Introduction
Thrombophilia is considered as a condition predisposing to the development of thrombosis. Arterial thrombosis usually occurs after the erosion or rupture of an atherosclerotic plaque and, through platelet-mediated thrombi, can cause ischaemic injuries especially in tissues with a terminal vascular bed. Indeed, cardiac ischaemia and stroke are the most severe clinical manifestations of atherothrombosis. Ischaemia can arise slowly from the progression of atherosclerotic disease (stable angina, claudication) or acutely in the case of vascular (atherosclerotic plaque rupture) or intracardiac (atrial fibrillation, mechanical valve prostheses) thromboembolisation.
Venous thromboembolism (VTE) is the most common vascular disease after acute myocardial infarction and stroke. It is represented by two main clinical events: deep venous thrombosis (DVT) and pulmonary embolism (PE), which often constitute an unique clinical picture in which PE follows DVT. Although VTE is a common disease, the underlying pathogenic mechanisms are only partially known, particularly in comparison to those of atherothrombosis. During the past decades, progress has been made in the identification and characterisation of the cellular and molecular mechanisms that interdependently influence Virchow’s triad. It is now accepted that the combination of stasis and hypercoagulability, much more than endothelial damage, is crucial for the occurrence of VTE; venous thrombi are mainly constituted by fibrin and red blood cells, and less by platelets. In contrast, platelets are essential for primary haemostasis, repair of damaged endothelium and play a pivotal role in the development of atherosclerosis. Inflammation, lipids and the immune system, through a complex interplay, are also important determinants of arterial and, albeit to a lesser extent, of venous thrombosis. Pathophysiological and epidemiological findings have enabled the definition of the main risk factors for atherothrombosis and VTE, listed in Tables I and II. This review summarises the recent epidemiological data on the main risk factors for venous and arterial thrombosis, and considers the mechanisms by which they mediate the disease.
Table I.
Classical risk factors for cardiovascular disease35.
| Risk factor | OR (99%CI) |
|---|---|
| Hyperlipidaemia | 3.25 (2.81–3.76) |
| Smoking | 2.87 (2.58–3.19) |
| Diabetes | 2.37 (2.07–2.71) |
| Hypertension | 1.91 (1.74–2.10) |
| Abdominal obesity | 1.62 (1.45–1.80) |
OR: odds ratio; CI: confidence intervals.
Table II.
Classical risk factors for venous thromboembolism.
| Strong risk factors (odds ratio >10) |
| trauma or fractures |
| major orthopaedic surgery |
| oncological surgery |
| Moderate risk factors (odds ratio 2–9) |
| non-oncological surgery |
| oral contraceptives and hormone replacement therapy |
| pregnancy and puerperium |
| hypercoagulability |
| previous venous thromboembolism |
| Weak risk factors (odds ratio <2) |
| age |
| bed rest (> 3 days) |
| prolonged travel |
| metabolic syndrome |
| air pollution |
Age
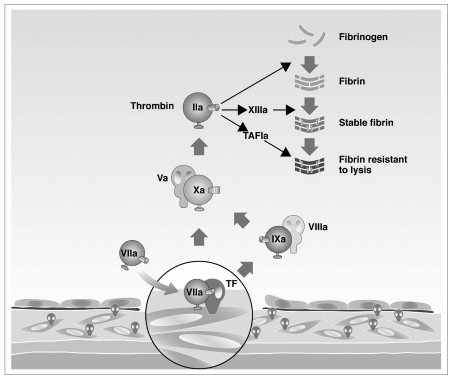
There is an exponential increase in the risk of both arterial and venous thrombotic events with age1,2, and the increase in life expectancy in the second half of the 20th century is a major cause of the current epidemic of both arterial and venous thrombosis1,3. Possible mechanisms include cumulative effects of risk factors on the arterial wall, decreased regular exercise, increasing immobility resulting in venous stasis, and increasing systemic activation of blood coagulation4,5. Plasma concentrations of some coagulation factors (factors V, VII, VIII, and IX, fibrinogen) increase progressively with age6,7. The same is true for von Willebrand factor (vWF), a key protein in platelet-vessel wall interactions8. For instance, the Framingham study showed that plasma levels of fibrinogen increased from a mean value of 280 mg/dL in individuals aged 47–54 years to more than 300 mg/dL in those aged 65–79 years9, with an increase of 10 mg/dL for each decade of age. High plasma levels of fibrinogen may play a causative role in the high incidence of cardiovascular events observed in elderly people, perhaps by enhancing the bridging of platelets via their glycoprotein IIb-IIIa receptor, by serving as a direct substrate of the clot and/or by increasing blood viscosity10. Alternatively, high fibrinogen levels may simply be a marker of the chronic inflammatory state typical of aging, without directly contributing to the risk10. A similar trend was shown for another acute phase protein, coagulation factor VIII, which increases progressively with age, up to more than 200 U/dL in the seventh decade of life4. Coagulation factor VII, both as a zymogen and as the activated protease, also increases with age11. The role of tissue factor (TF) and factor VII as key components of blood coagulation and thrombus formation is well established (Figure 1). TF, a protein localised in the membrane of vascular cells, monocytes and circulating microparticles, is considered a key initiator of blood coagulation. When it is exposed in its active form at the vessel wall (e.g. after endothelial activation or during chronic inflammation, both conditions typical of aging), TF activates factor VII. This complex produces small amounts of thrombin and promotes thrombus formation through the activation of coagulation reactions on the membrane surfaces of activated platelets and microparticles12. During aging, an increasing number of individuals develop a laboratory picture of enhanced activity of coagulation enzymes, expressed by high levels of the activation peptides that are cleaved from prothrombin, factor IX, factor X and fibrinogen [prothrombin fragment 1+2 (F1+2), thrombin-antithrombin complex (TAT), factor IX activation peptide, factor X activation peptide, fibrinopeptide A] when these zymogens are converted into their corresponding active enzymes13,14. An impairment of fibrinolytic activity also occurs with aging. There is an increase of plasminogen activator inhibitor type 1 (PAI-1), the major inhibitor of fibrinolysis15, and a corresponding age-dependent decrease in fibrinolytic activity16. An increase in platelet reactivity with aging has also been demonstrated, and activated platelets greatly accelerate thrombin generation. Platelets of 60-year-old or older individuals aggregate more in response to adenosine diphosphate (ADP) and collagen than platelets from younger individuals17. Furthermore, a positive correlation has been observed between age and markers of platelet activation such as plasma β-thromboglobulin (a protein stored in the α granules of platelets) and platelet membrane phospholipids18. Because the vascular endothelium plays an important role in the normal process of haemostasis, any structural or functional change in the vascular wall (involving the extracellular matrix, vascular smooth muscle or endothelium) that occurs during aging may contribute to the increased risk of thrombosis in the elderly, particularly atherothrombosis. Advanced age is characterised by stiffness and dilation of the arteries, due to degeneration of elastic fibres and an increase in collagen and calcium content, and by a decrease in prostacyclin and nitric oxide with a related reduction in endothelium-dependent dilation19. There is also increased binding of platelet-derived growth factor to arteries, caused by changes in the glycosaminoglycan content of the vessel wall, which enhances the progression of atherosclerosis and indirectly contributes to atherothrombosis20. In conclusion, there are several alterations of the haemostatic system in the elderly. A causal association between these alterations and thrombosis is likely but has not been formally proven, because of the lack of prospective studies demonstrating the development of clinical manifestations of thrombosis in comparison with aged healthy individuals.
Figure 1.
Role of tissue factor (TF) and coagulation factor VII in the activation of the coagulation cascade leading to thrombin formation.
TAFI = thrombin activatable fibrinolysis inhibitor; “a” = “activated”.
Thrombophilia abnormalities
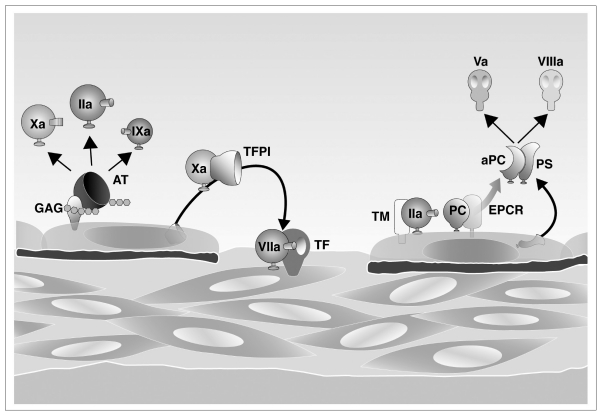
Normally, the coagulation process is under the control of several inhibitors that limit clot formation near the damaged vessel wall, thus avoiding thrombus propagation (Figure 2). This delicate balance can be interrupted whenever the procoagulant activity of one of the coagulation factors is increased or the activity of one of the naturally occurring inhibitors decreases, leading to thrombus formation. This occurs with inherited deficiencies of natural inhibitors, as well as with inherited gain-of-function mutations of some coagulation factors21 (Table III). Inherited antithrombin, protein C and protein S deficiencies are rare but strong risk factors for venous thrombosis; they have little or no effect on arterial thrombosis. Antithrombin directly inhibits several activated coagulation factors, particularly thrombin and activated factor X, and the inhibitory effect is amplified by its binding to glycosaminoglycans of the endothelial surface which carry heparin-like activity. Antithrombin deficiency results in significantly reduced inhibition of thrombin and activated factor X and an increased tendency to clot formation, particularly in the venous system where the coagulation pathway (as distinct from platelets) plays a major role in thrombus formation21. The protein C anticoagulant pathway, localised on the surface of the endothelium, is essential in the down-regulation of thrombin generation. Thrombin activates protein C; the presence of thrombomodulin, together with endothelial protein C receptor (EPCR), accelerates the catalytic efficiency of this activation. Activated protein C proteolytically inactivates factor Va and factor VIIIa, the two most important activated co-factors of the coagulation cascade, dramatically slowing the rate of thrombin and fibrin formation. The inhibitory effect of activated protein C is accelerated by its main co-factor, protein S22. The inherited deficiency of one of these inhibitors leads to a critical reduction of the natural anticoagulant system and enhances thrombin generation, increasing susceptibility to VTE21.
Figure 2.
Anticoagulant mechanisms of blood coagulation. Antithrombin (AT) inhibits mainly activated factors II (IIa) and X (Xa) through its binding to glycosaminoglycans (GAG); protein C (PC), with its co-factor protein S (PS), is activated by thrombomodulin (TM) and inhibits activated factors V (Va) and VIII (VIIIa) through its binding to endothelial protein C receptor (EPCR).
TFPI = tissue factor pathway inhibitor; “a” = “activated”.
Table III.
Inherited, acquired and mixed coagulation or metabolic risk factors for thrombosis.
| Inherited | Acquired | Mixed |
|---|---|---|
| Antithrombin deficiency | Antiphospholipid syndrome | Hyperhomocysteinaemia |
| Protein C deficiency | Increased fibrinogen levels | |
| Protein S deficiency | Increased factor VIII levels | |
| Factor V Leiden | Increased factor IX levels | |
| Prothrombin G20210A | Increased factor XI levels |
The two most common genetic risk factors for VTE are the G1691A mutation in the factor V gene (factor V Leiden) and the G20210A mutation in the prothrombin gene. The factor V Leiden gain-of-function mutation consists of the substitution of an arginine by glutamine at position 506 of coagulation factor V (R506Q), which is the cleavage site for activated protein C in the factor V molecule23. Mutant factor V is partially resistant to inactivation by activated protein C, leading to a hypercoagulable state. Factor V Leiden explains more than 90% of cases of activated protein C resistance24. The G20210A mutation in the prothrombin gene is a G to A transition at nucleotide position 20210 in the 3′-untranslated region of the coagulation factor II (prothrombin) gene, which increases plasma prothrombin levels25. These two mutations also increase the risk of atherothrombosis, but to a lesser extent26. The prevalences of inherited thrombophilia in the general population and in patients with VTE are shown in Table IV.
Table IV.
Prevalence (%) of inherited risk factors for VTE in the general population and in patients.
| Abnormality | General population | Patients with VTE | Patients with recurrent VTE or age < 45 years |
|---|---|---|---|
| Antithrombin deficiency | 0.02 - 0.17 | 1.1 | 0.5 - 4.9 |
| Protein C deficiency | 0.14 - 0.5 | 3.2 | 1.4 - 8.6 |
| Protein S deficiency | ? | 2.2 | 1.4 - 7.5 |
| Heterozygous factor V Leiden | 3.6 - 6.0 | 21.0 | 10 - 64 |
| Heterozygous prothrombin G20210A | 1.7 - 3.0 | 6.2 | 18 |
The antiphospholipid antibody syndrome is one of the most important acquired risk factors for thrombosis. Characterised by the presence of circulating antiphospholipid antibodies in plasma, it is associated with arterial or venous thrombosis and/or pregnancy complications, including foetal loss. The clinically relevant antiphospolipid antibodies include lupus anticoagulant, anticardiolipin and anti-β2-glycoprotein I antibodies. The term “antiphospolipid antibodies” is widely used even if it is not correct, because antibodies are not directed against phospholipids per se, but against a wide variety of protein co-factors acting on phospholipid membrane surfaces (β2-glycoprotein I, prothrombin, protein C, protein S, annexin V, coagulation factor XII and others). The resulting complexes interact with several cell types, including endothelial cells, monocytes and platelets, all of which play important roles in haemostasis and thrombogenesis. The indirect activation of these cells results in the release of prothrombotic and pro-inflammatory mediators (e.g. TF-bearing microparticles, interleukin-6, proteins of the complement system), leading to the activation of platelet and coagulation pathways27. Recent observations show that antiphospholipid antibodies interact directly with vessel wall and cause alterations of plasma lipoprotein [i.e. high density lipoprotein (HDL)] function leading to increased atherothrombotic risk28.
Hyperhomocysteinaemia is a mild risk factor for thrombosis due to an impairment of the metabolic pathway that transforms the amino acid methionine into cysteine, leading to an abnormal elevation of plasma concentrations of homocysteine, an intermediate product of this pathway. Genetic factors (e.g., gene mutations in methylenetetrahydrofolate reductase and cystathionine β-synthase) and acquired factors (e.g., deficiencies of folate, vitamin B12 or vitamin B6, advanced age, chronic renal failure, and the use of anti-folic drugs) interact to determine plasma homocysteine concentrations, so that hyperhomocysteinaemia is a “mixed” (i.e., genetic and/or acquired) risk factor for both arterial and venous thrombosis29. The possible mechanisms by which hyperhomocysteinaemia contributes to thrombosis are multiple and still under study; they include a toxic effect on endothelial cells, smooth-muscle-cell proliferation and intimal thickening, impaired generation of nitric oxide and prostacyclin, increased platelet adhesion, activation of factor V, interference with protein C activation and thrombomodulin expression, induction of tissue factor activity and inhibition of tissue plasminogen activator (t-PA)30.
An association between increased plasma levels of some coagulation factors (VIII, IX, XI, and fibrinogen) and an increased risk of VTE has been demonstrated 31. The plasma levels of these factors are influenced by age and inflammation, but are also under genetic control. The mechanisms by which increased coagulation factors plasma levels enhance the risk of thrombosis are unknown, but a shift in the balance of the coagulation process towards a procoagulant state is plausible. High levels of fibrinogen are associated with an increased risk of atherothrombosis, while the effect of factor VIII is dependent on vWF, which plays the most important role in the increased risk of thrombosis associated with the factor VIII/vWF complex.
Metabolic syndrome and smoking
One of the most widely used definitions of the metabolic syndrome was proposed in 2001 by the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) and is based on the presence of at least three of the following diagnostic criteria: elevated waist circumference (abdominal obesity), elevated triglycerides, reduced HDL cholesterol, elevated blood pressure and elevated fasting glucose32. There is increasing evidence for an association between atherothrombosis and the metabolic syndrome32–34. The INTERHEART study identified nine risk factors that collectively accounted for more than 90% of the risk of acute myocardial infarction. Risk predictors included life-style factors such as smoking, co-morbidities (hypertension, diabetes, abdominal obesity, abnormal lipid profiles), as well as psychosocial factors35. Meta-analyses of randomised controlled trials on blood pressure36 and cholesterol reduction37, and observational studies on smoking cessation38 confirmed that these three risk factors play causative roles in arterial disease, partly through atherogenesis and partly through a systemic activation of blood coagulation and inflammation39. Available evidence from multiple randomised trials supports life-styles that promote weight reduction, cigarette smoking cessation and regular moderate exercise in order to reduce platelet reactivity and coagulability, and to promote fibrinolysis. The overall effects can be expected to translate into an improved cardiovascular prognosis or other beneficial clinical outcomes in healthy individuals and those with cardiovascular risk factors or established coronary heart disease40. Nevertheless, a high residual risk for secondary ischemic events, despite life-style modifications, is evident from the results of recent clinical practice registries, which justifies the need for adding pharmacotherapy.
From a biological point of view, the metabolic syndrome is frequently accompanied by a prothrombotic state. This includes elevated plasma levels of PAI-1, thrombin-activatable fibrinolysis inhibitor (TAFI), vWF, coagulation factors VIII, VII, and XIII and fibrinogen, TF, increased release of endothelial cell microparticles and decreased protein C levels. Moreover, patients with the metabolic syndrome exhibit endothelial dysfunction (mainly decreased production of nitric oxide and prostacyclin) and heightened platelet reactivity33. The activation of the haemostatic system related to the metabolic syndrome has been mainly attributed to the action of pro-inflammatory and pro-atherogenic mediators (e.g. leptin, tumour necrosis factor-α, interleukin-6) released by adipose cells33, to a triggering effect of very low density lipoproteins (VLDL) and remnant lipoproteins on platelet activation and PAI-1 gene expression41, to the adverse effects of chronic hyperglycaemia on fibrin structure and function (generating a clot more resistant to fibrinolysis)42 and to increased circulating microparticles that sustain blood coagulation by exposure of anionic phospholipids and TF 43.
This inflammatory and hypercoagulable state may explain the biological role of the major cardiovascular risk factors and could also be involved in VTE. Patients with idiopathic VTE have a higher prevalence of atherosclerosis than patients with VTE secondary to known risk factors and control subjects34. In addition, the long-term incidence of cardiovascular disease is higher in patients with idiopathic VTE than in those with secondary VTE44,45. These studies support the hypothesis of VTE as the first symptomatic cardiovascular event. Several epidemiological studies showed associations between obesity, metabolic syndrome or type 2 diabetes and VTE46–50. A meta-analysis50 of 63,552 patients showed that the relative risk of VTE was 2.33 (95% CI 1.68–3.24) for obesity, 1.42 (95% CI 1.12–1.77) for diabetes and 1.51 (95% CI 1.23–1.85) for hypertension (Table V). Obesity may confer an increased risk of VTE independently of the metabolic syndrome, because a high body weight can cause mechanical impairment of the valve system in the deep veins of the lower limbs, favouring venous stasis. In the same meta-analysis a non-significant increased risk for smoking was found50, while in a large population-based case-control study [Multiple Environmental and Genetic Assessment (MEGA) study], the relative risk of VTE was 1.42 (95% CI 1.28–1.58) in current smokers and 1.23 (95% CI 1.10–1.37) in past smokers, compared to the risk in individuals who had never smoked51.
Table V.
Associations between classic cardiovascular risk factors and VTE.
| Risk factor | OR (95% CI) |
|---|---|
| Obesity (BMI) | 2.33 (1.68–3.24)50 |
| Diabetes | 1.42 (1.12–1.77)50 |
| Hypertension | 1.51 (1.23–1.85)50 |
| Smoking | 1.42 (1.28–1.58)51 |
BMI = body mass index; OR = odds ratio; CI = confidence intervals.
Finally, also dyslipidaemia may exert a mild influence on the risk of VTE52,53, as determined by a recent meta-analysis in which patients with VTE had high triglyceride and low HDL cholesterol levels, while no effect of total cholesterolaemia on VTE was seen50. Moreover, preliminary evidence shows that statins may be protective against VTE54,55, supporting the hypothesis of dyslipidaemia influencing the risk of VTE.
In conclusion, despite the discrepancy between the estimated relative risks of VTE and atherothrombosis associated with cardiovascular risk factors, the latter may represent a link between two clinical entities which have classically been considered distinct.
Previous thrombosis
Despite the unambiguous benefit achieved with life-style modification, blood pressure control and the use of statins, angiotensin II-active and antiplatelet agents, the residual risk of recurrent acute events in patients with established atherothrombotic disease remains substantial56,57. The residual risk is likely related to progression of atherosclerosis despite the use of statins and angiotensin II-active agents, to insufficient inhibition of platelet activation by aspirin and thienopyridines, and to other factors not yet identified58. Studies that investigated the effect of novel approaches with or beyond statins on progression of atherosclerosis showed inconsistent results59–64. Response variability to antiplatelet therapy may contribute to the residual risk of thrombotic events. Sufficient evidence supports the hypothesis that a persistent enhanced platelet reactivity despite the use of aspirin65 and/or clopidogrel66 is associated with adverse clinical outcomes. A number of factors may influence response to antiplatelet therapy, including drug dose and absorption, patient’s compliance and genetic polymorphisms66. Novel approaches to limit platelet-mediated thrombosis, such as prasugrel, ticagrelor and cangrelor, appear promising. Nevertheless, current antiplatelet agents and those still in development, inhibit the thromboxane A2 or ADP platelet activation pathways but do not interfere with a number of other platelet activation pathways that may contribute to thrombotic events. In addition, dual antiplatelet therapy is associated with increased bleeding. These considerations underline the urgent need for novel therapies that provide more complete platelet inhibition and, therefore, greater protection against thrombotic events, possibly without increasing the bleeding risk.
The presence of a residual thrombus after a first episode of DVT is an independent risk factor for recurrence67. After a first episode of VTE, patients are 40 times more likely to develop a recurrent event compared to previously unaffected individuals68. Previous VTE represents the most important risk factor for recurrence of DVT or PE (OR 15.5; 95% CI 6.77–35.99) and the risk is higher in individuals with previous idiopathic VTE than in those with secondary VTE69. The risk of recurrence varies over time, being higher during the first 6–12 months after the index event70. In a study involving 355 patients, the incidence of recurrent VTE was 8.6% at 6 months and 17.5% after 2 years71. After 8 years, the rate of recurrence was as high as 30.3%71. Moreover, recurrent DVT or PE is associated with an increased risk of post-thrombotic syndrome and chronic thromboembolic pulmonary hypertension72. Secondary prevention of VTE is, therefore, crucial to reduce the burden of these diseases significantly and to date the most effective strategy is represented by anticoagulant therapy.
A potential mechanism by which the residual thrombus increases the risk of recurrence is impaired venous outflow, resulting in blood stasis and clot formation. However, because some patients develop recurrent thrombosis in the initially unaffected leg and others develop isolated PE, other mechanisms must be implicated. Residual thrombosis is perhaps a marker for a more generalized procoagulant diathesis. Indeed, elevated plasma D-dimer levels after withdrawal of oral anticoagulation (a marker of hypercoagulability) are an independent risk factor for recurrent venous thrombosis73,74.
Trauma, surgery and immobilisation
These transient conditions are associated with an increased risk of venous thrombosis. The incidence of DVT associated with major trauma is up to 58%, PE occurs in 2% of these individuals and is the third cause of death among patients who survive the first 24 hours after trauma75,76. Considering minor trauma, the incidence of DVT is 28% when lower limbs are involved and the risk is higher in proximal than in distal fractures77. In a large, population-based, case-control study the relative risk of VTE associated with previous minor injury was 3.1 (95% CI 2.5–3.8)78. Minor injuries in a leg were strongly associated with VTE (OR 5.1; 95% CI 3.9–6.7), whereas minor injuries in other parts of the body were not78. The presence of factor V Leiden in patients with a leg injury increased the risk up to 50-fold78. The risk of thrombosis associated with surgery varies from 15% to 60% in the case of a laparotomic approach79, while the risk associated with laparoscopic and arthroscopic surgery is less defined. Major orthopaedic surgery involving the lower extremity is a major risk factor for VTE. Rates of DVT without prophylaxis range from 40% to 60% in the 2 weeks after major orthopaedic surgery80. Patients undergoing total hip arthroplasty are in the highest risk category for developing post-operative VTE. Evaluation of the natural history of this population of patients reveals that the incidence of DVT without appropriate prophylaxis is as high as 32% to 60%, and that of PE is as high as 16%, with a 0.3% to 3.4% occurrence of fatal PE81. Current data suggest an overall DVT rate of 9.9% and a proximal DVT rate of 2.1% after knee arthroscopy without thromboprophylaxis82. Even with appropriate thromboprophylaxis, total hip or knee replacement will lead to symptomatic VTE in 1% to 3% of patients83. The mechanism by which these conditions lead to VTE is a combination of stasis and local accumulation of TF (i.e., hypercoagulability). Blood flow is relatively static in the pockets of venous valves, particularly those of the lower limbs. This effect is accentuated by immobilization. Stasis locally concentrates haemostasis activation factors (cytokines and other mediators of inflammation), favours cellular margination and interaction of circulating blood cells with endothelium, and is responsible for local hypoxia which is one of the principal mechanisms of endothelial activation84. Studies in animals have shown that stasis alone does not provoke thrombosis85. TF is expressed by cells in the subendothelial compartment. Thus, physical disruption of the endothelium, as occurs in trauma or surgery, may lead to exposure of blood to extravascular TF. However, the vast majority of venous thrombi occur in the context of an intact endothelium. In these cases, TF may be expressed on the surface of activated endothelial cells and/or mononuclear cells which have been stimulated by any number of inflammatory mediators including cytokines, chemokines (interleukins 1, 6 and 8, tumour necrosis factor-α, monocyte chemoattractant protein-1), vascular endothelial growth factor, factors derived by complement activation (C5a and complex of membrane attachment), immunocomplexes and antibodies, P-selectin, haemodynamic stress, hypoxia, and cell-cell interactions84,86. In addition to expressing TF on their cell surface, activated cells (e.g., endothelial cells, monocytes, leucocytes and platelets) may release TF- and phospholipid-rich microparticles that circulate in the bloodstream87. Microparticles can interact with other cells through the action of adhesive proteins. For example, P-selectin glycoprotein ligand 1 facilitates the transfer of P-selectin from platelets or endothelial cells to microparticles of monocyte origin88. These properties may facilitate thrombus propagation and activate coagulation in various sites. Finally, leucocytes and platelets can further enhance thrombosis through their expression of TF under inflammatory stimuli (C5a, bacterial formylated peptides, P–selectin) and platelet agonists (ADP, collagen, thrombin), respectively89.
Although VTE is the most frequent thrombotic complication of surgery, surgical iatrogenic injuries can also lead to arterial occlusion. Moreover, arterial thrombosis secondary to surgery can represent the first manifestation of heparin-induced thrombocytopenia, an autoimmune disease triggered by exposure to the heparin that is commonly given as antithrombotic prophylaxis of post-operative VTE. The clinical picture is characterised by transient thrombocytopenia (in more than 90% of cases the platelet count is >15,000/μL) and both arterial and venous thromboses, especially of the lower limbs, are described in surgical patients90.
Cancer
Cancer is one of the most important acquired risk factors for VTE91. Some authors estimate an annual incidence of VTE of 1 in 200 patients with cancer92, and 20% of VTE cases occur in patients with cancer93. Conversely, of all patients with cancer, 15% will develop symptomatic VTE93, 50% asymptomatic VTE94, and 50% will have VTE diagnosed at autopsy91. The risk of VTE is higher at diagnosis (OR 53.5; 95% CI 8.6–334.3) and in patients with distant metastases (OR 19.8; 95% CI 2.6–149.1)95. If a patient with cancer survives an initial VTE event, he or she has an increased risk of recurrence (OR 1.72; 95% CI 1.31–2.25) compared with that in a patient without cancer. The cancer patient with VTE also has a significantly increased risk of death (OR 8.1; 95% CI 3.6–18.1), which persists for as long as the malignancy persists71. In addition, VTE is the second leading cause of death in hospitalised patients with cancer, after infections96.
The pathophysiology of VTE in patients with cancer is even more complex than that in patients without. Three sets of factors have been linked to the increased risk of VTE in these patients: those related to the tumour, those related to the host, and those to the therapies the patient is receiving97. Tumour mass may create stasis by compression and invasion of vessels. Tumour cells may promote the release of TF from the affected organs during expansion and the metastatic processes. Importantly, cancer cells themselves may release TF-rich microparticles. These microparticles can then adhere to (and be incorporated into) monocytes and other cells, in particular those activated by hypoxia, and promote fibrin formation98,99. Finally, tumour cell-derived inflammatory and pro-angiogenic cytokines (e.g., tumour necrosis factor-α, interleukin 1 and mostly vascular endothelial growth factor) may induce TF expression as a host response in endothelial cells and monocyte-macrophages100. Data from several epidemiological investigations demonstrated significant heterogeneity in the risk of VTE according to the different cancer histology. Pancreatic cancer, lymphoma, and brain cancer have a relative risk for VTE greater than 25, whereas the VTE risk associated with cancer of the ovary, stomach, kidney, colon, rectum, and lung is lower (> 17), compared with that of individuals without cancer101. In the MEGA study, patients with haematological malignancies had the highest risk of VTE (OR 28.0; 95% CI 4.0–199.7), followed by that in patients with lung cancer and gastrointestinal cancer95.
Considering the therapeutic factors that influence the cancer-related risk of VTE, it has been shown that patients with cancer face double the risk of developing VTE because of oncological surgery, compared with the risk in patients without cancer undergoing similar surgery91. Chemotherapy increases the risk of thrombosis 6.5-fold102. The proposed mechanisms for chemotherapy-related risk of VTE probably include both direct drug-induced damage of the endothelium and an increased expression of TF procoagulant activity by macrophages and monocytes, thus inducing a procoagulant response by host cells103. Another prothrombotic mechanism of antitumour therapy is likely related to the direct hepatotoxicity of radio- and chemo-therapy, which can cause a reduction in the plasma levels of natural anticoagulant proteins (antithrombin, protein C and protein S)100. Women who are treated with tamoxifen for breast cancer have a 2- to 5-fold increased risk of VTE, and the risk is even higher after menopause and when tamoxifen is associated with chemotherapy104,105. Cancer patients with a central venous catheter or transvenous pacemaker have a 6-fold increase in upper-extremity DVT106. Finally, the presence of factor V Leiden in cancer patients increases the risk of VTE nearly 12-fold compared to those individuals without cancer and who have the wild-type factor V Leiden; similar results have been observed in cancer patients carrying the prothrombin G20210A variant95.
Oral contraceptives and hormone therapy
Various clinical studies have investigated the risk of thrombosis with hormone-based oral contraceptives. However, due to the variety of preparations and the heterogeneity of study populations, these studies showed different or even contradictory results. Evidence on oral contraceptive-related risk of VTE has mostly been derived from case-control and nested case-control studies, attributing a relative risk of VTE to oral contraceptive use (compared with non-use) ranging from 1.12 (95% CI 0.4–2.9) to 22.1 (95% CI 5.9–84.2)107. Overall, a 2- to 6-fold increased relative risk of VTE is observed in oral contraceptive users compared with non-users, for an absolute risk of 1 to 3 cases of VTE per 10,000 women-years107. An increased risk of VTE was reported among women using third-generation oral contraceptives, i.e., those containing desogestrel or gestodene, compared to those using second-generation products containing levonorgestrel108–111. A meta-analysis confirmed that third-generation oral contraceptives were associated with a significantly increased risk of VTE (RR 1.9; 95% CI 1.5–2.2) compared with that associated with second-generation oral contraceptives112. Another meta-analysis confirmed an overall adjusted odds ratio for third-versus second-generation oral contraceptives of 1.7 (95% CI 1.4–2.0)113. Moreover, the odds ratios for short-term users compared with those of longer term users were 2.5 (95% CI 1.6–4.1) and 2.0 (95% CI 1.4–2.7), respectively113. However, some authors emphasised that the difference in VTE risk according to whether second- or third-generation oral contraceptives are used is minimal, and probably related to underlying congenital or acquired thrombophilic states114. Second- and third-generation oral contraceptives (as well as pregnancy/post-partum) increase the risk of VTE in carriers of factor V Leiden by 3.3- fold and 4.2-fold, respectively, whereas other risk factors have a minor effect115.
An unequivocal mechanism for explaining the thrombogenicity of oral contraceptives (especially oestrogen compounds) has not been identified, as several metabolic abnormalities might be triggered to induce a mild prothrombotic state. Mechanisms include a direct effect of oestrogens on the vascular wall, changes in factors that promote endothelial dysfunction, and changes in coagulation factors. Studies in animals suggest a loss of the normal elastic configuration of the aorta, significant intimal thickening and an increase in endothelial permeability after administration of oral contraceptives116. There are also a few reports of increased venous distensibility and reduced blood flow in women taking oral contraceptives117. A possible explanation might be an oestrogen-induced dose-dependent increase in the expression of matrix metalloproteinases that cleave collagen and elastin in the vascular intima. The loss of venous tone, with the accompanying tendency to venous stasis, increases the risk of venous thrombosis. Oral contraceptives may increase the risk of arterial thrombosis by promoting endothelial dysfunction. However, this is a poorly investigated area and it has not been established how much these changes matter in the pathophysiology of thrombosis in oral contraceptive users118. Another mechanism increasing the risk of thrombosis, particularly that of atherothrombosis in women taking oral contraceptives, is linked to changes in lipids and lipoprotein metabolism. Oral contraceptives increase total cholesterol, mainly by increasing LDL cholesterol. In addition, oestrogens decrease HDL cholesterol and increase triglyceride levels, affect lipoprotein metabolism by increasing the hepatic synthesis of apolipoproteins, and may induce changes in hormones affecting lipoprotein metabolism such as cortisol, thyroxine or growth hormone119,120. Progestogen-only oral contraceptives have generally no or little effect on plasma lipoprotein levels.
Oral contraceptives modify the plasma levels of several coagulation factors (Table VI). However, these changes are often modest and concentrations of coagulation factors usually remain within the normal range. Oral contraceptive-mediated alterations in coagulation factor levels may result in synergistic or opposing effects on the risk of venous thrombosis. Levels of the anticoagulant proteins antithrombin and protein S decrease during oral contraceptive use, whereas protein C levels may increase121,122. The greatest effects are seen with preparations containing the highest oestrogen doses. Important effects of oral contraceptives on blood coagulation are an acquired resistance to activated protein C and the reduction of protein S plasma levels123. These and other changes in coagulation factors appear to be more pronounced in women using third-generation compounds than in those using second-generation compounds124,125, although the difference is debated126. Oral contraceptives also affect the fibrinolytic system by reducing t-PA levels and increasing levels of TAFI, PAI-1 and D-dimer. An overall increase in thrombin generation in women on oral contraceptives has recently been demonstrated by means of the endogenous thrombin potential test, i.e., the area under the thrombin generation curve, which is able to identify a global hypercoagulable state and has been found to be higher in oral contraceptive users than in non-users118,121,122,127.
Table VI.
Haemostatic changes during oral contraceptive (OC) use and pregnancy.
| Change during OC use | Change during pregnancy | |
|---|---|---|
| Procoagulant factors | ||
| fibrinogen, V, VII, VIII, IX, X, XII | ↑ | ↑ |
| XI | = or ↑ | ↓ |
| von Willebrand factor | = | ↑ |
| Anticoagulant proteins | ||
| antithrombin | ↓ | = |
| protein C | = or ↑ | = or ↑ |
| protein S | ↓ | ↓ |
| resistance to activated protein C (ratio) | ↓ | ↓ |
| Markers of thrombin formation | ||
| F1+2, TAT complexes, fibrinopeptide A | ↑ | ↑ |
| D-dimer | ↑ | ↑ |
| Fibrinolytic factors | ||
| TAFI, PAI 1 and 2 | ↑ | ↑ |
| t-PA | ↓ | ↓ |
↑ increase, ↓ decrease, = no change, compared to non-use of oral contraceptives and to the non-pregnant state.
Pregnancy
VTE remains the major cause of maternal mortality world-wide (the rate of maternal deaths from VTE is 0.12 per 10,000 live births and stillbirths)128. Results from studies in which either all or most pregnant women underwent accurate diagnostic testing for VTE report an incidence of VTE ranging from 0.6 to 1.3 events per 1,000 deliveries, confirming a 5- to 10-fold increased risk in pregnant women compared to that in non-pregnant women of comparable age129. The MEGA study showed that the risk of VTE is nearly 5-fold increased during pregnancy and up to 60-fold during the first 3 months after delivery130. A 14-fold increased risk of DVT of the legs and a 6-fold increased risk of PE were reported, with this risk being higher in the third trimester (OR 3.3; 95% CI 2.2–5.0) and during puerperium (OR 11.0; 95% CI 8.1–15.1), and highest in the 2 days before and the day after delivery (OR 77.6; 95% CI 52.4–114.8)131. The major risk factors for VTE during pregnancy include the presence of thrombophilic abnormalities, Caesarean section, advanced maternal age, obesity and pre-eclampsia, which are identified in nearly 70% of women with pregnancy- or puerperium-related VTE. It has been reported that the risk of pregnancy-related VTE might be 11- to 52-fold increased in factor V Leiden carriers, 3- to 31-fold in carriers of the prothrombin G20210A mutation, and more than 10-fold in those with deficiencies of antithrombin (7-fold for mild deficiency and 64-fold for severe deficiency), protein C (3.6-fold for mild deficiency and 7.2 fold for severe deficiency), or protein S (5-fold for mild deficiency) compared to non-pregnant women without thrombophilia130,133–135. An association between thrombophilia and adverse obstetric outcomes such as recurrent miscarriage, pre-eclampsia, placental abruption, foetal growth retardation, stillbirth and foetal death has been observed136–138, although it is not certain139.
From a biological point of view, normal pregnancy is characterised by a hypercoagulable state. Pregnancy is associated with haemostatic changes that include increased concentrations of most procoagulant factors, decreased concentrations of some of the natural anticoagulants and reduced fibrinolytic activity (Table VI). These changes help to maintain placental function during pregnancy and minimise blood loss at delivery. However, they may also predispose to maternal thrombosis and placental vascular complications. Plasma concentrations of coagulation factors V, VII, VIII, IX, X, and XII, fibrinogen and vWF rise significantly during pregnancy, while factor XI levels tend to decrease. Total and free protein S decrease, whereas protein C and antithrombin remain substantially unchanged140–141. Activated protein C resistance, likely caused by increasing factors V and VIII and decreasing protein S, is frequently observed in pregnancy142. The activation of coagulation is demonstrated by increasing levels of F1+2, TAT complexes, fibrinopeptide A and D-dimer141,142. These changes occur during the whole gestational period but are more pronounced in the third trimester. The fibrinolytic system is also impaired during pregnancy, as shown by increased plasma levels of TAFI, PAI-1 and -2 (the latter of placental origin) and decreased t-PA activity142,143. TF is largely expressed in the placenta and is markedly increased in the amniotic fluid but not in plasma143, and, together with thrombomodulin, is involved not only in haemostasis, but also in the differentiation of placental blood vessels144. Placental detachment at delivery with the ensuing release of trophoblastic substances at the site of separation is responsible, together with post-partum haemoconcentration, for the particularly high risk of VTE in the post-partum period145. Three weeks after delivery, blood coagulation and fibrinolysis have generally returned to normal146.
Air pollution
Air pollution consists of gaseous and particulate-matter pollutants. The former include carbon monoxide (CO), nitrogen dioxide (NO2), sulphur dioxide (SO2) and ozone (O3). The latter include particulate matter (PM) with a cut-off of less than 10 μm in aerodynamic diameter (PM10), fine particles of less than 2.5 μm (PM2.5) and ultrafine particles of less than 0.1 μm (PM0.1)147. As compared with PM10 and PM2.5, ultrafine particles have a larger total surface area and hence a greater potential for carrying toxic substances, including metals, elemental and organic carbon and others. Because of their small size, ultrafine particles are deposited deep in the lung alveoli and can reach the blood stream. Particulate matter is the type of air pollutant that causes the most numerous and serious effects on human health, because of the broad range of different toxic substances that it contains148,149. Over the last decade, a growing body of epidemiological and clinical evidence has led to a heightened concern about the deleterious effects of air pollution on the cardiovascular system150–152. Observations from studies across North America and Europe have shown higher rates of hospital admissions for all cardiovascular causes, and a direct association was also identified with the incidence of ischaemic heart disease and failure153. The correlation between PM2.5 levels and onset of symptoms in 772 patients with myocardial infarction was studied in a case-crossover study: elevated odds ratios were associated with an increase of 25 μg/m3 PM2.5 during a 2-h period before the event (OR 1.48; 95% CI 1.09–2.02) and an increase of 20 μg/m3 PM2.5 was observed in the 24-h period before the event (OR 1.69; 95% CI 1.13–2.34)154. In a study on air pollution and emergency admissions, an association was found between NO2 (12.7% increase), PM2.5 (8.6% increase) and the risk of hospitalisation for myocardial infarction155. In another crossover study, an increase in ambient particulates of 10 μg/m3 was associated with a 4.5% increased risk of acute coronary syndromes (unstable angina and myocardial infarction)156. The association between traffic-related air pollutants and acute myocardial infarction is also supported by the results of the European HEAPSS (Health Effects of Air Pollution among Susceptible Subpopulations) study157.
Potential mechanisms leading to cardiovascular disease include autonomic dysfunction, systemic and local inflammation, endothelial injury, and alterations in the coagulation cascade150,151. Changes in heart rate and heart-rate variability, arrhythmias, increase in markers of inflammation and tissue damage such as C-reactive protein, cytokines, interleukins and serum lipids are conditions induced by air pollution that affect the cardiovascular system151. Experimental and epidemiological studies evaluating plasma concentrations of coagulation factors in association with air pollution exposure have produced different results. While some studies found increased levels of factor VII, fibrinogen and vWF158–161, others showed decreased levels or no change162. More recently, a novel association between air pollution and hypercoagulability was observed both in healthy individuals and in patients with DVT163,164. Air pollution is associated with a shortened prothrombin time in healthy subjects162 and increased total plasma homocysteine levels in smokers163. A large case-control study164 showed that high mean PM10 levels in the year before venous thrombosis were associated with a significantly shortened prothrombin time, and that each increase of 10 μg/m3 in PM10 was associated with a 70% increase in risk of VTE. This effect was absent in women who used oral contraceptives. As the aforementioned coagulation changes induced by air pollution are similar in characteristics and degree to those observed in oral contraceptive users, it may be that coagulation is already activated by oral contraceptives so that no further enhancing effect is observed after exposure to PM10.
Travel
Over the past decades, several studies have investigated the relationship between thrombosis and travel, but whether or not long-distance travel and symptomatic VTE are truly associated is still debated, as most travellers who develop DVT or PE also have one or more other predisposing risk factors165. Considering an analysis of three large case-control studies on patients with clinically suspected DVT and PE, the resulting pooled odds ratio for the association between a median travel time of 7 hours and symptomatic VTE was negligible (OR 0.9; 95% CI 0.6–1.4)166. However, a further analysis of the duration of travel yielded an increased odds ratio of 2.5 (95% CI 1.0–6.2) in the category of 10 to 15 hours of travel166. A more recent review that summarised available data on this topic concluded that long-distance travel is associated with an up to 4-fold increased risk of VTE167. The absolute risk of a symptomatic event within 4 weeks of flights longer than 4 hours was 1 in 4600 flights, whereas the risk of acute PE increased with duration of travel, being up to 4.8 per million in flights longer than 12 hours167. Taken together, these data are consistent with the hypothesis that medium- to long-distance travellers have a 2- to 4-fold increased relative risk of VTE compared to non-travellers. Among the several plausible explanations for this increased risk are immobilisation and a sitting position. Tall individuals are particularly vulnerable because of cramped seating, and short individuals because their feet do not touch the floor and they, therefore, undergo extra compression of the popliteal veins168. Thrombin generation among travellers has been evaluated in several studies through measurements of F1+2 and its inhibitor complex TAT. Several studies investigating the effect of prolonged immobilisation on thrombin generation and on the fibrinolytic system have yielded conflicting results167 and the vast majority of these reports lacked a control group. The only controlled study published to date169 failed to find a difference in F1+2, TAT and D-dimer between travellers and non travellers. The effect of hypoxia (due to decreased cabin pressure) on coagulation has been investigated in both hypobaric and normobaric conditions. The results during hypobaric, but not normobaric, hypoxia support activation of the coagulation and fibrinolytic systems, reflected in a shortened activated partial thromboplastin time, decreased levels of fibrinogen and factor VIII170, factor VII antigen and TF pathway inhibitor (TFPI)171, increased levels of D-dimer170, F1+2, TAT and factor VIIa-TF complex171,172. However, two other studies found no difference in markers of thrombin generation during hypobaric or normobaric hypoxia173,174. Fibrinolysis was more activated during air travel than during immobilisation or deambulation, as shown in a crossover study175.
Conclusions
A large body of evidence over the past 20 years has improved our understanding of the biochemical mechanisms involved in the pathogenesis of thrombus formation, in arteries as well as in veins. We begin to understand that changes in blood coagulation, inflammation, and immune response are intricately linked and interdependent. Heightened generation of thrombin, the ultimate enzyme involved in coagulation, platelet activation and also cell-signalling effector molecules, is crucial not only in the development of VTE, but also of atherothrombosis. On the other hand, traditional cardiovascular risk factors may also play a role in VTE. Thus, pathogenesis of thrombosis has to be considered within a multifaceted perspective, as confirmed by the amount of epidemiological data on both genetic and environmental thrombotic risk factors. Nevertheless, a significant proportion of arterial and venous thrombotic episodes, especially among young individuals, occur without a plausible explanation. Further basic and clinical research is needed to reach a correct identification of new factors associated with VTE and/or arterial thrombosis, in order to assess the individual risk of thrombosis and promote more targeted prophylactic and therapeutic options.
Acknowledgments
We thank Dr. Mario Colucci for his help in designing the figures.
References
- 1.Hume M, Sevitt S, Thomas DP. Venous Thrombosis and Pulmonary Embolism. Harvard University Press; Cambridge, MA: 1970. [Google Scholar]
- 2.Gordon T, Kannel WB. Predisposition to atherosclerosis in the head, heart and legs. The Framingham Study. J Am Med Ass. 1972;221:661. [PubMed] [Google Scholar]
- 3.Nieto FJ. Cardiovascular disease and risk factor epidemiology: a look back at the epidemic of the 20th century. Am J Pub Health. 1999;89:292–94. doi: 10.2105/ajph.89.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lowe GDO, Rumley A, Woodward M, et al. Epidemiology of coagulation factors, inhibitors and activation markers: the Third Glasgow MONICA Survey I. Illustrative reference ranges by age, sex and hormone use. Br J Haematol. 1997;97:775–84. doi: 10.1046/j.1365-2141.1997.1222936.x. [DOI] [PubMed] [Google Scholar]
- 5.Rumley A, Emberson JR, Wannamethee SG, et al. Effects of older age on fibrin D-dimer, C-reactive protein and other hemostatic and inflammatory variables in men aged 60–79 years. J Thromb Haemost. 2006;4:982–7. doi: 10.1111/j.1538-7836.2006.01889.x. [DOI] [PubMed] [Google Scholar]
- 6.Abbate R, Prisco D, Rostagno C, et al. Age-related changes in the hemostatic system. Int J Clin Lab Res. 1993;23:1–3. doi: 10.1007/BF02592271. [DOI] [PubMed] [Google Scholar]
- 7.Franchini M. Hemostasis and aging. Crit Rev Oncol Hematol. 2006;60:144–151. doi: 10.1016/j.critrevonc.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Coppola R, Mari D, Lattuada A, Franceschi C. von Willebrand factor in Italian centenarians. Haematologica. 2003;88:39–43. [PubMed] [Google Scholar]
- 9.Kannell WB, Wolf PA, Castelli WP, et al. Fibrinogen and risk of cardiovascular disease. The Framingham Study. JAMA. 1987;258:1183–6. [PubMed] [Google Scholar]
- 10.Tracy RP. Hemostatic and inflammatory markers as risk factors for coronary disease in the elderly. Am J Geriatr Cardiol. 2002;11:93–100. doi: 10.1111/j.1076-7460.2002.00997.x. [DOI] [PubMed] [Google Scholar]
- 11.Ofosu FA, Craven S, Dewar L, et al. Age-related changes in factor VII proteolysis in vivo. Br J Haematol. 1996;94:407–12. doi: 10.1046/j.1365-2141.1996.d01-1793.x. [DOI] [PubMed] [Google Scholar]
- 12.Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008;359:938–49. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 13.Bauer KA, Weiss LM, Sparrow D, et al. Aging-associated changes in indices of thrombin generation and protein C activations in humans. Normative Aging Study. J Clin Invest. 1987;80:1527–34. doi: 10.1172/JCI113238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mari D, Mannucci PM, Coppola R, et al. Hypercoagulability in centenarians: the paradox of successful aging. Blood. 1995;85:3144–9. [PubMed] [Google Scholar]
- 15.Wilkerson WR, Sane DC. Aging and thrombosis. Sem Thromb Hemost. 2002;28:555–67. doi: 10.1055/s-2002-36700. [DOI] [PubMed] [Google Scholar]
- 16.Gleerup G, Winther K. The effect of ageing on platelet function and fibrinolytic activity. Angiology. 1995;46:715–8. doi: 10.1177/000331979504600810. [DOI] [PubMed] [Google Scholar]
- 17.Kasjanovova D, Balaz V. Age-related changes in human platelet function in vitro. Mech Ageing Dev. 1986;37:175–82. doi: 10.1016/0047-6374(86)90074-6. [DOI] [PubMed] [Google Scholar]
- 18.Bastyr EJ, 3rd, Kadrofske MM, Vinik AI. Platelet activity and phosphoinositide turnover increase with advancing age. Am J Med. 1990;88:601–6. doi: 10.1016/0002-9343(90)90525-i. [DOI] [PubMed] [Google Scholar]
- 19.Taddei S, Virdis A, Ghiadoni L, et al. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38:274–9. doi: 10.1161/01.hyp.38.2.274. [DOI] [PubMed] [Google Scholar]
- 20.Feyzi E, Saldeen T, Larsson E, et al. Age-dependent modulation of heparan sulfate structure and function. J Biol Chem. 1998;273:13395–8. doi: 10.1074/jbc.273.22.13395. [DOI] [PubMed] [Google Scholar]
- 21.Seligsohn U, Lubetsky A. Genetic susceptibility to venous thrombosis. N Engl J Med. 2001;344:1222–31. doi: 10.1056/NEJM200104193441607. [DOI] [PubMed] [Google Scholar]
- 22.Lane DA, Philippou H, Huntington JA. Directing thrombin. Blood. 2005;106:2605–12. doi: 10.1182/blood-2005-04-1710. [DOI] [PubMed] [Google Scholar]
- 23.Bertina RM, Koeleman BP, Koster T, et al. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature. 1994;369:64–7. doi: 10.1038/369064a0. [DOI] [PubMed] [Google Scholar]
- 24.Dahlbäck B, Carlsson M, Svensson PJ. Familial thrombophilia due to a previously unrecognized mechanism characterized by poor anticoagulant response to activated protein C: prediction of a cofactor to activated protein C. Proc Natl Acad Sci USA. 1993;90:1004–8. doi: 10.1073/pnas.90.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poort SR, Rosendaal FR, Reitsma PH, et al. A common genetic variation in the 3′-untranslated region of the prothrombin gene is associated with elevated plasma prothrombin levels and an increase in venous thrombosis. Blood. 1996;88:3698–703. [PubMed] [Google Scholar]
- 26.Ye Z, Liu EH, Higgings JP, et al. Seven hemostatic gene polymorphisms in coronary disease: meta-analysis of 66,155 cases and 91,307 controls. Lancet. 2006;367:1729–30. doi: 10.1016/S0140-6736(06)68263-9. [DOI] [PubMed] [Google Scholar]
- 27.Urbanus RT, Derksen RHMW, de Groot PG. Current insight into diagnostics and pathophysiology of the antiphospholipid syndrome. Blood Rev. 2008;22:93–105. doi: 10.1016/j.blre.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Charakida M, Besler C, Batuca JR, et al. Vascular abnormalities, paraoxonase activity, and dysfunctional HDL in primary antiphospholipid syndrome. JAMA. 2009;302:1210–7. doi: 10.1001/jama.2009.1346. [DOI] [PubMed] [Google Scholar]
- 29.Cattaneo M. Hyperhomocysteinemia, atherosclerosis and thrombosis. Thromb Haemost. 1999;81:165–76. [PubMed] [Google Scholar]
- 30.D’Angelo A, Selhub J. Homocysteine and thrombotic disease. Blood. 1997;90:1–11. [PubMed] [Google Scholar]
- 31.Franco RF, Reitsma PH. Genetic risk factors of venous thrombosis. Hum Genet. 2001;109:369–84. doi: 10.1007/s004390100593. [DOI] [PubMed] [Google Scholar]
- 32.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 33.Franchini M, Targher G, Montagnana M, et al. The metabolic syndrome and the risk of arterial and venous thrombosis. Thromb Res. 2008;122:727–35. doi: 10.1016/j.thromres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 34.Prandoni P, Bilora F, Marchioli A, et al. An association between atherosclerosis and venous thrombosis. N Engl J Med. 2003;348:1435–41. doi: 10.1056/NEJMoa022157. [DOI] [PubMed] [Google Scholar]
- 35.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case control study. Lancet. 2004;364:937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 36.Turnbull F. Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362:1527–35. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 37.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–78. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 38.Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ. 2004;328:1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wannamethee SG, Lowe GD, Shaper AG, et al. The metabolic syndrome and insulin resistance: relationship to haemostatic and inflammatory markers in older non-diabetic men. Atherosclerosis. 2005;181:101–8. doi: 10.1016/j.atherosclerosis.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 40.Lee KW, Lip GY. Effects of lifestyle on hemostasis, fibrinolysis, and platelet reactivity: a systematic review. Arch Intern Med. 2003;163:2368–92. doi: 10.1001/archinte.163.19.2368. [DOI] [PubMed] [Google Scholar]
- 41.Olufadi R, Byrne CD. Effects of VLDL and remnant particles on platelets. Pathophysiol Haemost Thromb. 2006;35:281–91. doi: 10.1159/000093221. [DOI] [PubMed] [Google Scholar]
- 42.Grant PJ. Diabetes mellitus as a prothrombotic condition. J Intern Med. 2007;262:157–72. doi: 10.1111/j.1365-2796.2007.01824.x. [DOI] [PubMed] [Google Scholar]
- 43.Arteaga RB, Chirinos JA, Soriano AO, et al. Endothelial microparticles and platelet and leukocyte activation in patients with the metabolic syndrome. Am J Cardiol. 2006;98:70–4. doi: 10.1016/j.amjcard.2006.01.054. [DOI] [PubMed] [Google Scholar]
- 44.Becattini C, Agnelli G, Prandoni P, et al. A prospective study on cardiovascular events after acute pulmonary embolism. Eur Heart J. 2005;26:77–83. doi: 10.1093/eurheartj/ehi018. [DOI] [PubMed] [Google Scholar]
- 45.Prandoni P, Ghirarduzzi A, Prins MH, et al. Venous thromboembolism and the risk of subsequent symptomatic atherosclerosis. J Thromb Haemost. 2006;4:1891–6. doi: 10.1111/j.1538-7836.2006.02058.x. [DOI] [PubMed] [Google Scholar]
- 46.Goldhaber SZ, Grodstein F, Stampfer MJ, et al. A prospective study of risk factors for pulmonary embolism in women. J Am Med Ass. 1997;277:642–5. [PubMed] [Google Scholar]
- 47.Hansson PO, Eriksson H, Welin L, et al. Smoking and abdominal obesity. Risk factors for VTE among middle-aged men. “The study of men born in 1913”. Arch Int Med. 1999;159:1886–90. doi: 10.1001/archinte.159.16.1886. [DOI] [PubMed] [Google Scholar]
- 48.Tsai A, Cushman M, Rosamond W, et al. Cardiovascular risk factors and VTE incidence. Arch Int Med. 2002;162:1182–9. doi: 10.1001/archinte.162.10.1182. [DOI] [PubMed] [Google Scholar]
- 49.Ageno W, Prandoni P, Romauldi E, et al. The metabolic syndrome and the risk of venous thrombosis: a case-control study. J Thromb Haemost. 2006;4:1914–8. doi: 10.1111/j.1538-7836.2006.02132.x. [DOI] [PubMed] [Google Scholar]
- 50.Ageno W, Becattini C, Brighton T, et al. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation. 2008;117:93–102. doi: 10.1161/CIRCULATIONAHA.107.709204. [DOI] [PubMed] [Google Scholar]
- 51.Pomp ER, Rosendaal FR, Doggen CJ. Smoking increases the risk of venous thrombosis and acts synergistically with oral contraceptive use. Am J Hematol. 2008;83:97–102. doi: 10.1002/ajh.21059. [DOI] [PubMed] [Google Scholar]
- 52.Vaya A, Mira Y, Ferrando F, et al. Hyperlipidemia and venous thromboembolism in patients lacking thrombophilic risk factors. Br J Haematol. 2002;118:255–9. doi: 10.1046/j.1365-2141.2002.03563.x. [DOI] [PubMed] [Google Scholar]
- 53.Deguchi H, Pecheniuk NM, Elias DJ, et al. High-density lipoprotein deficiency and dyslipoproteinemia associated with venous thrombosis in men. Circulation. 2005;112:893–9. doi: 10.1161/CIRCULATIONAHA.104.521344. [DOI] [PubMed] [Google Scholar]
- 54.Ray J. Dyslipidemia, statins and venous thromboembolism: a potential risk factor and a potential treatment. Curr Opin Pulm Med. 2003;9:378–84. doi: 10.1097/00063198-200309000-00007. [DOI] [PubMed] [Google Scholar]
- 55.Glynn RJ, Danielson E, Fonseca FA, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009;360:1851–61. doi: 10.1056/NEJMoa0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Antithrombotic Trialists’ Collaboration Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cannon CP, Steinberg BA, Murphy SA, et al. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol. 2006;48:438–45. doi: 10.1016/j.jacc.2006.04.070. [DOI] [PubMed] [Google Scholar]
- 58.Pepine CJ. Residual risk for secondary ischemic events in patients with atherothrombotic disease: opportunity for future improvements in patient care. Ann Med. 2010;42:19–35. doi: 10.3109/07853890903260898. [DOI] [PubMed] [Google Scholar]
- 59.Kastelein JJ, van Leuven SI, Burgess L, et al. Effect of torcetrapib on carotid atherosclerosis in familial hypercholesterolemia. N Engl J Med. 2007;356:1620–30. doi: 10.1056/NEJMoa071359. [DOI] [PubMed] [Google Scholar]
- 60.Nissen SE, Tardif JC, Nicholls SJ, et al. Effect of torcetrapib on the progression of coronary atherosclerosis. N Engl J Med. 2007;356:1304–16. doi: 10.1056/NEJMoa070635. [DOI] [PubMed] [Google Scholar]
- 61.Kastelein JJ, Akdim F, Stroes ES, et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008;358:1431–43. doi: 10.1056/NEJMoa0800742. [DOI] [PubMed] [Google Scholar]
- 62.Crouse J, 3rd, Raichlen JS, Riley WA, et al. Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: the METEOR Trial. JAMA. 2007;297:1344–53. doi: 10.1001/jama.297.12.1344. [DOI] [PubMed] [Google Scholar]
- 63.Smilde TJ, van Wissen S, Wollersheim H, et al. Effect of aggressive versus conventional lipid lowering on atherosclerosis progression in familial hypercholesterolaemia (ASAP): a prospective, randomised, double-blind trial. Lancet. 2001;357:577–81. doi: 10.1016/s0140-6736(00)04053-8. [DOI] [PubMed] [Google Scholar]
- 64.Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–22. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 65.Krasopoulos G, Brister SJ, Beattie WS, et al. Aspirin ‘resistance’ and risk of cardiovascular morbidity: systematic review and meta-analysis. BMJ. 2008;336:195–8. doi: 10.1136/bmj.39430.529549.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, et al. Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives. J Am Coll Cardiol. 2007;49:1505–16. doi: 10.1016/j.jacc.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 67.Prandoni P, Lensing AWA, Prins MH, et al. Residual venous thrombosis as a predictive factor of recurrent venous thromboembolism. Ann Intern Med. 2002;137:955–60. doi: 10.7326/0003-4819-137-12-200212170-00008. [DOI] [PubMed] [Google Scholar]
- 68.Kearon C. Natural history of venous thromboembolism. Circulation. 2003;107:122–30. doi: 10.1161/01.CIR.0000078464.82671.78. [DOI] [PubMed] [Google Scholar]
- 69.Samama MM. An epidemiologic study of risk factors for deep vein thrombosis in medical outpatients: the Sirius study. Arch Intern Med. 2000;160:3415–20. doi: 10.1001/archinte.160.22.3415. [DOI] [PubMed] [Google Scholar]
- 70.Fowkes FJ, Price JF, Fowkes FG. Incidence of diagnosed deep vein thrombosis in the general population: systematic review. Eur J Vasc Endovasc Surg. 2003;25:1–5. doi: 10.1053/ejvs.2002.1778. [DOI] [PubMed] [Google Scholar]
- 71.Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125:1–7. doi: 10.7326/0003-4819-125-1-199607010-00001. [DOI] [PubMed] [Google Scholar]
- 72.Baglin T. What happens after venous thromboembolism? J Thromb Haemost. 2009;7:287–90. doi: 10.1111/j.1538-7836.2009.03409.x. [DOI] [PubMed] [Google Scholar]
- 73.Eichinger S, Minar E, Bialonczyk C, et al. D-dimer levels and risk of recurrent venous thromboembolism. JAMA. 2003;290:1071–4. doi: 10.1001/jama.290.8.1071. [DOI] [PubMed] [Google Scholar]
- 74.Palareti G, Cosmi B, Legnani C, et al. for the PROLONG Investigators D-dimer testing to determine the duration of anticoagulation therapy. N Engl J Med. 2006;355:1780–9. doi: 10.1056/NEJMoa054444. [DOI] [PubMed] [Google Scholar]
- 75.Rogers FB. Venous thromboembolism in trauma patients: a review. Surgery. 2001;130:1–12. doi: 10.1067/msy.2001.114558. [DOI] [PubMed] [Google Scholar]
- 76.Rocha AT, Tapson VF. Venous thromboembolism in intensive care patient. Clinical Chest Med. 2003;24:103–22. doi: 10.1016/s0272-5231(02)00056-4. [DOI] [PubMed] [Google Scholar]
- 77.Abelseth G, Buckley RE, Pineo GE, et al. Incidence of DVT in patients with fractures of the lower extremity distal to the hip. J Orthop Trauma. 1996;10:230–5. doi: 10.1097/00005131-199605000-00002. [DOI] [PubMed] [Google Scholar]
- 78.van Stralen KJ, Rosendaal FR, Doggen CJ. Minor injuries as a risk factor for venous thrombosis. Arch Intern Med. 2008;168:21–6. doi: 10.1001/archinternmed.2007.5. [DOI] [PubMed] [Google Scholar]
- 79.Geerts WH, Pineo GP, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:338–400S. doi: 10.1378/chest.126.3_suppl.338S. [DOI] [PubMed] [Google Scholar]
- 80.White RH, Zhou H, Romano PS. Incidence of symptomatic venous thromboembolism after different elective or urgent surgical procedures. Thromb Haemost. 2003;90:446–55. doi: 10.1160/TH03-03-0152. [DOI] [PubMed] [Google Scholar]
- 81.Pineo GF, Hull RD. Prophylaxis of venous thromboembolism following orthopaedic surgery: mechanical and pharmacological approaches and the need for extended prophylaxis. Thromb Haemost. 1999;82:918–24. [PubMed] [Google Scholar]
- 82.Ilahi OA, Reddy J, Ahmad I. Deep venous thrombosis after knee arthroscopy: a meta-analysis. Arthroscopy. 2005;21:727–30. doi: 10.1016/j.arthro.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 83.Rosendaal FR. Venous thrombosis: the role of genes, environment, and behavior. Hematology (Am Soc Hematol Educ Program) 2005. pp. 1–12. [DOI] [PubMed]
- 84.Arnout J, De Gaetano G, Hoylaerts M, et al. Thrombosis Fundamental and Clinical Aspects. Leuven: Leuven University Press; 2003. [Google Scholar]
- 85.Day SM, Reeve JL, Pedersen B, et al. Macrovascular thrombosis is driven by tissue factor derived primarily from the blood vessel wall. Blood. 2005;105:192–8. doi: 10.1182/blood-2004-06-2225. [DOI] [PubMed] [Google Scholar]
- 86.Mackman N. Role of tissue factor in hemostasis, thrombosis, and vascular development. Arterioscl Thromb Vasc Biol. 2004;24:1015–22. doi: 10.1161/01.ATV.0000130465.23430.74. [DOI] [PubMed] [Google Scholar]
- 87.Lynch SF, Ludlam CA. Plasma microparticles and vascular disorders. Br J Haematol. 2007;137:36–48. doi: 10.1111/j.1365-2141.2007.06514.x. [DOI] [PubMed] [Google Scholar]
- 88.Chirinos JA, Heresi GA, Velasquez H, et al. Elevation of endothelial microparticles, platelets and leukocyte activation in patients with venous thromboembolism. J Am Coll Cardiol. 2005;45:1467–71. doi: 10.1016/j.jacc.2004.12.075. [DOI] [PubMed] [Google Scholar]
- 89.Maugeri N, Brambilla M, Camera M, et al. Human polymorphonuclear leukocytes produce and express functional tissue factor upon stimulation. J Thromb Haemost. 2006;4:1323–1330. doi: 10.1111/j.1538-7836.2006.01968.x. [DOI] [PubMed] [Google Scholar]
- 90.Greinacher A. Heparin-induced thrombocytopenia. J Thromb Haemost. 2009;7:9–12. doi: 10.1111/j.1538-7836.2009.03385.x. [DOI] [PubMed] [Google Scholar]
- 91.Falanga A, Zacharski L. Deep vein thrombosis in cancer: the scale of the problem and approaches to management. Ann Oncol. 2005;16:696–701. doi: 10.1093/annonc/mdi165. [DOI] [PubMed] [Google Scholar]
- 92.Lee AY, Levine MN. Venous thromboembolism and cancer: risks and outcomes. Circulation. 2003;107:I17–I21. doi: 10.1161/01.CIR.0000078466.72504.AC. [DOI] [PubMed] [Google Scholar]
- 93.Caine GJ, Stonelake PS, Lip GY, et al. The hypercoagulable state of malignancy: pathogenesis and current debate. Neoplasia. 2002;4:465–73. doi: 10.1038/sj.neo.7900263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Johnson MJ, Walker ID, Sproule MW, et al. Abnormal coagulation and deep venous thrombosis in patients with advanced cancer. Clin Lab Haematol. 1999;21:51–4. doi: 10.1046/j.1365-2257.1999.00163.x. [DOI] [PubMed] [Google Scholar]
- 95.Blom JW, Doggen CJM, Osanto S, et al. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293:715–22. doi: 10.1001/jama.293.6.715. [DOI] [PubMed] [Google Scholar]
- 96.Ambrus JL, Ambrus CM, Mink IB, et al. Causes of death in cancer patients. J Med. 1975;6:61–4. [PubMed] [Google Scholar]
- 97.Kessler CM. The link between cancer and venous thromboembolism. Am J Clin Oncol. 2009;32:S3–S7. doi: 10.1097/COC.0b013e3181b01b17. [DOI] [PubMed] [Google Scholar]
- 98.Prandoni P, Falanga A, Piccioli A. Cancer and venous thromboembolism. Lancet Oncol. 2005;6:401–10. doi: 10.1016/S1470-2045(05)70207-2. [DOI] [PubMed] [Google Scholar]
- 99.Hron G, Kollars M, Weber H, et al. Tissue factor-positive microparticles: cellular origin and association with coagulation activation in patients with colorectal cancer. Thromb Haemost. 2007;97:119–123. [PubMed] [Google Scholar]
- 100.Key N, Makris M, O’Shaughnessy D, et al. Practical Hemostasis and Thrombosis. 2nd ed. Oxford, UK: Wiley-Blackwell; 2009. [Google Scholar]
- 101.Heit JA. Cancer and venous thromboembolism: scope of the problem. Cancer Control. 2005;12:5–10. doi: 10.1177/1073274805012003S02. [DOI] [PubMed] [Google Scholar]
- 102.Lyman GH, Khorana AA, Falanga A, et al. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol. 2007;25:5490–505. doi: 10.1200/JCO.2007.14.1283. [DOI] [PubMed] [Google Scholar]
- 103.Palumbo A, Rajkumar SV, Dimopoulos MA, et al. Prevention of thalidomide- and lenalinomide-associated thrombosis in myeloma. Leukemia. 2008;22:414–23. doi: 10.1038/sj.leu.2405062. [DOI] [PubMed] [Google Scholar]
- 104.Fisher B, Costantino J, Redmond C, et al. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen receptor-positive tumors. N Engl J Med. 1989;320:479–84. doi: 10.1056/NEJM198902233200802. [DOI] [PubMed] [Google Scholar]
- 105.Pritchard KI, Paterson AH, Paul NA, et al. Increased thromboembolic complications with concurrent tamoxifen and chemotherapy in a randomized trial of adjuvant therapy for women with breast cancer. National Cancer Institute of Canada Clinical Trials Group Breast Cancer Site Group. J Clin Oncol. 1996;14:2731–7. doi: 10.1200/JCO.1996.14.10.2731. [DOI] [PubMed] [Google Scholar]
- 106.Hutten BA, Prins MH, Gent M, et al. Incidence of recurrent thromboembolic and bleeding complications among patients with venous thromboembolism in relation to both malignancy and achieved international normalized ratio: a retrospective analysis. J Clin Oncol. 2000;18:3078–83. doi: 10.1200/JCO.2000.18.17.3078. [DOI] [PubMed] [Google Scholar]
- 107.Gomes MP, Deitcher SR. Risk of venous thromboembolic disease associated with hormonal contraceptives and hormone replacement therapy: a clinical review. Arch Intern Med. 2004;164:1965–76. doi: 10.1001/archinte.164.18.1965. [DOI] [PubMed] [Google Scholar]
- 108.World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception Venous thromboembolic disease and combined oral contraceptives: results of an international multicentre case-control study. Lancet. 1995;346:1575–82. [PubMed] [Google Scholar]
- 109.Jick H, Jick SS, Gurewich V, et al. Risk of idiopathic cardiovascular death and nonfatal venous thromboembolism in women using oral contraceptives with differing progestagen components. Lancet. 1995;346:1589–93. doi: 10.1016/s0140-6736(95)91928-7. [DOI] [PubMed] [Google Scholar]
- 110.Bloemenkamp KW, Rosendaal FR, Helmerhorst FM, et al. Enhancement by factor V Leiden mutation of risk of deep-vein thrombosis associated with oral contraceptives containing a third-generation progestagen. Lancet. 1995;346:1593–6. doi: 10.1016/s0140-6736(95)91929-5. [DOI] [PubMed] [Google Scholar]
- 111.Spitzer WO, Lewis MA, Heinemann LA, et al. Third generation oral contraceptives and risk of venous thromboembolic disorders: an international case-control study. BMJ. 1996;312:83–8. doi: 10.1136/bmj.312.7023.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Farley TM, Meirik O, Collins J. Cardiovascular disease and combined oral contraceptives: reviewing the evidence and balancing the risks. Hum Reprod Update. 1999;5:721–35. doi: 10.1093/humupd/5.6.721. [DOI] [PubMed] [Google Scholar]
- 113.Kemmeren JM, Algra A, Grobbee DE. Third generation oral contraceptives and risk of venous thrombosis: meta-analysis. BMJ. 2001;323:131–4. doi: 10.1136/bmj.323.7305.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mammen EF. Oral contraceptive pills and hormonal replacement therapy and thromboembolic disease. Hematol Oncol Clin North Am. 2000;14:1045–59. doi: 10.1016/s0889-8588(05)70170-2. [DOI] [PubMed] [Google Scholar]
- 115.Simioni P, Sanson BJ, Prandoni P, et al. Incidence of venous thromboembolism in families with inherited thrombophilia. Thromb Haemost. 1999;81:198–202. [PubMed] [Google Scholar]
- 116.Almen T, Härtel M, Nylander G, et al. The effect of estrogen on the vascular endothelium and its possible relation to thrombosis. Surg Gynecol Obstet. 1975;140:938–40. [PubMed] [Google Scholar]
- 117.Fawer R, Dettling A, Weihs D, et al. Effect of the menstrual cycle, oral contraception and pregnancy on forearm blood flow, venous distensibility and clotting factors. Eur J Clin Pharmacol. 1978;13:251–7. doi: 10.1007/BF00716359. [DOI] [PubMed] [Google Scholar]
- 118.Godsland IF, Winkler U, Lidegaard Ø, et al. Occlusive vascular diseases in oral contraceptive users. Epidemiology, pathology and mechanisms. Drugs. 2000;60:721–869. doi: 10.2165/00003495-200060040-00003. [DOI] [PubMed] [Google Scholar]
- 119.Wynn V, Doear J, Mills G, et al. Fasting serum triglyceride, cholesterol, and lipoprotein levels during oral-contraceptive therapy. Lancet. 1969;2:756–60. doi: 10.1016/s0140-6736(69)90476-0. [DOI] [PubMed] [Google Scholar]
- 120.Wynn V, Doear J, Mills G, et al. Some effects of oral contraceptives on serum-lipid and lipoprotein levels. Lancet. 1966;2:720–3. doi: 10.1016/s0140-6736(66)92979-5. [DOI] [PubMed] [Google Scholar]
- 121.Kluft C, Lansink M. Effect of oral contraceptives on hemostasis variables. Thromb Haemost. 1997;78:315–26. [PubMed] [Google Scholar]
- 122.Winkler UH. Blood coagulation and oral contraceptives: a critical review. Contraception. 1998;57:203–9. doi: 10.1016/s0010-7824(98)00020-1. [DOI] [PubMed] [Google Scholar]
- 123.van Vliet HA, Bertina RM, Dahm AE, et al. Different effects of oral contraceptives containing different progestogens on protein S and tissue factor pathway inhibitor. J Thromb Haemost. 2008;6:346–51. doi: 10.1111/j.1538-7836.2008.02863.x. [DOI] [PubMed] [Google Scholar]
- 124.Rosing J, Middeldorp S, Curvers J, et al. Low-dose oral contraceptives and acquired resistance to activated protein C: a randomized cross-over study. Lancet. 1999;354:2036–40. doi: 10.1016/s0140-6736(99)06092-4. [DOI] [PubMed] [Google Scholar]
- 125.Tans G, Curvers J, Middeldorp S, et al. A randomized cross-over study in the effects of levonorgestrel- and desogestrel-containing oral contraceptives on the anticoagulant pathways. Thromb Haemost. 2000;84:15–21. [PubMed] [Google Scholar]
- 126.Winkler UH. Hemostatic effects of third- and second-generation oral contraceptives: absence of a causal mechanism for a difference in risk of venous thromboembolism. Contraception. 2000;62:11S–20S. doi: 10.1016/s0010-7824(00)00148-7. [DOI] [PubMed] [Google Scholar]
- 127.Tchaikovski SN, van Vliet HA, Thomassen MC, et al. Effect of oral contraceptives on thrombin generation measured via calibrated automated thrombography. Thromb Haemost. 2007;98:1350–6. [PubMed] [Google Scholar]
- 128.Tan JY. Thrombophilia in pregnancy. Ann Acad Med Singapore. 2002;31:328–34. [PubMed] [Google Scholar]
- 129.Bates SM, Greer IA, Pabinger I, et al. Venous thromboembolism, thrombophilia, antithrombotic therapy, and pregnancy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2008;133:844S–886S. doi: 10.1378/chest.08-0761. [DOI] [PubMed] [Google Scholar]
- 130.Pomp ER, Lenselink AM, Rosendaal FR, et al. Pregnancy, the postpartum period and prothrombotic defects: risk of venous thrombosis in the MEGA study. J Thromb Haemost. 2008;6:632–637. doi: 10.1111/j.1538-7836.2008.02921.x. [DOI] [PubMed] [Google Scholar]
- 131.Ros HS, Lichtenstein P, Bellocco R, et al. Pulmonary embolism and stroke in relation to pregnancy: how can high-risk women be identified? Am J Obstet Gynecol. 2002;186:198–203. doi: 10.1067/mob.2002.119177. [DOI] [PubMed] [Google Scholar]
- 132.McColl MD, Ramsay JE, Tait RC, et al. Risk factors for pregnancy associated venous thromboembolism. Thromb Haemost. 1997;78:1183–8. [PubMed] [Google Scholar]
- 133.Martinelli I, De Stefano V, Taioli E, et al. Inherited thrombophilia and first venous thromboembolism during pregnancy and puerperium. Thromb Haemost. 2002;87:791–5. [PubMed] [Google Scholar]
- 134.Gerhardt A, Scharf RE, Beckmann MW, et al. Prothrombin and factor V mutations in women with a history of thrombosis during pregnancy and the puerperium. N Engl J Med. 2000;342:374–80. doi: 10.1056/NEJM200002103420602. [DOI] [PubMed] [Google Scholar]
- 135.Gerhardt A, Scharf RE, Zotz RB. Effect of hemostatic risk factors on the individual probability of thrombosis during pregnancy and the puerperium. Thromb Haemost. 2003;90:77–85. [PubMed] [Google Scholar]
- 136.Gammaro L, Lippi G, Bottini E, et al. Severe preeclampsia in antithrombin III deficiency with no history of venous thromboembolism. J Nephrol. 2001;14:312–5. [PubMed] [Google Scholar]
- 137.Brenner B. Thrombophilia and fetal loss. Semin Thromb Hemost. 2003;29:165–70. doi: 10.1055/s-2003-38831. [DOI] [PubMed] [Google Scholar]
- 138.Rodger MA, Paidas M. Do thrombophilias cause placenta-mediated pregnancy complications? Semin Thromb Hemost. 2007;33:597–603. doi: 10.1055/s-2007-985756. [DOI] [PubMed] [Google Scholar]
- 139.Rodger MA, Paidas M, McLintock C, et al. Inherited thrombophilia and pregnancy complications revisited. Obstet Gynecol. 2008;112:320–4. doi: 10.1097/AOG.0b013e31817e8acc. [DOI] [PubMed] [Google Scholar]
- 140.Bloom AL, Forbes CD, Thomas DP, et al. Hemostasis and Thrombosis. 3rd ed. Edinburgh, UK: Churchill Livingstone; 1994. [Google Scholar]
- 141.Brenner B. Hemostatic changes in pregnancy. Thromb Res. 2004;114:409–14. doi: 10.1016/j.thromres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 142.Clark P, Brennard J, Conkie JA, et al. Activated protein C sensitivity, protein C, protein S and coagulation in normal pregnancy. Thromb Haemost. 1998;79:1166–70. [PubMed] [Google Scholar]
- 143.Franchini M. Haemostasis and pregnancy. Thromb Haemost. 2006;95:401–13. doi: 10.1160/TH05-11-0753. [DOI] [PubMed] [Google Scholar]
- 144.Lockwood CJ, Krikun G, Runic R, et al. Progestin epidermal growth factor regulation of tissue factor expression during decidualization of human endometrial stromal cells. J Clin Endocrinol Metab. 2000;85:297–301. doi: 10.1210/jcem.85.1.6292. [DOI] [PubMed] [Google Scholar]
- 145.Lanir N, Aharon A, Brenner B. Hemostatic mechanisms in human placenta. Best Pract Res Clin Haematol. 2003;16:183–95. doi: 10.1016/s1521-6926(02)00098-1. [DOI] [PubMed] [Google Scholar]
- 146.Dalhamn T, Hellgren M, Blomback M. Changes in blood coagulation and fibrinolysis in the normal puerperium. Gynecol Obstet Invest. 1985;20:37–44. doi: 10.1159/000298969. [DOI] [PubMed] [Google Scholar]
- 147.WHO Working Group Health Aspects of Air Pollution with Particulate Matter, Ozone and Nitrogen Dioxide. Accessed on 13/04/10 at http:/www.euro.who.int/air/activities/20050222_2).
- 148.Schwarze PE, Ovrevik J, Lag M, et al. Particulate matter properties and health effects: consistency of epidemiological and toxicological studies. Hum Exp Toxicol. 2006;25:559–79. doi: 10.1177/096032706072520. [DOI] [PubMed] [Google Scholar]
- 149.Bathnagar A. Environmental cardiology. Studying mechanistic links between pollution and heart disease. Circ Res. 2006;99:692–705. doi: 10.1161/01.RES.0000243586.99701.cf. [DOI] [PubMed] [Google Scholar]
- 150.Franchini M, Mannucci PM. Short-time effect of air pollution on cardiovascular diseases outcomes and mechanisms. J Thromb Haemost. 2007;5:2169–74. doi: 10.1111/j.1538-7836.2007.02750.x. [DOI] [PubMed] [Google Scholar]
- 151.Brook RD, Franklin B, Cascio W, et al. Air pollution and cardiovascular disease. A statement for health case professionals from the expert panel on population and prevention science of the American Heart Association. Circulation. 2004;109:2655–71. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 152.Vermylen J, Nemmar A, Nemery B, et al. Ambient air pollution and acute myocardial infarction. J Thromb Haemost. 2005;3:1955–1961. doi: 10.1111/j.1538-7836.2005.01471.x. [DOI] [PubMed] [Google Scholar]
- 153.Hoek G, Brunekreef B, Fischer P, et al. The association between air pollution and heart failure, arrhythmia, embolism, thrombosis, and other cardiovascular causes of death in a time series study. Epidemiology. 2001;12:355–7. doi: 10.1097/00001648-200105000-00017. [DOI] [PubMed] [Google Scholar]
- 154.Peters A, Dockery DW, Muller JE, et al. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103:2810–5. doi: 10.1161/01.cir.103.23.2810. [DOI] [PubMed] [Google Scholar]
- 155.Zanobetti A, Schwartz J. Air pollution and emergency admissions in Boston, MA. J Epidemiol Community Health. 2006;60:890–5. doi: 10.1136/jech.2005.039834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pope CA, 3rd, Muhlestein JB, May HT, et al. Ischemic heart disease events triggered by short-term exposure to fine particulate air pollution. Circulation. 2006;114:2443–8. doi: 10.1161/CIRCULATIONAHA.106.636977. [DOI] [PubMed] [Google Scholar]
- 157.Lanki T, Pekkanen J, Aalto P, et al. Associations of traffic related air pollutants with hospitalisation for first acute myocardial infarction: the HEAPSS study. Occup Environ Med. 2006;63:844–51. doi: 10.1136/oem.2005.023911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Seaton A, Soutar A, Crawford V, et al. Particulate air pollution and the blood. Thorax. 1999;54:1027–32. doi: 10.1136/thx.54.11.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Liao D, Heiss G, Chinchilli VM, et al. Association of criteria pollutants with plasma hemostatic/inflammatory markers: a population-based study. J Expo Anal Environ Epidemiol. 2004;15:319–28. doi: 10.1038/sj.jea.7500408. [DOI] [PubMed] [Google Scholar]
- 160.Pekkanen J, Brunner EJ, Anderson HR, et al. Daily concentrations of air pollution and plasma fibrinogen in London. Occup Environ Med. 2000;57:818–22. doi: 10.1136/oem.57.12.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ruckerl R, Ibald-Mulli A, Koenig W, et al. Air pollution and markers of inflammation and coagulation in patients with coronary heart disease. Am J Respir Crit Care Med. 2006;173:432–41. doi: 10.1164/rccm.200507-1123OC. [DOI] [PubMed] [Google Scholar]
- 162.Baccarelli A, Zanobetti A, Martinelli I, et al. Effects of exposure to air pollution on blood coagulation. J Thromb Haemost. 2007;5:252–260. doi: 10.1111/j.1538-7836.2007.02300.x. [DOI] [PubMed] [Google Scholar]
- 163.Baccarelli A, Zanobetti A, Martinelli I, et al. Air pollution, smoking and plasma homocysteine. Environ Health Perspect. 2007;115:176–81. doi: 10.1289/ehp.9517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Baccarelli A, Martinelli I, Zanobetti A, et al. Exposure to particulate air pollution and risk of deep vein thrombosis. Arch Int Med. 2008;168:920–7. doi: 10.1001/archinte.168.9.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gallus AS. Travel, venous thromboembolism, and thrombophilia. Semin Thromb Hemost. 2005;31:90–6. doi: 10.1055/s-2005-863810. [DOI] [PubMed] [Google Scholar]
- 166.Ten Wolde M, Kraaijenhagen RA, Schiereck J, et al. Travel and the risk of symptomatic venous thromboembolism. Thromb Haemost. 2003;89:499–505. [PubMed] [Google Scholar]
- 167.Kuipers S, Schreijer AJM, Cannegieter SC, et al. Travel and venous thrombosis: a systematic review. J Intern Med. 2007;262:615–34. doi: 10.1111/j.1365-2796.2007.01867.x. [DOI] [PubMed] [Google Scholar]
- 168.Cannegieter SC, Doggen CJM, van Houwelingen HC, et al. Travel-related venous thrombosis: results from a large population-based case control study (MEGA study) PLoS Med. 2006;3:1258–65. doi: 10.1371/journal.pmed.0030307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tardy B, Tardy-Poncet B, Bara L, et al. Effect of long travels in sitting position in elderly volunteers on biological markers of coagulation activation and fibrinolysis. Thromb Res. 1996;83:153–60. doi: 10.1016/0049-3848(96)00116-8. [DOI] [PubMed] [Google Scholar]
- 170.Colucci G, Stricker H, Roggiani W, et al. Venous stasis and thrombin generation. J Thromb Haemost. 2004;2:1008–9. doi: 10.1111/j.1538-7836.2004.00748.x. [DOI] [PubMed] [Google Scholar]
- 171.Simons R, Krol J. Jet leg, pulmonary embolism, and hypoxia. Lancet. 1996;348:416. doi: 10.1016/s0140-6736(05)65046-5. [DOI] [PubMed] [Google Scholar]
- 172.Hodkinson PD, Hunt BJ, Parmar K, et al. Is mild normobaric hypoxia a risk factor for venous thromboembolism? J Thromb Haemost. 2003;1:2131–3. doi: 10.1046/j.1538-7836.2003.00407.x. [DOI] [PubMed] [Google Scholar]
- 173.Toff WD, Jones CI, Ford I, et al. Effect of hypobaric hypoxia, simulating conditions during long-haul air travel, on coagulation, fibrinolysis, platelet function, and endothelial activation. JAMA. 2006;295:2251–61. doi: 10.1001/jama.295.19.2251. [DOI] [PubMed] [Google Scholar]
- 174.Schobersberger W, Schobersberger B, Mittermayr M, et al. Air travel, hypobaric hypoxia, and prothrombotic changes. JAMA. 2006;296:2313–4. doi: 10.1001/jama.296.19.2313-b. [DOI] [PubMed] [Google Scholar]
- 175.Schreijer AJM, Cannegieter SC, Meijers JCM, et al. Activation of coagulation system during air travel: a crossover study. Lancet. 2006;367:832–8. doi: 10.1016/S0140-6736(06)68339-6. [DOI] [PubMed] [Google Scholar]