Abstract
Although the effects of cannabis on perception are well documented, little is known about their neural basis or how these may contribute to the formation of psychotic symptoms. We used functional magnetic resonance imaging (fMRI) to assess the effects of Delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) during visual and auditory processing in healthy volunteers. In total, 14 healthy volunteers were scanned on three occasions. Identical 10 mg THC, 600 mg CBD, and placebo capsules were allocated in a balanced double-blinded pseudo-randomized crossover design. Plasma levels of each substance, physiological parameters, and measures of psychopathology were taken at baseline and at regular intervals following ingestion of substances. Volunteers listened passively to words read and viewed a radial visual checkerboard in alternating blocks during fMRI scanning. Administration of THC was associated with increases in anxiety, intoxication, and positive psychotic symptoms, whereas CBD had no significant symptomatic effects. THC decreased activation relative to placebo in bilateral temporal cortices during auditory processing, and increased and decreased activation in different visual areas during visual processing. CBD was associated with activation in right temporal cortex during auditory processing, and when contrasted, THC and CBD had opposite effects in the right posterior superior temporal gyrus, the right-sided homolog to Wernicke's area. Moreover, the attenuation of activation in this area (maximum 61, −15, −2) by THC during auditory processing was correlated with its acute effect on psychotic symptoms. Single doses of THC and CBD differently modulate brain function in areas that process auditory and visual stimuli and relate to induced psychotic symptoms.
Keywords: cannabis, delta-9-tetrahydrocannabinol, cannabidiol, visual, auditory, sensory
INTRODUCTION
Cannabis is the world's most commonly used illicit substance (Hall and Babor, 2000). Along with effects on cognition and mood (Isbell et al, 1967; D'Souza et al, 2004), it has marked effects on sensory experiences, ranging from heightened subjective sensory awareness and appreciation to vivid mental imagery, illusions, and frank hallucinations (Tart, 1970). In some individuals, acute intoxication with cannabis can mimic sensory processing abnormalities associated with psychotic disorders (Koethe et al, 2006), and cannabis use has been implicated in the onset of psychotic disorders (Murray et al, 2007).
Both, chronic cannabis use and the experimental administration of Delta-9-tetrahydrocannabinol (THC – the main psychoactive constituent of cannabis) can affect regional brain activation during tasks that engage learning (Jager et al, 2007; Bhattacharyya et al, 2009b), motor response inhibition (Borgwardt et al, 2008), interference inhibition (Gruber and Yurgelun-Todd, 2005), and emotional processing (Fusar-Poli et al, 2009). However, there have been relatively few studies of the effects of cannabinnoids on the neural correlates of sensory processing. O'Leary et al, 2002, using [15O]H2O positron emission tomography to measure the effects of inhaled marijuana cigarettes on blood flow during an auditory attention task, reported that this was associated with increased perfusion in paralimbic regions, and reduced perfusion in the lateral temporal cortex bilaterally. However, in addition to delta-9-THC, marijuana cigarettes contain other cannabinoids, in particular cannabidiol (CBD, Mechoulam and Gaoni, 1967), the second most abundant constituent of Cannabis sativa. Although administration of THC can induce psychotic symptoms, anxiety, and cognitive impairments in healthy subjects (D'Souza et al, 2004), CBD has anxiolytic (Crippa et al, 2004) and possibly antipsychotic properties (Zuardi et al, 2006), and does not impair cognitive performance (Zuardi, 2008). The first aim of the present study was to assess and compare the respective effects of THC and CBD on neural activation during auditory and visual processing. A secondary aim was to examine the relationship between the neural effects of THC and the induction of ‘psychotic-like' experiences. In schizophrenia, functional changes in the auditory and visual processing pathways have been implicated in the pathophysiology of auditory and visual hallucinations, respectively (Oertel et al, 2007; Allen et al, 2008). As transient auditory and visual hallucinations are occasionally experienced under the influence of cannabis (Tart, 1970; Hall and Solowij, 1998), and appear to be mediated by THC (D'Souza et al, 2004), we investigated whether the acute induction of psychotic symptoms by THC was related to its effects on the areas involved in auditory and visual processing.
We used functional magnetic resonance imaging (fMRI) in conjunction with a paradigm that engaged both auditory and visual processing to study the acute effects of THC and CBD on regional brain activation in healthy volunteers.
Our first hypothesis was that both THC and CBD would alter activation in the lateral temporal and occipital cortices during auditory and visual processing, respectively. The second hypothesis was the effects of THC (but not CBD) on activation during sensory processing would be related to the acute induction of psychotic symptoms.
MATERIALS AND METHODS
Design
A double-blind, placebo-controlled, pseudo-randomized, within-subject study was conducted over three sessions, with each participant scanned three times (placebo, delta-9-THC, CBD), with a 1-month interval between sessions. The order of drug administration across sessions was pseudo-randomized across subjects, such that equal numbers followed each drug sequence.
Participants
In total, 18 subjects initially took part in the study, recruited from advertisements in local media. Three withdrew from the study because they experienced frank psychotic symptoms following THC administration, and could not tolerate the scanning procedure. Data from one further subject was corrupted, leaving a final sample of 14 subjects who completed all parts of the study.
These 14 subjects were healthy white right-handed men, aged from 20 to 42 years (average 26.7 years, SD 5.7). Their mean total years of education was 16.5 (SD 3.9), and their mean estimated IQ (National Adult Reading Test (Willshire et al, 1991) was 98.67 (SD 7.0)). All participants were native English speakers who had used cannabis at least once but fewer than 15 times in their lifetime, with no cannabis use in the past month, and minimal previous exposure to other illicit substances as assessed by the Structured Clinical Interview for DSM Disorders (Spitzer et al, 1992), and the Addiction Severity Index (McLellan et al, 1992). Participants were told to abstain from illicit drug use for the duration of the study and from alcohol and caffeine intake for 24 and 12 h, respectively, before each study day. Following a light standardized breakfast, a urine sample was collected for screening for amphetamines, benzodiazepines, cocaine, methamphetamine, opiates, and THC using immunometric assay kits. None of the participants tested positive on any of the sessions. Participants were carefully screened using a semi-structured clinical interview to exclude psychiatric neurological or serious physical illness or a family history of psychiatric illness. The study had local Ethics Committee approval and all participants gave their informed consent after the study procedure had been explained to them in detail.
Procedure
At 1 h before each scanning session, participants were given a capsule of 10 mg delta-9-THC, 600 mg CBD, or placebo (flour). These doses of THC and CBD were selected on the basis of previous research (Agurell et al, 1981; Chesher et al, 1990; Leweke et al, 1999; Koethe et al, 2006) to produce an effect on regional brain function without provoking severe toxic, psychiatric, or physical symptoms. The capsules were identical in appearance and taste and neither the participants nor the experimenters were aware of which drug was being administered. An intravenous line was inserted at the start of the testing session to monitor drug levels. All participants were physically examined before testing, and their heart rate and blood pressure were monitored at regular intervals (5 min per 1 h) throughout each session.
Symptom Ratings, Physiological, and Biochemical Variables
Subjects were asked to rate their subjective experiences at baseline, immediately before scanning (1 h), immediately after scanning (2 h), and 1 h postscanning (3 h) using the Visual Analog Mood Scale (Folstein and Luria, 1973), the Spielberger State Trait Anxiety Inventory (Spielberger et al, 1983), and a Visual Analog Intoxication Scale (Mathew et al, 1999). The presence of psychotic phenomena was assessed using the Positive and Negative Syndrome Scale (PANSS, Kay et al, 1987) at the same time points along with measures of blood pressure, heart rate, and blood levels of THC and CBD. Concentrations of THC and CBD in whole blood were measured by immunoassay with positives confirmed by gas chromatography–mass spectrometry (Tricho-Tech, Cardiff, UK).
Functional MRI Paradigm
Participants performed a number of cognitive tasks during each scanning session. For the current study, we used a sensory stimulation paradigm that has previously been shown to elicit robust responses in the auditory and visual cortices (Williams et al, 1997). During auditory stimulation blocks, each lasting 24 s, subjects listened passively through headphones to neutral words read at 30, 60, or 90 words per minute. During visual stimulation blocks, subjects viewed a radial checkerboard with flicker rates of 2, 4, or 8 Hz for a period of 16 s. In between blocks, subjects viewed a fixation cross. The order of presentation of the auditory and visual blocks in each of three load conditions was pseudo-randomly varied between subjects, such that each subject was presented with a total of four repetitions of each load level of visual stimuli, and three repetitions of each load level of auditory stimuli.
Image Acquisition and Analysis
Images were acquired on a 1.5Tesla Signa scanner (General Electric, Milwaukee, Wisconsin) at the Maudsley Hospital, London and analyzed using XBAM software developed at the Institute of Psychiatry (http://brainmap.it). Image acquisition parameters and preprocessing details are further described in Supplementary Material.
Following preprocessing we examined the main effect of each task condition (processing auditory and visual stimuli, separately) relative to viewing a fixation cross, independent of sensory load. We then examined the main effect of the drug (THC vs placebo and CBD vs placebo), independent of sensory load, in each task condition (auditory and visual processing), using nonparametric-repeated measures analyses of variance (ANOVAs), and the interaction of drug and load using 2 (drug) × 3 (load) factorial ANOVAs. Results of load × drug interactions are reported in Supplementary Material.
All results are reported at a voxelwise threshold of p<0.05 and, with the clusterwise threshold set such that the total number of false-positive clusters per brain volume was <1 (maximum p<0.01).
To investigate the association between changes in activation following administration of THC and concurrently induced psychotic symptoms, Spearman's product-moment correlations were calculated between the mean change in PANSS-positive and PANSS-total ratings, and the difference in the change in the median SSQ ratios in the voxel mean of each cluster between the placebo and THC scans. Two-tailed significance tests were used. When a significant or trend level correlation was identified for a given cluster, sensitivity testing was performed using Cook's D calculations, and symptom specificity was tested post hoc by analyzing the correlation with the change in other symptom ratings (PANSS subscores, STAI, AIS, and VAMS scores).
Behavioral and Symptom Rating Analyses
Measures of self report data, symptom ratings, physiological data, and drug levels were analyzed using repeated-measures ANOVAs to compare drug conditions in SPSS v15.0. When significant differences were found, the Tukey test for pairwise comparisons was applied.
RESULTS
Physiological Parameters
Mean whole blood levels of THC at 1 and 2 h after taking the drug were 3.9 (SD 7.3) ng/ml and 5.1 (SD 5.6) ng/ml, respectively; CBD levels at the same time points were 4.7 (SD 7.0) and 17.0 (SD 29.0) ng/ml, respectively. There was no significant drug effect on blood pressure; however, we did identify a (nonsignificant) trend for an increase in heart rate with THC+1.93 beats/min (SD 5.74) and +8.79 beats/min (SD 16.31) at 1 and 2 h after baseline.
Symptom Scores
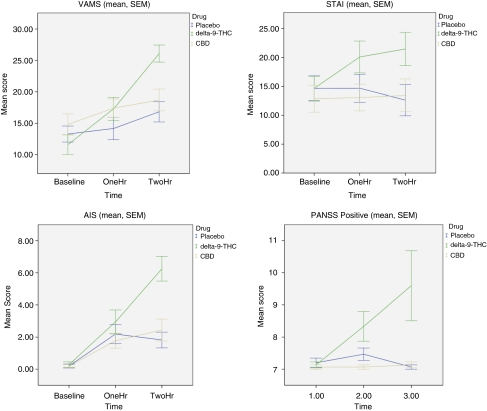
No significant differences were observed between the drug conditions at baseline for any symptom variables. Pairwise comparisons between baseline and 2 h postingestion revealed significant increases in ratings over time for mental sedation (VAMS, p<0.01), intoxication (AIS, p<0.01), anxiety (STAI, p<0.01), and positive psychotic symptoms (PANSS-P, p<0.01) for THC vs placebo (Figure 1). There were no significant changes in the corresponding ratings for the CBD condition and no significant effects of order of drug (p>0.1).
Figure 1.
Symptom measures across time points and drug conditions; VAMS, Visual Analog Mood Scale; STAI, Speiberger State Trait Anxiety Scale; AIS, Analog Intoxication Scale; PANSS, Positive and Negative Syndrome Scale.
Functional MRI Results
Effect of task
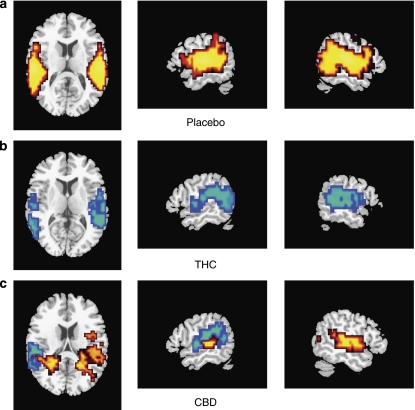
In the placebo condition, auditory processing was associated with bilateral activation in the superior temporal gyri (STG) and middle temporal gyri (MTG), insulae and supramarginal gyri, and in the left inferior frontal gyrus (IFG, Supplementary Table 1, Figure 2a). Visual stimulation was associated with bilateral activation in the fusiform, lingual, and middle occipital gyri, cuneus, and cerebellum (Supplementary Table 1, Figure 4a).
Figure 2.
Brain activation maps during auditory stimulation: (a) task effect (task > fixation cross under placebo conditions), (b) THC< placebo (Blue) (c) CBD>placebo (red); CBD < placebo (blue). L on figure, L side of brain.
Main Effect of Drug During Auditory Processing
THC vs placebo
During auditory stimulation, relative to placebo, THC reduced activation in the temporal cortex bilaterally in the anterior and posterior STG and MTG and insulae, the supramarginal gyri, and in the right IFG and left cerebellum (Table 1, Figure 2b). There were no regions in which THC significantly increased activation relative to placebo.
Table 1. Talairach Coordinates of Illustrative Peak Areas of Activation for the Main Effect of Drug During Auditory Stimulation- Areas with Identical p Values are Drawn from Contiguous Clusters.
| Cerebral region | Side | Size | Tal(x) | Tal(y) | Tal(z) | p |
|---|---|---|---|---|---|---|
| THC>placebo | ||||||
| Nonsignificant effect | ||||||
| THC<placebo | ||||||
| Superior temporal gyrus | L | 140 | −58 | −30 | 15 | 0.0006 |
| Superior temporal gyrus | R | 145 | 61 | −19 | −2 | 0.0006 |
| Middle temporal gyrus | L | 83 | −58 | −41 | 4 | 0.0006 |
| Transverse temporal gyrus | R | 47 | 61 | −19 | 9 | 0.0006 |
| Insula | L | 37 | −51 | −37 | 20 | 0.0006 |
| Supramarginal gyrus | R | 49 | 61 | −19 | 15 | 0.0006 |
| Supramarginal gyrus | L | 6 | −58 | −37 | 26 | 0.0006 |
| Inferior frontal gyrus | R | 18 | 47 | 26 | −7 | 0.0006 |
| Cerebellum | L | 12 | −43 | −63 | −18 | 0.0006 |
| Angular gyrus | L | 7 | −43 | −70 | 20 | 0.0006 |
| CBD>placebo | ||||||
| Superior temporal gyrus | R | 80 | 51 | −22 | 4 | 0.0009 |
| Middle temporal gyrus | R | 105 | 51 | −22 | −2 | 0.0009 |
| Middle temporal gyrus | L | 45 | −51 | −22 | −2 | 0.007 |
| Caudate | L | 40 | −22 | −41 | 9 | 0.007 |
| Caudate | L | 21 | −18 | −37 | 15 | 0.007 |
| Parahippocampal gyrus | L | 16 | −29 | −56 | −2 | 0.007 |
| Parahippocampal gyrus | R | 31 | 22 | −44 | 9 | 0.0009 |
| Insula/TTG | R | 41 | 47 | −22 | 9 | 0.0009 |
| Insula/STG | L | 28 | −43 | −22 | 4 | 0.007 |
| Hippocampus | R | 13 | 29 | −44 | 4 | 0.0009 |
| Insula | R | 11 | 32 | −26 | 15 | 0.0009 |
| CBD<placebo | ||||||
| Superior temporal gyrus | L | 89 | −54 | −37 | 15 | 0.002 |
| Insula | L | 18 | −51 | −19 | 4 | 0.002 |
| Supramarginal gyrus | L | 11 | −47 | −44 | 26 | 0.002 |
| Middle Temporal gyrus | L | 49 | −54 | −48 | 4 | 0.002 |
| THC>CBD | ||||||
| No significant regions | ||||||
| CBD>THC | ||||||
| Middle temporal gyrus | R | 134 | 58 | −19 | −2 | 0.0003 |
| Superior temporal gyrus | R | 76 | 51 | −22 | 4 | 0.0003 |
| Supramarginal gyrus/Insula | R | 10 | 51 | −22 | 15 | 0.0003 |
| Insula/TTG | R | 53 | 47 | −22 | 9 | 0.0003 |
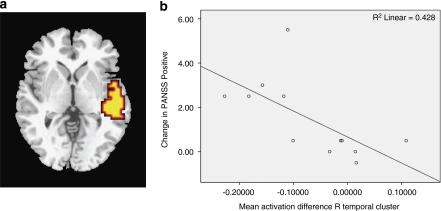
The attenuation of activation in the right temporal cluster (cluster maximum x=61, y=−15, z=−2, STG) following THC was correlated with the concurrent increase in PANSS total (r=−0.534 P=0.049) score relative to placebo, although not with the change in PANSS-positive score (r=−0.437, p=0.118). Following the removal of two outliers identified using Cook's D calculations with a threshold of 4/n, these correlations were strengthened, and the PANSS positive correlation also became significant (PANSS total r=−0.773, p=0.004, PANSS positive r=−0.754 p=0.004, Figure 3b). There were no significant correlations with changes in other PANSS subscores, or in any other symptom measures (STAI, AIS, VAMS). Drug-load interactions are reported in Supplementary Material (Supplementary Figures 1A–C).
Figure 3.
(a) Brain activation map during auditory stimulation: CBD > THC (b) Scatter plot showing change in activation of R temporal cluster (max 61,−15, −2) against change in PANSS – total due to THC relative to placebo (THC – placebo). L on figure, L side of brain.
CBD vs placebo
During auditory processing, CBD increased activation relative to placebo in the temporal cortex bilaterally extending medially to the insulae and caudally to the parahippocampal gyri and hippocampi bilaterally (Table 1, Figure 2c). CBD reduced activation relative to placebo in a posterolateral region of the left STG-incorporating parts of the insula, posterior middle temporal gyrus, and supramarginal gyrus (Table 2; Figure 2c, 3b and d).
Table 2. Talairach Coordinates of Peak Areas of Activation for the Main Effect of Drug During Visual Stimulation – Areas with Identical p Values are Drawn from Contiguous Clusters.
| Cerebral region | Side | Size | Tal(x) | Tal(y) | Tal(z) | BA | p |
|---|---|---|---|---|---|---|---|
| THC>placebo | |||||||
| Lingual gyrus | R | 38 | 14 | −89 | −7 | 17 | 0.0053 |
| Middle occipital gyrus | R | 19 | 25 | −85 | −2 | 18 | 0.0053 |
| Lingual gyrus | L | 7 | −18 | −74 | −7 | 18 | 0.0053 |
| Cerebellum | L | 81 | −7 | −78 | −18 | 71 | 0.0053 |
| Cerebellum | R | 23 | 14 | −81 | −13 | 71 | 0.0053 |
| THC<placebo | |||||||
| Middle occipital gyrus | R | 20 | 22 | −89 | 9 | 18 | 0.009 |
| Lingual gyrus | L | 46 | −4 | −85 | −2 | 18 | 0.009 |
| Lingual gyrus | R | 20 | 14 | −74 | −7 | 18 | 0.009 |
| Cuneus | R | 3 | 18 | −89 | 4 | 17 | 0.009 |
| Cerebellum | R | 25 | 14 | −74 | −13 | 71 | 0.009 |
| Cerebellum | L | 4 | −22 | −78 | −18 | 71 | 0.009 |
| CBD>placebo | |||||||
| Lingual gyrus | R | 81 | 11 | −89 | −2 | 18 | 0.0065 |
| Inferior occipital gyrus | R | 14 | 18 | −89 | −7 | 17 | 0.0065 |
| Middle occipital gyrus | R | 11 | 22 | −85 | 15 | 19 | 0.0065 |
| Cerebellum | R | 10 | 18 | −78 | −18 | 71 | 0.0065 |
| Middle occipital gyrus | R | 6 | 29 | −78 | 9 | 19 | 0.0065 |
| Cuneus | R | 10 | 25 | −67 | 20 | 31 | 0.0065 |
| CBD<placebo | |||||||
| No significant areas | |||||||
| THC>CBD | |||||||
| Lingual gyrus | L | 72 | −4 | −85 | −13 | 18 | 0.007 |
| Cerebellum | L | 85 | −22 | −67 | −18 | 71 | 0.007 |
| CBD>THC | |||||||
| Lingual gyrus | R | 121 | 18 | −89 | −2 | 0.0045 | |
| Lingual gyrus | L | 24 | −11 | −85 | −13 | 0.0045 | |
| Cerebellum | R | 76 | 29 | −78 | −13 | 0.0045 | |
| Cerebellum | L | 114 | −7 | −56 | −24 | 0.0045 |
THC vs CBD
As THC and CBD appeared to have opposing effects on temporal activation during auditory processing, we directly compared activation during the THC and CBD conditions. There was a significant difference in the right superior and middle temporal gyri; in this region, CBD increased activation relative to THC during auditory processing (Table 1 Figure 3a). There were no areas in which CBD reduced activation relative to THC.
Main Effect of Drug During Visual Processing
THC vs placebo
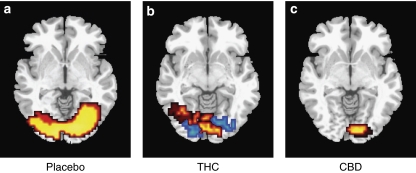
As found in the auditory task, THC reduced activation in areas that were activated under placebo conditions, predominantly in the extrastriate (secondary) visual cortex. (Table 2 Figure 4b). However, unlike during auditory processing, THC also increased activation relative to placebo in some regions. In the right hemisphere, THC increased activation in the lingual and middle occipital gyri (corresponding to the primary visual cortex); in the left hemisphere, THC increased activation extending anteriorally into parts of the lingual and fusiform gyri that had not been activated under placebo (Table 2 Figure 4b). Drug–load interaction in this region is reported in Supplementary Material (Supplementary Figures 1D and E).
Figure 4.
Brain activation map during visual stimulation: (a) task Effect (task > fixation cross under placebo conditions), (b) THC > placebo (Red) and THC < placebo (blue) (c) CBD > placebo (red). L on figure, L side of brain.
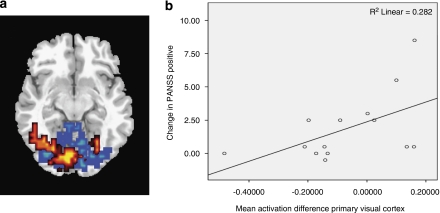
The increase in activation by THC relative to placebo across the visual cortex was correlated with the concomitant increase in PANSS positive score relative to the placebo condition (r=0.536 p=0.048, Figure 5b), but not with the change in PANSS total score (r=0.362 p=0.204). Following removal of two significant outliers identified using Cook's D calculations with a threshold of 4/n this correlation was nonsignificant (r=0.493, p=0.103). There were no correlations with other PANSS subscales or symptom measures. There were no correlations between the attenuation of activation in the primary visual cortex following THC and the change in PANSS positive or total scores.
Figure 5.
(a) Brain activation map during visual stimulation: (a) THC > CBD (red) and CBD>THC (blue) (b) Scatter plot showing change in activation (THC – placebo) in primary visual cortex cluster against change in PANSS Pos (THC – placebo). L on figure, L side of brain.
CBD vs placebo
During visual processing, CBD increased activation relative to placebo in the right occipital lobe, with maxima in the middle and inferior occipital gyri, the lingual gyrus, and cuneus (Table 2, Figure 4c). There were no areas in which CBD reduced activation relative to placebo.
THC vs CBD
Direct comparison of the effects of THC and CBD revealed that THC augmented activation relative to CBD in the left lingual and middle occipital gyri (corresponding to the primary visual cortex; Table 2 Figure 5a). THC attenuated activation relative to CBD in widespread occipital regions bilaterally (Table 2 Figure 5a). Mixed effects were seen within the cerebellum (Table 2).
DISCUSSION
We used fMRI to investigate how the two main psychoactive constituents of cannabis (THC and CBD) modulate brain function during auditory and visual processing. We used a double-blinded pseudo-randomized repeated measure crossover design measuring the BOLD response, whereas subjects passively experienced auditory and visual stimulation of differing levels of intensity. Consistent with our hypotheses, we found that both THC and CBD modulated activation in the auditory and visual cortices, and that the effects of THC on temporal lobe activation during auditory processing, and on occipital cortical activation during visual processing were correlated with its acute induction of psychotic symptoms.
In our study, 8 of the original 18 subjects (44.4%) experienced transient frank psychotic symptoms and of these 3 withdrew from the study. This rate is consistent with a growing literature on transient psychosis related to cannabis use. In a review article of self-reported cannabis effects to open-ended questions, about 51% is reported to feel paranoid and 20% experienced hallucinations (Green et al, 2003), whereas in a survey of adverse affects of cannabis, 15% reported to experience psychotic symptoms (Thomas, 1996).
Auditory stimulation was associated with activation in primary auditory cortex (including Heschl's gyrus and planum temporale), the left posterior superior temporal gyrus (corresponding to Wernicke's area), and its right-sided homolog, bilateral supramarginal and angular gyri, and a region of the left inferior frontal gyrus corresponding to Broca's area (Price et al, 1996). During visual processing we observed activation in primary visual cortex on the medial occipital surface surrounding the calcarine fissure, and secondary extrastriate visual cortices medially, and on the lateral occipital surface.
During auditory processing, THC attenuated activation in primary and secondary auditory regions bilaterally, relative to placebo. These findings are consistent with previous studies that used [15O2] H2O PET to measure the effect of marijuana cigarettes on rCBF during a dichotic auditory attention task (O'Leary et al, 2000; O'Leary et al, 2002; O'Leary et al, 2007). In these studies, inhalation of marijuana cigarettes (which included THC and CBD) relative to placebo cigarettes (without THC, but including CBD) was associated with reduced blood flow in the temporal cortices bilaterally, and increased blood flow in the orbital frontal cortex, anterior cingulate, temporal pole, insula, and cerebellum. These increases in activation were not seen in the present study, which in contrast utilized pure cannabinoids and a different task and imaging technique.
The attenuation of temporal activation by THC was associated with an increase in psychotic symptoms in the same subjects, suggesting that the induction of psychotic symptoms by THC could be mediated by an effect on temporal cortical function. Altered temporal lobe structure and function are robust findings in patients with psychotic disorders, and there is evidence of a negative relationship between left STG activation and severity of auditory hallucinations in schizophrenia (reviewed in Allen et al, 2008). Woodruff et al (1997) report reduced activation of the left and right temporal cortex to external speech in patients experiencing auditory verbal hallucinations and suggest that internally generated hallucinations and external speech compete for a common neurophysiological substrate.
In the left-posterior STG (corresponding to Wernicke's area), both THC and CBD attenuated the response to auditory stimuli. In contrast, in the homologous right-sided region, THC and CBD had opposite effects, and the attenuation in activation in this area due to THC was correlated with its induction of psychotic symptoms; THC also attenuated activation in the right homolog to Broca's area. Although left-sided language areas are thought to process linguistic stimuli analytically in dealing with routine, dominant, and frequent meanings, their right-sided homologs are thought to have a role in resolving ambiguity in language (Harpaz et al, 2009), modulating affective components and prosody, and perceiving infrequent subordinate meanings (reviewed in Jung-Beeman, 2005). An effect of THC on these aspects of language processing could thus contribute to its psychotogenic properties and account for the correlation with psychotic symptoms in the right temporal cortex. Equally, the opposite effects of CBD on activation in this region may contribute to its reported antipsychotic properties (Zuardi et al, 2006). CBD also increased activation in a smaller region corresponding to the left primary auditory cortex, and in the parahippocampal cortex bilaterally. Augmentation of activation in these areas by CBD has previously been reported in the same subjects when they were performing a verbal memory task (Bhattacharyya et al, 2009b).
THC and CBD have previously been found to have opposing effects on activation in a number of regions during tasks that engage verbal memory (Bhattacharyya et al, 2009b), response inhibition (Borgwardt et al, 2008), and emotional processing (Fusar-Poli et al, 2009). The present data extend these findings by showing that, although the two cannabinoids also have opposite effects on regional brain responses during auditory and visual processing in some regions, they have similar effects in others. The molecular basis of their similar and opposing effects is unclear: CBD may function as an inverse agonist/ antagonist at CB1 receptors even at low concentrations (Pertwee, 2008). On the other hand, THC is known to be a partial agonist at CB1 receptors (Pertwee, 2008). This may explain their opposite effects in some brain areas. However, CBD may function through a number of other mechanisms as well, including hydrolysis of anandamide, enhancement of adenosine signaling, as an agonist at 5HT1A and vanilloid receptors, and through retrograde inhibition of a number of neurotransmitters predominately GABA and glutamate, but also through acetylcholine, dopamine, and others (Bhattacharyya et al, 2009a).
During visual processing, THC attenuated bilateral regions of the occipital lobe compared with the placebo condition. Although less extensive than the auditory literature, studies of patients with visual hallucinations report reductions of activation in visual areas during visual stimulation (eg, ffytche et al, 1998). This is thought to result from increased levels of spontaneous activity, again consistent with the notion that hallucinations may develop through competition between internally and externally generated visual stimuli for a common neural substrate.
However, in contrast to its effects during auditory stimulation, THC also augmented the response of some occipital regions during visual processing. In the left hemisphere, these effects included regions anterior to those activated under placebo conditions. The significance of this additional recruitment of visual cortical areas is unclear; however, this region is thought to mediate geometric hallucinations induced by flashing lights (Purkinje patterns, (ffytche 2008)), and also corresponds to areas activated during the experience of spontaneous geometric hallucinations in subjects with Charles Bonnet syndrome (ffytche et al, 1998). The enhanced activation under THC in primary visual cortex correlated with the rise in concurrently induced psychotic symptoms, but this correlation did not survive sensitivity analysis.
The study has some limitations. The symptom rating scales we used were not intended for use in healthy subjects, and provided limited information on the subjective nature of the sensory experiences induced by THC and CBD. This limited the amount of variance in the PANSS and correlations with brain activation should be interpreted accordingly. As in previous functional neuroimaging studies, the sample size was modest, reflecting the logistical demands of these types of studies, however, greater drug effects may have been detected with a larger sample. We also administered THC and CBD orally, rather than via more rapid intravenous or inhaled routes, in order to provide a sustained level of drug throughout the scanning protocol.
CONCLUSIONS
Single-acute doses of THC and CBD significantly modulate brain function in areas that process auditory and visual stimuli. These results are the first to demonstrate how the two major constituents of cannabis (THC and CBD) function on the sensory cortices, and how their effect are related to the induction of psychotic symptoms by cannabis. The data also provide further evidence that THC and CBD have distinct effects on regional brain activation, sometimes in opposite directions.
Acknowledgments
Funding/support: This study was supported by grants from the Psychiatry Research Trust, London, England. Dr Winton-Brown is supported by a Wellcome Trust Research Training Fellowship . Dr Fusar-Poli is supported by the Guy's & St Thomas' Charitable Foundation New Services and Innovations in Health Care. Dr Crippa and Dr Zuardi are recipients of Con-selho Nacional de Desenvolvimento Científico e Tecno- ló gico (CNPq) (Brazil) fellowships. Dr Bhattacharyya is supported by a joint Medical Research Council/Priory Clinical Research Training Fellowship Award from the Medical Research Council.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Agurell S, Carlsson S, Lindgren JE, Ohlsson A, Gillespie H, Hollister L. Interactions of delta 1-tetrahydrocannabinol with cannabinol and cannabidiol following oral administration in man. Assay of cannabinol and cannabidiol by mass fragmentography. Experientia. 1981;37:1090–1092. doi: 10.1007/BF02085029. [DOI] [PubMed] [Google Scholar]
- Allen P, Laroi F, McGuire PK, Aleman A. The hallucinating brain: a review of structural and functional neuroimaging studies of hallucinations. Neurosci Biobehav Rev. 2008;32:175–191. doi: 10.1016/j.neubiorev.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Crippa JA, Martin-Santos R, Winton-Brown T, Fusar-Poli P. Imaging the neural effects of cannabinoids: current status and future opportunities for psychopharmacology. Curr Pharm Des. 2009a;15:2603–2614. doi: 10.2174/138161209788957465. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Fusar-Poli P, Borgwardt S, Martin-Santos R, Nosarti C, O'Carroll C, et al. Modulation of mediotemporal and ventrostriatal function in humans by Delta9-tetrahydrocannabinol: a neural basis for the effects of Cannabissativa on learning and psychosis. Arch Gen Psychiatry. 2009b;66:442–451. doi: 10.1001/archgenpsychiatry.2009.17. [DOI] [PubMed] [Google Scholar]
- Borgwardt SJ, Allen P, Bhattacharyya S, Fusar-Poli P, Crippa JA, Seal ML, et al. Neural basis of Delta-9-tetrahydrocannabinol and cannabidiol: effects during response inhibition. Biol Psychiatry. 2008;64:966–973. doi: 10.1016/j.biopsych.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Chesher GB, Bird KD, Jackson DM, Perrignon A, Starmer GA. The effects of orally administered delta 9-tetrahydrocannabinol in man on mood and performance measures: a dose-response study. Pharmacol Biochem Behav. 1990;35:861–864. doi: 10.1016/0091-3057(90)90371-n. [DOI] [PubMed] [Google Scholar]
- Crippa JA, Zuardi AW, Garrido GE, Wichert-Ana L, Guarnieri R, Ferrari L, et al. Effects of cannabidiol (CBD) on regional cerebral blood flow. Neuropsychopharmacology. 2004;29:417–426. doi: 10.1038/sj.npp.1300340. [DOI] [PubMed] [Google Scholar]
- D'Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu YT, et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29:1558–1572. doi: 10.1038/sj.npp.1300496. [DOI] [PubMed] [Google Scholar]
- ffytche DH. The hodology of hallucinations. Cortex. 2008;44:1067–1083. doi: 10.1016/j.cortex.2008.04.005. [DOI] [PubMed] [Google Scholar]
- ffytche DH, Howard RJ, Brammer MJ, David A, Woodruff P, Williams S. The anatomy of conscious vision: an fMRI study of visual hallucinations. Nat Neurosci. 1998;1:738–742. doi: 10.1038/3738. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Luria R. Reliability, validity, and clinical application of the Visual Analogue Mood Scale. Psychol Med. 1973;3:479–486. doi: 10.1017/s0033291700054283. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Crippa JA, Bhattacharyya S, Borgwardt SJ, Allen P, Martin-Santos R, et al. Distinct effects of {delta}9-tetrahydrocannabinol and cannabidiol on neural acti. Arch Gen Psychiatry. 2009;66:95–105. doi: 10.1001/archgenpsychiatry.2008.519. [DOI] [PubMed] [Google Scholar]
- Green B, Kavanagh D, Young R. Being stoned: a review of self-reported cannabis effects. Drug Alcohol Rev. 2003;22:453–460. doi: 10.1080/09595230310001613976. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Yurgelun-Todd DA. Neuroimaging of marijuana smokers during inhibitory processing: a pilot investigation. Brain Res Cogn Brain Res. 2005;23:107–118. doi: 10.1016/j.cogbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Hall W, Babor TF. Cannabis use and public health: assessing the burden. Addiction. 2000;95:485–490. doi: 10.1046/j.1360-0443.2000.9544851.x. [DOI] [PubMed] [Google Scholar]
- Hall W, Solowij N. Adverse effects of cannabis. Lancet. 1998;352:1611–1616. doi: 10.1016/S0140-6736(98)05021-1. [DOI] [PubMed] [Google Scholar]
- Harpaz Y, Levkovitz Y, Lavidor M. Lexical ambiguity resolution in Wernicke's area and its right homologue. Cortex. 2009;45:1097–1103. doi: 10.1016/j.cortex.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Isbell H, Gorodetzsky CW, Jasinski D, Claussen U, von Spulak F, Korte F. Effects of (—)delta-9-trans-tetrahydrocannabinol in man. Psychopharmacologia. 1967;11:184–188. doi: 10.1007/BF00401256. [DOI] [PubMed] [Google Scholar]
- Jager G, Van Hell HH, De Win MM, Kahn RS, Van Den Brink W, Van Ree JM, et al. Effects of frequent cannabis use on hippocampal activity during an associative memory task. Eur Neuropsychopharmacol. 2007;17:289–297. doi: 10.1016/j.euroneuro.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Jung-Beeman M. Bilateral brain processes for comprehending natural language. Trends in Cognitive Sciences. 2005;9:512–518. doi: 10.1016/j.tics.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Koethe D, Gerth CW, Neatby MA, Haensel A, Thies M, Schneider U, et al. Disturbances of visual information processing in early states of psychosis and experimental delta-9-tetrahydrocannabinol altered states of consciousness. Schizophr Res. 2006;88:142–150. doi: 10.1016/j.schres.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Leweke FM, Schneider U, Thies M, Münte TF, Emrich HM. Effects of synthetic delta9-tetrahydrocannabinol on binocular depth inversion of natural and artificial objects in man. Psychopharmacology (Berl) 1999;142:230–235. doi: 10.1007/s002130050884. [DOI] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH, Chiu NY, Turkington TG, Degrado TR, Coleman RE. Regional cerebral blood flow and depersonalization after tetrahydrocannabinol administration. Acta Psychiatr Scand. 1999;100:67–75. doi: 10.1111/j.1600-0447.1999.tb10916.x. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Gaoni Y. Recent advances in the chemistry of hashish. Fortschr Chem Org Naturst. 1967;25:175–213. doi: 10.1007/978-3-7091-8164-5_6. [DOI] [PubMed] [Google Scholar]
- Murray RM, Morrison PD, Henquet C, Di Forti M. Cannabis, the mind and society: the hash realities. Nat Rev Neurosci. 2007;8:885–895. doi: 10.1038/nrn2253. [DOI] [PubMed] [Google Scholar]
- O'Leary DS, Block RI, Flaum M, Schultz SK, Boles Ponto LL, Watkins GL, et al. Acute marijuana effects on rCBF and cognition: a PET study. Neuroreport. 2000;11:3835–3841. doi: 10.1097/00001756-200011270-00047. [DOI] [PubMed] [Google Scholar]
- O'Leary DS, Block RI, Koeppel JA, Flaum M, Schultz SK, Andreasen NC, et al. Effects of smoking marijuana on brain perfusion and cognition. Neuropsychopharmacology. 2002;26:802–816. doi: 10.1016/S0893-133X(01)00425-0. [DOI] [PubMed] [Google Scholar]
- O'Leary DS, Block RI, Koeppel JA, Schultz SK, Magnotta VA, Ponto LB, et al. Effects of smoking marijuana on focal attention and brain blood flow. Hum Psychopharmacol. 2007;22:135–148. doi: 10.1002/hup.832. [DOI] [PubMed] [Google Scholar]
- Oertel V, Rotarska-Jagiela A, van de Ven VG, Haenschel C, Maurer K, Linden DE. Visual hallucinations in schizophrenia investigated with functional magnetic resonance imaging. Psychiatry Res. 2007;156:269–273. doi: 10.1016/j.pscychresns.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Wise RJ, Warburton EA, Moore CJ, Howard D, Patterson K, et al. Hearing and saying. The functional neuro-anatomy of auditory word processing. Brain. 1996;119 (Part 3:919–931. doi: 10.1093/brain/119.3.919. [DOI] [PubMed] [Google Scholar]
- Spielberger CG, Lushene RL, R Vagg PR. State-Trait Anxiety Inventory For Adults. Consulting Psychologists Press: Palo Alto, CA; 1983. [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. The structured clinical interview for DSM-III-R (SCID). I: history, rationale, and description. Arch Gen Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Tart CT. Marijuana intoxication common experiences. Nature. 1970;226:701–704. doi: 10.1038/226701a0. [DOI] [PubMed] [Google Scholar]
- Thomas H. A community survey of adverse effects of cannabis use. Drug Alcohol Depend. 1996;42:201–207. doi: 10.1016/s0376-8716(96)01277-x. [DOI] [PubMed] [Google Scholar]
- Williams SA, Simmons A, Bullmore E, Brammer M. Multiplexed stimuli presentation for functional MRI studies. Neuroimage. 1997;3:S41. [Google Scholar]
- Willshire D, Kinsella G, Prior M. Estimating WAIS-R IQ from the National Adult Reading Test: a cross-validation. J Clin Exp Neuropsychol. 1991;13:204–216. doi: 10.1080/01688639108401038. [DOI] [PubMed] [Google Scholar]
- Woodruff PW, Wright IC, Bullmore ET, Brammer M, Howard RJ, Williams SC, et al. Auditory hallucinations and the temporal cortical response to speech in schizophrenia: a functional magnetic resonance imaging study. Am J Psychiatry. 1997;154:1676–1682. doi: 10.1176/ajp.154.12.1676. [DOI] [PubMed] [Google Scholar]
- Zuardi AW. Cannabidiol: from an inactive cannabinoid to a drug with wide spectrum of action. Rev Bras Psiquiatr. 2008;30:271–280. doi: 10.1590/s1516-44462008000300015. [DOI] [PubMed] [Google Scholar]
- Zuardi AW, Crippa JA, Hallak JE, Moreira FA, Guimarães FS. Cannabidiol, a Cannabis sativa constituent, as an antipsychotic drug. Braz J Med Biol Res. 2006;39:421–429. doi: 10.1590/s0100-879x2006000400001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.