Abstract
Background. Autophagy is critical to maintaining cell homeostasis and is implicated in neurodegenerative diseases. This research examined the role of autophagy in human immunodeficiency virus type 1 (HIV-1)–associated encephalitis, the pathologic hallmark of neuroAIDS.
Methods. The frontal cortex from 32 HIV-infected persons (12 without evidence HIV-1 encephalitis or clinical signs of central nervous system impairment and 20 with histopathological findings of HIV-1 encephalitis) and 8 persons without HIV infection and any neuropathology were examined postmortem. Green fluorescent protein-labeled (GFP) light chain 3 (LC3)–expressing neuroblastoma SK-N-SH cells treated with gp120 from CXCR4 and CCR5 viruses were also examined. Autophagic markers were assessed by means of Western blot analysis, transmission electron microscopy (TEM), and confocal microscopy.
Results. Autophagic proteins Beclin 1, Autophagy-related gene (Atg)-5, Atg-7, and LC3-II were significantly increased in brains with HIV-1 encephalitis (P < .05). These findings were confirmed by TEM and immunostaining of brain tissue. Additionally, levels of autophagic proteins and autophagosomes were increased in neuronal cells treated with both CXCR4- or CCR5-tropic HIV-1 gp120. No increase in the level of autophagy was observed in the brains of HIV-infected persons without HIV-1 encephalitis compared with the level in brains of HIV-uninfected persons.
Conclusions. Postmortem brains with HIV-1 encephalitis exhibit increased markers of autophagy compared with brains from HIV-infected persons without HIV-1 encephalitis or HIV-uninfected control brains, which suggests that dysregulation of autophagy may be important in the pathogenesis of neuroAIDS.
The central nervous system (CNS) is an important target of human immunodeficiency virus type 1 (HIV-1) and is associated with both subtle and severe neurological impairment. With the increased use of highly active antiretroviral therapy, severe HIV-associated dementia is less common; however, minor cognitive motor disorder remains an important part of infection, affecting ∼30% of persons living with HIV-1 infection [1–4].
The neuropathological hallmarks of HIV-1 infection in the brain are encephalitis featured with widespread reactive astrocytosis, myelin pallor, microglial nodules, activated resident microglia, multinucleated giant cells, and infiltration predominantly by monocytoid cells, including blood-derived macrophages [5]. Macrophages and microglia are the primary cells infected by HIV-1, can support viral replication, and provide the primary ongoing source of virus in the CNS [6]. Although HIV-1 does not directly infect the neuron, infection of macrophages and microglia with the release viral proteins and inflammatory mediators have been implicated in neuronal and astrocytic dysfunction, and are thought to drive the pathogenesis of HIV-associated dementia [7, 8].
Autophagy is induced in response to nutrient depletion and is a nonapoptotic pathway of programmed cell death. The hallmark of autophagy is a double-membraned autophagosome that ultimately fuses with a lysosome, thereby generating a single-membraned autolysosome that is capable of degrading the contents, which can then be recycled by the cell [9, 10]. There is a basal homeostatic level of autophagy in brain neurons that when altered can result in neuronal dysfunction without cell death [11]. Numerous examples exist for autophagy playing an important role in neurological disorders including degenerative CNS diseases such as Alzheimer's disease, Huntington's disease, and Parkinson's disease [12–19]. Markers of autophagy include the following: Beclin 1, a Bcl-2-interacting coiled-coil protein, which mainly engages hVps34 in the autophagic pathway and in autophagosome formation [20–23]; and microtubule-associated protein (MAP) light chain 3 (LC3), in which the conversion of LC3-I (18 kDa) to LC3-II (16 kDa) is a specific autophagic marker [24–26].
The alterations of cellular processes induced by viral infection generally favor viral replication and spread [27–29]. Recent data indicate that exposure of T cells to HIV-1 gp120 can lead to bystander cell death through induction of autophagy associated with gp120/CXCR4 interactions [30, 31]. In contrast to the effect on bystander cells, during permissive HIV-1 infection, autophagy is inhibited [32]. In the research presented here, we have examined the possible role of autophagy in the development of neuroAIDS. Our findings indicate that postmortem brains with evidence of HIV-1 encephalitis demonstrate increased markers of autophagy. Moreover, in an in vitro model using a neuronal cell line, we demonstrate that gp120 from CCR5- or CXCR4-using virus can induce autophagy.
MATERIALS AND METHODS
Brain Tissue and Reagents
Postmortem brain tissues of frontal cortex were obtained from 32 HIV-infected persons from the HIV Neurobehavioral Research Center and California NeuroAIDS Tissue Network at the University of California San Diego. Table 1 summarizes the demographic data available on persons from whom brain samples were obtained. HIV-infected persons had neuromedical and neuropsychological examinations a median of 12 months before death. Autopsies were performed within 24 h of death. Persons with a history of CNS opportunistic infections or non-HIV-related developmental, neurologic, psychiatric, or metabolic conditions that might affect CNS function (eg, loss of consciousness exceeding 30 min in duration, psychosis, or substance dependence) were excluded. Neuropathological assessment was performed on paraffin sections from the frontal cortex, parietal cortex, temporal cortex, hippocampus, basal ganglia, and brain stem, which were stained with hematoxylin and eosin or immunolabeled with antibodies against p24 and glial fibrillary acidic protein (GFAP). The diagnosis of HIV encephalitis was based on the presence of microglial nodules, astrogliosis, HIV p24–positive cells, and myelin pallor. Among HIV-1-positive brains, there were 12 without evidence of HIV encephalitis and/or clinical signs of CNS impairment (HIV-positive only group) and 20 with histopathological findings consistent with HIV encephalitis (10 with previously diagnosed HIV dementia). Additionally, brain samples from 8 persons without HIV-1 infection and any known neurological symptoms or neuropathology were used as HIV-negative controls.
Table 1.
Summary of Subject Demographic Characteristics and Clinical History
| HIV status, subject no. | Age, years | Sex | Neurological diagnosis during life | Neuropathology | Cause of death | Receiving ART at time of death |
| Uninfected | ||||||
| 1 | 26 | Male | None | NPC | Hodgkin's lymphoma | No |
| 2 | 53 | Male | None | NPC | Cardiac failure | No |
| 3 | 43 | Male | None | NPC | Coccidiomycosis | No |
| 4 | 48 | Male | None | NPC | Steatosis, liver failure | No |
| 5 | 47 | Female | None | NPC | CHF | No |
| 4 | 53 | Male | None | NPC | Cholangiocarcinoma | No |
| 7 | 48 | Female | None | NPC | Respiratory failure | No |
| 8 | 55 | Female | None | NPC | Pneumonia | No |
| Infected without HIVE | ||||||
| 1 | 46 | Male | None | NPC | AIDS-NSDx | Yes |
| 2 | 55 | Male | None | NPC | Renal failure | Yes |
| 3 | 53 | Male | None | NPC | AIDS-NSDx | Yes |
| 4 | 45 | Male | None | NPC | Acute aspiration | Yes |
| 5 | 59 | Male | None | NPC | Pneumonia | Yes |
| 6 | 54 | Male | None | NPC | Cardiac infarction | Yes |
| 7 | 43 | Male | MCMD | NPC | Pulmonary embolism | Yes |
| 8 | 67 | Male | MCMD | NPC | Renal failure | Yes |
| 9 | 39 | Male | MCMD | NPC | Pneumonia | Yes |
| 10 | NA | NA | None | NPC | AIDS-NSDx | Yes |
| 11 | NA | NA | None | NPC | AIDS-NSDx | Yes |
| 12 | NA | NA | None | NPC | AIDS-NSDx | Yes |
| Infected with HIVE | ||||||
| 1 | 34 | Male | None | HIVE | PCP, CMV infection, KS | Yes |
| 2 | 37 | Male | None | HIVE | PCP | No |
| 3 | 59 | Female | None | HIVE | PCP | No |
| 4 | 43 | Male | None | HIVE | MAC infection, pneumonia | Yes |
| 5 | 35 | Male | MCMD | HIVE | AIDS-NSDx | Yes |
| 6 | 37 | Male | MCMD | HIVE | Pneumonia | Yes |
| 7 | 42 | Male | MCMD | HIVE | AIDS-NSDx | Yes |
| 8 | 46 | Male | MCMD | HIVE | Sepsis | No |
| 9 | 39 | Male | MCMD | HIVE | Multi-organ failure | No |
| 10 | NA | NA | None | HIVE | Aseptic meningitis | Yes |
| Infected with HAD and HIVE | ||||||
| 1 | 38 | Female | HAD | HIVE | Hypotension | No |
| 2 | 57 | Male | HAD | HIVE | Disseminated MAC infection | No |
| 3 | 34 | Male | HAD | HIVE | Heart failure | No |
| 4 | 43 | Male | HAD | HIVE | Pneumonia | No |
| 5 | 55 | Male | HAD | HIVE | Cardiac arrhythmia | Yes |
| 6 | 31 | Male | HAD | HIVE | Renal failure | No |
| 7 | 39 | Male | HAD | HIVE | Sepsis | Yes |
| 8 | 29 | Male | HAD | HIVE | Pneumonia | No |
| 9 | 40 | Male | HAD | HIVE | Electrolyte imbalance | No |
| 10 | NA | NA | HAD | HIVE | AIDS-NSDx | No |
NOTE. AIDS-NSDx, AIDS—no specific cause of death noted; ART, antiretroviral therapy; CHF, congestive heart failure; CMV, cytomegalovirus; HAD, HIV-associated dementia; HIV, human immunodeficiency virus; HIVE, HIV-associated encephalitis; KS, Kaposi sarcoma; MAC, Mycobacterium avium complex; MCMD, minor cognitive motor disorder; NA, not available; NPC, no neuropathological changes; PCP, pneumocystis pneumonia.
HIV-1 gp120 (expressed in Chinese hamster ovary cells; CXCR4-using virus strain HIV-1MN) was obtained from Immunodiagnostics. HIV-1BAL gp120 (CCR5-using virus) was obtained from the AIDS Research and Reference Program. Antibodies against Beclin 1, Atg-7, Atg-5, lysosomal membrane protein 1 (LAMP-1), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were purchased from Santa Cruz Biotechnology. The rabbit LC3 antibodies were purchased from Abgent.
Double Immunolabeling and Confocal Laser Microscopy
To evaluate the colocalization of neurons and the autophagosome marker LC3, double immunocytochemical analysis was performed. Vibratome sections were immunolabeled with a monoclonal antibody against NeuN, and LC3 was detected with fluorescein isothiocyanate–conjugated and Cy3-conjugated secondary antibodies. Sections were processed under the same conditions, and experiments were repeated to ensure reproducibility. Sections were imaged with a Zeiss 63X (numerical aperture, 1.4) objective on an Axiovert 35 microscope (Zeiss) with an attached MRC1024 laser scanning confocal microscopy system (BioRad). To confirm the specificity of primary antibodies, control experiments were performed in which sections were incubated overnight in the absence of primary antibody or pre-immune serum and primary antibody alone.
Electron Microscopic Analysis
Vibratome sections were fixed in .25% glutaraldehyde and 3% paraformaldehyde in .1 mol/L cacodylate buffer (pH, 7.4) and then pre-embedded with 50% Durcupan epoxy resin and 50% ethanol (dry) for 30 min. Tissues were then embedded in Durcupan mix epoxy resin, polymerized under a vacuum at 60°C for 48 h, mounted into plastic cylinders, sectioned with an ultra microtome (Reichert Ultracut E) at 60-nm thickness, and collected in copper grids for ultrastructural analysis. Grids were analyzed with a Zeiss EM10 electron microscope, and electron micrographs were obtained at a magnification of ×20,000. Electron microscopic quantitative analyses of lysosomes and autophagosomes were performed on pyramidal neurons in the frontal cortex of HIV-uninfected brains, HIV-infected brains without evidence of HIV encephalitis, and brains with HIV encephalitis (n = 5 cases per group). The mean and standard deviation of the number of lysosomes and autophagosomes was determined for a total of 25 neurons per case.
Treatment of Neuroblastoma Cells With HIV-1 gp120
The GFP-LC3 expression vector was from T. Yoshimori (National Institute of Genetics, Shizuoka, Japan) [30]. Cells were transfected with each expression plasmid by use of the Lipofectamine 2000 reagent (Invitrogen) [25, 33, 34]. SK-N-SH cells (ATCC) were transfected with GFP-LC3-expressing plasmids and maintained in G418-containing Dulbecco modified Eagle medium with fetal bovine serum. Approximately 1 × 106 SK-N-SH cells in 6-well flat-bottom plates were cultured in complete medium with or without gp120 or rapamycin. For protein analysis, cells were harvested at specified time points and treated with M-Per protein extraction buffer according to the manufacturer's instructions (Pierce).
Treatment of Neuroblastoma Cells With Bafilomycin A1
GFP-LC3-expressing SK-N-SH cells were cultured on coverslips (Grace Bio-Labs) with culture medium only, HIV-1MN gp120 (100 ng/mL), HIV-1BAL gp120 (100 ng/mL), or rapamycin (200 nmol/L), with or without pretreatment with bafilomycin A1 (100 nmol/L) for 30 min (Calbiochem), for 6 h [35]. Cells were stained with Lysotracker red (Invitrogen) at 37°C with 5% carbon dioxide for 45 min, fixed with 3.7% formaldehyde for 15 min at room temperature, and washed with phosphate-buffered saline. Slides were mounted with ProLong Gold Antifade reagent with 4′,6-diamidino-2-phenylindole (Invitrogen), examined using an Olympus confocal microscope, and analyzed with FV10-ASW Viewer software (version 2.1; Olympus). The colocalization of images was analyzed with ImageJ software (National Institutes of Health) and the Co-localization Colormap plug-in (Adam Gorlewicz; http://rsbweb.nih.gov/ij/notes.html) [35].
Western Blot Analysis of Autophagic Proteins
Brain tissue and SK-N-SH cells were homogenized and lysed to obtain tissue lysates. Western blots were performed as described elsewhere [32]. Rabbit polyclonal antibodies against human LC3 or cleaved LC3 and goat polyclonal antibodies against human Beclin 1, Atg-7, Atg-5, or GAPDH were used for primary antibodies. The blot membrane was exposed to x-ray film, developed, scanned, and analyzed with ImageJ software (National Institutes of Health).
Quantification of the GFP-LC3 Puncta
GFP-LC3-transfected SK-N-SH cells were grown to subconfluence on poly-l-lysine-coated glass coverslips (Sigma) under specified treatment conditions. The samples were mounted and imaged for green fluorescent protein GFP-LC3–labeled autophagosomes by use of an Olympus confocal disk-spinning microscope. The fluorescence of GFP-LC3 puncta was quantified with Slidebook software (Intelligent Imaging Innovations) in at least 3 independent experiments [32].
Statistical Analysis
All analyses were conducted on coded samples with the clinical and pathological diagnoses unknown to the investigators. For brain tissue, comparisons between 2 groups were performed using the unpaired Student t test. Results for which P < .05 (2-sided test) were considered significant.
RESULTS
Autophagic Protein Levels Are Increased in Brain Tissues of Persons With HIV Encephalitis
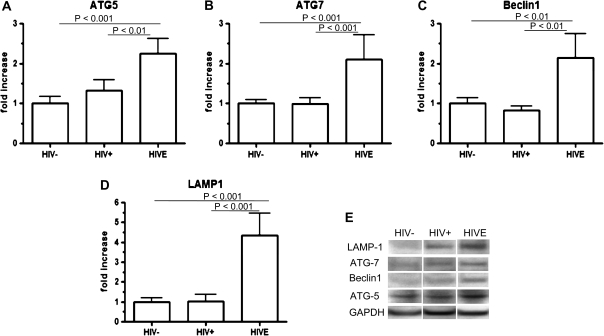
Using Western blot analyses, levels of autophagic proteins Atg-5, Atg-7, and Beclin 1 were found to be significantly increased in the brains of persons with HIV encephalitis in comparison to HIV-positive only brains and control brains, whereas there was no difference between HIV-positive only brains and uninfected control brains (Figure 1). The mean (+SE) autophagic protein expression was increased for HIV encephalitis brains compared with the levels obtained from HIV-positive only brains with no evidence of HIV encephalitis: 2.25-fold (± .37-fold; P < .01) for Atg-5, 2.11-fold (± .62-fold; P < .01) for Atg-7, and 2.15-fold (± .59-fold; P < .001) for Beclin 1P. No significant differences were observed for autophagic protein expression between the subgroups of brains with HIV encephalitis with previously documented HIV-associated dementia and those with HIV encephalitis without previously documented HIV-associated dementia (P >.10 for all comparisons; data not shown).
Figure 1.
Quantification of autophagic proteins and lysosomal membrane protein 1 (LAMP-1) in postmortem brain tissue from patients with human immunodeficiency virus (HIV) infection. Autophagic proteins and LAMP-1 were detected with Western blotting. The density of the protein bands on Western blots was analyzed and quantitated using ImageJ software (National Institutes of Health). The autophagic proteins were normalized with glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The ratio of the mean protein level of the brains from each group of HIV-infected patients (HIV –positive only (HIV+), n = 12 patients; HIV encephalitis [HIVE], n = 20 patients) to that of the brains from HIV-uninfected persons (HIV−; n = 8 persons) was calculated and expressed as the fold increase. A, Atg-5; B, Atg-7; C, Beclin 1; D, LAMP-1. E, Western blot bands from a representative patient’s brain. The band sizes of the proteins are 23 kDa (Atg-5), 60 kDa (Beclin 1), 71 kDa (Atg-7), and 120 kDa (LAMP-1). Error bars show the standard error of the mean.
LAMP-1 Expression Is Increased in Brains of Persons With HIV Encephalitis
LAMP-1 is a commonly used marker for the presence of lysosomes and autolysosomes in the cytoplasm of cells [36]. By Western blot analysis, the level of LAMP-1 was found to be increased 4.35-fold (± .48-fold) in brains with HIV encephalitis above the level in control brains, compared with a modest increase of 1.42-fold (± 1.13-fold) in HIV-positive only brains (P < .01) (Figure 1D). No differences were observed between HIV encephalitis brains with HIV-associated dementia and those without HIV-associated dementia (data not shown), nor between control brains from HIV-uninfected persons and HIV-positive only brains.
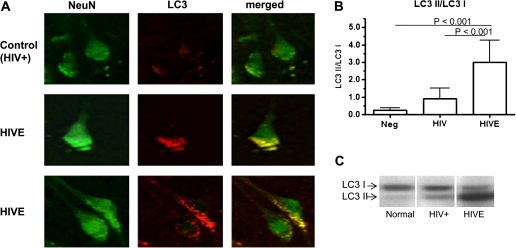
LC3 Puncta and LC3-II/LC3-I Ratio Are Increased in Brain Tissue With HIV Encephalitis
Using double labeling for the neuronal cell-specific marker NeuN and for the autophagosome marker LC3, the relative number of LC3 puncta in neuronal cells was assessed by means of immunofluorescence. Brains demonstrating HIV encephalitis were consistently found to exhibit LC3 puncta in neurons, whereas the neurons in brains obtained from HIV-positive only patients rarely showed any LC3 staining (Figure 2A). To further evaluate the relative level of autophagy, we assessed the conversion of LC3-I to LC3-II that occurs during the process of autophagosome formation [30–32]. Our findings indicate that the mean (+SE) LC3-II/LC3-I ratio is significantly increased in HIV encephalitis brains (3.0 ± 1.3) compared with that in HIV-positive only brains (.92 ± .59; P < .001) (Figure 2B). The quantity of LC3-II as measured by the LC3-II/GAPDH ratio (1.40 ± .01) is also significantly increased in HIV encephalitis brains compared with that in HIV-positive only brains (P <.05; data not shown). Again, no differences were observed between brains of persons with HIV encephalitis with documented HIV-associated dementia or those from persons without dementia, or between control HIV-negative brains and HIV-positive only brains (data not shown).
Figure 2.
Double labeling of frontal cortex for neuN/light chain 3 (LC3) and ratio of LC3-II to LC3-I proteins in postmortem brain tissue from patients with human immunodeficiency virus (HIV) infection. A, Representative brain from a patient with HIV encephalitis (HIVE) and representative brain from an HIV-positive (HIV+) patient without any evidence of HIV encephalitis, double-labeled with NeuN and LC3. Whereas neurons can be clearly seen to express LC3 in the frontal cortex of the brain with HIV encephalitis, LC3 expression was not observed in the HIV-positive only brain. B, LC3 protein quantification. LC3 protein was detected by Western blot analysis and quantitated using ImageJ software (National Institutes of Health). The mean ratio of LC3-II to LC3-I for the HIV-infected groups of patients is compared with that for the HIV-uninfected group (Neg). C, Representative Western blot.
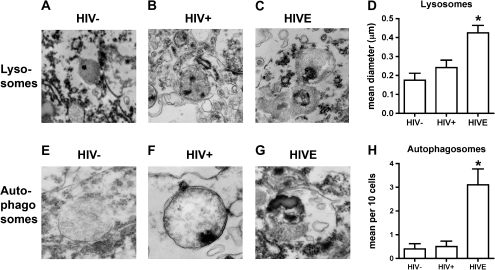
Levels of Autophagosomes and Lysosomes Are Increased in Brains With HIV Encephalitis
Maturation of autophagosomes involves fusion of autophagosomes and lysosomes resulting in the release of sequestered contents for degradation and recycling by enzymes within the lysosome. To identify whether autophagosomes and lysosomes are detectable within the brains of persons with HIV encephalitis, pyramidal neurons in the frontal cortex were examined by transmission electron microscopy (TEM). Twenty-five neurons from each case were analyzed, with 5 cases in each group (persons with HIV encephalitis, HIV-positive persons without evidence of HIV encephalitis, and HIV-negative persons). In HIV encephalitis cases, the lysosomes were enlarged and contained abundant electrodense material. Figure 3A–3C illustrates the ultrastructural characteristics of the lysosomes that contain electrodense granules. The mean diameter of the lysosomes present in the HIV encephalitis brains was significantly larger than that in the other 2 groups of brains (P < .05) (Figure 3D). Additionally, whereas only rare autophagosomes were detected in the HIV-infected brains with no HIV encephalitis and HIV-uninfected brains, in the HIV encephalitis brains, the lysosomes were more abundant and larger and contained electrodense material (Figure 3E–3G). Additionally, the number of autophagosomes in neuronal cell bodies was significantly increased (P < .05) (Figure 3H).
Figure 3.
Electron microscopic analysis of lysosomes and autophagy in human immunodeficiency virus (HIV)–associated encephalitis. Panels were obtained postmortem from pyramidal neurons in the frontal cortex of HIV-negative persons (HIV−), HIV-positive persons (HIV+), and persons with HIV encephalitis (HIVE; n = 5 persons per group). On average, a total of 25 neurons were analyzed in each case. A–C, Utrastructural characteristics of the lysosomes, which contain electrodense granules. In HIV encephalitis cases, the lysosomes were enlarged and contained abundant electrodense material. D, Analysis of mean lysosme diameter. E–G, Utrastructural characteristics of the autophagosomes. In HIV-negative control cases, only rare autophagosomes were detected; in most of them the membranes were thin, and in most instances the double membrane was detectable. In HIV encephalitis cases, the autophagosomes were enlarged, were more abundant, and contained electrodense material. H, Ultrastructural analysis of autophagosome frequency in neuronal cell bodies; *P < .05 for comparison of HIV encephalitis brains with HIV-negative brains or HIV-positive brains with no evidence of HIV encephalitis.
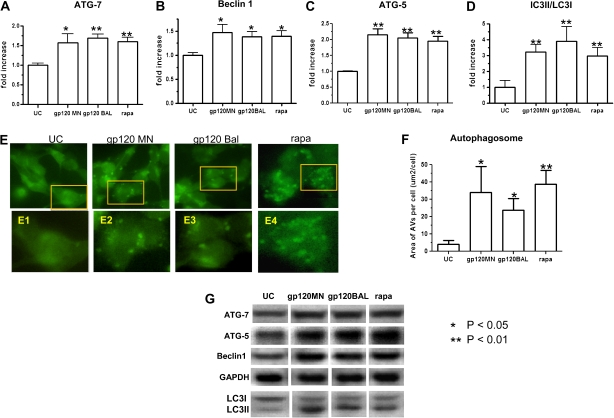
SK-N-SH Cells Treated With HIV-1MN and HIV-1BAL gp120 Exhibit Increased Autophagy
To assess the effects of HIV-1 gp120 on neurons, we used the human neuroblastoma SK-N-SH cell line that is restricted for HIV-1 replication [37]. SK-N-SH cells, like neurons in vivo, do not express CD4 but constitutively express CXCR4, CCR5, and N-methyl-D-aspartic acid (NMDA) receptors, each of which is capable of binding HIV-1 gp120 [38–40]. In these experiments, the SK-N-SH cells were exposed to gp120 for 6 h, and levels of the autophagic proteins Atg-5, Atg-7 and Beclin 1 were analyzed by Western blotting (Figure 4). The level of each of the autophagy proteins was significantly elevated following exposure to either HIVMN or HIVBAL gp120 (P < .01 for Atg-5 and Atg-7; P <.05 for Beclin 1) (Figure 4A–4C). These results indicate that the HIV-1 envelope protein gp120 from both CCR5- and CXCR4-using viruses can induce autophagy.
Figure 4.
Expression of autophagic proteins and autophagosome formation in human immunodeficiency virus Type 1 (HIV-1) gp120–treated neuroblastoma SK-N-SH cells. SK-N-SH cells transfected with pGFP–light chain 3 (LC3) were exposed to HIV-1MN gp120 (100 ng/mL), HIV-1BAL gp120 (100 ng/mL), or rapamycin (rapa; 100 nmol/L) for 6 h. Autophagic proteins were detected by Western blot analysis, normalized with glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and analyzed with ImageJ software (National Institutes of Health). The mean (± SE) levels of expression of Atg-7 in HIV-1MN gp120–treated cells (gp120 MN) and HIV-1BAL gp120–treated cells (gp120 BAL) were 1.57-fold (± .23-fold) and 1.69-fold (± .11-fold; P < .01) above those of unconditioned media controls (UC), respectively (A). The mean (± SE) levels of Beclin 1 were 1.30-fold (± .10-fold; P < .05) and 1.40-fold (± .13-fold; P < .05) for gp120 MN and gp120 BAL, respectively (B). The mean (± SE) Atg-5 levels were 2.08-fold (± .22-fold; P < .01) and 2.04-fold (± .20-fold; P < .01) for gp120 MN and gp120 BAL, respectively (C). D, LC3 proteins detected by Western blotting and the ratio of LC3-II to LC3-I. E, Autophagosome formation in HIV-1 gp120–treated SK-N-SH cells. GFP-LC3–expressing SK-N-SH cells grown on glass coverslips were mounted and examined with an Olympus Spinning Disk confocal microscope. With activation, the free-form LC3-I protein is converted to membrane-bond LC3-II, which specifically localizes on the autophagosome membrane and is thus considered to be a specific autophagosome marker. GFP-LC3 puncta are visualized as high-density particles representing autophagosomes. Green GFP-LC3 was evenly distributed in the cytoplasm of unstimulated SK-N-SH cells (UC). Concentrated GFP-LC3 particles representing autophagosomes (or autophagic vacuoles) were seen to be increased in number in the cytoplasm of cells treated with HIV-1MN gp120 and HIV-1BAL gp120. Panels E1, E2, E3, and E4 show enlarged micrographs with 100x objective lens gated for the different treatments, demonstrating the presence of autophagosomes. The mean surface area of autophagic vacuoles (AVs) was calculated for each cell with at least 100 random cells counted for each sample (F). G, Bands of Atg-7 (71 kDa), Atg-5 (23 kDa), Beclin 1 (60 kDa), LC3-II/LC3-I, and GAPDH as detected by Western blot analysis. Results are representative of 5 separate experiments.
To confirm that the level of autophagy was increased in gp120-exposed SK-N-SH cells, the presence of autophagosomes was assessed in treated versus untreated cells transfected with a GFP-LC3 expression plasmid, pGFP-LC3. In these transfected cells, GFP-LC3 is expressed as LC3-I, the original free C-terminus state of LC3. With activation, GFP-LC3-I was broadly distributed throughout the cytoplasm, whereas the converted GFP-LC3-II as a specific autophagosome marker was seen as high-density green particles (GFP-LC3 puncta) anchored to newly formed autophagosomes (Figure 4E). After the transfected cells were treated with gp120 either from X4 (HIVMN) or R5 (HIVBAL) virus, the number of fluorescent-staining autophagosomes was markedly increased (Figure 4F). Similarly, the mean (± SE) level of LC3-II protein was also increased following gp120 exposure, with a 3-fold increase (± .48-fold) for HIVMN gp120 and 3.9-fold increase (± .9-fold) for HIVBAL gp120 (Figure 4D).
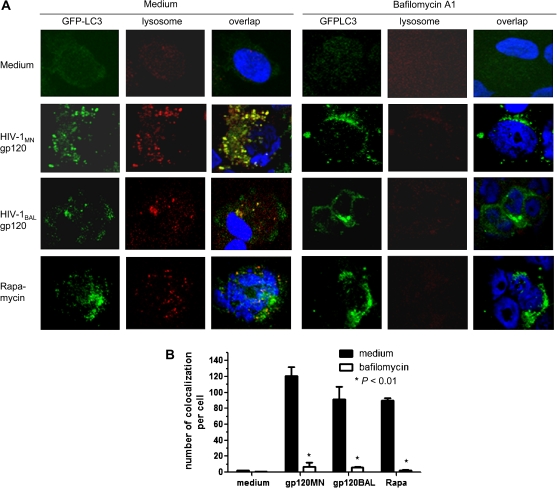
Bafilomycin A1 Affects the Colocalization of Autophagosomes and Lysosomes
As an assessment of autophagic flux, SK-N-SH cells were pretreated with bafilomycin A1, an inhibitor of the vacuolar hydrogen adenosine triphosphatase and autophagosome-lysosome fusion [35, 41, 42]. GFP-LC3-expressing SK-N-SH cells were cultured with medium only, HIV-1MN gp120 (100 ng/mL), HIV-1BAL gp120 (100 ng/mL), or rapamycin (200 nmol/L) with or without pretreatment with bafilomycin A1 (100 nmol/L; 30 min) for 6 h. Autophagosomes, represented by GFP-LC3 puncta, were detected in cells pretreated with bafilomycin A1 followed by either HIV-1MN or HIVBAL gp120, or rapamycin. However, the absolute number of lysosomes or autophagosomes colocalized with lysosomes was markedly decreased in bafilomycin A1 pretreated cells (P <.01) (Figure 5A and 5B). These findings suggest that autophagy induced by HIV-1 gp120 promotes the fusion of autophagosomes with lysosomes, which was inhibited by bafilomycin A1.
Figure 5.
Colocalization of autophagosomes and lysosomes in bafilomycin A1–treated cells. SK-N-SH cells were cultured with medium only, HIV-1MN gp120 (gp120MN; 100 ng/mL), HIV-1BAL gp120 (gp120BAL; 100 ng/mL), and rapamycin (Rapa; 200 nmol/L) with or without bafilomycin A1 (100 nmol/L) for 6 h. A, Cells stained for GFP-LC3 puncta representing autophagosomes (green), lysosomes using Lysotracker red (red), and nuclei using 4′,6-diamidino-2-phenylindole (blue). Yellow particles represent GFP-LC3 puncta colocalized with lysosomes. B, Mean (± SE) number of colocalized particles per cell for each of the different treatment regimens performed in triplicate.
DISCUSSION
Despite much research on the pathogenesis of neurological complications associated with HIV-1 infection, the mechanisms associated with the development of CNS impairment remain unclear. Recently, we proposed that alterations in neuronal autophagy may play an important role in HIV-related CNS impairment [43]. Autophagy is involved with both neuroprotection and neurodegeneration, and has been implicated in a number of neurological diseases including Alzheimer's disease, Parkinson's disease, and Huntington's disease [44]. In these conditions, an accumulation of autophagosomes is found in brains of persons who died of the disorders as well as in mouse models that represent an increase in autophagy associated with a deficiency in autophagosome-lysosome fusion [44–46]. A common pathway for these neurodegenerative diseases appears to be the inadequate or inefficient removal of protein aggregrates by autophagy [47]. Additionally, there is increasing evidence to suggest that enhanced autophagy leads to neurite degeneration. In contrast, inhibition of autophagy results in a decrease in the number of autophagosomes and protects neurites against degeneration [48]. However, although autophagy protects cells from stress-related toxicity, overinduction of autophagy can lead to cell death. Thus, a delicate balance exists in which autophagy protects neurons from external toxins but overinduction can lead to cell destruction.
In our previous research, we have demonstrated that permissive HIV-1 infection of CD4+ lymphocytes and macrophages leads to a down-regulation of autophagy, whereas induction of autophagy can inhibit HIV-1 replication [32] (Campbell and Spector, submitted). In the findings presented here, we demonstrate that within the CNS, neurons that are nonpermissive for HIV-1 show an increase in autophagic activity. We have used a number of different approaches to confirm this increase in the brains with histopathologically confirmed HIV-1 encephalitis. First, because Beclin 1 is required for autophagy, we examined the levels of Beclin 1 by Western blot analysis. Brains with HIV-1 encephalitis consistently expressed higher levels of Beclin 1 protein compared with those of HIV-positive only brains and uninfected control brains. In additional experiments, levels of Atg proteins Atg-5 and Atg-7 were found to be increased in HIV encephalitis brains. Autophagosome formation requires Beclin 1 for nucleation, whereas vesicle elongation is regulated by the covalent binding of Atg-5 to Atg-12—a process catalyzed by Atg-7 and Atg-10. These findings suggested to us that the level of autophagy is, in fact, likely increased. Because conjugation of LC3-I to LC3-II is mediated by Atg-7, Atg-3, and Atg-4, we compared LC3-II to LC3-I and found the ratio to be increased HIV encephalitis brains. Unlike the Atg-12–Atg-5–Atg-16 complex, which is only transiently associated with the autophagosome, LC3-II remains associated with mature autophagosomes and is degraded together with the cargo following fusion of the autophagosome with a lysosome. This finding combined with the increase in LC3 puncta in HIV encephalitis brains further supports an increase in autophagic activity.
Since an increase in autophagic activity is known to be associated with increased numbers of lysosomes and autophagosomes, we also examined the brain tissues for evidence of the expression of LAMP-1. Similar to the increase in the level of autophagic proteins in HIV encephalitis brains, we found an increase in the level of LAMP-1 protein in the same brain tissues. An increase in the numbers of autophagosomes and lysosomes in HIV encephalitis brains detected by TEM provides additional support for enhanced autophagic activity.
Of interest, we observed no differences in autophagic markers between the brains obtained from persons with documented HIV-associated dementia prior to death and those from persons with HIV encephalitis diagnosed postmortem. The brains available for this study were obtained from persons who had neurocognitive evaluations a median of 12 months prior to death. In the interim, it is likely that impaired CNS function would have been detected if evaluations were performed at shorter intervals. Additionally, consistent with our findings of autophagic markers, no differences were observed in the extent of HIV encephalitis between the 2 groups.
A limitation of our study was that only postmortem brain tissues were evaluated. Therefore, it is possible that the changes observed postmortem might not be present during life. However, given that all specimens were obtained postmortem, it is unlikely that the increase in autophagy-related proteins would be differentially altered in 1 group of patients. Another potential limitation to our study is that the cause of death and some other demographic data were not available for all the patients whose brain tissues were evaluated. However, any brains that demonstrated evidence of infection with pathogens other than HIV-1 or were found to have any HIV-unrelated neuropathological disease were excluded from the study.
Recently, Alirezaei et al [49] found evidence for impaired autophagy in a limited number of primate brains with simian immunodeficiency virus encephalitis (SIVE) infection and brains obtained postmortem from HIV-infected persons with HIV-associated dementia. However, in an addendum, the investigators briefly noted that exposure of SK-N-SH cells to microglial supernatant resulted in increased autophagy [50]. Our research demonstrates that SK-N-SH cells exposed to gp120 from either CXCR4- or CCR5-using viruses results in induction of autophagy. Although it is unclear why Alirezaei et al [49, 50] have observed a decrease in autophagy in brains of persons who died of HIV-associated dementia, we believe that our findings of increased levels of autophagic markers in brains with documented HIV encephalitis and/or HIV-associated dementia are consistent with the in vitro findings of Alirezaei et al [49, 50] and with those of Espert et al [30], who observed increased autophagy in gp120-treated CD4+ T cells. In the study by Espert et al [30], autophagic proteins Atg-7 and Beclin 1 were examined; both were found to be increased in CD4+ cells following exposure to gp120.
It is important to note that our findings of increased markers of autophagy in brains of persons with HIV encephalitis may represent either an absolute increase of autophagy within neurons or ineffective autophagy with an accumulation of autophagic products. In this regard, our findings in this and previous research suggest a possible model for the development and progression of HIV-related CNS impairment. In this model, during permissive infection of susceptible cells, HIV-1 down-regulates autophagy to facilitate viral replication. In contrast, cells that bind HIV-1 gp120 and other products of infection that are nonpermissive to HIV-1 replication enhance autophagy in an attempt to remove toxic stress and maintain cell survival. In this scenario, our data suggest that although autophagy may help to sustain neuron survival, the increase in autophagic activity contributes to the development of HIV encephalitis and cognitive impairment. Therefore, when combined with antiretrovirals, drugs that reduce autophagic activity to homeostatic levels may help to prevent or reverse the CNS deficits associated with HIV-1 infection.
In summary, our findings indicate that postmortem brains with evidence of HIV encephalitis have increased markers of autophagy compared with brains from HIV-infected persons without evidence of HIV encephalitis or HIV-uninfected control brains. These findings provide support for the hypothesis that the dysregulation of autophagy during HIV infection is important in the pathogenesis of neuroAIDS.
Funding
This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant numbers AI068632 and R21 AI084573 to the International Maternal Pediatric Adolescent AIDS Clinical Trials [IMPAACT] Network).
Acknowledgments
We thank Jennifer Meerloo and Kersi Pestonjamasp of the University of California San Diego Microscopy Center for technical support.
References
- 1.Ances BM, Ellis RJ. Dementia and neurocognitive disorders due to HIV-1 infection. Semin Neurol. 2007;27:86–92. doi: 10.1055/s-2006-956759. [DOI] [PubMed] [Google Scholar]
- 2.McArthur JC. HIV dementia: an evolving disease. J Neuroimmunol. 2004;157:3–10. doi: 10.1016/j.jneuroim.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 3.Robertson KR, Smurzynski M, Parsons TD, et al. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS. 2007;21:1915–21. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- 4.Joska JA, Gouse H, Paul RH, Stein DJ, Flisher AJ. Does highly active antiretroviral therapy improve neurocognitive function? A systematic review. J Neurovirol. 2010;16:101–14. doi: 10.3109/13550281003682513. [DOI] [PubMed] [Google Scholar]
- 5.Gray F, Adle-Biassette H, Chretien F, Lorin de la Grandmaison G, Force G, Keohane C. Neuropathology and neurodegeneration in human immunodeficiency virus infection: pathogenesis of HIV-induced lesions of the brain, correlations with HIV-associated disorders and modifications according to treatments. Clin Neuropathol. 2001;20:146–55. [PubMed] [Google Scholar]
- 6.Gorry PR, Ong C, Thorpe J, et al. Astrocyte infection by HIV-1: mechanisms of restricted virus replication, and role in the pathogenesis of HIV-1-associated dementia. Curr HIV Res. 2003;1:463–73. doi: 10.2174/1570162033485122. [DOI] [PubMed] [Google Scholar]
- 7.Garden GA. Microglia in human immunodeficiency virus-associated neurodegeneration. Glia. 2002;40:240–51. doi: 10.1002/glia.10155. [DOI] [PubMed] [Google Scholar]
- 8.Merrill JE, Chen IS. HIV-1, macrophages, glial cells, and cytokines in AIDS nervous system disease. FASEB J. 1991;5:2391–7. doi: 10.1096/fasebj.5.10.2065887. [DOI] [PubMed] [Google Scholar]
- 9.Kelekar A. Autophagy. Ann N Y Acad Sci. 2005;1066:259–71. doi: 10.1196/annals.1363.015. [DOI] [PubMed] [Google Scholar]
- 10.Komatsu M, Ueno T, Waguri S, Uchiyama Y, Kominami E, Tanaka K. Constitutive autophagy: vital role in clearance of unfavorable proteins in neurons. Cell Death Differ. 2007;10:887–94. doi: 10.1038/sj.cdd.4402120. [DOI] [PubMed] [Google Scholar]
- 11.Lee JA. Autophagy in neurodegeneration: two sides of the same coin. BMB Rep. 2009;42:324–30. doi: 10.5483/bmbrep.2009.42.6.324. [DOI] [PubMed] [Google Scholar]
- 12.He C, Klionsky DJ. Autophagy and neurodegeneration. ACS Chem Biol. 2006;1:211–3. doi: 10.1021/cb600182h. [DOI] [PubMed] [Google Scholar]
- 13.Jellinger KA, Stadelmann C. Mechanisms of cell death in neurodegenerative disorders. J Neural Transm Suppl. 2000;59:95–114. doi: 10.1007/978-3-7091-6781-6_13. [DOI] [PubMed] [Google Scholar]
- 14.Jellinger KA, Stadelmann C. Problems of cell death in neurodegeneration and Alzheimer's disease. J Alzheimers Dis. 2001;3:31–40. doi: 10.3233/jad-2001-3106. [DOI] [PubMed] [Google Scholar]
- 15.Jellinger KA. Challenges in neuronal apoptosis. Curr Alzheimer Res. 2006;3:377–91. doi: 10.2174/156720506778249434. [DOI] [PubMed] [Google Scholar]
- 16.Kanthasamy A, Anantharam V, Ali SF, Kanthasamy AG. Methamphetamine induces autophagy and apoptosis in a mesencephalic dopaminergic neuronal culture model: role of cathepsin-D in methamphetamine-induced apoptotic cell death. Ann N Y Acad Sci. 2006;1074:234–44. doi: 10.1196/annals.1369.022. [DOI] [PubMed] [Google Scholar]
- 17.Ravikumar B, Vacher C, Berger Z, et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 2004;36:585–95. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- 18.Rubinsztein DC, Huntington JA. Paradoxical aggregation versus oligomerisation properties of mutant and wild-type huntingtin fragments. Exp Neurol. 2006;199:243–4. doi: 10.1016/j.expneurol.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Yamashita S, Nishino I, Nonaka I, Goto Y. Genotype and phenotype analyses in 136 patients with single large-scale mitochondrial DNA deletions. J Hum Genet. 2008;53:598–606. doi: 10.1007/s10038-008-0289-8. [DOI] [PubMed] [Google Scholar]
- 20.Zeng X, Overmeyer JH, Maltese WA. Functional specificity of the mammalian Beclin-Vps34 PI 3-kinase complex in macroautophagy versus endocytosis and lysosomal enzyme trafficking. J Cell Sci. 2006;119:259–70. doi: 10.1242/jcs.02735. [DOI] [PubMed] [Google Scholar]
- 21.Liang XH, Jackson S, Seaman M, et al. Induction of autophagy and inhibition of tumorigenesis by Beclin 1. Nature. 1999;402:672–6. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 22.Liang XH, Kleeman LK, Jiang HH, et al. Protection against fatal Sindbis virus encephalitis by Beclin, a novel Bcl-2-interacting protein. J Virol. 1998;72:8586–96. doi: 10.1128/jvi.72.11.8586-8596.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pattingre S, Tassa A, Qu X, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–39. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Asanuma K, Tanida I, Shirato I, et al. MAP-LC3, a promising autophagosomal marker, is processed during the differentiation and recovery of podocytes from PAN nephrosis. FASEB J. 2003;17:1165–7. doi: 10.1096/fj.02-0580fje. [DOI] [PubMed] [Google Scholar]
- 25.Kabeya Y, Mizushima N, Ueno T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–8. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kabeya Y, Mizushima N, Yamamoto A, Oshitani-Okamoto S, Ohsumi Y, Yoshimori T. LC3, GABARAP and GATE16 localize to autophagosomal membrane depending on form-II formation. J Cell Sci. 2004;117:2805–12. doi: 10.1242/jcs.01131. [DOI] [PubMed] [Google Scholar]
- 27.Espert L, Codogno P, Biard-Piechaczyk M. Involvement of autophagy in viral infections: antiviral function and subversion by viruses. J Mol Med. 2007;85:811–23. doi: 10.1007/s00109-007-0173-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee HK, Iwasaki A. Autophagy and antiviral immunity. Curr Opin Immunol. 2008;20:23–9. doi: 10.1016/j.coi.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munz C. Viral evasion of autophagy. Cell Host Microbe. 2007;1:9–11. doi: 10.1016/j.chom.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Espert L, Denizot M, Grimaldi M, et al. Autophagy and CD4+ T lymphocyte destruction by HIV-1. Autophagy. 2007;3:32–4. doi: 10.4161/auto.3275. [DOI] [PubMed] [Google Scholar]
- 31.Levine B, Sodora DL. HIV and CXCR4 in a kiss of autophagic death. J Clin Invest. 2006;116:2078–80. doi: 10.1172/JCI29447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou D, Spector SA. Human immunodeficiency virus type-1 infection inhibits autophagy. AIDS. 2008;22:695–9. doi: 10.1097/QAD.0b013e3282f4a836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–73. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- 34.Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15:1101–11. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaskolski F, Mulle C, Manzoni OJ. An automated method to quantify and visualize colocalized fluorescent signals. J Neurosci Methods. 2005;146:42–9. doi: 10.1016/j.jneumeth.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 36.Klionsky DJ, Abeliovich H, Agostinis P, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–75. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsia K, Spector DH, Spector SA. Molecular analysis of the differential restriction of human immunodeficiency virus type 1 replication in neuronal cell lines. J Gen Virol. 1997;78:3255–64. doi: 10.1099/0022-1317-78-12-3255. [DOI] [PubMed] [Google Scholar]
- 38.Geeraerts T, Deiva K, M'Sika I, Salim H, Hery C, Tardieu M. Effects of SDF-1alpha and gp120IIIB on apoptotic pathways in SK-N-SH neuroblastoma cells. Neurosci Lett. 2006;399:115–20. doi: 10.1016/j.neulet.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 39.Pizzi M, Boroni F, Bianchetti A, et al. Expression of functional NR1/NR2B-type NMDA receptors in neuronally differentiated SK-N-SH human cell line. Eur J Neurosci. 2002;16:2342–50. doi: 10.1046/j.1460-9568.2002.02403.x. [DOI] [PubMed] [Google Scholar]
- 40.Schroder HC, Perovic S, Kavsan V, Ushijima H, Muller WE. Mechanisms of prionSc- and HIV-1 gp120 induced neuronal cell death. Neurotoxicology. 1998;19:683–8. [PubMed] [Google Scholar]
- 41.Klionsky DJ, Elazar Z, Seglen PO, Rubinsztein DC. Does bafilomycin A1 block the fusion of autophagosomes with lysosomes? Autophagy. 2008;4:849–950. doi: 10.4161/auto.6845. [DOI] [PubMed] [Google Scholar]
- 42.Pivtoraiko VN, Harrington AJ, Mader BJ, et al. Low-dose bafilomycin attenuates neuronal cell death associated with autophagy-lysosome pathway dysfunction. J Neurochem. 2010;114:1193–204. doi: 10.1111/j.1471-4159.2010.06838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spector SA, Zhou D. Autophagy: an overlooked mechanism of HIV-1 pathogenesis and neuroAIDS? Autophagy. 2008;4:704–6. doi: 10.4161/auto.6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cherra SJ, Chu CT. Autophagy in neuroprotection and neurodegeneration: a question of balance. Future Neurol. 2008;3:309–23. doi: 10.2217/14796708.3.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–5. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- 46.Huang J, Klionsky DJ. Autophagy and human disease. Cell Cycle. 2007;6:1837–49. doi: 10.4161/cc.6.15.4511. [DOI] [PubMed] [Google Scholar]
- 47.Yu WH, Cuervo AM, Kumar A, et al. Macroautophagy—a novel beta-amyloid peptide-generating pathway activated in Alzheimer's disease. J Cell Biol. 2005;171:87–98. doi: 10.1083/jcb.200505082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang Y, Fukui K, Koike T, Zheng X. Induction of autophagy in neurite degeneration of mouse superior cervical ganglion neurons. Eur J Neurosci. 2007;26:2979–88. doi: 10.1111/j.1460-9568.2007.05914.x. [DOI] [PubMed] [Google Scholar]
- 49.Alirezaei M, Kiosses WB, Flynn CT, Brady NR, Fox HS. Disruption of neuronal autophagy by infected microglia results in neurodegeneration. PLoS One. 2008;3:e2906. doi: 10.1371/journal.pone.0002906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alirezaei M, Kiosses WB, Fox HS. Decreased neuronal autophagy in HIV dementia: a mechanism of indirect neurotoxicity. Autophagy. 2008;4:963–6. doi: 10.4161/auto.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]