Abstract
Nonalcoholic fatty liver disease (NAFLD) has been referred to as the hepatic manifestation of the metabolic syndrome. There is a lower prevalence of metabolic syndrome in individuals with higher health-related fitness (HRF) and physical activity (PA) participation. The relationship between NAFLD severity and HRF or PA is unknown. Our aim was to compare measures of HRF and PA in patients with a histological spectrum of NAFLD severity. Thirty-seven patients with liver biopsy–confirmed NAFLD (18 women/19 men; age = 45.9 ± 12.7 years) completed assessment of cardiorespiratory fitness (CRF, VO2peak), muscle strength (quadriceps peak torque), body composition (%fat), and PA (current and historical questionnaire). Liver histology was used to classify severity by steatosis (mild, moderate, severe), fibrosis stage (stage 1 versus stage 2/3), necroinflammatory activity (NAFLD Activity Score; ≤4 NAS1 versus ≥5 NAS2) and diagnosis of NASH by Brunt criteria (NASH versus NotNASH). Analysis of variance and independent t tests were used to determine the differences among groups. Fewer than 20% of patients met recommended guidelines for PA, and 97.3% were classified at increased risk of morbidity and mortality by %fat. No differences were detected in VO2peak (x = 26.8 ± 7.4 mL/g/min) or %fat (x = 38.6 ± 8.2%) among the steatosis or fibrosis groups. Peak VO2 was significantly higher in NAS1 versus NAS2 (30.4 ± 8.2 versus 24.4 ± 5.7 mL/kg/min, P = 0.013) and NotNASH versus NASH (34.0 ± 9.5 versus 25.1 ± 5.7 mL/kg/min, P = 0.048).
Conclusion
Patients with NAFLD of differing histological severity have suboptimal HRF. Lifestyle interventions to improve HRF and PA may be beneficial in reducing the associated risk factors and preventing progression of NAFLD.
Nonalcoholic fatty liver disease (NAFLD) is now the most common cause of abnormal liver enzymes in adults in the United States and the leading cause of referral to hepatology clinics.1 Prevalence estimates in the general populations of the United States, Japan, and Italy are 25%, 29%, and 20%, respectively.2-4 The term “NAFLD” is used by convention to describe a diverse range of histological abnormalities such as steatosis, steatohepatitis (mixed macro- and microvesicular steatosis, lobular necroinflammation with fibrosis), and cirrhosis.5 Although the exact pathophysiology of NAFLD has yet to be elucidated, insulin resistance is now recognized as playing a central role.6 Other features of the metabolic syndrome (abdominal obesity, type 2 diabetes mellitus, and dyslipidemia) are also common in patients with NAFLD.7-9 Hepatic insulin resistance has been hypothesized as a common feature of both NAFLD and the metabolic syndrome.10
The metabolic syndrome is associated with significantly increased morbidity and mortality, specifically the risk of cardiovascular disease death.11 Epidemiological studies report a lower prevalence of metabolic syndrome in individuals with higher participation in physical activity (PA), higher muscle strength, and higher cardiorespiratory fitness (CRF; a measure of oxygen transport to the working muscles, measured by peak oxygen uptake; VO2peak).12-15 Higher CRF has also been shown to attenuate the metabolic syndrome profile and associated increase in all-cause and cardiovascular disease mortality.16 Church et al.17 were the first to suggest a similar inverse relationship between CRF and the prevalence of NAFLD,17 and Perseghin et al.18 demonstrated an inverse relationship between habitual PA and intrahepatic fat content. However, health-related fitness (HRF) (CRF, muscle strength, body composition) and PA participation have not been evaluated in patients with a histological spectrum of NAFLD. Furthermore, it is unknown whether these HRF components or PA participation correlate with NAFLD disease severity. These data are crucial, as most therapeutic guidelines for persons with NAFLD suggest weight loss through lifestyle changes that include diet modifications and exercise participation.5 However, assessment of HRF is not addressed, and precise recommendations for exercise do not exist.
The aims of this study were to assess the HRF and PA participation of patients with a histological spectrum of NAFLD. Also, we aimed to determine whether objective measures of HRF and PA differ with severity of NAFLD.
Patients and Methods
This was a cross-sectional study of adult patients (≥18 years) with NAFLD recruited from the Non-Alcoholic Steatohepatitis (NASH) Clinical Research Network NAFLD Database at the University of California, San Francisco. Consecutive patients were invited to participate. Inclusion criteria included histopathology confirming NAFLD and alcohol use history of 20/10 g/day or less (men/women). All subjects had standard laboratory testing to exclude other causes of liver disease. Subjects with histopathological findings suggestive of another liver disease were excluded. Patients were also excluded if they had a history of total parenteral nutrition (previous 3 months), short bowel syndrome, bariatric surgery preceding the diagnosis of NAFLD, or evidence of decompensated liver disease. Patients with advanced pulmonary or cardiovascular disease, orthopedic limitations that precluded exercise testing, or any absolute contraindications to exercise testing as established by the American College of Cardiology/American Heart Association or the American College of Sports Medicine19,20 were also excluded. All HRF and body composition testing was performed in the Exercise Physiology and Body Composition Laboratory of the University of California, San Francisco CTSI Clinical Research Center. All details of this study were approved by the local Institutional Review Board, and all patients gave written informed consent.
Histological Assessment of NAFLD
Histology slides from a standard-of-care liver biopsy were obtained to confirm the diagnosis of NAFLD for all subjects. A single pathologist, blinded to the patients’ clinical status, reviewed the slides. This included assessment of steatosis, lobular inflammation, hepatocellular ballooning, and fibrosis as described by Kleiner et al.,21 also allowing determination of the NAFLD Activity Score (NAS). The composite NAFLD Activity Score (NAS – the unweighted sum of the scores for steatosis, lobular inflammation, and hepatocellular ballooning, ranging from 0-8) were used to assess disease activity, a NAS ≤ 4 reflecting low activity and NAS ≥ 5 reflecting high activity. In addition, the histological diagnosis of NASH was made according to Brunt et al.,22 with modifications made a priori: NASH was defined based on the presence of at least grade 1 steatosis with hepatocyte ballooning or at least grade 1 steatosis and with perisinusoidal fibrosis (but not isolated periportal fibrosis) with lobular inflammation. Categorized component scores, steatosis (mild, moderate, and severe), fibrosis [stage 1 mild (Fibrosis1) versus stage 2, 3 moderate–severe (Fibrosis2)], NAS scores [NAS ≤ 4 (NAS1) versus NAS ≥ 5 (NAS2)], as well as diagnosis of NASH (NASH versus NotNASH) were used in this study to assess NAFLD disease severity and activity. All of the following study assessments took place within 4 months (mean, 2.0 ± 1.6 mo.) of the liver biopsy without any interim therapeutic interventions.
Demographic Data and Laboratory Data
Medical record review and patient interviews were used to obtain medical health histories. The National Cholesterol Education Program’s Adult Treatment Panel III report criteria were used to classify patients with metabolic syndrome.23 Standard clinical, anthropometric, and biochemical measurements were obtained. Laboratory evaluation included aspartate aminotransferase (AST), alanine aminotransferase, fasting blood glucose, and insulin and standard lipid measurements. Surrogate measurements of insulin resistance, insulin sensitivity, and beta-cell insulin sensitivity were calculated according to the original homeostasis model assessment (HOMA1-IR; normal = 1) method,24 as well as the updated HOMA2 insulin sensitivity and HOMA2 beta-cell insulin sensitivity (normal insulin sensitivity and beta-cell insulin sensitivity = 100%) computer model.25
Health-Related Fitness Measures
Cardiorespiratory Fitness
Symptom-limited graded exercise testing was performed on a treadmill using a branching protocol19 that established a comfortable walking speed, after which the speed and grade were increased to achieve a 1 to 2 metabolic equivalent (MET) increment between stages. The work rate increased every 2 minutes until the subject was unable to continue (volitional fatigue) or until there was an indication to discontinue the test (that is, electrocardiographic changes, inappropriate blood pressure response).19 Blood pressure and a continuous 12-lead electrocardiogram monitoring were performed. Ratings of perceived exertion (subjective rating of effort) were measured at each stage on a 6-20 scale.26 Oxygen consumption (VO2) was determined using an open-circuit spirometry system (Quinton metabolic cart, Bothell, WA), calibrated against known gases before each test. Respiratory gases were analyzed for volume and fractions of oxygen and carbon dioxide, and VO2 was calculated. Oxygen uptake at the highest tolerated intensity of exercise (VO2peak) was determined and expressed relative to body weight (milliliters of O2 per kilogram of body weight per minute). Age-predicted VO2peak was determined using formulas reported for sedentary normal individuals by Bruce et al.27: VO2peak for men = 57.8 − (0.445 × age), and VO2peak for women = 42.3 − (0.356 × age).
Muscle Strength
Quadricep muscle strength was measured using an isokinetic muscle function testing system (Biodex III, Shirley, NY). The right leg was secured over the thigh and attached to a dynamometer distal to the calf, allowing for isolation of the quadriceps muscle group. The patient performed maximal effort knee extension at a controlled speed of 180 per second for 20 repetitions. The variables measured for analysis were peak torque (highest torque produced during the set of repetitions) and peak torque relative to body weight. Normative values are presented in data from Biodex and by Akima et al.28
Body Composition and Distribution
Body composition and distribution was determined using dual energy x-ray absorptiometry (Lunar Prodigy; GE Healthcare, Madison, WI). The instrument was calibrated daily per the manufacturer’s guidelines. Lean body mass (kg) and fat mass (kg) were determined from a whole body scan, and percent fat (%fat) was calculated. A waist-to-hip fat ratio was determined between the fat tissue in the android (central) and gynoid (hip and thigh) software-defined regions.
Physical Activity Participation
Physical activity was assessed for current and historical participation. Current PA was classified as inactive, somewhat active, or active in cardiovascular exercise using a series of questions, allowing classification according to the Surgeon General’s report guidelines.11 The classification of active required cardiovascular exercise 3 or more times per week for at least 30 minutes per session at an intensity described as “moderate effort” or greater. Participation in “some” cardiovascular exercise (that is, 2 times per week or for less than 30 minutes) was categorized as “some activity,” and “inactive” represented those that performed less than “some” exercise. The Historical Physical Activity Survey assessed PA participation over the previous 10 years,29 reflecting the long natural history of NAFLD. The instrument includes 21 types of physical and occupational activities—the outcome variables were average hours per week of leisure-time PA participation and average intensity of the activity (MET; 1 MET is equivalent to the energy expenditure for a person at rest) over the past 10 years.
Data Analysis
Descriptive statistics (means, standard deviations) were calculated for all continuous variables, and frequencies and percentages were generated for the noncontinuous variables. Chi-squared analyses were used to test for differences among groups in nominal categorical outcomes. Kendall’s Tau was used to measure the relationship between ordinal categorical variables. Primary analysis used one-way analysis of variance with one between-subjects factor (group: steatosis: mild, moderate, severe) to examine differences among steatosis groups in CRF (VO2peak), total body fat (%fat), body fat distribution (android: gynoid ratio), muscle strength (peak torque per body weight), and 10-year past participation in PA (hours of activity and METs). When appropriate (significant analysis of variance F value), post-hoc pairwise contrasts using the Tukey criteria were examined to determine specifically where group differences occurred. For secondary analyses, the disease severity indices were dichotomized into clinically meaningful groups. Three secondary analyses were conducted to examine the differences in HRF and physical activity outcomes between (1) fibrosis stages (Fibrosis1 versus Fibrosis2), (2) NAFLD Activity Score scores (NAS1 versus NAS2), and (3) the presence or absence of a NASH diagnosis (NASH versus NotNASH) with independent t tests.
In addition, one-way analysis of variance with one between-subjects factor (group: active, some activity, inactive) was used to examine differences among the determinants of HRF and PA participation in our NAFLD sample. For all analyses, statistical significance was set at an alpha level of 0.05.
Results
Subjects
Forty-three patients were recruited for the study, and 6 patients declined participation. Thirty-seven patients completed testing (Table 1). Forty-nine percent of the sample was white, 27% Hispanic, 19% Asian, 3% African American, and the remaining reported more than 1 ethnicity. All 12 patients with hypertension were receiving pharmacological treatment, but only 1 of 3 with diabetes and 4 of 10 with hyperlipidemia were being treated. Fiftyone percent of our sample met the The National Cholesterol Education Program’s Adult Treatment Panel III criteria for metabolic syndrome.
Table 1.
Demographic Characteristics By Steatosis Grade
| All (n = 37) | Mild Steatosis (n = 11) | Moderate Steatosis (n = 14) | Severe Steatosis (n = 12) | |
|---|---|---|---|---|
| Age (yr) | 45.9 ± 12.7 | 50.4 ± 13.1 | 42.5 ± 11.9 | 46.0 ± 12.8 |
| Sex (M/F) | 19/18 | 4/7 | 10/4 | 4/8 |
| Comorbidities n (%) | ||||
| Hyperlipidemia | 10 (27.0) | 3 (27.2) | 8 (57.1) | 7 (58.3) |
| Hypertension | 12 (32.4) | 3 (27.2) | 5 (35.7) | 4 (33.3) |
| Type 2 diabetes mellitus | 3 (8.1) | 1 (9.0) | 0 | 2 (16.6) |
| Laboratory test | ||||
| ALT (U/L) | 91.6 ± 50.8 | 81.7 ± 41.6 | 89.9 ± 62.7 | 102.3 ± 47.2 |
| AST (U/L) | 68.3 ± 41.7 | 61.2 ± 38.7 | 48.5 ± 19.9 | 93.8 ± 49.5b |
| Fasting glucose (mg/dL) | 96.2 ± 16.0 | 92.6 ± 11.1 | 96.9 ± 18.0 | 98.7 ± 18.1 |
| Fasting insulin (μU/mL) | 22.2 ± 24.4 | 15.2 ± 7.1 | 20.9 ± 13.7 | 28.4 ± 38.8 |
| HOMA1-IR | 4.4 ± 2.9 | 3.6 ± 1.9 | 5.1 ± 3.4 | 7.7 ± 12.6 |
| HOMA2-%S | 62.3 ± 48.0 | 66.6 ± 52.4 | 54.6 ± 34.9 | 69.6 ± 60.8 |
| HOMA2-%B | 152.3 ± 84.5 | 126.8 ± 54.6 | 168.7 ± 101.5 | 152.3 ± 84.5 |
| Metabolic syndrome n(%) | 19 (51.4) | 3 (27.3) | 8 (57.1) | 8 (66.7) |
| Histopathology | ||||
| Steatosis | 2.0 ± 0.8 | 1.0 ± 0.0 | 2.0 ± 0.0 | 3.0 ± 0.0 |
| Lobular inflammation | 1.5 ± 0.7 | 1.0 ± 0.9a | 1.6 ± 0.5 | 1.7 ± 0.6 |
| Hepatocyte ballooning | 0.9 ± 0.7 | 0.9 ± 0.9 | 0.9 ± 0.5 | 1.0 ± 0.6 |
| NAFLD activity score | 4.5 ± 1.6 | 2.9 ± 1.6a,c | 4.6 ± 0.6b | 5.7 ± 0.9 |
| Fibrosis | 2.2 ± 1.6 | 1.9 ± 1.9 | 1.9 ± 1.1 | 2.7 ± 1.7 |
| NASH n (%) | 30 (81.1) | 7 (63.6) | 12 (85.7) | 11 (91.7) |
Values are mean ± SD.
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; HDL, high-density lipoprotein cholesterol; HOMA, homeostasis model assessment; IR, insulin resistance; %S, insulin sensitivity; %B, β-cell function.
Mild versus moderate, P < 0.05.
Moderate versus severe, P < 0.05.
Mild versus severe, P < 0.05.
Primary Analysis: Comparison of the Steatosis Groups
Demographic characteristics of the subjects by severity of steatosis (mild, moderate, severe) are shown in Table 1. There were no differences detected in age, ethnicity, or prevalence of the metabolic syndrome among the 3 steatosis groups. A significant difference was found among the steatosis groups in levels of AST (F2,32 = 4.468, P = 0.019). AST was significantly higher in the severe steatosis group as compared with the moderate group (P = 0.017). Other laboratory values, including measures of insulin resistance, did not differ significantly among the steatosis groups. Histopathological differences among the steatosis groups included lobular inflammation score (F2,34 = 4.154; P = 0.024) and NAFLD activity score (F2,34 = 18.602; P < 0.0001). Not unexpectedly, the mean lobular inflammation was greater in the severe steatosis group compared with the moderate group (P = 0.031).
Cardiorespiratory Fitness
All patients tolerated the CRF testing without adverse events. Patients reported terminating the exercise test because of exertional shortness of breath (51%), leg fatigue (24%), and overall fatigue (19%). All HRF outcome data by steatosis grade are presented in Table 2. Two tests were stopped by the examiners, one because of significant S-T segment depression (asymptomatic, resolved in recovery) and the second because of low back pain. The exercise tests were “near maximal” effort based on standard criteria. There were no differences detected in any of the physiological measures among the steatosis groups, and all groups demonstrated below average age-predicted CRF, as assessed by VO2peak.
Table 2.
Comparison of Health-Related Fitness Components By Steatosis Grade
| All (n = 37) | Mild Steatosis (n = 11) | Moderate Steatosis (n = 14) | Severe Steatosis (n = 12) | |
|---|---|---|---|---|
| Cardiorespiratory fitness | ||||
| Rest | ||||
| Heart rate (bpm) | 86.3 ± 12.4 | 84.0 ± 19.2 | 90.6 ± 13.5 | 83.4 ± 13.7 |
| Systolic BP (mmHg) | 130.1 ± 12.9 | 128.2 ± 9.4 | 135.6 ± 16.1 | 126.3 ± 10.0 |
| Diastolic BP (mmHg) | 78.1 ± 11.3 | 76.7 ± 10.2 | 78.9 ± 14.6 | 78.3 ± 8.2 |
| Peak exercise | ||||
| Heart rate (bpm) | 167.0 ± 20.9 | 160.6 ± 18.2 | 174.5 ± 15.1 | 162.8 ± 27.7 |
| Systolic BP (mmHg) | 193.9 ± 27.1 | 182.2 ± 27.3 | 201.2 ± 25.4 | 193.8 ± 27.1 |
| Diastolic BP (mmHg) | 77.9 ± 11.2 | 74.9 ± 11.5 | 78.3 ± 11.9 | 79.8 ± 10.4 |
| VE (L/min) | 86.8 ± 34.7 | 85.5 ± 40.5 | 100.6 ± 33.4 | 70.6 ± 24.8 |
| VO2peak (mL/kg/min) | 26.8 ± 7.4 | 26.6 ± 8.9 | 27.6 ± 5.7 | 26.1 ± 8.1 |
| Age-predicted VO2peak (%) | 87.4 ± 20.1 | 94.9 ± 18.6 | 79.2 ± 14.4 | 90.1 ± 25.0 |
| Muscle strength—quadricep | ||||
| Peak torque (ft · lbs) | 73.8 ± 27.0 | 59.3 ± 24.3a | 89.7 ± 22.4 | 67.9 ± 26.4 |
| Peak torque/body weight | 37.0 ± 10.7 | 30.9 ± 9.9a | 42.2 ± 8.0 | 36.5 ± 10.7 |
| Body composition and distribution: | ||||
| BMI (kg/m2) | 31.7 ± 6.3 | 31.3 ± 6.6 | 33.6 ± 6.7 | 30.0 ± 5.4 |
| Lean mass (kg) | 54.6 ± 23.0 | 48.0 ± 18.4 | 66.2 ± 29.7 | 47.1 ± 10.6 |
| Fat mass (kg) | 34.0 ± 11.9 | 34.1 ± 13.8 | 36.4 ± 12.6 | 31.0 ± 9.4 |
| Body fat (%) | 38.6 ± 8.2 | 39.3 ± 9.9 | 37.2 ± 8.0 | 39.5 ± 7.0 |
| Android:gynoid ratio | 1.2 ± 0.2 | 1.2 ± 0.2 | 1.3 ± 0.2 | 1.1 ± 0.1 |
| Waist:hip ratio | 0.96 ± 0.07 | 0.94 ± 0.04 | 1.0 ± 0.59 | 0.94 ± 0.08 |
Values are mean ± SD.
Abbreviations: bpm, beats per minute; VE, peak ventilation; VO2peak, volume of oxygen consumed at the highest tolerated level of exercise; BMI, body mass index.
Mild versus moderate, P < 0.05.
Muscle Strength
A significant difference was found in quadriceps peak torque (F2,33 = 5.317, P = 0.01) and peak torque adjusted for body weight (F2,33 = 4.027, P = 0.027) among the groups. The moderate steatosis group demonstrated greater peak torque (P = 0.01) and peak torque per body weight (P = 0.02) as compared with the mild group.
Body Composition and Distribution
There were no differences detected in any of the body composition variables assessed among the steatosis groups. Approximately 84% of all patients were classified as overweight or obese (body mass index > 25.0 or 30, respectively), and 97.3% of patients had excessive total body fat (>25% men, >30% women).30
Physical Activity Participation
Current physical activity participation
There were no differences found in the proportion of patients classified as active, somewhat active, or inactive using self-report among the 3 steatosis groups (Table 3). Less than 20% of the mild and moderate steatosis groups and approximately 33% of the severe group reported meeting the Surgeon General’s PA recommendations.11 Overall, 62.2% of the patients reported participating in “some” PA, and 18.9% of the sample reported no regular PA participation at all.
Table 3.
Comparison of Current and Past Physical Activity Participation by Steatosis Grade
| All (n = 37) | Mild Steatosis (n = 11) | Moderate Steatosis (n = 14) | Severe Steatosis (n = 12) | |
|---|---|---|---|---|
| Current PA participation | ||||
| Self-report | ||||
| Active n (%) | 7 (18.9) | 2 (18.2) | 1 (7.1) | 4 (33.3) |
| Some activity n (%) | 23 (62.2) | 6 (54.5) | 10 (71.4) | 7 (58.3) |
| Inactive n (%) | 7 (18.9) | 3 (27.3) | 3 (21.4) | 1 (8.3) |
| Past 10yr PA participation | ||||
| Average MET intensity | 4.6 ± 1.7 | 3.5 ± 2.4a,c | 5.2 ± 1.1 | 5.1 ± 1.0 |
| Total leisure time PA (hours/week) | 15.5 ± 9.5 | 14.3 ± 12.1 | 16.1 ± 6.6 | 15.5 ± 9.5 |
| Low-intensity (<4.5 METs) | 11.6 ± 7.5 | 10.4 ± 9.1 | 11.3 ± 6.8 | 12.9 ± 6.9 |
| Moderate + vigorous (>4.5 METs) | 4.0 ± 4.2 | 3.9 ± 5.1 | 4.9 ± 4.4 | 3.0 ± 2.8 |
Values are mean ± SD.
Abbreviations: PA, physical activity; MET, metabolic rate or energy expenditure.
Mild versus moderate, P < 0.05.
Moderate versus severe, P < 0.05.
Mild versus severe, P < 0.05.
Past physical activity participation
A significant difference was found in average MET intensity (activity effort) on the Historical Physical Activity Survey questionnaire (F2,34 = 4.303, P = 0.022) among the groups. The mild steatosis group demonstrated a lower average activity effort as compared with the moderate (P = 0.030) and severe (P = 0.049) groups. No differences were detected in the total average hours per week of physical activity participation or in either the time spent in low or moderate to vigorous physical activities. Approximately 74% of the reported activity was classified as low-intensity activities (<4.5 METs) and 26% as moderate or vigorous-intensity activities (>4.5 METs).
Secondary Analysis 1: Comparison by Fibrosis Groups
The demographic characteristics of Fibrosis1 (n = 25) versus Fibrosis2 (n = 12) were comparable. The only difference in laboratory measurements was a higher AST in the Fibrosis2 group (93.1 ± 56.5 U/L) as compared with Fibrosis1 (55.4 ± 24.2 U/L; P = 0.046). More extensive hepatocellular ballooning (1.3 ± 0.49 versus 0.76 ± 0.66; P = 0.012), higher NAS scores (5.3 ± 1.3 versus 4.1 ± 1.6; P = 0.032), and a greater percent of NASH (100% versus 72%, P = 0.042) was found in Fibrosis2 versus Fibrosis1.
There were no differences detected in CRF, muscle strength, or the current or past (10 year) PA participation assessed between the 2 fibrosis groups. The only body composition difference between the 2 groups was the dual energy x-ray absorptiometry waist-to-hip fat measure, (android:gynoid ratio), which was significantly greater in the Fibrosis1 group (1.24 ± 0.18 versus 1.11 ± 0.16; P = 0.038).
Secondary Analysis 2: Comparison by NAFLD Activity Score
Demographic variables were similar between the 2 NAS groups (NAS1, n = 15 versus NAS2, n = 22). AST was significantly higher in the NAS2 (97.1 ± 50.5 versus 83.3 ± 51.9 U/L; P = 0.046), and histology affirmed the NAS2 group to have greater steatosis (P < 0.001), fibrosis (P = 0.022), lobular inflammation (P < 0.001), hepatocellular ballooning (P = 0.014), and a greater percentage of NASH diagnoses (100% versus 53%, P < 0.001). No differences were detected in muscle strength, body composition, or current or past (10 years) PA participation assessed between the 2 NAS groups. However, VO2 peak was significantly higher (P = 0.013) in the NAS1 group (30.4 ± 8.2 mL/kg/min) versus the NAS2 group (24.4 ± 5.7 mL/kg/min) (Fig. 1).
Fig. 1.
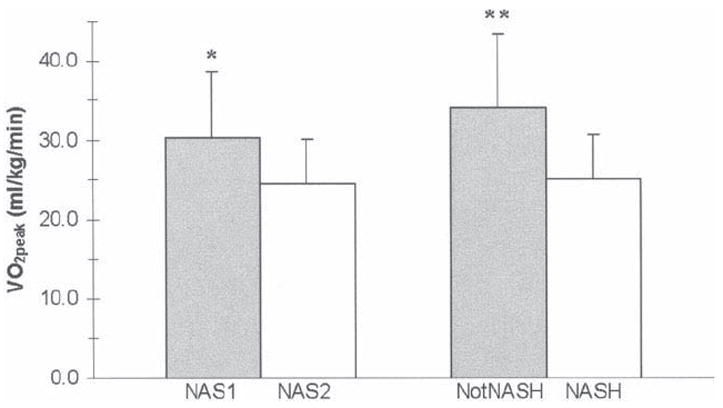
Cardiorespiratory fitness assessed by peak oxygen consumption (VO2peak) according to NAFLD activity score (NAS1 = NAS ≤ 4, NAS2 = NAS ≥ 5) and disease severity (NASH diagnosis). *P < 0.05 compared to NAS2; **P < 0.05 compared to NASH.
Secondary Analysis 3: Comparison by NASH Diagnosis
Demographic and laboratory values were similar between the 2 NASH groups (NASH versus NotNASH), and lobular inflammation, fibrosis, and a higher NAS were more common in the NASH group (P ≤ 0.001). No differences were detected in muscle strength, body composition, or current PA between the 2 NASH groups. Peak VO2 was significantly higher (P = 0.048) in the NotNASH group (34.0 ± 9.5 mL/kg/min) versus the NASH group (25.1 ± 5.7 mL/kg/min) (Fig. 1). Time spent in moderate to vigorous PA over the past 10 years was greater (P = 0.005) for the NotNASH group (7.8 ± 5.1 hours/week) as compared with the NASH group (3.1 ± 3.4 hours/week).
Additional analyses (that is, lobular inflammation and ballooning versus HRF) were also performed, and no statistically significant differences were detected between these dichotomized groups for any of the HRF variables.
Health-Related Fitness and Physical Activity Participation Among Activity Groups
A significant difference was found in peak torque per body weight (F2,33 = 3.953, P = 0.029) among the 3 activity groups (active, some activity, inactive). The active group demonstrated greater peak torque per body weight (P = 0.028) as compared with the inactive group. Similarly, a significant difference was found in %fat (F2,34 = 3.619, P = 0.038) among the 3 activity groups. The active group had lower %fat (P = 0.033) as compared with the inactive group. In addition, a difference was detected in average 10-year METs (F2,34 = 3.480, P = 0.042) among the 3 activity groups was found. The active group participated in higher MET activities (P = 0.039) over the past 10 years than the inactive group.
Discussion
This study is the first to define and compare health-related fitness (CRF, body composition, muscle strength) and PA participation in patients with a histological spectrum of NAFLD using objective, scientifically valid assessments. Although we did not detect significant differences among the individual steatosis and fibrosis severity groups in the components of HRF or PA participation, we did find worse CRF associated with increasing NAFLD activity and with disease severity by NASH diagnosis. The consistent trend for both of these measures is suggestive of a true association. Additionally we found that patients across a spectrum of NAFLD severity have suboptimal CRF, muscle strength, body composition, and PA participation.
Cardiorespiratory fitness, assessed by VO2peak, has been shown to have an inverse relationship with the prevalence of metabolic syndrome.15 Age-adjusted VO2peak values of less than 29.1 mL/kg/min in men were shown to have a 6.4-fold greater likelihood of having metabolic syndrome than those with values ≥35.5 mL/kg/min.15 Conversely, VO2peak ≥ 38.8 mL/kg/min in men has been shown to attenuate the metabolic syndrome profile and associated all-cause and cardiovascular disease mortality.16 A similar attenuation of all-cause mortality has also been demonstrated in women with CRF greater than 39.9 mL/kg/min.31 Our study revealed no differences in VO2peak among the steatosis or fibrosis severity groups, but did demonstrate higher CRF in the NAS1 and the NotNASH groups as compared with the NAS2 and NASH groups, respectively. This provocative finding raises the question of a cause-or-effect phenomenon: Does CRF attenuate NAFLD or does increasing NAFLD severity result in a decline in CRF?
The mean VO2peak of 26.8 ± 7.4 mL/kg/min falls below sedentary age-predicted norms (for both men and women) and is in the increased risk category of metabolic syndrome. Only one other study by Church et al.17 has reported CRF in NAFLD patients (n = 24), albeit with higher values (mean = 34.6 ± 7.4 mL/kg/min) than we observed, but the VO2peak was estimated from treadmill walking time, not directly measured. Importantly, their study population was defined by surrogate markers of NAFLD such as alanine aminotransferase and imaging that are now typically considered lacking in specificity and sensitivity.
Associations between muscle strength and the metabolic syndrome have been reported.13 A study of 8570 men aged 20 to 75 years found a significant inverse correlation between age-specific muscular strength score adjusted for body weight and the prevalence of metabolic syndrome, which remained significant even after adjustment for CRF. Our study is the first to document the muscle strength of patients with NAFLD. Mean quadricep peak torque adjusted for body weight for all subjects was below a normative value range (women, 58%; men, 77%).
Our combined incidence of overweight and obese patients with NAFLD (84%) falls in the range reported by others (73%-93%).32-34 Body composition assessment that quantifies percent fat provides important metabolic information. Approximately 97% of our total sample met the %fat criteria for being at increased risk of cardiovascular disease and diabetes (>25% in men, >30% women).30 Only one other study35 has reported the %fat of NAFLD patients of 35.3% ± 13%, similar to the 38.6% ± 8.2% we report.
Physical inactivity is an important factor in the development of obesity, cardiovascular disease, and type 2 diabetes mellitus.36 The current study assessed both current and 10-year past PA participation. Fewer than 20% of our sample reported currently participating in regular PA that would classify them as “active.” Furthermore, approximately 20% reported participating in absolutely no leisure-time PA. These data may help explain the low CRF and muscle strength, as well as the high %fat observed in this group. This study is the first to examine the historical (10-year) physical activity participation of NAFLD patients. Surprisingly, our data revealed that the mild steatosis group reported significantly lower average intensity of activity (MET intensity) than the moderate or severe steatosis groups over the past 10 years. This finding may be attributable to recall error.
Two other studies have examined the relationship between recent PA participation and NAFLD. Perseghin and colleagues18 compared PA participation with intrahepatic fat by 1H magnetic resonance spectroscopy and demonstrated a significantly higher PA index total in normal controls as compared with the fatty liver group. In addition, they found that intrahepatic fat content was lowest in the most physically active quartile of the total sample (P < 0.001). Another study reported the estimated energy expenditure from a 7-day PA recall questionnaire and showed higher values in those with low global NASH scores compared with those with high global NASH scores.37 These studies suggest the beneficial impact of PA participation on NAFLD. Differences in results between our study and these others may lie in measurement tools used or classification of NAFLD.
Our study is arguably limited by several aspects of design. Based on our sample size, our results may not be generalizable to all NAFLD patients. However, this study is the first to examine a spectrum of patients, with outcomes based on strict histological criteria. And, although the possible role of sampling variability of the biopsy may affect interpretation, the impact of sampling error appears to be small in the assessment of steatosis and disease activity by NAS.38 Although there was a relationship between the presence of NASH and HRF, the lack of similar findings with the severity of both steatosis and fibrosis is difficult to explain. Such a relationship may exist, but may not have emerged in such a sample or a sample containing few patients with severe disease. Though our finding of low HRF in subjects with NASH could be related to concomitant comorbidities, we did not find a difference in the presence of metabolic syndrome between the NASH groups, suggesting that controlling for these comorbidities likely would not alter the findings. Lastly, in terms of our PA participation data, we cannot rule out recall errors and bias. However, the Historical Physical Activity Survey was interviewer-administered, and we used cognitive interviewing techniques (memory cues and think-aloud techniques) to improve recall.
In summary, this study demonstrates suboptimal HRF and PA participation of patients with NAFLD across a histological spectrum of disease. Despite our study limitations, we believe the objective demonstration of low CRF and muscle strength with a high incidence of obesity illustrates the potential clinical relevance of these measurements both before and after interventions. The finding that severity of NAS and a diagnosis of NASH may be associated with CRF and past PA raises the possibility of a defined therapeutic role for prevention and exercise intervention. Future studies should include a randomized controlled trial of exercise training that is well defined, monitored, and precisely quantified to elucidate the direct effects on NAFLD histopathology. In the meantime, it would appear rational and prudent for healthcare providers to recommend exercise training to improve health-related fitness as an integral role in the care of patients with NAFLD.
Acknowledgments
This study was carried out in part in the General Clinical Research Center, Moffitt Hospital, University of California, San Francisco, with funds provided by the National Center for Research Resources (NCRR), MO1RR-0079 and UL 1 RR024131-01, a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. This study was also supported in part as an ancillary study of the NIH Nonalcoholic Steatohepatitis (NASH) Clinical Research Network, U01 DK61738 (N.M.B.). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Abbreviations
- AST
aspartate aminotransferase
- CRF
cardiorespiratory fitness
- HOMA
homeostasis model assessment
- HRF
health-related fitness
- MET
metabolic equivalent
- NAFLD
nonalcoholic fatty liver disease
- NAS
NAFLD activity score
- NASH
nonalcoholic steatohepatitis
- PA
physical activity
- VO2peak
peak oxygen uptake
Footnotes
Potential conflict of interest: Nothing to report.
References
- 1.Farrell G, George J, Hall PM, McCullough AJ. Overview: an introduction to NASH and related fatty liver diorders. In: Farrell GC, George J, Hall PM, McCullough AJ, editors. Fatty Liver Disease: NASH and Related Disorders. Malden, MA: Blackwell Publishing; 2005. p. 23. [Google Scholar]
- 2.Lonardo A, Bellini M, Tartoni P, Tondelli E. The bright liver syndrome: prevalence and determinants of a “bright” liver echopattern. Ital J Gastroenterol Hepatol. 1997;29:351. [PubMed] [Google Scholar]
- 3.Jimba S, Nakagami T, Takahashi M, Wakamatsu T, Hirota Y, Iwamoto Y, et al. Prevalence of non-alcoholic fatty liver disease and its association with impaired glucose metabolism in Japanese adults. Diabet Med. 2005;22:1141. doi: 10.1111/j.1464-5491.2005.01582.x. [DOI] [PubMed] [Google Scholar]
- 4.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- 5.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 6.Choudhury J, Sanyal AJ. Insulin resistance and the pathogenesis of nonalcoholic fatty liver disease. Clin Liver Dis. 2004;8:575. doi: 10.1016/j.cld.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Schaffler A, Scholmerich J, Buchler C. Mechanisms of disease: adipocytokines and visceral adipose tissue: emerging role in nonalcoholic fatty liver disease. Nat Clin Pract Gastroenterol Hepatol. 2005;2:273. doi: 10.1038/ncpgasthep0186. [DOI] [PubMed] [Google Scholar]
- 8.Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450. doi: 10.1016/s0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- 9.Assy N, Kaita K, Mymin D, Levy C, Rosser B, Minuk G. Fatty infiltration of liver in hyperlipidemic patients. Dig Dis Sci. 2000;45:1929. doi: 10.1023/a:1005661516165. [DOI] [PubMed] [Google Scholar]
- 10.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 11.US Department of Health and Human Services. Physical activity and health: a report of the Surgeon General. Atlanta: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; 1996. [Google Scholar]
- 12.Irwin ML, Ainsworth BE, Mayer-Davis EJ, Addy CL, Pate RR, Durstine JL. Physical activity and the metabolic syndrome in a tri-ethnic sample of women. Obes Res. 2002;10:1030. doi: 10.1038/oby.2002.140. [DOI] [PubMed] [Google Scholar]
- 13.Jurca R, Lamonte MJ, Church TS, Earnest CP, Fitzgerald SJ, Barlow CE, et al. Associations of muscle strength and fitness with metabolic syndrome in men. Med Sci Sports Exerc. 2004;36:1301. doi: 10.1249/01.mss.0000135780.88930.a9. [DOI] [PubMed] [Google Scholar]
- 14.Farrell SW, Cheng YJ, Blair SN. Prevalence of the metabolic syndrome across cardiorespiratory fitness levels in women. Obes Res. 2004;12:824. doi: 10.1038/oby.2004.99. [DOI] [PubMed] [Google Scholar]
- 15.Lakka TA, Laaksonen DE, Lakka HM, Männikkö N, Niskanen LK, Rauramaa R, et al. Sedentary lifestyle, poor cardiorespiratory fitness, and the metabolic syndrome. Med Sci Sports Exerc. 2003;35:1279. doi: 10.1249/01.MSS.0000079076.74931.9A. [DOI] [PubMed] [Google Scholar]
- 16.Katzmarzyk PT, Church TS, Blair SN. Cardiorespiratory fitness attenuates the effects of the metabolic syndrome on all-cause and cardiovascular disease mortality in men. Arch Intern Med. 2004;164:1092. doi: 10.1001/archinte.164.10.1092. [DOI] [PubMed] [Google Scholar]
- 17.Church TS, Kuk JL, Ross R, Priest EL, Biltoff E, Blair SN. Association of cardiorespiratory fitness, body mass index, and waist circumference to nonalcoholic fatty liver disease. Gastroenterology. 2006;130:2023. doi: 10.1053/j.gastro.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 18.Perseghin G, Lattuada G, De Cobelli F, Ragogna F, Ntali G, Esposito A, et al. Habitual physical activity is associated with intrahepatic fat content in humans. Diabetes Care. 2007;30:683. doi: 10.2337/dc06-2032. [DOI] [PubMed] [Google Scholar]
- 19.American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. 7. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 20.Gibbons RJ, Balady GJ, Beasley JW, Bricker JT, Duvernoy WF, Froelicher VF, et al. ACC/AHA Guidelines for Exercise Testing. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing) J Am Coll Cardiol. 1997;30:260. doi: 10.1016/s0735-1097(97)00150-2. [DOI] [PubMed] [Google Scholar]
- 21.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 22.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 23.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 24.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 25.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21:2191. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- 26.Borg G, Linderholm H. Perceived exertion and pulse rate during graded exercise in various age groups. Acta Med Scand. 1967;(Suppl 472):194. [Google Scholar]
- 27.Bruce RA, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J. 1973;85:546. doi: 10.1016/0002-8703(73)90502-4. [DOI] [PubMed] [Google Scholar]
- 28.Akima H, Kano Y, Enomoto Y, Ishizu M, Okada M, Oishi Y, et al. Muscle function in 164 men and women aged 20-84 yr. Med Sci Sports Exerc. 2001;33:220. doi: 10.1097/00005768-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Kriska AM, Sandler RB, Cauley JA, LaPorte RE, Hom DL, Pambianco G. The assessment of historical physical activity and its relation to adult bone parameters. Am J Epidemiol. 1988;127:1053. doi: 10.1093/oxfordjournals.aje.a114881. [DOI] [PubMed] [Google Scholar]
- 30.Blair SN, Brodney S. Effects of physical inactivity and obesity on morbidity and mortality: current evidence and research issues. Med Sci Sports Exerc. 1999;31:S646. doi: 10.1097/00005768-199911001-00025. [DOI] [PubMed] [Google Scholar]
- 31.Farrell SW, Braun L, Barlow CE, Cheng YJ, Blair SN. The relation of body mass index, cardiorespiratory fitness, and all-cause mortality in women. Obes Res. 2002;10:417. doi: 10.1038/oby.2002.58. [DOI] [PubMed] [Google Scholar]
- 32.Sreenivasa Baba C, Alexander G, Kalyani B, Pandey R, Rastogi S, Pandey A, et al. Effect of exercise and dietary modification on serum aminotransferase levels in patients with nonalcoholic steatohepatitis. J Gastroenterol Hepatol. 2006;21:191. doi: 10.1111/j.1440-1746.2005.04233.x. [DOI] [PubMed] [Google Scholar]
- 33.Rocha R, Cotrim HP, Carvalho FM, Siqueira AC, Braga H, Freitas LA. Body mass index and waist circumference in non-alcoholic fatty liver disease. J Hum Nutr Diet. 2005;18:365. doi: 10.1111/j.1365-277X.2005.00634.x. [DOI] [PubMed] [Google Scholar]
- 34.Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 35.Huang MA, Greenson JK, Chao C, Anderson L, Peterman D, Jacobson J, et al. One-year intense nutritional counseling results in histological improvement in patients with non-alcoholic steatohepatitis: a pilot study. Am J Gastroenterol. 2005;100:1072. doi: 10.1111/j.1572-0241.2005.41334.x. [DOI] [PubMed] [Google Scholar]
- 36.Lees SJ, Booth FW. Physical inactivity is a disease. World Rev Nutr Diet. 2005;95:73. doi: 10.1159/000088274. [DOI] [PubMed] [Google Scholar]
- 37.Kang H, Greenson JK, Omo JT, Chao C, Peterman D, Anderson L, et al. Metabolic syndrome is associated with greater histologic severity, higher carbohydrate, and lower fat diet in patients with NAFLD. Am J Gastroenterol. 2006;101:2247. doi: 10.1111/j.1572-0241.2006.00719.x. [DOI] [PubMed] [Google Scholar]
- 38.Merriman RB, Ferrell LD, Patti MG, Weston SR, Pabst MS, Aouizerat BE, et al. Correlation of paired liver biopsies in morbidly obese patients with suspected nonalcoholic fatty liver disease. Hepatology. 2006;44:874. doi: 10.1002/hep.21346. [DOI] [PubMed] [Google Scholar]


