Abstract
Analysis of 36 individuals over age 60 years who were immunized with Zostavax revealed varicella zoster virus (VZV) DNA in swabs of skin inoculation sites obtained immediately after immunization in 18 (50%) of 36 subjects (copy number per nanogram of total DNA, 28 to 2.1 × 106) and in saliva collected over 28 days in 21 (58%) of 36 subjects (copy number, 20 to 248). Genotypic analysis of DNA extracted from 9 random saliva samples identified vaccine virus in all instances. In some immunized individuals over age 60, vaccine virus DNA is shed in saliva up to 4 weeks.
Varicella zoster virus (VZV) is a neurotropic alphaherpesvirus. Primary infection usually causes varicella (chicken pox) in children. Airborne VZV enters the nasopharynx and replicates in tonsillar T cells followed by viremia and skin lesions [1, 2]. After primary infection, VZV becomes latent in neurons of cranial nerve ganglia, dorsal root ganglia, and autonomic ganglia along the entire neuraxis. Decades later, VZV reactivates in elderly and immunocompromised individuals to produce zoster (shingles), a syndrome characterized by pain and a vesicular rash on an erythematous base in 1–3 dermatomes. Zoster is common, with ∼1,000,000 cases annually in the United States. Importantly, zoster is often followed by chronic pain (postherpetic neuralgia [PHN]) as well as by meningoencephalitis, cerebellitis, cranial nerve palsies, vasculopathy, myelopathy, and multiple inflammatory diseases of the eye [3].
To prevent zoster and its attendant neurological complications, Zostavax vaccine (Merck) was approved by the Food and Drug Administration for use in individuals at least 60 years of age. Over a 3-year period, Zostavax effectively reduced the risk of zoster by 51% and PHN by 66% in nearly 20,000 healthy adults age 60 years or older [4]. Zostavax contains live attenuated VZV, and the package insert warns newly vaccinated individuals to avoid contact for an unspecified time with newborn infants, immunosuppressed individuals, and pregnant women who have not had chicken pox or have not been immunized for chicken pox. Because VZV DNA is present in saliva of zoster patients for at least 2 weeks [5] and VZV in saliva can also be infectious [6], we examined the inoculation site and saliva of Zostavax-vaccinated subjects for the presence of VZV DNA for 4 weeks after immunization.
METHODS
After informed consent and with approval of the University of Texas Health Science Center's Institutional Review Board, 36 immunocompetent subjects (19 men and 17 women), age 60–89 years (mean ± SD, 71.1 ± 7.6 years) were inoculated intramuscularly in the deltoid area with Zostavax (Merck).
Skin. Skin over the inoculation site was disinfected with alcohol before but not after immunization. Within 10 minutes after immunization, the inoculation site was swabbed with a cotton-tipped applicator (Fisher Scientific), and the skin swab sample was immediately placed in 1 mL nuclease-free sterile water overnight at 4°C and concentrated to 200 μL with a Microsep 100 K filtration unit (Filtron Technology Corporation) by centrifugation at 8000 rpm for 30 minutes. Samples were stored frozen at −20°C until processed for DNA extraction as described below.
Saliva. On day 0 before immunization and on days 1–3, 7, 14, 21, and 28 after immunization, saliva was collected upon arising and before eating or drinking using a Salivette tube (Sarstedt Inc). The polymerase chain reaction (PCR) technologist was blinded to the source of all saliva samples. Saliva was processed, and DNA was extracted and quantified by real-time PCR using Taqman 7900 (Applied Biosystems) as described elsewhere [7]. All PCR assays were performed in triplicate. VZV DNA copy number was determined by comparing the cycle threshold value (Ct) of the test samples to Ct values obtained by PCR on dilutions of VZV DNA extracted from virus nucleocapsids [8]. The sensitivity of detection was 10 copies of VZV DNA per nanogram of salivary DNA. A Ct value >35 indicated the presence of VZV DNA but at a concentration too low to quantify. Such values are noted as “+” on Table 1 followed by the Ct number. DNA extracted from 9 random saliva samples was analyzed for single nucleotide polymorphism sites in VZV open reading frames 38, 54, and 62 using real-time PCR as described elsewhere [9–11].
Table 1.
VZV DNA at Skin Inoculation Sites and in Saliva After Immunization With Zostavax Vaccine
| Age sex | Skin swab | Saliva before immunization | Saliva after immunization, days |
||||
| 13 | 7 | 14 | 21 | 28 | |||
| 65 F | 366 (28)a | 0 | 193 (32) b | 100 (32) | 30 (33) | +c (36) | 0 |
| 84 M | + (38) | 0 | 0 | 0 | 0 | 0 | 0 |
| 84 F | 0 | 0 | 64 (30) b | 26 (35) | 53 (26) | + (36) | 0 |
| 66 F | 28 (33) | 0 | 0 | 0 | 0 | 0 | 0 |
| 61 M | + (35) | 0 | + (37) | 56 (28) b | 0 | 68 (29) | 0 |
| 71 F | 0 | 0 | + (36) | 31 (33) | + (38) | + (37) | 0 |
| 71 F | 0 | 0 | 167 (32) b | 34 (29) | 0 | 0 | 0 |
| 62 M | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 64 F | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 65 F | + (39) | 0 | 0 | 0 | 0 | 0 | 0 |
| 63 F | 1178 (22) | 0 | 0 | 0 | 0 | 0 | 0 |
| 66 F | 0 | 0 | 47 (29) | 30 (32) | 53 (31) | + (38) | 0 |
| 66 M | + (39) | 0 | 110 (31) | 97 (27) b | 0 | 20 (34) | 0 |
| 75 F | + (39) | 0 | 111 (32) | 44 (29) | 31 (32) | 0 | 0 |
| 67 M | 0 | 0 | 248 (32) | 22 (34) | 0 | 0 | 0 |
| 65 F | 112426 (24) | 0 | 0 | 0 | 0 | 0 | 0 |
| 83 F | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 70 F | 0 | 0 | 58 (31) | 117 (30) | 24 (34) | 0 | + (39) |
| 64 F | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 76 M | + (38) | 0 | 79 (28) | + (36) | 0 | 0 | 0 |
| 60 M | 2124138 (18) | 0 | 150 (28) | + (35) | + (35) | 0 | 0 |
| 69 M | 0 | 0 | 67 (29) | + (35) | 0 | 24 (34) | 0 |
| 73 M | 262 (32) | 0 | 40 (34) | + (36) | 0 | + (38) | 0 |
| 89 M | + (38) | 0 | 0 | 0 | 0 | 0 | 0 |
| 79 M | + (39) | 0 | 57 (32) b | + (36) | 0 | 0 | 0 |
| 77 F | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 66 M | + (38) | 0 | + (38) | 27 (33) | 80 (29) | 0 | 0 |
| 72 M | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 85 M | 0 | 0 | + (37) | 47 (30) b | 117 (30) | 0 | 0 |
| 79 F | 0 | 0 | + (39) | 22 (29) | 0 | 0 | 0 |
| 70 M | 207 (27) | 0 | 0 | 0 | 0 | 0 | 0 |
| 70 M | 40191 (27) | 0 | + (35) | 30 (34) | 0 | 0 | 0 |
| 76 M | 19602 (31) | 0 | 30 (34) | 111 (33) b | 67 (32) | 36 (28) | 0 |
| 69 F | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 62 M | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 77 M | 0 | 0 | 50 (32) b | + (36) | 44 (32) | + (38) | + (35) |
| No.positive/total | 18/36 | 0/36 | 21/36 | 21/36 | 11/36 | 10/36 | 2/36 |
| Percent positive | 50 | 0 | 58 | 58 | 31 | 28 | 6 |
Note. aVZV DNA copies per nanogram total DNA (Ct value).
Vaccine strain VZV DNA verified.
VZV DNA present, but too few copies to quantify.
RESULTS
Inoculation site samples taken within 10 minutes after vaccination were positive for Zostavax VZV DNA in 18 (50%) of 36 subjects. The VZV DNA copy number per nanogram of total DNA ranged from 28 to 2.1 × 106 (Table 1), possibly reflecting the presence of infectious virus since no alcohol or other agent was used to wipe the skin after inoculation.
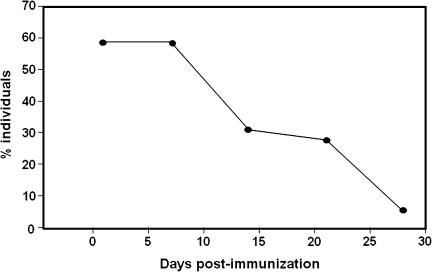
No saliva specimen collected immediately before immunization contained VZV DNA. During the first week after immunization, VZV DNA was detected in saliva of 21 (58%) of 36 subjects (13 men and 8 women). During the 28-day study period, VZV DNA was found in 11 (31%) of 36 subjects (5 men and 6 women) at day 14, in 10 (28%) of 36 subjects (6 men and 4 women) at day 21, and in 2 (6%) of 36 subjects (1 man and 1 woman) at day 28. Figure 1 shows the percent of immunized subjects who shed VZV DNA during the 28-day study period. VZV DNA copy numbers per nanogram of total DNA ranged from 20 to 248 (Table 1). Genotypic analysis of DNA from 9 random saliva samples revealed vaccine virus DNA in all instances (Table 1, bold); wild-type VZV DNA was not detected. In 15 (42%) of 36 vaccine recipients (6 men, 9 women), VZV DNA was not detected in saliva at any time during the 28-day study period.
Figure 1.
Percent individuals over age 60 whose saliva contained VZV DNA during the 28-day study period after Zostavax immunization.
DISCUSSION
In 2006, Zostavax vaccine was approved by the Food and Drug Administration and recommended by the Advisory Council for Immunization Practices for use in healthy individuals over 60 years of age. The development of a vaccine to prevent zoster is a major step in reducing the morbidity associated with this important public health threat. Transmission of the Oka/Merck strain of VZV has been reported after chicken pox vaccination [12, 13], but not after Zostavax immunization [13].
Herein, we detected Zostavax VZV DNA sequences in 18 (50%) of 36 skin swab samples taken within 10 minutes after immunization. We also detected Zostavax VZV DNA sequences in saliva of 21 (58%) of 36 subjects throughout the first week after immunization and in 6% of immunized subjects at 28 days. The detection of VZV DNA in saliva is one more indication of its potential use in diagnosis of VZV persistence in various clinical situations. VZV DNA has been found not only in saliva of patients with zoster at all levels of the neuraxis [5] but also in saliva of asymptomatic astronauts [7].
Importantly, earlier studies revealed the presence of wild-type VZV in the vesicular fluid of a patient with zoster who had been immunized twice with varicella vaccine [14] and the presence of 2 different VZV genotypes isolated from an immunocompetent man during 2 different episodes of zoster [15]. These findings revealed that a superinfecting VZV became latent. Although we did not study latency, our finding of VZV vaccine virus in saliva supports the notion that a superinfecting VZV spreads systemically.
Finally, while transmission of vaccine virus has not been found among vaccine recipients, the detection of VZV DNA in saliva of Zostavax recipients for up to 28 days suggests that contact with saliva of recently immunized individuals represents a potential source of transmission. Because astronaut saliva contains infectious virus [6], saliva (and skin) of immunized individuals should also be studied for infectious virus.
Funding
This work was supported by the National Institutes of Health (grant AG032958 to D. G. and R. J. C.; grant AG006127 to D. G.; and grant NS067070 to M. A. N.) and National Aeronautics and Space Administration (grant SMO-015 to D. L. P). National Institutes of Health (grant AG032958 to D. G. and R. J. C.; grant AG006127 to D. G.; and grant NS067070 to M. A. N.) and National Aeronautics and Space Administration (SMO-015 to D. L. P.).
Acknowledgments
We thank Marina Hoffman for editorial assistance and Cathy Allen for manuscript preparation.
References
- 1.Satyaprakash AK, Stelter AA, Tremaine AM, et al. Viremia in acute herpes zoster. J Infect Dis. 2009;200:26–32. doi: 10.1086/599381. [DOI] [PubMed] [Google Scholar]
- 2.Arvin AM, Moffat JF, Sommer M, et al. Varicella-zoster virus T cell tropism and the pathogenesis of skin infection. Curr Top Microbiol Immunol. 2010;342:189–209. doi: 10.1007/82_2010_29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilden D, Cohrs RJ, Mahalingam R, Nagel MA. Neurological disease produced by varicella zoster virus reactivation without rash. Curr Top Microbiol Immunol. 2010;342:243–53. doi: 10.1007/82_2009_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–84. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 5.Mehta SK, Tyring SK, Gilden DH, et al. Varicella-zoster virus in the saliva of patients with herpes zoster. J Infect Dis. 2008;197:654–7. doi: 10.1086/527420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohrs RJ, Mehta SK, Schmid DS, Gilden DH, Pierson DL. Asymptomatic reactivation and shed of infectious varicella zoster virus in astronauts. J Med Virol. 2008;80:1116–22. doi: 10.1002/jmv.21173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta SK, Cohrs RJ, Forghani B, Zerbe G, Gilden DH, Pierson DL. Stress-induced subclinical reactivation of varicella zoster virus in astronauts. J Med Virol. 2004;72:174–9. doi: 10.1002/jmv.10555. [DOI] [PubMed] [Google Scholar]
- 8.Gilden DH, Shtram Y, Friedmann A, et al. Extraction of cell-associated varicella-zoster virus DNA with triton X-100-NaCl. J Virol Methods. 1982;4:263–75. doi: 10.1016/0166-0934(82)90073-8. [DOI] [PubMed] [Google Scholar]
- 9.Loparev VN, McCaustland K, Holloway BP, Krause PR, Takayama M, Schmid DS. Rapid genotyping of varicella-zoster virus vaccine and wild-type strains with fluorophore-labeled hybridization probes. J Clin Microbiol. 2000;38:4315–9. doi: 10.1128/jcm.38.12.4315-4319.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loparev VN, Rubtcova E, Seward JF, Levin MJ, Schmid DS. DNA sequence variability in isolates recovered from patients with post vaccination rash or herpes zoster caused by Oka varicella vaccine. J Infect Dis. 2007;195:502–10. doi: 10.1086/510532. [DOI] [PubMed] [Google Scholar]
- 11.Lopez AS, Burnett-Hartman A, Nambiar R, et al. Transmission of a newly characterized strain of varicella-zoster virus from a patient with herpes zoster in a long-term-care facility, West Virginia, 2004. J Infect Dis. 2008;197:646–53. doi: 10.1086/527419. [DOI] [PubMed] [Google Scholar]
- 12.Chaves SS, Haber P, Walton K, et al. Safety of varicella vaccine after licensure in the United States: experience from reports to the vaccine adverse event reporting system, 1995–2005. J Infect Dis. 2008;197(Suppl 2):S170–7. doi: 10.1086/522161. [DOI] [PubMed] [Google Scholar]
- 13.Harpaz R, Ortega-Sanchez IR, Seward JF. Prevention of herpes zoster: recommendations of the Advisory Committee on immunization Practices (ACIP) MMWR Recomm Rep. 2008;57:1–30. [PubMed] [Google Scholar]
- 14.Hammerschlag MR, Gershon AA, Steinberg SP, Clarke L, Gelb LD. Herpes zoster in an adult recipient of live attenuated varicella vaccine. J Infect Dis. 1989;160:535–7. doi: 10.1093/infdis/160.3.535. [DOI] [PubMed] [Google Scholar]
- 15.Taha Y, Scott FT, Parker SP, Syndercombe Court D, Quinlivan ML, Breuer J. Reactivation of 2 genetically distinct varicella-zoster viruses in the same individual. Clin Infect Dis. 2006;43:1301–2. doi: 10.1086/508539. [DOI] [PubMed] [Google Scholar]