) were crossed to generate floxed homozygotes.
Animals and treatment.
Mice were bred and maintained under specific-pathogen-free conditions in University of Washington facilities under a 12-hour light-dark cycle, and they were provided with irradiated food and autoclaved water ad libitum. The α4flox/flox females were bred to Mx.cre+ males (27), and the progeny were screened for the cre transgene by slot blot analysis. Mx.cre+α4flox/+ mice were intercrossed, and the Mx.cre+ progeny were genotyped with respect to the α4 allele. Six-to-eight-week-old gender-matched Mx.cre+α4+/+and Mx.cre+α4flox/flox mice were used for all experiments. Cre recombinase was induced by three intraperitoneal injections of 300 μg of poly(I)-poly(C) (Sigma Chemical Company, St. Louis, Mo.) at 2-day intervals, and the mice were then analyzed at least 14 days after the final injection.
The capacity of Mx.cre+α4+/+ and Mx.cre+α4Δ/Δ leukocytes and progenitor cells to recover from cytotoxic stress was assessed after intravenous injection of 150 mg of 5-fluorouracil (5FU) per kg of body weight 3 weeks after poly(I)poly(C) treatment. Animals were killed 4, 8, 12, or 16 days later, and the progenitor content was assessed by using the assay for colony-forming cells (CFU-C) described below. The capacity of α4+/+ and α4Δ/Δ mice to recover from acute hemolysis was assessed after two daily intraperitoneal injections of 60 mg of phenylhydrazine (PHZ; Sigma Chemical Company) per kg. Reticulocyte and platelet numbers were determined in peripheral blood (PB) samples obtained retro-orbitally 2, 4, 6, 8, and 10 days later with an automated cell counter. In a separate cohort, mice were euthanized 2 days after the last injection, and hematopoietic tissues were analyzed for CFU-C content in methylcellulose and plasma clot assays.
CFU-C assays.
To quantitate committed progenitors of all lineages, CFU-C assays were performed using methylcellulose semisolid media (Stemgenix, Amherst, N.Y.) supplemented with an additional 50 ng of stem cell factor (Peprotech, Rocky Hill, N.J.) per ml. Next, 0.1 ml of lysed PB, 50,000 BM cells, and 500,000 spleen cells were plated on duplicate 35-mm culture dishes and then incubated at 37°C in a 5% CO2-95% air mixture in a humidified chamber for 7 days. Colonies generated by that time were counted by using a dissecting microscope, and all colony types (i.e., burst forming units-erythroid [BFU-e], CFU-granulocyte-macrophage [CFU-GM], and CFU-mixed [CFU-GEMM]) were pooled and reported as total CFU-C. CFU-erythroid (CFU-e) cells were assessed only in plasma clot cultures, as their evaluation in methylcellulose cultures is inaccurate. In plasma clots, BFU-e can also be concurrently evaluated. Plasma clots were prepared as described previously (44).
Fluorescence-activated cell sorter (FACS) analysis.
Hematopoietic cells were analyzed on a FACSCalibur (BD Immunocytometry Systems, San Jose, Calif.) by using the CELLQuest program. Staining was performed by using antibodies conjugated to fluorescein isothiocyanate (FITC), phycoerythrin (PE), or allophycocyanin. The following BD Pharmingen (San Diego, Calif.) antibodies were used for cell surface staining: allophycocyanin-conjugated c-kit (2B8) and CD45 (30F-11); PE-conjugated CD4 (RM4-5), B220 (RA3-6B2), Mac1 (M1/70), Ter119 (Ly-76), α2 integrin (HMa2), α5 integrin (MFR5), α6 integrin (GoH3), and CD11a (2D7); and FITC-conjugated CD3 (145-2C11), β1 integrin (Ha2/5), and β2 integrin (c71/16). PE-conjugated anti-α4 integrin (PS/2) was from Southern Biotechnology, Birmingham Ala. Irrelevant isotype-matched antibodies were used as controls.
Retroviral vectors and transduction of BM cells.
Stable producer lines were generated by infection of GpE86 packaging cells (34) with viral supernatants produced by transient transfection of BOSC23 cells with plasmids encoding MSCViresGFP or MSCV-cre-iresGFP (46). Following infection, transduced GpE86 cells were purified on the basis of green fluorescent protein (GFP) positivity. Mx.cre+α4flox/flox animals were injected intraperitoneally with 150 mg of 5FU per kg, and BM cells were recovered 2 days later. These were incubated at a concentration of 4 × 106 cells/ml for 48 h in Iscove’s modified Dulbecco medium with 10% fetal bovine serum (HyClone, Logan, Utah), 5% murine interleukin 3 culture supplement (Collaborative Biomedical Products, Bedford, Mass.), 50 ng of human interleukin 6 (Amgen, Thousand Oaks, Calif.) per ml, and 50 ng of murine stem cell factor per ml. BM cells were plated on irradiated monolayers of each producer line and cocultured for 48 h prior to analysis.
Homing assays and transplantation studies.
In homing assays, donor animals (Mx.cre+α4+/+ or Mx.cre+α4Δ/Δ) were sacrificed, and single-cell suspensions of the BM were prepared, counted, and used for transplantation at appropriate concentrations. An aliquot was also plated in methylcellulose cultures to quantify committed progenitor cells of various lineages present in donor cells. In all homing experiments, 20 × 106 to 30 × 106 cells were injected through the tail vein into mice subjected to 1,150-cGy whole-body irradiation with 137Cs. In certain experiments, donor cells were incubated for 30 min at 4°C in the presence of low-endotoxin, azide-free unconjugated antibody and then washed before intravenous infusion. Twenty-four hours later, recipients of fresh or antibody-treated cells were sacrificed under anesthesia, and the BM, spleen, and PB were collected. Single-cell suspensions of BM and spleen were prepared and cultured in duplicate to assess donor CFU-C recovery (CFU-GM, CFU-GEMM, and BFU-e) in the recipient animals. The number of donor CFU-C recovered after 24 h in BM, PB, or spleen was expressed as a percentage of total CFU-C infused. For estimating total BM recovery, the femur content was assumed to represent 6.7% of total BM (5).
For evaluating short-term engraftment, irradiated recipients were transplanted with 0.5 × 106, 1.0 × 106, or 5.0 × 106 donor cells (α+/+ or α4−/−) and analyzed 2 weeks after transplantation. Evaluation of total cell and total CFU-C content in BM, spleen, and PB was done as above.
Statistical analysis.
Results are expressed as the mean ± standard error of the mean. Data were analyzed using the unpaired two-tailed Student's t test. P values that were ≤0.05 were considered significant.
RESULTS
Generation of conditional α4 integrin-knockout mice.
To study the roles of α4 integrin in adult hematopoiesis, we used conditional gene inactivation by cre-loxP-mediated recombination to produce a conditional α4 integrin-knockout mouse. A targeting vector was constructed in which a neomycin-resistance cassette flanked by loxP sequences was introduced into the promoter of the α4 integrin gene (α4), and an additional loxP was inserted downstream of the second exon (Fig. 1A). This was designed so that the regulatory elements required for expression and the start of the coding region would be deleted by Cre recombinase expression to produce a null allele similar to that reported previously (72). The targeting vector was introduced into ES cells, and those clones surviving G418 selection were screened for homologous recombination. Appropriate targeting occurred in 3 of the 750 clones screened (Fig. 1B). Pups resulting from the injection of cells from each clone into blastocysts included a high percentage of coat color chimeras. Chimeric males were bred to C57BL/6 females to establish hybrid strains carrying a floxed α4 integrin allele (α4flox). Mice heterozygous and homozygous for the α4flox allele were observed at the expected ratio, suggesting that introduction of the neo cassette and loxP sites had not disrupted α4 integrin expression by transcriptional interference. The α4flox/flox mice were phenotypically normal and fertile, and FACS analysis confirmed that the α4 integrin expression levels in the BM of α4+/+, α4flox/+, and α4flox/flox littermates were indistinguishable (Fig. 1C). Deletion of the α4flox allele in these mice was tested for by using transduction with a retrovirus-expressing Cre recombinase. BM cells from α4flox/flox mice were transduced either with a control retrovirus encoding GFP (MSCViresGFP) or one in which the gene for Cre recombinase was located upstream of an internal ribosomal entry site (MSCV-cre-iresGFP). FACS analysis showed that only GFP+ cells transduced by MSCV-cre-iresGFP were α4 integrin deficient (data not shown). The resulting populations of transduced cells were sorted and plated in methylcellulose, and colonies were then analyzed a week later by Southern blotting. This analysis confirmed that the loss of α4 integrin expression after MSCV-cre-iresGFP transduction resulted from total excision between the loxP sites flanking the proximal end of the α4 gene to give a deleted allele (α4Δ) of the appropriate size (Fig. 1B).
FIG. 1.
Targeting of the murine α4 locus. (A) Maps of the targeting construct containing three loxP elements (grey bars) and positive (NEO) and negative (DT-A) selection cassettes; the α4 locus (α4+) including the first two exons (black boxes); the locus after homologous recombination (α4flox) and after Cre-mediated excision (α4Δ). Sites for restriction enzymes HindIII (H), KpnI (K), SphI (S), and XbaI are shown; sites introduced after homologous recombination are in bold typeface. The XbaI restriction fragments that are diagnostic for each of the three alleles are shown. (B) Homologous recombination and Cre-mediated excision at the α4 locus. The α4+ allele in ES cells migrates as a 12-kb SphI (lane 1) or 7.1-kb XbaI (lane 3) fragment, and the α4flox allele migrates as a 5.5-kb SphI (lane 2) or 5.7-kb XbaI (lane 4) fragment. BM cells from α4flox/flox mice were transduced with retroviruses encoding Cre and/or GFP, sorted, and cultured in methylcellulose. XbaI-digested DNAs from MSCViresGFP-transduced α4+/+ colonies (lane 5) are compared to those from CFU-C transduced by MSCViresGFP (lane 6) or MSCV-cre-iresGFP (lane 7). The location of the probe used is indicated by the hatched bar in panel A. (C) BM cells from α4+/+, α4flox/+, and α4flox/flox littermates were stained with PE-conjugated anti-α4 integrin antibody (PS/2) and analyzed by FACS (black histograms). An isotype control FACS profile (dotted line) is included for comparison. Typical FACS profiles for each group of animals are shown.
Phenotypic analysis of adult BM and blood after α4 integrin deletion.
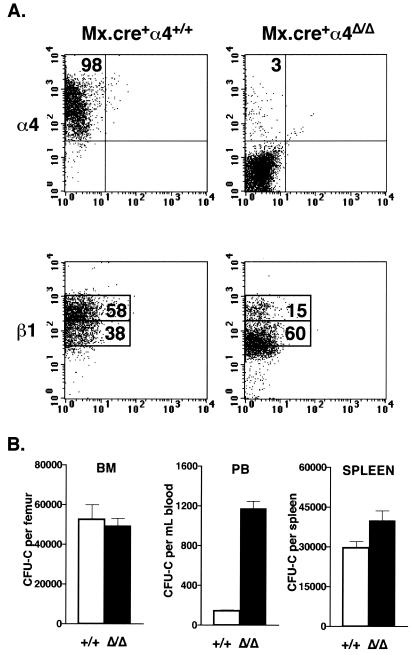
To produce an inducible α4 integrin knockout, our α4flox/flox mice were bred to Mx.cre transgenic animals, in which the interferon-inducible Mx promoter regulates Cre recombinase expression (27). To induce Cre expression, adult Mx.cre+α4+/+ and Mx.cre+α4flox/flox mice were injected three times with poly(I)poly(C), a synthetic RNA molecule that stimulates endogenous interferon expression; the mice were euthanized 2 weeks after the last injection. In an analysis of 31 mice of each genotype, only 3.7% ± 0.2% of BM cells isolated from the α4Δ/Δ mice expressed α4 integrin, compared to 93.0% ± 0.9% in α4+/+ mice (P < 0.001), as illustrated by the typical FACS profiles shown in Fig. 2A. Concurrent FACS analysis of the α2, α5, α6, and β2 integrins revealed that their expression patterns in BM cells did not alter (data not shown). However, deletion of the α4 gene reduced the levels of β1 integrin expressed in BM cells, as indicated by reduced intensity of fluorescence after antibody staining and a marked decrease in frequency of cells expressing high levels of β1 (Fig. 2A).Polymerase chain reaction and FACS analysis of 25 randomly selected individual colonies derived from the BM CFU-C subset of α4Δ/Δ mice confirmed that these had undergone deletion of the gene in vivo and were α4 integrin deficient (data not shown).
FIG. 2.
Deletion of α4 integrin expression in the BM alters progenitor distribution. (A) Mx.cre+α4+/+ and Mx.cre α4flox/flox animals were injected with poly(I)poly(C), and their BM nucleated cells were examined by FACS 2 weeks later for α4 and β1 integrin expression levels. Typical FACS profiles for both groups of animals are shown. (B) Progenitor cell numbers in the BM (n = 16 per genotype), PB (n = 45 per genotype), and spleen (n = 10 per genotype) of Mx.cre+α4+/+and Mx.cre+α4Δ/Δ mice were assessed 2 weeks after deletion by using CFU-C assays. White bars, Mx.cre+α4+/+ mice; black bars, Mx.cre+α4Δ/Δ mice.
The lineage composition of control and α4 integrin-deficient BM 2 weeks after deletion was examined through the expression of lineage-affiliated cell surface markers. No differences in the number of myeloid (Mac1+) or erythroid (Ter119+) cells in the BM were noted (n = 16 per genotype). However, small but significant reductions in the proportion of the B220+ B cells (24.9% ± 1.1% in α4+/+ versus 20.8% ± 1.0% in α4Δ/Δ; n = 16; P < 0.01) and CD4+ T-cell populations (1.7% ± 0.1% in α4+/+ versus 1.0% ± 0.05% in α4Δ/Δ; n = 16; P < 0.0002) were noted. Differential analysis of PB 2 weeks after poly(I)poly(C) treatment showed significantly increased (P < 0.001) white blood cell counts in the mice with induced deletion of α4 ([12.8 ± 0.5] × 106 per ml compared to [7.5 ± 0.3] × 106 per ml in control mice; n = 45 per genotype). This leukocytosis was due primarily to increased numbers of circulating lymphocytes and persisted for at least 50 weeks after induced deletion. Red blood cell and platelet numbers, however, were unaffected by deletion.
Progenitor distribution in the absence of α4 integrin.
Assays of clonogenic progenitors performed using BM cells from 16 Mx.cre+α4+/+ and 16 Mx.cre+α4Δ/Δ mice showed that hematopoietic progenitor numbers (total CFU-C) were not affected 2 weeks after deletion (52,488 ± 7,543 per femur in α4+/+ mice; 49,554 ± 3,615 per femur in α4Δ/Δ mice [Fig. 2B]). Circulating levels of progenitors in the blood of 45 Mx.cre+α4+/+and 45 Mx.cre+α4Δ/Δ mice were also evaluated 2 weeks after poly(I)poly(C) treatment. A dramatic increase in circulating progenitor cell numbers was noted in the absence of α4 integrin, from 142 ± 13 CFU-C per ml in control mice to 1,177 ± 71 CFU-C per ml in mice with induced deletion (P < 0.001). In addition to their increase in the PB, the number of progenitor cells within the spleen also increased following deletion, from 29,538 ± 2,549 in controls to 39,994 ± 3,680 per spleen in deletion-induced mice(n = 10 per genotype; P < 0.05).
The above data suggested that the distribution of progenitor cells is altered soon after α4 integrin deletion, most likely as a result of their ongoing release from the BM. To pursue these changes further and to characterize the kinetics of circulating CFU-C, we followed progenitor cell number and distribution in four additional Mx.cre+α4+/+ and Mx.cre+α4Δ/Δ mice over time. We found that circulating CFU-C levels remained elevated for the entire 28-week observation period, although their frequency gradually decreased before stabilizing (Fig. 3A). The spleens of these deletion-induced mice showed a progressive increase in progenitor content over time, from the 39,994 ± 3,680 CFU-C per spleen observed 2 weeks after deletion (Fig. 2B) to 79,096 ± 14,428 CFU-C 28 weeks after deletion (Fig. 3A) (P < 0.05). In contrast, the levels of circulating CFU-C and splenic CFU-C did not change in control animals (Fig. 2B and 3A). The calculated combined progenitor content of the PB, total BM (see Materials and Methods), and spleen had increased by 50% as a consequence of α4 integrin deletion, from 916,566 ± 19,742 CFU-C in the α4+/+ mice to 1,364,325 ± 68,312 in the α4Δ/Δ mice (P < 0.005). FACS analysis of BM cells and CFU-C-derived cells from these Mx.cre+α4Δ/Δ mice revealed no reemergence of α4 integrin-expressing cells in the 28 weeks following deletion.
FIG. 3.
α4 integrin-null progenitor numbers in the PB, BM, and spleen over time. (A) PB progenitor levels in Mx.cre+α4+/+ and Mx.cre+α4Δ/Δ mice (n = 4 per genotype) were monitored over time. The animals were euthanized 28 weeks after induced deletion, and the progenitor contents of the BM and spleen were assessed in CFU-C assays. White bars, Mx.cre+α4+/+ mice; black bars, Mx.cre+α4Δ/Δ mice. (B) PB progenitor levels of nonsplenectomized and splenectomized Mx.cre+α4Δ/Δ mice (n = 4 per group) were monitored over time. Black bars, nonsplenectomized Mx.cre+α4Δ/Δ mice; striped bars, splenectomized Mx.cre+α4Δ/Δ mice. (C) The BM progenitor content of splenectomized Mx.cre+α4+/+ and Mx.cre+α4Δ/Δ mice (n = 4 per genotype) was assessed 50 weeks after induced deletion. White bar, splenectomized Mx.cre+α4+/+ mice; striped bar, splenectomized Mx.cre+α4Δ/Δ mice.
As the progenitor content in the spleen was increased shortly after deletion and continued to increase thereafter, we explored the role of the spleen in altering the CFU-C distribution by assessing mice splenectomized a month after poly(I)poly(C) treatment. Thirteen weeks after deletion, the PB CFU-C content in four splenectomized Mx.cre+α4Δ/Δ mice was appreciably higher than that in a cohort of nonsplenectomized Mx.cre+α4Δ/Δ animals (P < 0.05), supporting the notion that the spleen continuously sequesters many circulating α4 integrin-deficient progenitors (Fig. 3B). The PB progenitor cell frequency in splenectomized deletion-induced mice also decreased over time, albeit at a much lower rate than that observed in nonsplenectomized deletion-induced animals. Furthermore, 50 weeks after deletion was induced, the progenitor content of the BM of splenectomized α4Δ/Δ mice was significantly less (P < 0.01) than that of splenectomized α4+/+ mice (38,159 ± 2,246 CFU-C per femur [n = 4] and 50,965 ± 733 CFU-C per femur [n = 4], respectively [Fig. 3C]). This was in direct contrast to the BM of nonsplenectomized α4Δ/Δ animals analyzed 50 weeks after deletion, in which progenitor numbers had increased compared to α4+/+ mice (83,078 ± 8,652 CFU-C per femur [n = 4] and 52,285 ± 3,336 CFU-C per femur [n = 4], respectively; P < 0.05). Preliminary evaluation of cycling cells of α4-deleted BM in the presence or absence of the spleen showed no statistically significant differences. (Percentage of cycling cells in BM of α4+/+ splenectomized animals was 24.2% ± 3.59%, whereas it was 29.7% ± 2.0% in α4−/− splenectomized and 34.1 ± 2.4% in α4−/− nonsplenectomized mice [n = 4 per group].) Whether the rate of apoptosis is different in the BM of splenectomized animals was not explored.
α4 integrin is required for efficient recovery from hemolytic anemia.
Because of the impact of α4 integrins during erythropoiesis shown previously (3, 15), we tested the responses of recently deletion-induced animals to anemia by inducing acute hemolysis with PHZ, a scenario in which recovery depends on the erythroid progenitor reserve. Induction of anemia produced a drop in hematocrit from 50.8% ± 0.7% to 22.3% ± 1.8% in Mx.cre+ α4+/+ mice and from 50.3% ± 1.0% to 23.4% ± 1.3% in Mx.cre+α4Δ/Δ mice 2 days after the second of two daily PHZ injections (n = 6 per genotype). At this time, reticulocytes were 29.3% ± 2.3% of the α4+/+ PB and 18.5% ± 1.9% in α4Δ/Δ PB (P < 0.05), suggesting an impaired generation of reticulocytes in α4 integrin-deficient mice (Fig. 4A). Consistent with this hypothesis, red blood cell levels were lower in α4Δ/Δ mice throughout the period of recovery examined (P < 0.05 6 days after PHZ; P < 0.01 10 days after PHZ). Also, PHZ exposure induced a rapid, transient thrombocytosis in control mice similar in nature to that previously reported (12), but thrombocytosis was not observed in animals in which the α4 integrin had been deleted. The mechanisms involved in platelet release from megakaryocytes residing in the BM or spleen after the induction of anemia are currently unclear; absence of rebound thrombocytosis in α4 integrin-deficient animals, however, suggests a role for α4 integrin in this process.
FIG. 4.
Response to PHZ-induced anemia in the absence of α4 integrin. Responses to hemolytic anemia were tested in Mx.cre+α4+/+ and Mx.cre+α4Δ/Δ mice (n = 4 per genotype) by PB differential analyses performed 2, 4, 6, 8, and 10 days after PHZ treatment. (A) Reduced reticulocyte levels were observed in the PB of the deletion-induced mice 4 to 6 days after PHZ administration. The α4 integrin-deficient platelet levels also failed to increase above baseline levels in response to PHZ from days 2 to 6. *, significant difference. White bars, Mx.cre+α4+/+ mice; black bars, Mx.cre+α4Δ/Δ mice. (B) Erythroid progenitor numbers in BM and spleen were evaluated 2 days after the last PHZ treatment by using plasma clot assays. BFU-e numbers were significantly reduced in the BM and spleen of deletion-induced animals. In contrast, CFU-e levels were reduced in the BM but not in the spleen.
To explore the mechanisms of delayed erythroid regeneration in the absence of α4 integrin, five additional Mx.cre+α4+/+and Mx.cre+α4Δ/Δ animals were also analyzed 2 days after the last PHZ treatment. FACS analysis of α4Δ/Δ BM cells showed that Ter119+ cell numbers were reduced to (7.6 ± 0.4) × 106 per femur compared with (11.2 ± 0.7) × 106 in control mice (P < 0.005). Concomitantly, CFU-e and especially BFU-e numbers were reduced in the α4Δ/Δ BM compared to control BM (P < 0.1 and 0.05, respectively) (Fig. 4B). BFU-e numbers were also reduced in the spleens of deletion-induced mice, from 125,363 ± 25,528 in control spleens to 46,902 ± 17,915 (P < 0.05). In the PB, an 11-fold difference in CFU-e number was observed in α4 integrin-deleted animals (83 ± 29 per ml in α4+/+ mice versus 964 ± 296 per ml in α4Δ/Δ mice; P < 0.05). In contrast, PB BFU-e levels did not change (855 ± 191 per ml in α4+/+ mice and 1,213 ± 383 per ml in α4Δ/Δ mice; P > 0.1). The number of α4Δ/Δ megakaryocytic progenitors did not differ from controls in any of the tissues examined (data not shown). The combined number of BFU-e in the PB, BM, and spleen of deletion-induced mice was significantly less than that of control mice ([0.8 ± 0.2] × 105 and [2.9 ± 0.6] × 105 BFU-e, respectively; P < 0.05), and this reduced number was likely responsible for the observed delayed recovery from anemia in α4 integrin-deleted mice. Interestingly, this delay in progenitor recovery occurred despite their greater number prior to PHZ treatment and did not impair their preferential release into the blood during recovery.
Response of α4 integrin-deficient BM cells to hematopoietic stress by 5FU.
Exposure to 5FU selectively kills cycling hematopoietic cells (30, 60), with recovery involving the recruitment of quiescent stem and progenitor cells into the cell cycle (51). To test the ability of myeloid progenitor cells to recover from hematopoietic stress in the absence of α4 integrin, we treated four groups of five Mx.cre+α4+/+ and five Mx.cre+α4Δ/Δ mice with 5FU and then assayed the PB and BM cell and progenitor content during the recovery phase 4, 8, 12, and 16 days later. BM cellularity was severely affected by the fourth day in both groups of mice; progenitor numbers were reduced to 670 ± 136 CFU-C per α4+/+ femur and to 1,371 ± 699 CFU-C per α4Δ/Δ femur (Fig. 5). By day 8, BM cellularity had doubled in control mice, whereas it was unchanged in deletion-induced mice (P > 0.1). At this time, CFU-C numbers were 23,390 ± 6,144 per α4Δ/Δ femur compared to 37,815 ± 10,393 per α4+/+ femur. Thus, between days 4 and 8, the progenitor content of the α4+/+ BM increased by an average of 56-fold, compared to 17-fold in α4Δ/Δ mice (P < 0.05). By day 12, progenitor numbers had recovered to near-normal levels in the BM of both groups of mice.
FIG. 5.
Response to cytotoxic stress induced by 5FU in the absence of α4 integrin. Individual groups of Mx.cre+α4+/+ and Mx.cre+α4Δ/Δ mice (n = 4 per genotype) were treated with 5FU, and analyzed 4, 8, 12, or 16 days later to determine the level of myeloid recovery in the BM and PB (nucleated cells and progenitor cells). White bars, Mx.cre+α4+/+mice; black bars, Mx.cre+α4Δ/Δ mice.
Consistent with a delayed recovery of α4Δ/Δ BM cells between days 4 and 8, white blood cell numbers in the PB of deletion-induced animals dropped lower than those of controls (P < 0.1), reaching a nadir of 12.7% ± 14.4% of α4Δ/Δ steady-state levels compared with 40.5% ± 18.6% in controls (Fig. 5). By day 16, leukocyte numbers in the deletion-induced mice increased to two-thirds of their steady-state level, whereas levels in α4+/+ mice had completely recovered. Similarly, the kinetics of progenitor cell release into the PB is delayed in the absence of α4 integrin expression. PB analysis 8 days after 5FU exposure showed that the number of circulating progenitor cells in controls increased to four times the normal level (390 ± 161 CFU-C per ml compared to 101 ± 2 CFU-C per ml). However, at the same time, progenitor numbers in deletion-induced mice were lower than their baseline levels (101 ± 4 CFU-C per ml compared to 1,030 ± 90 CFU-C per ml; P < 0.001). PB progenitor numbers in both groups of mice peaked on day 12, when BM recovery was nearing completion (3,414 ± 696 CFU-C per ml in controls compared to 10,958 ± 4,286 CFU-C per ml in deletion-induced mice; P < 0.05). The latter data are consistent with an enhanced mobilization of progenitor cells observed in primates during the early post-5FU recovery period by concurrent administration of function blocking α4 integrin antibodies(9).
α4 integrin-null cells have reduced BM homing and short-term engraftment.
To examine the ability of α4 integrin-deleted adult hematopoietic cells to home, we studied homing to hematopoietic tissues after injection of BM cells from Mx.cre+α4+/+or Mx.cre+ α4Δ/Δ donor mice into lethally irradiated recipient mice (n = 5 recipients per donor BM genotype). To assess homing, the number of donor clonogenic progenitors (CFU-C) recovered in PB, BM, and spleen 24 h after their intravenous injection was estimated as a fraction of the total number of CFU-C injected. Previous experiments have indicated that BM homing levels are not significantly different from 3 to 24 h after injection and that they are largely unaffected as yet by proliferation (57). Total BM progenitor homing (calculated from the femur data, as described in Materials and Methods) was estimated at 14.1% ± 0.8% of injected α4+/+ CFU-C but only at 9.3% ± 0.9% of injected α4Δ/Δ CFU-C (P < 0.005) (Fig. 6A). Concurrently, fourfold-higher numbers of progenitor cells remained circulating in the PB in the absence of α4 integrin (P < 0.001). Increased CFU-C accumulation was observed in the spleens of these recipients, with 13.2% ± 0.9% of injected CFU-C recovered from animals receiving deletion-induced BM cells compared to 8.7% ± 0.5% of injected CFU-C using control BM (P < 0.005). These data confirm the selective inhibition of BM homing observed earlier using function blocking antibodies (42).
FIG. 6.
α4 integrin-deficient progenitors have reduced BM homing. BM cells from Mx.cre+α4+/+ or Mx.cre+α4Δ/Δ mice were injected without prior treatment (A) or after incubation with an anti-LFA-1 antibody (B) into irradiated recipient mice (n = 5 recipients per donor genotype). These animals were euthanized 24 h later, and the progenitor content of the BM, PB, and spleen was assessed by CFU-C assays.
As only partial inhibition of BM homing was seen with α4 integrin-deficient cells, the participation of additional molecules in this process was suggested. We previously identified a synergistic contribution by β2 integrins in homing by using blocking antibodies to both α4 and β2 integrin or using CD18-deficient donor BM cells treated with anti-α4 integrin antibody (45). To test the behavior of our α4 integrin-deleted cells, we treated BM cells from Mx.cre+α4+/+ and Mx.cre+α4Δ/Δ animals with anti-leukocyte function-associated antigen 1 (LFA-1) antibody prior to injection. Marrow homing in irradiated recipient mice (n = 5 recipients per donor BM sample) was dramatically impaired in the combined absence of α4 and β2 integrin function, as only 1.1% ± 0.1% of treated α4Δ/Δ CFU-C were recovered from the total BM compared to 9.7% ± 0.8% of the injected α4+/+ CFU-C (P < 0.001) (Fig. 6B). Again, higher CFU-C numbers were observed in the spleens of α4Δ/Δ recipient mice (20.6% ± 0.7% of injected α4Δ/Δ CFU-C compared to 4.3% ± 0.6% of injected α4+/+ CFU-C; P < 0.001), and in the PB (0.214% ± 0.027% and 0.015% ± 0.003%, respectively; P < 0.005).
In addition to homing, we studied the kinetics of early BM repopulation by homed α4 integrin-expressing and α4 integrin-deficient progenitors. Normal α4+/+ recipient animals were irradiated and each infused with 0.5 × 106 cells from Mx.cre+α4+/+or Mx.cre+α4Δ/Δ BM (n = 5 recipient mice per donor BM genotype). All mice were alive 12 days later, at which point they were sacrificed for analysis. The recipients of α4Δ/Δ cells had reduced BM cellularity ([1.9 ± 0.2] × 106 cells per femur compared to [3.7 ± 0.3] × 106 cells per femur in recipients of control cells; P < 0.005). Moreover, a fivefold difference in the BM progenitor content of these animals was observed, with the recipients of α4+/+ cells having 264 ± 99 CFU-C per femur and recipients of α4Δ/Δ cells having only 49 ± 5 CFU-C per femur (P < 0.05) (Fig. 7A). The total CFU-C content of the spleen was unaffected, averaging 12,245 ± 1,629 CFU-C in the five recipients of control BM and 11,856 ± 1,754 CFU-C in the five recipients of α4Δ/Δ BM (P > 0.1). Furthermore, comparable numbers of donor-derived macroscopically visible colonies in the spleen were observed in both groups of recipient mice, demonstrating normal CFU-S12 development within the spleen in the absence of α4 integrin.
FIG. 7.
Short-term engraftment of α4-deficient cells in recipients with and without spleens. A total of 0.5 × 106 BM cells from Mx.cre+α4+/+or from Mx.cre+α4Δ/Δ animals was injected into irradiated spleen-containing recipients (A), or 106 α4+/+ or +α4Δ/Δ cells were injected into irradiated splenectomized recipients (B); progenitor content (as determined by CFU-C assays) in BM, PB, and spleen was analyzed 2 weeks later (n = 5 mice per donor genotype).
To test whether short-term recovery after transplantation is different in the absence of the spleen, splenectomized recipient animals (n = 5 recipient mice per donor BM genotype) were lethally irradiated, infused with donor BM (106 control cells and either 1 × 106 or 5 × 106 α4 integrin-deficient cells), and sacrificed 12 days after transplant for analysis. At that time, recipients of 106 α4+/+ BM cells generated 12,166 ± 6,146 CFU-C per femur, whereas recipients of α4Δ/Δ cells had only 697 ± 431 CFU-C per femur. Even when a dose of 5 × 106 deletion-induced cells was used, progenitor numbers recovered from the BM were lower than those in recipients of 106 non-deletion-induced cells (7,889 ± 2,625 CFU-C per femur in controls compared to 12,166 ± 6,146 CFU-C per femur in recipients of deletion-induced cells). Interestingly, there was a significant reduction of circulating α4-deficient progenitors in splenectomized versus nonsplenectomized recipients, reflecting the drastic decrease in total progenitor content in these mice (Fig. 7B). The data in aggregate confirm results with nonsplenectomized animals and strongly support the argument that α4 integrin-deficient cells have a profound impairment in BM-dependent progenitor cell expansion shortly after transplantation over and above the expected levels from the partial BM homing deficit.
DISCUSSION
Hematopoietic progenitor distribution is altered in the absence of α4 integrins.
Deletion of the α4 integrin gene resulted in an efflux of deletion-induced hematopoietic progenitor cells from the BM into the circulation, which continued for as long as the mice were tested (more than 50 weeks). As the circulating pool of progenitors accounts for only a small proportion of the total BM progenitor population and is likely to have a short turnover time (67), static quantitative measurements of the α4 integrin-deficient progenitor cells in the PB likely underestimate the true number of progenitors leaving the BM compartment as a result of deletion. Although the kinetics of circulating progenitors may also be perturbed in α4 integrin-deleted animals, our analyses suggest that their homing to tissues other than the BM is not expected to be perturbed.
Sustained egress of progenitors into the circulation for over 12 months strongly suggested that α4 integrin was deleted in primitive multipotential progenitor cells responsible for the generation of all those progenitors found in the circulation and that no compensatory mechanism(s) had emerged to correct this phenotype. Nevertheless, a decrease in circulating progenitor numbers was seen over time before a plateau was eventually established. What caused this downward trend? The following possibilities were entertained: (i) that a stem cell escaping α4 integrin deletion slowly expanded to generate descendants that express α4 integrin and consequently remained within the BM or (ii) that the spleen avidly siphoned off many of the circulating progenitor cells. Studies of α4 integrin expression levels in total BM cells, as well as in CFU-C-derived cells, provided no evidence of an increasing contribution by stem or progenitor cells with nondeleted α4 integrin over time. The role of the spleen in the reduction of circulating progenitor cells was studied in parallel in a cohort consisting of splenectomized and nonsplenectomized animals. In nonsplenectomized α4Δ/Δ animals, there was an increase in progenitor numbers within the spleen, suggesting that the spleen increasingly takes up many of these cells, although their subsequent expansion within the spleen remains a possibility. The presence of increased numbers of circulating progenitors in splenectomized α4 integrin-deficient mice compared to matched nonsplenectomized animals is not only consistent with the above interpretation but also suggests that the circulating progenitor cells originate in the marrow. Nevertheless, a gradual decrease in their numbers in the PB was also documented in splenectomized Mx.cre+α4Δ/Δ animals over time, suggesting that factors in these mice in addition to splenic uptake contribute to the observed decline. Studies of the BM from splenectomized and nonsplenectomized animals were informative. The progenitor content 28 weeks after deletion was significantly higher in nonsplenectomized Mx.cre+ α4Δ/Δ mice than in normal controls and splenectomized Mx.cre+α4Δ/Δ animals. Although increased progenitor proliferation in the absence of α4 integrin could be implicated in the increase in BM progenitors on the basis of prior in vitro data (33, 53, 73), we found no statistically significant differences in the in vivo cell cycling in the BM of these mice. Other possibilities considered were a decrease in apoptosis of α4-deleted cells, which was unlikely, and a limited continuous reentry of circulating progenitors into the BM, especially under conditions of enhanced mobilization (according to a recent study [1]). Although the latter hypothesis will be prospectively tested using parabiotic pairs, it should have also occurred in splenectomized mice. Since this was not the case, either a “rehoming” to BM is not occurring or, if it is, it obscures a dysregulated balance between proliferation and apoptosis in these mice, as the BM cellularity was lower in splenectomized animals. Further studies of PB and BM in splenectomized animals may shed light on this issue. Other unknown parameters could also be responsible. Furthermore, data addressing the self-renewal of long-term repopulating cells with competitive repopulation experiments in serial transplantations are also needed to resolve some of these issues.
Progenitor expansion following stress is impaired in α4 integrin-deficient mice.
Certain aspects of stem or progenitor cell deficiencies, such as those described for several knockout mice (i.e., mpl, Tpo, metalloproteinase 9, and placental growth factor), may be concealed during steady-state hematopoiesis (8, 11, 16, 17, 24, 64). Perturbation of the steady state by induction of hematological stress has the potential to uncover defects that are not normally apparent. Despite the total increase in hematopoietic progenitors in our mice, a delayed recovery in regeneration was noted following 5FU treatment. The fact that this delay was not a consequence of a greater sensitivity of deletion-induced cells to the killing effects of 5FU (by killing more cycling cells) was demonstrated when these mice were treated with PHZ, which causes severe anemia by destroying only mature red cells. As α4 integrin-deficient animals have more progenitor cells than control mice, the former would have been expected to recover more rapidly from PHZ-induced anemia. Instead, they exhibited a protracted recovery, as shown by the kinetics of reticulocyte release into the circulation. Further assessment revealed a significant impairment in acute erythroid progenitor cell (BFU-e) expansion in the BM and spleen. This result is reminiscent of the antiproliferative effects on FL erythropoiesis observed following administration of an anti-α4 integrin antibody (15). As erythropoietic recovery from PHZ-induced anemia is critically dependent upon the increase of kit ligand (KL) and is drastically impaired in Sl/Sld mice with decreased levels of KL (7), it can be speculated that, despite the expected elevated levels of KL in both control and α4 integrin-deleted PHZ-treated mice, a pivotal interaction between its receptor, c-kit, and α4 integrin was perturbed in α4 integrin-deleted mice. Our experiments may have therefore uncovered a previously speculated (22), but not directly demonstrated, critical in vivo interaction between α4 integrin and c-kit that is needed for exponential expansion of BFU-e.
Homing and short-term engraftment defects in α4 integrin-deficient cells.
Partial inhibition of homing to the BM was found when α4 integrin-deleted cells were used in homing assays. In parallel, increased numbers of circulating donor cells were detected 24 h after injection along with an increased uptake by the spleen. This inhibition was comparable to those previously described by us and which used BM incubated with an anti-α4 integrin antibody as a source of donor cells or which used recipient mice treated with an anti-VCAM-1 antibody (42). The ineffectiveness of anti-α4 integrin antibody treatment in α4Δ/Δ mice in comparison to control animals (Scott et al., unpublished data) provided corroborative evidence about the specificity of the prior antibody data. The partial nature of the inhibition, however, suggested that other adhesion molecules contribute to the homing process. Indeed, we have shown that treatment of BM cells with anti-β2 integrin or anti-selectin antibodies, which do not inhibit homing on their own, provided synergistic inhibition when used in conjunction with an anti-α4 integrin antibody (45). Synergism was also observed when β2 integrin-deficient cells were treated only with anti-α4 integrin antibody (45), or as in the present study, when α4Δ/Δ BM cells were incubated with an antibody to LFA-1, one of the β2 integrin dimerization partners. LFA-1 is expressed by progenitors and has been implicated in transendothelial migration of hematopoietic progenitors in vitro (74).
In addition to the homing impairment, an even more pronounced effect on the initial expansion of α4 integrin-null progenitor cells was observed in the first 2 weeks after their transplantation. This impairment in expansion was again restricted to the BM compartment and not to the spleen. Although some reduction in progenitor number could be anticipated in the absence of α4 integrin because of their decreased BM homing ability, the impairment observed was out of proportion to the homing deficit. This result is highly consistent with recently published data showing that treatment with anti-α4 and anti-α5 integrin antibodies inhibits engraftment of cells delivered directly into the BM microenvironment to bypass any homing defects (69). We can only speculate about the molecular basis of these effects. Adhesion of hematopoietic cells to the BM stroma is thought to maintain the cells' quiescence rather than stimulate proliferation (19, 39), and this factor may be important for their survival in the immediate posttransplantation period. However, integrins also work in concert with various growth factors to influence hematopoietic cell proliferation (32, 54), which is needed at later stages of recovery following transplantation. This growth factor-integrin cross talk is particularly strong with receptor tyrosine kinases, such as c-kit, and the fibroblast and platelet-derived growth factor receptor family members (22, 37). Although the levels of many cytokines and chemokines increase following transplantation or other conditions of hematopoietic stress (48, 50), their ability to protect cell survival and enhance their subsequent proliferation in these situations was apparently curtailed in our mice following α4 integrin deletion. Further studies with different cell types will be required to fully uncover the networks operating under such conditions and to determine whether the balance between proliferation and survival has been altered in our mice. Nevertheless, it is fair to suggest that both the effects on homing and on short-term engraftment observed in recipient mice with normal hemopoietic microenvironment are consequent to hemopoietic cell-intrinsic defects rather than to alterations in microenvironment cells.
Comparison of the α4 integrin-deleted phenotype with that of α4 integrin-null chimeras and conditional β1 integrin or VCAM-1 knockouts.
The data summarized herein revealed several differences from previously described studies, although there were some similarities. Arroyo et al. have emphasized a critical role for α4 integrin in normal fetal erythropoiesis, with knockout animals at the time of death having smaller, paler livers than those of age-matched controls (3). Similarly, using chimeras derived from α4 integrin-null ES cells, these authors noted impaired terminal erythroid differentiation in the fetus and a demise in postnatal hematopoiesis contributed by the α4 integrin-null fetal progenitors (2-4). As in vitro erythroid differentiation was not affected, these workers concluded that defective development of α4 integrin-deficient cells in the FL and postnatal BM resulted from impaired interaction of α4 integrin-deficient (fetal) progenitors with their respective environments. In agreement with the data of Arroyo et al., we did not detect any impairment in in vitro erythroid differentiation in the absence of α4 integrin. However, in contrast to their data with chimeric mice, our adult α4 integrin-deleted mice did not display perturbations in steady-state erythropoiesis. As our data concerned adult cells interacting with an adult BM environment, it is likely that erythroid progenitor requirements within the adult BM and/or spleen are distinct from those observed with fetal cells. Such an explanation was recently suggested to interpret the different outcomes of stem cell leukemia gene deletion in fetal versus adult hematopoiesis (14, 35). Despite the presence of normal steady-state hematopoiesis, we found that the expansion of early erythroid progenitors was impaired in the absence of α4 integrin after induced hemolytic anemia. If one interprets the dramatic expansion of progenitors occurring during fetal development as being physiologically equivalent to stress, then some similarities with the previous data can be envisioned. Nevertheless, additional significant differences between our data and those previously published were present. Homing and engraftment defects were present in the α4 integrin-deleted mice, although similar defects were not described in α4 integrin-null chimeras. This may be because no formal homing or transplantation experiments were performed using cells of these animals. More importantly, the differential requirements for α4 integrin in the adult BM versus the spleen contrast with the profound hematopoietic impairments observed in both the neonatal BM and the spleen of the α4 integrin-null chimeric mice (3, 4). It should be kept in mind, however, that we induced deletion in adult animals once hematopoiesis had been well established in these tissues, whereas in α4 integrin-null chimeric mice, integrin-deficient cells failed to compete with α4+/+ cells in establishing hematopoiesis.
Drastic homing and migration deficits have been noted when BM cells from β1 integrin-conditional knockouts were deleted in vitro and used for transplantation (49). In this study, homing to all hematopoietic tissues (BM, thymus, and spleen) was abolished. Concurrent absence of several α integrins that partner with β1 integrin may have been responsible for the extreme inhibition of homing to the BM and the spleen. Inhibition of homing to the spleen in particular, although consistent with decade-old data showing inhibition of short-term (12-day) engraftment in the BM and spleen following treatment with polyclonal anti-β1 integrin antibodies (66), presents a significant departure from the results we obtained with α4 integrin-deleted BM cells. A disparity in homing to the BM versus to the spleen has been the rule in several other studies (29, 42, 59, 65), and the splenic architecture and its microenvironment, represented by stromal and endothelial cells, have been suggested as underlying factors in this difference. However, the extent to which defects are cell intrinsic rather than dictated by the microenvironment and the nature of molecular interactions remain to be determined.
Despite the devastating homing defect in β1 integrin-deleted cells, deletion of the β1 integrin gene in adult animals allowed for normal hematopoiesis (6). Brakebusch and coworkers transplanted non-deletion-induced cells into wild-type recipients and then induced deletion of the floxed β1 integrin alleles after engraftment was complete in order to overcome both homing defects and putative defects in nonhematopoietic microenvironmental recipient cells. It is difficult to compare these experiments with our transplantation experiments, in which cells were deprived of α4 before their transplantation in normal irradiated recipients. Further transplantation experiments using either normal or α4-deleted animals as recipients may provide additional insight. However, we believe that the homing and short-term engraftment defects we observed in normal recipients are likely attributable to cell-intrinsic defects. Similar experiments have not been carried out with conditionally deletion-induced β1 cells before transplantation. Finally, studies of conditionally deletion-induced VCAM-1 adult mice uncovered abnormalities in lymphocyte trafficking, but myeloid cell migration and homing were not explored (25, 31) for comparison.
We believe that our α4 integrin-conditional knockout model will be useful for elucidating molecular interactions between several classes of hematopoietic cells and their microenvironment, which are necessary for maintaining hematopoietic homeostasis and for responding to acute demands as well as for exploring combinatorial integrin deficiencies or synergistic interactions with other molecules.
Acknowledgments
We acknowledge the technical assistance of Denise Farrar, Betty Nakamoto, Alex Rohde, and Vivian Zafiropoulos. We are grateful to Ken Peterson for his contribution in the early stages of this work. James Downing (St. Jude's Children's Research Hospital, Memphis, Tenn.) kindly provided the MSCV-cre-iresGFP plasmid.
This work was supported by National Institutes of Health grant numbers HL46557 and HL58734, which were awarded to T.P.
REFERENCES
- 1.Abkowitz, J. L., A. E. Robinson, S. Kale, M. W. Long, and J. Chen. 2003. The mobilization of hematopoietic stem cells during homeostasis and after cytokine exposure. Blood 102:1249-1253. [DOI] [PubMed] [Google Scholar]
- 2.Arroyo, A., J. T. Yang, H. Rayburn, and R. O. Hynes. 1996. Differential requirements for α4 integrins during fetal and adult hematopoiesis. Cell 85:997-1008. [DOI] [PubMed] [Google Scholar]
- 3.Arroyo, A. G., J. T. Yang, H. Rayburn, and R. O. Hynes. 1999. α4 integrins regulate the proliferation/differentiation balance of multilineage hematopoietic progenitors in vivo. Immunity 11:555-566. [DOI] [PubMed] [Google Scholar]
- 4.Arroyo, A. G., D. Taverna, C. A. Whittaker, U. G. Strauch, B. L. Bader, H. Rayburn, D. Crowley, C. M. Parker, and R. O. Hynes. 2000. In vivo roles of integrins during leukocyte development and traffic: insights from the analysis of mice chimeric for α5, αv, and α4 integrins. J. Immunol. 165:4667-4675. [DOI] [PubMed] [Google Scholar]
- 5.Boggs, D. R. 1984. The total marrow mass of the mouse: a simplified method of measurement. Am. J. Hematol. 16:277-286. [DOI] [PubMed] [Google Scholar]
- 6.Brakebusch, C., S. Fillatreau, A. J. Potocnik, G. Bungartz, P. Wilhelm, M. Svensson, P. Kearney, H. Korner, D. Gray, and R. Fassler. 2002. β1 integrin is not essential for hematopoiesis but is necessary for the T cell-dependent IgM antibody response. Immunity 16:465-477. [DOI] [PubMed] [Google Scholar]
- 7.Broudy, V. C., N. L. Lin, G. V. Priestley, K. Nocka, and N. S. Wolf. 1996. Interaction of stem cell factor and its receptor c-kit mediates lodgment and acute expansion of hematopoietic cells in the murine spleen. Blood 88:75-81. [PubMed] [Google Scholar]
- 8.Carmeliet, P., L. Moons, A. Luttun, V. Vincenti, V. Compernolle, M. De Mol, Y. Wu, F. Bono, L. Devy, H. Beck, D. Scholz, T. Acker, T. DiPalma, M. Dewerchin, A. Noel, I. Stalmans, A. Barra, S. Blacher, T. Vandendriessche, A. Ponten, U. Eriksson, K. H. Plate, J. M. Foidart, W. Schaper, D. S. Charnock-Jones, D. J. Hicklin, J. M. Herbert, D. Collen, and M. G. Persico. 2001. Synergism between vascular endothelial growth factor and placental growth factor contributes to angio-genesis and plasma extravasation in pathological conditions. Nat. Med. 7:575-583. [DOI] [PubMed] [Google Scholar]
- 9.Craddock, C. F., B. Nakamoto, R. Andrews, G. V. Priestley, and T. Papayannopoulou. 1997. Antibodies to VLA-4 integrin mobilize long-term repopulating cells and augment cytokine-induced mobilization in primates and mice. Blood 90:4779-4788. [PubMed] [Google Scholar]
- 10.Fassler, R., and M. Meyer. 1995. Consequences of lack of β1 integrin gene expression in mice. Genes Dev. 9:1896-1908. [DOI] [PubMed] [Google Scholar]
- 11.Fox, N., G. Priestley, T. Papayannopoulou, and K. Kaushansky. 2002. Thrombopoietin expands hematopoietic stem cells after transplantation. J. Clin. Investig. 110:389-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freedman, M. L., and S. Karpatkin. 1975. Heterogeneity of rabbit platelets. IV. Thrombocytosis with absolute megathrombocytosis in phenylhydrazine-induced hemolytic anemia in rabbits. Thromb. Diath. Haemorrh. 33:335-340. [PubMed] [Google Scholar]
- 13.Gurtner, G. C., V. Davis, H. Li, M. J. McCoy, A. Sharpe, and M. I. Cybulsky. 1995. Targeted disruption of the murine VCAM1 gene: essential role of VCAM-1 in chorioallantoic fusion and placentation. Genes Dev. 9:1-14. [DOI] [PubMed] [Google Scholar]
- 14.Hall, M. A., D. J. Curtis, D. Metcalf, A. G. Elefanty, K. Sourris, L. Robb, J. R. Gothert, S. M. Jane, and C. G. Begley. 2003. The critical regulator of embryonic hematopoiesis, SCL, is vital in the adult for megakaryopoiesis, erythropoiesis, and lineage choice in CFU-S12. Proc. Natl. Acad. Sci. USA 100:992-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamamura, K., H. Matsuda, Y. Takeuchi, S. Habu, H. Yagita, and K. Okumura. 1996. A critical role of VLA-4 in erythropoiesis in vivo. Blood 87:2513-2517. [PubMed] [Google Scholar]
- 16.Hattori, K., B. Heissig, Y. Wu, S. Dias, R. Tejada, B. Ferris, D. J. Hicklin, Z. Zhu, P. Bohlen, L. Witte, J. Hendrikx, N. Hackett, R. G. Crystal, M. A. Moore, Z. Werb, D. Lyden, and S. Rafifi. 2002. Placental growth factor reconstitutes hematopoiesis by recruiting VEGFR1+ stem cells from bone-marrow microenvironment. Nat. Med. 8:841-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heissig, B., K. Hattori, S. Dias, M. Freidrich, B. Ferris, N. R. Hackett, R. G. Crystal, P. Besmer, D. Lyden, M. A. Moore, Z. Werb, and S. Rafii. 2002. Recruitment of stem and progenitor cells from the BM niche requires MMP-9 mediated release of kit-ligand. Cell 109:625-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirsch, E., A. Iglesias, A. J. Potocnik, U. Hartmann, and R. Fassler. 1996. Impaired migration but not differentiation of haematopoietic stem cells in the absence of β1 integrins. Nature 380:171-175. [DOI] [PubMed] [Google Scholar]
- 19.Hurley, R. W., J. B. McCarthy, and C. M. Verfaillie. 1995. Direct adhesion to bone marrow stroma via fibronectin receptors inhibits hematopoietic progenitor proliferation. J. Clin. Investig. 96:511-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hynes, R. O. 2002. Integrins: bidirectional, allosteric signaling machines. Cell 110:673-687. [DOI] [PubMed] [Google Scholar]
- 21.Jakubowski, A., M. D. Rosa, S. Bixler, R. Lobb, and L. C. Burkly. 1995. Vascular cell adhesion molecule (VCAM)-Ig fusion protein defines distinct affinity states of the very late antigen-4 (VLA-4) receptor. Cell Adhes. Commun. 3:131-142. [DOI] [PubMed] [Google Scholar]
- 22.Kapur, R., R. Cooper, L. Zhang, and D. A. Williams. 2001. Cross-talk between α4β1/α5β1 and c-kit results in opposing effects on growth and survival of hematopoietic cells via the activation of focal adhesion kinase, mitogen-activated protein kinase, and Akt signaling pathways. Blood 97:1975-1981. [DOI] [PubMed] [Google Scholar]
- 23.Kikuta, T., C. Shimazaki, E. Ashihara, Y. Sudo, H. Hirai, T. Sumikuma, N. Yamagata, T. Inaba, N. Fujita, T. Kina, and M. Nakagawa. 2000. Mobilization of hematopoietic primitive and committed progenitor cells into blood in mice by anti-vascular adhesion molecule-1 antibody alone or in combination with granulocyte colony-stimulating factor. Exp. Hematol. 28:311-317. [DOI] [PubMed] [Google Scholar]
- 24.Kimura, S., A. W. Roberts, D. Metcalf, and W. S. Alexander. 1998. Hematopoietic stem cell deficiencies in mice lacking c-mpl, the receptor for thrombopoietin. Proc. Natl. Acad. Sci. USA 95:1195-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koni, P. A., S. K. Joshi, U. A. Temann, D. Olson, L. Burkly, and R. A. Flavell. 2001. Conditional vascular cell adhesion molecule 1 deletion in mice: impaired lymphocyte migration to bone marrow. J. Exp. Med. 193:741-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kovach, N. L., N. Lin, T. Yednock, J. M. Harlan, and V. C. Broudy. 1995. Stem cell factor modulates avidity of α4β1 and α5β1 integrins expressed on hematopoietic cell lines. Blood 85:159-167. [PubMed] [Google Scholar]
- 27.Kuhn, R., F. Schwenk, M. Aguet, and K. Rajewsky. 1995. Inducible gene targeting in mice. Science 269:1427-1429. [DOI] [PubMed] [Google Scholar]
- 28.Kwee, L., H. S. Baldwin, H. M. Shen, C. Stewart, C. Buck, C. A. Buck, and M. A. Labow. 1995. Defective development of the embryonic and extraembryonic circulatory systems in vascular cell adhesion molecule (VCAM-1) deficient mice. Development 121:489-503. [DOI] [PubMed] [Google Scholar]
- 29.Lanzkron, S. M., M. I. Collector, and S. J. Sharkis. 1999. Hematopoietic stem cell trafficking in vivo: a comparison of short-term and long-term repopulating cells. Blood 93:1916-1921. [PubMed] [Google Scholar]
- 30.Lerner, C., and D. E. Harrison. 1990. 5-Fluorouracil spares hematopoietic stem cells responsible for long-term repopulation. Exp. Hematol. 18:114-118. [PubMed] [Google Scholar]
- 31.Leuker, C. E., M. Labow, W. Muller, and N. Wagner. 2001. Neonatally induced inactivation of the vascular cell adhesion molecule 1 gene impairs B cell localization and T cell-dependent humoral immune response. J. Exp. Med. 193:755-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levesque, J. P., D. I. Leavesley, S. Niutta, M. Vadas, and P. J. Simmons. 1995. Cytokines increase human hematopoietic cell adhesiveness by activation of very late antigen (VLA)-4 and VLA-5 integrins. J. Exp. Med. 181:1805-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levesque, J. P., D. N. Haylock, and P. J. Simmons. 1996. Cytokine regulation of proliferation and cell adhesion are correlated events in human CD34+ hematopoietic progenitors. Blood 88:1168-1176. [PubMed] [Google Scholar]
- 34.Markowitz, D., S. Goff, and A. Bank. 1988. Construction and use of a safe and efficient amphotropic packaging cell line. Virology 167:400-406. [PubMed] [Google Scholar]
- 35.Mikkola, H. K., J. Klintman, H. Yang, H. Hock, T. M. Schlaeger, Y. Fujiwara, and S. H. Orkin. 2003. Haematopoietic stem cells retain long-term repopulating activity and multipotency in the absence of stem-cell leukemia SCL/tal-1 gene. Nature 421:547-551. [DOI] [PubMed] [Google Scholar]
- 36.Miyake, K., I. L. Weissman, J. S. Greenberger, and P. W. Kincade. 1991. Evidence for a role of the integrin VLA-4 in lymphohemopoiesis. J. Exp. Med. 173:599-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyamoto, S., H. Teramoto, J. S. Gutkind, and K. M. Yamada. 1996. Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J. Cell Biol. 135:1633-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oostendorp, R. A., and P. Dormer. 1997. VLA-4-mediated interactions between normal human hematopoietic progenitors and stromal cells. Leuk. Lymphoma 24:423-435. [DOI] [PubMed] [Google Scholar]
- 39.Oostendorp, R. A., E. Spitzer, G. Reisbach, and P. Dormer. 1997. Antibodies to the β1-integrin chain, CD44, or ICAM-3 stimulate adhesion of blast colony-forming cells and may inhibit their growth. Exp. Hematol. 25:345-349. [PubMed] [Google Scholar]
- 40.Papayannopoulou, T. 2001. Very late activation/β1 integrins in hematopoiesis, p. 337-342. In L. I. Zon (ed.), Hematopoiesis. Oxford University Press, New York, N.Y.
- 41.Papayannopoulou, T., and M. Brice. 1992. Integrin expression profiles during erythroid differentiation. Blood 79:1686-1694. [PubMed] [Google Scholar]
- 42.Papayannopoulou, T., C. Craddock, B. Nakamoto, G. V. Priestley, and N. S. Wolf. 1995. The VLA4/VCAM-1 adhesion pathway defines contrasting mechanisms of lodgment of transplanted murine hemopoietic progenitors between bone marrow and spleen. Proc. Natl. Acad. Sci. USA 92:9647-9651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Papayannopoulou, T., and B. Nakamoto. 1993. Peripheralization of hemopoietic progenitors in primates treated with anti-VLA4 integrin. Proc. Natl. Acad. Sci. USA 90:9374-9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Papayannopoulou, T., P. Nute, S. Kurachi, and G. Stamatoyannopoulos. 1978. Consistent activation of fetal hemoglobin synthesis in cultured adult bone marrow cells. Blood 51:671-679. [PubMed] [Google Scholar]
- 45.Papayannopoulou, T., G. V. Priestley, B. Nakamoto, V. Zafiropoulos, and L. M. Scott. 2001. Molecular pathways in bone marrow homing: dominant role of α4β1 over β2-integrins and selectins. Blood 98:2403-2411. [DOI] [PubMed] [Google Scholar]
- 46.Pear, W. S., G. P. Nolan, M. L. Scott, and D. Baltimore. 1993. Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. USA 90:8392-8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peled, A., O. Kollet, T. Ponomaryov, I. Petit, S. Franitza, V. Grabovsky, M. M. Slav, A. Nagler, O. Lider, R. Alon, D. Zipori, and T. Lapidot. 2000. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34+ cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood 95:3289-3296. [PubMed] [Google Scholar]
- 48.Ponomaryov, T., A. Peled, I. Petit, R. S. Taichman, L. Habler, J. Sandbank, F. Arenzana-Seisdedos, A. Magerus, A. Caruz, N. Fujii, A. Nagler, M. Lahaz, M. Szyper-Kravitz, D. Zipori, and T. Lapidot. 2000. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J. Clin. Investig. 106:1331-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Potocnik, A. J., C. Brakebusch, and R. Fassler. 2000. Fetal and adult hematopoietic stem cells require β1 integrin function for colonizing fetal, spleen, and bone marrow. Immunity 12:653-663. [DOI] [PubMed] [Google Scholar]
- 50.Psenak, O., L. Sefc, V. Sykora, K. T. Chang, and E. Necas. 2003. Cytokine gene expression in regenerating hematopoietic tissues of mice after cyclophosphamide treatment. Acta Hematol. 109:68-75. [DOI] [PubMed] [Google Scholar]
- 51.Randall, T. D., and I. L. Weissman. 1997. Phenotypic and functional changes induced at the clonal level in hematopoietic stem cells after 5-fluorouracil treatment. Blood 89:3596-3606. [PubMed] [Google Scholar]
- 52.Roy, V., and C. M. Verfaillie. 1999. Expression and function of cell adhesion molecules on fetal liver, cord blood and bone marrow hematopoietic progenitors: implications for anatomical localization and developmental stage specific regulation of hematopoiesis. Exp. Hematol. 27:302-312. [DOI] [PubMed] [Google Scholar]
- 53.Schofield, K. P., M. J. Humphries, E. de Wynter, N. Testa, and J. T. Gallagher. 1998. The effect of α4β1-integrin binding sequences of fibronectin on growth of cells from human hematopoietic progenitors. Blood 91:3230-3238. [PubMed] [Google Scholar]
- 54.Schofield, K. P., G. Rushton, M. J. Humphries, T. M. Dexter, and J. T. Gallagher. 1997. Influence of interleukin-3 and other growth factors on α4β1 integrin-mediated adhesion and migration of human hematopoietic progenitor cells. Blood 90:1858-1866. [PubMed] [Google Scholar]
- 55.Schwartz, M. A., and M. H. Ginsberg. 2002. Networks and crosstalk: integrin signalling spreads. Nat. Cell Biol. 4:E65-E68. [DOI] [PubMed] [Google Scholar]
- 56.Soligo, D., R. Schiro, R. Luksch, G. Manara, N. Quirici, C. Parravicini, and G. L. Deliliers. 1990. Expression of integrins in human bone marrow. Br. J. Haematol. 76:323-332. [DOI] [PubMed] [Google Scholar]
- 57.Srour, E. F., A. Jetmore, F. M. Wolber, P. A. Platt, R. Abonour, M. C. Yoder, and C. M. Orschell-Traycoff. 2001. Homing, cell cycle kinetics and fate of transplanted hematopoietic stem cells. Leukemia 15:1681-1684. [DOI] [PubMed] [Google Scholar]
- 58.Stephens, L. E., A. E. Sutherland, I. V. Klimanskaya, A. Andrieux, J. Meneses, R. A. Pedersen, and C. H. Damsky. 1995. Deletion of β1 integrins in mice results in inner cell mass failure and peri-implantation lethality. Genes Dev. 9:1883-1895. [DOI] [PubMed] [Google Scholar]
- 59.Szilvassy, S. J., M. J. Bass, G. Van Zant, and B. Grimes. 1999. Organ-selective homing defines engraftment kinetics of murine hematopoietic stem cells and is compromised by ex vivo expansion. Blood 93:1557-1566. [PubMed] [Google Scholar]
- 60.Van Zant, G. 1984. Studies of hematopoietic stem cells spared by 5-fluorouracil. J. Exp. Med. 159:679-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Verfaillie, C. M. 2000. Anatomy and physiology of hematopoiesis, p. 139-154. In R. Hoffman, E. J. Benz, S. J. Shattil, B. Furie, H. J. Cohen, L. E. Silberstein, and P. McGlave (ed.), Hematology: basic principles and practice. Churchill Livingstone, Ltd., Philadelphia, Pa.
- 62.Vermeulen, M., F. Le Pesteur, M. C. Gagnerault, J. Y. Mary, F. Sainteny, and F. Lepault. 1998. Role of adhesion molecules in the homing and mobilization of murine hematopoietic stem and progenitor cells. Blood 92:894-900. [PubMed] [Google Scholar]
- 63.Voura, E. B., F. Billia, N. N. Iscove, and R. G. Hawley. 1997. Expression mapping of adhesion receptor genes during differentiation of individual hematopoietic precursors. Exp. Hematol. 25:1172-1179. [PubMed] [Google Scholar]
- 64.Vu, T. H., J. M. Shipley, G. Bergers, J. E. Berger, J. A. Helms, D. Hanahan, S. D. Shapiro, R. M. Senior, and Z. Werb. 1998. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell 93:411-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wiesmann, A., and G. J. Spangrude. 1999. Marrow engraftment of hematopoietic stem and progenitor cells is independent of Gαi-coupled chemokine receptors. Exp. Hematol. 27:946-955. [DOI] [PubMed] [Google Scholar]
- 66.Williams, D. A., M. Rios, C. Stephens, and V. P. Patel. 1991. Fibronectin and VLA-4 in haematopoietic stem cell-microenvironment interactions. Nature 352:438-441. [DOI] [PubMed] [Google Scholar]
- 67.Wright, D. E., A. J. Wagers, A. P. Gulati, F. L. Johnson, and I. L. Weissman. 2001. Physiological migration of hematopoietic stem and progenitor cells. Science 294:1933-1936. [DOI] [PubMed] [Google Scholar]
- 68.Yagi, T., S. Nada, N. Watanabe, H. Tamemoto, N. Kohmura, Y. Ikawa, and S. Aizawa. 1993. A novel negative selection for homologous recombinants using diphtheria toxin A fragment gene. Anal. Biochem. 214:77-86. [DOI] [PubMed] [Google Scholar]
- 69.Yahata, T., K. Ando, T. Sato, H. Miyatake, Y. Nakamura, Y. Mugurama, S. Kato, and T. Hotta. 2003. A highly sensitive strategy for SCID-repopulating cell assay by direct injection of primitive human hematopoietic cells into NOD/SCID mice bone marrow. Blood 101:2905-2913. [DOI] [PubMed] [Google Scholar]
- 70.Yanai, N., C. Sekine, H. Yagita, and M. Obinata. 1994. Roles for integrin very late activation antigen-4 in stroma-dependent erythropoiesis. Blood 83:2844-2850. [PubMed] [Google Scholar]
- 71.Yang, F. C., S. J. Atkinson, Y. Gu, J. B. Borneo, A. W. Roberts, Y. Zheng, J. Pennington, and D. A. Williams. 2001. Rac and cdc42 GTPases control hematopoietic stem cell shape, adhesion, migration, and mobilization. Proc. Natl. Acad. Sci. USA 98:5614-5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang, J. T., H. Rayburn, and R. O. Hynes. 1995. Cell adhesion events mediated by α4 integrins are essential in placental and cardiac development. Development 121:549-560. [DOI] [PubMed] [Google Scholar]
- 73.Yokota, T., K. Oritani, H. Mitsui, K. Aoyama, J. Ishikawa, H. Sugahara, I. Matsumura, S. Tsai, Y. Tomiyama, Y. Kanakura, and Y. Matsuzawa. 1998. Growth-supporting activities of fibronectin on hematopoietic stem/progenitor cells in vitro and in vivo: structural requirement for fibronectin activities of CS1 and cell-binding domains. Blood 91:3263-3272. [PubMed] [Google Scholar]
- 74.Yong, K. L., M. Watts, T. N. Shaun, A. Sullivan, S. Ings, and D. C. Linch. 1998. Transmigration of CD34+ cells across specialized and nonspecialized endothelium requires prior activation by growth factors and is mediated by PECAM-1 (CD31). Blood 91:1196-1205. [PubMed] [Google Scholar]
- 75.Zanjani, E. D., A. Flake, G. Almeida-Porada, N. Tran, and T. Papayannopoulou. 1999. Homing of human cells in the fetal sheep model: modulation by antibodies activating or inhibiting VLA-4 dependent function. Blood 94:2515-2522. [PubMed] [Google Scholar]