Abstract
There has been significant controversy whether stressful life events (SLEs) experienced over the lifespan may elevate the risk of depression in individuals who are homozygous for the short (S) allele of the repeat length polymorphism (5-HTTLPR) in the regulatory region of the serotonin transporter gene (SLC6A4), compared with individuals homozygous for the long (L) allele. On the basis of the hypothesis that age may be a critical variable, by which such a gene-by-environment interaction may be present in younger adults, but not in older adults and in children, aim of this study was to investigate the role of 5-HTTLPR and SLEs on the endocrine stress response in multiple age cohorts. A total of 115 children (8–12 years), 106 younger adults (18–31 years), and 99 older adults (54–68 years) were subjected to the Trier Social Stress Test (TSST) and structured interviews on SLEs. The TSST induced significant endocrine stress responses in all groups. There was a main effect of genotype in younger and older adults with individuals homozygous for the more active L allele showing a significantly larger cortisol response to the TSST than individuals carrying at least one of the low-expressing S alleles. As predicted, there was a significant interaction of 5-HTTLPR genotype and SLEs, but this interaction was only significant in younger adults and only when the measured SLEs had occurred during the first 5 years of life, suggesting that both age and the specific type of SLE has a role in whether a significant gene–environment interaction is observed.
Keywords: serotonin transporter gene, 5-HTTLPR, stress, stressful life events, hypothalamic–pituitary–adrenal axis, cortisol
INTRODUCTION
There has been considerable debate over the possible interaction of genetic polymorphisms and life stress history predicting susceptibility to psychopathology. Specifically, Caspi et al (2003) found that the development of depression was modulated by an interaction between a polymorphism in the serotonin transporter-linked promoter region (5-HTTLPR), which had previously been associated with elevated levels of neuroticism (Lesch et al, 1996), affective disorders including depression (Collier et al, 1996) and stressful life events (SLEs) (Caspi et al, 2003). Carriers of the S allele who experienced SLEs (such as childhood abuse, threat, loss, or humiliation) showed an increased risk for major depression or suicidal ideation, suggesting a gene-by-environment interaction (G × E). In contrast, individuals homozygous for the L allele were not at greater risk for depression irrespective of the number of SLEs they had experienced. Subsequent replication studies, however, produced inconsistent results (Bennett et al, 2002; Eley et al, 2004; Gillespie et al, 2005; Kendler et al, 2005; Neumeister et al, 2002; Ohara et al, 1998; Surtees et al, 2006). Indeed, the investigators of a recent meta-analysis concluded that adding the 5-HTTLPR genotype does not improve the prediction of depression in relation with exposure to negative life events (Risch et al, 2009).
Yet, this meta-analysis did not take into account many of the potentially important methodological differences across studies that may have affected study outcomes. For example, Uher and McGuffin (2008, 2010) suggested that study outcome may have been affected by the age of the selected cohort and by the method of SLE assessment (Lewis et al, 2010; Uher and McGuffin, 2008): they pointed out that positive results were reported in studies using younger cohorts (whereas studies using children and older adults produced mostly negative results), and in studies that assessed the occurrence of SLEs with structured interviews, rather than self-report measures (Lewis et al, 2010; Uher and McGuffin, 2008). Furthermore, they suggested that studies using biological endophenotypes, such as those that investigate stress-induced HPA activation, might be more strongly associated with a specific polymorphism than a psychiatric disorder (Lewis et al, 2010; Uher and McGuffin, 2008).
In order to directly test these hypotheses, we conducted a study of multiple age cohorts (children, younger adults, and older adults), using structured interviews to assess SLEs, in a social stress paradigm in which the biological endophenotype of interest was cortisol reactivity. The use of this particular paradigm was motivated by findings that 5-HTTLPR genotype is associated with individual differences in HPA axis activity (Barr et al, 2004; Gotlib et al, 2008). Our primary goal was to assess whether cortisol reactivity would vary as a function of 5-HTTLPR × SLE interaction. On the basis of the hypothesis of Uher and McGuffin (2008) that 5-HTTLPR genotype-mediated vulnerability might operate in a developmental period of young adulthood (Uher and McGuffin, 2008), we hypothesized that the 5-HTTLPR × SLE interaction would be evident in younger adults, but not in children or in older adults.
PARTICIPANTS AND METHODS
Participants
A sample of 115 children (50 boys; sample mean age 9.3 years, SD=1.03, range 8–11 years) was recruited from schools in Dresden. A sample of initially 114 younger adults was recruited among students of the Technische Universitaet Dresden. Owing to missing data regarding some female participants' menstrual cycle status, the final sample size was reduced to 106 younger adults (56 males; sample mean age 23.8 years, SD=2.55, range 19–31 years). There were no smokers in this sample and none of the female participants used hormonal contraceptives. A sample of 99 older adults (39 males; sample mean age 61.1 years, SD=2.70, range 54–68 years) was recruited from Dresden. Participants in this sample were also nonsmokers. All participants were of German/Middle European ancestry.
Participants were informed about the aims of the study and gave written informed consent (consent in the children's sample was obtained from their parents). The Ethics Committee of the German Psychological Association approved the study design.
Procedure—TSST
All participants were scheduled for a laboratory session in the afternoon. The Trier Social Stress Test (TSST) was used to induce psychosocial stress. This standardized laboratory stressor consists of a free speech and a mental arithmetic task in front of an audience. After their arrival, participants were given a rest period of at least 20 min and then introduced to the TSST. The TSST has been found to elicit the strongest and most reliable cortisol responses to laboratory stress, as well as significant cardiovascular, immune, and participative responses compared with other protocols (Dickerson and Kemeny, 2004; Kudielka et al, 2007). For participating children, an age appropriate version of the TSST was adapted (‘Trier Social Stress Test for Children' TSST-C), which has been described in detail elsewhere (Buske-Kirschbaum et al, 1997). To assess cortisol levels, salivary cortisol samples were obtained repeatedly 2 min before the TSST (t1) as well as 2 (t2), 10 (t3), 20 (t4), and 30 (t5) minutes after the termination of the TSST using ‘Salivettes' (Sarstedt; Rommelsdorf, Germany). All samples were collected for determination of the unbound and biologically active fraction of cortisol and were kept at −20 °C until analysis. Salivary cortisol samples were prepared for biochemical analysis by centrifuging at 3000 r.p.m. for 5 min, which resulted in a clear supernatant of low viscosity. Salivary-free cortisol concentrations were determined employing a chemiluminescence immunoassay with high sensitivity of 0.16 ng/ml (IBL; Hamburg, Germany). Intra- and interassay coefficients of variation were below 8%.
Psychological and Sociodemographic Assessments
Participants of the younger adult sample, as well as parents of participating children, underwent a semi-structured screening for psychiatric or neurological disorders or treatment and a structured interview for the assessment of SLEs. This interview was based on the life history calendar (LHC), which collects detailed retrospective data about SLEs, for example, death of close relatives or friends, serious illness or injury, relationship stressors, major difficulties at work or in school, financial problems, and experienced disasters (Axinn et al, 1999; Freedman et al, 1988). Validity and reliability of the LHC is enhanced through its use of memory cues, relating one event to other events that occurred about the same time. It elicits easily recalled memories of a personal matter and uses this information to aid the retrieval of less easily recalled information. The LHC uses a calendar format, which makes it easier to assess consistency and to correct discrepancies. The interview combines chronological and theme-based structures that fit to the organization of autobiographical memories and support sequencing as well as parallel retrieval approaches. Freedman et al (1988) reported agreement ranging from 72 to 92% between retrospectively obtained LHC data in comparison with data obtained 5 years earlier about the respondent's current situation (Freedman et al, 1988). In addition, Caspi et al (2003) found 90% agreement over a 3-year period (Caspi and Moffitt, 2006). For the older adult sample, the interview used was based on the Life Events Questionnaire (LEQ), which measures negative life events concerning self or significant others (ie, parents, siblings, partner, children, and important people such as a close friend or a confidant) (Kraaij and de Wilde, 2001). This interview is a lifetime instrument since the incidence of all events is being questioned for different developmental periods, that is, childhood, adulthood, late adulthood, and the year before the interview.
Genotyping
To obtain DNA samples, Oragene DNA Extraction kits (DNA Genotek, Ottawa, Ontario, Canada) or BuccalAmp DNA Extraction kits and protocol were used. Participants were genotyped for the 43-bp insertion/deletion polymorphism in the regulatory promoter region of the serotonin transporter gene (5-HTTLPR) and the A/G SNP as previously described (Lesch et al, 1996; Wendland et al, 2006). Carriers of S and LG alleles were grouped together (S group: S/S, S/LG, LG/LG, S/LA, LG/LA genotypes) because of the comparable expression profiles of S and LG (Hu et al, 2005; Hu et al, 2006; Wendland et al, 2006) and compared with LA/LA homozygotes (L group). Frequencies of the two 5-HTTLPR groups including the A/G SNP for all three samples are shown in Table 1.
Table 1. Genotype Frequencies and Percentages of the 5-HTTLPR (including A/G SNP (dbSNP: rs25531) Genotype Groups S (S/S, S/LG, LG/LG, S/LA, LG/LA) vs L (LA/LA).
| N | % | |
|---|---|---|
| 5-HTTLPR groups in children | ||
| S group (S/S, S/LG, LG/LG, S/LA, LG/LA) | 87 | 75.7 |
| L group (LA/LA) | 28 | 24.3 |
| 5-HTTLPR classification in younger adults | ||
| S group (S/S, S/LG, LG/LG, S/LA, LG/LA) | 78 | 73.6 |
| L group (LA/LA) | 28 | 26.4 |
| 5-HTTLPR classification in older adults | ||
| S group (S/S, S/LG, LG/LG, S/LA, LG/LA) | 72 | 72.7 |
| L group (LA/LA) | 27 | 27.3 |
As genetic association studies yield inconsistent analyses regarding 5-HTTLPR classifications, tri-allelic genotypes were classified and analyzed as well (see Supplementary Information).
Statistical Analyses
The average cortisol levels for all time points were computed and tested for univariate normality in all samples (Kolmogorov–Smirnov test). Although cortisol levels were normally distributed in the younger adult sample (p>0.05), cortisol data in the children's and older adult's samples had to be log-transformed before conducting subsequent analyses.
Cortisol responses were then analyzed with separate repeated measurements (ANOVAs) to ensure successful stress induction by the TSST and to look for potential gender influences in each of the three samples. As among younger adults women varied in their menstrual cycle stage, this information was included in the factor gender (men vs women in luteal phase vs women in non-luteal phase). Univariate ANOVAs with cortisol levels as a repeated-measures within-subject factor and 5-HTTLPR genotype (S vs L group) as a between-subject factor were conducted separately for each of the three samples. Cortisol increase from baseline (2 min before the TSST) to the highest peak level after the TSST was entered as independent variable. As shown in Table 2, the highest peak after the TSST was reached after 20 min in children and in older adults, and after 10 min in younger adults.
Table 2. Mean Cortisol (nmol/l; SEM) for Total Sample and 5-HTTLPR Groups.
| Sample | N |
Time |
||||
|---|---|---|---|---|---|---|
| 2 min before TSST | 2 min after TSST | 10 min after TSST | 20 min after TSST | 30 min after TSST | ||
| Children | ||||||
| S group | 87 | 2.53 (0.35) | 3.55 (0.35) | 7.11 (0.69) | 7.58 (0.88) | 6.45 (0.84) |
| L group | 28 | 2.56 (0.64) | 4.01 (0.83) | 8.79 (1.49) | 8.49 (1.56) | 6.91 (1.29) |
| Younger adults | ||||||
| S group | 78 | 6.37 (0.55) | 9.56 (0.71) | 13.55 (0.96) | 12.91 (0.84) | 10.41 (0.68) |
| L group | 28 | 7.17 (0.85) | 12.30 (1.16) | 17.65 (1.90) | 17.35 (1.80) | 14.33 (1.46) |
| Older adults | ||||||
| S group | 72 | 5.12 (0.36) | 8.32 (0.55) | 13.02 (0.91) | 13.81 (1.08) | 11.59 (0.94) |
| L group | 27 | 4.58 (0.47) | 9.38 (1.38) | 16.03 (2.23) | 18.06 (2.52) | 15.13 (1.99) |
S group= S/S, S/LG, LG/LG, S/LA, LG/LA genotypes; L group = LA/LA genotype.
In subsequent analyses, main effects of, and interactions between, 5-HTTLPR genotype and SLEs on the endocrine stress response were examined in all samples. ANOVAs were conducted with the stress-induced alteration levels to the TSST (and TSST-C, respectively), as a within-subject factor and genotype (S vs L group) as well as SLEs as between-subjects factors. The total number of SLEs (SLE-T) reported was used for determining life stress. All samples were divided into three SLE-T groups (low, moderate, and high life stress). For children and younger adults, a second measure of cumulative stress was analyzed, that is, accumulated stress over the first 5 years of life (SLE-5). Both samples were divided into three SLE-5 groups (none, moderate, and high life stress). Since this measure was unfortunately not available for older adults, we analyzed accumulated stress over the first 15 years of life (SLE-15) for older and younger adults. Since in the children sample, age ranged between 8 and 11 years we did not include this measure here. All SLE groups did not differ with regard to genotype (S vs L group) and gender (all p⩾0.08).
Bonferroni-adjusted post hoc analyses and follow-up ANOVAs for group comparisons were conducted where appropriate. For determination of effect size, partial Eta2 for significant effects was estimated. Degrees of freedom were Greenhouse–Geisser corrected where appropriate. Level of significance was α=0.05 for all analyses. All statistical analyses were performed using SPSS for Windows (Version 15 and 18, Chicago, Illinois, USA).
We have completed a procedure to control for type I error in our planned comparisons using a modified Bonferroni correction suggested by Keppel and Wickens (2004) to control for family-wise error.
All analyses regarding main effects of, and interactions between, 5-HTTLPR genotype and SLEs on the endocrine stress response have been computed with the tri-allelic genotype classification of 5-HTTLPR (S'/S' vs S'/L' vs L'/L') for every sample as well (see Supplementary Information).
RESULTS
Stress Response to Public Speaking
A repeated-measures ANOVA revealed that cortisol increased significantly in response to the psychosocial stressor in children (F1.46, 114=94.85, p=0.000, η2=0.45), younger adults (F2.10, 105=86.96, p<0.001, η2=0.45) and older adults (F1.72, 93=141.07, p<0.001, η2=0.59).
Main Effects of TSST, SLE, and Genotype on Stress Reactivity in Children
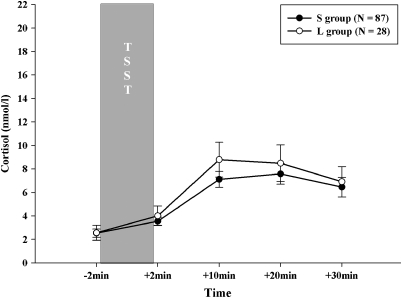
The two genotype groups did not differ with regard to gender (χ2115=0.006, p=0.94) but age (t115=–2.41, p=0.017) with the L'/L' genotype group being slightly older. Thus, for the following analyses age was used as a covariate. ANOVAS did not show a significant main effect of SLE-5 (F2, 103=0.35, p=0.707), SLE-T (F2, 103=0.48, p=0.618), or 5-HTTLPR (F1, 113=0.74, p=0.392) (as illustrated in Figure 1) on the cortisol response. There was no effect of gender on the endocrine stress response (F1, 113=0.15, p=0.701).
Figure 1.
Cortisol responses (mean±standard error of mean) to TSST shown for the two genotype groups (S including SS, SLG, LGLG, SLA, LGLA genotypes and L group including LA/LA) in children (mean age 9.3 years, range 8–11 years).
Interaction of Genotype × SLE on Stress Reactivity in Children
In children, analyses of 5-HTTLPR and SLE-T and SLE-5 did not show any significant interaction impacting on the endocrine stress response (all p⩾0.69).
Main Effects of TSST, SLE, and Genotype on Stress Reactivity in Younger Adults
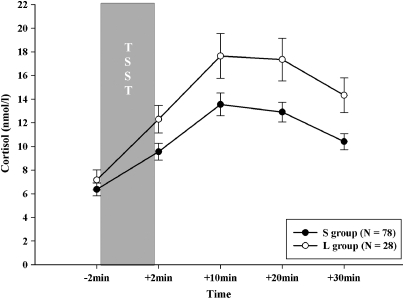
In younger adults, genotype groups did not differ in gender (χ2106=2.07, p=0.355) or age (t106=0.19, p=0.229). ANOVAS did not show a significant main effect of SLE-5 (F2, 97=0.82, p=0.446), SLE-15 (F2, 102=0.32, p=0.726), or SLE-T (F2, 97=2.08, p=0.130) on the cortisol response. However, there was a significant main effect for 5-HTTLPR (F1, 100=7.94, p=0.006, η2=0.07) on the cortisol response with the L group showing a significant higher stress response than the S group (p<0.05) as illustrated in Figure 2. We also found a significant main effect of gender on the endocrine stress response (F2, 103=4.13, p<0.05, η2=0.07) with male participants showing a higher endocrine stress response in comparison with women in the non-luteal phase (p<0.05), which is in line with previously reported findings (Kirschbaum et al, 1999). No differences were observed between women in the luteal vs non-luteal phase or between men and women in the luteal phase, respectively (all p>0.19).
Figure 2.
Cortisol responses (mean±standard error of mean) to TSST shown for the two genotype groups (S including SS, SLG, LGLG, SLA, LGLA genotypes and L group including LA/LA) in younger adults (mean age 23.9 years, range 19–31 years).
Interaction of Genotype × SLE on Stress Reactivity in Younger Adults
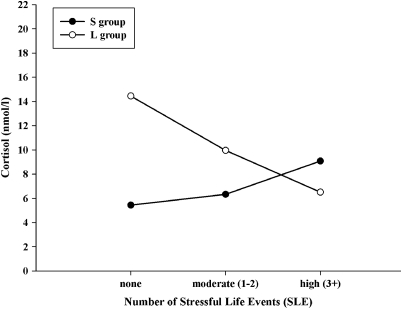
As a result of the very small cell sizes, we could not include gender as an additional factor in our main ANOVA in younger adults but added it as a covariate. Here, analyses concerning 5-HTTLPR and SLE-15 or SLE-T showed no significant interaction on the endocrine stress response (all p⩾0.84). However, there was a significant interaction of 5-HTTLPR and SLE-5 (F2, 99=3.71; p=0.028, η2=0.07) on the endocrine stress response: for the L group, the endocrine stress response was negatively correlated with SLEs, whereas for the S group, the endocrine stress response was positively correlated with SLEs. This interaction effect was so strong that it contributed to dramatically different patterns of cortisol reactivity: in the absence of any early SLEs, individuals in the L group exhibited a significantly higher endocrine stress response than individuals in the S group; yet, this pattern was reversed for individuals who had experienced three or more early SLEs (see Figure 3). After the control for type I error in our planned comparisons using a modified Bonferroni correction suggested by Keppel and Wickens (2004) to control for family-wise error, this effect was still significant.
Figure 3.
Stress-induced alteration levels of cortisol to the TSST shown in dependence of number of SLE (low, moderate, and high) during the first 5 years of life and the two genotype groups (S including SS, SLG, LGLG, SLA, LGLA genotypes and L group including LA/LA) in younger adults (mean age 23.8 years, range 19–31 years). Among the 78 participants in the S group, 28, 35, and 15 study members experienced 0, 1 to 2, and 3 or more stressful events, respectively. Among the 28 participants in the S group, 6, 16, and 6 study members experienced 0, 1 to 2, 3 three or more stressful events, respectively.
Main Effects of TSST, SLE, and Genotype on Stress Reactivity in Older Adults
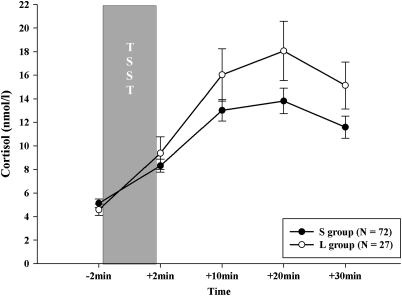
In older adults, genotype groups did not differ in gender distribution (χ299=1.19, p=0.275) or age (t99=0.31, p=0.757). ANOVAS did not show a significant effect of SLE-T (F2, 96=1.03, p=0.360) on the cortisol response but there was a trend toward an effect for SLE-15 (F2, 96=2.82, p=0.064). The highest endocrine stress response was found for individuals with a low number of SLEs during first 15 years of life compared with individuals with a high number of SLEs during first 15 years of life who showed the lowest endocrine stress response. We also found a main effect of 5-HTTLPR on the cortisol response with the L group showing a higher cortisol response than the S group (F1, 97=4.04, p=0.047, η2=0.04) as illustrated in Figure 4. There was a tendency toward an effect of gender on the endocrine stress response (F1, 97=3.03, p=0.085) with men showing a slightly higher endocrine stress response than women.
Figure 4.
Cortisol responses (mean±standard error of mean) to TSST shown for the two genotype groups (S including SS, SLG, LGLG, SLA, LGLA genotypes and L group including LA/LA) in older adults (mean age 61.1 years, range 54–68 years).
Interaction of Genotype × SLE on Stress Reactivity in Older Adults
In older adults, interactions between 5-HTTLPR and SLE-15 or SLE-T showed no significant interaction effect on the cortisol stress response (all p⩾0.41).
All analyses have been conducted for tri-allelic genotypes as well (see Supplementary Information and Supplementary Table 2).
DISCUSSION
The existing literature on gene–environment interactions for 5-HTTLPR genotype and life stress has produced conflicting results, possibly due to methodological heterogeneity across studies with respect to subject recruitment and life stress assessment (Uher and McGuffin, 2008; Uher and McGuffin, 2010). We have now conducted the first study to assess such a gene–environment interaction across multiple age cohorts, using a consistent and highly sensitive life stress assessment methodology, and a quantitatively measured biologically based endophenotype. On the basis of three age cohorts of children, younger adults, and older adults, and with each cohort similarly powered to detect a gene–environment interaction, we now report evidence for a significant interaction in the cohort of younger adults only.
Our analyses suggest that, indeed, subject age and SLE assessment both influence whether a significant interaction between 5-HTTLPR genotype and SLE will be observed. When the measure of SLE was the total number of such events (SLE-T), accumulated over a lifetime, none of the three age cohorts exhibited a significant gene–environment interaction. Similarly, when the measure of SLE was the number of such events during the first 15 years of life (SLE-15), neither younger nor older adults exhibited a significant gene–environment interaction (no SLE-15 data were available for the cohort of children studied here). However, when the focus was on SLE that had occurred during the first 5 years of life (SLE-5), there was a significant interaction between 5-HTTLPR genotype and SLE, but only in the younger adults, not in children (no SLE-5 data were available for older adults). Thus, this study is consistent with the hypothesis (Lewis et al, 2010; Uher and McGuffin, 2008) that age but also SLE assessment may be critical variables in gene–environment interactions involving 5-HTTLPR genotype (Uher and McGuffin, 2008; Uher and McGuffin, 2010). Future work will also need to address why significant G × E interactions were absent in adolescents but present in younger adults. We speculate that the emergence of significant G × E interactions over this period may reflect developmental processes in brain maturation that may be epigenetically regulated (Bennett-Baker et al, 2003; Munoz-Najar and Sedivy, 2010).
Among younger adults, the interaction between 5-HTTLPR genotype and life stress was significant only when the analysis focused on SLEs that had occurred in the first 5 years of life: individuals homozygous for the LA allele exhibited a negative correlation between the number of SLEs during the first 5 years of life and peak cortisol response to social stress, whereas carriers of the S allele exhibited a positive correlation. Strikingly, a very similar pattern was reported by Canli et al (2006), who in an imaging study observed a negative correlation between SLEs and amygdala activation in homozygous L allele carriers and a positive correlation in S allele carriers (Canli et al, 2006), although these analyses did not separate early SLEs from other SLEs.
The interesting ‘crossover' found in the cortisol response when analyzing is similar G × E and seems to be similar to what has been observed for depression and suicide studies showing that the S allele may be associated with enhanced cortisol release after early exposure to stress (Caspi et al, 2003; Roy et al, 2007).
These data suggest that the short variant of the 5-HTTLPR does not constitute a ‘vulnerability' allele, but rather that the endophenotype associated with the short allele may be optimal or not, depending on the environment: if one operationalizes optimal socioemotional functioning in terms of low cortisol reactivity to a social stressor, then individuals in the S group outperform individuals in the L group, if both groups had experienced low numbers of early SLEs. Indeed, Taylor et al (2006) made a similar observation with respect to homozygous S allele carriers, noting that in benign environments, their genotype assumes properties that protect from depression, relative to L allele carriers (Taylor et al, 2006). Contradictory, recent results showed that newborns with two S alleles have been found to show the highest response after a physical stressor (Mueller et al, 2010).
The data from this study, as in Canli et al (2006), also show that responsiveness to environmental influences is not a special attribute of the short variant of the 5-HTTLPR, as some have argued in favor of the short allele as a ‘plasticity gene' (Belsky et al, 2009; Belsky and Pluess, 2009) providing a genetic basis to improved performance in an array of cognitive tasks and increased social conformity (Homberg and Lesch, 2010). Instead, both studies have shown that homozygous long allele carriers exhibit markedly different behavioral and neural responses to social stressors, visual stimuli, and in the scanner at rest, depending on their early life stress history. This may explain why some studies associated the L allele with less favorable phenotypes, such as increased cardiovascular reactivity, greater risk of myocardial infarction (Coto et al, 2003; Fumeron et al, 2002; Williams et al, 2001), increased risk of psychosis (Goldberg et al, 2009), and increased risk of chronic PTSD (Grabe et al, 2009; Lee et al, 2005; Thakur et al, 2009). In light of these results, it is possible that, in the absence of early SLEs, homozygous L allele carriers are susceptible to multiple physical and psychological adverse outcomes.
The interaction of 5-HTTLPR genotype and SLEs may explain, at least in part, why cortisol studies that have not taken life stress history into account have produced inconsistent findings. For example, Jabbi et al (2007) and Gotlib et al (2008) found larger cortisol responses to a stressor in homozygous carriers of the S allele (Gotlib et al, 2008; Jabbi et al, 2007). We recently reported that newborns with two S alleles exhibit the highest cortisol response after a physical stressor (Mueller et al, 2010). In contrast, Alexander et al (2009) and Wüst et al (2009) did not find significant differences in cortisol reactivity between 5-HTTLPR groups after a stressful task. Contrary to these previous studies, we now report that homozygous carriers of the L allele exhibited the largest cortisol response to a stressor. However, this vigorous reactivity has to be viewed in the context of early SLEs: it was only present in those individuals who had not experienced any early SLEs (see Figure 4). Indeed, the cortisol response of the L group with no early adversity was almost thrice as high as the cortisol response of the S group. However, in individuals who had experienced three or more early SLEs, the pattern was reversed: the cortisol response of the L group with early SLEs was almost one-third below the cortisol response of the S group. Thus, SLEs likely represent a major confounding variable that needs to be included in future analyses of cortisol reactivity as a function of 5-HTTLPR genotype. Indeed, the fact that among younger adults in our study the L group exhibited greater cortisol reactivity than the S group can therefore be largely attributed to the fact that the majority of L group members (N=12 (out of 28)) had not experienced any early SLEs.
Only one previous study had measured cortisol reactivity as a function of SLEs: Alexander et al (2009) reported higher cortisol levels in males homozygous for the S allele with a history of SLEs (Alexander et al, 2009). Our sample was too small to formally conduct the same analysis, but inspection of the subsample of men in the younger sample revealed higher cortisol reactivity in the L group, in contrast to Alexander et al's (2009) findings. These different outcomes might at least be partly due to the use of different stressors across the two studies. Future work with much larger samples will need to address these and other factors that may have driven these conflicting gender effects.
Our study is limited by its reliance on self-report: although the LHC and LEQ have been shown to reliably elicit memories, biases cannot be entirely ruled out. Furthermore, our participants may have differed in their ability to remember events accurately despite the use of memory cues and the calendar format of the LHC method. Also, because the number of SLE during the first 5 years of life was not available for older adults because of the nature of the assessment method, analyses between early life stress and genotype could not been carried out for older adults. Another limitation is that recruiting strategies differed between the three groups: children were recruited at schools in Dresden, and younger adults were recruited via flyers from campus, but older adults were recruited via flyers in a fitness center for older people and an advertisement in a local newspaper. Thus, our sample of older adults may represent a cohort of healthier and more active individuals, compared with the other cohorts tested in this study. Finally, our current sample is too small to conduct analyses in regard to possible quadruple interactions between 5-HTTLPR genotype, SLEs, age, and gender. We plan to continue increasing our sample to be able to conduct these analyses in the future.
In sum, our findings support the notion that the effects of functional genetic variations are further modulated by environmental factors, particularly by those occurring during the early 5 years of life. Instead of a simple additive effect, by which early life stress may exacerbate a genetically predisposed individual to respond to social stressors in later life, we observed an interaction of 5-HTTLPR genotype and early life SLEs. On the basis of this interaction, characterizing an individual's stress response on genotype alone leads to potentially erroneous conclusions. For example, in our younger adult data set, the cortisol response of homozygous L allele carriers may be substantially greater or smaller than that of S allele carriers, depending on the number of SLEs they experienced. Fascinatingly, a high number of early SLEs in homozygous L carriers are associated with reduced cortisol stress reactivity, suggesting positive adaptation in the face of early environmental stressors. This is the first study, to our knowledge, to investigate the influence of 5-HTTLPR and SLEs in a cross-sectional design. We find that 5-HTTLPR is associated with individual differences in stress reactivity that may endow individuals with variable stress reactivity, with early SLEs modulating the direction of this response.
All results for tri-allelic genotypes have been discussed as well (see Supplementary Information).
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (KI 537/20-1, 20-3, SFB 581/B9, and TRR 58/A1, A5). TC was supported by NIA Grant 1 R01 AG034578-01 and NSF Grant BCS-0843346. We would also like to thank Nicole Steigerwald for her excellent technical assistance in DNA sample processing and genotyping and Gabriele Arnold for conducting cortisol analyses. Furthermore, we thank all student assistants for their support during data collection.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Alexander N, Kuepper Y, Schmitz A, Osinsky R, Kozyra E, Hennig J. Gene-environment interactions predict cortisol responses after acute stress: implications for the etiology of depression. Psychoneuroendocrinology. 2009;34 (9:1294–1303. doi: 10.1016/j.psyneuen.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Axinn W, Pearce L, Ghimire D. Innovations in life history calendar applications. Social Sci Res. 1999;28:243–264. [Google Scholar]
- Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, et al. Rearing condition and rh5-HTTLPR interact to influence limbic-hypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biol Psychiatry. 2004;55:733–738. doi: 10.1016/j.biopsych.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. Vulnerability genes or plasticity genes. Mol Psychiatry. 2009;14 (8:746–754. doi: 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol Bull. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE, et al. Early experience and serotonin transporter gene variation interact to influence primate CNS function. Mol Psychiatry. 2002;7:118–122. doi: 10.1038/sj.mp.4000949. [DOI] [PubMed] [Google Scholar]
- Bennett-Baker PE, Wilkowski J, Burke DT. Age-associated activation of epigenetically repressed genes in the mouse. Genetics. 2003;165:2055–2062. doi: 10.1093/genetics/165.4.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Jobst S, Psych D, Wustmans A, Kirschbaum C, Rauh W, et al. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosom Med. 1997;59:419–426. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- Canli T, Qiu M, Omura K, Congdon E, Haas BW, Amin Z, et al. Neural correlates of epigenesis. Proc Natl Acad Sci USA. 2006;103:16033–16038. doi: 10.1073/pnas.0601674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE. Gene-environment interactions in psychiatry: joining forces with neuroscience. Nat Rev Neurosci. 2006;7:583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Collier DA, Stober G, Li T, Heils A, Catalano M, DiBella D, et al. A novel functional polymorphism within the promoter of the serotonin transporter gene: possible role in susceptibility to affective disorders. Mol Psychiatry. 1996;1:453–460. [PubMed] [Google Scholar]
- Coto E, Reguero JR, Alvarez V, Morales B, Batalla A, Gonzalez P, et al. 5-Hydroxytryptamine 5-HT2A receptor and 5-hydroxytryptamine transporter polymorphisms in acute myocardial infarction. Clin Sci (Lond) 2003;104:241–245. doi: 10.1042/CS20020246. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Eley TC, Sugden K, Corsico A, Gregory AM, Sham P, McGuffin P, et al. Gene-environment interaction analysis of serotonin system markers with adolescent depression. Mol Psychiatry. 2004;9:908–915. doi: 10.1038/sj.mp.4001546. [DOI] [PubMed] [Google Scholar]
- Freedman D, Thornton A, Camburn D, Alwin D, Young-demarco L. The life history calendar: a technique for collecting retrospective data. Sociol Methodol. 1988;18:37–68. [PubMed] [Google Scholar]
- Fumeron F, Betoulle D, Nicaud V, Evans A, Kee F, Ruidavets JB, et al. Serotonin transporter gene polymorphism and myocardial infarction: Etude Cas-Temoins de l'Infarctus du Myocarde (ECTIM) Circulation. 2002;105:2943–2945. doi: 10.1161/01.cir.0000022603.92986.99. [DOI] [PubMed] [Google Scholar]
- Gillespie NA, Whitfield JB, Williams B, Heath AC, Martin NG. The relationship between stressful life events, the serotonin transporter (5-HTTLPR) genotype and major depression. Psychol Med. 2005;35:101–111. doi: 10.1017/s0033291704002727. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Kotov R, Lee AT, Gregersen PK, Lencz T, Bromet E, et al. The serotonin transporter gene and disease modification in psychosis: evidence for systematic differences in allelic directionality at the 5-HTTLPR locus. Schizophr Res. 2009;111:103–108. doi: 10.1016/j.schres.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA axis reactivity: a mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biol Psychiatry. 2008;63:847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabe HJ, Spitzer C, Schwahn C, Marcinek A, Frahnow A, Barnow S, et al. Serotonin transporter gene (SLC6A4) promoter polymorphisms and the susceptibility to posttraumatic stress disorder in the general population. Am J Psychiatry. 2009;166 (8:926–933. doi: 10.1176/appi.ajp.2009.08101542. [DOI] [PubMed] [Google Scholar]
- Homberg JR, Lesch KP.2010Looking on the bright side of serotonin transporter gene variation Biol Psychiatry(in press). [DOI] [PubMed]
- Hu X, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78:815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Oroszi G, Chun J, Smith TL, Goldman D, Schuckit MA. An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcohol Clin Exp Res. 2005;29:8–16. doi: 10.1097/01.alc.0000150008.68473.62. [DOI] [PubMed] [Google Scholar]
- Jabbi M, Korf J, Kema IP, Hartman C, van der Pompe G, Minderaa RB, et al. Convergent genetic modulation of the endocrine stress response involves polymorphic variations of 5-HTT, COMT and MAOA. Mol Psychiatry. 2007;12:483–490. doi: 10.1038/sj.mp.4001975. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: a replication. Arch Gen Psychiatry. 2005;62:529–535. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- Keppel G, Wickens TD.2004Design and Analysis: A Researcher's Handbook4th edn. Chap. 6,Pearson Education: Upper Saddle River, NJ [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kraaij V, de Wilde EJ. Negative life events and depressive symptoms in the elderly: a life span perspective. Aging Ment Health. 2001;5:84–91. doi: 10.1080/13607860020020681. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, Kirschbaum C.2007Ten years of research with the Trier Social Stress Test—RevisitedIn: Harmon-Jones E, Winkielman P (eds).Social Neuroscience: Integrating Biological and Psychological Explanations of Social Behavior Guilford Press: New York; 56–83. [Google Scholar]
- Lee HJ, Lee MS, Kang RH, Kim H, Kim SD, Kee BS, et al. Influence of the serotonin transporter promoter gene polymorphism on susceptibility to posttraumatic stress disorder. Depress Anxiety. 2005;21:135–139. doi: 10.1002/da.20064. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Lewis CM, Ng MY, Butler AW, Cohen-Woods S, Uher R, Pirlo K, et al. Genome-wide association study of major recurrent depression in the UK population. Am J Psychiatry. 2010;167 (8:949–957. doi: 10.1176/appi.ajp.2010.09091380. [DOI] [PubMed] [Google Scholar]
- Mueller A, Brocke B, Fries E, Lesch KP, Kirschbaum C. The role of the serotonin transporter polymorphism for the endocrine stress response in newborns. Psychoneuroendocrinology. 2010;35 (2:289–296. doi: 10.1016/j.psyneuen.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Munoz-Najar UM, Sedivy JM. Epigenetic control of aging. Antioxid Redox Signal. 2010;14 (2:241–259. doi: 10.1089/ars.2010.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumeister A, Konstantinidis A, Stastny J, Schwarz MJ, Vitouch O, Willeit M, et al. Association between serotonin transporter gene promoter polymorphism (5HTTLPR) and behavioral responses to tryptophan depletion in healthy women with and without family history of depression. Arch Gen Psychiatry. 2002;59:613–620. doi: 10.1001/archpsyc.59.7.613. [DOI] [PubMed] [Google Scholar]
- Ohara K, Nagai M, Tsukamoto T, Tani K, Suzuki Y. Functional polymorphism in the serotonin transporter promoter at the SLC6A4 locus and mood disorders. Biol Psychiatry. 1998;44:550–554. doi: 10.1016/s0006-3223(98)00112-7. [DOI] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, et al. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Hu XZ, Janal MN, Goldman D. Interaction between childhood trauma and serotonin transporter gene variation in suicide. Neuropsychopharmacology. 2007;32:2046–2052. doi: 10.1038/sj.npp.1301331. [DOI] [PubMed] [Google Scholar]
- Surtees PG, Wainwright NW, Willis-Owen SA, Luben R, Day NE, Flint J. Social adversity, the serotonin transporter (5-HTTLPR) polymorphism and major depressive disorder. Biol Psychiatry. 2006;59:224–229. doi: 10.1016/j.biopsych.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Way BM, Welch WT, Hilmert CJ, Lehman BJ, Eisenberger NI. Early family environment, current adversity, the serotonin transporter promoter polymorphism, and depressive symptomatology. Biol Psychiatry. 2006;60:671–676. doi: 10.1016/j.biopsych.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Thakur GA, Joober R, Brunet A. Development and persistence of posttraumatic stress disorder and the 5-HTTLPR polymorphism. J Trauma Stress. 2009;22 (3:240–243. doi: 10.1002/jts.20405. [DOI] [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: review and methodological analysis. Mol Psychiatry. 2008;13:131–146. doi: 10.1038/sj.mp.4002067. [DOI] [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the etiology of depression: 2009 update. Mol Psychiatry. 2010;15:18–22. doi: 10.1038/mp.2009.123. [DOI] [PubMed] [Google Scholar]
- Wendland JR, Martin BJ, Kruse MR, Lesch KP, Murphy DL. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Mol Psychiatry. 2006;11:224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- Williams RB, Marchuk DA, Gadde KM, Barefoot JC, Grichnik K, Helms MJ, et al. Central nervous system serotonin function and cardiovascular responses to stress. Psychosom Med. 2001;63:300–305. doi: 10.1097/00006842-200103000-00016. [DOI] [PubMed] [Google Scholar]
- Wüst S, Kumsta R, Treutlein J, Frank J, Entringer S, Schulze TG, et al. Sex-specific association between the 5-HTT gene-linked polymorphic region and basal cortisol secretion. Psychoneuroendocrinology. 2009;34:972–982. doi: 10.1016/j.psyneuen.2009.01.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.