Abstract
Lithium has been used extensively for mood stabilization, and it is particularly efficacious in the treatment of bipolar mania. Like other drugs used in the treatment of psychiatric diseases, it has little effect on the mood of healthy individuals. Our previous studies found that mice with a mutation in the Clock gene (ClockΔ19) have a complete behavioral profile that is very similar to human mania, which can be reversed with chronic lithium treatment. However, the cellular and physiological effects that underlie its targeted therapeutic efficacy remain unknown. Here we find that ClockΔ19 mice have an increase in dopaminergic activity in the ventral tegmental area (VTA), and that lithium treatment selectively reduces the firing rate in the mutant mice with no effect on activity in wild-type mice. Furthermore, lithium treatment reduces nucleus accumbens (NAc) dopamine levels selectively in the mutant mice. The increased dopaminergic activity in the Clock mutants is associated with cell volume changes in dopamine neurons, which are also rescued by lithium treatment. To determine the role of dopaminergic activity and morphological changes in dopamine neurons in manic-like behavior, we manipulated the excitability of these neurons by overexpressing an inwardly rectifying potassium channel subunit (Kir2.1) selectively in the VTA of ClockΔ19 mice and wild-type mice using viral-mediated gene transfer. Introduction of this channel mimics the effects of lithium treatment on the firing rate of dopamine neurons in ClockΔ19 mice and leads to a similar change in dopamine cell volume. Furthermore, reduction of dopaminergic firing rates in ClockΔ19 animals results in a normalization of locomotor- and anxiety-related behavior that is very similar to lithium treatment; however, it is not sufficient to reverse depression-related behavior. These results suggest that abnormalities in dopamine cell firing and associated morphology underlie alterations in anxiety-related behavior in bipolar mania, and that the therapeutic effects of lithium come from a reversal of these abnormal phenotypes.
Keywords: circadian rhythms, ventral tegmental area, bipolar disorder, dopaminergic activity
INTRODUCTION
Bipolar disorder is a prevalent and severe disease that can often lead to suicide. According to the DSM IV, the depressive state is defined by periods of sadness, guilt, sleep disturbances, and anhedonia; whereas the manic state usually consists of hyperactivity, little need for sleep, euphoria, increased risk taking behavior, impulsivity, and increased reward seeking. A large number of clinical studies over many years have found disruptions in the circadian rhythms of sleep, blood pressure, hormone secretions, and body temperature in individuals with bipolar disorder (reviewed by McClung (2007)). Moreover, mood-related episodes in bipolar patients are clearly linked to disruptions in their normal sleep wake and social schedule. These studies have led to the suggestion that instability in the circadian system underlies the development and progression of bipolar disorder. Indeed, in the past few years, several human genetic studies have identified single-nucleotide polymorphisms (SNPs) in a number of genes that regulate circadian rhythms that are significantly associated with bipolar disorder (Kripke et al, 2009; Mansour et al, 2009; McGrath et al, 2009; Nievergelt et al, 2006; Soria et al, 2010).
The basic molecular loop that regulates circadian rhythms consists of a set of transcription factors that regulate their own expression over a 24 h cycle (Ko and Takahashi, 2006). The circadian locomotor output cycles kaput (CLOCK) protein binds to brain and muscle ARNT-like protein 1 (BMAL1). Then, this heterodimer regulates the expression of the Period (Per) and Cryptochrome (Cry) genes, which bind together as proteins, enter the nucleus, and inhibit the activity of CLOCK and BMAL1. To better understand the neurobiology of mood regulation, and the association between circadian rhythms and mood, we tested mice with a mutation in the Clock gene (ClockΔ19 mutants) in a variety of behavioral measures of mood, anxiety, activity, and reward, and found that they had a complete behavioral profile that was strikingly similar to bipolar patients in the manic state (McClung et al, 2005; Roybal et al, 2007). These included decreased depression-like behavior, hyperactivity, decreased anxiety, increased preference for sucrose and cocaine, and increased rates of intracranial self stimulation (McClung et al, 2005; Roybal et al, 2007). Moreover, when we treated these mice with the mood-stabilizing drug, lithium, we found that their behavioral responses returned to wild-type levels (Roybal et al, 2007). These studies make the ClockΔ19 mice a compelling model of human mania, which we can utilize to help understand why these phenotypes develop and how lithium is able to reverse them.
As the ClockΔ19 mice were hyperactive, hyperhedonic, and showed lower-depression-related behavioral responses, we suspected that there may be alterations in dopaminergic signaling in these mice in the ventral tegmental area (VTA). Indeed, dopaminergic transmission is known to be keenly involved in the rewarding responses to drugs of abuse, changes in the levels of anxiety, and in depression-related behavior in response to chronic stress (Krishnan et al, 2007; Nestler and Carlezon, 2006; Wise, 1998). When we performed in vivo recordings from VTA dopamine neurons, we found that the ClockΔ19 mice had an increase in both dopaminergic cell firing and bursting compared with wild-type littermates (McClung et al, 2005). However, the importance of dopamine in both the development of manic-like behaviors and in the reversal of these phenotypes by lithium remains unclear. Furthermore, the cellular and molecular mechanisms that underlie the increased dopaminergic activity in these mice also remain unknown. Previous studies have shown that changes in dopamine neuronal size are associated with differences in reward-related behavior, depression-related behavior, and hyperactivity (Krishnan et al, 2008a; Russo et al, 2007; Viggiano and Sadile, 2000). Here we find that changes in dopamine cell morphology and neuronal activity are important for the expression of hyperactivity and anxiety-related phenotypes of the ClockΔ19 mice, and that chronic lithium likely restores proper behavior in part through the modulation of this system.
METHODS
Mice
ClockΔ19 mice were created by N-ethyl-N-nitrosourea mutagenesis on a BALB/cJ background and produce a dominant-negative CLOCK protein as described previously (King et al, 1997; Vitaterna et al, 2006). Male Clock mutant (ClkΔ19/ClkΔ19) and wild-type (+/+) littermate controls, 8–10 weeks old, were utilized in all studies. Mice were group housed on a 12/12 light/dark cycle (lights on 0700 hours, lights off 1900 hours) with food and water ad libitum. All assays were performed between ZT3 and ZT7. All animal use was approved by the institutional animal care and use committee at UT Southwestern.
Lithium Treatment
Adult male mice were treated with 600 mg/l LiCl in drinking water for 10 days as published previously (Roybal et al, 2007). This treatment has been shown to produce a stable serum concentration in the low therapeutic range for human beings (0.41±0.06 mmol/l), with little to no adverse health consequence (Roybal et al, 2007).
VTA Slice Cultures
The preparations were obtained as described previously (Krishnan et al, 2007, 2008a). Acute brain slices (250 μm) containing the VTA were obtained for standard electrophysiology (see the next section). For the viral studies, herpes simplex virus (HSV) vectors were pipetted onto the VTA area of the slice surface after an 1 h incubation. Slices were maintained 1–2 days at 34°C. The culture medium used in this study was MEM (Invitrogen, Gaithersburg, MD) containing 30 mM HEPES, 20 mM -glucose, 5% B27, 5.0 mM -glutamine, and 25 U/ml streptomycin/penicillin.
Electrophysiology
All recordings were carried out blind to the experimental conditions of the viral vectors or drug treatment. To minimize possible stress effects on the recordings, we anesthetized the mice immediately after it was delivered to the dissection area and its brain was removed quickly in ice-cold artificial cerebrospinal fluid (ACSF), which contained 128 mM NaCl, 3 mM KCl, 1.25 mM NaH2PO4, 10 mM -glucose, 24 mM NaHCO3, 2 mM CaCl2, and 2 mM MgCl2 (oxygenated with 95% O2 and 5% CO2, pH 7.35, 295–305 mOsm). Acute brain slices containing the VTA were cut using a microslicer (DTK-1000, Ted Pella) in sucrose-ACSF, which was derived by fully replacing NaCl with 254 mM sucrose, and saturated by 95% O2 and 5% CO2. Slices were maintained in the holding chamber for 1 h at 37°C. The recording external solution (flow rate 2.5 ml/min) was ACSF. Single unit extracellular potential was carried out by use of cell-attach configuration with AxoClamp 2B (Axon Instruments), as described previously (Han et al, 2006; Krishnan et al, 2007). Recordings were obtained from VTA dopamine neurons identified by their location and well-established electrophysiological criteria (Grace and Onn, 1989; Marinelli and White, 2000; Ungless et al, 2004): regular spontaneous firing, action potential (AP) with triphasic waveforms (positive, negative, positive), and AP width (from start to trough) >1.1 ms. Patch pipettes (3–5 M) were filled with an internal solution containing 115 mM potassium gluconate, 20 mM KCl, 1.5 mM MgCl2, 10 mM phosphocreatine, 10 mM HEPES, 2 mM magnesium ATP, and 0.5 mM GTP (pH 7.2, 285 mOsm). VTA cells were visualized with an upright microscope using infrared differential interference contrast illumination, and viral-infected cells were visualized by GFP in the VTA. The firing rate was recorded in the amplifier's bridge mode, and data acquisition and online analysis of firing rate were collected using a Digidata 1322A digitizer and pClamp 8.2 (Axon Instruments).
Stereotaxic Surgery
Stereotaxic surgery was performed according to Mukherjee et al (2010). Mice were anesthetized with a mixture of ketamine (50 mg/kg body weight) and xylazine (10 mg/kg body weight) in saline (0.9% NaCl). Bilateral stereotaxic injections of purified high titer HSV-Kir2.1 or HSV-GFP were injected into the VTA (from bregma: angle 7°, AP −3.2 mm, Lat +1.0, DV −4.6) using a 33-gauge Hamilton syringe (Hamilton, Reno, NV). Injection speed was 0.1 μl/min, and the needle was kept in place for an additional 5 min before it was slowly withdrawn. Behavioral experiments were performed 24 h after the HSV injection. Preparation and packaging of these viruses have been published previously (Dong et al, 2006). After behavioral testing was completed, immunohistochemistry was performed to determine the extent of infection and correct viral placement. Brains in which a minimum of 25% of dopamine cells were infected by virus were included in the study.
Behavioral Tests
Locomotor response to novelty
Mice were individually placed in novel, unexplored automated locomotor activity chambers equipped with infrared photobeams (San Diego Instruments, San Diego, CA) and measurements began immediately. Activity of the animal was continuously measured and the data were collected in 5-min blocks over a period of 2 h.
Elevated plus maze
Mice were placed in the center of an elevated plus maze (arms are 30 cm × 5 cm, with 25 cm tall walls on the closed arms) under low light levels and their behavior was monitored for 5 min. The time spent on the closed and open arms, as well as the number of explorations of open and closed arms, were determined by video tracking software, Ethovision 3.0 (Noldus, Leesburg, VA). Time spent on the open arm and the percent of entries into the open arm are both negatively correlated with anxiety-like behavior. The apparatus was cleaned and allowed to dry in between every mouse.
Dark/light test
The dark/light apparatus consisted of two-chambered boxes (25 cm × 26 cm for each side; Med Associates, St Albans, VT). One side was kept dark (room light entry limited) and the other side was brightly lit by a fluorescent bulb across the top. Mice were first placed in dark side for 2 min, and then the automatic door between the compartments was opened and they were allowed to freely explore either the light or dark side for 10 min. Anxiety-like behavior was measured as the time spent in the lit side during the final 10 min.
Forced swim test
The forced swim test (FST) was performed as described in Krishnan et al (2007). Mice were videotaped while they were in a 4 l Pyrex glass beaker containing 3 l of water at 24±1°C for 6 min. After a 2 min lead time, latency to immobility (latency) was determined as the first cessation of all movement. The videotape was scored manually by a trained and blinded observer. Total immobility was measured as the time spent without any motion, except for single limb paddling to maintain flotation.
Learned helplessness
Mice were placed in a chamber (Med Associates) where they received foot shocks (5 s duration for 30 s) per min for 1 h and the door of the chamber was closed so that they cannot escape. After 1 h, they were returned to their home cage. This was repeated for 2 days. On the third day, the automatic door was opened simultaneously after each foot shock (one 0.3 mA 30 s shock for 30 min) letting the mice escape. The latency to escape and the number of failures was measured.
Immunohistochemistry
After anesthetizing the mice by an injection of a mixture of xylazine and ketamine, they were perfused for 5 min with a solution of 1 × PBS to allow for exsanguination. Then, perfusion continued for 15 min with a 4% paraformaldehyde solution in 1 × PBS for tissue fixation. Brains were then collected and kept in fixative solution overnight at 4°C. After equilibration in 20% glycerol, brains were sectioned with a microtome. Sections including the VTA or the subiculum were identified with a neuroanatomy atlas (Franklin and Paxinos, 1997). Sections were stored at 4°C in 1 × PBS with 0.05% sodium azide.
Upon staining, sections were first rinsed in 1 × PBS to remove sodium azide. Sections were then incubated with primary antibodies. For the lithium treatment group: VTA sections were identified by using anti-tyrosine hydroxylase (TH) antibody (T2928; Sigma, St Louis, MO), a marker of DA cells, and also stained histone H3 (4069, Cell Signaling, Danvers, MA) to highlight the nucleus. For subiculum sections: NeuN (MAB377; Millipore, Billerica, MA) was used to stain only the nuclei of neurons. Subsequently, sections were incubated with secondary antibodies. The NeuN-stained sections were then incubated with NeuroTrace (N-21480; Invitrogen, Carlsbad, CA) to stain the cytoplasm. For the surgery group, sections were incubated first with these primary antibodies: anti-TH as done above and with GFP antibody (ab290; Abcam, Cambridge, MA), and then with secondary antibodies. Sections were then mounted on slides using Vectashield (H-1200; Vector Lboratories, Burlingame, CA).
Cell Volume Measurements
Z-stacks of stained sections described above were acquired with a Zeiss LSM510 META confocal microscope. Volume reconstruction of the soma of DA in the VTA and of neurons from the subiculum area of the hippocampus was performed with IMARIS (Bitplane, Saint Paul, MN). With the Surpass function of IMARIS, wireframes were created along the x, y, and z axes, around the soma of stained cells. Each wireframe was used to create successive volumes enclosing a given cell soma, isolating it from close neighboring cells. A digital isosurface was created, based on the fluorescence intensity of the cytoplasm, that completely encloses a cell soma, and surface area was measured in microns square. The experimenter was blind to conditions until after all measurements were made. Between 29 and 58, cells were analyzed per condition.
High-Performance Liquid Chromatography
Nucleus accumbens (NAc) dopamine measurements were performed as described previously (Goldberg et al, 2003). Briefly, fresh brains were dissected from adult animals and 1 mm coronal slices taken using a mouse brain matrix. One millimeter tissue punches of the NAc were placed in 1.5 ml microcentrifuge tubes, weighed, and frozen on dry ice. The samples were stored at −80°C until further processing. The tissue punches were homogenized by sonicating in 49 volumes of 0.1 N perchloric acid and 0.2 mM sodium bisulfite. The homogenate was centrifuged at 20 000 g for 10 min at 4°C to pellet the debris. Twenty-five microliters of the resulting homogenate was loaded into an autosampler connected to an high-performance liquid chromatography with an electrochemical detector (ESA CoulArray with Model 5014B Microdialysis Cell) to measure the levels of dopamine and dopamine metabolites homovanillic acid (HVA), 3,4-dihydroxyphenylacetic acid (DOPAC), and 3-methoxytyramine (3-MT). Neurotransmitter levels were normalized to tissue weight.
Statistical Analysis
All comparisons for statistical differences between groups were carried out by ANOVA, followed by post hoc t-tests. In cases where only two groups were compared, then t-tests only were performed. P<0.05 was considered significant for all experiments. All error bars represent the standard error of the mean (S.E.M.).
RESULTS
ClockΔ19 Mice have an Increase in VTA Dopamine Cell Firing In Vitro, which is Reversed by Chronic Lithium Treatment
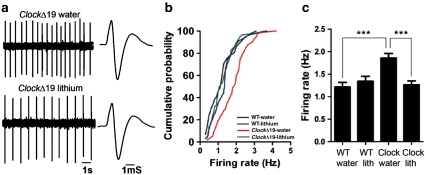
Previously we found using in vivo recordings of anesthetized animals that there was an increase in dopaminergic activity and bursting events in the VTA of ClockΔ19 mice compared with wild-type littermates (McClung et al, 2005). To determine if the increased activity remains in the absence of input from other regions, we utilized patch-clamp recordings in coronal slices containing the VTA. In agreement with our previous findings, there is an increase in the firing rate of individual dopaminergic neurons and cumulative probability of firing rate frequency in slices from the ClockΔ19 mice compared with wild-type controls, suggesting that the increased firing rates are the result of local changes in the VTA (Figure 1b and c).
Figure 1.
Chronic treatment with lithium normalizes the increased excitability in the ventral tegmental area (VTA) dopamine neurons in ClockΔ19 mice. (a) Sample traces and spikes for extracellular recordings in the VTA slice. (b) Cumulative probability of frequency, and (c) the quantitative data show that VTA firing rate were significantly increased in ClockΔ19 mice, which was decreased by treatment with lithium. Treatment with lithium did not change the VTA firing rate in wild-type mice. ***P<0.0001 (one-way analysis of variance (ANOVA), n=34–58 cells from 5 to 7 mice per group).
To determine if chronic lithium treatment restores proper dopamine cell firing rates to ClockΔ19 mice, we treated these mice, along with wild-type littermates, with 10 days of lithium chloride (600 mg/l) as performed previously (Roybal et al, 2007). We then took slices containing the VTA and measured dopamine cell activity. We found that chronic lithium treatment significantly reduced the firing rates of individual dopamine neurons to wild-type levels in the ClockΔ19 mice, but had no significant effect on the firing rates of dopamine neurons in wild-type littermates (Figure 1a-c). These results show that ClockΔ19 mice have an increase in dopaminergic activity in the VTA, which is restored to wild-type levels by chronic lithium treatment.
Lithium Treatment Reduces NAc Dopamine Levels in ClockΔ19 Mice
We measured the levels of dopamine and its metabolites, DOPAC, 3-MT, and HVA in NAc tissue of ClockΔ19 and wild-type mice either with or without treatment with chronic lithium. We found that ClockΔ19 mice have a ∼19% increase in dopamine levels compared with wild-type littermates (Table 1). In agreement with the electrophysiological data, treatment with chronic lithium significantly decreased the levels of dopamine in the ClockΔ19 mice, but had no effect on wild-type mice (Table 1). Furthermore, lithium treatment leads to a significant reduction in the levels of DOPAC, 3-MT, and HVA only in the ClockΔ19 mice.
Table 1. NAc Dopamine and Metabolite Levels.
| Group | DA (% ctrl) | Dopamine | HVA | DOPAC | 3-MT |
|---|---|---|---|---|---|
| WT H2O | 100 | 88.14±4.57 | 7.43±0.85 | 9.66±1.72 | 5.73±0.67 |
| WT lithium | 102 | 89.89±6.58 | 7.72±0.31 | 9.58±1.73 | 6.05±0.42 |
| ClockΔ19 H2O | 119 | 104.87±5.68* | 8.82±0.64 | 8.8±0.75 | 6.49±0.57 |
| ClockΔ19 lithium | 69 | 60.85±5.94* | 6.00±0.59* | 5.38±0.86* | 3.83±0.62* |
ClockΔ19 and wild-type (WT) littermates were treated with either water (H2O) alone or chronic lithium. Levels of dopamine (DA), HVA, 3-MT, and DOPAC were measured in NAc tissue by HPLC. Values shown are the average pM/mg tissue±the standard error. Dopamine levels were elevated in the ClockΔ19 mice (water alone) compared with wild-type controls (water alone) (*P=0.05; two-tailed t-test, n=5 per group). There were no significant differences between ClockΔ19 mice (water alone) and WT mice (water alone) in any of the metabolites. ClockΔ19 mice treated with lithium had significantly reduced levels of DA, HVA, 3-MT, and DOPAC compared with ClockΔ19 mice treated with water alone, or to WT mice treated with lithium (*P<0.05; two-tailed t-test, n=5 per group).
ClockΔ19 Mice have a Decrease in Dopamine Cell Volume, which is Rescued by Chronic Lithium Treatment
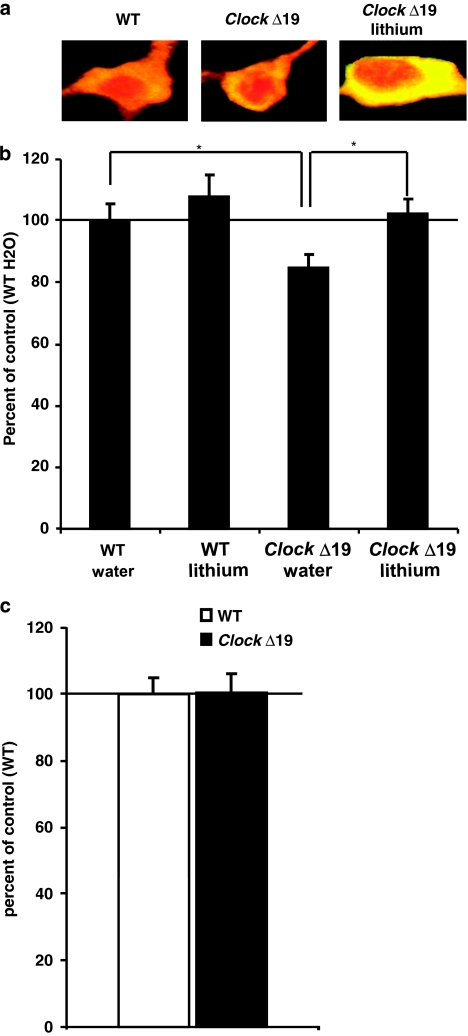
We wanted to determine if there were any cellular or morphological phenotypes that are perhaps responsible for producing the increased dopaminergic activity and manic-like behavior in the ClockΔ19 mice, which are also reversed by chronic lithium treatment. We performed immunohistochemistry on VTA containing sections of ClockΔ19 mice and wild-type littermates either with or without 10 days of lithium treatment (600 mg/l) in the drinking water to stain for TH to identify dopamine neurons and histone H3 to stain the nucleus. We then used confocal microscopy and stereology software to measure various properties of the cells. We found that the soma of dopamine cells in the VTA of ClockΔ19 mice had a reduced volume (∼20%) compared with the soma of dopamine cells from wild-type littermates (Figure 2a and b). Interestingly, treatment with chronic lithium restored the volume of the cells to wild-type levels. To determine if these changes were indicative of a decrease in the volume of all neurons in the brain, we measured the volume of a non-dopaminergic population of neurons. We selected the subiculum of the hippocampus as there are no dopaminergic neurons in this region, and it is anatomically distinct and identifiable in individual brain sections. We found no difference in the volume of neurons in the subiculum of the hippocampus between ClockΔ19 mice and wild-type littermates (Figure 2c), suggesting that the change in volume is not universal and perhaps is specific to dopamine neurons.
Figure 2.
ClockΔ19 mutant mice have smaller dopamine cell soma size, which is restored with lithium treatment (a) Representative dopamine neurons stained with histone H3 (red) and TH antibodies (yellow: red+green). (b) The soma of dopamine neurons of ClockΔ19 mutant mice are significantly smaller than the cell somas of the same neuron type in wild-type littermates (*P<0.05 by analysis of variance (ANOVA), n=5–8 mice per group, 7–10 cells per animal). Under lithium treatment, the size of the somas of cells from Clock mutant mice significantly goes back to untreated wild-type level (*P<0.05 by ANOVA, n=5 mice per group, 7–10 cells per animal). There is no significant difference between wild-type mice treated with water or lithium. (b) Non-dopaminergic neurons of the subiculum of the hippocampus have the same volume in ClockΔ19 mutant mice compared with wild-type littermates (n=5 mice per group, 10 cells per animal).
Expression of HSV-Kir2.1 in the VTA Reverses the Firing Rate and Morphological Abnormalities of Dopamine Neurons in ClockΔ19 Mice
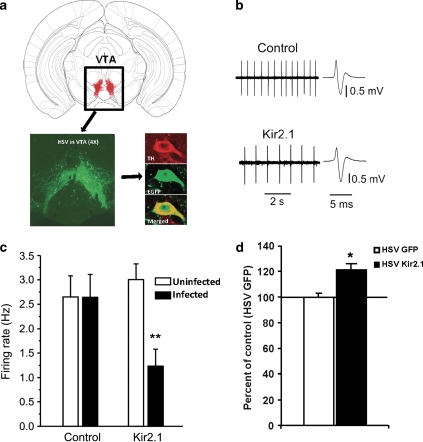
As chronic lithium treatment reverses both the increased dopamine cell firing and manic-like behaviors in the ClockΔ19 mice, this suggests that changes in dopaminergic activity may regulate these behavioral responses. However, lithium is known to have many effects in many different brain regions, and these changes in dopaminergic activity may not be relevant to lithium's actions. To test the importance of dopaminergic activity in mood- and anxiety-related behavior directly, we utilized an HSV vector, which contains the Kir2.1 potassium channel subunit. This virus has been used as a tool in previous studies by other groups to manipulate the firing rate of neurons in distinct brain regions including the VTA (Dong et al, 2006; Krishnan et al, 2007). When expressed in the VTA of ClockΔ19 mice, this virus should reduce the firing rate of dopamine neurons, and mimic the effects of chronic lithium treatment on these mice. Indeed, when we expressed this virus directly into the VTA of adult ClockΔ19 mice (Figure 3a) and measured dopamine cell activity (Figure 3b and c), we found that there was a significant decrease in the firing rate that is similar in magnitude to previously published studies using wild-type animals (Krishnan et al, 2007), and this reduction in firing is similar to that produced by lithium treatment (as shown in Figure 1).
Figure 3.
Expression of herpes simplex virus (HSV)-Kir2.1 decreases the firing rate of dopamine neurons and restores proper cell size in the ventral tegmental area (VTA) of the ClockΔ19 mice. (a) Schematic diagram showing a coronal section of mouse brain containing the VTA, along with a representative image (bottom left) showing a × 4 magnification of the VTA-specific targeting of HSV by stereotaxic surgery after 4 days. On the bottom right are representative dopamine neurons immunostained with enhanced green fluorescent protein (EGFP) and anti-tyrosine hydroxylase (TH) antibodies and images were merged to see colocalization using a confocal microscope. (b) Sample traces and spikes for extracellular recordings in the VTA slice. (c) HSV-Kir2.1 infection reduces the firing rate of dopamine neurons when compared with both ClockΔ19 animals infected with HSV-GFP and neighboring uninfected neurons in ClockΔ19 animals infected with the HSV-Kir2.1. **P<0.01 (one-way ANOVA, n=7–15 cells from 5 to 7 mice per group). (d) The size of the soma of dopamine cells of ClockΔ19 mutant mice injected with HSV-Kir2.1 in the VTA are larger than the cells of mutants that are injected with HSV-GFP (**P<0.01 by t-test, n=4–5 mice per group, 25–35 cells per group).
To determine the effects of the HSV-Kir2.1 channel on dopamine cell morphology in the ClockΔ19 mice, we performed immunohistochemistry followed by stereology measures of ClockΔ19 brain slices expressing either the HSV-Kir2.1 channel or HSV-GFP. Viral-infected dopamine neurons were readily detectable by their co-labeling of TH and GFP. Similar to chronic lithium treatment, we found that expression of the Kir2.1 gene lead to an increase in the volume of dopamine neurons in the ClockΔ19 mice (Figure 3d). These results show that HSV-Kir2.1 expression produces a change in both dopamine cell morphology and activity that effectively mimics the actions of lithium on the ClockΔ19 mice.
Reduced Dopaminergic Activity in the ClockΔ19 Mice is Associated with a Decreased Locomotor Response to Novelty
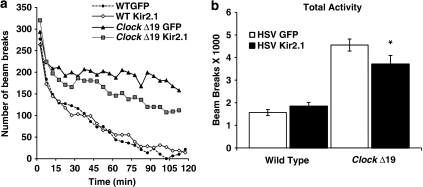
To determine if a reduction in the firing rate of dopamine neurons in the VTA of ClockΔ19 mice is sufficient to alter their hyperactivity, we injected the HSV-Kir2.1 virus into the VTA of the mutant mice and measured their locomotor response to a novel environment. We found that ClockΔ19 mice injected with the HSV-Kir2.1 had less locomotor activity over a 2 h period in response to a novel environment (Figure 4b). When examining the beam breaks over the full 2 h, it is apparent that the locomotor response is no different than the HSV-GFP-infected mice over the first 20 min; however, the Kir2.1-infected ClockΔ19 mice appear to habituate to the environment more readily over the course of the experiment than mice injected with an HSV-GFP control virus (Figure 4a). Wild-type mice infected with either the HSV-GFP or HSV-Kir2.1 virus were less active overall compared with ClockΔ19 mice, as has been described previously (Easton et al, 2003; Roybal et al, 2007), and the Kir2.1 virus had no effect on their locomotor activity (Figure 4). Importantly, when the ClockΔ19 mice injected with the HSV-Kir2.1 virus are tested on the rotorod, they are indistinguishable from mice injected with the control virus, which shows that they do not have a deficit in motor coordination (data not shown), but have a selective reduction in exploratory activity.
Figure 4.
Dopaminergic cell firing rates directly correlate with the hyperactive response to novelty in ClockΔ19 mice. (a) Locomotor activity and the habituation to novelty was determined by number of beam breaks made by herpes simplex virus (HSV)-injected ClockΔ19 and wild-type mice in a novel environment measured in 5 min bins over 2 h. (b) HSV-Kir2.1-injected ClockΔ19 mice exhibited significantly lower levels of locomotor activity than the HSV-green fluorescent protein-injected mice (**P<0.001 analysis of variance (ANOVA), n=5–10). There is a significant difference in locomotor activity between wild-type and ClockΔ19 mice injected with HSV-GFP (P<0.001).
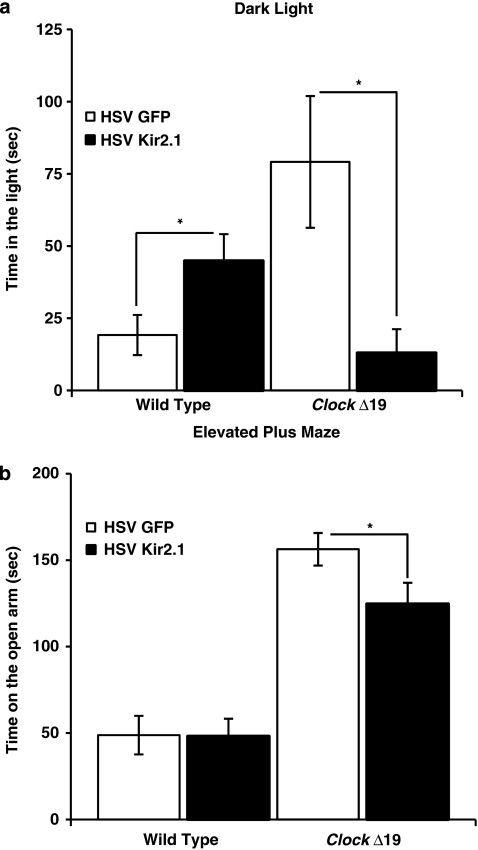
Normalization of Dopaminergic Activity in the ClockΔ19 Mice Leads to a Reduction in Anxiety-Related Behavior
We wanted to determine if HSV-Kir2.1 expression in ClockΔ19 mice would lead to a normalization of anxiety-related behavior, which was similar to chronic lithium treatment (Roybal et al, 2007). Here, we utilized both the dark/light and elevated plus-maze tests, which are standard measures of anxiety-related behavior, and both have been validated in several previous studies with anxiolytic medications. Similar to our previous results, we found that the HSV-GFP-injected ClockΔ19 mice show greater exploratory or anxiolytic behavior in both of these tests compared with wild-type mice (Figure 5). In addition, we found that the ClockΔ19 mice injected with the HSV-Kir2.1 spent less time on light side of the dark/light test (Figure 5a) and less time on the open arm of the elevated plus-maze (Figure 5b) than HSV-GFP-injected ClockΔ19 mice, suggesting that a reduction in dopaminergic activity is anxiogenic in these mice. We have great confidence that these results represent true changes in anxiety-related behavior and are not due to changes in locomotor activity as they are consistent between both measures, and because our tests of the locomotor response to novelty (as mentioned above) found no difference in the initial locomotor response to a new environment between the HSV-Kir2.1- and HSV-GFP-injected animals. Both of these anxiety-related measurements were performed within 10–15 min, a time when there is no difference between groups, and the HSV-Kir2.1-injected animals display even a slight, although nonsignificant, increase in the locomotor response to novelty. Interestingly, HSV-Kir2.1 expression in wild-type animals had no significant effect in the time in the open arms of the elevated plus maze (Figure 5b); however, there was a significant increase in the time spent in the light in the dark/light test (Figure 5a), suggesting that decreased dopaminergic activity in wild-type animals perhaps leads to a slight anxiolytic response.
Figure 5.
Decreasing the firing rate of dopaminergic cells leads to decreased ‘risk taking' behavior in ClockΔ19 mice. (a) Herpes simplex virus (HSV)-injected ClockΔ19 and wild-type (WT) mice were subjected to a dark/light test and the time spent on the light side was measured. HSV-Kir2.1-injected mutant mice spent less time in the light side as compared with HSV-green fluorescent protein-injected mice. Wild-type HSV-Kir2.1-injected mice spent significantly more time in the light side than WT HSV-GFP-injected mice (n=9–15, *P<0.05 by analysis of variance (ANOVA)). There is a significant difference in time spent in the lighted area between wild-type and ClockΔ19 mice injected with HSV-GFP (P<0.05). (b) HSV-injected ClockΔ19 and WT mice were subjected to the elevated plus-maze test. The time spent on the open arms was determined by video tracking software. HSV-Kir2.1-injected mutant mice spent significantly less time in the open arm as compared with HSV-GFP-injected mice (n=10–20, *P<0.05 by ANOVA). There is a significant difference in time spent on the open arm in between wild-type and ClockΔ19 mice injected with HSV-GFP (P<0.001).
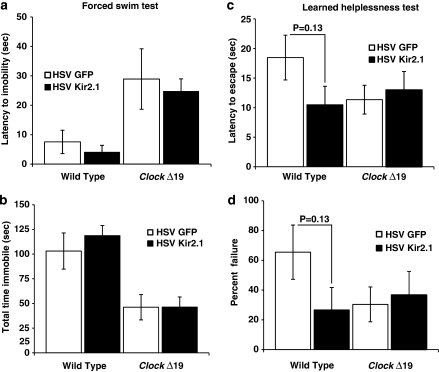
Reduced Dopaminergic Activity in the VTA of ClockΔ19 Mice is Not Sufficient to Restore Proper Mood-Related Behavior
To determine if a reduction in dopamine cell firing in the VTA of ClockΔ19 mice will recapitulate the effects of chronic lithium on depression-related behaviors in the ClockΔ19 mice, we subjected the HSV-Kir2.1 mice and HSV-GFP-injected mice to the FST and learned helplessness (LH) paradigms. These tests are both measures of behavioral despair or ‘helpless' behavior in a stressful environment and have been extensively validated with antidepressant medications. In the FST, both the latency to immobility and total time immobile were calculated, and in the LH both the latency to escape and failure to escape were calculated. Although the expression of HSV-Kir2.1 was sufficient to alter the locomotor response to novelty- and anxiety-related behavior in the ClockΔ19 mice (Figures 4 and 5), it was not sufficient to alter depression-related behavior in the FST or LH in the same animals (Figure 6). Interestingly, although results did not reach statistical significance, Kir2.1 expression in wild-type animals produced a trend towards an antidepressant response in the LH test (Figure 6).
Figure 6.
Depression-like behavior of ClockΔ19 mice is not affected by herpes simplex virus (HSV)-Kir2.1 infection (a, b) HSV-injected ClockΔ19 and wild-type (WT) mice were subjected to the forced swim test (FST). (a) Latency to immobility was determined when the first cessation of all movements occurred for 3 s. There was no difference in latency to immobility observed between HSV-Kir2.1- and HSV-green fluorescent protein-infected mice (n=9–20). (b) Total immobility in the FST was measured and there were no difference in total time immobile between HSV-Kir2.1- and HSV-GFP-injected mice (n=10–20). Both latency to immobility and total time immobile is significantly different between wild-type and ClockΔ19 mice injected with HSV-GFP (P<0.01 by two-way analysis of variance (ANOVA)). (c, d) ClockΔ19 and WT mice were subjected to the learned helplessness paradigm. (c) The latency to escape was not significantly different between the HSV-Kir2.1- and HSV-GFP-injected mice. (d) The number of escape failures was calculated and there was no significant difference between HSV-Kir2.1- and HSV-GFP-injected mice. (n=6–10).
DISCUSSION
Our results show that ClockΔ19 mice have an increase in dopaminergic firing rates in the VTA in slice preparations, suggesting that the activity is altered in these mutants because of intrinsic changes in dopamine neurons themselves. Furthermore, chronic lithium treatment is able to restore dopamine cell firing to near wild-type levels. ClockΔ19 mice also have an increase in NAc dopamine levels. There was no significant difference in the levels of dopamine metabolites in the ClockΔ19 mice and interpretations of these data are complicated in light of previous studies, which found that mutations in circadian genes can lead to reductions in MAOA activity (Hampp et al, 2008). However, the results of chronic lithium treatment are clear in that there is a significant reduction in dopamine cell firing and the levels of dopamine and metabolites in the ClockΔ19 mice following lithium treatment.
Interestingly, this particular lithium treatment paradigm has no significant effect on the firing rates of dopamine neurons or levels of dopamine in wild-type animals. This is very intriguing as our previous results show that this lithium treatment paradigm does not produce significant behavioral effects in wild-type mice in most measures of locomotor activity, anxiety, or depression-related behavior (Roybal et al, 2007). Previous studies with larger concentrations of lithium in wild-type mice have generally found that lithium produces an anxiolytic and antidepressant effect, which opposes the anxiogenic and pro-depressant effects that we find in ClockΔ19 mice (O'Donnell and Gould, 2007). Therefore, lithium is having a very specific set of actions in the ClockΔ19 mice leading to decreased dopaminergic activity and a reversal of manic-like behavior. This is important as the response to lithium in human populations is very different between those with bipolar disorder and individuals with other mood disorders or healthy individuals (Barton et al, 1993; Malhi et al, 2009). The largest therapeutic effects of lithium are found in the treatment of bipolar mania, and these effects are well documented in an extensive literature (Grandjean and Aubry, 2009; Malhi et al, 2009; Nierenberg, 2008). The effects of lithium on bipolar depression are not as clear, and the strongest conclusion is that lithium can augment the effects of an antidepressant (Nierenberg, 2008). When lithium is given to healthy human volunteers, it generally has no effect on their mood, or can have a slight variable effect on mood (Barton et al, 1993). Therefore, it stands to reason that lithium would have very selective effects in a mouse that has a manic-like phenotype vs a wild-type mouse. This also suggests that the changes that we find in response to lithium in the ClockΔ19 mice might be very relevant for the treatment of mania.
As lithium treatment decreased the firing rate of dopamine neurons and reduced levels of striatal dopamine in the ClockΔ19 mice, we wanted to determine if this decrease was important in the regulation of manic-like behavior. Interestingly, when we caused a selective decrease in VTA cell firing using the HSV-Kir2.1 virus as a tool in ClockΔ19 mice, we found that this was sufficient to reverse some of the increased exploratory behavior- and anxiety-related abnormalities in these mice, but not the depression-related abnormalities in the mice. This was a surprise as lithium treatment will normalize both of these behavioral phenotypes (Roybal et al, 2007). Even though the anxiety studies and depression studies were largely performed in the same animals, it is possible that we simply did not infect enough neurons of the VTA with the virus, or express the Kir2.1 channel at a sufficient level to alter depression-like behavior. Another possibility is that VTA cell firing is not involved in depression-related behavior; however, many previous studies suggest that this is unlikely (Krishnan and Nestler, 2008b; Nestler and Carlezon, 2006). What is more likely is that the role of dopamine in mood regulation is a complex interaction with other brain regions. The role of Clock expression in the VTA in mood-associated behavior is also complex. A knockdown of Clock gene expression using RNA interference in the VTA recapitulates the hyperactivity and anxiolytic effects, as well as the increased dopamine cell firing that is seen in the ClockΔ19 mice; however, it also leads to a large increase in depression-related behavior (Mukherjee et al, 2010). Restoration of a functional Clock gene into the VTA of ClockΔ19 mice rescues anxiety-related behavioral phenotypes, but is not sufficient to rescue depression-related phenotypes (Roybal et al, 2007; unpublished observations). Clearly, additional studies are needed to determine the exact role for dopamine in the regulation of depression-like behavior. What is clear from all of these studies is that anxiety-related behavior and depression-related behavior are regulated by separate mechanisms. Recently, we found that the ClockΔ19 mice have a defect in the phasic entrainment of δ and γ oscillations in the NAc neurons, which can be rescued by chronic lithium treatment (Dzirasa et al, 2010). It is very likely that it is not merely the increase or decrease in firing rates within the VTA-NAc circuit that is important in regulating mood, but the patterns of firing, oscillations, and coherence between and within structures is what dictates the behavioral- and mood-related outputs.
Similar to our findings with chronic lithium treatment (Roybal et al, 2007), wild-type mice were not consistently affected by the overexpression of Kir2.1. The effects that were seen tended to be opposite to those seen in ClockΔ19 mice in that they were anxiolytic and perhaps antidepressant. Krishnan et al (2007) found that wild-type mice, which have been subjected to chronic social defeat have an increase in dopamine cell firing in the VTA compared with non-defeated mice (Krishnan et al, 2007). Interestingly, overexpression of this same HSV-Kir2.1 channel promoted resilient behavior in wild-type animals, suggesting that a reduction in dopamine cell firing in wild-type animals can have antidepressant effects in animals with elevated dopamine owing to chronic stress (Krishnan et al, 2007). The lack of consistent effect that we found in these behavioral tests is likely due to the fact that these wild-type animals were not chronically stressed (thus had only basal levels of dopamine), and were tested at a time of day when dopamine levels in the VTA are normally quite low (Hood et al, 2010). If the animals had been chronically stressed, or were tested during the night, the effects would certainly be more pronounced. These trends that we see in wild-type animals, however, further confirm the idea that ClockΔ19 mice have specific defects in the dopamine circuit that cause them to respond in different ways to pharmacological treatments or manipulation of neuronal activity.
To attempt to elucidate the cellular mechanisms that underlie changes in dopamine cell firing and these specific behavioral responses, we measured morphological properties of the dopamine neurons in ClockΔ19 mice with and without lithium treatment or expression of the Kir2.1 channel. ClockΔ19 mice have dopamine neurons that are smaller in volume when compared with wild-type mice. Smaller neurons are generally associated with increased rates of firing as they have lowered membrane resistance, and mice with increased dopaminergic activity following chronic social defeat or morphine treatment have dopamine neurons that are reduced in size (Krishnan et al, 2008a; Russo et al, 2007). Lithium treatment and Kir2.1 expression both restore proper cell size and reduce cell firing, suggesting that there is a distinct connection between the cell size and firing rate. It is possible that at least part of the therapeutic actions of lithium come from its ability to increase the size of these dopamine neurons. It is also possible that changes in cell size serve as a marker for changes in dopamine cell firing. The mechanism by which lithium leads to a change in cell size is unclear. Several studies have found that lithium can inhibit the activity of glycogen synthase kinase 3-β (GSK3-β) (reviewed by Young (2009)). This enzyme normally inhibits nuclear factors that turn on cell growth and protection; thus, one possibility is that lithium is increasing cell volume through its inhibition of this enzyme. Indeed, lithium treatment in animals upregulates neurotrophins such as brain-derived neurotrophic factor and nerve growth factor among others. Furthermore, GSK3-β is part of the AKT signaling pathway, which plays an important role in mood- and reward-related behaviors, as well as cell size. Decreased AKT signaling through pharamacological or viral manipulations leads to reduced dopamine cell volume and increased dopaminergic activity (Russo et al, 2007; Krishnan et al, 2008a). As AKT normally inhibits GSK3-β, it is possible that lithium treatment would be similar to an activation of AKT signaling, thus increasing dopamine cell size and decreasing firing rates. Future experiments will determine if these proteins (or one or more of lithium's other molecular targets) are mediating the effects on cell morphology and behavior specifically in the ClockΔ19 mice.
In conclusion, the increased exploratory drive and hyperactivity in response to novelty in the ClockΔ19 mouse model of mania are likely due in large part to the increase in dopaminergic activity in these mice. There are clear defects in cellular morphology in these mutants that may underlie the increase in dopaminergic activity. Lithium treatment is able to reverse nearly all of their behavioral abnormalities, including both anxiety- and depression-related behaviors, and at least the reversal of the anxiety-related behaviors may depend on the ability of lithium to normalize changes in dopamine cell volume and ultimately dopaminergic activity in the VTA. Depression-related behavior is more complex and likely involves the interaction between the VTA and other brain regions. Future studies will determine the molecular actions of lithium that induce these cellular changes with the hope of developing more targeted mood-related medications in the future.
Acknowledgments
We thank Dr Eric Nestler for assistance with the HSV viral vectors. We thank Dr Joe Takahashi for the ClockΔ19 mice. We also thank Kole Roybal, Dr Michelle Mazie-Robinson, Dr Scott Russo, Charles Taylor, Dr Andrea Gillman, and Kristen A Ketcherside for technical help and useful discussions. This study was funded by grants to Dr McClung from the Blue Gator Foundation, NARSAD, the McKnight Foundation, NINDS (R21 NS058339), NIDA (R01DA023988), and NIMH (R01MH082876).
Dr McClung has received research funding from GlaxoSmithKline and Pfizer on unrelated project, and honoraria and consulting fees from GlaxoSmithKline, Pfizer, Servier, and Orphagen Pharmaceuticals. All other authors have nothing to disclose.
References
- Barton CD, Jr, Dufer D, Monderer R, Cohen MJ, Fuller HJ, Clark MR, et al. Mood variability in normal subjects on lithium. Biol Psychiatry. 1993;34:878–884. doi: 10.1016/0006-3223(93)90055-i. [DOI] [PubMed] [Google Scholar]
- Dong Y, Green T, Saal D, Marie H, Neve R, Nestler EJ, et al. CREB modulates excitability of nucleus accumbens neurons. Nat Neurosci. 2006;9:475–477. doi: 10.1038/nn1661. [DOI] [PubMed] [Google Scholar]
- Dzirasa K, Coque L, Sidor MM, Kumar S, Dancy EA, Takahashi JS, et al. Lithium ameliorates nucleus accumbens phase-signaling dysfunction in a genetic mouse model of mania. J Neurosci. 2010;30:16314–16323. doi: 10.1523/JNEUROSCI.4289-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton A, Arbuzova J, Turek FW. The circadian Clock mutation increases exploratory activity and escape-seeking behavior. Genes Brain Behav. 2003;2:11–19. doi: 10.1034/j.1601-183x.2003.00002.x. [DOI] [PubMed] [Google Scholar]
- Franklin K, Paxinos G. Academic Press: San Diego; 1997. The mouse brain in stereotaxic coordinates. [Google Scholar]
- Goldberg M, Fleming SM, Palacin JJ, Cepeda C, Hoa LA, Bhatangar A, et al. Parkin-deficient mice exhibit nigrostriatal deficits but not loss of dopaminergic neurons. J Biol Chem. 2003;278:43628–43635. doi: 10.1074/jbc.M308947200. [DOI] [PubMed] [Google Scholar]
- Grace AA, Onn SP. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J Neurosci. 1989;9:3463–3481. doi: 10.1523/JNEUROSCI.09-10-03463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean EM, Aubry JM. Lithium: updated human knowledge using an evidence-based approach: Part I: clinical efficacy in bipolar disorder. CNS Drugs. 2009;23:225–240. doi: 10.2165/00023210-200923030-00004. [DOI] [PubMed] [Google Scholar]
- Hampp G, Ripperger JA, Houben T, Schmutz I, Blex C, Perreau-Lenz S, et al. Regulation of monoamine oxidase A by circadian-clock components implies clock influence on mood. Curr Biol. 2008;18:678–683. doi: 10.1016/j.cub.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Han MH, Bolanos CA, Green TA, Olson VG, Neve RL, Liu RJ, Aghajanian GK, et al. Role of cAMP response element-binding protein in the rat locus ceruleus: regulation of neuronal activity and opiate withdrawal behavior. J Neurosci. 2006;26:4624–4629. doi: 10.1523/JNEUROSCI.4701-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood S, Cassidy P, Cossette M-P, Weigl Y, Verwey M, Robinson B, et al. Endogenous dopamine regulates the rhythm of expression of the clock protein Per2 in the rat dorsal striatum via daily activation of D2 dopamine receptors. J Neurosci. 2010;30:14046–14058. doi: 10.1523/JNEUROSCI.2128-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, et al. Positional cloning of the mouse circadian clock gene. Cell. 1997;89:641–653. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15 (Spec No. 2:R271–R277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- Kripke DF, Nievergelt CM, Joo E, Shekhtman T, Kelsoe JR. Circadian polymorphisms associated with affective disorders. J Circadian Rhythms. 2009;7:2. doi: 10.1186/1740-3391-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Mazei-Robison M, Iniguez SD, Ables JL, Vialou V, et al. AKT signaling within the ventral tegmental area regulates cellular and behavioral responses to stressful stimuli. Biol Psychiatry. 2008a;64:691–700. doi: 10.1016/j.biopsych.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008b;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi GS, Adams D, Berk M. Is lithium in a class of its own? A brief profile of its clinical use. Aust N Z J Psychiatry. 2009;43:1096–1104. doi: 10.3109/00048670903279937. [DOI] [PubMed] [Google Scholar]
- Mansour HA, Talkowski ME, Wood J, Chowdari KV, McClain L, Prasad K, et al. Association study of 21 circadian genes with bipolar I disorder, schizoaffective disorder, and schizophrenia. Bipolar Disord. 2009;11:701–710. doi: 10.1111/j.1399-5618.2009.00756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli M, White FJ. Enhanced vulnerability to cocaine self-administration is associated with elevated impulse activity of midbrain dopamine neurons. J Neurosci. 2000;20:8876–8885. doi: 10.1523/JNEUROSCI.20-23-08876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA. Circadian genes, rhythms and the biology of mood disorders. Pharmacol Ther. 2007;114:222–232. doi: 10.1016/j.pharmthera.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, et al. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci USA. 2005;102:9377–9381. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath CL, Glatt SJ, Sklar P, Le-Niculescu H, Kuczenski R, Doyle AE, et al. Evidence for genetic association of RORB with bipolar disorder. BMC Psychiatry. 2009;9:70. doi: 10.1186/1471-244X-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Coque L, Cao JL, Kumar J, Chakravarty S, Asaithamby A. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Coque L, Cao JL, Kumar J, Chakravary S, Asaithamby A, et al. Knockdown of Clock in the ventral tegmental area through RNA interference results in a mixed state of mania and depression-like behavior. Biol Psych. 2010;68:503–11. doi: 10.1016/j.biopsych.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WAJr, et al. The mesolimbic dopamine reward circuit in depression. Biol Psych. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Nierenberg AA. Effective agents in treating bipolar depression. J Clin Psychiatry. 2008;69:e29. doi: 10.4088/jcp.1008e29. [DOI] [PubMed] [Google Scholar]
- Nievergelt CM, Kripke DF, Barrett TB, Burg E, Remick RA, Sadovnick AD, et al. Suggestive evidence for association of the circadian genes PERIOD3 and ARNTL with bipolar disorder. Am J Med Genet B. 2006;141B:234–241. doi: 10.1002/ajmg.b.30252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell KC, Gould TD. The behavioral actions of lithium in rodent models: leads to develop novel therapeutics. Neurosci Biobehav Rev. 2007;31:932–962. doi: 10.1016/j.neubiorev.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, et al. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci USA. 2007;104:6406–6411. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Bolanos CA, Theobald DE, DeCarolis NA, Renthal W, Kumar A, et al. IRS2-Akt pathway in midbrain dopamine neurons regulates behavioral and cellular responses to opiates. Nat Neurosci. 2007;10:93–99. doi: 10.1038/nn1812. [DOI] [PubMed] [Google Scholar]
- Soria V, Martinez-Amoros E, Escaramis G, Valero J, Perez-Egea R, Garcia C, et al. Differential association of circadian genes with mood disorders: CRY1 and NPAS2 are associated with unipolar major depression and CLOCK and VIP with bipolar disorder. Neuropsychopharmacology. 2010;35:1279–1289. doi: 10.1038/npp.2009.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303:2040–2042. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- Viggiano D, Sadile AG. Hypertrophic A10 dopamine neurones in a rat model of attention-deficit hyperactivity disorder (ADHD) Neuroreport. 2000;11:3677–3680. doi: 10.1097/00001756-200011270-00018. [DOI] [PubMed] [Google Scholar]
- Vitaterna MH, Ko CH, Chang AM, Buhr ED, Fruechte EM, Schook A, et al. The mouse Clock mutation reduces circadian pacemaker amplitude and enhances efficacy of resetting stimuli and phase–response curve amplitude. Proc Natl Acad Sci USA. 2006;103:9327–9332. doi: 10.1073/pnas.0603601103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Drug-activation of brain reward pathways. Drug Alcohol Depend. 1998;51:13–22. doi: 10.1016/s0376-8716(98)00063-5. [DOI] [PubMed] [Google Scholar]
- Young W. Review of lithium effects on brain and blood. Cell Transplant. 2009;18:951–975. doi: 10.3727/096368909X471251. [DOI] [PubMed] [Google Scholar]