Abstract
Nicotine improves cognitive performance and attention in both experimental animals and in human subjects, including patients affected by neuropsychiatric disorders. However, the specific molecular mechanisms underlying nicotine-induced behavioral changes remain unclear. We have recently shown in mice that repeated injections of nicotine, which achieve plasma concentrations comparable to those reported in high cigarette smokers, result in an epigenetically induced increase of glutamic acid decarboxylase 67 (GAD67) expression. Here we explored the impact of synthetic α4β2 and α7 nAChR agonists on GABAergic epigenetic parameters. Varenicline (VAR), a high-affinity partial agonist at α4β2 and a lower affinity full agonist at α7 neuronal nAChR, injected in doses of 1–5 mg/kg/s.c. twice daily for 5 days, elicited a 30–40% decrease of cortical DNA methyltransferase (DNMT)1 mRNA and an increased expression of GAD67 mRNA and protein. This upregulation of GAD67 was abolished by the nAChR antagonist mecamylamine. Furthermore, the level of MeCP2 binding to GAD67 promoters was significantly reduced following VAR administration. This effect was abolished when VAR was administered with mecamylamine. Similar effects on cortical DNMT1 and GAD67 expression were obtained after administration of A–85380, an agonist that binds to α4β2 but has negligible affinity for α3β4 or α7 subtypes containing nAChR. In contrast, PNU–282987, an agonist of the homomeric α7 nAChR, failed to decrease cortical DNMT1 mRNA or to induce GAD67 expression. The present study suggests that the α4β2 nAChR agonists may be better suited to control the epigenetic alterations of GABAergic neurons in schizophrenia than the α7 nAChR agonists.
Keywords: schizophrenia, varenicline, DNA methyltransferase-1, GAD67, A–85380, PNU–282987
INTRODUCTION
Protracted and repeated administration of nicotine to laboratory animals, and the inhalation of large amounts of nicotine from tobacco smoking in healthy human subjects and schizophrenia (SZ) patients results in an increased attention span and in an improvement of sensory/cognitive functions (Freedman et al, 2008; Evans and Drobes, 2009; Hasselmo and Sarter, 2011). However, the precise molecular correlates of the behavioral actions of nAChR stimulation have not been extensively studied.
It has now been established that when the postmortem brain of SZ patients is compared with that of non-psychiatric subjects, a decrease of high- (α4β2) and low (α7)-affinity nAChR subtypes is detected in the hippocampus and cortex (Freedman et al, 1995; Olincy et al, 1997; Breese et al, 2000) along with GABAergic neuropathologies in the same brain areas (Akbarian et al, 1995; Guidotti et al, 2000, 2005; Lewis et al, 2005; Benes et al, 2007). These neuropathologies are characterized by the decreased expression of glutamic acid decarboxylase 67 (GAD67) and the increased expression of DNA methyltransferases (DNMTs) (Ruzicka et al, 2007; Veldic et al, 2005; Zhubi et al, 2009; Guidotti et al, 2010). DNMTs are a family of enzymes that catalyze the methylation of the carbon 5′ of cytosines embedded in cytosine phosphodiester guanine (CpG) islands of many gene promoters (Van Emburgh and Robertson, 2008). Evidence suggests that GABAergic promoter hypermethylation, mediated by the overexpression of DNMTs, may be an underlying pathophysiological mechanism leading to a downregulation of GABAergic transmission in SZ patients (Guidotti et al, 2010). Downregulation of GABAergic transmission may in turn lead to an impairment of the cortical gamma oscillations related to sensory/cognitive deficits in these patients (Lewis and Gonzalez-Burgos, 2008).
We have recently shown in mice that repeated injections of nicotine, which achieve plasma concentrations comparable to those reported by heavy cigarette smokers, result in: (1) a downregulation of DNMT expression, (2) a decrease of GAD67 promoter methylation, and (3) an increased expression of GAD67 in cortical or hippocampal but not in striatal GABAergic neurons (Satta et al, 2008).
As these effects of nicotine are blocked by mecamylamine but not by hexamethonium, the data support the notion that nAChR ligands active at central α4β2 and α7 nAChR may represent an effective pharmacological tool to correct the epigenetic GABAergic neuropathology present in SZ patients.
Nicotine, acting on a variety of central and peripheral nAChRs, also elicits unwanted side effects such as cardiovascular toxicity, gastrointestinal toxicity, tremor, and addiction. Furthermore, if nicotine is administered by smoking, serious comorbid health conditions are likely to develop. Hence, the potential of α4β2 and/or α7 nAChR full or partial agonists for increasing attention and cognitive performance (Potter et al, 1999; Wilens et al, 2006; Wilens and Decker, 2007; Dunbar et al, 2007; Howe et al, 2010; Olincy et al, 2006; Freedman et al, 2008) with minimal adverse side effects may represent an innovative and efficient way to improve cognitive deficits in patients with neuropsychiatric disorders (Freedman et al, 2008; Smith et al, 2009; Hasselmo and Sarter, 2011).
To test whether the epigenetic GABAergic events induced by the administration of high and repeated doses of nicotine can be replicated with specific α4β2 and/or α7 nAChR full or partial agonists endowed with a lower toxic liability than nicotine, we compared the action of varenicline (VAR) (a high-affinity partial agonist at α4β2 and a lower affinity full agonist at α7 neuronal nAChR) with that of A–85380 (a high-affinity full agonist at α4β2 nAChR) and PNU–282987 (a high-affinity full agonist at α7 nAChR) on GABAergic epigenetic parameters (Rollema et al, 2007; Mihalak et al, 2006; Smith et al, 2007; Bodnar et al, 2005).
VAR has been recently approved for smoking cessation treatment (Faessel et al, 2010). Unlike nicotine, VAR fails to increase sympathetic tone, such as the increase of respiratory rate, locomotor activity, and tremor, but at the same time it reduces the level of smoking addiction in patients, and blocks nicotine intake in rats with access to nicotine self-administration (Rollema et al, 2007; George et al, 2010).
Post-marketing reports of neuropsychiatric symptoms, including agitation, depressed mood, and suicidal ideation in subjects attempting to quit smoking using VAR have led to the addition of a ‘boxed warning' to the product labeling (Faessel et al, 2010; Gunnell et al, 2009), nevertheless in a large clinical study (Gunnell et al, 2009), there was no evidence that VAR was associated with an increased risk of self-harm or suicidal ideation. Hence, despite early negative reports, VAR may represent an innovative and relatively safe way to control cognitive dysfunction, anxiety, and mood disorders through nAChR stimulation in psychotic patients (Smith et al, 2009).
We recently reported that 8 weeks of VAR treatment improves cognitive function while decreasing DNMT1 mRNA expression in the peripheral blood lymphocytes of SZ patients (Smith et al, 2010).
This has provided a rationale for testing whether VAR or the more selective α4β2 (ie, A–85380) or α7 (ie, PNU–282987) nAChR agonists with reduced nicotine-like side-effect liability, elicit an epigenetic-GABAergic gene upregulation in cortical or in hippocampal GABAergic neurons.
MATERIALS AND METHODS
Animals and Drug Administration
Male Swiss albino mice (Harlan Breeders), 60–80 days old and weighing 20–25 g were used in this study. The following drugs were used: mecamylamine hydrochloride and 3-(2(S)-azetidinyl methoxy)pyridine dihydrochloride (A–85380), from Sigma–Aldrich. N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]-4-chlorobenzamide hydrochloride (PNU–282987) and VAR were a generous gift from Dr Mihaly Hajos (Department of Neuroscience, Pfizer Global Research and Development, Groton, CT). All drugs were dissolved in a volume of saline solution corresponding to 0.05 ml/10 g body weight and were injected s.c. or i.p. as indicated.
We studied the effect of VAR, A–85830, and PNU–289987 in Swiss albino mice because in a previous study (Satta et al, 2008), we observed that nicotine injected into these mice in doses ranging from 0.75 to 3.5 mg/kg elicited a significant dose-related decrease of DNMT1 and an increase of GAD67 expression in the frontal cortex (FC) and hippocampus if injected protractedly and repeatedly for at least 4 days. Therefore, in this study, to mimic the central effects of nicotine, selective nAChR agonists and antagonists were administered for 5 days. Unless otherwise indicated, five mice per group were used for the analyses.
Frontal Cortex Dissection
To collect FC samples free of striatal tissue, brains were sectioned coronally 2 mm anterior to the bregma. The tissue anterior to the section was frozen immediately and kept at −80°C until assays were carried out.
Fear-Conditioning Apparatus
The fear-conditioning apparatus consisted of a transparent acrylic chamber measuring 25 cm wide, 18 cm high and 21 cm deep (San Diego Instruments, San Diego, CA). The cage floor was composed of stainless steel rods connected to a shock generator (San Diego Instruments). The chamber was surrounded by a frame with 16 infrared photo beams. A computer controlled the delivery of foot shocks (unconditioned stimulus (US)) and auditory stimuli (CS), and recorded beam interruptions and latencies to beam interruptions (freezing time). A small fan was located on the top wall of the enclosure. A speaker placed on a sidewall of the conditioning chamber delivered the CS.
Training Test
Animals were placed into a training chamber (San Diego Instruments) and allowed to explore it for 2 min. After this time, they received an acoustic CS (30 s, 85 dB with noise) followed by a US (electric shock 2 s, 0.5 mA) three times every 2 min. After the last shock, animals were allowed to explore the context for an additional minute before removal from the training chamber.
Contextual Test
After 24 h, the animals were placed in the contextual cage and freezing behavior was measured for 5 min (Freeze Monitor System, San Diego Instrument) without foot shock presentation.
Freezing behavior was measured for 5 min without foot shock presentation.
Cue Test
After 24 h, mice were tested for freezing responses to the CS. For this purpose, the conditioning chamber was modified (by locating a smaller Plexiglas box, measuring 18 cm wide, 9 cm high, and 11 cm deep in the contextual chamber and adding a few drops of lemon scent). Mice were allowed to explore the new environment for 2 min. After this time, an acoustic CS (30 s, 85 dB with noise) was presented and the expression of freezing behavior was measured during the following 5 min.
Freezing was defined by the absence of any movement except for those related to respiration while the animal was in a stereotypical crouching posture (for further details see Pibiri et al, 2008).
Open Field Test
To assess the general behavior and locomotor activity level, each mouse was placed in an open field apparatus lined with photobeams (40 × 40 × 30 cm; Accuscan Instruments, Columbus, OH) and partitioned into quadrants. We had three fields and six available quadrants allowing us to run six animals simultaneously. Horizontal and vertical activity was recorded over a 10-min period.
RNA Isolation and Quantitative RT-PCR Analysis
Total RNA was extracted by cesium chloride density gradient centrifugation as described (Ruzicka et al, 2007). DNMT1 and NSE mRNA content were measured in each sample by competitive RT-PCR. The following amplification primers were used: DNMT1, forward, base pairs 4231–4254; reverse, base pairs 4667–4644; GenBank accession no. X14805.1 and NSE, forward, base pairs 382–405; reverse, base pairs 769–792; GenBank accession no. M22349.1. This technique is based on the simultaneous amplification of the target mRNA and a specific internal standard having the same sequence as the target template except for a deletion of 58 bp for DNMT1 (between 4461 and 4518 bp) and 85 bp for NSE (between 552 and 636 bp) (Ruzicka et al, 2007; Auta et al, 2007).
Western Blot Analysis
Extraction of GAD65/67
Briefly, proteins were extracted directly from the brain tissue in Laemmli buffer (100 μl/10 mg tissue) and were separated by 4–12%. SDS/PAGE (Invitrogen), blotted onto nitrocellulose membranes (Invitrogen) and developed overnight at 4°C with anti-GAD65/67 rabbit polyclonal antibody diluted to 1 : 2000 (Chemicon). Membranes were then washed and re-blotted with a β-actin mAb (1 : 5000; Sigma-Aldrich) for 2 h (Tremolizzo et al, 2005).
Changes in GAD65/67 expression induced by various treatments were expressed as the ratio of the OD of GAD65/67 vs the OD of β-actin. Approximately, 3 to 5 serial dilutions were run for every sample to identify the linear range for protein quantification.
MeCP2 Chip Assay
To indirectly assess the cytosine methylation level of GAD67 promoters, we used the MeCP2 ChIP assay method as previously described (Dong et al, 2005, 2008).
Approximately 10 mg of FC tissue were used for this procedure. Tissue slices (0.3 × 0.3 mm) were incubated at 37°C for 15 min with 400 μl of PBS containing 1% formaldehyde supplemented with protease inhibitors (1 mM PMSF, 1 μg/ml aprotinin, and 1 μg/ml pepstatin) (Upstate Cell Signaling Solutions, Lake Placid, NY) to crosslink MeCP2 with the target-methylated genomic DNAs. After being washed six times with cold PBS containing protease inhibitors, slices were homogenized in 200–400 μl of SDS lysis buffer (supplied by ChIP kit; Upstate Cell Signaling Solutions) with minor modifications as previously described (Dong et al, 2005).
Genomic DNA was extracted from the mouse FC and sonicated to produce fragment sizes of 200–600 bp. An aliquot of the solution (50 μl) was removed and stored at −20°C to be used as input. The remaining solution was incubated overnight at 4°C with MeCP2 antibody (Upstate Cell Signaling Solutions). The DNA–antibody complex was then added to 50 μl of protein A agarose beads (Invitrogen) and incubated on a rotating platform for 2 h at 4°C. The resulting DNA–antibody–bead complex was isolated after reverse crosslink and the DNA was released by proteinase K digestion. After phenol–chloroform extraction and ethanol precipitation, the DNA pellet was resuspended in 20 μl of diethylpyrocarbonate water.
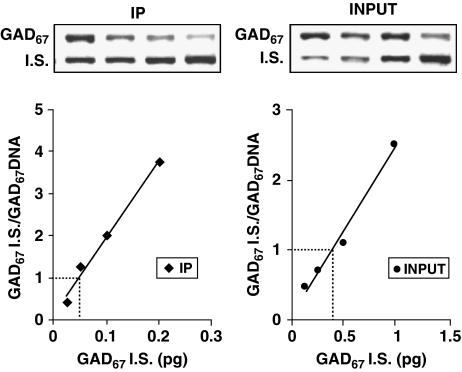
A CpG-rich GAD67 promoter fragment (base pairs –760 to −311) (Dong et al, 2008) was measured by quantitative PCR analysis. The following amplification primers were used: forward, base pairs 760–737; reverse, base pairs 311–334 (Dong et al, 2008). The internal standard used for the quantification was an oligonucleotide with the same sequence as the target except for a deletion of 101 bases (between 390 and 490 bp) (Dong et al, 2008). The level of methylation of the GAD67 promoter is expressed as percentage of the input DNA that is immunoprecipitated by the MeCP2 antibody. In Figure 1, a representative example of a competitive PCR for the quantitative measurement of GAD67 promoter in a sample immunoprecipitated with an antibody directed against MeCP2 and the corresponding input is depicted.
Figure 1.
Example of competitive PCR analysis for glutamic acid decarboxylase 67 (GAD67) promoter in a frontal cortex (FC) sample immunoprecipitated with an antibody directed against MeCP2 (IP) and in the corresponding INPUT. For each sample, four solutions were prepared, each containing the same amount of DNA and increasing amounts of GAD67 internal standard (IS). The upper panels show ethidium bromide gel electrophoresis for the IP and INPUT samples. The two bands in the upper panel correspond to the amplification products of GAD67 IS and GAD67 DNA promoter fragments in each solution. The ratio between the optical density of IS and GAD67 promoters is plotted (lower panels) vs the IS concentrations. At the equivalence point (ratio=1), the amount of GAD67 promoter fragment in the sample is equal to the amount of GAD67 IS. The ratio of IP/INPUT represents the percentage of GAD67 promoter immunoprecipitated by the MeCP2 antibody. For details of the method, see Dong et al (2007).
Statistical Analyses
A one-way repeated ANOVA was used to analyze the treatment effects. Post-hoc analyses of variance were determined by the Student–Newman–Keuls test with multiple range comparisons. Data are expressed as mean±SE.
RESULTS
Varenicline Reproduces the Action of Nicotine on Cortical DNMT and GAD67 Expression
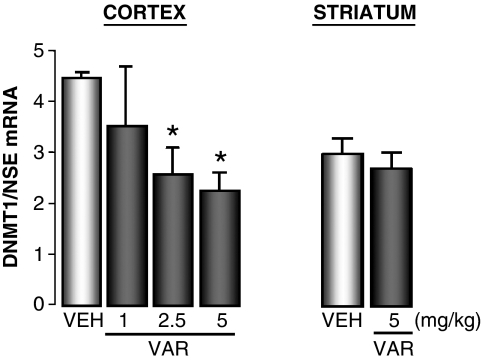
In the doses used in this study (1–5 mg/kg, i.p.), VAR fails to produce the tremor, piloerection or loss of motor coordination that are observed (Satta et al, 2008) after 0.75–3.5 mg/kg of nicotine administration. Furthermore, VAR (5 mg/kg i.p., 2 h before testing) fails to alter locomotor activity. The distance traveled was 2010±228 counts/10 min in vehicle (VEH)-treated mice and 1879±238 counts/10 min in VAR-treated mice; n=10; nonsignificant. A single injection of VAR (5 mg/kg i.p., 2 h or 24 h before testing) fails to change cortical DNMT1 mRNA levels. However, like nicotine, repeated VAR injections (1–5 mg/kg i.p., twice daily for 5 days) elicit a significant 30–40% decrease of cortical DNMT1 mRNA (Figure 2), but fail to significantly decrease DNMT1 mRNA expression in the striatum (Figure 2).
Figure 2.
Varenicline (VAR) reduces DNA methyltransferase (DNMT)1 mRNA expression in the FC but not in the striatum. VAR was injected in mice i.p. twice a day for 5 days. FC samples were collected 2 h after the last VAR injection. Data are expressed as fmol DNMT1 mRNA/0.1 pmol NSE mRNA. Overall one-way ANOVA (F3,14=5.0) provided a p<0.01; *p<0.01 for Student–Newman–Keuls multiple comparison between vehicle (VEH)- and VAR-treated groups.
In addition to DNMT1, DNMT3a has been identified in the mouse brain (Satta et al, 2008). Unlike DNMT1, FC levels of DNMT3a mRNA failed to change following VAR administration (fmol DNMT1/0.1 pmol NSE mRNA: 4.6±0.5 in VEH and 4.8±0.35 in VAR (5 mg/kg s.c. twice daily for 5 days) treated mice; n=5). DNMT3b was not expressed in significant amounts in the FC of adult mice.
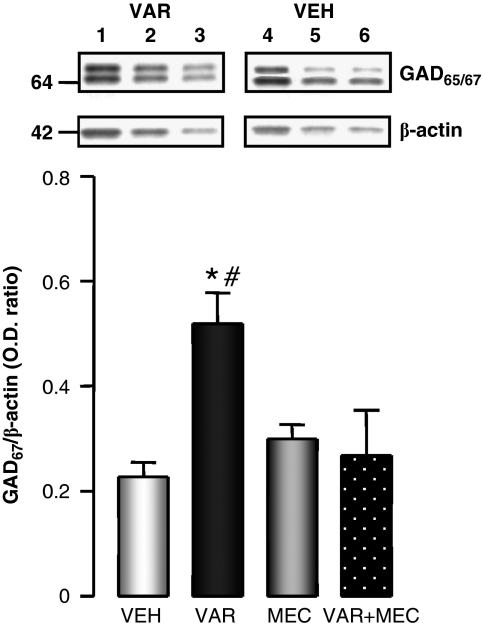
In the same mice in which VAR induced a decrease of cortical DNMT1 mRNA, the expression of GAD67 mRNA (fmol GAD67/0.1 pmol NSE mRNA: 2.4±0.38 in VEH- and 4.1±0.48 in VAR-treated mice) and protein (Figure 3) was significantly increased. The upregulation of GAD67 induced by VAR was abolished by the nAChR antagonist mecamylamine (2 mg/kg twice a day just before VAR administration) (Figure 3).
Figure 3.
Varenicline (VAR) increases glutamic acid decarboxylase 67 (GAD67) protein expression in mouse frontal cortex (FC). The effect of VAR (5 mg/kg, i.p. twice a day for 5 days) was blocked by co-administration of mecamylamine (MEC) (2 mg/kg, i.p.). A one-way ANOVA for GAD67 in vehicle (VEH), VAR MEC, and VAR+MEC (F3,16=4.8) provided a p<0.01. *p<0.01 for Student–Newman–Keuls multiple comparison between VEH- and VAR-treated groups. #p<0.01 for Student–Newman–Keuls multiple comparison between VAR- and VAR+MEC-treated groups. In the insert is a typical Western immunoblot of GAD65/67 and β-actin after 4–12% SDS-PAGE. Comparison between FC extracts of VAR- (lanes 1–3 serial dilution of the same sample) and VEH- (lanes 4–6 of the same sample) treated mice.
In the same sample, we failed to observe a significant increase of GAD65 protein expression; the GAD65 β-actin OD ratio expressed as a percentage of the control is 112±15; n=10; nonsignificant.
A typical example of Western blot of GAD65/67 and β-actin after 4–12% SDS-PAGE from VEH- and VAR-treated mice is shown in the insert of Figure 3.
Varenicline Reduces the Binding of MeCP2 to a CpG-Rich GAD67 Promoter Region
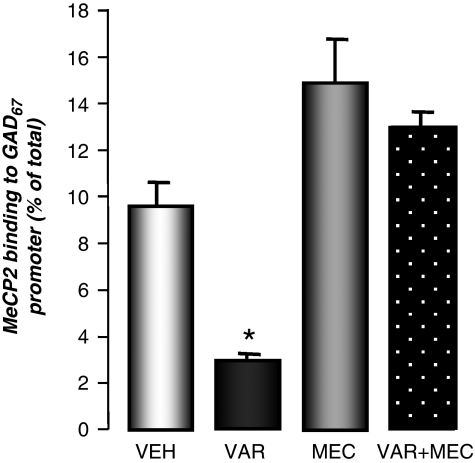
To test the hypothesis that the VAR-induced increase of GAD67 expression is a consequence of the reduction of DNMT1 and the reduction of GAD67 promoter methylation, we studied the effect of VAR on the methylation status of a specific GAD67 promoter region enriched with CpG dinucleotides (see Materials and Methods section). In mouse FC extracts, we quantified the fraction of the GAD67 promoter, immunoprecipitated by a specific MeCP2 antibody (Dong et al, 2005, 2007, 2008), using competitive PCR with a genomic internal standard, as detailed in Figure 1. We showed that protracted VAR treatment (5 mg/kg i.p., twice daily for 5 days) significantly reduces the level of MeCP2 bound to the GAD67 promoter. This effect was abolished when VAR was administered with the nAChR antagonist mecamylamine (Figure 4).
Figure 4.
Varenicline (VAR) reduces MeCP2 binding to GAD67 promoters in the mouse frontal cortex (FC). The effect of VAR (5 mg/kg, i.p. twice a day for 5 days) was blocked by the co-administration of mecamylamine (MEC) (2 mg/kg, i.p.). A one-way ANOVA for GAD67 promoter methylation in vehicle (VEH), VAR, MEC, and VAR+MEC (F3,8=5.6) provided a p<0.01. *p<0.01 for Student–Newman–Keuls multiple comparison between VEH- and VAR- treated mice.
A–85380 Reproduces the Action of Varenicline on Cortical DNMT And GAD67 Expression
As mentioned, in small concentrations (2 μM), VAR is a partial agonist at α4β2 and in higher concentrations (18 μM), is a full agonist at α7 nAChRs (Mihalak et al, 2006). Hence, the experiments with high doses of VAR do not allow the identification of the nAChR subtype that is responsible for the downregulation of cortical DNMT1 or the increase of cortical GAD67 expression.
To establish whether α4β2 nAChR stimulation is responsible for the epigenetic modifications in mice caused by nicotine and VAR, we administered A–85380, an agonist that binds with subnanomolar affinity to α4β2 but has negligible affinity for α3β4- or α7 subtype-containing receptors (Smith et al, 2007; Rueter et al, 2006). In pharmacologically relevant antidepressant, anti-nociceptive, and anti-allodynic doses (0.1–1 mg/kg), A–85380 shows considerably lower toxicity than nicotine and epibatidine (Curzon et al, 1998; Rueter et al, 2003, 2006; Buckley et al, 2004). This drug easily penetrates the blood–brain barrier, and in mice has a relatively long half-life (more than 1 h) (Curzon et al, 1998) compared with nicotine that has a half-life of few minutes (Damaj et al, 2007).
In the doses used in this study, ie, 0.5 to 2.5 mg/kg s.c. twice a day, A–85380 fails to produce the hypercholinergic peripheral symptomatology of nicotine. However, similar to nicotine (Satta et al, 2008) or VAR (Figure 2), A–85380 elicits a 30–40% decrease of cortical DNMT1 mRNA and fails to significantly decrease DNMT1 mRNA in the striatum (Table 1).
Table 1. A–85380, A Selective α4β2 nAChR Agonist, Reduces DNMT1 mRNA Expression In The Frontal Cortex But Not In The Striatum.
| Drug | Dose (mg/kg) |
DNMT1 mRNAa |
|
|---|---|---|---|
| FC | Striatum | ||
| VEH | — | 3.8±0.3 | 3.0±0.3 |
| A–85380 | 0.5 | 2.4±0.1* | 2.5±0.1 |
| 1.0 | 2.7±0.2* | 2.6±0.1 | |
| 2.5 | 2.5±0.1* | 2.2±0.2 | |
| PNU–282987 | 1.0 | 3.4±0.2 | 2.7±0.5 |
| 2.5 | 3.8±0.4 | 3.1±0.5 | |
| 5.0 | 3.9±0.4 | 3.3±0.5 | |
Mice received s.c. injections of A–85380 or PNU–282987 for 5 days, twice daily and were killed 2 h after the last injection. Overall one-way ANOVA for DNMT1 mRNA in VEH-, A–85380-, and PNU–282987-treated mice (F6,28=5.4) provided a p<0.01 in the FC.
*p<0.01 for Student–Newman–Keuls multiple comparison between VEH- and A–85380-treated mice.
DNMT1 mRNA is expressed as fmol DNMT1mRNA/0.1 pmol NSE mRNA.
Mice exposed to A–85380 in doses that reduce the expression of DNMT1 mRNA exhibit a significant increase of GAD67 but fail to increase GAD65 protein expression in the FC (Table 2).
Table 2. A–85380 But Not PNU–282987 Increases GAD67 Protein Expression in Mouse Frontal Cortex.
| A–85380 (mg/kg) | GAD67a | GAD65a |
|---|---|---|
| VEH | 0.23±0.05 | 0.45±0.07 |
| 0.5 | 0.52*±0.05 | 0.47±0.10 |
| 1 | 0.37*±0.03 | 0.41±0.05 |
| 2.5 | 0.35*±0.02 | 0.53±0.08 |
| PNU–282987 (mg/kg) | GAD67a | GAD65a |
| VEH | 0.21±0.03 | 0.47±0.06 |
| 1 | 0.26±0.02 | 0.41±0.03 |
| 2.5 | 0.17±0.01 | 0.54±0.05 |
| 5 | 0.25±0.02 | 0.38±0.04 |
Mice were treated s.c. with A–85380 or PNU–282987 two times a day for 5 days. GAD67 and GAD65 were measured 2 h after the last injection of drug. Overall one-way ANOVA for GAD67 in VEH-, A–85380-, and PNU–282987-treated mice (F6, 6=5.4) yielded a p<0.01 in the FC.
*p<0.01 for Student–Newman–Keuls multiple comparison between VEH- and A–85380- treated mice.
Data is expressed as GAD67 or GAD65/β-actin OD ratio.
PNU–282987 Fails to Decrease Cortical DNMT mRNA or to Induce GAD67 Expression in the Mouse FC
PNU–282987 is a benzamide derivative functioning as a selective agonist of the homomeric α7 nAChR (Bodnar et al, 2005). It evokes a whole-cell current sensitive to the selective α7 nAChR antagonist methylcaconitine and enhances GABAergic synaptic activity when applied to rat hippocampal slices or dissociated rat hippocampal neurons in culture (Hajós et al, 2005). In rats injected with PNU–282987 at doses of 1 mg/kg i.v., the disruption of auditory gating elicited by amphetamine is reversed (Hajós et al, 2005).
PNU–282987 has a half-life of several hours in rodents (Hajós et al, 2005). On the basis of this information, we administered PNU–282987 every 12 h.
Because most of the experiments with PNU–282987 were conducted in rats, to optimize the pharmacological intervention in mice with PNU–282987, we first studied the effect of PNU–282987 injected in doses of 0.5 to 5 mg/kg s.c. on contextual fear conditioning.
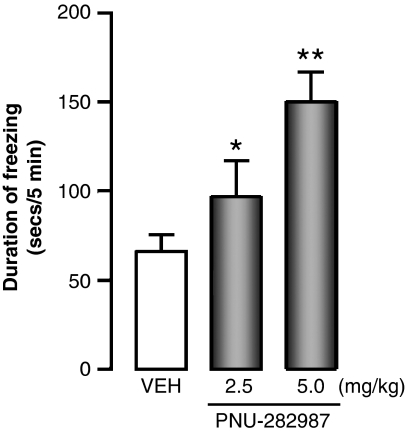
It is important that PNU–282987, despite its lack of effects on gross behavior and locomotor activity, enhances cortico-limbic-dependent forms of learning (LeDoux, 2000), inducing a dose-dependent enhancement of the contextual fear condition (Figure 5). There was no effect of PNU–282987 on freezing from the acoustic CS. In these experiments, PNU282987 was injected s.c. at 2 h before the training test.
Figure 5.
PNU–282987 increases the contextual fear conditioning response in mice. PNU 282987 was injected s.c. 2 h before the training session. Total duration of freezing time was measured 24 h after a training session. The training session consisted of a conditioned stimulus (CS) (acoustic tone, 30 s, 85 dB) paired with an unstimulated stimulus (US) (electric foot shock, 2 s, 0.5 mA) three times every 2 min. Each value is the mean±SEM of five animals. An overall one-way ANOVA in VEH- and PNU–282987-treated mice (F3,12=6) provided a p<0.01. *p<0.05 for Student–Newman–Keuls multiple comparison between VEH- and PNU–282987- (2.5 mg/kg) treated mice. **p<0.01 for Student–Newman–Keuls multiple comparison between VEH- and PNU–282987- (5 mg/kg) treated mice.
In doses ranging from 1 to 5 mg/kg, PNU–282987 fails to induce significant changes in DNMT1 mRNA expression in the FC (Table 1). Furthermore, no significant GAD67 or GAD65 protein increase was observed in the FC of PNU–282987-treated mice (Table 2).
DISCUSSION
We have recently reported that mice treated protractedly with nicotine show a cortical and hippocampal decrease of DNMT, reduced GAD67 promoter methylation and increased GAD67 expression (Satta et al, 2008).
The present study (Figures 2, 3, 4) shows that VAR injected in mice in doses that failed to alter gross behavior or locomotor activity but acted as a partial agonist at the α4β2 nAChR subtype and as a full agonist at the α7 nAChR subtype (Mihalak et al, 2006) decreased DNMT mRNA, reduced the binding of MeCP2 to GAD67 promoters, and increased the levels of GAD67 in the FC in a manner similar to that of nicotine (Figure 1). These changes are apparently mediated through central nAChR stimulation because they are blocked by mecamylamine.
The downregulation of DNMT expression induced by nAChR stimulation in GABAergic neurons of the FC appears to be brain region-specific because it fails to occur in the GABAergic medium spiny neurons of the striatum. These neurons primarily express muscarinic AChR (Zhou et al, 2002).
The decrease of DNMT and the increase of GAD67 induced by VAR in FC can be mimicked by injections of A–85380, an α4β2 nAChR-selective agonist that fails to replicate the unwanted peripheral side effects of nicotine. In contrast, PNU–282987, an α7 nAChR-selective agonist injected in doses that enhance the cortico-limbic-dependent form of learning (Figure 5), is inactive on DNMT1 and GAD67 expression. Hence, the data suggest that VAR may regulate DNMT and GAD67 expression in cortical and hippocampal GABAergic neurons acting at α4β2 nAChR subtypes.
Several lines of investigation suggest that DNMT, which is highly expressed in telencephalic GABAergic neurons, is responsible for the epigenetic regulation of selective GABAergic gene promoters, including GAD67 and reelin (Veldic et al, 2007; Ruzicka et al, 2007; Zhubi et al, 2009; Grayson et al, 2009). In fact, the effect of α4β2 nAChR stimulation on GABAergic gene promoter regulation is not generalized to all genes because in the same mice in which VAR and A–85380 induce an increase of FC GAD67 expression, GAD65 expression is not increased.
At present time, the cascade of molecular events that through nAChR stimulation downregulates DNMT1 and upregulates GAD67 expression in cortical and hippocampal GABAergic neurons is unknown. However, there is ample evidence obtained in in vitro (Grayson et al, 2009) and in vivo (Day and Sweatt 2010; Tremolizzo et al, 2005; Zhang et al, 2010; Meaney, 2010) experiments that changes in DNMT levels are cause-related to changes in target gene expression, including the expression of GAD67.
Given that DNMT promoters contain consensus sequences for inducible transcription factors such as c-jun and c-fos (Bigey et al, 2000; Slack et al, 2001), it could be hypothesized that nAChR stimulation can regulate DNMT expression by altering the availability of these transcription factors. These factors could include the ‘growth arrest and DNA damage-inducible protein 45b,' an inducible immediate early gene funtioning as a molecular factor in the DNA demethylation process in the brain (Ma et al, 2009).
Although the data indicate a decrease of DNMT as the cause of decreased GAD67 promoter methylation, we cannot exclude that nAChR stimulation reduces the GAD67 promoter methylation activating DNA demethylation processes. Studies of the characterization of DNA demethylase are presently in progress in our laboratory (Dong et al, 2010).
Current research in SZ suggests that the overexpression of DNMT in telencephalic GABAergic neurons is responsible for the epigenetic hypermethylation of specific GABAergic gene promoters, including GAD67 and reelin (Veldic et al, 2007; Ruzicka et al, 2007). The expression downregulation of these genes in SZ brains likely leads to a GABAergic transmission defect, which presumably has an important role in the pathogenetic mechanisms that underlie the cognitive, behavioral, and auditory gating system impairments expressed in psychotic patients (Guidotti et al, 2005; Lewis et al, 2005). This evidence suggests that a reversal of the epigenetically induced transcriptional downregulation of GAD67 and other genes in cortical GABAergic neurons of SZ patients should be attempted by using drugs that directly or indirectly target DNMT.
The present study and independent investigations (Martin et al, 2004; Adams and Stevens, 2007; Ochoa and Lasalde-Dominicci, 2007; Hasselmo and Sarter, 2011) suggest that full and partial α4β2 nAChR agonists are promising pharmacological agents that deserve to be tested for the treatment of cognitive deficits in SZ and in related psychiatric disorders.
Hence, the use of VAR to selectively downregulate DNMT in GABAergic interneurons of the cortex but not in the striatum may represent an innovative attempt to control the hypermethylation of GAD67 and other gene promoters operative in selected populations of telencephalic GABAergic neurons of SZ patients while leaving the function of DNMT intact in cells that do not express nAChRs.
Interestingly, VAR repeatedly administered to patients, with SZ or schizoaffective disorders, who are tobacco smokers produced significant anti-smoking effects and improvements in some cognitive test scores, primarily associated with verbal learning and memory (Smith et al, 2009). Contrary to early reports that VAR may increase suicidal ideation or depression (Gunnell et al, 2009), subsequent studies have shown that VAR is well tolerated in animals and humans, and is an effective smoking cessation agent (Faessel et al, 2010; Jorenby et al, 2006).
Varenicline, unlike nicotine, fails to produce profound tachyphylaxis and it is only partially reinforcing in animal studies (Rollema et al, 2007; George et al, 2010). Hence, one may infer that this drug represents a better pharmacological tool than nicotine to selectively increase GAD67 expression in cortico-limbic inhibitory interneurons.
On the basis of our results, we suggest that the possible molecular mechanism underlying the cognitive-enhancing action of nAChR stimulation in SZ patients is the control of the epigenetic alteration of cortico-limbic GABAergic neurons. Furthermore, the present study suggests that α4β2 nAChR agonists may be better suited than the α7 nAChR agonists to control epigenetic alterations of GABAergic neurons in SZ. This difference is in keeping with studies showing that selective α4β2 nAChR agonists produce more robust and perhaps more clinically relevant increases in attention and cognitive performance than the α7 nAChR agonists, which show rapid tachyphylaxis on cognition (Olincy et al, 2006; Hasselmo and Sarter, 2011).
The authors declare no conflict of interest.
References
- Adams CE, Stevens KE. Evidence for a role of nicotinic acetylcholine receptors in schizophrenia. Front Biosci. 2007;12:4755–4772. doi: 10.2741/2424. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- Auta J, Chen Y, Ruzicka WB, Grayson DR, Baker G, Dunn S, et al. 2007The handbook of neurochemistry and molecular neurobiology, nucleic acid quantitation using the competitive polymerase chain reactionIn: Baker G, Dunn S, Holt A, Lajtha A (eds).Practical Neurochemistry Methods(Springer, New York; ), pp341–361. [Google Scholar]
- Benes FM, Lim B, Matzilevich D, Walsh JP, Subburaju S, Minns M. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proc Natl Acad Sci USA. 2007;104:10164–10169. doi: 10.1073/pnas.0703806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigey P, Ramchandani S, Theberge J, Araujo FD, Szyf M. Transcriptional regulation of the human DNA methyltransferase (dnmt1) gene. Gene. 2000;242:407–418. doi: 10.1016/s0378-1119(99)00501-6. [DOI] [PubMed] [Google Scholar]
- Bodnar AL, Cortes-Burgos LA, Cook KK, Dinh DM, Groppi VE, Hajos M, et al. Discovery and structure-activity relationship of quinuclidine benzamides as agonists of alpha7 nicotinic acetylcholine receptors. J Med Chem. 2005;48:905–908. doi: 10.1021/jm049363q. [DOI] [PubMed] [Google Scholar]
- Breese CR, Lee MJ, Adams CE, Sullivan B, Logel J, Gillen KM, et al. Abnormal regulation of high affinity nicotinic receptors in subjects with schizophrenia. Neuropsychopharmacology. 2000;23:351–364. doi: 10.1016/S0893-133X(00)00121-4. [DOI] [PubMed] [Google Scholar]
- Buckley MJ, Surowy C, Meyer M, Curzon P. Mechanism of action of A–85380 in an animal model of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:723–730. doi: 10.1016/j.pnpbp.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Curzon P, Nikkel AL, Bannon AW, Arneric SP, Decker MW. Differences between the antinociceptive effects of the cholinergic channel activators A–85380 and (+/−)-epibatidine in rats. J. Pharmacol Exp Ther. 1998;287:847–853. [PubMed] [Google Scholar]
- Damaj MI, Fonck C, Marks MJ, Deshpande P, Labarca C, Lester HA, et al. Genetic approaches identify differential roles for alpha4beta2* nicotinic receptors in acute models of antinociception in mice. J. Pharmacol Exp Ther. 2007;321:1161–1169. doi: 10.1124/jpet.106.112649. [DOI] [PubMed] [Google Scholar]
- Day JJ, Sweatt JD. DNA methylation and memory formation. Nat Neurosci. 2010;13:1319–1323. doi: 10.1038/nn.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E, Agis-Balboa RC, Simonini MV, Grayson DR, Costa E, Guidotti A. Reelin and glutamic acid decarboxylase67 promoter remodeling in an epigenetic methionine-induced mouse model of schizophrenia. Proc Natl Acad Sci USA. 2005;102:12578–12583. doi: 10.1073/pnas.0505394102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E, Chen Y, Gavin DP, Grayson DR, Guidotti A. Valproate induces DNA demethylation in nuclear extracts from adult mouse brain. Epigenetics. 2010;5:730–735. doi: 10.4161/epi.5.8.13053. [DOI] [PubMed] [Google Scholar]
- Dong E, Guidotti A, Grayson DR, Costa E. Histone hyperacetylation induces demethylation of reelin and 67-kDa glutamic acid decarboxylase promoters. Proc Natl Acad Sci USA. 2007;104:4676–4681. doi: 10.1073/pnas.0700529104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong E, Nelson M, Grayson DR, Costa E, Guidotti A. Clozapine and sulpiride but not haloperidol or olanzapine activate brain DNA demethylation. Proc Natl Acad Sci USA. 2008;105:13614–13619. doi: 10.1073/pnas.0805493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar GC, Inglis F, Kuchibhatla R, Sharma T, Tomlinson M, Wamsley J. Effect of ispronicline, a neuronal nicotinic acetylcholine receptor partial agonist, in subjects with age associated memory impairment (AAMI) J Psychopharmacol. 2007;21:171–178. doi: 10.1177/0269881107066855. [DOI] [PubMed] [Google Scholar]
- Evans DE, Drobes DJ. Nicotine self-medication of cognitive-attentional processing. Addict Biol. 2009;14:32–42. doi: 10.1111/j.1369-1600.2008.00130.x. [DOI] [PubMed] [Google Scholar]
- Faessel HM, Obach RS, Rollema H, Ravva P, Williams KE, Burstein AH. A review of the clinical pharmacokinetics and pharmacodynamics of varenicline for smoking cessation. Clin Pharmacokinet. 2010;49:799–816. doi: 10.2165/11537850-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Freedman R, Hall M, Adler LE, Leonard S. Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol Psychiatry. 1995;38:22–33. doi: 10.1016/0006-3223(94)00252-X. [DOI] [PubMed] [Google Scholar]
- Freedman R, Olincy A, Buchanan RW, Harris JG, Gold JM, Johnson L, et al. Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am J Psychiatry. 2008;165:1040–1047. doi: 10.1176/appi.ajp.2008.07071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Lloyd A, Carroll FI, Damaj MI, Koob GF.2010Varenicline blocks nicotine intake in rats with extended access to nicotine self-administration Psychopharmacology;(cited 16 December 2010) Available from: http://www.ncbi.nlm.nih.gov/pubmed/20924754 . [DOI] [PMC free article] [PubMed]
- Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull. 2008;34:944–961. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson DR, Chen Y, Dong E, Kundakovic M, Guidotti A. From trans-methylation to cytosine methylation: evolution of the methylation hypothesis of schizophrenia. Epigenetics. 2009;4:144–149. doi: 10.4161/epi.4.3.8534. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Chen Y, Davis JM, Dong E, Gavin DP, et al. 2010Epigenetic GABAergic targets in schizophrenia and bipolar disorder Neuropharmacology(cited 16 December 2010) Available from: http://www.ncbi.nlm.nih.gov/pubmed/21074545 . [DOI] [PMC free article] [PubMed]
- Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Dong E, Grayson DR, Veldic M, et al. GABAergic dysfunction in schizophrenia: new treatment strategies on the horizon. Psychopharmacology. 2005;180:191–205. doi: 10.1007/s00213-005-2212-8. [DOI] [PubMed] [Google Scholar]
- Gunnell D, Irvine D, Wise L, Davies C, Martin RM. Varenicline and suicidal behaviour: a cohort study based on data from the general practice research database. BMJ. 2009;339:b3805. doi: 10.1136/bmj.b3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajós M, Hurst RS, Hoffmann WE, Krause M, Wall TM, Higdon NR, et al. The selective alpha7 nicotinic acetylcholine receptor agonist PNU–282987 [N-[(3R)-1-Azabicyclo[2.2.2]oct-3-yl]-4-chlorobenzamide hydrochloride] enhances GABAergic synaptic activity in brain slices and restores auditory gating deficits in anesthetized rats. J. Pharmacol Exp Ther. 2005;312:1213–1222. doi: 10.1124/jpet.104.076968. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Sarter M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology. 2011;36:52–73. doi: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe WM, Ji J, Parikh V, Williams S, Mocaer E, Trocme-Thibierge C, et al. Enhancement of attentional performance by selective stimulation of [alpha]4[beta]2[ast] nAChRs: underlying cholinergic mechanisms. Neuropsychopharmacology. 2010;35:1391–1401. doi: 10.1038/npp.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lewis DA, González-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 2008;33:141–165. doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- Lewis SW, Tarrier N, Drake RJ. Integrating non-drug treatments in early schizophrenia. Br J Psychiatry Suppl. 2005;48:s65–s71. doi: 10.1192/bjp.187.48.s65. [DOI] [PubMed] [Google Scholar]
- Ma DK, Jang M, Guo JU, Kitabatake Y, Chang M, Pow-Anpongkul N, et al. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science. 2009;323:1074–1077. doi: 10.1126/science.1166859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LF, Kem WR, Freedman R. Alpha-7 nicotinic receptor agonists: potential new candidates for the treatment of schizophrenia. Psychopharmacology. 2004;174:54–64. doi: 10.1007/s00213-003-1750-1. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Epigenetics and the biological definition of gene × environment interactions. Child Dev. 2010;81:41–79. doi: 10.1111/j.1467-8624.2009.01381.x. [DOI] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70:801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- Ochoa ELM, Lasalde-Dominicci J. Cognitive deficits in schizophrenia: focus on neuronal nicotinic acetylcholine receptors and smoking. Cell Mol Neurobiol. 2007;27:609–639. doi: 10.1007/s10571-007-9149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olincy A, Harris JG, Johnson LL, Pender V, Kongs S, Allensworth D, et al. Proof-of-concept trial of an alpha7 nicotinic agonist in schizophrenia. Arch Gen Psychiatry. 2006;63:630–638. doi: 10.1001/archpsyc.63.6.630. [DOI] [PubMed] [Google Scholar]
- Olincy A, Young DA, Freedman R. Increased levels of the nicotine metabolite cotinine in schizophrenic smokers compared to other smokers. Biol Psychiatry. 1997;42:1–5. doi: 10.1016/S0006-3223(96)00302-2. [DOI] [PubMed] [Google Scholar]
- Pibiri F, Nelson M, Guidotti A, Costa E, Pinna G. Decreased corticolimbic allopregnanolone expression during social isolation enhances contextual fear: a model relevant for posttraumatic stress disorder. Proc Natl Acad Sci USA. 2008;105:5567–5572. doi: 10.1073/pnas.0801853105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter A, Corwin J, Lang J, Piasecki M, Lenox R, Newhouse PA. Acute effects of the selective cholinergic channel activator (nicotinic agonist) ABT-418 in Alzheimer's disease. Psychopharmacology. 1999;142:334–342. doi: 10.1007/s002130050897. [DOI] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, et al. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Rueter LE, Kohlhaas KL, Curzon P, Surowy CS, Meyer MD. Peripheral and central sites of action for A–85380 in the spinal nerve ligation model of neuropathic pain. Pain. 2003;103:269–276. doi: 10.1016/s0304-3959(02)00455-4. [DOI] [PubMed] [Google Scholar]
- Rueter LE, Donnelly-Roberts DL, Curzon P, Briggs CA, Anderson DJ, Bitner RS. A–85380: a pharmacological probe for the preclinical and clinical investigation of the alphabeta neuronal nicotinic acetylcholine receptor. CNS Drug Rev. 2006;12:100–112. doi: 10.1111/j.1527-3458.2006.00100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzicka WB, Zhubi A, Veldic M, Grayson DR, Costa E, Guidotti A. Selective epigenetic alteration of layer I GABAergic neurons isolated from prefrontal cortex of schizophrenia patients using laser-assisted microdissection. Mol Psychiatry. 2007;12:385–397. doi: 10.1038/sj.mp.4001954. [DOI] [PubMed] [Google Scholar]
- Satta R, Maloku E, Zhubi A, Pibiri F, Hajos M, Costa E, et al. Nicotine decreases DNA methyltransferase 1 expression and glutamic acid decarboxylase 67 promoter methylation in GABAergic interneurons. Proc Natl Acad Sci USA. 2008;105:16356–16361. doi: 10.1073/pnas.0808699105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack A, Pinard M, Araujo FD, Szyf M. A novel regulatory element in the dnmt1 gene that responds to co-activation by Rb and c-Jun. Gene. 2001;268:87–96. doi: 10.1016/s0378-1119(01)00427-9. [DOI] [PubMed] [Google Scholar]
- Smith JW, Mogg A, Tafi E, Peacey E, Pullar IA, Szekeres P, et al. Ligands selective for alpha4beta2 but not alpha3beta4 or alpha7 nicotinic receptors generalise to the nicotine discriminative stimulus in the rat. Psychopharmacology (Berl.) 2007;190:157–170. doi: 10.1007/s00213-006-0596-8. [DOI] [PubMed] [Google Scholar]
- Smith RC, Lindenmayer J, Davis JM, Cornwell J, Noth K, Gupta S, et al. Cognitive and antismoking effects of varenicline in patients with schizophrenia or schizoaffective disorder. Schizophr Res. 2009;110:149–155. doi: 10.1016/j.schres.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Smith RC, Zhubi A, Maloku E, Sershen H, Lajtha A, Davis JM, et al. Varenicline treatment decreases DNMT1 mRNA expression in lymphocytes of schizophrenic patients who are cigarette smokers. Schizophr Res. 2010;119:269–270. doi: 10.1016/j.schres.2010.02.1064. [DOI] [PubMed] [Google Scholar]
- Tremolizzo L, Doueiri M, Dong E, Grayson DR, Davis J, Pinna G, et al. Valproate corrects the schizophrenia-like epigenetic behavioral modifications induced by methionine in mice. Biol Psychiatry. 2005;57:500–509. doi: 10.1016/j.biopsych.2004.11.046. [DOI] [PubMed] [Google Scholar]
- Van Emburgh BO, Robertson KD.2008DNA methyltransferases and methyl CpG binding proteins as multifunctional regulators of chromatin structure and development in mammalian cellsIn Tost J. (ed).EpigeneticsChapter 2, Caister Academic Press: Norwich, UK; 23–62. [Google Scholar]
- Veldic M, Guidotti A, Maloku E, Davis JM, Costa E. In psychosis, cortical interneurons overexpress DNA-methyltransferase 1. Proc Natl Acad Sci USA. 2005;102:2152–2157. doi: 10.1073/pnas.0409665102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldic M, Kadriu B, Maloku E, Agis-Balboa RC, Guidotti A, Davis JM, et al. Epigenetic mechanisms expressed in basal ganglia GABAergic neurons differentiate schizophrenia from bipolar disorder. Schizophr Res. 2007;91:51–61. doi: 10.1016/j.schres.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Decker MW. Neuronal nicotinic receptor agonists for the treatment of attention-deficit/hyperactivity disorder: focus on cognition. Biochem Pharmacol. 2007;74:1212–1223. doi: 10.1016/j.bcp.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Verlinden MH, Adler LA, Wozniak PJ, West SA. ABT-089, a neuronal nicotinic receptor partial agonist, for the treatment of attention-deficit/hyperactivity disorder in adults: results of a pilot study. Biol Psychiatry. 2006;59:1065–1070. doi: 10.1016/j.biopsych.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Zhang T, Hellstrom IC, Bagot RC, Wen X, Diorio J, Meaney MJ. Maternal care and DNA methylation of a glutamic acid decarboxylase 1 promoter in rat hippocampus. J Neurosci. 2010;30:13130–13137. doi: 10.1523/JNEUROSCI.1039-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Wilson CJ, Dani JA. Cholinergic interneuron characteristics and nicotinic properties in the striatum. J Neurobiol. 2002;53:590–605. doi: 10.1002/neu.10150. [DOI] [PubMed] [Google Scholar]
- Zhubi A, Veldic M, Puri NV, Kadriu B, Caruncho H, Loza I, et al. An upregulation of DNA-methyltransferase 1 and 3a expressed in telencephalic GABAergic neurons of schizophrenia patients is also detected in peripheral blood lymphocytes. Schizophr Res. 2009;111:115–122. doi: 10.1016/j.schres.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]