Abstract
BIIB 513 and EMD 85131 are selective inhibitors of the Na+/H+ exchanger-1 (NHE-1) that are benzoylguanidine derivatives of the clinically employed diuretic amiloride. Prior studies have suggested a role for NHE-1 activity in platelet activation and aggregation using amiloride or its non-benzoylguanidines derivatives. However, the concentrations employed in these prior studies were at levels known to exert effects on other ion transport systems besides the NHE-1. Therefore, the purpose of this study was to examine the effects of more selective NHE-1 inhibitors, BIIB 513 and EMD 85131, on platelet aggregation and in vivo cyclic flow following arterial injury. BIIB 513 and EMD 85131 effects on ex vivo canine and human platelet aggregation in response to various agents was monitored via platelet aggregation. For analysis of in vivo thrombus formation, a femoral artery crush injury model was employed and a flow meter was used to monitor the effect of BIIB 513 on cyclic blood flow. Treatment of either canine or human platelets with up to 1 mM of BIIB 513 had no effect on aggregation induced by platelet activating factor (PAF), thrombin receptor activator peptide (TRAP), or adenosine diphosphate (ADP). Additionally, the structurally related compound EMD 85131 at up to 1 mM failed to inhibit TRAP induced platelet aggregation. In vivo administration of up to 9 mg/kg of BIIB 513 intravenously failed to affect cyclic flow in a canine model of femoral artery injury. These data demonstrate that the specific and selective NHE-1 inhibitors BIIB 513 or EMD 85131 have no effect on ex vivo platelet aggregation or in vivo cyclic flow following arterial injury.
Keywords: Sodium-hydrogen exchanger, Platelet aggregation, Animal model, Arterial thrombosis, Human, Blood flow
Introduction
The role of the Na+/H+ exchanger-1 (NHE-1) in platelet activation and aggregation previously has been studied using the clinically employed diuretic amiloride or its derivatives [2, 9, 17, 21–23]. We previously reported the characterization of benzamide-N-(aminoiminomethyl)-4-[4-(2-furanylcarbonyl)-1-piperazinyl]-3-(methylsulfonyl) methanesulfonate (BIIB 513) and 2-methyl-5-methylsulfonyl-1-(1-pyrrollyl)-benzoyl-guanidine (EMD 85131), both specific and selective benzoylguanidine inhibitors of the NHE isoform 1 (NHE-1), compounds that confer marked cardioprotection in vivo, as measured by a reduction in arrhythmias and myocardial infarct size [3–8]. While early studies suggested that inhibition of NHE activity with amiloride or non-benzoylguanidine derivatives blocked platelet aggregation induced by a variety of agents [21] including thrombin [9, 17, 22], ADP [2], serotonin [23], PAF [1, 23, 24], and collagen [23], these studies employed amiloride or amiloride derivatives at concentrations significantly higher than that required to inhibit NHE-1 activity and known to exert effects on other ion transport systems besides NHE [11, 18]. Given the known contribution of platelets and their products to myocardial infarction, and the previous literature suggesting the involvement of NHE-1 activity in platelet activation and aggregation, the purpose of this study was to examine the effects of specific and selective NHE-1 inhibitors on ex vivo platelet aggregation and in vivo cyclic flow following arterial injury. The results of this study demonstrate that the selective and specific inhibitors of NHE-1, BIIB 513 and EMD 85131, have no effect on ex vivo platelet aggregation or in vivo cyclic flow following arterial injury.
Materials and methods
Test compounds
Benzamide-N-(aminoiminomethyl)-4-[4-(2-furanylcarbonyl) 1-piperazinyl]-3-(methylsulfonyl) methanesulfonate (BIIB 513) and 2-methyl-5-methylsulfonyl-1-(1-pyrrollyl)-benzoyl-guanidine (EMD 85131) were provided by Boehringer Ingelheim and Merck KGaA respectively. Both have been previously characterized as selective and specific inhibitors of NHE-1 [3–8].
Canine and human platelet aggregation studies
Informed consent was obtained prior to human blood collection and the study was approved by the Committee on Human Research at the Blood Center of Southeastern Wisconsin. The investigation involving dogs also conformed to the Guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health, and was approved by the Institutional Animal Care and Use Committee at the Medical College of Wisconsin.
Whole canine blood was collected from a femoral vein catheter into sodium citrate. Human blood was collected by venipuncture into sodium citrate. Blood was centrifuged at 2009g for 15 min and the platelet rich plasma (PRP) supernatant was transferred to a new tube. Platelet poor plasma was prepared by centrifuging 1 ml of PRP at 2,0009g for 1 min. Platelets were counted using a hemocytometer and adjusted to 3 × 108/ll with platelet poor plasma. Platelet aggregation was monitored using a whole blood aggregometer (Chronolog Corp., Haverston, PA, Model 560-VS). The NHE-1 exchange inhibitor BIIB 513 was diluted in DMSO and added at concentrations from 100 lM to 1 mM. Final DMSO concentration did not exceed 0.5%. PRP was incubated with the designated concentration of BIIB 513 or EMD 85131 for 5 min at 37°C prior to induction of aggregation. Aggregation was induced by the addition of the following substances to yield the designated final concentrations: TRAP (0.07 lM), ADP (1.4 × 10 −5 M), or platelet activating factor (2 lg/ml).
In vivo cyclic flow protocol
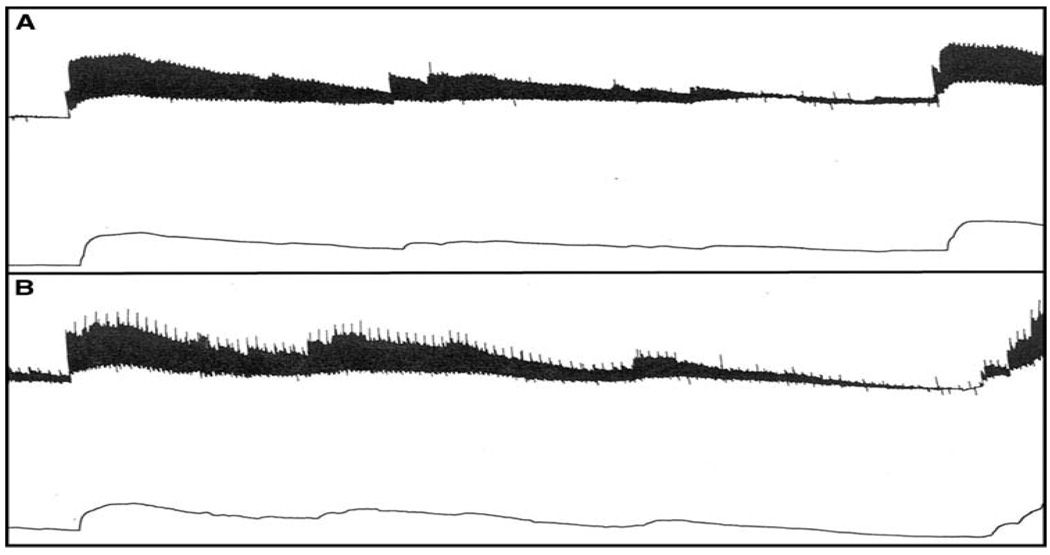
Adult mongrel dogs (n = 2) were subjected to an experimental preparation similar to that referenced earlier [3–8]. For analysis of cyclic flow, the left femoral artery also was isolated and the endothelium damaged by gently squeezing the vessel three times with a hemostat followed by placement of a plastic constrictor around the site of injury. A calibrated electromagnetic flow probe (Statham SP 7515, Gould-Statham) was placed around the vessel distal to the constrictor site and a flow meter (Statham 2202) was used to monitor blood flow. Hemodynamics, heart rate and femoral blood flow were monitored and recorded by a polygraph (Model 7, Grass Instrument) throughout the experiment. This protocol triggers the development of cyclic variations in blood flow caused by repeated spontaneous accumulation and dislodgment of platelet-rich thrombi at the site of vessel injury and stenosis [26]. Once spontaneous cyclic variation in blood flow was established, femoral blood flow was monitored for 3 h. A cyclic variation in blood flow was specifically defined in this study as a slow decrease followed by an abrupt increase in femoral blood flow with an amplitude of [50% of the poststenotic blood flow value. Each dog served as its own control. After monitoring the cyclic variations in blood flow for at least 30 min, 9.0 mg/kg of BIIB 513 was infused intravenously and the cyclic variations in blood flow were monitored an additional 3 h.
Results
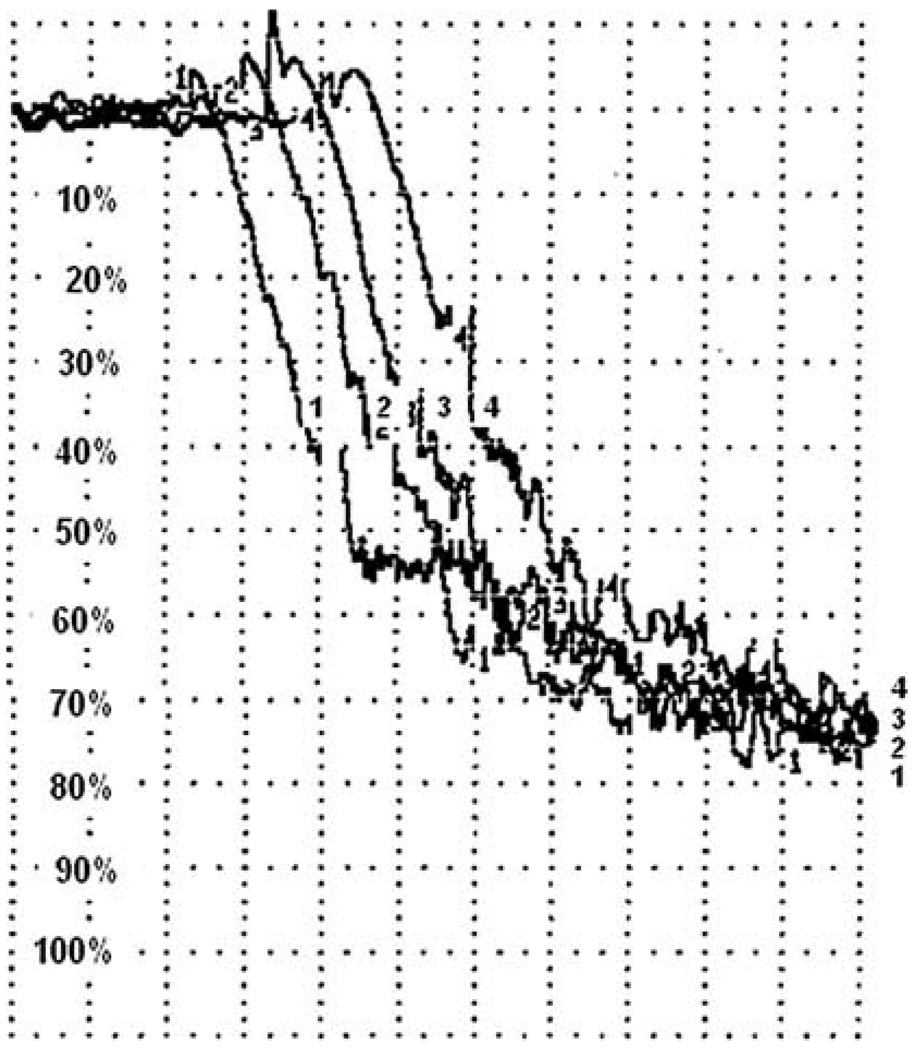
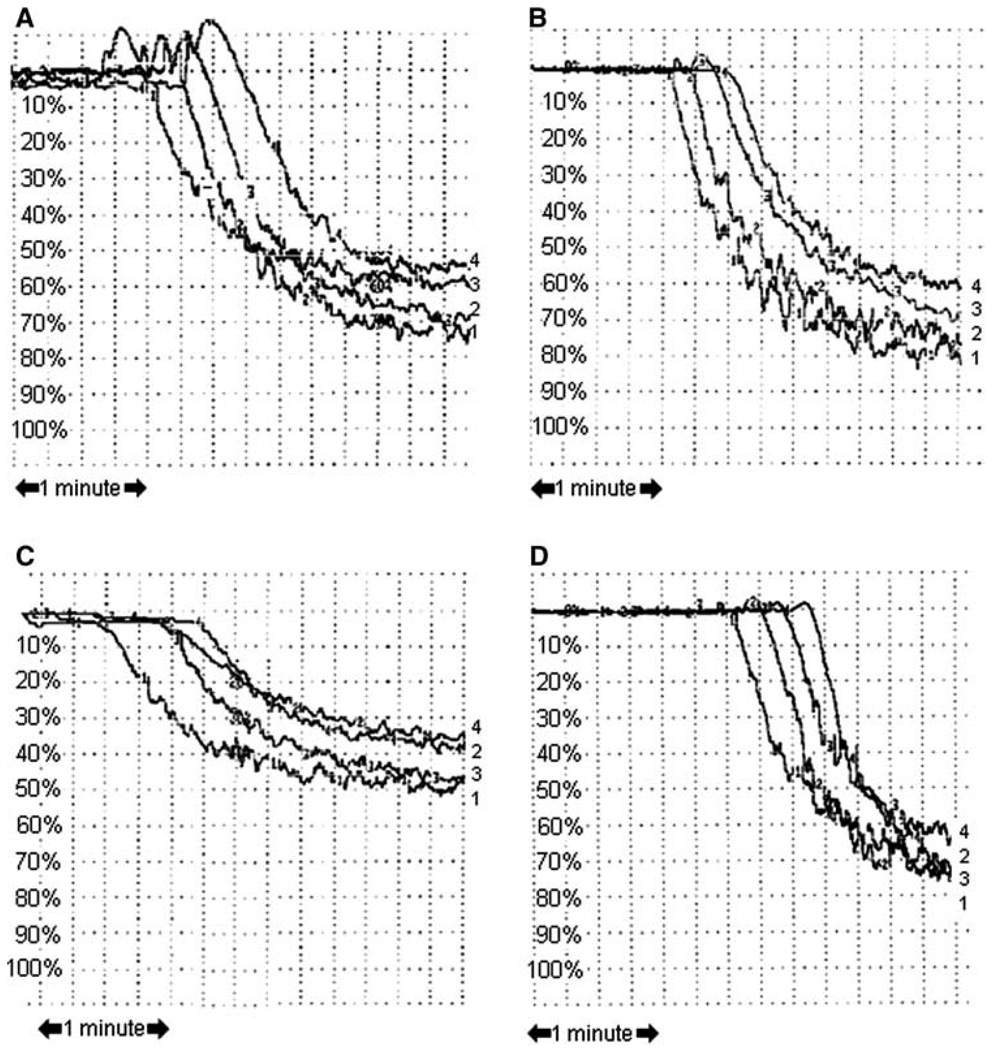
Treatment of canine platelets with up to 1 mM of the selective NHE-1 inhibitor, BIIB 513, had no effect on aggregation induced by either platelet activating factor (Fig. 1) or ADP (data not shown). Up to 1 mM of BIIB 513 had no effects on human platelet aggregation induced by platelet activating factor (PAF), thrombin receptor activator peptide (TRAP), or adenosine diphosphate (ADP) (Fig. 2a). Similarly, 1 mM EMD 85131 had no effects on human platelet aggregation induced by TRAP (Fig. 2b) In vivo administration of up to 9 mg/kg of BIIB 513 intravenously failed to affect cyclic flow in a canine model of femoral artery injury or induce thrombosis (Fig. 3).
Fig. 1.
Effect of NHE-1 inhibition on canine platelet aggregation. Canine platelets were isolated and stimulated with platelet activating factor as outlined in “Materials and methods.” Aggregation curves are shown. 1, 4 = control; 2, 3 = BIIB 513 1 mM
Fig. 2.
Effect of NHE-1 inhibition on human platelet aggregation. Human platelets were isolated and stimulated with a TRAP, b platelet activating factor, or c ADP in the presence of BIIB 513 or d TRAP in the presence of EMD 85131 as outlined in “Materials and methods.” Aggregation curves are shown. a–c 1, 4 = control; 2, 3 = BIIB 513 1 mM. d 1, 4 = control; 2, 3 = EMD 85131 1 mM
Discussion
Previous studies using less specific and less selective inhibitors of NHE-1 have demonstrated that inhibition of NHE results in an attenuation of platelet aggregation in response to various agents including thrombin [9, 17, 22], ADP [2], serotonin [23], PAF [1, 23, 24], and collagen [23]. However, the concentrations employed in these studies were significantly higher than those required to inhibit NHE-1 activity. The current study employed a specific and selective inhibitors of NHE-1, BIIB 513 and EMD 85131 (Fig. 1), to determine if NHE-1 activity is involved in platelet aggregation. Prior work has demonstrated that the specific and selective NHE-1 inhibitors cariporide [12, 15], eniporide [14, 15], zoniporide [13], T-162559 [15] and KR-32570 [16] inhibit platelet NHE-1 activity. Furthermore, the structural analogue BIIB722, which contain a 3-methylfluoronyl group in place of the 3-methylsulfonyl group in BIIB 513, also has been shown to inhibit platelet NHE-1 activity as measured by the platelet swelling assay [27]. However, to date, no evaluation of the effect of specific NHE-1 inhibitors on ex vivo platelet aggregation or in vivo platelet aggregation or thrombosis has been reported. We have demonstrated that ex vivo treatment of canine platelets (Fig. 1) and human platelets (Fig. 2), with up to 1 mM of BIIB 513 or EMD 85131 had no effect on platelet aggregation stimulated by a variety of agents (TRAP, PAF, ADP). Furthermore, there was no inhibition of ex vivo aggregation observed in platelets isolated from animals treated with up to 9 mg/kg of BIIB 513 (data not shown). Finally, in a canine model of in vivo thrombosis induced by femoral artery injury, no effect on the frequency or duration of variations in flow was observed in animals treated with up to 9 mg/kg of BIIB 513 (Fig. 3).
The current data contradict several reports using less specific inhibitors of NHE-1 which suggested that inhibition of NHE activity affects platelet aggregation [1, 2, 9, 17, 21–24]. Review of the concentrations of drugs employed in these previous studies revealed that between 0.5 and 1 mM of amiloride was required to inhibit platelet aggregation. Amiloride and its derivatives have nonspecific effects at these concentrations [11, 18]. One study has demonstrated that the amiloride derivative EIPA at low concentrations has no effect on platelet aggregation [10]. Alternatively, at higher concentrations of these nonselective compounds the Na+/Ca2+ exchanger (NCX) may be inhibited. Indeed, inhibition of NCX activity with 30,40-dichlorobenzamil or bepridil have been shown to inhibit platelet aggregation induced by ADP, collagen, epinephrine and thrombin, suggesting a direct role for NCX activity in the regulation of platelet aggregation [19, 20]. Furthermore, KB-R7943, a specific NCX inhibitor, inhibits aggregation of rabbit and human platelets induced by the combination of adrenaline plus 5-HT [25]. Future studies are needed to examine the effect of inhibition of the Na+/Ca2+ exchanger on platelet aggregation ex vivo and in vivo.
The current report demonstrates that treatment with the selective inhibitors of NHE-1, BIIB 513 or EMD 85131, does not affect ex vivo platelet aggregation or in vivo cyclic flow following arterial injury. Therefore, direct effect on platelets can be excluded as contributing to the previously reported antiarrhythmic activity and myocardial protection observed in vivo with BIIB 513-or EMD 85131 mediated NHE-1 inhibition [3–8].
Acknowledgments
The authors thank Ms. Jeannine Moore and Anna Hsu for their excellent technical assistance with the experiments reported. This study was supported by NIH grants HL-08311 and HL-074314 and grants from Boehringer Ingelheim Pharma KG and Merck KGaA. RJG currently supported by NIH grants HL-096038 and HL-094703.
Abbreviations
- NHE
Sodium/hydrogen exchanger
- NCX
Sodium/calcium exchanger
- PAF
Platelet activating factor
- ADP
Adenosine diphosphate
Contributor Information
Richard J. Gumina, Division of Cardiovascular Medicine, Department of Internal Medicine, The Ohio State University, 473 W. 12th Avenue, Suite 200, DHLRI, Columbus, OH 43210, USA richard.gumina@osumc.edu
Peter J. Newman, Blood Center of Southeastern Wisconsin, Milwaukee, WI, USA
Garrett J. Gross, Department of Pharmacology and Toxicology, Medical College of Wisconsin, Milwaukee, WI, USA
References
- 1.Borin ML, Pinelis VG, Azizova OA, Kudinov YV, Markov CM, Cragoe EJ, Khodorov BI. Na+/H+ exchange in PAF-stimulated platelets. J Lipid Mediat. 1989;1(5):257–272. [PubMed] [Google Scholar]
- 2.Connolly TM, Limbird LE. Removal of extraplatelet Na+ eliminates indomethacin-sensitive secretion from human platelets stimulated by epinephrine, ADP, and thrombin. Proc Natl Acad Sci USA. 1983;80(17):5320–5324. doi: 10.1073/pnas.80.17.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gumina RJ, Buerger E, Eickmeier C, Moore J, Daemmgen J, Gross GJ. Inhibition of the Na(+)/H(+) exchanger confers greater cardioprotection against 90 min of myocardial ischemia than ischemic preconditioning in dogs. Circulation. 1999;100(25):2519–2526. doi: 10.1161/01.cir.100.25.2519. discussion 2469–2572. [DOI] [PubMed] [Google Scholar]
- 4.Gumina RJ, Mizumura T, Beier N, Schelling P, Schultz JJ, Gross GJ. A new sodium/hydrogen exchange inhibitor, EMD 85131, limits infarct size in dogs when administered before or after coronary artery occlusion. J Pharmacol Exp Ther. 1998;286(1):175–183. [PubMed] [Google Scholar]
- 5.Gumina RJ, Auchampach J, Wang R, Buerger E, Eickmeier C, Moore J, Daemmgen J, Gross GJ. Na(+)/H(+) exchange inhibition-induced cardioprotection in dogs: effects on neutrophils versus cardiomyocytes. Am J Physiol Heart Circ Physiol. 2000;279(4):H1563–H1570. doi: 10.1152/ajpheart.2000.279.4.H1563. [DOI] [PubMed] [Google Scholar]
- 6.Gumina RJ, Beier N, Schelling P, Gross GJ. Inhibitors of ischemic preconditioning do not attenuate Na+/H+ exchange inhibitor mediated cardioprotection. J Cardiovasc Pharmacol. 2000;35(6):949–953. doi: 10.1097/00005344-200006000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Gumina RJ, Daemmgen J, Gross GJ. Inhibition of the Na(+)/H(+) exchanger attenuates phase 1b ischemic arrhythmias and reperfusion-induced ventricular fibrillation. Eur J Pharmacol. 2000;396(2–3):119–124. doi: 10.1016/s0014-2999(00)00200-4. [DOI] [PubMed] [Google Scholar]
- 8.Gumina RJ, El Schultz J, Moore J, Beier N, Schelling P, Gross GJ. Cardioprotective-mimetics reduce myocardial infarct size in animals resistant to ischemic preconditioning. Cardiovasc Drugs Ther. 2005;19(5):315–322. doi: 10.1007/s10557-005-3693-8. [DOI] [PubMed] [Google Scholar]
- 9.Horne WC, Simons ER. Effects of amiloride on the response of human platelets to bovine alphathrombin. Thromb Res. 1978;13(4):599–607. doi: 10.1016/0049-3848(78)90149-4. [DOI] [PubMed] [Google Scholar]
- 10.Hunyady L, Sarkadi B, Cragoe EJ, Jr, Spat A, Gardos G. Activation of sodium-proton exchange is not a prerequisite for Ca2+ mobilization and aggregation in human platelets. FEBS Letters. 1987;225(1–2):72–76. doi: 10.1016/0014-5793(87)81133-x. [DOI] [PubMed] [Google Scholar]
- 11.Kleyman TR, Cragoe EJ., Jr Amiloride and its analogs as tools in the study of ion transport. J Membr Biol. 1988;105(1):1–21. doi: 10.1007/BF01871102. [DOI] [PubMed] [Google Scholar]
- 12.Klinkhardt U, Kuczka K, Harder S. Effects of the NHE-1 inhibitor cariporide alone or together with the P2Y12 antagonist AR-C 69331 MX on CD62p expression and formation of platelet-leukocyte aggregates. Thromb Res. 2003;111(4–5):251–257. doi: 10.1016/j.thromres.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Knight DR, Smith AH, Flynn DM, MacAndrew JT, Ellery SS, Kong JX, Marala RB, Wester RT, Guzman-Perez A, Hill RJ, Magee WP, Tracey WR. A novel sodium-hydrogen exchanger isoform-1 inhibitor, zoniporide, reduces ischemic myocardial injury in vitro and in vivo. J Pharmacol Exp Ther. 2001;297(1):254–259. [PubMed] [Google Scholar]
- 14.Kovar A, Peters T, Beier N, Derendorf H. Pharmacokinetic/pharmacodynamic evaluation of the NHE inhibitor eniporide. J Clin Pharmacol. 2001;41(2):139–148. doi: 10.1177/00912700122009944. [DOI] [PubMed] [Google Scholar]
- 15.Kusumoto K, Igata H, Abe A, Ikeda S, Tsuboi A, Imamiya E, Fukumoto S, Shiraishi M, Watanabe T. In vitro and in vivo pharmacology of a structurally novel Na+-H+ exchange inhibitor, T-162559. Br J Pharmacol. 2002;135(8):1995–2003. doi: 10.1038/sj.bjp.0704647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee K-S, Park J-W, Jin Y-R, Jung I-S, Cho M-R, Yi K-Y, Yoo S-E, Chung H-J, Yun Y-P, Park T-K, Shin H-S. Antiplatelet activity of [5-(2-methoxy-5-chlorophenyl)furan-2-ylcarbonyl] guanidine (KR-32570), a novel sodium/hydrogen exchanger-1 and its mechanism of action. Arch Pharm Res. 2006;29(5):375–383. doi: 10.1007/BF02968587. [DOI] [PubMed] [Google Scholar]
- 17.Luzzatto G, Kroll MH, Zavoico GB, Schafer AI. Regulation of the phosphoinositide cycle by Na+/H+ exchange and intracellular pH in human platelets. Biochim Biophys Acta. 1991;1084(1):78–86. doi: 10.1016/0005-2760(91)90058-p. [DOI] [PubMed] [Google Scholar]
- 18.Pierce GN, Cole WC, Liu K, Massaeli H, Maddaford TG, Chen YJ, McPherson CD, Jain S, Sontag D. Modulation of cardiac performance by amiloride and several selected derivatives of amiloride. J Pharmacol Exp Ther. 1993;265(3):1280–1291. [PubMed] [Google Scholar]
- 19.Shiraga M, Tomiyama Y, Honda S, Kashiwagi H, Kosugi S, Handa M, Ikeda Y, Kanakura Y, Kurata Y, Matsuzawa Y. Affinity modulation of the platelet integrin alpha IIb beta 3 by alpha-chymotrypsin: a possible role for Na+/Ca2+ exchanger. Blood. 1996;88(7):2594–2602. [PubMed] [Google Scholar]
- 20.Shiraga M, Tomiyama Y, Honda S, Suzuki H, Kosugi S, Tadokoro S, Kanakura Y, Tanoue K, Kurata Y, Matsuzawa Y. Involvement of Na+/Ca2+ exchanger in inside-out signaling through the platelet integrin IIbbeta3. Blood. 1998;92(10):3710–3720. [PubMed] [Google Scholar]
- 21.Siffert W. Regulation of platelet function by sodium-hydrogen exchange. Cardiovasc Res. 1995;29(2):160–166. [PubMed] [Google Scholar]
- 22.Siffert W, Akkerman JW. Activation of sodium-proton exchange is a prerequisite for Ca2+ mobilization in human platelets. Nature. 1987;325(6103):456–458. doi: 10.1038/325456a0. [DOI] [PubMed] [Google Scholar]
- 23.Siffert W, Gengenbach S, Scheid P. Inhibition of platelet aggregation by amiloride. Thromb Res. 1986;44(2):235–240. doi: 10.1016/0049-3848(86)90139-8. [DOI] [PubMed] [Google Scholar]
- 24.Sweatt JD, Schwartzberg MS, Frazer M, Cragoe EJ, Jr, Blair IA, Reed PW, Limbird LE. Evidence for a role for Na+-H+ exchange in activation of human platelets by PAF. Circ Res. 1987;61(5 Pt 2):II6–II11. [PubMed] [Google Scholar]
- 25.Takano S, Kimura J, Ono T. Inhibition of aggregation of rabbit and human platelets induced by adrenaline and 5-hydroxytryptamine by KB-R7943, a Na(+)/Ca(2+) exchange inhibitor. Br J Pharmacol. 2001;132(7):1383–1388. doi: 10.1038/sj.bjp.0703928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torr SR, Haskel EJ, VonVoigtlander PF, Bergmann SR, Abendschein DR. Inhibition of cyclic flow variations and reocclusion after thrombolysis in dogs by a novel antagonist of platelet-activating factor. J Am Coll Cardiol. 1991;18(7):1804–1810. doi: 10.1016/0735-1097(91)90524-d. [DOI] [PubMed] [Google Scholar]
- 27.Touret N, Tanneur V, Godart H, Seidler R, Taki N, Burger E, Dammgen J, Counillon L. Characterization of sabiporide, a new specific NHE-1 inhibitor exhibiting slow dissociation kinetics and cardioprotective effects. Eur J Pharmacol. 2003;459(2–3):151–158. doi: 10.1016/s0014-2999(02)02824-8. [DOI] [PubMed] [Google Scholar]