Abstract
Background. Substantial morbidity occurs during the first year of antiretroviral therapy (ART) in persons with advanced human immunodeficiency virus (HIV) disease despite HIV suppression. Biomarkers may identify high-risk groups.
Methods. Pre-ART and 1-month samples from an initial ART trial were evaluated for biomarkers associated with AIDS events or death within 1–12 months. Case patients (n = 63) and control patients (n = 126) were 1:2 matched on baseline CD4 cell count, hepatitis status, and randomization date. All had ≥1 log10 HIV RNA level decrease at 1 month.
Results. Case patients had more frequent prior AIDS events, compared with control patients (P = .004), but similar HIV RNA levels at baseline. Pre-ART and 1-month C-reactive protein (CRP), D-dimer, and interleukin 6 (IL-6) levels and pre-ART hyaluronic acid (HA) levels were associated with new AIDS events or death (P ≤ .01). Patients who experienced immune reconstitution inflammatory syndrome (IRIS) events had higher pre-ART tumor necrosis factor α (TNF-α) and HIV RNA levels and significant 1-month increases in CRP, D-dimer, IL-6, interleukin 8, CXCL10, TNF-α, and interferon-γ levels, compared with patients who experienced non-IRIS events (P ≤ .03). Individuals with baseline CRP and HA levels above the cohort median (>2.1 mg/L and >50.0 ng/mL, respectively) had increased risk of AIDS or death (OR, 4.6 [95% CI, 2.0–10.3]; P < .001) and IRIS (OR, 8.7 [95% CI, 2.2–34.8] P = .002).
Conclusions. Biomarkers of Inflammation (CRP, IL-6), coagulation (D-dimer), and tissue fibrosis (HA) measured pre-ART and at 1 month are associated with higher risk of AIDS events, IRIS, or death, warranting additional study as risk stratification strategies.
Antiretroviral therapy (ART) has substantially reduced morbidity and mortality in human immunodeficiency virus (HIV)-infected persons [1–4]. Yet even with adequate HIV virologic suppression, substantial morbidity and mortality still occurs during the first year of ART as a result of immune reconstitution inflammatory syndrome (IRIS), progression of preexisting or new infections, toxic effects of medication, or noninfectious events [5–9]. Although many studies have confirmed persistently high morbidity and mortality during the first year and particularly the first 6 months of ART, identifying patients prior to starting ART who are at high subsequent risk remains a clinical challenge. Certain clinical variables, such as hemoglobin level, preexisting AIDS-defining illness, CD4 T-cell count, and injection drug use, have been identified as predictors of morbidity or mortality in the first months after ART initiation [10–13]. Biomarkers could also be important in guiding intense monitoring of selected subgroups of high-risk patients and could be used to elucidate pathogenesis pathways amenable to targeted therapeutic interventions.
Data from the Strategies for the Management of Antiretroviral Therapy (SMART) study have shown that elevated levels of interleukin 6 (IL-6), D-dimer, and C-reactive protein (CRP) are strong predictors of all-cause mortality [14]. More recently using SMART samples, sCD14, a marker of monocyte activation, was also associated with mortality [15]. In another SMART nested case-control study focusing on AIDS events, elevated CRP and IL-6 levels at baseline and proximal to the event were significant predictors [16]. The majority of participants in SMART were receiving ART, and it is unclear whether the same markers would have similar predictive value among people starting ART for the first time. In a small study of ART-naive patients starting therapy, pre-ART CRP and D-dimer levels also showed promise as predictors of IRIS [17].
We hypothesized that biomarkers can be used to identify persons at high risk for clinical events who may require closer monitoring upon ART initiation. We sought to determine whether inflammatory, coagulation, and tissue fibrosis markers could predict AIDS or death. Secondarily, we assessed whether any biomarkers were predictive of future IRIS events. We focused on biomarkers identified in the SMART study [16], as well as inflammatory biomarkers hypothesized as related to IRIS pathogenesis, including type-1 T-helper (Th1) and inflammatory cytokines and chemokines [18]. To that end, we conducted a nested case-control study using samples from a clinical trial of first-line ART (the Flexible Initial Retrovirus Suppression Therapies [FIRST] trial) [8].
METHODS
Study Design
The FIRST trial randomized 1397 ART-naive US persons during the period 1999 through 2002 to 1 of 3 first-line ART strategies using 2 or 3 classes of antiretrovirals [8]. Most participants had advanced HIV disease (median plasma HIV RNA level, 143,844 copies/mL; median CD4 T-cell count, 163 cells/μL; 38% had a prior AIDS-defining illness). Participants were recruited from the Community Programs for Clinical Research on AIDS (CPCRA) network and gave written informed consent. Plasma samples were prospectively stored at –70°C at months 0, 1, and 4 and then every 4 months thereafter. The CPCRA058 ClinicalTrials.gov registration number is NCT00000922.
In the FIRST study, AIDS events were defined as the first occurrence of events meeting 1993 Centers for Disease Control and Prevention criteria (with the exception of CD4 T-cell count <200 cells/μL) or as a recurrence of Pneumocystis jiroveci pneumonia (PCP), Candida esophagitis, herpes simplex infection, disseminated herpes zoster infection, or Salmonella septicemia. A clinical events committee blinded to randomization arm reviewed all AIDS events and deaths during the study [8]. For the current project, we considered only virologic responders: those participants who did not experience an AIDS event or death during the first month of ART and who did have a >1-log10 reduction in HIV RNA level 1 month after ART initiation. Of the 1157 persons classified as virologic responders in the FIRST trial, 63 had an AIDS event or died during months 1–12 of ART, 149 had an AIDS event or died only after >12 months, and 945 did not experience an AIDS event or death through a median 60-month period of follow-up. We excluded 17 AIDS or death events that occurred during the first month of ART, because a virologic response prior to the event could not be confirmed.
We performed a nested case-control study with 1:2 matching among virologic responders. A case was defined as an AIDS event or death during months 1–12 of ART. Each of the 63 case patients was matched with 2 control patients who did not have an AIDS event or death during months 1–12 of ART. Matching occurred on the basis of date of randomization (within ±3 months), CD4 T-cell count at randomization (within ±50 cells/μL), and hepatitis status. Hepatitis status was defined by hepatitis B surface antigen and/or hepatitis C antibody positivity.
After the case patients and control patients were determined for this study and before the biomarkers were measured, participant records of all case patients were retrospectively reviewed events by 3 coauthors (D. R. B., P. R. B., I. S.) to determine possible IRIS. IRIS was defined, in accordance with other published IRIS case definitions, by (1) receipt of effective ART; (2) a new and/or recurrent inflammatory event, temporarily associated with starting ART; and (3) exclusion of other etiologies [19–21]. All case patients were classified as experiencing either an unmasking IRIS event, a paradoxical IRIS event, or a non-IRIS event. A case patient was classified as experiencing an unmasking IRIS event if the event occurred within 6 months of ART initiation and was due to a new opportunistic infection associated with unmasking IRIS. Case patients were classified as experiencing paradoxical IRIS if they had a known history of an AIDS event prior to ART initiation and if the event that occurred during ART was a culture-negative clinical recurrence not due to the expected clinical course of the existing opportunistic infection(s), a new infection, drug adverse events, or an alternative explained cause. All IRIS events were considered possible IRIS events, because confirmation was difficult to establish retrospectively.
Biomarker Measurement
We analyzed cryopreserved plasma specimens from the baseline visit (pre-ART) and the 1-month visit after ART initiation. Levels of proinflammatory (interleukin-1β, IL-6, tumor necrosis factor α [TNF-α]), Th1 (interferon-γ [IFN-γ] and interleukin-12), Th2 (interleukin-4 and interleukin-13), Th17 (interleukin-17), and regulatory (interleukin-10) cytokines, proliferation/differentiation cytokines (interleukin-2 and interleukin-15), and chemokines (interleukin 8 [IL-8], CXCL10, CXCL1 [Gro-a], CCL3 [MIP1α], CCL4 [MIP1β], CCL11 [Eotaxin-1], CCL13 [MCP4], CCL17 [TARC], CCL22 [MDC], and CCL26 [Eotaxin-3, MIP-4α], which attract leukocytes to sites of inflammation) were measured with multiplex enzyme-linked immunosorbent assay (ELISA)–based assays (Meso Scale Discovery). Plasma D-dimer was quantified by means of enzyme linked fluorescent assay on a VIDAS instrument (bioMérieux). We also quantified by means of ELISA the plasma levels of CRP (Meso Scale Discovery), hyaluronic acid (Corgenix), intestinal fatty acid binding protein (Hycult Biotechnology BV), high-mobility group B1, and sCD14 (R&D Systems) according to the manufacturers’ protocols. Hyaluronic acid is a marker of increased extracellular matrix metabolism and tissue fibrosis [22], and sCD14 binds bacterial lipopolysaccharide [23]. Cytomegalovirus DNA was quantitated by means of real-time polymerase chain reaction as described by Yun et al [24].
Statistics
Demographic and other baseline characteristics were compared with the χ2 and Wilcoxon rank sum tests. For each biomarker, the unadjusted percent differences at baseline and at month 1 between case patients (AIDS or death) and control patients were estimated after loge transformation. To assess the risk of AIDS events or death, adjusted odds ratios (ORs) with 95% confidence intervals (CIs) for case patients compared with control patients were estimated with a conditional logistic regression model for each biomarker, which accounted for the matching. The ORs cited are per interquartile range (IQR) higher of the biomarker after loge transformation and were adjusted for HIV-RNA level and history of AIDS events prior to ART initiation. The models were repeated for biomarker values at the 1-month visit, with additional adjustment for the baseline log-transformed value of the biomarker and randomized treatment assignment in the FIRST study. All models were repeated for the subset of case patients classified as experiencing IRIS events, with their associated matched control patients. Multivariate conditional logistic regression models were also performed to assess the risk of AIDS or death, death alone, and IRIS using categories (above or below the overall cohort median) for selected baseline biomarkers. Among the case patients, the biomarker distributions were further compared between those who experienced IRIS and non-IRIS events. Those models were also adjusted for HIV RNA levels and prior AIDS events and were further adjusted for other baseline factors (CD4 cell count and triglyceride levels) that were different between the two groups.
All reported P values are 2 sided. Although there were no adjustments made for the multiple biomarker comparisons, we focus the results and discussion on biomarkers that were significant at the P ≤ .01 level.
RESULTS
Cohort Description
There were 63 prospectively identified and adjudicated case patients who experienced AIDS-defining events (41 patients) or deaths (22 patients) during months 1–12 of HIV therapy among 1157 virologic responders. The median CD4 cell count of case patients was 44 cells/μL (IQR, 15–153 cells/μL). The causes of 5 of the 22 deaths were confirmed as AIDS-related, 6 as possibly AIDS-related, 4 as sepsis, 2 as pneumonia, 2 as cardiac, 1 as kidney failure, 1 as liver failure, and 1 as pancreatitis. Of the 5 confirmed AIDS-related deaths, 2 were due to lymphoma, and 1 each to PCP, Kaposi's sarcoma, and histoplasmosis. The other AIDS events included Mycobacterium avium complex (MAC) (n = 9), Candida esophagitis (n = 7), lymphoma (n = 3), PCP (n = 4), Kaposi's sarcoma (n = 2), cryptococcosis (n = 3), varicella zoster (n = 3), cytomegalovirus (n = 3), both MAC and Candida esophagitis (n = 1), and 1 each of cryptosporidiosis, herpes simplex virus infection, isosporiasis, bacterial pneumonia, toxoplasmosis, and tuberculosis. Three of the PCP events, 3 of the Candida esophagitis events, and none of the varicella zoster events or herpes simplex virus events were recurrences.
The demographic characteristics of the 63 case patients and 126 matched control patients were generally similar (Table 1). Case patients had a higher proportion of prior AIDS-defining events, compared with control patients (46 [73%] vs 64 [51%]; P = .004). HIV RNA levels did not differ significantly between case patients and control patients at baseline (P = .61) or after 1 month of ART (P = .17). The use of PCP and MAC prophylaxis did not differ between case patients and control patients (Table 1). Of those who should have been receiving PCP prophylaxis according to US guidelines (a history of PCP or a CD4 cell count of <200 cells/μL), 79% of 63 case patients and 82% of 126 control patients were receiving appropriate prophylaxis prior to the initiation of ART (P = .64). Similarly, for those who should have been receiving MAC prophylaxis (CD4 cell count, <50 cells/μL), 51% (22/43) of case patients and 57% (38/67) of control patients were receiving appropriate prophylaxis (P = .61). Case patients and control patients were matched on pre-ART CD4 T-cell count; after 1 month of ART, the CD4 T-cell count distributions were not different (P = .84). Of the 63 case patients, 28 (44%) were classified retrospectively as experiencing possible IRIS events (21 unmasking events and 7 paradoxical events). The median time to a first IRIS event was 9 weeks (IQR, 6–14 weeks), whereas the median time to a first non-IRIS event was 37 weeks (IQR, 27–45 weeks). The characteristics of case patients classified as experiencing IRIS and non-IRIS events are also provided in Table 1. There were significant differences between those with IRIS and non-IRIS events for baseline CD4 T-cell count both prior to (P = .001) and 1 month after ART initiation (P = .03), HIV RNA levels both prior to (P = .002) and 1 month after ART initiation (P = .002), and triglyceride levels (P = .001).
Table 1.
Baseline Characteristics of All Case Patients (With AIDS or Death), Matched Control Patients, and Subsets of Case Patients Who Experienced Immune Reconstitution Inflammatory Syndrome (IRIS) and Non-IRIS Events
| Characteristic | IRIS case patientsa (N = 28) | Non-IRIS case patientsa (N = 35) | All case patients (N = 63) | All control patients (N = 126) | P b |
| Age, median years (IQR) | 38 (34–42) | 42 (33–46) | 40 (33–45) | 38 (33–46) | .87 |
| Female sex | 3 (10.7) | 9 (25.7) | 12 (19) | 19 (15) | .49 |
| Race/ethnicity | .52 | ||||
| Latino | 10 (36) | 6 (17) | 16 (25) | 24 (19) | |
| African American | 13 (46) | 20 (57) | 33 (52) | 63 (50) | |
| White/other | 5 (18) | 9 (26) | 14 (22) | 39 (31) | |
| Injection drug use | 4 (14) | 11 (31) | 15 (24) | 25 (20) | .53 |
| Hepatitis B | 4 (14) | 2 (6) | 6 (9.8) | 9 (7.8) | .64 |
| Hepatitis C | 5 (18) | 10 (29) | 15 (24) | 33 (26) | .72 |
| Hepatitis B or C | 9 (32) | 12 (34) | 21 (33) | 41 (33) | NAc |
| Prior AIDS event | 23 (82) | 23 (66) | 46 (73) | 64 (51) | .004 |
| Triglycerides, median mg/dL (IQR) | 185 (143–249) | 122 (106–162) | 149 (110–220) | 138 (105–197) | .39 |
| CD4 T-cell count, median cells/μL (IQR) | |||||
| Prior to ART initiation | 18 (10–63) | 102 (25–238) | 44 (15–153) | 53 (19–150) | NAc |
| 1 month after ART initiation | 97 (30–193) | 187 (84–359) | 132 (41–235) | 131 (60–256) | .84 |
| HIV RNA, median log10 copies/mL (IQR) | |||||
| Prior to ART initiation | 5.5 (5.4–6.0) | 5.1 (4.6–5.6) | 5.4 (4.9–5.8) | 5.3 (4.8–5.7) | .61 |
| 1 month after ART initiation | 2.9 (2.1–3.3) | 3.0 (2.2–3.5) | 3.0 (2.2–3.4) | 2.6 (2.1–3.1) | .17 |
| Use of PCP prophylaxis | |||||
| Prior to ART initiation | 23 (82) | 20 (57) | 43 (68) | 90 (71) | .65 |
| 1 month after ART initiation | 24 (86) | 22 (63) | 46 (73) | 95 (75) | .72 |
| Use of MAC prophylaxis | |||||
| Prior to ART initiation | 13 (46) | 9 (26) | 22 (35) | 38 (38) | .67 |
| 1 month after ART initiation | 14 (50) | 10 (29) | 24 (38) | 52 (41) | .67 |
NOTE. Data are no. (%) of participants unless otherwise specified. ART, antiretroviral therapy; IQR, interquartile range; MAC, Mycobacterium avium complex; PCP, Pneumocystis jiroveci pneumonia.
Retrospective subset of all cases.
Comparing all case patients with all matched control patients. Cases occurred within 1–12 months following ART initiation. All case patients and control patients had ≥1 log10 reduction in HIV RNA level between baseline and 1 month.
Matching factor.
Risk of AIDS Events or Death
The distributions of selected biomarkers prior to ART initiation, as well as 1 month after ART initiation, are provided in Table 2 for all case patients and control patients along with the adjusted odds ratios. Risk of AIDS or death increased with higher levels of D-dimer, CRP, and IL-6 both prior to ART initiation and 1 month after ART initiation. Detectable hyaluronic acid was associated with an AIDS event or death at baseline (OR, 2.7 [95% CI, 1.3–5.5]; P = .006) but not at month 1 (OR, 1.9 [95% CI, 0.8–4.6]; P = .13). At baseline, unadjusted hyaluronic acid levels were 33% higher (95% CI, 4% –56%; P = .002) in case patients than in control patients, whereas after 1 month of ART hyaluronic acid levels were 16% higher (95% CI, 2%–32%; P = .04) in case patients than in control patients. Detectable hyaluronic acid levels were present in 56% of 62 individuals with hepatitis B or C and 27% of 127 individuals without hepatitis B or C. Although baseline levels of IL-8, IFN-γ, and TNF-α were not associated with subsequent AIDS events or deaths, after 1 month of ART there was an increased risk associated with higher levels of IL-8 (OR, 2.5 [95% CI, 1.4–4.5]; P = .002), IFN-γ (OR, 2.8 [95% CI, 1.5–5.2]; P = .001) and TNF-α (OR, 3.2 [95% CI, 1.5–6.6]; P = .002) per each IQR higher. In comparing the percent differences, after 1 month of ART IL-8 was 78% higher (95% CI, 37%–131%; P < .001), IFN-γ was 97% higher (95% CI, 40%–177%; P < .001), and TNF-α was 38% higher (95% CI, 14%–68%; P = .004) in case patients than in control patients. The median untransformed baseline values and adjusted odds ratios for all other biomarkers tested are shown in Supplemental Table 1 for case patients and control patients.
Table 2.
Distribution of Biomarker Values and Adjusted Odds Ratios for Case Patients (With AIDS or Death) and Associated Control Patients
| Biomarker | Case patients (N = 63) | Control patients (N = 126) | Adjusteda ORb (95% CI) | P | |
| Baseline (pre-ART) | |||||
| D-dimer, mg/L | 1.3 (0.6–2.1) | 0.7 (0.4–1.2) | 2.4 (1.4–3.9) | .001 | |
| CRP, mg/L | 4.8 (1.2–12.4) | 1.5 (0.5–4.7) | 2.1 (1.3–3.3) | .001 | |
| IL-6, pg/mL | 3.9 (2.2–6.6) | 2.3 (1.5–4.5) | 1.8 (1.1–2.7) | .01 | |
| IL-8, pg/mL | 7.1 (4.5–13.7) | 6.1 (3.8–10.4) | 1.5 (1.0–2.3) | .08 | |
| IL-10, pg/mL | 8.3 (5.0–13.9) | 6.0 (3.9–9.1) | 1.5 (1.1–2.1) | .02 | |
| IFN-γ, pg/mL | 2.7 (1.5–6.3) | 2.6 (1.4–5.2) | 1.1 (0.7–1.7) | .80 | |
| TNF-α, pg/mL | 17.3 (11.9–26.4) | 15.6 (12.5–20.0) | 1.2 (0.9–1.6) | .32 | |
| HA, ng/mL | 78.8 (49.9–128) | 49.9 (49.9–78.4) | 2.7 (1.3–5.5) | .006 | |
| CXCL10, mg/L | 0.6 (0.3–1.1) | 0.6 (0.4–0.9) | 0.9 (0.6–1.4) | .66 | |
| sCD14, x106 pg/mL | 2.6 (2.3–3.1) | 2.3 (1.9–2.8) | 1.2 (0.9–1.7) | .21 | |
| Month 1 | |||||
| D-dimer, mg/L | 1.2 (0.7–2.0) | 0.5 (0.3–1.0) | 2.3 (1.2–4.4) | .01 | |
| CRP, mg/L | 14.0 (2.8–39.7) | 3.0 (1.0–8.5) | 2.9 (1.5–5.8) | .002 | |
| IL-6, pg/mL | 4.6 (2.4–10.7) | 1.9 (1.1–3.5) | 4.9 (2.2–10.8) | <.001 | |
| IL-8, pg/mL | 8.5 (3.8–15.5) | 4.5 (3.0–7.3) | 2.5 (1.4–4.5) | .002 | |
| IL-10, pg/mL | 7.1 (5.1–11.1) | 5.0 (3.1–7.8) | 1.4 (0.9–2.0) | .12 | |
| IFN-γ, pg/mL | 2.7 (1.4–9.5) | 1.7 (1.0–4.0) | 2.8 (1.5–5.2) | .001 | |
| TNF-α, pg/mL | 17.2 (11.2–25.3) | 13.3 (9.7–17.6) | 3.2 (1.5–6.6) | .002 | |
| HA, ng/mL | 49.9 (49.9–65.4) | 49.9 (49.9–49.9) | 1.9 (0.8–4.6) | .13 | |
| CXCL10, mg/L | 0.4 (0.1–0.9) | 0.3 (0.2–0.5) | 1.9 (1.1–3.4) | .03 | |
| sCD14, x106 pg/mL | 2.7 (2.3–3.0) | 2.3 (2.0–2.7) | 1.2 (0.8–1.7) | .32 | |
NOTE. Data are median (IQR) unless otherwise specified. CI, confidence interval; CRP, C-reactive protein; HA, hyaluronic acid; IFN-γ, interferon-γ; IL-6, interleukin 6; IL-8, interleukin 8; IL-10, interleukin 10; IQR, interquartile range; OR, odds ratio; TNF-α, tumor necrosis factor α.
The odds ratios take into account the matching factors and are further adjusted for a history of an AIDS event and HIV RNA levels prior to ART initiation. The models for month 1 are also adjusted for the log-transformed baseline value of the biomarker and randomization group for the study.
For HA, the odds ratio is for individuals with detectable (≥50 ng/mL) values, compared with individuals with undetectable values. Undetectable HA values are entered as 49.9 ng/mL. For other biomarkers, the odds ratios are compared per IQR after log transformation. The assay lower limits of detection are for D-dimer, 0.045 mg/L; CRP, 0.016 mg/L; IFN-γ, 0.6 pg/mL; IL-6, 0.6 pg/mL; IL-8, 0.1 pg/mL; IL-10, 0.4 pg/mL; TNF-α, 0.6 pg/mL; HA, 50 ng/mL; CXCL10, 0.6 pg/mL; sCD14, 125 pg/mL.
Risk of IRIS Events
For the subset of case patients retrospectively classified as experiencing possible IRIS, Table 3 presents the distribution of biomarkers and the adjusted odds ratios. Baseline CD4 T-cell counts did not differ (P = .34) between IRIS case patients and their associated control patients. At baseline, the strongest predictor of IRIS was TNF-α (OR, 3.7 [95% CI, 1.4–10.1]; P = .01). After 1 month of ART, the risk for an IRIS event was significantly greater with increased levels of CRP (OR, 12.1 [95% CI, 2.3–63.5]; P = .003), IL-6 (OR, 11.5 [95% CI, 2.1–62.8]; P = .005), IL-8 (OR, 4.1 [95% CI, 1.3–12.4]; P = .01), and IFN-γ (OR, 8.6 [95% CI, 1.9–28.6]; P = .005) per each IQR increase of the biomarker.
Table 3.
Distribution of Biomarker Values and Adjusted Odds Ratios for Immune Reconstitution Inflammatory Syndrome (IRIS) Case Patients and Associated Control Patients
| Biomarker | IRIS case patients (N = 28) | IRIS control patients (N = 56) | Adjusteda ORb (95% CI) | P |
| Baseline (pre-ART) | ||||
| D-dimer, mg/L | 1.4 (0.6–2.1) | 0.7 (0.4–1.2) | 2.8 (1.1–7.3) | .03 |
| CRP, mg/L | 4.4 (1.8–11.7) | 1.8 (0.6–6.2) | 2.1 (1.0–4.4) | .06 |
| IL-6, pg/mL | 4.3 (2.9–7.5) | 2.7 (1.7–4.5) | 2.0 (0.9–4.5) | .08 |
| IL-8, pg/mL | 10.5 (5.2–15.3) | 6.5 (4.6–11.8) | 1.6 (0.8–3.0) | .19 |
| IL-10, pg/mL | 8.9 (5.7–16.2) | 5.8 (3.5–8.5) | 2.0 (1.1–3.7) | .03 |
| IFN-γ, pg/mL | 5.0 (2.0–8.7) | 2.7 (1.6–4.6) | 1.8 (0.7–4.9) | .24 |
| TNF-α, pg/mL | 21.9 (16.3–27.9) | 15.0 (11.9–19.5) | 3.7 (1.4–10.1) | .01 |
| HA, ng/mL | 79.2 (52.1–158) | 49.9 (49.9–77.5) | 3.4 (1.1–10.7) | .03 |
| CXCL10, mg/L | 0.6 (0.4–1.0) | 0.6 (0.4–0.9) | 2.8 (1.1–7.3) | .03 |
| sCD14, x106 pg/mL | 2.9 (2.5–3.2) | 2.3 (2.1–2.8) | 2.0 (0.9–4.1) | .08 |
| Month 1 | ||||
| D-dimer, mg/L | 1.4 (1.0–2.3) | 0.6 (0.4–1.2) | 6.5 (1.4–29.6) | .02 |
| CRP, mg/L | 39.6 (9.1–109) | 3.1 (1.2–8.5) | 12.1 (2.3–63.5) | .003 |
| IL-6, pg/mL | 7.8 (3.1–16.5) | 2.3 (1.2–4.0) | 11.5 (2.1–62.8) | .005 |
| IL-8, pg/mL | 10.4 (5.2–21.9) | 5.2 (4.0–10.6) | 4.1 (1.3–12.4) | .01 |
| IL-10, pg/mL | 7.9 (6.1–20.5) | 5.9 (3.1–10.0) | 0.9 (0.5–1.6) | .70 |
| IFN-γ, pg/mL | 4.5 (2.5–19.6) | 1.9 (1.0–5.0) | 8.6 (1.9–38.6) | .005 |
| TNF-α, pg/mL | 22.7 (16.9–37.9) | 14.0 (11.0–18.4) | 434 (2 to >1000) | .03 |
| HA, ng/mL | 49.9 (49.9–79.5) | 49.9 (49.9–49.9) | 1.7 (0.5–6.6) | .42 |
| CXCL10, mg/L | 0.5 (0.3–1.6) | 0.3 (0.2–0.5) | 4.0 (1.1–14.9) | .04 |
| sCD14, x106 pg/mL | 2.9 (2.4–3.2) | 2.4 (2.1–2.7) | 1.6 (0.9–2.5) | .09 |
NOTE. Data are median (IQR) unless otherwise specified. IRIS events included 21 unmasking events: MAC (n = 11), cytomegalovirus (n = 2), KS (n = 2), PCP (n = 1), varicella zoster (n = 2), histoplasmosis (n = 1), isosporiasis (n = 1), and death due to acute hepatitis of unknown etiology (n = 1). Possible paradoxical events (n = 7) included 2 people with recurrent PCP, 2 persons with clinical deterioration and history of MAC at entry (1 died), cytomegalovirus, cryptococcal meningitis, tuberculosis (patient died), and 1 person who died with new lymphadenitis with a history of both cryptococcosis and Mycobacterium avium complex before initiation of antiretroviral therapy (ART). CI, confidence interval; CRP, C-reactive protein; HA, hyaluronic acid; IFN-γ, interferon-γ; IL-6, interleukin 6; IL-8, interleukin 8; IL-10, interleukin 10; IQR, interquartile range; OR, odds ratio; TNF-α, tumor necrosis factor α.
The odds ratios take into account the matching factors and are further adjusted for a history of an AIDS event and human immunodeficiency virus RNA levels prior to ART initiation. The models for month 1 are also adjusted for the log-transformed baseline value of the biomarker and randomization group for the study.
For HA, the odds ratio is for those with detectable (≥50 ng/mL) values compared with those with undetectable values. For other biomarkers the odds ratios are per IQR after log transformation.
Multiple Biomarkers and Risk of Clinical Events
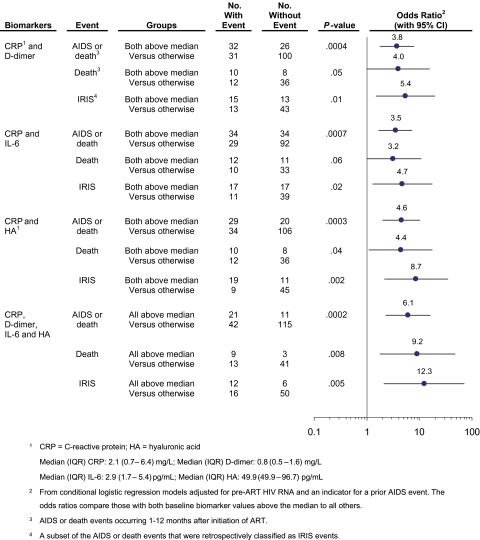
The risk for any AIDS event or death, death alone, or IRIS was further assessed with adjusted odds ratios in 4 multivariate models that considered combinations of baseline biomarker levels that were significant (P < −.01) when considered individually (Figure 1). The first 3 models evaluated the risk for those with high (greater than the cohort median) values of 2 biomarkers (CRP and D-dimer, CRP and IL-6, or CRP and detectable hyaluronic acid), compared with those without high values of both biomarkers. The final model evaluated the risk for those with levels of all 4 biomarkers above the cohort median, compared with those without all 4 values above the median. For all event types and for all biomarker combinations, there was increased risk for those with high biomarker values. Those with values above the median for CRP, D-dimer, IL-6, and hyaluronic acid had a 6-fold increased risk of an AIDS event or death (OR, 6.1 [95% CI, 2.3 – 16.1]; P =< .001).
Figure 1.
Adjusted multivariate odds ratios (with 95% confidence intervals) for AIDS or death, death alone, and immune reconstitution inflammatory syndrome (IRIS) events for selected combinations of biomarkers measured prior to the initiation of antiretroviral therapy (ART). Each odds ratio compares participants with all biomarker values considered above the cohort medians to participants without all biomarker values above the cohort medians.
Differences Between IRIS and Non-IRIS Case Patients
Table 4 presents the distribution of biomarker values and the percent differences (unadjusted and adjusted) between case patients (AIDS or death) that were retrospectively classified as experiencing IRIS, compared with the non-IRIS case patients. The distributions were similar at baseline except for TNF-α, for which the adjusted percent difference was 42% higher among the IRIS case patients (95% CI, 7%–88%; P = .02). At month 1, the adjusted percent differences for D-dimer, CRP, IL-6, and CXCL10 were significantly higher among IRIS case patients, compared with non-IRIS case patients. The differences for CRP and IL-6 were driven by increases from baseline to month 1 for the IRIS case patients but not the non-IRIS case patients.
Table 4.
Distribution of Biomarker Values and Percent Differences (Unadjusted and Adjusted) Between Case Patients With Immune Reconstitution Inflammatory Syndrome (IRIS) Events and Non-IRIS Events
| Biomarker | IRIS case patients (N = 28) | Non-IRIS case patients (N = 35) | Unadjusted difference, % | Adjusted difference, a % (95% CI) | P |
| Baseline (pre-ART) | |||||
| D-dimer, mg/L | 1.4 (0.6–2.1) | 1.2 (0.6–2.6) | 7.8 | –9.1 (–45.6 to 51.9) | .72 |
| CRP, mg/L | 4.4 (1.8–11.7) | 5.4 (1.1–17.6) | 8.2 | 26.2 (–53.3 to 241.0) | .65 |
| IL-6, pg/mL | 4.3 (2.9–7.5) | 3.5 (2.0–5.8) | 24.6 | 25.6 (–20.5 to 98.3) | .33 |
| IL-8, pg/mL | 10.5 (5.2–15.3) | 6.4 (4.2–12.1) | 37.1 | 35.8 (–13.7 to 113.6) | .19 |
| IL-10, pg/mL | 8.9 (5.7–16.2) | 8.3 (4.9–13.4) | 27.9 | 19.4 (–36.2 to 123.7) | .58 |
| IFN-γ, pg/mL | 5.0 (2.0–8.7) | 2.3 (1.3–5.1) | 49.1 | –2.4 (–45.6 to 75.1) | .94 |
| TNF-α, pg/mL | 21.9 (16.3–27.9) | 14.8 (11.1–21.5) | 32.7 | 41.6 (6.7–88.0) | .02 |
| HA, ng/mL | 79.2 (52.1–158) | 49.9 (49.9–125) | 12.9 | 11.6 (–23.3 to 62.3) | .57 |
| CXCL10, mg/L | 0.6 (0.4–1.0) | 0.6 (0.3–1.5) | 4.6 | –13.7 (–45.6 to 36.9) | .53 |
| sCD14, x106 pg/mL | 2.9 (2.5–3.2) | 2.4 (1.9–3.0) | 36.6 | 31.7 (0.6–72.5) | .05 |
| Month 1 | |||||
| D-dimer, mg/L | 1.4 (1.0–2.3) | 0.9 (0.5–1.6) | 116.0 | 122.1 (38.4–256.7) | .002 |
| CRP, mg/L | 39.6 (9.1–109) | 6.3 (1.9–17.5) | 364.3 | 280.0 (70.4–747.7) | .002 |
| IL-6, pg/mL | 7.8 (3.1–16.5) | 3.4 (2.2–5.7) | 136.5 | 175.7 (49.6–408.3) | .002 |
| IL-8, pg/mL | 10.4 (5.2–21.9) | 7.2 (3.7–11.5) | 74.3 | 59.0 (–3.5 to 161.9) | .07 |
| IL-10, pg/mL | 7.9 (6.1–20.5) | 6.2 (4.6–9.6) | 23.4 | 15.7 (–28.7 to 87.5) | .56 |
| IFN-γ, pg/mL | 4.5 (2.5–19.6) | 1.7 (1.3–3.4) | 174.2 | 145.4 (22.2–392.9) | .01 |
| TNF-α, pg/mL | 22.7 (16.9–37.9) | 13.1 (9.5–21.9) | 77.2 | 29.3 (–8.6 to 83.1) | .15 |
| HA, ng/mL | 49.9 (49.9–79.5) | 49.9 (49.9–61.8) | –4.7 | –4.8 (–26.5 to 23.3) | .71 |
| CXCL10, mg/L | 0.5 (0.3–1.6) | 0.2 (0.2–0.6) | 90.6 | 133.8 (33.1–310.4) | .005 |
| sCD14, x106 pg/mL | 2.9 (2.4–3.2) | 2.6 (2.0–2.9) | 22.8 | 27.0 (–63 to 72.0) | .13 |
NOTE. Data are median (IQR) unless otherwise specified. CI, confidence interval; CRP, C-reactive protein; HA, hyaluronic acid; IFN-γ, interferon-γ; IL-6, interleukin 6; IL-8, interleukin 8; IL-10, interleukin 10; IQR, interquartile range; OR, odds ratio; TNF-α, tumor necrosis factor α.
Adjusted for baseline CD4 T-cell count, human immunodeficiency virus RNA level, triglyceride levels, and prior AIDS events. The models for month 1 are also adjusted for the baseline biomarker level.
DISCUSSION
In the present study, we determined that biomarkers measured in ART-naive patients immediately prior to ART initiation and 1 month after are associated with adverse outcomes (AIDS events or death) during the first year of ART. Our results suggest that D-dimer, CRP, IL-6, and hyaluronic acid could be useful in identifying ART-naive patients at higher risk of AIDS or death after ART initiation. In addition, levels of TNF-α prior to ART and levels of CRP, IFN-γ, IL-8, and IL-6 at 1 month after ART initiation could be helpful in recognizing a subgroup of patients at risk for IRIS events.
This study builds on the emerging field of biomarkers, which is now being explored in order to improve the care of HIV-infected individuals. Data from the SMART study suggested that markers of activation of the coagulation pathway (D-dimer) and IL-6 were the strongest predictors of all-cause mortality after controlling for CD4 T-cell counts and HIV viral load [14]. Most of the events that occurred in the SMART study were noninfectious, which suggests that the chronic inflammation and activation of coagulation may be driving some of these events independent of the degree of immunodeficiency. In a separate nested case-control study of AIDS events in SMART, CRP was a stronger predictor than were IL-6 and D-dimer [16]. IL-6 generally induces hepatic CRP production; however, IL-6 and CRP do not always correlate, because IL-6 induces CRP production only in the absence of IFN-α [25], which may be induced during viral diseases. Yet even in the absence of clinical illness or any effective CD4 T-cell recognition, antigen-presenting cells produce IL-6 and TNF-α in response to foreign antigen. Thus, TNF-α, IL-6, and downstream CRP may serve as biomarkers for occult infections that are unmasked during immune reconstitution with ART (ie, unmasking IRIS). Our findings are thus consistent with data published from the SMART study and more recently from 2 other studies showing that markers of acute inflammation (such as soluble TNF receptor 1, sCD27, and sCD40L) appear to be important prognostic indicators in ART-naive patients upon therapy initiation [14, 16, 26]. In contrast to a recent SMART case-control study that found a strong association between mortality and sCD14 [15], an association of events with sCD14 was not observed in this study. This could be due to the fact that only a small number of deaths were evaluated here and most events were AIDS events occurring shortly after ART initiation.
Many studies have now shown that tissue fibrosis (eg, lymphatics, liver, gut, bone marrow) occurs in untreated HIV infection and may both hamper immune restoration and lead to organ dysfunction [27]. Hyaluronic acid, a polysaccharide component of the extracellular matrix, provides cellular support for adhesion, orientation, migration, and differentiation of cells. Hyaluronic acid accumulation in plasma reflects a loss of balance between biosynthesis and degradation and thereby is a marker of tissue fibrosis that has been shown in chronic liver disease to predict liver-related mortality with and without HIV [28]. Our data suggest that hyaluronic acid may be useful before the initiation of ART to identify patients at risk for AIDS events or death, even in the absence of chronic liver disease. It is unclear whether the high hyaluronic acid levels in chronic HIV infection represent higher production or decreased metabolism and clearance. Plasma levels of inflammatory cytokines, such as IFN-γ, TNF-α, interleukin 1, tissue growth factor (TGF)-β, and lipopolysaccharide, are increased in untreated HIV infection, and each of these is known to stimulate hyaluronic acid production [29, 30].
In our study, we also considered the subgroup of case patients identified as experiencing possible IRIS by means of retrospective record review. The pathogenesis of IRIS remains unclear, with some data pointing to the possibility of exuberant Th1 responses [31, 32]. Regardless of the underlying pathogen, it is thought that unmasking IRIS happens in the context of subclinical occult infections or neoplasms. It is thus plausible that patients with biomarker abnormalities could be targeted for further screening evaluation to investigate for subclinical disease. This concept is supported in other reversible immunosuppressant conditions, such as severe malnutrition, where CRP is useful for predicting the unmasking of subclinical infections during refeeding [33]. Our data support a Th1-bias and proinflammatory component being associated with IRIS with higher levels of IFN-γ, IL-6, and downstream Th1 inflammatory chemokines, such as CXCL10, which has been reported as elevated in tuberculosis-IRIS, cryptococcal IRIS, and hepatitis B IRIS events [34–36]. In addition, pre-ART TNF-α levels were higher in those who developed IRIS. This is in agreement with a recent study showing that soluble TNF receptor 1 was higher in ART-naive patients who went on to develop AIDS events soon after ART initiation [26] and with an older study that described high serum TNFα and CRP levels in HIV-infected patients with pulmonary mycobacterial infection [37]. With the exception of TNF-α, the distinction of IRIS predilection became more evident at 1 month of ART when many of the inflammatory indices started decreasing in patients with uneventful immune recovery while remaining elevated or increasing in patients with impending IRIS events. It also appears that the level of CRP at 1 month may be a useful clinical marker for upcoming IRIS events, since only 2 possible IRIS case patients out of the 28 had levels below the sampled population's median of 2.1 mg/L. The elevated 1-month inflammatory biomarker levels could be a consequence of an event pathogenesis already in development but still represent an early indicator preceding the clinical event. Whereas the AIDS events and deaths were prospectively identified, the retrospective evaluation of IRIS limited our ability to recognize all cases of, particularly, paradoxical IRIS events, which typically lack microbiologic confirmation. Thus, the cases identified represent severe manifestations associated with AIDS events and could only be classified as possible IRIS cases. This limitation, however, may have enhanced the specificity of our findings. Because this was a case-control study, determining specific cut points for risk was not feasible to determine the diagnostic performance (eg, sensitivity and positive predictive value) in the overall cohort; this should be explored in future studies.
In summary, our data show that pre-ART plasma levels of CRP, IL-6, D-dimer, and hyaluronic acid may be useful in risk stratification of patients who are about to initiate ART. The presence of elevated levels of these biomarkers at baseline, as well as persistently high or increasing levels after 1 month of ART, could assist in selecting patients who could benefit from closer monitoring or additional workup to unveil occult infections. In addition, these biomarkers support a central role for inflammation, coagulation pathways, and tissue fibrosis in HIV pathogenesis.
Supplementary Data
Supplementary data are available online at http://jid.oxfordjournals.org.
Funding
This work was supported in part through the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health through grants U01AI042170, U01AI046362, and U01AI068641 for the FIRST Study (CPCRA 058) and INSIGHT, as well as through the Intramural Research Program of NIAID. In addition, this project has been supported in part by federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. D. R. B. is supported by NIAID grant K23AI073192-02, and J. V. B. by grant K12RR023247-05.
Supplementary Material
References
- 1.Palella FJ, Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 3.Mocroft A, Ledergerber B, Katlama C, et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet. 2003;362:22–9. doi: 10.1016/s0140-6736(03)13802-0. [DOI] [PubMed] [Google Scholar]
- 4.Murphy EL, Collier AC, Kalish LA, et al. Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Ann Intern Med. 2001;135:17–26. doi: 10.7326/0003-4819-135-1-200107030-00005. [DOI] [PubMed] [Google Scholar]
- 5.Castelnuovo B, Manabe YC, Kiragga A, Kamya M, Easterbrook P, Kambugu A. Cause-specific mortality and the contribution of immune reconstitution inflammatory syndrome in the first 3 years after antiretroviral therapy initiation in an urban African cohort. Clin Infect Dis. 2009;49:965–72. doi: 10.1086/605500. [DOI] [PubMed] [Google Scholar]
- 6.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22:1897–08. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Sighem AI, van de Wiel MA, Ghani AC, et al. Mortality and progression to AIDS after starting highly active antiretroviral therapy. AIDS. 2003;17:2227–36. doi: 10.1097/00002030-200310170-00011. [DOI] [PubMed] [Google Scholar]
- 8.MacArthur RD, Novak RM, Peng G, et al. A comparison of three highly active antiretroviral treatment strategies consisting of non-nucleoside reverse transcriptase inhibitors, protease inhibitors, or both in the presence of nucleoside reverse transcriptase inhibitors as initial therapy (CPCRA 058 FIRST Study): a long-term randomised trial. Lancet. 2006;368:2125–35. doi: 10.1016/S0140-6736(06)69861-9. [DOI] [PubMed] [Google Scholar]
- 9.Lundgren JD, Mocroft A. The impact of antiretroviral therapy on AIDS and survival. J HIV Ther. 2006;11:36–8. [PubMed] [Google Scholar]
- 10.Mocroft A, Kirk O, Barton SE, et al. Anaemia is an independent predictive marker for clinical prognosis in HIV-infected patients from across Europe. AIDS. 1999;13:943–50. doi: 10.1097/00002030-199905280-00010. [DOI] [PubMed] [Google Scholar]
- 11.Mocroft A, Gatell J, Reiss P, et al. Causes of death in HIV infection: the key determinant to define the clinical response to anti-HIV therapy. AIDS. 2004;18:2333–7. doi: 10.1097/00002030-200411190-00018. [DOI] [PubMed] [Google Scholar]
- 12.Lewden C, Raffi F, Cuzin L, et al. Factors associated with mortality in human immunodeficiency virus type 1-infected adults initiating protease inhibitor-containing therapy: role of education level and of early transaminase level elevation (APROCO-ANRS EP11 study) J Infect Dis. 2002;186:710–4. doi: 10.1086/342047. [DOI] [PubMed] [Google Scholar]
- 13.MacArthur RD, Perez G, Walmsley S, Baxter JD, Mullin CM, Neaton JD. Comparison of prognostic importance of latest CD4+ cell count and HIV RNA levels in patients with advanced HIV infection on highly active antiretroviral therapy. HIV Clin Trials. 2005;6:127–35. doi: 10.1310/A9B9-RQD7-U8KA-503U. [DOI] [PubMed] [Google Scholar]
- 14.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandler NG, Wand H, Roque A, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780–90. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodger AJ, Fox Z, Lundgren JD, et al. Activation and coagulation biomarkers are independent predictors of the development of opportunistic disease in patients with HIV infection. J Infect Dis. 2009;200:973–83. doi: 10.1086/605447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porter BO, Ouedraogo GL, Hodge JN, et al. d-Dimer and CRP levels are elevated prior to antiretroviral treatment in patients who develop IRIS. Clin Immunol. 2010;136:42–50. doi: 10.1016/j.clim.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.French MA. HIV/AIDS: immune reconstitution inflammatory syndrome: a reappraisal. Clin Infect Dis. 2009;48:101–7. doi: 10.1086/595006. [DOI] [PubMed] [Google Scholar]
- 19.Meintjes G, Lawn SD, Scano F, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis. 2008;8:516–23. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haddow LJ, Colebunders R, Meintjes G, et al. Cryptococcal immune reconstitution inflammatory syndrome in HIV-1-infected individuals: proposed clinical case definitions. Lancet Infect Dis. 2010;10:791–802. doi: 10.1016/S1473-3099(10)70170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.French MA, Price P, Stone SF. Immune restoration disease after antiretroviral therapy. AIDS. 2004;18:1615–27. doi: 10.1097/01.aids.0000131375.21070.06. [DOI] [PubMed] [Google Scholar]
- 22.Trocme C, Leroy V, Sturm N, et al. Longitudinal evaluation of a fibrosis index combining MMP-1 and PIIINP compared with MMP-9, TIMP-1 and hyaluronic acid in patients with chronic hepatitis C treated by interferon-alpha and ribavirin. J Viral Hepat. 2006;13:643–51. doi: 10.1111/j.1365-2893.2006.00730.x. [DOI] [PubMed] [Google Scholar]
- 23.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–3. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 24.Yun Z, Lewensohn-Fuchs I, Ljungman P, Ringholm L, Jonsson J, Albert J. A real-time TaqMan PCR for routine quantitation of cytomegalovirus DNA in crude leukocyte lysates from stem cell transplant patients. J Virol Methods. 2003;110:73–9. doi: 10.1016/s0166-0934(03)00103-4. [DOI] [PubMed] [Google Scholar]
- 25.Enocsson H, Sjowall C, Skogh T, Eloranta ML, Ronnblom L, Wettero J. Interferon-alpha mediates suppression of C-reactive protein: explanation for muted C-reactive protein response in lupus flares? Arthritis Rheum. 2009;60:3755–60. doi: 10.1002/art.25042. [DOI] [PubMed] [Google Scholar]
- 26.Kalayjian RC, Machekano RN, Rizk N, et al. Pretreatment levels of soluble cellular receptors and interleukin-6 are associated with HIV disease progression in subjects treated with highly active antiretroviral therapy. J Infect Dis. 2010;201:1796–805. doi: 10.1086/652750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Estes J, Baker JV, Brenchley JM, et al. Collagen deposition limits immune reconstitution in the gut. J Infect Dis. 2008;198:456–64. doi: 10.1086/590112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nunes D, Fleming C, Offner G, et al. Noninvasive markers of liver fibrosis are highly predictive of liver-related death in a cohort of HCV-infected individuals with and without HIV infection. Am J Gastroenterol. 2010;105:1346–53. doi: 10.1038/ajg.2009.746. [DOI] [PubMed] [Google Scholar]
- 29.Mohamadzadeh M, DeGrendele H, Arizpe H, Estess P, Siegelman M. Proinflammatory stimuli regulate endothelial hyaluronan expression and CD44/HA-dependent primary adhesion. J Clin Invest. 1998;101:97–108. doi: 10.1172/JCI1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sampson PM, Rochester CL, Freundlich B, Elias JA. Cytokine regulation of human lung fibroblast hyaluronan (hyaluronic acid) production: evidence for cytokine-regulated hyaluronan (hyaluronic acid) degradation and human lung fibroblast-derived hyaluronidase. J Clin Invest. 1992;90:1492–503. doi: 10.1172/JCI116017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bourgarit A, Carcelain G, Martinez V, et al. Explosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. AIDS. 2006;20:F1–7. doi: 10.1097/01.aids.0000202648.18526.bf. [DOI] [PubMed] [Google Scholar]
- 32.Elliott JH, Vohith K, Saramony S, et al. Immunopathogenesis and diagnosis of tuberculosis and tuberculosis-associated immune reconstitution inflammatory syndrome during early antiretroviral therapy. J Infect Dis. 2009;200:1736–45. doi: 10.1086/644784. [DOI] [PubMed] [Google Scholar]
- 33.Murray MJ, Murray AB, Murray NJ, Murray MB. Infections during severe primary undernutrition and subsequent refeeding: paradoxical findings. Aust N Z J Med. 1995;25:507–11. doi: 10.1111/j.1445-5994.1995.tb01496.x. [DOI] [PubMed] [Google Scholar]
- 34.Boulware DR, Meya DB, Bergemann TL, et al. Clinical features and serum biomarkers in HIV immune reconstitution inflammatory syndrome after cryptococcal meningitis: a prospective cohort study. PLoS Med. 2010;7:e1000384. doi: 10.1371/journal.pmed.1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crane M, Oliver B, Matthews G, et al. Immunopathogenesis of hepatic flare in HIV/hepatitis B virus (HBV)-coinfected individuals after the initiation of HBV-active antiretroviral therapy. J Infect Dis. 2009;199:974–81. doi: 10.1086/597276. [DOI] [PubMed] [Google Scholar]
- 36.Oliver BG, Elliott JH, Price P, et al. Mediators of innate and adaptive immune responses differentially affect immune restoration disease associated with Mycobacterium tuberculosis in HIV patients beginning antiretroviral therapy. J Infect Dis. 2010;202:1728–37. doi: 10.1086/657082. [DOI] [PubMed] [Google Scholar]
- 37.Benito N, Moreno A, Filella X, et al. Inflammatory responses in blood samples of human immunodeficiency virus-infected patients with pulmonary infections. Clin Diagn Lab Immunol. 2004;11:608–14. doi: 10.1128/CDLI.11.3.608-614.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.