Abstract
Background
Impairment in instrumental activities of daily living (IADL) leads to early loss in productivity and adds significant burden to caregivers. Executive dysfunction is thought to be an important contributor to functional impairment. The objective of this study was to investigate the relationship between executive function and IADL in a large cohort of well characterized normal older controls (NC), mild cognitive impairment (MCI) and mild Alzheimer’s disease (AD) patients, separately as well as across the entire sample, while accounting for demographic, cognitive, and behavioral factors.
Methods
Subjects with baseline clinical datasets (n=793) from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) study (228 NC, 387 MCI, 178 AD) were included in the analyses. A multiple regression model was used to assess the relationship between executive function and IADL.
Results
A multiple regression model, including diagnosis, global cognitive impairment, memory performance, and other covariates demonstrated a significant relationship between executive dysfunction and IADL impairment across all subjects (R2=0.60, p<0.0001 for model; Digit Symbol, partial β=−0.044, p=0.005; Trailmaking Test B – A, quadratic relation, p=0.01). An analysis using MCI subjects only also yielded a significant relationship (R2=0.16, p<0.0001 for model; Digit Symbol, partial β=−0.08, p=0.001).
Conclusions
These results suggest that executive dysfunction is a key contributor to impairment in IADL. This relationship was evident even after accounting for degree of memory deficit across the continuum of cognitive impairment and dementia.
Keywords: Alzheimer’s disease, executive function, instrumental activities of daily living, memory, mild cognitive impairment
1. Introduction
One of the key clinical features of Alzheimer’s disease (AD) is impairment in daily functioning. Instrumental activities of daily living (IADL) consist of preparing meals, handling the finances, driving or using public transportation, shopping, and many other everyday activities. IADL impairment leads to early loss of independence and the ability to be an active member of society, while shifting many daily responsibilities to caregivers and increasing their burden. IADL impairment in patients with clinical AD has been associated with global pathologic changes and frontal and posterior hypometabolism1–3.
As AD progresses, executive dysfunction becomes more prominent. Executive function consists of complex attention, working memory, verbal and visual organization, planning, judgment, and reasoning. Executive dysfunction and IADL impairment have been shown to predict progression from amnestic mild cognitive impairment (MCI) to clinical AD and are thought to be associated with each other and prefrontal dysfunction4–7. Most studies exploring the relationship between executive function and IADL have focused on either normal aging8, 9 or dementia4, 10. However, only a few studies with small numbers of subjects have explored the relationship between executive function and IADL across the continuum from normal aging to MCI and mild AD11, 12. MCI subjects are a heterogeneous group with variable pathology. Therefore, investigating the relationship between executive function and IADL more closely may be important for identifying subjects with MCI who are likely to progress to clinical AD. Early identification of those MCI subjects who are at risk for AD will allow them to take advantage of early treatment opportunities as they arise. Moreover, it is important to determine what the additional contribution of executive dysfunction is to impairment in IADL, when accounting for memory deficits and global cognitive impairment, as treatments specifically targeting executive dysfunction may also be beneficial in maintaining patient independence.
The objective of this study was to investigate the relationship between executive function and IADL in a large cohort of well characterized subjects, including normal older controls (NC), MCI, and mild AD patients. In particular, we sought to determine the influence of executive function on functional capacity both across the continuum of aging to early dementia, and specifically within the MCI group. We used novel statistical analyses to carefully tease apart the complex relations between IADL and measures of executive function. Moreover, we investigated the influence of several factors that were not all considered in the previous literature, including diagnostic group, age, education, memory performance, global cognitive impairment, depression, and apathy.
2. Methods
2.1. Subjects from ADNI database
Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (www.loni.ucla.edu\ADNI)13. ADNI is a large multi-center, longitudinal, observational trial taking place across North America, in which subjects with normal cognition, amnestic MCI, and mild AD are followed with periodic neuropsychological testing, multiple imaging techniques, and fluid biomarkers. The goals of ADNI are to standardize brain imaging across multiple sites, obtain a large longitudinal dataset for future research, and develop reliable biomarker surrogates for treatment trials. ADNI is the result of efforts of many co-investigators from a broad range of academic institutions and private corporations, and subjects have been recruited from over 50 sites across the U.S. and Canada.
Subjects with complete baseline clinical datasets (n=793) from the ADNI study (228 NC, 387 MCI, 178 AD) were included in the current analysis. Subjects were ages 55–91 (inclusive), in general good health or had stable medical problems at the time of screening, and had a study partner/caregiver able to provide an independent evaluation of the subject’s cognitive, behavioral, and functional status. Subjects had a Modified Hachinski Ischemic Score14 ≤ 4 and a Geriatric Depression Scale15 (GDS, short form) < 6. Subjects did not have other significant neurological conditions, significant active psychiatric disorders, or alcohol or substance abuse within two years of screening. 6 subjects who did not meet the specific ADNI diagnostic classification criteria stated below, but who were still listed in the ADNI database, were excluded from the current analyses.
Subjects were assigned to a diagnostic group (NC, MCI, or AD) by the site investigators at the screening visit and again at the baseline visit. Baseline diagnoses were used in the current analyses because at the end of the baseline visit, the site investigators had the advantage of reviewing the comprehensive neuropsychological testing (assessing memory, attention, executive function, language, and visuospatial function) and additional study partner questionnaires about behavior and daily functioning. In accordance with the ADNI protocol, both quantitative cut-off scores and qualitative clinical assessments were used by site investigators to determine the diagnosis of each subject. Diagnoses were ultimately based on the clinical judgment of the site investigator. The criteria for each diagnostic group are described below.
NC subjects had a global Clinical Dementia Rating scale16 (CDR) score = 0, CDR Memory Box score = 0, Mini-Mental State Examination17 (MMSE) of 25–30 (inclusive), and performance at an objective cut-off of 1.5 standard deviations above education adjusted cut-off scores on the Logical Memory IIa (LM-IIa) of the Weschler Memory Scale-Revised18 (WMS-R) (subjects who had ≥ 16 years of education, required a LM-IIa score > 8; 8–15 years, LM-IIa > 4; 0–7 years, LM-IIa > 2). Moreover, NC subjects had to be deemed cognitively normal based on an absence of significant impairment in cognitive functions or IADL following review of the screening and baseline data by the site investigator.
MCI subjects fulfilled criteria for amnestic MCI (single and multiple domain)19: Non-demented subjects with memory complaint (global CDR score = 0.5, with a Memory Box score ≥ 0.5), MMSE of 24–30 (inclusive), and essentially preserved IADL (there was no specified score on a test of IADL to determine this criterion; it was a qualitative clinical determination made by the investigator at each site). Subjects performed at an objective cut-off of 1.5 standard deviations below education-adjusted norms on the LM-IIa of the WMS-R (subjects who had ≥ 16 years of education, required a LM-IIa score ≤ 8; 8–15 years, LM-IIa ≤ 4; 0–7 years, LM-IIa ≤ 2).
AD subjects met the National Institute of Neurologic and Communicative Disorders and Stroke and the AD and Related Disorders Association Work Group (NINCDS-ADRDA) criteria for probable AD20 with mild dementia severity (global CDR score = 0.5 or 1), MMSE of 20–26 (inclusive), and the same objective cut-off scores on the LM-IIa of the WMS-R as MCI subjects.
The study was approved by the local Institutional Review Boards (IRB) of each participating site. Written informed consent was obtained from all subjects and study partners after all study procedures and risks were thoroughly explained in accordance with local IRB guidelines.
2.2. Clinical assessments
The Functional Activities Questionnaire21 (FAQ) was used to assess IADL impairment (higher scores indicate greater impairment, range 0–30). In one study FAQ scores of ≥ 6 were reported as consistent with functional impairment22; other studies do not provide an established cut-off score for the FAQ.
Trailmaking Test A23 (TMT-A, higher scores indicate greater impairment; range 17–150 seconds; 17 seconds was the lowest score obtained in this analysis; if subjects were not able to complete the task after 150 seconds, it was discontinued, and they were assigned a score of 150 seconds), Trailmaking Test B23 (TMT-B, higher scores indicate greater impairment; range 34–300 seconds; 34 seconds was the lowest score obtained in this analysis; if subjects were not able to complete the task after 300 seconds, it was discontinued, and they were assigned a score of 300 seconds), and the Wechsler Adult Intelligence Scale-Revised (WAIS-R) Digit Symbol24 (DSym, lower scores indicate greater impairment, possible range 0–110; in the current sample the highest score was 80) were used to assess executive function. TMT-A and TMT-B both depend on processing speed and visuomotor and perceptual-scanning skills, but TMT-B also requires considerable cognitive flexibility in shifting from number to letter sets under time pressure. DSym depends on multiple cognitive abilities including attention, psychomotor speed, complex scanning, visual tracking, and immediate memory. These tests were selected from the ADNI neuropsychological battery because they have been well-validated and have a wide range of scores across the continuum of normal aging to mild AD. Prior studies have used these tests, as well as other tests to assess the relationship between executive function and IADL8, 10, 12, 25, 26. For the analyses of the current study we used DSym and a difference score between TMT-B and TMT-A (TMT-B minus TMT-A, TMT-B-A), which corrects for processing speed and visuomotor scanning, resulting in a purer executive function measure of set shifting27.
Other assessments relevant to this analysis included the MMSE (a measure of global cognitive function; range 20–30 in the current analysis, but 0–30 in general; lower scores indicate greater impairment), the Rey Auditory Verbal Learning Test28 (RAVLT) 30 minute delayed recall task (a measure of memory performance; range 0–15; lower scores indicate greater impairment), GDS short form (a measure of depression; range 0–5 in the current analysis, but 0–15 in general; higher scores indicate greater impairment), the Neuropsychiatric Inventory brief questionnaire form29 (NPI-Q) Depression item (a measure of depression; range 0–3; higher scores indicate greater impairment), and the NPI-Q Apathy item (a measure of apathy; range 0–3; higher scores indicate greater impairment).
2.3. Data analysis
For our primary analysis, a linear and curvilinear (quadratic) multiple regression analysis (general linear model) was conducted using all subjects with FAQ as the dependent variable and the following predictors: diagnostic group, linear and quadratic terms for the measures of executive function (TMT-B-A (a difference score between TMT-B and TMT-A), DSym), for age, for education, for global cognitive impairment (MMSE), for memory performance (RAVLT delayed recall), for measures of depression (GDS, NPI-Q Depression), and for apathy (NPI-Q Apathy), and also the interaction of diagnosis with the linear/quadratic terms for these predictors. Starting with a saturated model with all predictive terms, the most non-significant terms were progressively removed, one at a time, in a backward elimination strategy until only individually significant predictors remained in the model. (Non-significant lower order counterparts of significant higher order terms were allowed to remain, e.g., a non-significant linear term corresponding to a significant quadratic predictor was retained.)
Two analyses with MCI subjects only were then performed. In the first analysis, a linear multiple regression analysis was conducted across MCI subjects with FAQ as the dependent variable and the following simultaneous predictors: executive function (TMT-B-A, DSym), age, education, MMSE, RAVLT delayed recall, GDS, NPI-Q Depression, and NPI-Q Apathy.
The second analysis was performed using a clinically relevant dichotomy between MCI subjects considered to be with and without executive dysfunction. All ADNI MCI subjects were required to meet amnestic MCI criteria. For this analysis, MCI subjects were then divided into subjects with impaired memory and executive dysfunction (MCI Executive) and subjects with impaired memory and no executive dysfunction (MCI Non-Executive). MCI Executive subjects performed at an objective cut-off of 1.5 standard deviations below reported normative means for TMT-B and DSym. The norms used were from the Uniform Data Set of the Alzheimer’s Disease Centers30, which has a similar population to ADNI. The relationship between FAQ and MCI Executive and MCI Non-Executive subjects was determined using independent samples t-test. Finally, a linear multiple regression analysis was conducted with FAQ as the dependent variable and the following simultaneous predictors: MCI Executive vs. MCI Non-Executive status, age, education, MMSE, RAVLT delayed recall, GDS, NPI-Q Depression, and NPI-Q Apathy.
3. Results
Table 1 provides the demographics for all subjects as a combined group, as well as the three diagnostic groups separately. As expected, there were significant differences in MMSE, RAVLT delayed recall, NPI-Q Depression item, NPI-Q Apathy item, FAQ, TMT-A, TMT-B, TMT-B-A, and DSym between NC, MCI, and AD groups. Additionally, age was greater in NC compared to MCI subjects (t=2.13, p=0.03). There was a significantly greater proportion of male subjects in the MCI group when compared to the NC and AD groups (omnibus chi-square test p=0.002; MCI vs. NC: p=0.003; MCI vs. AD: p=0.007). Mean years of education were less in the AD group when compared to the NC and MCI groups (AD vs. NC: t=4.62, p<0.0001; AD vs. MCI: t=3.89, p=0.0001). Table 2 provides correlations between FAQ (the dependent variable) and each of the predictors in the model across the entire sample and within each diagnostic group.
Table 1.
Demographics of subjects.
| Group | All subjects | NC | MCI | AD |
|---|---|---|---|---|
|
n |
793 | 228 | 387 | 178 |
|
Age |
75.4±6.8‡‡ | 76.0±5.0 | 75.0±7.4 | 75.6±7.4 |
|
Sex (% male) |
58.3‡ | 52.2 | 64.6 | 52.2 |
|
Education |
15.6±3.0†† | 16.1±2.9 | 15.7±3.0 | 14.6±3.2 |
|
MMSE |
26.8±2.6* | 29.1±1.0 | 27.1±1.8 | 23.4±2.0 |
|
RAVLT delayed recall |
3.7±4.0* | 7.4±3.7 | 2.9±3.3 | 0.7±1.6 |
|
GDS |
1.4±1.4† | 0.8±1.1 | 1.6±1.4 | 1.6±1.4 |
|
NPI-Q Depression |
0.2±0.5** | 0.1±0.3 | 0.2±0.5 | 0.4±0.6 |
|
NPI-Q Apathy |
0.2±0.6* | 0.01±0.1 | 0.2±0.5 | 0.5±0.8 |
|
FAQ |
4.8±6.4* | 0.1±0.6 | 3.8±4.4 | 12.7±6.7 |
|
TMT-A |
46.6±25.5* | 36.3±13.0 | 44.2±21.7 | 64.8±34.5 |
|
TMT-B |
134.5±80.2* | 89.3±44.3 | 130.8±73.2 | 200.5±86.6 |
|
TMT-B-A |
88.0±66.9* | 53.0±38.8 | 86.6±63.1 | 135.8±74.3 |
| DSym | 37.4±12.9* | 45.8±10.2 | 37.0±11.1 | 27.6±12.5 |
AD (Alzheimer’s disease), DSym (Digit Symbol), FAQ (Functional Activities Questionnaire), GDS (Geriatric Depression Scale), MCI (mild cognitive impairment), MMSE (Mini-Mental State Examination), NC (normal control), NPI-Q Apathy (Neuropsychiatric Inventory brief questionnaire form, Apathy item), NPI-Q Depression (Neuropsychiatric Inventory brief questionnaire form, Depression item), RAVLT (Rey Auditory Verbal Learning Test), TMT-A (Trailmaking Test A), TMT-B (Trailmaking Test B), TMT-B-A (Trailmaking Test B minus Trailmaking Test A).
All values represent mean ± standard deviation (except n and sex).
p<0.0001 for NC vs. MCI, NC vs. AD, and MCI vs. AD.
p<0.005 for NC vs. MCI, NC vs. AD, and MCI vs. AD.
p<0.0001 for NC vs. MCI and NC vs. AD.
p<0.005 for NC vs. AD and MCI vs. AD.
p<0.01 for NC vs. MCI and MCI vs. AD.
p<0.05 for NC vs. MCI.
Table 2.
Correlations between FAQ and predictors.
| Group | All subjects (n=793) |
NC (n=228) |
MCI (n=387) |
AD (n= 178) |
||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
|
Age |
0.02 | 0.64 | 0.08 | 0.23 | 0.002 | 0.97 | 0.07 | 0.33 |
|
Education |
−0.11 | 0.002 | 0.02 | 0.75 | 0.02 | 0.68 | 0.03 | 0.74 |
|
MMSE |
−0.63 | <0.0001 | −0.02 | 0.77 | −0.05 | 0.33 | −0.33 | <0.0001 |
|
RAVLT delayed recall |
−0.46 | <0.0001 | −0.01 | 0.85 | −0.22 | <0.0001 | −0.12 | 0.11 |
|
GDS |
0.13 | 0.0001 | 0.08 | 0.21 | 0.02 | 0.62 | 0.01 | 0.92 |
|
NPI-Q Depression |
0.23 | <0.0001 | 0.02 | 0.73 | 0.20 | <0.0001 | −0.02 | 0.81 |
|
NPI-Q Apathy |
0.38 | <0.0001 | 0.10 | 0.13 | 0.23 | <0.0001 | 0.29 | <0.0001 |
|
TMT-A |
0.39 | <0.0001 | 0.11 | 0.11 | 0.18 | 0.0003 | 0.15 | 0.05 |
|
TMT-B |
0.44 | <0.0001 | 0.05 | 0.47 | 0.17 | 0.0006 | 0.13 | 0.07 |
|
TMT-B-A |
0.38 | <0.0001 | 0.02 | 0.77 | 0.14 | 0.006 | 0.09 | 0.25 |
| DSym | −0.45 | <0.0001 | −0.10 | 0.12 | −0.22 | <0.0001 | −0.15 | 0.04 |
AD (Alzheimer’s disease), DSym (Digit Symbol), FAQ (Functional Activities Questionnaire), GDS (Geriatric Depression Scale), MCI (mild cognitive impairment), MMSE (Mini-Mental State Examination), NC (normal control), NPI-Q Apathy (Neuropsychiatric Inventory brief questionnaire form, Apathy item), NPI-Q Depression (Neuropsychiatric Inventory brief questionnaire form, Depression item), RAVLT (Rey Auditory Verbal Learning Test), TMT-A (Trailmaking Test A), TMT-B (Trailmaking Test B), TMT-B-A (Trailmaking Test B minus Trailmaking Test A).
Across the sample as a whole, there were significant negative correlations between TMT-B and DSym (r=−0.71, p<0.0001) and TMT-B-A and DSym (r=−0.60, p<0.0001), consistent with greater executive dysfunction being represented by higher scores on TMT-B and TMT-B-A and lower scores on DSym.
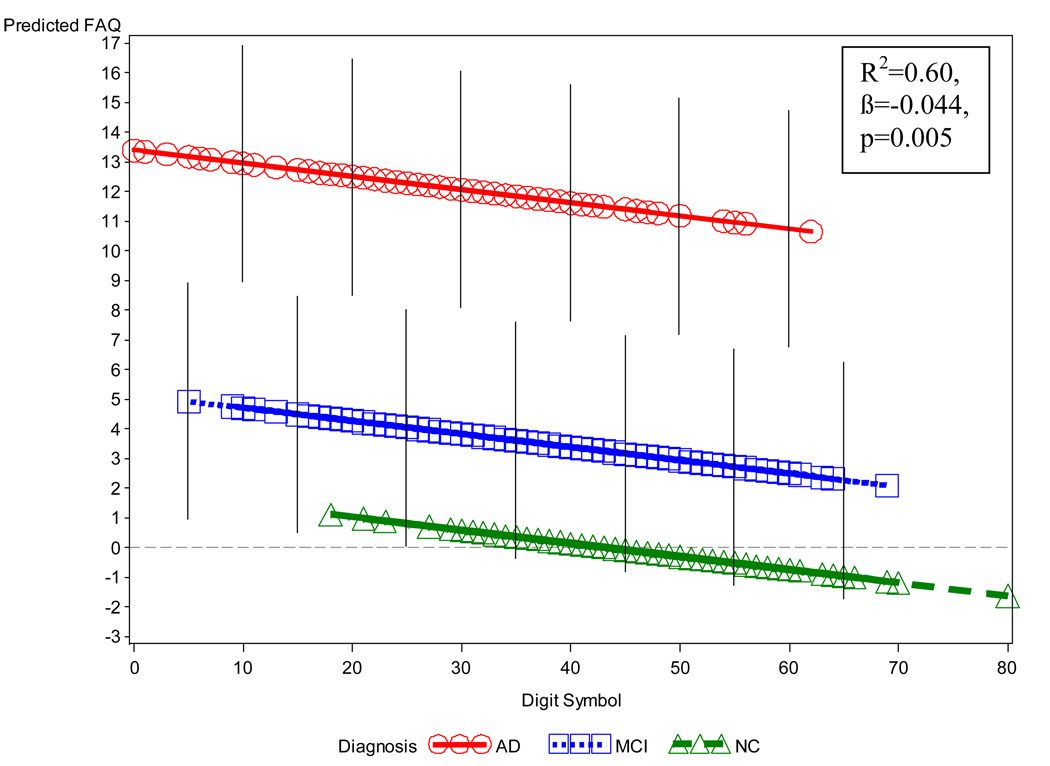
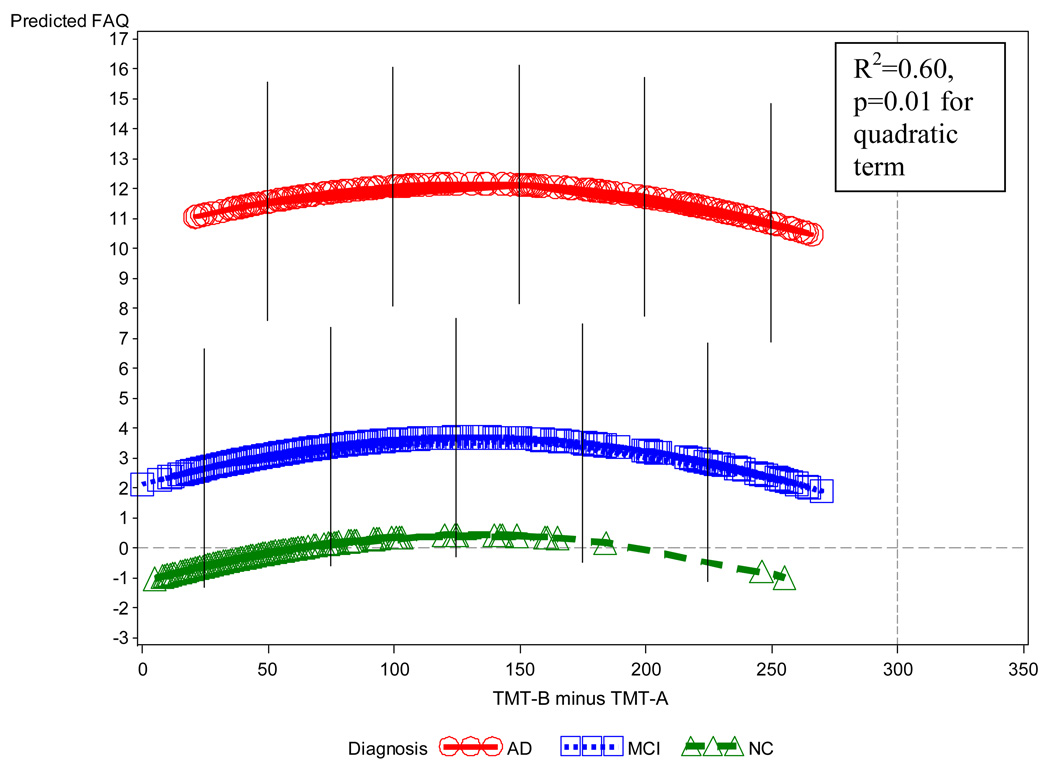
In the regression model for all subjects, after backward elimination, a significant (p<0.0001) overall regression model emerged, accounting for 60% of the variance of FAQ. Individually significant (p≤0.05) terms retained in this model showed relations generally consistent with predictions: (1) an expected main effect for diagnosis in which the AD group had overall more highly elevated FAQ scores (greater IADL impairment) than the MCI group, which in turn had higher scores than those for the NC group; (2) a negative linear relation for DSym within each diagnostic group (unstandardized partial regression coefficient (β)=−0.044, p=0.005, 95% confidence interval (CI) for β=−0.075, −0.013), i.e., lower DSym scores (greater executive dysfunction) were associated with higher FAQ scores (greater IADL impairment) (see Figure 1); (3) a quadratic relation for TMT-B-A within diagnosis in which FAQ scores increased (greater IADL impairment) with increasing TMT-B-A scores (greater executive dysfunction) up to a maximum at about TMT-B-A equal to 150 and then declining (p=0.01 for quadratic term) (see Figure 2); (4) a quadratic interaction of age and diagnosis in which age showed a U-shaped relation to FAQ within the AD group with a minimum value at approximately 75 years, but a flat relation for the other diagnostic groups; (5) a negative linear relation of MMSE within the AD group (an interaction with diagnosis), i.e., lower MMSE scores (greater global cognitive impairment) were associated with higher FAQ scores (greater IADL impairment) for AD (a flat line for the other groups); (6) a curvilinear relation of RAVLT delayed recall with FAQ mostly indicating that lower RAVLT scores (greater memory impairment) were associated with higher FAQ scores (greater IADL impairment); and (7) a curvilinear relation of NPI-Q Apathy item scores indicating that greater apathy was associated with acceleratingly higher FAQ scores (greater IADL impairment). (The distribution of the residuals from the regression model above was bell shaped in conformance to assumptions of significance tests.)
Figure 1.
Predicted values of FAQ vs. diagnostic group and DSym from the multiple regression model, which also included TMT-B-A, age, MMSE, curvilinear terms for RAVLT delayed recall and NPI-Q Apathy item, and an interaction of MMSE with diagnostic group. In this graph, other variables (age, MMSE, RAVLT, NPI-Q Apathy, TMT-B-A) are held constant at their grand mean values. The local density of the actual values is indicated by the density of symbols. Error bars indicate the root mean square above and below the predicted value (not shown for NC because of floor effect). Note the negative relation of DSym to FAQ within diagnostic groups, additive to the level effects of the diagnostic groups themselves. AD (Alzheimer’s disease), DSym (Digit Symbol), FAQ (Functional Activities Questionnaire), MCI (mild cognitive impairment), MMSE (Mini-Mental State Examination), NC (normal control), NPI-Q Apathy (Neuropsychiatric Inventory Questionnaire brief form, apathy item), RAVLT (Rey Auditory Verbal Learning Test), TMT-B-A (Trailmaking Test B minus Trailmaking Test A).
Figure 2.
Predicted values of FAQ vs. diagnostic group and TMT-B-A from the multiple regression model, which also included DSym, age, MMSE, curvilinear terms for RAVLT delayed recall and NPI-Q Apathy item, and an interaction of MMSE with diagnostic group. In this graph, other variables (age, MMSE, RAVLT, NPI-Q Apathy, DSym) are held constant at their grand mean values. The local density of the actual values is indicated by the density of symbols. Error bars indicate the root mean square above and below the predicted value (not shown for NC because of floor effect). Note the relation of TMT-B-A to FAQ within diagnostic groups, additive to the level effects of the diagnostic groups themselves. AD (Alzheimer’s disease), DSym (Digit Symbol), FAQ (Functional Activities Questionnaire), MCI (mild cognitive impairment), MMSE (Mini-Mental State Examination), NC (normal control), NPI-Q Apathy (Neuropsychiatric Inventory Questionnaire brief form, apathy item), RAVLT (Rey Auditory Verbal Learning Test), TMT-B-A (Trailmaking Test B minus Trailmaking Test A).
The downward bend in the relation of TMT-B-A to FAQ at high TMT-B-A scores (see Figure 2), was largely reflective of a proportionally large number of subjects with assigned ceiling scores on TMT-B (because they exceeded the time limit of 300 seconds) while having floor or relatively low scores on FAQ, a phenomenon which was especially common in the MCI group. This “bending” may be a subject selection artifact, i.e., subjects who were at ceiling on TMT-B and also scored poorly (high) on FAQ, may have been too impaired to be included in the MCI group, and perhaps even too demented to be included in ADNI, thus resulting in an over-representation in the poor (high) TMT-B / good (low) FAQ region; ergo the observed “bend”.
In terms of effect sizes, all terms involving diagnosis uniquely accounted for about 10% of the variance in FAQ, whereas all other significant terms each contributed only a few or a fraction of a percentage point. (The relatively large effect size for diagnosis is evident in the wide separation of diagnostic groups seen in Figure 1 and Figure 2). The remaining portion of the 60% of variance accounted for in FAQ is confounded covariation of the correlated significant predictors, which cannot be disentangled statistically but in unison predict a substantial portion of the FAQ variance.
In the first analysis of MCI subjects only employing the linear multiple regression model, including age, education, MMSE, RAVLT, GDS, NPI-Q Depression item, and NPI-Q Apathy item, a significant partialed negative linear relationship between IADL impairment and lower executive function (DSym) was seen (R2=0.16, p<0.0001 for the overall model; DSym β=−0.08, p=0.001; 95% CI for β=−0.13, −0.03). There was no significant relationship between IADL impairment and TMT-B-A, while there was a significant relationship between IADL impairment and worsening memory (lower RAVLT delayed recall scores), worsening apathy (higher NPI-Q Apathy scores), and worsening depression (higher NPI-Q Depression scores).
The second analysis dividing the amnestic MCI subjects based on the level of executive function impairment resulted in 83 (21.4%) subjects classified as MCI Executive and 304 (78.6%) as MCI Non-Executive. Table 3 provides the demographics for the two MCI subgroups. MCI Executive subjects, who had memory impairment and executive dysfunction, had significantly greater IADL impairment than MCI Non-Executive subjects, who had memory impairment and no executive dysfunction (t=2.99, p=0.003). The multiple regression model, including age, education, MMSE, RAVLT, GDS, NPI-Q Depression item, and NPI-Q Apathy item also demonstrated a significant partialed relationship between IADL impairment and the MCI subgroups (R2=0.15, p<0.0001 for the model; MCI subgroup β=−1.50, p=0.005; 95% CI for β=−2.56, −0.45; effect size (Cohen’s “d”31)=0.40).
Table 3.
Demographics of MCI subgroups. MCI Executive subjects have both memory impairment and executive dysfunction, while MCI Non-Executive subjects have memory impairment but no executive dysfunction.
| Group | All MCI | MCI Executive | MCI Non-Executive | t/X2 | p |
|---|---|---|---|---|---|
|
n |
387 | 83 | 304 | ||
|
Age |
75.0±7.4 | 74.5±7.4 | 75.1±7.4 | 0.73 | 0.47 |
|
Sex (% male) |
64.6 | 59.0 | 66.1 | 1.43 | 0.23 |
|
Education |
15.7±3.0 | 14.3±3.4 | 16.1±2.7 | 4.35 | <0.0001 |
|
MMSE |
27.1±1.8 | 26.4±1.7 | 27.2±1.8 | 3.84 | 0.0001 |
|
RAVLT delayed recall |
2.9±3.3 | 2.3±2.6 | 3.0±3.5 | 2.15 | 0.03 |
|
GDS |
1.6±1.4 | 1.7±1.4 | 1.5±1.4 | 1.14 | 0.26 |
|
NPI-Q Depression |
0.2±0.5 | 0.3±0.6 | 0.2±0.5 | 0.75 | 0.45 |
|
NPI-Q Apathy |
0.2±0.5 | 0.3±0.7 | 0.2±0.5 | 1.72 | 0.09 |
| FAQ | 3.8±4.4 | 5.2±4.9 | 3.4±4.2 | 2.99 | 0.003 |
FAQ (Functional Activities Questionnaire), GDS (Geriatric Depression Scale), MCI (mild cognitive impairment), MMSE (Mini-Mental State Examination), NPI-Q Apathy (Neuropsychiatric Inventory brief questionnaire form, Apathy item), NPI-Q Depression (Neuropsychiatric Inventory brief questionnaire form, Depression item), RAVLT (Rey Auditory Verbal Learning Test).
4. Discussion
These results demonstrate a significant relationship between executive dysfunction and IADL impairment independent of diagnosis, global cognitive impairment, memory performance, depression, and apathy. Moreover, executive dysfunction was linearly or quadratically related to IADL impairment within each diagnostic group (NC, MCI, and mild AD). This relationship has previously been demonstrated in AD and other dementias4, 10, but our findings also suggest that executive dysfunction impacts daily function in subjects with milder clinical impairment. In fact, these results demonstrate that a significant proportion of subjects with MCI and mild AD have executive dysfunction, earlier in the disease course than typically reported. This subset of MCI subjects with significant executive dysfunction have greater functional impairment and are bordering on dementia. It is very important to identify such individuals and treat them as early as possible in order to preserve their tenuous level of independence.
The current study assessed the relationship between executive dysfunction and IADL impairment in aging across a continuum of cognitive function from normal cognition to mild AD. Initially, the entire sample was assessed, followed by a focus on the MCI subjects alone. In the initial analysis, a general linear regression model was used, accounting for standard demographics, diagnosis, global cognitive impairment, memory performance, depression, and apathy. The model was also realistic in terms of curvilinear effects and interactions with diagnostic group. This model demonstrated that diagnosis accounts for more of the variance of IADL than executive dysfunction, but the latter was still independently predictive of IADL impairment. Additionally, the interaction of executive function and diagnosis in their relation to IADL was not significant. Both measures of executive function (TMT-B-A and DSym) were statistically significantly associated with IADL. Strictly speaking, the relationship with TMT-B-A was curvilinear reaching a peak and then declining because of a possible selection artifact: subjects with high TMT-B scores (worse executive function) and high FAQ scores (worse IADL) may have been too impaired to be included in the study; this in turn could have resulted in an over-representation in the high TMT-B scores (worse executive function) and low FAQ scores (better IADL), and the observed artifactual “bend” and decline instead of rise in the curve (see Figure 2).
Since the CDR, which closely relates to IADL in mildly impaired subjects, played a major role in determining diagnosis in the ADNI population, a strong relationship between diagnostic group and IADL impairment was expected. On the other hand, the CDR was one of many factors taken into account in assigning a diagnosis to the subjects participating in ADNI. The other factors included questionnaires assessing behavior, the MMSE, and neuropsychological tests assessing memory, attention, executive function, language, and visuospatial function.
When assessing MCI subject only, a linear regression model accounting for standard demographics, global cognitive impairment, memory performance, depression, and apathy, showed a significant association between IADL impairment and executive dysfunction for DSym only. TMT-B-A was not significantly associated with IADL impairment.
For the current study, we used two measures of executive function (TMT-B-A and DSym) to assess the relationship with IADL. TMT-B, with or without controlling for TMT-A, has been used widely in assessing the relationship between executive function and IADL5, 8, 26. For the analyses of the current study we used a difference score between TMT-B and TMT-A (TMT-B-A), thus correcting for processing speed and visuomotor scanning, resulting in a purer executive function measure of set shifting27. When assessing the entire sample (NC, MCI, and mild AD), TMT-B-A was significantly associated with IADL. However, when assessing the MCI subjects only, it was not. This may be due to a proportionally large number of subjects reaching the ceiling on TMT-B (exceeding the time limit of 300 seconds) while having relatively low or floor scores on FAQ. This was especially notable in the MCI group. The lack of a significant result for TMT-B-A may also be partly the result of lower power due to the smaller sample size. In the analysis of the full sample, strength is borrowed across diagnostic groups. On the other hand, DSym was significantly associated with IADL when assessing the entire sample, as well as MCI only. DSym is more of a processing speed task. Processing speed has been shown to relate to early cognitive decline in the elderly, well before dementia ensues32. This may partly explain why DSym retained its significant association even when assessing the MCI subjects alone. Additionally, some argue that most tests of executive function are associated with perceptual speed and reasoning33, thus reinforcing the use of DSym in the current analyses.
A previous meta-analysis of studies evaluating the relationship between executive dysfunction and IADL impairment showed that tests for screening global cognitive performance, such as the MMSE, related most strongly to IADL, significantly more so than any individual cognitive domain26. However, that analysis also suggested that among the various cognitive domains, executive function is most closely related to IADL. The current study showed an association between IADL impairment and global cognitive impairment measured by the MMSE for AD subjects only. It also demonstrated a relationship between IADL impairment and memory impairment measured by a word-list delayed recall test (RAVLT) for all subjects (NC, MCI, and mild AD); this was previously reported in non-demented subjects5. The current study showed an association between worsening apathy and IADL impairment for all subjects, which was previously reported in AD patients4, 6. The results of the current study were also in line with previous longitudinal studies, which have shown that baseline executive dysfunction predicts worsening IADL over time and progression to clinical AD5, 11. In addition to these findings, the current study demonstrated a strong relationship between executive dysfunction and IADL impairment, independent of global cognitive impairment, memory impairment, and apathy, thus supporting the distinct role of executive dysfunction in IADL impairment.
In this study, we also performed an analysis in which we divided amnestic MCI subjects into two groups based on presence or absence of significant executive dysfunction. We used a cut-off of 1.5 standard deviations below reported norms for executive function performance in order to obtain a clinically relevant dichotomy. This is a similar approach to that used in the ADNI standard research criteria for amnestic MCI when determining significant memory impairment. About one fifth of the amnestic MCI subjects had significant executive dysfunction in addition to memory impairment and were labeled MCI Executive, while the rest of the subjects had memory impairment but no significant executive dysfunction and were labeled MCI Non-Executive. This dichotomy should not be confused with the non-amnestic MCI model, in which MCI subjects have cognitive impairment in one or more domains that are not memory, such as executive function; in the current analysis all MCI subjects had memory impairment. The results of this analysis demonstrated that the MCI Executive group had significantly greater IADL impairment when compared to the MCI Non-Executive group, thus reinforcing the results of our primary analyses. Of note, the effect size (Cohen’s “d”) for this association was small (0.4), which makes it is difficult to interpret the results in a clinical context, as is often the case with such analyses of large databases. However, many relevant covariates were included in the regression model used, thus controlling for other clinically relevant factors.
In recent years, clinical trials assessing the efficacy of cholinesterase inhibitors in the treatment of MCI did not show a significant response when using less rigorous amnestic MCI criteria34, 35. Another trial evaluating donepezil for the treatment of MCI used more rigorous amnestic MCI criteria36, identical to those used in ADNI. This trial showed significant acute effects up to 18 months, but at 3 years donepezil did not significantly decrease the conversion rate from MCI to dementia. Therefore, using the approach presented here, which also accounts for executive dysfunction, may prove valuable in selecting subjects for clinical trials, who are more likely to represent prodromal AD and perhaps more likely to respond to treatment. It has been previously reported that memory impairment and executive dysfunction in non-demented subjects have been the best cognitive predictors of progression to AD5, which further supports our findings. However, using such restrictive criteria will also make it tougher to recruit subjects for clinical trials and might not be as easily generalizable to the larger community-dwelling elderly population. Furthermore, in amnestic MCI subjects with or without executive dysfunction, the annual conversion rate to clinical AD is already 10–15% (compared to 1–2% for normal elderly controls)37. We hypothesize that subjects with amnestic MCI plus executive dysfunction might account for the higher rate of progression, and will investigate this hypothesis in longitudinal datasets.
As illustrated in Table 3, the MCI Executive subjects were more impaired in global cognitive function (MMSE), memory (RAVLT delayed recall), and IADL (FAQ), as well as less educated than the MCI Non-Executive subjects. Thus, the MCI Executive subjects are especially likely to represent the gray zone between MCI and mild AD, which is often encountered clinically. As such, the ADNI protocol has an overlap in their criteria for MCI and mild AD, which reflects the continuum of these conditions. Furthermore, it could very well be that at the hands of different clinicians, some of the subjects in the MCI Executive group would have been diagnosed with mild AD.
The pathophysiological process underlying the clinical syndrome of MCI is known to be heterogeneous38–41. It remains unknown whether subjects with amnestic MCI plus executive dysfunction are more likely to harbor high levels of amyloid pathology, and thus might demonstrate a better response to treatment targeting underlying AD pathology. A clinical-biomarker correlation using cerebrospinal fluid markers or clinical-imaging correlation, using positron emission tomography (PET) amyloid imaging, may help clarify this question.
There are other treatment implications of the impact of executive dysfunction on the ability to perform IADL. Symptomatic treatments, such as stimulants or dopaminergic agents, specifically targeting attention and executive function systems, might have significant impact on maintaining independence and decreasing caregiver burden. Behavioral interventions, specifically targeted at executive dysfunction symptoms, may also help maintain independence in these individuals. Identifying executive dysfunction early in the course of MCI may be critical for sustaining quality of life in non-demented elderly individuals.
The current study and analyses have several limitations. Subjects participating in ADNI were very carefully selected to neatly fit into the diagnostic groups of normal cognition for age, amnestic MCI, and mild AD. Subjects with significant cerebrovascular disease, psychiatric disorders, or major health issues were excluded. The cognitive profile of the MCI subjects was also carefully selected in order to represent subjects who are more likely to have AD as the underlying etiology of their impairments. Therefore, the ADNI population may not represent the full continuum of the cognitively impaired older population, which limits the generalizability of the findings of this study. This issue is further discussed above in the case of the even more restrictive and less generalizable population represented by the MCI Executive group obtained from the dichotomous MCI analysis. That said, the ADNI population does specifically represent a spectrum of subjects who are either at risk for AD or who are at the early stages of AD, as opposed to other causes of dementia. Another issue that the dichotomous MCI analysis brings to the foreground is the overlap in some of the criteria utilized in the MCI and mild AD diagnoses in the ADNI protocol. The same minimum criteria for memory impairment are used in both diagnoses and there is an overlap in the global cognitive impairment range of the MMSE and the global rating of the CDR. This reflects the sometimes arbitrary division between MCI and mild AD, while it is likely that in fact this represents a continuum. This is demonstrated by the performance of the MCI Executive subjects, who are closing in on the performance of the mild AD subjects. The current study has focused on executive function and has not explored other cognitive domain impairments in MCI, which may be as informative as memory impairment and executive dysfunction in assessing the relationship with IADL. On the other hand, prior studies have emphasized these two cognitive domains and global cognitive impairment in the assessment of IADL5, 26, as well as relevant behavioral symptoms such as apathy4, which were assessed in the current study. Finally, a ceiling effect was noted in one of the executive function measures used in the current study, TMT-B. There was also a floor effect noted in the IADL measure (FAQ) in the NC subjects. These ceiling and floor effects were addressed by modeling linear and curvilinear interactions of variables of interest with diagnosis in order to reach down and accurately model diagnostic group level differences, as well as differences in within group linear/quadratic relations as needed, being sensitive to the complexities of the data. In addition, the residual distributions reasonably conformed to test assumptions.
Few longitudinal studies demonstrating that executive dysfunction predicts functional impairment and progression to dementia have been done5, 42. The initial three year follow-up phase of the ADNI longitudinal observational study is nearing its end. Future longitudinal analyses of the ADNI cohort and similar large well characterized cohorts will be necessary to determine whether executive dysfunction precedes IADL impairment in MCI and predicts progression to clinical AD. Functional impairment takes its toll on caregivers of AD patients and usually leads to the placement of the patient in an institution. Therefore, earlier detection and prediction of functional impairment with elements such as executive dysfunction is vital. The longitudinal studies described above will play a major role toward achieving this goal. This in turn will lead to better design of future clinical trials for the treatment of prodromal AD with a population of MCI subjects that are better characterized and suited for disease-modifying treatments targeting AD. Additionally, symptomatic treatment of executive dysfunction at early stages may also further delay IADL impairment and improve patients’ quality of life.
Acknowledgements
This study was supported by R01 AG027435, 1K23AG033634-01A1, the Rosalinde and Arthur Gilbert Foundation/AFAR New Investigator Awards in Alzheimer’s Disease, the Massachusetts Alzheimer’s Disease Research Center (P50 AG005134-24), and ADNI. The Foundation for the National Institutes of Health (www.fnih.org) coordinates the private sector participation of the $60 million ADNI public-private partnership that was begun by the National Institute on Aging (NIA) and supported by the National Institutes of Health (NIH). To date, more than $27 million has been provided to the Foundation for NIH by Abbott, AstraZeneca AB, Bayer Schering Pharma AG, Bristol-Myers Squibb, Eisai Global Clinical Development, Elan Corporation, Genentech, GE Healthcare, GlaxoSmithKline, Innogenetics, Johnson & Johnson, Eli Lilly and Co., Merck & Co., Inc., Novartis AG, Pfizer Inc., F. Hoffmann-La Roche, Schering-Plough, Synarc Inc., and Wyeth, as well as non-profit partners the Alzheimer's Association and the Institute for the Study of Aging.
Abbreviations
- (AD)
Alzheimer’s disease
- (ADNI)
Alzheimer’s Disease Neuroimaging Initiative
- (CDR)
Clinical Dementia Rating scale
- (CI)
confidence interval
- (DSym)
Digit Symbol
- (FAQ)
Functional Activities Questionnaire
- (GDS)
Geriatric Depression Scale
- (IADL)
instrumental activities of daily living
- (IRB)
Institutional Review Board
- (LM-IIa)
Logical Memory IIa
- (MCI)
mild cognitive impairment
- (MMSE)
Mini-Mental State Examination
- (NIA)
National Institute on Aging
- (NIH)
National Institutes of Health
- (NINCDS-ADRDA)
National Institute of Neurologic and Communicative Disorders and Stroke and the AD and Related Disorders Association Work Group
- (NPI-Q)
Neuropsychiatric Inventory brief questionnaire form
- (NC)
normal control
- (PET)
positron emission tomography
- (RAVLT)
Rey Auditory Verbal Learning Test
- (TMT-A)
Trailmaking Test A
- (TMT-B)
Trailmaking Test B
- (β)
unstandardized partialed regression coefficient
- (WAIS-R)
Wechsler Adult Intelligence Scale-Revised
- (WMS-R)
Weschler Memory Scale-Revised
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data used in the preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (www.loni.ucla.edu\ADNI). The authors are site investigators for ADNI at Brigham and Women’s Hospital and Massachusetts General Hospital. The other site investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. ADNI investigators include (complete listing available at http://www.loni.ucla.edu/ADNI/Collaboration/ADNI_Manuscript_Citations.pdf).
None of the authors have a competing interest to disclose.
References
- 1.Marshall GA, Fairbanks LA, Tekin S, et al. Neuropathologic correlates of activities of daily living in Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20:56–59. doi: 10.1097/01.wad.0000201852.60330.16. [DOI] [PubMed] [Google Scholar]
- 2.Rowe CC, Ng S, Ackermann U, et al. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68:1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- 3.Salmon E, Lespagnard S, Marique P, et al. Cerebral metabolic correlates of four dementia scales in Alzheimer's disease. J Neurol. 2005;252:283–290. doi: 10.1007/s00415-005-0551-3. [DOI] [PubMed] [Google Scholar]
- 4.Boyle PA, Malloy PF, Salloway S, et al. Executive dysfunction and apathy predict functional impairment in Alzheimer disease. Am J Geriatr Psychiatry. 2003;11:214–221. [PubMed] [Google Scholar]
- 5.Chen P, Ratcliff G, Belle SH, et al. Cognitive tests that best discriminate between presymptomatic AD and those who remain nondemented. Neurology. 2000;55:1847–1853. doi: 10.1212/wnl.55.12.1847. [DOI] [PubMed] [Google Scholar]
- 6.Chen ST, Sultzer DL, Hinkin CH, et al. Executive dysfunction in Alzheimer's disease: association with neuropsychiatric symptoms and functional impairment. J Neuropsychiatry Clin Neurosci. 1998;10:426–432. doi: 10.1176/jnp.10.4.426. [DOI] [PubMed] [Google Scholar]
- 7.Tabert MH, Albert SM, Borukhova-Milov L, et al. Functional deficits in patients with mild cognitive impairment: prediction of AD. Neurology. 2002;58:758–764. doi: 10.1212/wnl.58.5.758. [DOI] [PubMed] [Google Scholar]
- 8.Cahn-Weiner DA, Boyle PA, Malloy PF. Tests of executive function predict instrumental activities of daily living in community-dwelling older individuals. Appl Neuropsychol. 2002;9:187–191. doi: 10.1207/S15324826AN0903_8. [DOI] [PubMed] [Google Scholar]
- 9.Royall DR, Palmer R, Chiodo LK, Polk MJ. Declining executive control in normal aging predicts change in functional status: the Freedom House Study. J Am Geriatr Soc. 2004;52:346–352. doi: 10.1111/j.1532-5415.2004.52104.x. [DOI] [PubMed] [Google Scholar]
- 10.Razani J, Casas R, Wong JT, et al. Relationship between executive functioning and activities of daily living in patients with relatively mild dementia. Appl Neuropsychol. 2007;14:208–214. doi: 10.1080/09084280701509125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cahn-Weiner DA, Farias ST, Julian L, et al. Cognitive and neuroimaging predictors of instrumental activities of daily living. J Int Neuropsychol Soc. 2007;13:747–757. doi: 10.1017/S1355617707070853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pereira FS, Yassuda MS, Oliveira AM, Forlenza OV. Executive dysfunction correlates with impaired functional status in older adults with varying degrees of cognitive impairment. Int Psychogeriatr. 2008;20:1104–1115. doi: 10.1017/S1041610208007631. [DOI] [PubMed] [Google Scholar]
- 13.Mueller SG, Weiner MW, Thal LJ, et al. The Alzheimer's disease neuroimaging initiative. Neuroimaging Clin N Am. 2005;15:869–877. doi: 10.1016/j.nic.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosen WG, Terry RD, Fuld PA, et al. Pathological verification of ischemic score in differentiation of dementias. Ann Neurol. 1980;7:486–488. doi: 10.1002/ana.410070516. [DOI] [PubMed] [Google Scholar]
- 15.Sheikh JI, Yesavage JA. Clinical Gerontology: A Guide to Assessment and Intervention. New York: The Haworth Press; 1986. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version; pp. 165–173. [Google Scholar]
- 16.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR"Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 18.Weschler D. WMS-R Weschler Memory Scale Revised Manual. New York: The Psychological Corporation, Harcourt Brace Jovanovich, Inc; 1987. [Google Scholar]
- 19.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 20.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 21.Pfeffer RI, Kurosaki TT, Harrah CH, Jr, et al. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 22.Nitrini R, Caramelli P, Herrera E, Jr, et al. Incidence of dementia in a community-dwelling Brazilian population. Alzheimer Dis Assoc Disord. 2004;18:241–246. [PubMed] [Google Scholar]
- 23.Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 24.Wechsler D. WAIS-R manual. New York: The Psychological Corporation; 1981. [Google Scholar]
- 25.Bell-McGinty S, Podell K, Franzen M, et al. Standard measures of executive function in predicting instrumental activities of daily living in older adults. Int J Geriatr Psychiatry. 2002;17:828–834. doi: 10.1002/gps.646. [DOI] [PubMed] [Google Scholar]
- 26.Royall DR, Lauterbach EC, Kaufer D, et al. The cognitive correlates of functional status: a review from the Committee on Research of the American Neuropsychiatric Association. J Neuropsychiatry Clin Neurosci. 2007;19:249–265. doi: 10.1176/jnp.2007.19.3.249. [DOI] [PubMed] [Google Scholar]
- 27.Drane DL, Yuspeh RL, Huthwaite JS, Klingler LK. Demographic characteristics and normative observations for derived-trail making test indices. Neuropsychiatry Neuropsychol Behav Neurol. 2002;15:39–43. [PubMed] [Google Scholar]
- 28.Rey A. L'examen clinique en psychologie. Paris: Presses Universitaires de France; 1964. [Google Scholar]
- 29.Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12:233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- 30.Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer's Disease Centers' Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Lawrence Erlbaum Associated Publishers; 1988. [Google Scholar]
- 32.Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- 33.Salthouse TA. Relations between cognitive abilities and measures of executive functioning. Neuropsychology. 2005;19:532–545. doi: 10.1037/0894-4105.19.4.532. [DOI] [PubMed] [Google Scholar]
- 34.Feldman HH, Ferris S, Winblad B, et al. Effect of rivastigmine on delay to diagnosis of Alzheimer's disease from mild cognitive impairment: the InDDEx study. Lancet Neurol. 2007;6:501–512. doi: 10.1016/S1474-4422(07)70109-6. [DOI] [PubMed] [Google Scholar]
- 35.Winblad B, Gauthier S, Scinto L, et al. Safety and efficacy of galantamine in subjects with mild cognitive impairment. Neurology. 2008;70:2024–2035. doi: 10.1212/01.wnl.0000303815.69777.26. [DOI] [PubMed] [Google Scholar]
- 36.Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 37.Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 38.Bennett DA, Schneider JA, Bienias JL, et al. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005;64:834–841. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- 39.Jicha GA, Parisi JE, Dickson DW, et al. Neuropathologic outcome of mild cognitive impairment following progression to clinical dementia. Arch Neurol. 2006;63:674–681. doi: 10.1001/archneur.63.5.674. [DOI] [PubMed] [Google Scholar]
- 40.Petersen RC, Parisi JE, Dickson DW, et al. Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol. 2006;63:665–672. doi: 10.1001/archneur.63.5.665. [DOI] [PubMed] [Google Scholar]
- 41.Wolk DA, Price JC, Saxton JA, et al. Amyloid imaging in mild cognitive impairment subtypes. Ann Neurol. 2009;65:557–568. doi: 10.1002/ana.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson JK, Lui LY, Yaffe K. Executive function, more than global cognition, predicts functional decline and mortality in elderly women. J Gerontol A Biol Sci Med Sci. 2007;62:1134–1141. doi: 10.1093/gerona/62.10.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]