Abstract
Background
Hereditary amyloidosis due to mutations of apolipoprotein A-I (apoA-I) is a rare disease characterized by the deposition of amyloid fibrils constituted by the N-terminal fragment of apoA-I in several organs. L75P is a variant of apoA-I associated with systemic amyloidosis predominantly involving the liver, kidneys, and testis, identified in a large number of unrelated subjects. Objective of the present paper was to evaluate the impact of the L75P apoA-I variant on HDL subpopulations and cholesterol esterification in carriers.
Methods and results
Plasma samples were collected from 30 carriers of the amyloidogenic L75P apoA-I (Carriers) and from 15 non affected relatives (Controls). Carriers displayed significantly reduced plasma levels of HDL-cholesterol, apoA-I, and apoA-II compared to Controls. Plasma levels of LpA-I, but not LpA-I:A-II, were significantly reduced in Carriers. HDL subclass distribution was not affected by the presence of the variant. The unesterified to total cholesterol ratio was higher, and cholesterol esterification rate and LCAT activity were lower in Carriers than in Controls.
Conclusions
The L75P apoA-I variant is associated with hypoalphalipoproteinemia, a selective reduction of LpA-I particles, and a partial defect in cholesterol esterification.
Keywords: Apolipoprotein A-I, High density lipoproteins, HDL subpopulations, LCAT
1. Introduction
Hereditary systemic amyloidoses are autosomal dominant, late-onset disorders caused by mutations in genes coding for a series of plasma proteins, including transthyretin, lysozyme, fibrinogen Aα chain, gelsolin, apolipoprotein A-I (apoA-I) and apoA-II [1]. ApoA-I hereditary amyloidosis (OMIM #107680) is a rare disease characterized by progressive deposition of amyloid fibrils mainly constituted by N-terminal polypeptide fragments of this protein [2]. The clinical manifestations of apoA-I amyloidosis frequently involve liver, kidney, heart, larynx, skin, testis and adrenal glands [2]. The molecular mechanisms underlying the amyloidogenic properties of apoA-I mutants have not been widely investigated. However, it is likely that well-recognized, general mechanisms of in vivo amyloidogenesis, like misfolding of the native protein structure and generation of a critical concentration of the amyloidogenic precursor, may apply also to apoA-I amyloidosis. Indeed, amyloid deposits consisting mainly of N-terminal fragments of the precursor protein are also common in other types of amyloidosis [3].
Fourteen amyloidogenic missense/nonsense apoA-I mutations have been identified thus far, and most of these are private [2,4]. Few years ago we described a new apoA-I variant, L75P, associated with systemic amyloidosis predominantly involving the liver, kidneys, and testis [5]. The variant has been identified in a large number of unrelated subjects and is associated with a milder disease compared with all other known amyloidogenic apoA-I mutations [5]. In the present paper, the impact of the L75P apoA-I variant on high density lipoprotein (HDL) subpopulations and cholesterol esterification was evaluated in carriers in comparison with non carrier relatives.
2. Methods
2.1. Subjects
Thirty heterozygous carriers of the L75P apoA-I variant belonging to 18 Italian families volunteered for this study (Carriers). Fifteen control subjects were selected among non affected relatives (Controls). Carriers were divided into symptomatic and asymptomatic subjects according to the presence or absence of clinical signs of organ damage due to amyloid deposits, evaluated as increased plasma levels of alkaline phosphatase (ALP, liver), of follicle-stimulating hormone and luteinizing hormone (FSH and LH, testis) and as values of glomerular filtration rates (GFR) lower than 60 ml/min/1.73 m2 (kidney). The study was approved by the local institutional Ethic Committee, and all enrolled subjects gave written informed consent for participation in the study.
2.2. Lipoprotein profile
Fasting blood was collected into tubes containing Na2-EDTA (final concentration 1 mg/ml) and plasma prepared by low speed centrifugation at 4 °C. Aliquots were immediately frozen and stored at − 80 °C until assayed. Plasma levels of total and unesterified cholesterol, HDL-cholesterol (HDL-C), and triglycerides were determined by certified standard enzymatic techniques. LDL-cholesterol (LDL-C) was calculated using the Friedewald's formula. ApoA-I, apoA-II, and apoB levels were determined by immunoturbidimetry, using commercially available polyclonal antibodies.
Plasma levels of lecithin:cholesterol acyltransferase (LCAT) were measured by a specific competitive ELISA [6]. LCAT ability to esterify cholesterol within endogenous lipoproteins (cholesterol esterification rate, CER) or within a standardized substrate (LCAT activity) was determined as previously described [7].
2.3. HDL subpopulations
Plasma levels of HDL particles containing only apoA-I (LpA-I) and of particles containing both apoA-I and apoA-II (LpA-I:A-II) were determined by electroimmunodiffusion in agarose gel [8]. HDL subclass distribution according to particle size was determined by non-denaturing polyacrylamide gradient gel electrophoresis (GGE, 4–30%) of the d < 1.21 g/ml plasma total lipoprotein fraction; the protein-stained gels were scanned with an imaging densitometer to determine particle size and HDL were divided into small (Ø < 8.2 nm), medium (8.2 < Ø<8.8 nm) and large (Ø > 8.8 nm) particles [8]. Plasma content of preβ-HDL was assessed by non-denaturing two-dimensional (2-D) electrophoresis, in which agarose gel electrophoresis was followed by non-denaturing GGE and immunoblotting against apoA-I [9]. Plasma content of preβ-HDL was expressed as percentage of total apoA-I.
2.4. Statistical analyses
Results are reported as mean ± SD. Group differences in continuous variables were evaluated by one-way ANOVA, with post hoc analysis by the Neuman–Keuls test. For categorical variables, group differences were examined with the use of 2×2 contingency tables and a χ2 test of significance. Group differences with a p value < 0.05 were considered statistically significant.
3. Results
3.1. Clinical features
Carriers of the L75P apoA-I variant were comparable to Controls for gender distribution and average age (Table 1). Fifteen Carriers were asymptomatic and 15 showed clinical signs of amyloidosis (symptomatic). Asymptomatic Carriers (11 females and 4 males) were prevalently healthy subjects; only one had a previous acute myocardial infarction. Symptomatic Carriers were mainly males (11 out of 15) and had ALP elevation (n = 9), GFR reduction (n = 9) and FSH/LH elevation (n = 9); ten had hypertension, one was obese, one diabetic and one had a previous acute myocardial infarction. On average, symptomatic subjects were older than asymptomatic ones (54.7 ± 11.0 vs 45.4 ± 10.6, respectively, p = 0.026).
Table 1.
Lipid and apolipoprotein levels in Carriers and Controls.
| Carriers | Controls | P | |
|---|---|---|---|
| Gender (f/m) | 15/15 | 7/8 | 0.899 |
| Age (y) | 50.1 ± 11.6 | 51.1 ± 11.2 | 0.784 |
| Total cholesterol (mg/dl) | 187.9 ± 42.5 | 231.7 ± 34.5 | 0.001 |
| Unesterified cholesterol (mg/dl) | 57.4 ± 11.7 | 63.8 ± 10.2 | 0.079 |
| Unesterified/total cholesterol | 0.31 ± 0.06 | 0.28 ± 0.04 | 0.029 |
| LDL-cholesterol (mg/dl) | 132.5 ± 39.1 | 158.6 ± 34.3 | 0.034 |
| HDL-cholesterol (mg/dl) | 33.0 ± 11.1 | 47.0 ± 9.3 | < 0.001 |
| Triglycerides (mg/dl) | 109.6 ± 49.6 | 130.6 ± 44.5 | 0.174 |
| Apolipoprotein B (mg/dl) | 101.9 ± 26.1 | 110.6 ± 21.4 | 0.271 |
| Apolipoprotein A-I (mg/dl) | 93.7 ± 19.3 | 117.7 ± 15.1 | < 0.001 |
| Apolipoprotein A-II (mg/dl) | 29.3 ± 5.1 | 33.9 ± 5.5 | 0.008 |
| CER (nmol/ml/h) | 27.8 ± 8.0 | 38.4 ± 13.2 | 0.002 |
| LCAT activity (nmol/ml/h) | 23.8 ± 7.9 | 41.7 ± 12.1 | < 0.001 |
| LCAT (μg/ml) | 3.69 ± 1.40 | 4.24 ± 0.78 | 0.308 |
Data are expressed as mean ± SD, except for gender distribution; n = 30 for Carriers and n = 15 for Controls.
According to their clinical features, Carriers were taking hypertensive drugs (n = 9), testosterone replacement therapy (n = 3), aspirin and clopidogrel (n = 2), atorvastatin (n = 2) and insulin (n = 1).
3.2. Lipid profile
Carriers displayed significantly lower plasma levels of total and LDL-cholesterol than Controls (Table 1). The L75P apoA-I variant was associated with a significant reduction (− 30%) in HDL-C; apoA-I and apoA-II levels were also significantly reduced in Carriers compared to Controls (Table 1). Interestingly, the plasma unesterified to total cholesterol ratio was higher in Carriers than in Controls (Table 1). The evaluation of the cholesterol esterification process in Carriers showed a significant reduction in cholesterol esterification rate and LCAT activity in the presence of normal plasma LCAT levels (Table 1).
3.3. HDL subpopulations
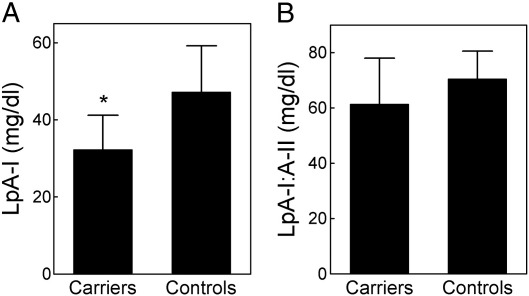
The hypoalphalipoproteinemia observed in Carriers of the L75P apoA-I variant was associated with a marked and significant reduction in plasma LpA-I levels (− 31.5% vs Controls, p < 0.001, Fig. 1), while plasma LpA-I:A-II levels were similar to those observed in Controls. HDL subclass distribution was not affected by the presence of the L75P apoA-I variant; indeed, the percentage of small, medium, and large HDL, and the mean particle diameter of HDL2 and HDL3 were comparable between Carriers and Controls (Table 2). Plasma content of discoidal preβ-HDL tended to be higher in Carriers than in Controls, although the difference was not statistically significant (Table 2). This was associated with an increased unesterified to total cholesterol ratio in the HDL fraction in Carriers compared to Controls (0.27 ± 0.04 vs. 0.20 ± 0.05, P = 0.014).
Fig. 1.
Plasma levels of LpA-I (panel A) and LpA-I:A-II (panel B) in Carriers of the L75P apoA-I variant (n = 30) and in Controls (n = 15). Data are expressed as mean ± SD. *p < 0.05 vs. Controls.
Table 2.
HDL subpopulations in Carriers and Controls.
| Carriers | Controls | P | |
|---|---|---|---|
| Small HDL (%) | 14.0 ± 5.5 | 14.8 ± 5.2 | 0.642 |
| Medium HDL (%) | 17.8 ± 5.0 | 17.2 ± 4.7 | 0.701 |
| Large HDL (%) | 68.2 ± 9.5 | 68.0 ± 9.2 | 0.947 |
| HDL2 (nm) | 11.3 ± 0.3 | 11.4 ± 0.6 | 0.458 |
| HDL3 (nm) | 8.9 ± 0.4 | 8.9 ± 0.2 | 1.000 |
| Preβ HDL (%) | 19.1 ± 4.3 | 17.0 ± 4.0 | 0.122 |
Data are expressed as mean ± SD; n = 30 for Carriers and n = 15 for Controls.
3.4. Impact of disease progression on lipid profile and HDL subclass distribution
Plasma HDL-C levels were nearly significantly reduced, and apoA-I and apoA-II levels tended to be lower in symptomatic compared to asymptomatic Carriers (Table 3). No differences were detected between asymptomatic and symptomatic Carriers in other lipid parameters (Table 3).
Table 3.
Lipid/apolipoprotein levels and HDL subpopulations in asymptomatic and symptomatic Carriers.
| Asymptomatic Carriers | Symptomatic Carriers | P | |
|---|---|---|---|
| Total cholesterol (mg/dl) | 187.0 ± 49.6 | 188.8 ± 35.7 | 0.910 |
| Triglycerides (mg/dl) | 95.5 ± 50.2 | 123.7 ± 46.5 | 0.122 |
| Unesterified cholesterol (mg/dl) | 55.7 ± 9.0 | 59.1 ± 13.9 | 0.433 |
| Unesterified/total cholesterol | 0.31 ± 0.05 | 0.32 ± 0.06 | 0.700 |
| LDL-Cholesterol (mg/dl) | 130.5 ± 47.1 | 134.5 ± 30.8 | 0.785 |
| Apolipoprotein B (mg/dl) | 95.4 ± 24.8 | 108.4 ± 26.6 | 0.177 |
| HDL-cholesterol (mg/dl) | 36.5 ± 12.4 | 29.5 ± 8.8 | 0.085 |
| Apolipoprotein A-I (mg/dl) | 97.7 ± 22.7 | 89.7 ± 14.9 | 0.264 |
| Apolipoprotein A-II (mg/dl) | 30.4 ± 6.3 | 28.4 ± 4.0 | 0.308 |
| LpA-I (mg/dl) | 34.8 ± 7.0 | 29.7 ± 10.0 | 0.117 |
| LpA-I:A-II (mg/dl) | 62.9 ± 19.5 | 59.9 ± 13.5 | 0.628 |
| Small HDL (%) | 13.8 ± 6.5 | 14.3 ± 4.7 | 0.811 |
| Medium HDL (%) | 17.3 ± 5.1 | 18.3 ± 5.1 | 0.596 |
| Large HDL (%) | 69.0 ± 10.8 | 67.4 ± 8.3 | 0.653 |
| HDL2 (nm) | 11.3 ± 0.3 | 11.2 ± 0.3 | 0.369 |
| HDL3 (nm) | 8.9 ± 0.4 | 9.0 ± 0.4 | 0.499 |
| Preβ-HDL (%) | 18.1 ± 3.7 | 20.0 ± 4.7 | 0.229 |
| CER (nmol/ml/h) | 34.1 ± 15.2 | 30.3 ± 19.3 | 0.554 |
| LCAT activity (nmol/ml/h) | 26.2 ± 10.8 | 22.4 ± 6.2 | 0.473 |
| LCAT (μg/ml) | 4.14 ± 1.38 | 3.39 ± 1.40 | 0.151 |
Data are expressed as mean ± SD; n = 15 in each group.
4. Discussion
The L75P is an amyloidogenic apoA-I variant associated with systemic amyloidosis predominantly involving the liver, kidneys, and testis [5]. The variant has been identified in a large number of subjects, all living in the same area but not related. Carriers of the L75P apoA-I variant present with a moderate hypoalphalipoproteinemia (30% reduction in HDL-C), which tend to worsen with disease progression. Plasma apoA-I concentrations are also significantly reduced in carriers, similar to what is observed for other amyloidogenic apoA-I mutants. Moreover, in carriers' plasma, the wild-type protein is far more abundant than the mutant, the last accounting for less than 10% of total apoA-I [5,10]. This unbalance, shared by other amyloidogenic apoA-I variants [11,12], is at least in part due to an impaired secretion of the mutant [10], although an enhanced plasma clearance, already reported for the G26R mutant [13], cannot be excluded. The worsening of the hypoalphalipoproteinemia in symptomatic carriers suggests that the low plasma levels of the variant might be also due to a faster uptake in target tissues.
The hypoalphalipoproteinemia observed in carriers of the L75P apoA-I variant is mainly due to a marked reduction in LpA-I concentration with no significant changes in LpA-I:A-II levels. This is somehow surprising, since carriers of apoA-I variants leading to reduced HDL-C levels generally show a decrease of both LpA-I and LpA-I:A-II particles [13–16], or a selective reduction of LpA-I:A-II particles [17,18]. The selective reduction of LpA-I concentrations in carriers could be explained by a disturbed association of the L75P apoA-I with distinct HDL subpopulations (LpA-I particles) or by a preferential hypercatabolism of the variant bound to LpA-I.
A modest but significant increase in the free to total cholesterol plasma ratio and a reduction in cholesterol esterification rate and LCAT activity were detected in carriers of the L75P apoA-I, similar to what is observed in carriers of apoA-I variants with impaired LCAT activation capacity [19,20]. The evaluation of the in vitro capacity of the L75P variant to activate LCAT is far beyond the scope of the present paper; however, it is likely that the L75P apoA-I has impaired LCAT activation ability, despite the mutation being far from the apoA-I central region involved in LCAT activation. Indeed, the majority of the apoA-I mutations causing impaired ability to activate LCAT are located within helices 5, 6, and 7 (residues 121–187) [21]. However, an N-terminal apoA-I mutation (S36A) with impaired in vitro capacity to activate LCAT and associated with impaired cholesterol esterification in carriers has been very recently described [22], suggesting that the N-terminal region of human apoA-I is likely necessary for LCAT activation.
In conclusion, the amyloidogenic L75P apoA-I variant is associated with hypoalphalipoproteinemia, a selective reduction of LpA-I particles, and a partial defect in cholesterol esterification. Despite the hypoalphalipoproteinemia there is no evidence of premature cardiovascular disease in the large kindred; moreover, the evaluation of pre-clinical atherosclerosis, assessed by the measurement of carotid intima-media thickness, showed no differences between carriers and unaffected relatives [23].
Abbreviations
- ALP
Alkaline phosphatase
- apoA-I
apolipoprotein A-I
- CER
cholesterol esterification rate
- FSH
follicle-stimulating hormon
- GFR
glomerular filtration rate
- GGE
gradient gel electrophoresis
- HDL
high density lipoprotein
- HDL-C
HDL-cholesterol
- LDL-C
LDL-cholesterol
- LCAT
lecithin:cholesterol acyltransferase
- LH
luteinizing hormone
- LpA-I
particles containing only apoA-I
- LpA-I:A-II
particles containing both apoA-I and apoA-II
Acknowledgments
This work was supported in part by a grant from Telethon-Italy (GGP08052 to M.G.).
References
- 1.Sipe J.D., Benson M.D., Buxbaum J.N. Amyloid fibril protein nomenclature: 2010 recommendations from the nomenclature committee of the International Society of Amyloidosis. Amyloid. 2010;17:101–104. doi: 10.3109/13506129.2010.526812. [DOI] [PubMed] [Google Scholar]
- 2.Obici L., Franceschini G., Calabresi L. Structure, function and amyloidogenic propensity of apolipoprotein A-I. Amyloid. 2006;13:191–205. doi: 10.1080/13506120600960288. [DOI] [PubMed] [Google Scholar]
- 3.Bohne S., Sletten K., Menard R. Cleavage of AL amyloid proteins and AL amyloid deposits by cathepsins B, K, and L. J Pathol. 2004;203:528–537. doi: 10.1002/path.1553. [DOI] [PubMed] [Google Scholar]
- 4.Eriksson M., Schonland S., Yumlu S. Hereditary apolipoprotein AI-associated amyloidosis in surgical pathology specimens: identification of three novel mutations in the APOA1 gene. J Mol Diagn. 2009;11:257–262. doi: 10.2353/jmoldx.2009.080161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Obici L., Palladini G., Giorgetti S. Liver biopsy discloses a new apolipoprotein A-I hereditary amyloidosis in several unrelated italian families. A new amyloidogenic apolipoprotein A-I mutation. Gastroenterology. 2004;126:1416–1422. doi: 10.1053/j.gastro.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Murakami T., Michelagnoli S., Longhi R. Triglycerides are major determinants of cholesterol esterification/transfer and HDL remodeling in human plasma. Arterioscler Thromb Vasc Biol. 1995;15:1819–1828. doi: 10.1161/01.atv.15.11.1819. [DOI] [PubMed] [Google Scholar]
- 7.Calabresi L., Pisciotta L., Costantin A. The molecular basis of lecithin:cholesterol acyltransferase deficiency syndromes: a comprehensive study of molecular and biochemical findings in 13 unrelated Italian families. Arterioscler Thromb Vasc Biol. 2005;25:1972–1978. doi: 10.1161/01.ATV.0000175751.30616.13. [DOI] [PubMed] [Google Scholar]
- 8.Franceschini G., Calabresi L., Colombo C., Favari E., Bernini F., Sirtori C.R. Effects of fenofibrate and simvastatin on HDL-related biomarkers in low-HDL patients. Atherosclerosis. 2007;195:385–391. doi: 10.1016/j.atherosclerosis.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 9.Favari E., Lee M., Calabresi L. Depletion of pre-beta-high density lipoprotein by human chymase impairs ATP-binding Cassette Transporter A1- but not Scavenger Receptor Class B Type I-mediated lipid efflux to high density lipoprotein. J Biol Chem. 2004;279:9930–9936. doi: 10.1074/jbc.M312476200. [DOI] [PubMed] [Google Scholar]
- 10.Marchesi M., Parolini C., Valetti C. The intracellular quality control system down-regulates the secretion of amyloidogenic apolipoprotein A-I variants: a possible impact on the natural history of the disease. Biochim Biophys Acta. 2011;1812:87–93. doi: 10.1016/j.bbadis.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Mangione P., Sunde M., Giorgetti S. Amyloid fibrils derived from the apolipoprotein AI Leu174Ser variant contain elements of ordered helical structure. Protein Sci. 2001;10:187–199. doi: 10.1110/ps.29201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy C.L., Wang S., Weaver K., Gertz M.A., Weiss D.T., Solomon A. Renal apolipoprotein A-I amyloidosis associated with a novel mutant Leu64Pro. Am J Kidney Dis. 2004;44:1103–1109. doi: 10.1053/j.ajkd.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 13.Rader D.J., Gregg R.E., Meng M.S. In vivo metabolism of a mutant apolipoprotein, apoA-IIowa, associated with hypoalphalipoproteinemia and hereditary systemic amyloidosis. J Lipid Res. 1992;33:755–763. [PubMed] [Google Scholar]
- 14.Cheung M.C., Nichols A.V., Blanche P.J., Gong E.L., Franceschini G., Sirtori C.R. Characterization of A-I-containing lipoproteins in subjects with A-IMilano variant. Biochim Biophys Acta. 1988;960:73–82. doi: 10.1016/0005-2760(88)90011-2. [DOI] [PubMed] [Google Scholar]
- 15.Miccoli R., Bertolotto A., Navalesi R. Compound heterozygosity for a structural apolipoprotein A-I variant, apo A-I(L141R)Pisa, and an apolipoprotein A-I null allele in patients with absence of HDL cholesterol, corneal opacifications, and coronary heart disease. Circulation. 1996;94:1622–1628. doi: 10.1161/01.cir.94.7.1622. [DOI] [PubMed] [Google Scholar]
- 16.Bruckert E., von Eckardstein A., Funke H. The replacement of arginine by cysteine at residue 151 in apolipoprotein A-I produces a phenotype similar to that of apolipoprotein A-IMilano. Atherosclerosis. 1997;128:121–128. doi: 10.1016/s0021-9150(96)05982-5. [DOI] [PubMed] [Google Scholar]
- 17.Tilly-Kiesi M., Zhang Q., Ehnholm S. ApoA-IHelsinki (Lys107–>0) associated with reduced HDL cholesterol and LpA-I:A-II deficiency. Arterioscler Thromb Vasc Biol. 1995;15:1294–1306. doi: 10.1161/01.atv.15.9.1294. [DOI] [PubMed] [Google Scholar]
- 18.Leren T.P., Bakken K.S., Daum U. Heterozygosity for apolipoprotein A-I(R160L)Oslo is associated with low levels of high density lipoprotein cholesterol and HDL- subclass LpA-I/A-II but normal levels of HDL-subclass LpA-I. J Lipid Res. 1997;38:121–131. [PubMed] [Google Scholar]
- 19.Franceschini G., Baio M., Calabresi L., Sirtori C.R., Cheung M.C., Apolipoprotein A-IMilano. Partial lecithin:cholesterol acyltransferase deficiency due to low levels of a functional enzyme. Biochim Biophys Acta. 1990;1043:1–6. doi: 10.1016/0005-2760(90)90102-4. [DOI] [PubMed] [Google Scholar]
- 20.Calabresi L., Franceschini G., Burkybile A., Jonas A. Activation of lecithin cholesterol acyltransferase by a disulfide-linked apolipoprotein A-I dimer. Biochem Biophys Res Commun. 1997;232:345–349. doi: 10.1006/bbrc.1997.6286. [DOI] [PubMed] [Google Scholar]
- 21.Sorci-Thomas M.G., Bhat S., Thomas M.J. Activation of lecithin:cholesterol acyltransferase by HDL ApoA-I central helices. Clin Lipidol. 2009;4:113–124. doi: 10.2217/17584299.4.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weers P.M., Patel A.B., Wan L.C. Novel N-terminal mutation of human apolipoprotein A-I reduces self-association and impairs LCAT activation. J Lipid Res. 2011;52:35–44. doi: 10.1194/jlr.M007500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muiesan M.L., Salvetti M., Paini A. Carotid artery structural alterations in patients with apolipoprotein A-I amyloidosis (Leu75Pro) J Hyperten. 2009;27(Suppl. 4):S323. [Google Scholar]