Abstract
Background. The worldwide burden of the Group A Streptococcus (GAS) primary infection and sequelae is considerable, although immunization programs with broad coverage of the hyper variable GAS are still missing. We evaluate the streptococcal hemoprotein receptor (Shr), a conserved streptococcal protein, as a vaccine candidate against GAS infection.
Methods. Mice were immunized intraperitoneally with purified Shr or intranasally with Shr-expressing Lactococcus lactis. The resulting humoral response in serum and secretions was determined. We evaluated protection from GAS infection in mice after active or passive vaccination with Shr, and Shr antiserum was tested for bactericidal activity.
Results. A robust Shr-specific immunoglobulin (Ig) G response was observed in mouse serum after intraperitoneal vaccination with Shr. Intranasal immunization elicited both a strong IgG reaction in the serum and a specific IgA reaction in secretions. Shr immunization in both models allowed enhanced protection from systemic GAS challenge. Rabbit Shr antiserum was opsonizing, and mice that were administrated with Shr antiserum prior to the infection demonstrated a significantly higher survival rate than did mice treated with normal rabbit serum.
Conclusions. Shr is a promising vaccine candidate that is capable of eliciting bactericidal antibody response and conferring immunity against systemic GAS infection in both passive and active vaccination models.
Streptococcus pyogenes, or Group A Streptococcus (GAS), is a versatile pathogen capable of producing a spectrum of illness ranging from mild infections, such as pharyngitis and impetigo, to invasive diseases, including myositis and necrotizing faciitis [1]. GAS infections can also trigger a number of disabling sequelae. For example, pharyngitis, an ordinary childhood disease of which there are hundreds of millions of cases per year worldwide, can lead to acute rheumatic fever (RF) in ∼3% of the untreated patients [2]. RF is thought to result from cross-reaction of antibodies and T-cell receptors with tissues of the heart, synovium, and/or neurons of the basal ganglia in the brain [3, 4]. In addition to the induction of heart disease, the damage inflicted by autoimmune reactions is hypothesized to produce a number of neuropsychiatric disorders, including Sydenhym chrea and obsessive-compulsive disorder [5, 6]. All together, GAS costs billions of dollars in the United States alone and >500,000 deaths per year globally. An effective GAS vaccine is therefore highly desirable, especially for the developing parts of the world where RF and rheumatic heart disease are leading causes of disability and mortality in children [2, 7].
GAS M protein has been extensively studied as a vaccine candidate since early observations that it elicits lasting immunity [8–10]. However, the antiphagocytic M protein is a highly variable antigen [11, 12]. The N-terminal domain, the molecule's most outward facing and least conserved region, evokes an M type–specific antibody [13]. More than 150 M types are known, and the number and identity of the prevalent strains varies significantly in different parts of the globe [14–16]. In addition to the complications arising from extensive antigenic variation, M-based vaccination programs suffer from safety concerns. Several M serotypes were implicated in RF development, and cross-reactivity has been found between some M epitopes and human tissues.
Recent studies suggest that a safe and effective M-base vaccine for GAS may be in reach. Two vaccines based on peptides derived from the N-terminal domain of the M protein were found to be protective in clinical trials without adverse outcome [17, 18]. In its current formulation, M vaccine provides protection against 26 serotypes, covering ∼85% of GAS strains in the United States [14]. Nevertheless, this vaccine is expected to have fairly limited coverage in developing countries, and there are concerns that it may trigger a shift in serotype prevalence [19]. Therefore, there is a significant interest in identifying additional protective antigens that may facilitate broad immunization programs. A number of GAS components have been investigated, including antigens derived from virulence factors or surface components, such as the C5a peptidase [20], the conserved C-terminal region of the M protein [21], group A carbohydrates [22], lipoteichoic acid [23, 24], and several fibronectin-binding proteins [25–27]. Antigens that were identified in silico and/or by proteomics or genomics methods have been also studied [28, 29]. Although a protective response was observed with some antigens in ≥1 model, difficulties arising from limited expression among GAS isolates or the high concentration required for effective antibody response were reported. Other than the N-terminus peptides of the M protein, no additional GAS antigens have reached human trails since the 1970s.
The streptococcal hemoprotein receptor (Shr) is highly conserved in GAS genomes. It binds to several hemoproteins and extracellular matrix components and is implicated in iron acquisition and adherence [30, 31]. Shr is available on the bacterial surface for antibody binding, and recent analysis confirmed the expression of Shr in 15 of 17 tested clinical isolates representing 12 M types [31]. Here, we investigate the ability of Shr to elicit protective immunity. We show that Shr is highly immunogenic and that vaccination with Shr in both active and passive models protected mice from systemic GAS challenge.
MATERIALS AND METHODS
Bacterial Strains and Growth Conditions
Escherichia coli DH5α harboring plasmid pCB1, which expresses a recombinant Shr protein (His6-Xpress-Shr, rShr [30]), were grown in Luria-Bertani broth at 37°C. The GAS strains MGAS5005 (M type 1 [32]), MGAS315 (M type 3 [33]), and ZE491 (M type 49, mtsR- [34]) were grown at 37°C in Todd-Hewitt broth (Difco Laboratories) with 0.2% w/v yeast extract (THY). The Lacococcus lactis MG1363 and MG1363 harboring plasmid pXL14 (expressing the native Shr protein [31]) were cultivated in M17 (Difco Laboratories) supplemented with 0.5% w/v glucose (GM17) at 30°C. When necessary, ampicillin (100 μg/mL) or kanamycin (70 μg/mL) was added to the medium.
Mouse Vaccinations
CD-1 female mice were used (weight, 20–22 g; Charles River Laboratories). For systemic immunization, rShr was purified as described elsewhere [31] and was quantified using the Modified Lowry Protein Assay Kit (Thermo Scientific). Purified rShr protein (40 μg) was administered intraperitoneally (ip) on days 0 (emulsified in Complete Freund's adjuvant, CFA), 14, 28, and 42 (emulsified in Incomplete Freund's adjuvant, IFA). For mock vaccination, phosphate-buffered saline (PBS) mixed with CFA or IFA was administrated. Serum samples were collected on days 0, 7, 21, 35, and 49, and the anti-Shr immunoglobulin (Ig) G response in individual animals was determined. Mucosal immunization was done as described elsewhere [35]. L. lactis cells (MG1363/pXL14 or MG1363) were briefly washed and resuspended in saline to give 1 × 108 CFU/μL. Mice were vaccinated intranasally by 4 administrations of 2 × 109 CFU (20 μL) into the nostril, every 10 days for 3 consecutive days (ie, on days 1, 2, 3, 14, 15, 16, 27, 28, and 29 and on days 40, 41, and 42). The anti-Shr IgG titer in serum samples collected on day 50 was determined. Lung lavage specimens were collected on day 52, as described elsewhere [35], and the anti-Shr IgA level was determined. For passive immunization, 100 μL of rabbit rShr-antiserum [30] or serum samples from naive animals (normal rabbit serum [NRS]) were mixed with 400 μL of PBS and administrated intraperitoneally. All of the vaccination and mouse challenge experiments were repeated at least twice.
GAS Infection Model
GAS cells at the mid-logarithmic phase (optical density at 600 nm, 0.7) were harvested, washed, and resuspended in saline. Cell concentration was determined by microscopic counts and verified by plating. Mice were infected with 0.1 mL of cell suspension (lethal dose, 80%) and were observed 4 times per day after challenge. Animals exhibiting signs of severe distress were euthanized and counted as dead. All experiments involving animals were conducted according to protocols approved by the Georgia State University Institutional Animal Care and Use Committee.
Shr Antibody Detection
The antibody response was measured by enzyme-linked immunosorbent assay (ELISA) [35]. In brief, microtiter plates (Costar; Corning) were coated with rShr (50 ng per well), washed, and blocked with 1% w/v BSA in PBS-Tween. Serum or lung fluid samples were allowed to react with the coated plates for 1 h at 37°C. Antibody production was detected using anti-mouse IgG or anti-mouse IgA secondary antibodies (Sigma). The absorbance at 405 nm was measured after a 30-min incubation. Antibody titers were defined as the dilution producing the same OD405 at 2 times of the background level (ie, the reading obtained with serum or lung fluid samples for nonimmunized mice).
Opsonophagocytic Killing Assay
ZE491 GAS cells (OD600, ∼0.7) were harvested and washed. A total of 0.1 mL of diluted (1: 200,000) cell samples were added into 0.5 mL of fresh rabbit whole blood, together with 0.1 mL of rabbit Shr antiserum [30] or NRS (as a control), and incubated with shaking at 37°C for 3 h. Surviving bacteria were enumerated by plating for viable counts. Opsonization expressed as the percent reduction in mean colony-forming units (CFUs) was calculated as [1–(CFU in the presence of Shr antiserum)/(CFU in the presence of NRS)] × 100 [36].
Statistical Analysis
Data presented are average or representative of data from experiments repeated at least 2 times. The Student t test was used for testing significance when comparing 2 groups. Kaplan-Meier plots of survival and log-rank tests were used for comparison of protection by immunization.
RESULTS
Intraperitoneal Vaccination With Shr Elicits a Robust Humoral Response and Protection
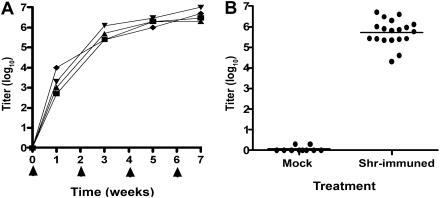
Previous studies demonstrated that Shr is expressed in vivo and suggested that it is required for GAS disease process [31], raising the possibility that the multifunctional Shr may serve as a vaccine target. To examine Shr antigenic potential, a recombinant protein (rShr, [30]) was expressed, purified, and administrated intraperitoneally to mice. The time course development of the anti-Shr response was monitored in serum samples from 4 representative mice (Figure 1A). All 4 animals developed a significant serum reaction to Shr after a single antigen administration (IgG titer range, 103–104). The anti-Shr IgG level continued to ascend after the second immunization and appeared to be saturated after the third immunization, demonstrating an anti-Shr titer range of (1.25–6.25) × 106. Endpoint titer analysis in the rest of the cohorts’ mice revealed a robust anti-Shr response in the immunized animals, with a mean titer of 5.6 × 105 (Figure 1B). In contrast to the significant anti-Shr response found in the experimental group (ie, Shr-immunized mice), Shr-specific antibody was not seen in serum samples obtained from the control group (ie, the mock vaccination group).
Figure 1.
Anti-streptococcal hemoprotein receptor (Shr) response in serum after intraperitoneal vaccination. Mice were administrated rShr (arrow heads), and the resulting serum immunoglobulin (Ig) G titers were determined by enzyme-linked immunosorbent assay performed in quadruplicates. Each datum point represents the average response in individual animals. A, Shr antibody development. Shr-specific Ig G levels were determined in serum samples collected from 4 representative mice on days 0, 7, 21, 35, and 49. B, Endpoint anti-Shr IgG response. The anti-Shr IgG titers found in serum samples collected on day 49 from mice administered rShr (Shr-immuned; n = 19) or with phosphate-buffered saline (mock; n = 10) are shown. The horizontal bar indicts the mean titer response.
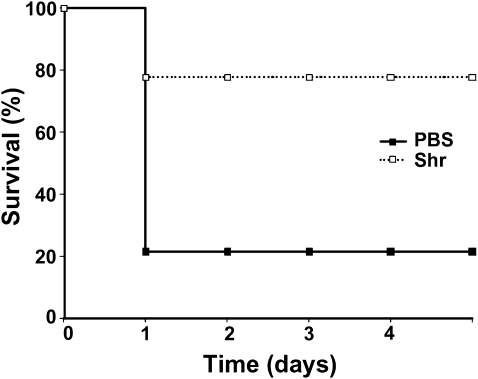
To test for protective immunity, vaccinated mice were challenged intraperitoneally with a lethal dose of MGAS5005 (M type 1) on day 49 of the vaccination experiment, and animal survival was monitored for 5 days. A rapidly progressing fatal infection developed in most of the mice in the control group (mock vaccination), resulting in a 21% survival rate at the end point. Similarly, an aggressive disease was previously reported in a murine model for systemic GAS infection with MGAS5005 [32]. A less severe disease developed after infection of mice immunized with rShr, and the end point survival rate for this group was 78%, 3.7-fold higher than that of the mock-vaccinated group (P = .002) ( Figure 2). Therefore, systemic immunization of mice with rShr provides protection from systemic GAS challenge.
Figure 2.
Protection of mice from systemic group A Streptococcus (GAS) challenge after intraperitoneal vaccination with streptococcal hemoprotein receptor (Shr). Immunized mice were infected intraperitoneally with 5 × 108 CFU. Kaplan-Meier survival curves of antigen-immunized mice (shr; n = 18) and mock-vaccinated mice (phosphate-buffered saline [PBS]; n = 14) are shown. The statistical significance (P = .002) was determined by the log-rank test. Two independent experiments producing similar results were conducted; the data shown are from a representative experiment.
Intranasal Vaccination With Shr Results in Strong Antibody Response and Enhanced Defense
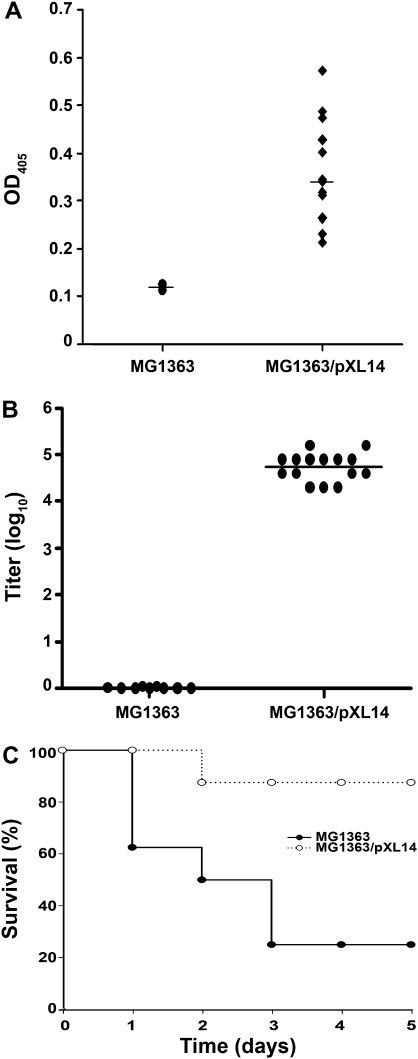
The ability of Shr to elicit immune response by mucosal vaccination was examined using the probiotic L. lactis as a delivery vector. Mice intranasally received recombinant bacteria expressing Shr on the surface (MG1363/pXL14, [31]) or with the native host cells (MG1363). The presence of anti-Shr IgA in undiluted lung lavage samples collected 10 days after the last antigen administration (day 52) was determined by ELISA. A measurable Shr-IgA activity was exhibited by all of the mice that were immunized with Shr-expressing bacteria (Figure 3A); 9 of 13 mice developed a Shr-specific IgA level that was at least 3 times higher than that seen in untreated animals (with an endpoint IgA titer (the titer is expressed as a dilution ration) that varied from 1:4 to 1:64; data not shown). Mice that were vaccinated with unmodified L. lactis cells showed only the background IgA level seen with untreated animals (P = .01). The induction of anti-Shr IgG in the serum after the course of mucosal vaccination (day 50) was tested as well (Figure 3B). All of the mice that received recombinant L. lactis (MG1363/pXL14) developed a significant IgG response to Shr, demonstrating a mean endpoint titer (± standard deviation) of 5.5 × 104 ± 2 × 104, whereas only background response was found in serum samples from pre-immune mice or mice vaccinated with native L. lactis (MG1363, P < .0001). Therefore, mucosal administration with L. lactis expressing Shr resulted in a measurable immune response in secretions and a strong reaction in the serum.
Figure 3.
Streptococcal hemoprotein receptor (Shr) intranasal vaccination and protective immune response. Mice were immunized with 2 × 109 CFU of Lacococcus lactis (MG1363) or L. lactis expressing Shr (MG1363/pXL14). Endpoint antibody response (on days 50 and 52, respectively) was determined by enzyme-linked immunosorbent assay preformed in quadruplicate. A, Shr-specific immunoglobulin (Ig) A level in undiluted lung lavage specimens from mice treated with MG1363 (n = 2) or MG1363/pXL14 (n = 13). Each datum point represents the mean response in individual animals. The statistical significance (P = .01) was determined by the Student t test. B, Shr-specific IgG titer in serum for individual mice treated with MG1363 (n = 15) or MG1363/pXL14 (n = 15). The statistical significance (P < .0001) was determined by the Student t test. C, Kaplan-Meier survival curves of vaccinated mice after systemic group A Streptococcus challenge. Mice immunized with MG1363 (n = 8) or MG1363/pXL14 (n = 8) were intraperitoneally received 1 × 108 CFU. The statistical significance (P =.014) was determined by the log-rank test. The data shown are pooled data from 2 independent experiments.
We next asked whether the anti-Shr immune response triggered by the mucosal immunization was sufficient to protect against GAS infection. Vaccinated mice were infected intraperitoneally (on day 52) with MGAS5005. Aggressive infections were seen after the challenge in mice that were immunized with the native L. lactis strain (MG1363), demonstrating only a 25% survival rate on the fifth day after infection. In contrast, most of the mice that were administrated with Shr-producing lactococci (MG1363/pXL14) were able to recover and demonstrated an 87% survival rate—3.48-fold higher than that of the control group (P = .014) (Figure 3C). Thus, mucosal immunization with Shr-producing bacteria resulted in protective immunity against systemic GAS challenge.
Passive Immunity With Rabbit Shr Antiserum
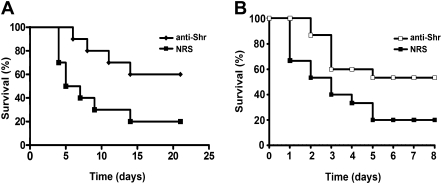
The studies described above show that Shr vaccination administered via both the intraperitoneal and intranasal routes resulted in significant serum anti-Shr IgG. To examine whether the observed humoral response to Shr could account for the protection exhibited by the vaccinated animals, we tested the ability of rabbit Shr anti-serum [30] to protect naive mice from upcoming GAS infection. Pretreatment of mice with Shr anti-serum (with a titer of 106) prior to intraperitoneal inoculation of MGAS5005 provided significant defense; the survival rate at the end point for mice administrated with the Shr antiserum was 60%, whereas only a 20% survival rate was observed with the control mice that were treated with NRS prior to the challenge (P = .0308) ( Figure 4A). The same Shr antiserum also defended mice from infection with the invasive M type 3 strain MGAS315, demonstrating 53% and 20% survival rates for mice treated with anti-Shr or NRS, respectively (P = .034) ( Figure 4B). These observations indicate that Shr antibodies are protective against multiple GAS serotypes and suggest that the defense from GAS obtained by vaccination is mediated at least in part by serum antibody to Shr.
Figure 4.
Protection of mice from systemic group A Streptococcus (GAS) infection with passive immunization with streptococcal hemoprotein receptor (Shr). Kaplan-Meier survival curves are shown for mice challenged with 5 × 107 CFU 4 h after intraperitoneal administration of rabbit Shr antiserum (anti-Shr) or with normal rabbit serum (NRS). A, MGAS5005 challenge. The results are representative of 2 independent experiments (n = 10 for both groups; P = .0308). B, MGAS315 challenge. The data shown are pooled from 3 independent experiments (n = 15 for both groups; P = .034). Statistical significance was determined by the log-rank test.
Rabbit Shr-antiserum Is Bactericidal
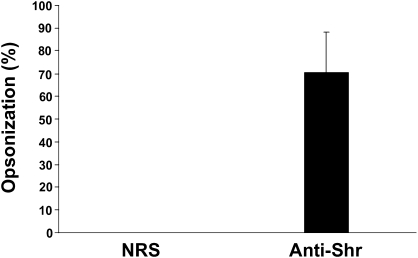
Because rabbit Shr-antiserum protects mice from GAS infection, we tested whether it contains opsonizing antibodies. The GAS metal-dependent repressor MtsR represses the expression of Shr in vitro [34]. To ensure Shr production under the experimental conditions, we used the mtsR mutant ZE491, in which Shr expression is deregulated [34]. ZE491 cells were allowed to grow in rabbit whole blood in the presence of Shr-antiserum or NRS, and the change in the bacterial load after 3 hours of incubation was determined by viable counts. A significant reduction in the bacterial number (mean ± standard deviation, 70% ± 17%) was observed in cultures treated with Shr anti-serum, comparison with those that were treated with NRS (Figure 5), indicating that the anti-Shr serum facilitates phagocytosis and bacterial killing in whole blood.
Figure 5.
Serum mediation of phagocytosis and killing of bacteria with anti–streptococcal hemoprotein receptor (Shr). ZE491 cells were incubated in rabbit whole blood in the presence of Shr antiserum or normal rabbit serum (NRS). Bacterial growth after 3 h of incubation with Shr-antiserum was compared with that of control NRS. The mean opsonization percentage (measured as the percentage reduction in CFU) derived from 4 independent experiments is shown; the standard deviation is represented by the error bar (P < .001).
DISCUSSION
An effective prevention program for GAS is greatly needed, especially in the developing world, where antibiotic therapy fails to control the occurrences of RF and rheumatic heart disease as it does in developed countries [2, 4, 16]. The increasing reports of drug-resistant GAS further underscore the need for GAS vaccine [37–40]. The hyper-variable M protein is the most prominent and promising GAS antigen [17, 18]. However, M-based vaccination would likely require unique formulations for different geographic areas, and the efficacy of the vaccine may be hampered by rapid changes in GAS populations. Conserved antigens, which can be used alone or in combination with M-epitopes to increase coverage and combat population shifts, are therefore required. Here, we show that a conserved protein, Shr, is a promising antigen that can elicit robust humoral response and provide defense from systemic GAS infections in both active and passive immunization models.
We previously reported that GAS infection led to serum conversion in most of the surviving mice [31]. In this study, we measured the serum immune response triggered by Shr vaccination using CFA/IFA or L. lactis as adjuvants in systemic and mucosal application, respectively. The vigorous Shr-specific IgG response developed in mice after intraperitoneal vaccination (Figure 1) and the high antibody levels observed in the intranasally immunized animals (Figure 3B) together suggest that Shr is a strong immunogen. Moreover, both vaccination protocols resulted in enhanced resistance to systemic GAS challenge, showing that the immune reaction to Shr is protective (Figures 2 and 3C). It is interesting to note, however, that although the mucosal antigen administration produced a lower anti-Shr IgG titer (5.5 × 104 ± 2 × 104) than did intraperitoneal vaccination with rShr (5.6 × 105), a similar level of mouse recovery from GAS infection was seen in both cases. Immunization with L. lactis typically leads to a T-helper 1 (Th1)–biased response [35, 41, 42]. In addition to supporting IgG2a production over other immunoglobulin subtypes, Th1 adaptive response promotes cellular immunity, which could complement the protection provided by serum IgG.
Mucosal immunity is an important prevention and control measure of mucosal pathogens such as GAS. Lactococcus species were previously used successfully to elicit mucosal defense against Streptococcus pneumonia, group B streptococci, and GAS [42, 43]. Here, we show that in addition to the strong production of serum antibody, intranasal immunization with L. lactis expressing Shr triggered anti-Shr IgA formation in secretions (Figure 3A). Shr-specific IgA was seen in the lung lavage specimens obtained from most mice, although the antibody titer was modest. Mucosal vaccination with M-derived peptides provided protection from intranasal challenge even in the absence of strong IgA response, suggesting a protective role for serum IgG [36, 44]. Nevertheless, IgA is important for GAS defense [26, 45, 46]; thus, the mucosal delivery of Shr needs improvement. Investigations of alternative mucosal adjuvants, such as the lipid core peptide [36], proteosomes [47], or GAS pilus–based UPTOP (for unhindered presentation of polypeptides on tips of pili) system [35], are warranted.
Mice that were administrated with anti-Shr rabbit serum before infection demonstrated significantly higher survival rates after challenge with MGAS5005 or MGAS315 (60% and 53%, respectively) than did mice treated with NRS (20%) (Figure 4). The observation that humoral response to Shr is protective suggests that the defense conferred by active vaccination with Shr is mediated, at least in part, by serum antibodies. Protection by serum antibodies is further supported by the findings that Shr antiserum is bacteriocidal (Figure 5). In addition to facilitating GAS clearance by phagocytes, Shr serum antibodies are likely to interfere with Shr iron acquisition and adherence [30, 31] and thus may act directly to limit GAS growth and spread. The observation that Shr confers passive immunity suggests that Shr antiserum may be useful in therapy of invasive diseases, such as streptococcal toxic shock syndrome (STSS). STSS is a rapidly progressing, superantigen-mediated disease that involves bacterimia, hypotension, and multiple-organ failure [48]. The absence of protective antibodies against GAS M protein and superantigens is found to be associated with higher risk for STSS. Effective GAS antibodies may be useful in STSS treatment as they may act in reducing the bacterial load in the blood and thus superantigen production.
Funding
This study was supported by a grant from the National Institute of Allergy and Infectious Disease, National Institutes of Health, to Z. E. (AI057877).
Acknowledgments
This manuscript is dedicated to the late Moshe Vicus for his help and enthusiastic support.
References
- 1.Cunningham MW. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev. 2000;13:470–511. doi: 10.1128/cmr.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steer AC, Danchin MH, Carapetis JR. Group A streptococcal infections in children. J Paediatr Child Health. 2007;43:203–13. doi: 10.1111/j.1440-1754.2007.01051.x. [DOI] [PubMed] [Google Scholar]
- 3.Guilherme L, Fae K, Oshiro SE, Kalil J. Molecular pathogenesis of rheumatic fever and rheumatic heart disease. Expert Rev Mol Med. 2005;7:1–15. doi: 10.1017/S146239940501015X. [DOI] [PubMed] [Google Scholar]
- 4.Guilherme L, Ramasawmy R, Kalil J. Rheumatic fever and rheumatic heart disease: genetics and pathogenesis. Scand J Immunol. 2007;66:199–207. doi: 10.1111/j.1365-3083.2007.01974.x. [DOI] [PubMed] [Google Scholar]
- 5.Snider LA, Swedo SE. Post-streptococcal autoimmune disorders of the central nervous system. Curr Opin Neurol. 2003;16:359–65. doi: 10.1097/01.wco.0000073938.19076.31. [DOI] [PubMed] [Google Scholar]
- 6.Snider LA, Swedo SE. PANDAS: current status and directions for research. Mol Psychiatry. 2004;9:900–7. doi: 10.1038/sj.mp.4001542. [DOI] [PubMed] [Google Scholar]
- 7.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5:685–94. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 8.Cohen-Poradosu R, Kasper DL. Group A streptococcus epidemiology and vaccine implications. Clin Infect Dis. 2007;45:863–5. doi: 10.1086/521263. [DOI] [PubMed] [Google Scholar]
- 9.Dale JB. Current status of group A streptococcal vaccine development. Adv Exp Med Biol. 2008;609:53–63. doi: 10.1007/978-0-387-73960-1_5. [DOI] [PubMed] [Google Scholar]
- 10.Steer AC, Batzloff MR, Mulholland K, Carapetis JR. Group A streptococcal vaccines: facts versus fantasy. Curr Opin Infect Dis. 2009;22:544–52. doi: 10.1097/QCO.0b013e328332bbfe. [DOI] [PubMed] [Google Scholar]
- 11.Facklam RF, Martin DR, Lovgren M, et al. Extension of the Lancefield classification for group A streptococci by addition of 22 new M protein gene sequence types from clinical isolates: emm103 to emm124. Clin Infect Dis. 2002;34:28–38. doi: 10.1086/324621. [DOI] [PubMed] [Google Scholar]
- 12.Beall B, Facklam R, Thompson T. Sequencing emm-specific PCR products for routine and accurate typing of group A streptococci. J Clin Microbiol. 1996;34:953–8. doi: 10.1128/jcm.34.4.953-958.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lancefield RC. Current knowledge of type-specific M antigens of group A streptococci. J Immunol. 1962;89:307–13. [PubMed] [Google Scholar]
- 14.Shulman ST, Tanz RR, Dale JB, et al. Seven-year surveillance of North American pediatric group a streptococcal pharyngitis isolates. Clin Infect Dis. 2009;49:78–84. doi: 10.1086/599344. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan EL, Wotton JT, Johnson DR. Dynamic epidemiology of group A streptococcal serotypes associated with pharyngitis. Lancet. 2001;358:1334–7. doi: 10.1016/S0140-6736(01)06415-7. [DOI] [PubMed] [Google Scholar]
- 16.Steer AC, Jenney AW, Kado J, et al. Prospective surveillance of streptococcal sore throat in a tropical country. Pediatr Infect Dis J. 2009;28:477–82. doi: 10.1097/INF.0b013e318194b2af. [DOI] [PubMed] [Google Scholar]
- 17.McNeil SA, Halperin SA, Langley JM, et al. Safety and immunogenicity of 26-valent group a streptococcus vaccine in healthy adult volunteers. Clin Infect Dis. 2005;41:1114–22. doi: 10.1086/444458. [DOI] [PubMed] [Google Scholar]
- 18.Kotloff KL, Corretti M, Palmer K, et al. Safety and immunogenicity of a recombinant multivalent group a streptococcal vaccine in healthy adults: phase 1 trial. Jama. 2004;292:709–15. doi: 10.1001/jama.292.6.709. [DOI] [PubMed] [Google Scholar]
- 19.Steer AC, Magor G, Jenney AW, et al. emm and C-repeat region molecular typing of beta-hemolytic streptococci in a tropical country: implications for vaccine development. J Clin Microbiol. 2009;47:2502–9. doi: 10.1128/JCM.00312-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji Y, Carlson B, Kondagunta A, Cleary PP. Intranasal immunization with C5a peptidase prevents nasopharyngeal colonization of mice by the group A Streptococcus. Infect Immun. 1997;65:2080–7. doi: 10.1128/iai.65.6.2080-2087.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pandey M, Batzloff MR, Good MF. Mechanism of protection induced by group A Streptococcus vaccine candidate J8-DT: contribution of B and T-cells towards protection. PLoS One. 2009;4:e5147. doi: 10.1371/journal.pone.0005147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sabharwal H, Michon F, Nelson D, et al. Group A Streptococcus (GAS) carbohydrate as an immunogen for protection against GAS infection. J Infect Dis. 2006;193:129–35. doi: 10.1086/498618. [DOI] [PubMed] [Google Scholar]
- 23.Dale JB, Baird RW, Courtney HS, Hasty DL, Bronze MS. Passive protection of mice against group A streptococcal pharyngeal infection by lipoteichoic acid. J Infect Dis. 1994;169:319–23. doi: 10.1093/infdis/169.2.319. [DOI] [PubMed] [Google Scholar]
- 24.Yokoyama Y, Harabuchi Y. Intranasal immunization with lipoteichoic acid and cholera toxin evokes specific pharyngeal IgA and systemic IgG responses and inhibits streptococcal adherence to pharyngeal epithelial cells in mice. Int J Pediatr Otorhinolaryngol. 2002;63:235–41. doi: 10.1016/s0165-5876(02)00021-6. [DOI] [PubMed] [Google Scholar]
- 25.McArthur J, Medina E, Mueller A, et al. Intranasal vaccination with streptococcal fibronectin binding protein Sfb1 fails to prevent growth and dissemination of Streptococcus pyogenes in a murine skin infection model. Infect Immun. 2004;72:7342–5. doi: 10.1128/IAI.72.12.7342-7345.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawabata S, Kunitomo E, Terao Y, et al. Systemic and mucosal immunizations with fibronectin-binding protein FBP54 induce protective immune responses against Streptococcus pyogenes challenge in mice. Infect Immun. 2001;69:924–30. doi: 10.1128/IAI.69.2.924-930.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Courtney HS, Hasty DL, Dale JB. Serum opacity factor (SOF) of Streptococcus pyogenes evokes antibodies that opsonize homologous and heterologous SOF-positive serotypes of group A streptococci. Infect Immun. 2003;71:5097–103. doi: 10.1128/IAI.71.9.5097-5103.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez-Ortega MJ, Norais N, Bensi G, et al. Characterization and identification of vaccine candidate proteins through analysis of the group A Streptococcus surface proteome. Nat Biotechnol. 2006;24:191–7. doi: 10.1038/nbt1179. [DOI] [PubMed] [Google Scholar]
- 29.Turner CE, Kurupati P, Wiles S, Edwards RJ, Sriskandan S. Impact of immunization against SpyCEP during invasive disease with two streptococcal species: Streptococcus pyogenes and Streptococcus equi. Vaccine. 2009;27:4923–9. doi: 10.1016/j.vaccine.2009.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bates CS, Montanez GE, Woods CR, Vincent RM, Eichenbaum Z. Identification and characterization of a Streptococcus pyogenes operon involved in binding of hemoproteins and acquisition of iron. Infect Immun. 2003;71:1042–55. doi: 10.1128/IAI.71.3.1042-1055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fisher M, Huang YS, Li X, McIver KS, Toukoki C, Eichenbaum Z. Shr is a broad-spectrum surface receptor that contributes to adherence and virulence in group A streptococcus. Infect Immun. 2008;76:5006–15. doi: 10.1128/IAI.00300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sumby P, Porcella SF, Madrigal AG, et al. Evolutionary origin and emergence of a highly successful clone of serotype M1 group a Streptococcus involved multiple horizontal gene transfer events. J Infect Dis. 2005;192:771–82. doi: 10.1086/432514. [DOI] [PubMed] [Google Scholar]
- 33.Beres SB, Sylva GL, Barbian KD, et al. Genome sequence of a serotype M3 strain of group A Streptococcus: phage-encoded toxins, the high-virulence phenotype, and clone emergence. Proc Natl Acad Sci U S A. 2002;99:10078–83. doi: 10.1073/pnas.152298499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bates CS, Toukoki C, Neely MN, Eichenbaum Z. Characterization of MtsR, a new metal regulator in group A streptococcus, involved in iron acquisition and virulence. Infect Immun. 2005;73:5743–53. doi: 10.1128/IAI.73.9.5743-5753.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quigley BR, Hatkoff M, Thanassi DG, Ouattara M, Eichenbaum Z, Scott JR. A foreign protein incorporated on the Tip of T3 pili in Lactococcus lactis elicits systemic and mucosal immunity. Infect Immun. 2010;78:1294–303. doi: 10.1128/IAI.01037-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olive C, Sun HK, Ho MF, et al. Intranasal administration is an effective mucosal vaccine delivery route for self-adjuvanting lipid core peptides targeting the group A streptococcal M protein. J Infect Dis. 2006;194:316–24. doi: 10.1086/505580. [DOI] [PubMed] [Google Scholar]
- 37.Malli E, Tatsidou E, Damani A, et al. Macrolide-resistant Streptococcus pyogenes in Central Greece: prevalence; mechanism and molecular identification. Int J Antimicrob Agents. 2010;35:614–5. doi: 10.1016/j.ijantimicag.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 38.Ikebe T, Wada A, Oguro Y, et al. Emergence of clindamycin-resistant Streptococcus pyogenes isolates obtained from patients with severe invasive infections in Japan. Jpn J Infect Dis. 2010;63:304–5. [PubMed] [Google Scholar]
- 39.Feng L, Lin H, Ma Y, et al. Macrolide-resistant Streptococcus pyogenes from Chinese pediatric patients in association with Tn916 transposons family over a 16-year period. Diagn Microbiol Infect Dis. 2010;67:369–75. doi: 10.1016/j.diagmicrobio.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 40.Ardanuy C, Domenech A, Rolo D, et al. Molecular characterization of macrolide- and multidrug-resistant Streptococcus pyogenes isolated from adult patients in Barcelona, Spain (1993–2008) J Antimicrob Chemother. 2010;65:634–43. doi: 10.1093/jac/dkq006. [DOI] [PubMed] [Google Scholar]
- 41.Repa A, Grangette C, Daniel C, et al. Mucosal co-application of lactic acid bacteria and allergen induces counter-regulatory immune responses in a murine model of birch pollen allergy. Vaccine. 2003;22:87–95. doi: 10.1016/s0264-410x(03)00528-0. [DOI] [PubMed] [Google Scholar]
- 42.Hanniffy SB, Carter AT, Hitchin E, Wells JM. Mucosal delivery of a pneumococcal vaccine using Lactococcus lactis affords protection against respiratory infection. J Infect Dis. 2007;195:185–93. doi: 10.1086/509807. [DOI] [PubMed] [Google Scholar]
- 43.Buccato S, Maione D, Rinaudo CD, et al. Use of Lactococcus lactis expressing pili from group B Streptococcus as a broad-coverage vaccine against streptococcal disease. J Infect Dis. 2006;194:331–40. doi: 10.1086/505433. [DOI] [PubMed] [Google Scholar]
- 44.Olive C, Clair T, Yarwood P, Good MF. Protection of mice from group A streptococcal infection by intranasal immunisation with a peptide vaccine that contains a conserved M protein B cell epitope and lacks a T cell autoepitope. Vaccine. 2002;20:2816–25. doi: 10.1016/s0264-410x(02)00205-0. [DOI] [PubMed] [Google Scholar]
- 45.D'Alessandri R, Plotkin G, Kluge RM, et al. Protective studies with group A streptococcal M protein vaccine. III. Challenge of volunteers after systemic or intranasal immunization with Type 3 or Type 12 group A Streptococcus. J Infect Dis. 1978;138:712–8. doi: 10.1093/infdis/138.6.712. [DOI] [PubMed] [Google Scholar]
- 46.Batzloff MR, Pandey M, Olive C, Good MF. Advances in potential M-protein peptide-based vaccines for preventing rheumatic fever and rheumatic heart disease. Immunol Res. 2006;35:233–48. doi: 10.1385/IR:35:3:233. [DOI] [PubMed] [Google Scholar]
- 47.Jones T, Allard F, Cyr SL, et al. A nasal Proteosome influenza vaccine containing baculovirus-derived hemagglutinin induces protective mucosal and systemic immunity. Vaccine. 2003;21:3706–12. doi: 10.1016/s0264-410x(03)00387-6. [DOI] [PubMed] [Google Scholar]
- 48.Johansson L, Thulin P, Low DE, Norrby-Teglund A. Getting under the skin: the immunopathogenesis of Streptococcus pyogenes deep tissue infections. Clin Infect Dis. 2010;51:58–65. doi: 10.1086/653116. [DOI] [PubMed] [Google Scholar]