Abstract
(See the editorial commentary by Schleiss, on pages 1513–6.)
Traditionally, vaccines have been utilized to generate immune responses to a pathogen in a naive population. In the setting of congenital cytomegalovirus (CMV) infection, a vaccine that, when administered to women already infected with CMV, could boost the mother's immunity to CMV would most likely be beneficial in diminishing in utero transmission of CMV. However, the ability to boost an immune response in a population of individuals seropositive for a pathogen of interest is not well studied. This study examines the ability of a recombinant CMV glycoprotein B vaccine with MF59 adjuvant to boost both antibody (neutralizing and enzyme-linked immunosorbent assay end point dilution titer) and CD4+ T-cell responses in previously CMV-seropositive women by way of natural infection. These data suggest that this vaccine is capable of boosting immunity in a population of CMV-infected women and warrants additional evaluation to determine whether these boosted responses may prevent mother to child transmission of CMV.
Congenital cytomegalovirus (CMV) infections are a major public health problem in the United States. Preexisting immunity against CMV in the mother before conception has been shown to provide substantial protection against congenital CMV infection in the newborn [1]. However, women who are seropositive for CMV whose CMV infection is reactivated [2] or who are reinfected with a different strain of CMV can sometimes transmit the virus during pregnancy, resulting in symptomatic congenital infection [3]. The ability of the immune system to mount an effective and protective secondary response that will survive long term after an encounter with a pathogen is the cornerstone of immunological memory and the basis for the development of vaccines [4]. Thus, the availability of a CMV vaccine capable of boosting immunity in a previously immune population of individuals may aid in the prevention of mother-to-child transmission of CMV.
Although there are scant data in vaccination regimens for immune populations, CD4+ T-cell–mediated immunity has been implicated in the prevention of herpes zoster, and the boosting of varicella zoster virus–specific immunity was demonstrated with the recently developed zoster vaccine [5]. A study attempting to understand the correlates of immune protection during the primary immune response to CMV determined that the formation of effector memory CD4+ T cells was necessary for recovery of infection [6]. Recently, a CMV glycoprotein B (gB) vaccine with MF59 administered to CMV-seronegative women was shown to prevent infection in women of childbearing age [7]. In these studies, we set out to analyze both the antibody and the CD4+ T-cell response after gB/MF59 vaccination in women with preexisting immunity to CMV.
MATERIALS AND METHODS
Study Population
The study enrolled women 14–40 years of age (median age for both vaccine and placebo groups, 26 years) who screened seropositive for CMV, using a commercial CMV immunoglobulin (Ig) G assay (Axsym CMV IgG; Abbott) as previously described [1]. A total of 150 women were enrolled in the study (120 received the vaccine and 30 received placebo). The 4:1 vaccine: placebo ratio allowed for additional power to detect safety, as is standard for phase I studies. To perform the CD4+ T-cell studies, the first 40 women were enrolled in this substudy; 32 women were vaccinated intramuscularly (IM), and 8 received placebo. In both the vaccine and placebo groups, 75% of the women enrolled were African American, and the remaining women were Caucasian. Informed consent was obtained from all subjects under the guidelines of the US Department of Health and Human services and the Institutional Review Board of the University of Alabama at Birmingham (UAB).
Vaccination and Blood Specimen Collection
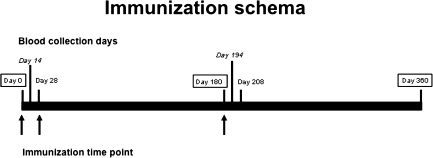
The CMV vaccine (gB/MF59) [7] was composed of 20 μg of gB and MF59 (squalene, sorbitan trioleate, and polysorbate 80 with citrate buffer) in 0.5 mL of buffered saline. The placebo was saline. Vaccinations were administered IM on day 0, at 1 month, and at 6 months. Blood specimens were collected at day 0 (prevaccination), day 14 (2 weeks after the first vaccination), day 180, day 194 (2 weeks after the third vaccination) and day 360 for T-cell assays. Serum specimens were collected at day 0 (prevaccination), day 28 (4 weeks after first vaccination), day 180 (prior to third vaccination), day 208 (4 weeks after third vaccination), and day 360 for antibody measurements (Figure 1). Peripheral blood mononuclear cells (PBMCs) were isolated by standard Histopaque (Sigma-Aldrich) density centrifugation and were cryopreserved as previously described [8]. The data analysis was done in a blinded fashion, with the code revealed only after the assays were completed.
Figure 1.
Immunization schedule. Arrows indicate day of vaccination (day 0, day 28, and day 180). Boxed dates are time points for blood collection days for both antibody titers and CD4+ T-cell assays. Bolded dates are time points for blood collection days for antibody titer measurements, and dates in italics are for CD4+ T-cell assays.
Antigens
Various antigens were used to stimulate T-cell responses, including the following: gB recombinant protein used as the vaccine antigen, 10 μg/mL, provided by Sanofi Pasteur for laboratory use; pp65 peptide pool (69, 18-mers, overlapping by 10 spanning the entire amino acid sequence of the CMV pp65 protein; New England Peptide) used as a CMV antigen not contained in the vaccine (2 μg/mL); and staphylococcal enterotoxin B (SEB; Sigma-Aldrich) at 1 μg/mL as a positive control.
Enzyme-Linked Immunosorbent Assay (ELISA) End Point Dilution Titers
Serological assays for quantitation of human IgG to CMV gB were performed at a contract laboratory under the management of Sanofi Pasteur according to the following ELISA procedure: 96-well microtiter plates (Corning) were coated with gB (Sanofi Pasteur) in bicarbonate buffer and incubated at 4°C overnight. The plates were then blocked with Invitrogen for 90 min at room temperature. Test samples were diluted in BSA buffer at a starting dilution of either 1:50 or 1:10. After serial dilutions were prepared, plates were covered and incubated for 45 min in a humidified incubator at 37°C. Anti-human IgG conjugate (KPL) was diluted to 1:50,000 and added to the wells. The plates were covered and incubated for 30 min in a humidified incubator at 37°C. Tetramethylbenzidine substrate (KPL) was added to the wells, and the plates were incubated at room temperature for 30 min. The color reaction was stopped using 1M phosphoric acid (Sigma). Absorbances were read and data were analyzed to calculate titers using Molecular Devices SoftMax software. Data presented in this manuscript are for the 40 women enrolled in the CD4+ T-cell substudy.
Neutralizing Antibody Titers
Quantitation of CMV-specific neutralizing antibodies was performed at Sanofi Pasteur's Global Clinical Immunology laboratory (Swiftwater, PA) according to the following procedure and based upon a method by Gonczol et al [9]. Serum sample or control was added to the culture medium in wells of a 96-well tissue culture plate (Nalge Nunc), and serial 2-fold dilutions were made. This was followed by addition of a CMV viral suspension and guinea pig complement (Rockland Immunochemicals for Research). After incubation for 1–1.5 h at 37°C in a CO2 incubator, MRC-5 human embryo lung fibroblasts (Coriell) were added, and the plates were incubated for 44–52 h at 37°C in a CO2 incubator. The end point titer was determined as the reciprocal of the highest serum dilution where no cytopathic effect was observed. Data presented in this manuscript for the neutralizing antibody are for the 40 women enrolled in the CD4+ T-cell substudy.
5,6-Carboxyflourescein Diacetate Succinimidyl Ester (CFSE) Proliferation
PBMCs were resuspended in phosphate-buffered saline (PBS; 5 × 106–107 cells/mL) and labeled with 1.25 μM CFSE (Molecular Probes) for 4 min at room temperature with constant gentle agitation. Cells were washed twice in PBS containing 10% fetal bovine serum. Cells were then resuspended in complete media (Roswell Park Memorial Institute medium [RPMI] plus 10% AB sera, 50 U/mL of penicillin-streptomycin, 2 mM L-glutamine, and 25 mM HEPES). Proliferation was monitored following stimulation with appropriate antigens for 5 days. Cells stimulated with SEB were used as the positive control, and unstimulated cells served as the negative control. Fluorochrome labeled anti-CD3-APC, anti-CD4-PE and anti-CD8-PerCP antibodies (BD Biosciences) were added to surface stain the cells for 20 min in the dark at room temperature. The cells were subsequently analyzed on an LSR II flow cytometer, and at least 100,000 CD3+ events were collected. Proliferation was measured off the CD3+CD4+ gate, and the media control values were used to set these gates, which were then applied to all samples from the same individual for each time point.
Intracellular Cytokine Staining Assay
Intracellular cytokine staining was performed as previously described [10]. Frozen PBMCs were washed once in RPMI containing 10% human AB sera by centrifugation at 250 × g for 10 min at room temperature. Costimulatory monoclonal antibodies (anti-CD28 and anti-CD49d; each at 1μg/mL) were added to each tube along with 50 U/mL of Benzonase (Novagen). For coculture, CD154–APC (BD Biosciences) was added to the cells at this step. Cells were pulsed with the appropriate antigen for 1 h before 10 μg/mL Monensin (Golgistop; BD Biosciences) was added, followed by a 5-h incubation at 37°C, 5% CO2. Cells were then labeled with a fluorescent LIVE/DEAD fixable Dead cell Stain (Molecular Probes). The surface phenotype of PBMC samples was determined by staining with anti-CD3-Pacific Blue, anti-CD4-PercpCy5.5 (BD Biosciences), and anti-CD127-PE antibodies (Beckman Coulter) for 20 min at room temperature. Cells were washed in PBS, followed by permeabilization with the Cytofix/cytoperm reagent (BD Biosciences) for 20 min at room temperature in the dark. Intracellular cytokine staining followed using anti–interleukin (IL)-2-FITC, anti–tumor necrosis factor (TNF) α Pecy7, and anti–interferon (IFN) γ Alexa700 conjugated antibodies (BD Biosciences). At least 100,000 CD3+ events were acquired from each sample using an LSR II flow cytometer (BD Biosciences). Data was analyzed using FlowJo software, version 8.5 (TreeStar). Lymphocytes were analyzed on the basis of forward- and side-scatter profiles after the exclusion of dead cells. Cytokines produced were measured off the CD3+CD4+ gates relative to the media control values, and these gates were applied to all samples from the same individual for each time point. CD3+CD4+ cells were also gated to determine the expression of CD127 on cytokine-positive (IFN-γ, IL-2, or TNF-α) antigen-specific T cells. Responders for the % CD27hi analysis included all individuals who had a CD4+IFN-γ+ response. This was determined by having a response at least 2× the unstimulated cells and 2× the response at day 0 for each individual.
Statistics
Statistical analyses were performed using the nonparametric Mann-Whitney U test for all comparisons except for the CD127 analysis using only responder individuals, for which the Wilcoxon signed-rank test was used. Analyses were done with Graphpad Prism software, version 4.0 for Mac. Differences were considered to be significant on the basis of 95% confidence intervals (CIs). For the antibody response, on both ELISA end point dilution titers and neutralizing antibody titers, geometric means and their 95% CIs were computed at every time point.
RESULTS
CMV-Specific Antibody Responses Were Boosted After gB Vaccination
ELISA end point dilution and neutralizing antibody titers were measured to determine whether this vaccine regimen was capable of boosting gB-specific responses previously generated by natural infection. Both of these types of antibody titers were significantly boosted when compared with the prevaccination time point (day 0) at every time point analyzed (Table 1). Importantly, the antibody titers at 6 months after the final vaccine dose (day 360) remained higher than the antibody titers at day 0. However, neither the binding nor neutralizing antibodies were significantly boosted beyond the first vaccination (day 28). In placebo recipients, no change was observed in ELISA end point dilution and neutralizing antibody titers during the study follow-up. In addition, gB-specific ELISA endpoint titer antibodies in vaccine recipients were increased relative to placebo on day 28 and day 208, time points corresponding to 4 weeks after vaccination (Table 1). Both gB ELISA and neutralizing antibody titers were significantly different between placebo recipients and vaccine recipients at all time points except day 180 for neutralizing titers when data was analyzed for the entire study (n = 150 women; data not shown).
Table 1.
Anti-glycoprotien B (gB) Enzyme-Linked Immunosorbent Assay (ELISA) End Point Dilution Titer and Anti-cytomegalovirus (CMV) Neutralizing Antibodies in Vaccinees and Placebos
| Vaccinated individuals |
Placebo recipients |
|||||||||
| gB ELISA antibody |
CMV neutralizing antibody |
gB ELISA antibody |
CMV neutralizing antibody |
|||||||
| Visit | N | GM | 95% CI | GM | 95% CI | N | GM | 95% CI | GM | 95% CI |
| Day 0 | 32 | 2521 | 1953–3253 | 38 | 27–55 | 8 | 2151 | 1026–4506 | 31 | 12–83 |
| Day 28 | 30 | 12497 | 9402–16611 | 146 | 119–180 | 7 | 2184 | 915–5214 | 39 | 11–138 |
| Day 180 | 25 | 8024 | 6200–10385 | 111 | 88–139 | 6 | 2363 | 747–7471 | 40 | 8–207 |
| Day 208 | 22 | 10375 | 7445–14459 | 134 | 109–163 | 6 | 2247 | 691–7308 | 38 | 8–190 |
| Day 360 | 22 | 7203 | 5385–9636 | 90 | 70–115 | 6 | 2069 | 709–6040 | 33 | 7–144 |
NOTE. CI, confidence interval; GM, geometric mean titer; N, number of vaccinated individuals with a sample available for measurement of antibody titers.
CD4+ Cell Proliferative Responses Were Generated to gB Protein
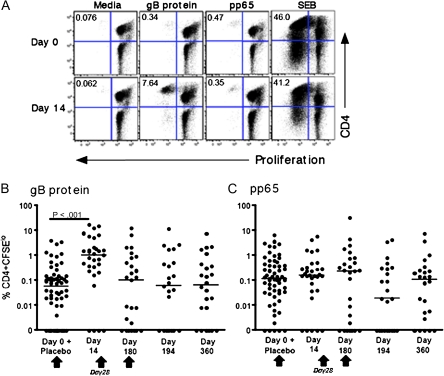
In vitro proliferation in response to various antigens was measured in CFSE-labeled PBMCs from CMV-seropositive women who were vaccinated with the gB/MF59 vaccine to determine whether this vaccine was capable of boosting a T-cell response that was previously generated by natural infection. A representative flow plot is shown in Figure 2A for CD4+ T-cell responses at day 0 (prevaccination) and day 14 (2 weeks after first vaccination with gB/MF59) to gB protein (vaccine antigen) and pp65 (CMV antigen not contained in the vaccine). SEB was used as a positive control. The CD4+ T-cell response to gB was boosted 2 weeks after first vaccination. In addition, detectable pp65 responses were present at both time points, and these were not affected by vaccination. Proliferative responses to gB protein and pp65 for the entire cohort mirrored this representative reactivity (Figures 2B and 2C). Day 0 and placebo group data were combined and used as the negative control (ie, individuals not vaccinated). Statistically significant differences were noted for responses to gB protein between individuals who were not vaccinated (individuals at day 0 and placebo recipients) and day 14 for the vaccinees. No other statistically significant differences were found for the other time points (Figure 2B). These differences remained even if the placebo recipients were withdrawn from the analysis (data not shown). Moreover, no statistically significant differences were noted for pp65 responses (antigen not included in the vaccine) between vaccinees and individuals before vaccination or individuals who were not vaccinated at all (individuals at day 0 and placebo recipients) (Figure 2C).
Figure 2.
CD4+ T cells proliferate in response to glycoprotein B (gB) protein. Peripheral blood mononuclear cells (PBMCs) were stimulated in vitro with gB protein, a pool of peptides from cytomegalovirus pp65, or staphylococcal enterotoxin B (SEB). A, The frequency of proliferating CD3+CD4+ T cells was determined by labeling PBMCs with 5, 6-carboxyflourescein diacetate succinimidyl ester (CFSE) at prevaccination (day 0) and 2 weeks after vaccination (day 14) and is shown for a selected individual. Percentage of CFSElo cells is indicated in the upper left hand quadrant. CD4+ T cell proliferative responses are shown in response to gB protein (B) or pP65 protein (C) at the indicated time points. Data for all vaccine-naive subjects (placebo recipients and individuals at the prevaccination time point of day 0) are combined. Net responses (ie, with unstimulated responses subtracted) are plotted. The median response is shown by the horizontal line. Vaccination time points are indicated by an arrow.
CD4+ gB-specific T cells From Healthy CMV-Seropositive Women Were Detected After Vaccination
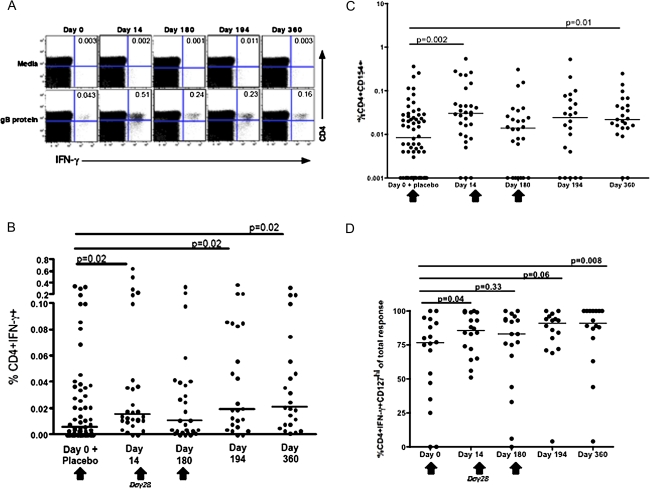
To further characterize the CD4+ T-cell response to this vaccine, intracellular cytokine staining was performed. A representative dot plot for gB-specific IFN-γ secretion at selected time points is shown (Figure 3A). A compilation of the CD4+IFN-γ+ responses for the entire cohort revealed significant boosting after vaccination, compared with the prevaccination and vaccine-naive time point (individuals at day 0 and placebo recipients) for all time points except day 180 (Figure 3B). However, responses peaked at day 14 and did not increase with subsequent vaccinations. Again, these same differences remained even if the placebo recipients were withdrawn from the analysis (data not shown). There were no statistically significant differences among the responses to pp65 at day 0 and all other time points (data not shown). In addition, no differences were noted for gB-specific responses as measured by the secretion of IL-2 and TNF-α (data not shown).
Figure 3.
Induction of glycoprotein B (gB)–specific CD4+ T cells measured by intracellular cytokine staining. A, interferon (IFN) γ secretion from CD4+ T cells from a vaccinated volunteer at day 0 (prevaccination), day 14 (2 weeks after the first vaccination), day 180 (5 months after the second vaccination), day 194 (2 weeks after the third vaccination), and day 360 (6 months after the third vaccination). Peripheral blood mononuclear cells (PBMCs) were stimulated in vitro with gB protein, and the frequency of IFN-γ producing CD3+CD4+ T cells was determined by intracellular cytokine staining assay. Percentage of CD4+ IFN-γ+ cells is indicated in the upper right hand quadrant. B, The generation of gB-specific CD4+ T cells from all vaccinees at the indicated time points is shown. Net responses (ie, with unstimulated responses subtracted) are plotted. C, Upregulation of CD154 on PBMCs from vaccinees in response to gB stimulation after vaccination. D, The proportion of gB-specific CD4+ T cells that are IFN-γ+CD127hi is shown. Only responder individuals, as defined by a positive response to gB by IFN-γ secretion, are shown. Net responses (ie, with unstimulated responses subtracted) are plotted. The median responses are shown by horizontal line. Vaccination time points are indicated by an arrow.
Cells expressing CD154 (CD40L) represent a subset of CD4+ T cells that have recently been activated. To corroborate our intracellular cytokine staining studies, using a different marker for measuring vaccine-specific responses, we chose to measure the expression of CD154 after vaccination [11, 12], when cells were stimulated in vitro with gB (vaccine antigen). An increase in gB-specific CD154+CD4+ T cells was observed at day 14 (P = .002) and day 360 (P = .01) after vaccination (Figure 3C). Just as with IFN-γ secretion, a significant increase in gB-specific cells (CD154+) was seen at day 14 without subsequent further expression (Figure 3C), and these same differences remained even if the placebo recipients were withdrawn from the analysis (data not shown). In addition, no statistically significant differences were observed between day 0 and all other time points to responses measured against pp65 (antigen not contained in the vaccine; data not shown).
To determine whether the populations of gB-specific cells induced by this vaccine are long-lived memory cells, we analyzed the expression of CD127hi on CD3+CD4+IFNγ+ T cells of responder individuals, as determined by IFN-γ secretion [13, 14]. CD4+IFNγ+CD127hi vaccine-specific responses (gB-specific responses) that were significantly different from the prevaccination time point were detected at all time points after vaccination except day 180 and day 194 (Figure 3D). Both CD127lo and CD127hi (long-lived memory cells) CD4+ T cells secrete IFN-γ in response to gB. However, the proportion of CD127hi cells (compared with CD127lo) in CD4+IFNγ+ cells increased relative to the prevaccination time point.
DISCUSSION
These studies demonstrate that both CMV-specific antibody and CD4+ T-cell responses can be boosted after vaccination with a CMV gB/MF59 vaccine in women who had chronic CMV infection. Although gB-specific T-cell proliferative responses were only boosted after the first vaccination, IFN-γ producing T cells with the same specificity were higher at most time points, relative to prevaccination, including at 6 months after the final vaccination. Importantly, the CD4+ T cell CMV-specific responses, detected by several different measurements, were clearly vaccine induced and were not attributable to prior infection, because responses to pp65, which is a CMV protein not present in the vaccine, were not boosted. ELISA end point dilution titer and neutralizing antibody responses were uniformly elevated after vaccination at all time points. When antibody responses were compared between vaccinees and placebo recipients at the various time points (Table 1), differences were only noted for gB ELISA antibodies at day 28 and day 208, suggesting that preexisting CMV responses that were attributable to natural infection required additional boosting. Antibody data for the entire cohort of 150 individuals did show differences at all time points for both gB ELISA and neutralizing antibody titers, except for neutralizing antibody at day 180, which indicated that increasing the number of responses measured resulted in statistically significant differences.
In addition to the production of IFN-γ, we also noted that vaccination with this gB/MF59 vaccine was able to increase the expression of the CD40 ligand (CD40L or CD154). CD154, the ligand for CD40, is expressed on CD4+ T cells that have been recently activated [11, 12]. In addition, the expression of CD40L by CD4+ T cells is necessary for the initiation of T cell–dependent antibody responses [15]. Nevertheless, no correlation was observed between any of the measured CD4+ T-cell responses (including CD40L) and gB-specific antibodies (binding or neutralizing) (data not shown). The lack of correlation may be explained by the fact that the CD4+ T-cell response to CMV is dominated by a Th1 response, exemplified by the secretion of IFN-γ [16–18]. This response has been mainly attributed to help for CD8+ T-cell responses, and it thus would not necessarily correlate with antibody responses. Had we measured IL-4 or IL-5, which are Th2 cytokines that support antibody responses, correlations might have been noted. In addition, 2 studies involving humans that studied CD4+ T cell and antibody responses to different human immunodeficiency virus vaccines also did not find a correlation between CD4+ T-cell responses and antibody titers in the individuals studied ([19, 20]).
Because the induction of T-cell memory is an important aspect of a protective vaccine, we measured the CD127 (the IL-7 receptor or IL-7R) expression on T cells. This T-cell surface receptor allows these cells to respond to IL-7, which is important for the survival and homeostatic proliferation of memory T cells [14, 21]. Studies performed on CD4+ T cells of various specificities in both mice and humans have demonstrated that CD127 also plays a critical role in the propagation of long-lived memory CD4+ T cells [13,22–26]. In studies involving CMV-infected individuals that analyzed central memory T cells, it was demonstrated that these CMV-specific cells were associated with an increase in the production of IFN-γ relative to responses generated to cleared antigens, such as tetanus toxoid and hepatitis B surface antigen [27, 28]. In addition, central memory CMV-specific CD4+ T cells expressed higher levels of CD127 than did CMV-specific effector T cells, which suggested that the former could survive long term under the influence of IL-7 [28]. The CD127 data presented here suggests that gB/MF59 vaccination increases the CD4+ T cell gB memory pool, and this pool is maintained for at least 1 year after the first vaccination is administered. Although gB-specific CD4+ T-cell responses are maintained for up to 1 year after all 3 vaccinations are administered, the response measured at day 180 (5 months after the second vaccination) was not different than that measured before vaccination (day 0). This finding suggests that, to prevent early waning of responses, at least 3 vaccinations are necessary. A potential caveat is the fact that gB/MF59 responses were not compared with responses to MF59 alone; however, this adjuvant has been extensively tested and is currently licensed, and these issues have already been resolved [29].
A recent preclinical study demonstrated that reinfection of Rhesus macaques with CMV readily occurs and is dependent on the viral machinery that down modulates the host major histocompatibility type I proteins [30]. Presumably such an effect weakens the ability of the CD8+ T cells to recognize CMV-infected cells and combat infection. Previous results [7] in CMV-seronegative women using this same vaccine have demonstrated that this vaccine could protect these women from CMV infection and that it induces gB-specific boostable antibodies, which suggests that these vaccine-induced responses may not be subject to the same limitations as those seen for CD8+ T cells. Unfortunately, a direct association between antibody titers and protection in humans has not been established. However, studies of congenital CMV infection in guinea pigs vaccinated with a recombinant gB vaccine from CMV demonstrated that vaccination resulted in a reduction in pup mortality. Pup mortality was significantly decreased in the group of animals who were born to mothers vaccinated with gB with Freud's adjuvant, and these animals also had significantly higher CMV-specific neutralizing antibody titers [31, 32].
Together with the data presented here from the substudy, which show the induction of CD4+ T-cell responses and boosting of gB-specific antibodies, these findings suggest that this vaccine is generating appropriate CD4+ T-cell responses that are likely helping to increase the baseline gB-specific antibody level. Based on these data, additional evaluations of this CMV vaccine in women with prior CMV infection should be considered.
Funding
Sanofi Pasteur (to S. S., R. F. P., and P. A. G.) and Public Health Service grant 5UL1 RR025777 from the National Institutes of Health National Center for Research Resources (to R. P.).
Acknowledgments
We thank the volunteers for their participation; Doug Ritter, for performing some of the CFSE assays; Michelle Bradley, for processing PBMC samples; Marion Spell, for help with the flow cytometric acquisition; and Dick Ward and Sanofi Pasteur Global Clinical Immunology Laboratory (Swiftwater, PA), for the antibody studies performed in this cohort.
References
- 1.Fowler KB, Stagno S, Pass RF. Maternal immunity and prevention of congenital cytomegalovirus infection. JAMA. 2003;289:1008–11. doi: 10.1001/jama.289.8.1008. [DOI] [PubMed] [Google Scholar]
- 2.Huang ES, Alford CA, Reynolds DW, Stagno S, Pass RF. Molecular epidemiology of cytomegalovirus infections in women and their infants. N Engl J Med. 1980;303:958–62. doi: 10.1056/NEJM198010233031702. [DOI] [PubMed] [Google Scholar]
- 3.Boppana SB, Rivera LB, Fowler KB, Mach M, Britt WJ. Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity. N Engl J Med. 2001;344:1366–71. doi: 10.1056/NEJM200105033441804. [DOI] [PubMed] [Google Scholar]
- 4.Welsh RM, Selin LK, Szomolanyi-Tsuda E. Immunological memory to viral infections. Annu Rev Immunol. 2004;22:711–43. doi: 10.1146/annurev.immunol.22.012703.104527. [DOI] [PubMed] [Google Scholar]
- 5.Levin MJ, Oxman MN, Zhang JH, et al. Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J Infect Dis. 2008;197:825–35. doi: 10.1086/528696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gamadia LE, Remmerswaal EB, Weel JF, Bemelman F, van Lier RA, Ten Berge IJ. Primary immune responses to human CMV: a critical role for IFN-gamma-producing CD4+ T cells in protection against CMV disease. Blood. 2003;101:2686–92. doi: 10.1182/blood-2002-08-2502. [DOI] [PubMed] [Google Scholar]
- 7.Pass RF, Zhang C, Evans A, et al. Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med. 2009;360:1191–9. doi: 10.1056/NEJMoa0804749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards BH, Bansal A, Sabbaj S, Bakari J, Mulligan MJ, Goepfert PA. Magnitude of functional CD8+ T-cell responses to the gag protein of human immunodeficiency virus type 1 correlates inversely with viral load in plasma. J Virol. 2002;76:2298–305. doi: 10.1128/jvi.76.5.2298-2305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonczol E, Furlini G, Ianacone J, Plotkin SA. A rapid microneutralization assay for cytomegalovirus. J Virol Methods. 1986;14:37–41. doi: 10.1016/0166-0934(86)90005-4. [DOI] [PubMed] [Google Scholar]
- 10.Sabbaj S, Heath SL, Bansal A, et al. Functionally competent antigen-specific CD127(hi) memory CD8+ T cells are preserved only in HIV-infected individuals receiving early treatment. J Infect Dis. 2007;195:108–17. doi: 10.1086/509510. [DOI] [PubMed] [Google Scholar]
- 11.Chattopadhyay PK, Yu J, Roederer M. A live-cell assay to detect antigen-specific CD4+ T cells with diverse cytokine profiles. Nat Med. 2005;11:1113–7. doi: 10.1038/nm1293. [DOI] [PubMed] [Google Scholar]
- 12.Frentsch M, Arbach O, Kirchhoff D, et al. Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat Med. 2005;11:1118–24. doi: 10.1038/nm1292. [DOI] [PubMed] [Google Scholar]
- 13.Kondrack RM, Harbertson J, Tan JT, McBreen ME, Surh CD, Bradley LM. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med. 2003;198:1797–806. doi: 10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–32. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 15.Blanchard D, Gaillard C, Hermann P, Banchereau J. Role of CD40 antigen and interleukin-2 in T cell-dependent human B lymphocyte growth. Eur J Immunol. 1994;24:330–5. doi: 10.1002/eji.1830240209. [DOI] [PubMed] [Google Scholar]
- 16.Casazza JP, Betts MR, Price DA, et al. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J Exp Med. 2006;203:2865–77. doi: 10.1084/jem.20052246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kannanganat S, Ibegbu C, Chennareddi L, Robinson HL, Amara RR. Multiple-cytokine-producing antiviral CD4 T cells are functionally superior to single-cytokine-producing cells. J Virol. 2007;81:8468–76. doi: 10.1128/JVI.00228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rentenaar RJ, Gamadia LE, van DerHoek N, et al. Development of virus-specific CD4(+) T cells during primary cytomegalovirus infection. J Clin Invest. 2000;105:541–8. doi: 10.1172/JCI8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bansal A, Jackson B, West K, et al. Multifunctional T-cell characteristics induced by a polyvalent DNA prime/protein boost human immunodeficiency virus type 1 vaccine regimen given to healthy adults are dependent on the route and dose of administration. J Virol. 2008;82:6458–69. doi: 10.1128/JVI.00068-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. doi: 10.1093/infdis/jiq105. Goepfert PA, Elizaga ML, Sato A, et al. Phase 1 safety and immunogenicity testing of DNA and recombinant modified vaccinia Ankara vaccines expressing HIV-1 virus-like particles. J Infect Dis 2011; 203:610–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Becker TC, Wherry EJ, Boone D, et al. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J Exp Med. 2002;195:1541–8. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cellerai C, Harari A, Vallelian F, Boyman O, Pantaleo G. Functional and phenotypic characterization of tetanus toxoid-specific human CD4+ T cells following re-immunization. Eur J Immunol. 2007;37:1129–38. doi: 10.1002/eji.200636885. [DOI] [PubMed] [Google Scholar]
- 23.Lenz DC, Kurz SK, Lemmens E, et al. IL-7 regulates basal homeostatic proliferation of antiviral CD4+T cell memory. Proc Natl Acad Sci U S A. 2004;101:9357–62. doi: 10.1073/pnas.0400640101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Huston G, Swain SL. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J Exp Med. 2003;198:1807–15. doi: 10.1084/jem.20030725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riou C, Yassine-Diab B, Van grevenynghe J, et al. Convergence of TCR and cytokine signaling leads to FOXO3a phosphorylation and drives the survival of CD4+ central memory T cells. J Exp Med. 2007;204:79–91. doi: 10.1084/jem.20061681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seddon B, Tomlinson P, Zamoyska R. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat Immunol. 2003;4:680–6. doi: 10.1038/ni946. [DOI] [PubMed] [Google Scholar]
- 27.Harari A, Vallelian F, Pantaleo G. Phenotypic heterogeneity of antigen-specific CD4 T cells under different conditions of antigen persistence and antigen load. Eur J Immunol. 2004;34:3525–33. doi: 10.1002/eji.200425324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stubbe M, Vanderheyde N, Pircher H, Goldman M, Marchant A. Characterization of a subset of antigen-specific human central memory CD4+ T lymphocytes producing effector cytokines. Eur J Immunol. 2008;38:273–82. doi: 10.1002/eji.200737611. [DOI] [PubMed] [Google Scholar]
- 29.Schultze V, D'Agosto V, Wack A, Novicki D, Zorn J, Hennig R. Safety of MF59 adjuvant. Vaccine. 2008;26:3209–22. doi: 10.1016/j.vaccine.2008.03.093. [DOI] [PubMed] [Google Scholar]
- 30.Hansen SG, Powers CJ, Richards R. Evasion of CD8+ T cells is critical for superinfection by cytomegalovirus. Science. 328;26:102–106. doi: 10.1126/science.1185350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schleiss MR, Bourne N, Bernstein DI. Preconception vaccination with a glycoprotein B (gB) DNA vaccine protects against cytomegalovirus (CMV) transmission in the guinea pig model of congenital CMV infection. J Infect Dis. 2003;188:1868–74. doi: 10.1086/379839. [DOI] [PubMed] [Google Scholar]
- 32.Schleiss MR, Bourne N, Stroup G, Bravo FJ, Jensen NJ, Bernstein DI. Protection against congenital cytomegalovirus infection and disease in guinea pigs, conferred by a purified recombinant glycoprotein B vaccine. J Infect Dis. 2004;189:1374–81. doi: 10.1086/382751. [DOI] [PubMed] [Google Scholar]