Introduction
Elective surgery is the most common cause of major bleeding, that is, loss of 20% or more of the volume of blood; cardiovascular surgery and orthopaedic operations (hip and knee replacements, spinal surgery), as well as liver transplants and resections are particularly strongly associated with substantial intra-operative bleeding1.
Post-partum haemorrhage is an important cause of maternal morbidity and mortality2. Primary postpartum haemorrhage is defined by the World Health Organization as the loss of more than 500 mL of blood in the first 24 hours after delivery3,4.
Management of the patient in the intra-operative period
The correct intra-operative management of the patient includes evaluation and monitoring of the following parameters5:
amount of blood lost;
haemoglobin (Hb) or haematocrit (Htc);
signs of inadequate perfusion and oxygenation of the vital organs;
platelet count;
prothrombin time (PT), activated partial thromboplastin time (aPTT), fibrinogen, antithrombin (AT), D-dimer.
The evaluation of blood loss should be based on the volume of blood removed from the surgical field by aspirators and that absorbed by gauzes and swabs.
This estimate can be difficult in obstetric cases if the blood lost remains within the uterus, in the broad ligament or in the peritoneal cavity, with modest or no signs of external bleeding: it is, therefore, suggested that particular attention should be paid to evaluating clinical signs of haemorrhagic shock (Grade of recommendation: 2C)6.
It has also been suggested that the evaluation of the patient is targeted to detect the presence of abnormal microvascular bleeding, a sign of coagulopathy (Grade of recommendation: 2C)5.
The intracellular partial pressure of oxygen (pO2) represents the decisional parameter “of choice” for evaluating tissue hypoxia7–9; it cannot, however, be used in clinical practice and for this reason Hb and Htc, which are “surrogate” parameters, are used10.
Tissue oxygenation depends on various factors10:
- the concentration of Hb;
- the saturation of Hb, which in its turn is dependent on the oxygen (O2) tension and the affinity of Hb for O2;
- the demand for O2, that is, the volume of O2 necessary for tissues to carry out their aerobic function.
The physiological mechanisms of adaptation to anaemia (increased cardiac output, increased coronary artery blood flow, redistribution of blood flow, increased O2 extraction, increased red blood cell 2,3-diphosphoglycerate) can be affected by7,10–16:
- limited increase in the cardiac output: hypovolaemia, coronary artery disease, disorders of heart valves, congestive heart disease, negative inotropes;
- impaired ability to increase O2 extraction: acute respiratory distress syndrome (ARDS), sepsis, systemic inflammatory response syndrome (SIRS), syndrome of ischaemia-reperfusion injury;
- altered gas exchange: chronic obstructive pulmonary disease (COPD), ARDS;
- increased O2 consumption: fever, pain, stress, sepsis, SIRS, hyperventilation syndromes.
The platelet count is one of the decisional parameters for platelet transfusion, along with the clinical evaluation of the patient (Grade of recommendation: 2C)17–19.
PT and aPTT are fundamental laboratory parameters which, in the presence of haemorrhage, guide the therapeutic decision regarding transfusion of fresh-frozen plasma (FFP)18.
The diagnosis of disseminated intravascular coagulation (DIC) can be made or excluded on the basis of an integrated dynamic evaluation of the clinical picture, laboratory data [PT, aPTT, fibrinogen, AT, D-dimer] and of the patient’s underlying pathology (Grade of recommendation: 2C)20.
In the case of suspected DIC, the fibrinogen level should be measured by the Clauss method (Grade of recommendation: 2C+)20–22.
Physiological transfusion threshold
Table I shows the clinical and instrumental parameters that, in the anaemic and normovolaemic patient, are indicative of inadequate perfusion and oxygenation of vital organs (physiological transfusion triggers)23–27.
Table I.
Clinical and instrumental parameters indicative of hypoxia in the anaemic, normovolaemic patient27§.
|
Cardiopulmonary symptoms
Electrocardiographic signs typical of ischaemia
Global indices of insufficient O2 release, evaluated by invasive methods
|
Notes
At term, pregnant women have about a 45% increase (about 1.5 L) in blood volume, with a greater increase in plasma than in red blood cells, leading to the so-called haemodilution anaemia of pregnancy which reduces the Htc by about 10%6.
Tachycardia may already be present at baseline in pregnant women or develop as the result of an infusion of a tocolytic6.
Placental perfusion in patients with hypertensive disorders during pregnancy, such as pre-eclampsia or haemolytic anaemia with elevated liver enzymes and low platelet count (HELLP) syndrome, can be inadequate at blood pressure values tolerated by other patients because of the increased peripheral resistance6.
Effect of anaesthesia on the cardiovascular response to acute anaemia
Acute normovolaemic anaemia in the conscious patient causes a physiological increase in cardiac output through effects of both increased systolic volume and heart rate15,28–31; in the anaesthetised patient, on the other hand, cardiac output only increases as a result of an increase in systolic volume15,30,32–35 and, for this reason, the onset of tachycardia, or a further increase in already existing tachycardia in pregnant women (in whom it is already present at baseline)6, in the presence of acute anaemia is an indicator of hypovolaemia, which must be corrected with crystalloids/colloids10,15.
The monitoring of adequate perfusion and oxygenation of vital organs should normally be based on evaluations of arterial blood pressure, heart rate, body temperature, O2 saturation, pH, volume of urine and electrocardiographic traces but, in particular conditions, may be based on the evaluation of echocardiographic findings, mixed venous O2 saturation or blood-gas analyses (Grade of recommendation: 2C)5.
Fluid therapy to restore intravascular blood volume
The main therapeutic strategy in the treatment of acute haemorrhage is to prevent or correct hypovolaemic shock10. In order to ensure tissue oxygenation it is essential to restore the circulating blood volume by an infusion of crystalloids/colloids, in sufficient amounts to maintain adequate blood flow and blood pressure.
Crystalloid and non-protein colloid solutions are the treatment of first choice36–39; 5% albumin is used as a second choice when crystalloid and non-protein colloids have already been used at maximum doses, without having produced an adequate clinical response and in cases in which non-protein colloids are contra-indicated (Grade of recommendation: 1A)36–39.
Albumin 5% and saline are clinically equivalent in terms of mortality and morbidity outcomes at 28 days in restoring intravascular blood volume in patients in intensive care40.
A meta-analysis in 2008 concluded that all colloids are equally safe41, without, however, excluding clinically significant differences between albumin and dextran.
Care is recommended when using artificial colloids in patients with impaired renal function (Grade of recommendation: 1C+)42,43.
High molecular weight hydroxyethyl starch solutions should not be used, in order to avoid alterations in haemostatis due to impaired platelet function (Grade of recommendation: 1C+)44–50.
Transfusion therapy
Effect of bleeding on haemostasis
Intra-operative transfusion support is aimed at correcting acute anaemia and treating clotting disorders and secondary forms of thrombocytopenia5. In fact, a massive, acute haemorrhage can cause hypovolaemic shock with consequent tissue hypoxia, acidosis, hypothermia and a systemic inflammatory response, which can trigger DIC51.
Furthermore, the reduction of the Htc can “mechanically” affect platelet function, because it shifts blood flow towards the centre of the vessel’s lumen and reduces the interactions between the platelets themselves and the endothelium51; anaemia also acts on haemostasis, causing vasodilatation and inhibition of platelet function because of reduced production of adenosine-diphosphate and thromboxane and the lesser availability of Hb to eliminate nitric oxide51.
The available blood components and plasma-derived drugs
The blood components that can be used for transfusion support are whole blood from predeposit or pre-operative normovolaemic haemodilution, autologous red cell concentrates (RCC), derived from predeposit or intra-operative salvage, allogeneic RCC and platelet concentrates, allogeneic or autologous FFP and cryoprecipitate and allogeneic or autologous blood components for topical use (fibrin glue)17,52,53; the plasma-derived drugs that can be used are albumin, AT and fibrinogen.
The pools of platelet concentrates from single units of whole blood and the platelet concentrates from apheresis contain approximately the same amount of platelets; comparative studies have shown that they are equivalent in terms of post-transfusion platelet increment and haemostatic efficacy, if transfused fresh, and also with regards incidence of side effects (Grade of recommendation: 1A)48,54–58.
Likewise, FFP prepared from units of whole blood and that obtained by apheresis are equivalent in terms of haemostasis and side effects (Grade of recommendation: 1A)59.
In cardiovascular surgery, the routine use of apheresis procedures in the immediate pre-operative period, in order to produce platelet concentrates or units of FFP from apheresis is not recommended because the documented superiority of benefits compared to the possible risks for the patient is marginal, because it is often impossible to produce therapeutic doses of autologous blood components and because of the costs of the procedure itself (Grade of recommendation: 1C+)60–70.
Transfusion therapy of acute anaemia
The decision to transfuse allogeneic or autologous RCC or autologous whole blood in the intra-operative period depends on the concentration of Hb, the amount and speed of the blood loss and the clinical condition of the patient, in particular whether he or she is showing signs and symptoms of reduced local or general oxygenation (Table I)5,8,10,15,23–27,71–94.
Rapid measurement of haematological parameters (Hb and Htc) by “point of care” (POC) analysers can raise the margins of safety and optimise transfusion support82. It is recommended that the POC instruments used for this purpose are automated systems that do not require the dilution of whole blood during the preanalytic phase (Grade of recommendation: 1C+)95.
A loss of less than 15% of the blood volume does not usually produce symptoms or require transfusion, providing there is not pre-existing anaemia (Table II) (Grade of recommendation: 1C+)10,73,76,80,81,85,91,96.
Table II.
Decisional criteria for transfusion in acute anaemia.
| Class of haemorrhage | Reduction of volaemia % | Blood loss (mL)* | Indication for transfusion of RCC | GoR |
|---|---|---|---|---|
| Class I | <15% | <750 | Non necessary, if no pre-existing anaemia | 1C+ |
| Class II | 15–30% | 750–1,500 | Non necessary, unless pre-existing anaemia and/or cardiopulmonary disease | 1C+ |
| Class III | 30–40% | 1,500–2,000 | Probably necessary | 1C+ |
| Class IV | >40% | >2,000 | Necessary | 1C+ |
Legend: RCC: red cell concentrate; GoR: grade of recommendation
*: in an adult person weighing 70 kg and with a circulating blood volume of 5,000 mL
When there is a loss of between 15% and 30% of the blood volume, compensatory tachycardia occurs and transfusion is indicated only in the presence of pre-existing anaemia or concomitant cardiopulmonary disease (Table II) (Grade of recommendation: 1C+)10,73,76,80,81,85,91,96,97.
Blood losses of more than 30% can cause shock and, when the blood loss exceeds 40%, the shock becomes severe. The probability of having to use transfusion therapy with red blood cells increases notably with losses of 30–40%, even though, in previously healthy subjects, volume replacement alone may be sufficient (Table II) (Grade of recommendation: 1C+)10,73,76,79,80,81,85,91,96.
Transfusion becomes a life-saving therapy when more than 40% of the patient’s blood is lost (Table II) (Grade of recommendation: 1C+)10,73,76,79,80,81,85,91,96.
Patients with acute haemorrhage can have normal, or even raised, values of Htc and Hb until the plasma volume is restored; in these cases, therefore, the clinical examination of the patient becomes extremely important (Grade of recommendation: 2C+)10,73,76,79 81,85,91,92,96,97.
However, as a guide, Hb values below 60 g/L almost always indicate the need for transfusion therapy (Table III) (Grade of recommendation: 1C+)5,10,27,70,73,78–81,83–85,89,92,96,97.
Table III.
Indications for transfusion therapy with RCC in patients with acute anaemia.
| Hb values | Presence of risk factors/mechanisms of compensation | TT with RCC | GoR |
|---|---|---|---|
| ≤ 60 g/L | Transfusion therapy is almost always necessary* | YES* | 1C+ |
| 60–80 g/L | Absence of risk factors/adequate mechanisms of compensation | NO | 1C+ |
| Presence of risk factors (for example, coronary arterydisease, heart failure, cerebrovascular disease/limited mechanisms of compensation) | YES | 1C+ | |
| Presence of symptoms indicative of hypoxia (physiological transfusion triggers: tachycardia, hypotension, electrocardiographic signs of ischemia, lactic acidosis, etc.) | YES | 1C+ | |
| 80–100 g/L | Presence of symptoms indicative of hypoxia (physiological transfusion triggers: tachycardia, hypotension (physiological transfusion triggers: tachycardia, hypotension, electrocardiographic signs of ischemia, lactic acidosis, etc.) | YES | 2C |
| >100 g/L | Transfusion therapy is required extremely rarely** | NO** | 1A |
Notes
- Hb values do not guarantee an adequate measure of the capacity to release O2 to tissues.
- In the presence of hypovolaemia the Htc does not reflect blood loss.
- The presence of individual risk factors may make it necessary to use transfusion triggers other than those indicated.
Legend: RCC: red cell concentrate; GoR: grade of recommendation; TT, transfusion therapy.
Values of Hb below 60 g/L can be tolerated provided an evaluation of the individual patient has excluded risk factors and inadequate mechanisms of compensation;
The individual patient must be evaluated to establish whether transfusion therapy is indicated to raise the values of Hb above 100 g/L.
In stable patients with Hb values between 60 and 100 g/L, an evaluation of the patients’ clinical status is necessary (Table III) (Grade of recommendation: 1C+)5,10,27,70,73,78–81,83–85,89,92,96,97.
It is very rarely necessary to transfuse patients whose Hb concentration is higher than 100 g/L (Table III) (Grade of recommendation: 1A)5,10,27,70,73,78–85,89,92,96,97.
Inappropriate indications
Inappropriate indications for the use of RCC or whole blood in the intra-operative period are10:
- anaemia with a Hb above 100 g/L (in the absence of specific risk factors related to the patient’s clinical characteristics);
- expansion of the blood volume.
Adverse events
Transfusion therapy with RCC or whole blood can cause adverse events which are classified according to their aetiopathogenesis and the time of onset with respect to the transfusion10.
- Immediate reactions with an immunological mechanism:
- - acute haemolytic reactions;
- - non-haemolytic febrile reactions;
- - allergic reactions (anaphylaxis, urticaria);
- - non-cardiogenic acute pulmonary oedema (Transfusion-Related Acute Lung Injury - TRALI), which develops within 6–8 hours after the transfusion.
- Delayed reactions with an immunological mechanism:
- - delayed haemolytic reactions;
- - Graft-versus-Host Disease;
- - post-transfusion purpura;
- - alloimmunisation.
- Immediate reactions with a non-immunological mechanism:
- - reactions to bacterial contamination;
- - circulatory overload;
- - non-immunological haemolysis.
- Delayed reactions with a non-immunological mechanism:
- - iron overload;
- - post-transfusion infections: viral or protozoan (in particular malaria) diseases are possible, but very rare.
Acute normovolaemic haemodiluition
Acute normovolaemic haemodilution is an autotransfusion procedure that was introduced in the 1970s98–100. It consists in removing at least three or four units of autologous blood while maintaining isovolaemia immediately before elective surgery16,66,101–104.
Volaemia should be maintained by infusing crystalloids at a dose of 2–3 mL for every 1 mL of blood removed, or colloids, at a 1:1 ratio with the volume of the blood removed (Grade of recommendation: 2C)103.
Acute normovolaemic haemodilution is generally performed after the induction of anaesthesia101, immediately before the surgical incision105. The responsibility for managing this activity and other procedures that do not involve the storage of blood components, such as intra-operative and post-operative blood salvage, is usually that of the anaesthetists53,66,106.
Candidates for acute normovolaemic haemodilution must have Hb values at least near the upper limit of the norm, must fulfil the same clinical criteria as those for suitability for autologous predeposit66,103 and the predicted blood loss during the operation must be more than 50% of the circulating volume or, at any rate, not less than 1,500 mL (Grade of recommendation: 1C+)16,27,82,101–104,107–109.
The rationale of acute normovolaemic haemodilution is to reduce the Htc before the intraoperative bleeding, in order to limit the loss of red blood cells103. The efficacy of acute normovolaemic haemodilution in reducing the need for allogeneic blood transfusion does, however, remain doubtful110. Various clinical studies, including prospective randomised ones, have demonstrated that acute normovolaemic haemodilution can reduce the use of allogeneic transfusion therapy in patients undergoing elective heart surgery, orthopaedic surgery (knee replacements), abdominal, vascular, urological, maxillo-facial and hepatic operations and even in patients undergoing surgery for burns111–122; other studies, however, did not show any substantial benefit or even found an increased use of allogeneic blood123–125.
The doubts on the real benefits of this procedure were further reinforced by some meta-analyses126–129. The relative risk of receiving allogeneic blood at any peri-operative moment were not, in fact, significantly reduced by using acute normovolaemic haemodilution and, likewise, there was no reduction in a comparison of acute normovolaemic haemodilution with other pharmacological techniques for blood saving, such as tranexamic acid which, in elective orthopaedic surgery, was found to be more effective than acute normovolaemic haemodilution in containing the use of allogeneic transfusion128,130. The relative risk of allogeneic transfusion was not reduced significantly in relation to the degree of haemodilution used, the type of surgery (orthopaedic, cardiac, urological, thoracic or vascular operations), the Htc taken as the transfusion threshold, the number of patients enrolled in the studies analysed or the year in which these were performed; indeed, the strategy seemed to be less effective in the more recent studies128. As regards secondary outcomes, such as intra-operative and post-operative bleeding and the incidence of adverse events, acute normovolaemic haemodilution reduced total bleeding (weighted mean difference = 91 mL), but increased intra-operative bleeding in heart surgery, liver surgery and thoracic surgery115,128,131–133; furthermore, there are reports of an increase in the relative risk of repeat surgery because of bleeding134, as well as a general lack of information on the safety of the procedure126,128,129,134.
The use of acute normovolaemic haemodilution cannot be recommended as a routine method for sparing the use of allogeneic blood (Grade of recommendation: 1A)70,104,126–129.
Acute normovolaemic haemodilution could be used in selected groups of patients (those with rare blood groups or multiple alloimmunisation), in whom it is absolutely necessary to avoid giving an allogeneic transfusion, and/or in the context of local protocols that integrate various different strategies (surgical, anaesthesiological, pharmacological) of saving blood (Grade of recommendation: 2B)70,102,104,110,126–129.
Furthermore, it has been suggested that “restrictive” transfusion protocols should be adopted in order to reduce the exposure of patients to autologous and/or homologous transfusion therapy (Grade of recommendation: 1C+)134.
Acute normovolaemic haemodilution may be taken into consideration in the management of Jehovah’s Witnesses, who can accept this technique provided that the blood removed remains within a closed circuit in continuity with the patient’s own blood circulation (Grade of recommendation: 2C)102,135.
The autologous units must be collected using procedures that comply with current legislation52, must be identified unequivocally and should be maintained at room temperature for no more than 6 hours (Grade of recommendation: 1C+)27,66,101,103,104,136.
The volume of blood to remove can be calculated using the formula proposed by Gross101,103,104,137.
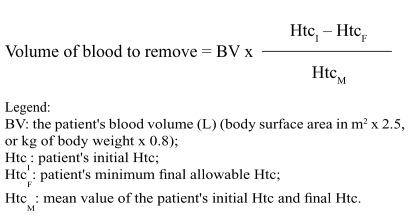 |
The units of blood should be re-infused in the reverse order from that of their withdrawal, since the first units have a higher Htc and greater content of clotting factors and platelets (Grade of recommendation: 2C)101,103,138,139.
Whenever, for selected types of operation and patients, acute normovolaemic haemodilution is combined with intra-operative blood salvage (IOBS), the salvaged units of red cells should be reinfused first, followed by the units of whole blood from acute normovolaemic haemodilution (Grade of recommendation: 2C)101,103,138–140.
Intra-operative blood salvage
IOBS is a blood-saving technique in which blood lost from the operating field is re-used; this blood is aspirated and anticoagulated before passing into a collection reservoir and from here, through filters for microaggregates of various diameter, into the bowl of the specific cell separators, to be concentrated by centrifugation and subsequently washed with saline before being reinfused into the patient81,135,140. A higher degree of flow turbulence of blood aspirated from the operating field is a cause of haemolysis and can be the result of high suction pressures135,140–145, or an incorrect method of aspiration (generation of foam, suction at the interface between blood and air, use of aspirators whose suction holes are too narrow)135–140.
It is suggested that the pressure of suction of the blood from the operating field should be kept between 80 and 120 mmHg, avoiding levels higher than 150 mmHg, unless for brief periods to clear the operating field if rapid and unexpected bleeding occurs (Grade of recommendation: 2C)135,140–143,145.
The formation of foam should be avoided during IOBS and the aspirator should be immersed directly in the blood; instruments with the widest possible suction holes should be used (Grade of recommendation: 2C)135.
If the aspiration field is shallow and flat, as for example, in surgery of the vertebral column, the formation of foam during the aspiration and mechanical stress to red blood cells can be reduced by irrigating the surgical field with physiological saline (Grade of recommendation: 2C+)146.
The blood recovered is anticoagulated with heparin, at a dose of 30,000 IU/L of saline, or with citrate-based solutions [citric acid/sodium citrate/dextrose (ACD), citrate/phosphate/dextrose/adenine (CPD-A)]; there is still controversy regarding the optimal anticoagulant135,140. Both the anticoagulant solutions are generally used, through the aspiration systems, at a fixed ratio of 15 mL per 100 mL of blood aspirated135,140,141.
The process of concentration by centrifugation of the bowls enables the plasma, platelets, and irrigating solutions to be removed, as well as 70–90% of the soluble contaminants present in the salvaged blood110,140.
The subsequent washing with saline removes the remaining soluble contaminants and the socalled “biochemical debris”104,129,134,140,141,145,147 (fibrinogen and fibrin degradation products, D-dimer, tissue plasminogen activator, activated fibrinolysis products such as plasmin, components of the activated complement cascade such as C3a and C5a, cytokines, proteolytic enzymes, cardiac or other enyzmes, cell stroma and cell fragments, activated leucocytes, free Hb, bacteria and endotoxins, fat, anticoagulant solutions, metal ions and fragments deriving from orthopaedic prostheses) and prevents the onset of a broad range of complications (DIC, multiorgan failure, microembolism, acute renal failure, respiratory or circulatory failure, electrolyte disturbances, immunosuppression, thrombosis, haemorrhage)81,110,129,134,135,140,141,145,147–149.
It is recommended that IOBS procedures are not used for autologous blood which will not be washed before reinfusion (Grade of recommendation: 1A)81,104,110,129,134,135,140,141,145,147–150.
IOBS should be used with caution in orthopaedic revision surgery of metal-metal prostheses because of the possible contamination by Co or Cr ions or by metal particles, despite appropriate washing prior to reinfusion (Grade of recommendation: 2C)151.
The process of washing can generally be considered completed if the fluid exiting from the cell separator appears clear and transparent and the volume of fluid used for the washing (1–2 litres) is at least three times the volume of the bowl (Grade of recommendation: 2C)104,140,145.
The Htc of autologous RCC obtained by IOBS depends on the washing programme used by the cell separator and can vary from 55% to 80%16,104,152. The recirculation of blood salvaged and returned to the patient through IOBS makes it difficult to calculate the real blood loss that occurs during the surgical procedure135. However, the following formula can be used to estimate this loss153:
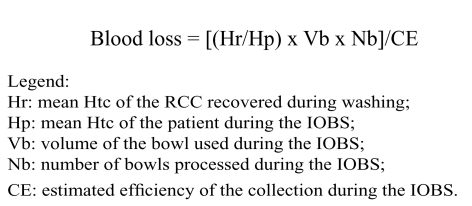 |
The accuracy of the calculation of blood loss depends on the efficiency of the collection. The efficiency of the IOBS varies depending on several factors including, mainly, the size of the suction holes of the aspirator, the precision of the operator responsible for the IOBS, the duration of the contact between the blood and the operating field; however, if the IOBS procedure is carried out carefully, its efficiency can reach 60–70%135,140.
If the estimated collection efficiency is not considered reliable, using the same formula, the blood loss can in any case be determined to lie within a range between a minimum volume (maximum collection efficiency) and maximum volume (minimum collection efficiency)135.
Autologous RCC obtained by IOBS are usually transfused immediately; in special circumstances they can be stored, for a maximum of 6 hours, at 4±2 °C, but must be identified unequivocally (Grade of recommendation: 1C+)27,66,81.
Indications
IOBS may be indicated in many types of elective and emergency surgery, when blood loss is expected to be at least 800–1,000 mL of 20% of more of the patient’s circulating blood volume16,154.
The fields of use (heart surgery, orthopaedic surgery, vascular surgery, emergency surgery, neurosurgery, obstetrics, cancer surgery [in selected cases], liver transplantation) should be identified according to local protocols that take into account the patients’ characteristics (haemorrhagic risk, multiple alloimmunisation) and expectations, type of operation, requirements of the surgical and anaesthetic teams, a cost-efficiency evaluation carried out in the setting of the individual hospital, as well as any local programmes aimed at achieving self-sufficiency in blood components (Grade of recommendation: 2C)140.
IOBS can be taken into consideration in the management of Jehovah’s Witnesses, who can accept the technique provided that the blood collection circuit, the cell separator and the units of RCC salvaged remain within a closed circuit in continuity with the patient’s own blood circulation (Grade of recommendation: 2C)16,155.
Heart surgery
Between 2004 and 2006, three systematic reviews analysed the role of intra- and post-operative blood recovery in reducing allogeneic transfusion needs in adults patients undergoing elective heart surgery, orthopaedic surgery and vascular surgery129,134,150. In heart surgery and orthopaedic surgery the relative risk of receiving allogeneic transfusion therapy was reduced more by using IOBS procedures in which the blood returned was washed. The reduction in the relative risk of allogeneic transfusion in vascular operations was not statistically significant. The authors concluded that the use of blood salvage techniques that involve washing the collected blood is justified in heart surgery and in orthopaedic surgery, although the trials included in the reviews were not of high methodological quality and the number of patients enrolled was limited. The efficacy of the salvage decreased where the use of transfusion therapy was guided by specific guidelines and protocols.
A meta-analysis in 2009156 confirmed that IOBS guarantees the safety and quality of the recovered blood157 and significantly reduces the need for allogeneic transfusions during heart surgery; it is, therefore, recommended that this strategy is used for recovering blood lost throughout the operation, whether this involves beating heart surgery or for operations with cardiopulmonary bypass (before, during and after this), as well as for washing residual blood in the heart-lung machine in the case of cardiopulmonary bypass operations (Grade of recommendation: 1A)70,129,134,150,156,157.
There are systems for aspirating blood that is shed into the thoracic cavity. Such systems, which were introduced in the 1960s, are designed to return the blood lost from the operating field into the cardiopulmonary bypass158; given the principle on which they are based and the construction of the equipment, these systems are very haemolytic159–161 and a possible cause of acute renal damage, thrombocytopenia and secondary platelet defects162,163. Furthermore, the salvaged blood contains large amounts of cell debris and lipid microparticles164,165, which, in animal models, are responsible for cerebral microemboli166. The use of such blood, even when washed with a cell separator specific for IOBS, has been associated with greater bleeding in the post-operative period and a greater need for FFP156,167,168.
It is, therefore, recommended that the use of blood recovered with suction systems from the operating field during cardiopulmonary bypass is limited to emergency circumstances, even if the blood is washed with a cell separator specific for IOBS (Grade of recommendation: 1C+)70,156–168.
Other intra-operative techniques for saving blood during heart surgery
Retrograde priming of the cardiopulmonary bypass circuit with autologous blood (up to 1,100 mL) can be used as a complementary intra-operative method of saving blood (Grade of recommendation: 2B)70,105,169–176. This strategy is used with the purpose of replacing the crystalloids used to fill the circuitry of the heart-lung machine and, thereby, avoid the risk of excessive haemodilution and minimise transfusion needs, particularly of patients with small intravascular blood volumes.
Beating heart surgery for coronary artery bypass, if feasible in the light of the patient’s characteristics and the experience of the surgical and anaesthetic team, is a recommended strategy for reducing the incidence of clotting disorders and transfusion needs, particularly if combined with IOBS (Grade of recommendation: 1C+)70,105,110,177–184.
Orthopaedic surgery
The meta-analyses currently available show that both autologous predeposit and IOBS significantly reduce allogeneic transfusion needs in elective surgery129,149,150,185–194; however, the use of predeposited blood leads to an increase in the total number of units (autologous and/or allogeneic) transfused185,186.
The use of IOBS and the consequent reinfusion of washed RCC reduces the relative risk of receiving allogeneic transfusion therapy129,134,150.
In major orthopaedic surgery, IOBS can be a cost-effective procedure and better than autologous predeposit in reducing allogeneic transfusion, especially if the predoposit, when indicated, is not carried out respecting optimal time intervals187–195.
IOBS should be used in major orthopaedic surgery in all those cases in which the use of predeposit is not indicated, not practicable for religious reasons, or not possible with respect to the time needed for the patient’s haematological recovery: in these cases IOBS should be used, also on the basis of local protocols that include integrated recourse to other techniques of saving blood (pharmacological, surgical and anaesthesiological), taking into consideration the characteristics of the individual patients (haemorrhagic risk, multiple alloimmunisation) and the experience of the surgical and anaesthetic team (Grade of recommendation: 2C+)105,110,129,134,149,150,194–198.
Vascular surgery
The use of IOBS in vascular surgery does not significantly reduce the relative risk of requiring allogeneic transfusion129,134,150.
In abdominal vascular surgery (aorto-femoral bypass and infrarenal aneurysms of the abdominal aorta) there is not sufficient evidence to recommend systematic adoption of IOBS with the purpose of containing allogeneic transfusion needs199. Observational or retrospective studies indicate that it can reduce the use of allogeneic transfusion and be cost-effective, particularly for operations with an intra-operative blood loss of ≥1,000 mL which allow the re-infusion of at least two or three units of RCC, or for emergency operations for a ruptured aneurysm, always on the basis of the pre-operative assessment of the individual patient carried out by the surgical team200–203 (Grade of recommendation: 2B)129,134,150,199–203.
In the subgroup of operations for infrarenal abdominal aortic aneurysm, a recent meta-analysis showed that IOBS can significantly reduce the relative risk of allogeneic transfusion, although the confidence interval is wide and the number of patients limited (Grade of recommendation: 2B)204–207.
Emergency surgery
IOBS can be used in cases of abdominal trauma with liver and/or splenic lesions, in cases of thoracic trauma, for haemoperitoneum following an ectopic pregnancy and for unexpected bleeding during laparoscopic surgery (Grade of recommendation: 2C)140,154,208–217.
Neurosurgery
IOBS should be used in neurosurgery (vertebral column fusion operations or intracranial surgery for giant aneurysms of the basilar artery) in the context of local protocols which, with the aim of minimising allogeneic transfusion needs and optimising the cost-efficacy ratio, take into account the characteristics of the individual patient (haemorrhagic risk, multiple alloimmunisation) and the experience of the surgical and anaesthetic team (Grade of recommendation: 2C)140,218–220.
Obstetrics
The use of IOBS in obstetrics does not seem to be related to an increased incidence of amniotic fluid embolism, infectious complications or DIC221–223.
Amniotic fluid embolism is currently considered a syndrome with an immunological pathogenesis; indeed, in 1995224 the new name of “anaphylactoid syndrome of pregnancy” was proposed104,224–227.
The risk of Rh isoimmunisation deriving from contamination of the salvaged blood by foetal red blood can be prevented by administering the mother an adequate dose228–230 of immunoglobulin G (IgG) anti-D [20–25 μg (100–125 UI) of IgG per mL of Rh(D)-positive red blood cells or per 2 mL of whole Rh(D) positive blood] (Grade of recommendation: 1A)104,154,231,232.
The use of leucodepletion filters when reinfusing RCC efficiently removes squamous cells and other contaminants derived from the amniotic fluid16,104,154,223,233.
In the context of obstetrics it is suggested that IOBS be used for the management of haemorrhagic emergencies or for cases with a high risk of bleeding (e. g. placenta previa, or other cases of abnormal placentation such as placenta accreta or percreta), provided that it possible to use leucodepletion filters for the transfusion of the RCC recovered (Grade of recommendation: 2B)16,104,154,221–227,231–235.
Cancer surgery
Various prospective and retrospective studies have shown that IOBS can be used with the purpose of reducing the use of allogeneic transfusion, without increasing the risk of recurrences, in surgery of urological malignancies (radical prostatectomy, radical cystectomy)236–243, gynaecological operations244, in liver resections245or in liver transplants246, in thoracic surgery247 and in combined oncological and cardiovascular surgery248.
A high percentage of these patients have malignant cells in the circulation, without this, however, being related to a worse survival after the surgical procedure154,249; in fact, only a limited percentage of these cells has the capacity to metastasise (from 1/104 to 1/108)154,250.
Leucodepletion filters have been used to reduce the number of malignant cells in the blood recovered during cancer surgery154,243,244,246,247,251–253. Such filtration has been successfully combined with irradiation of the RCC obtained by IOBS16,251,253–257.
In cancer surgery, in the context of local protocols that take into account the characteristics of the individual patient (haemorrhagic risk, multiple alloimmunisation) and of the experience of the surgical and anaesthetic team, is it suggested that IOBS be used, provided that the recovered RCC are filtered through leucodepletion filters and irradiated (25 Gy) prior to transfusion (Grade of recommendation: 2C)16,27,154,236–257.
Liver transplantation
IOBS should be used in liver transplant operations, with the purpose of limiting the use of allogeneic transfusion therapy (Grade of recommendation: 2B)92,246,258–261.
Leucodepletion filters
Units of RCC obtained from IOBS are often contaminated by bacteria even when the type of operation is not one in which contamination is predicted262.
RCC obtained from IOBS should be transfused through leucodepletion filters in order to reduce bacterial contamination (Grade of recommendation: 2C)16,154,263.
Contraindications
Recent studies in obstetrics16,104,154,221–227,231–235 and cancer surgery154,236–250 have re-evaluated the possibility of using IOBS in these settings; RCC salvaged from the operating field must be filtered through leucodepletion filters16,104,154,223,233,243,244,246,247,251–253 and, in the case of cancer surgery, must also be irradiated16,251,253–257.
There are, however, numerous contraindications to the use of IOBS (Table IV), most of which are related to the presence of possible contaminants or solutions/drugs in the operating field, which can cause haemolysis16,140,154.
Table IV.
Contraindications to intra-operative recovery of blood.
| Condition | Cause or contaminant |
|---|---|
| Presence of solutions and drugs in the operating field | Haemostatic agents for topical use |
| Synthetic resins (methylmetacrylate) | |
| Irrigating solutions of disinfectants for topical use (oxygenated water, betadine, distilled water, alcohol) | |
| Anticoagulant drugs | |
| Presence of contaminants in the operating field | Urine |
| Bone fragments | |
| Adipose tissue | |
| Faeces | |
| Infection of areas of the operating field | |
| Amniotic fluid* | |
| Malignant tumours | Neoplastic cells** |
| Haematological diseases | Sickle cell anaemia |
| Thalassaemia | |
| Miscellaneous | Carbon monoxide (electrocautery smoke) |
| Catecholamines (phaeochromocytoma) | |
| Decongestant drugs (oxymetazoline) |
In these circumstances it has been suggested using a supplementary aspirator in order to remove contaminants and prevent their entry into the reservoir (Grade of recommendation: 2C)140,154,222,264,265.
Complications
The main complication of IOBS is gas embolism which can occur when the primary reinfusion bag is directly connected to the patient’s vascular access135,140,266. Massive haemolysis can be caused by the mistaken use of distilled water as a washing solution135,140.
Treatment of thrombocytopenia and platelet disorders
Intra-operative transfusion of platelets is indicated for the treatment of bleeding17,18, in patients with thrombocytopenia or primary or secondary platelet function disorders.
The decision to transfuse platelet concentrates must not be based exclusively on the platelet count, but must also take into account the patient’s clinical condition, in particular fever above 38.5 °C, plasma coagulation disorders, recent haemorrhages and neurological deficits (Grade of recommendation: 2C)17,18.
An absolute indication for transfusion of platelets is severe thrombocytopenia together with clinically relevant bleeding. Human leucocyte antigen (HLA)- and/or human platelet antigen (HPA)-compatible platelets can be used in the treatment of immunised patients18.
Following a validated procedure of leucodepletion, platelet concentrates from apheresis are an acceptable alternative to cytomegalovirus-negative platelet concentrates for the prevention of infections by cytomegalovirus18.
Indications for the intra-operative transfusion of platelet concentrates
The need for platelet concentrates, in the presence of thrombocytopenia (platelets <100x109/L) or functional defects (including iatrogenic ones) of the platelets, depends on the type and site of the bleeding, the presence or absence of clotting disorders, intercurrent treatments, as well as the clinical condition of the patient18.
The following approach is recommended:
- In the surgical patient with ongoing bleeding, transfusion of platelets if the count is <50x109/L; if, however, the count is >100x109/L a transfusion should not be given, except in particular circumstances (Grade of recommendation: 2C)18,54,58,85,96,267–270.
- During massive transfusions a transfusion threshold of 75x109/L is suggested to prevent the platelet count from falling below 50x109/L, the critical threshold for haemostasis (Grade of recommendation: 2C)18,54,91; for patients with multiple trauma from high velocity accidents or with lesions involving the central nervous system, it is suggested that a higher transfusion threshold is adopted (Grade of recommendation: 2C)18,54,91.
- In acute DIC, in the presence of substantial haemorrhage and thrombocytopenia, it is advised that the platelet count is maintained around 50x109/L (Grade of recommendation: 2C)18,20,54,85,271.
- Extracorporeal circulation: platelet transfusion is recommended for patients who, at the end of the operation, have bleeding that is not related to the surgery or other clotting disorders (Grade of recommendation: 1C+)18,54. In these patients, given the secondary functional platelet disorders, the decision to transfuse platelets should be based on clinical criteria (microvascular bleeding and excessive post-surgical anaemia) (Grade of recommendation: 2C)18,54.
- Platelet function disorders (congenital or acquired): transfusion of platelets is indicated, independently of the platelet count, in the presence of haemorrhage (Grade of recommendation: 2C)18,54,85,269.
Transfusion practice
In anaemic and thrombocytopenic patients (platelet count ≤20x109/L), without ongoing bleeding, an increase of about 30% in the Htc can reduce the risk of haemorrhage (Grade of recommendation: 1C+)18,51,58,272–283.
Mean dose of platelets for each transfusion54,85
About 3x1011 platelets (1 platelet concentrate from apheresis or 1 platelet concentrate from pools of 5–8 platelet concentrates from whole blood or from buffy coat pools).
Calculation of the dose of platelets to transfuse
The dose of platelets to transfuse can be calculated using the following formula18:
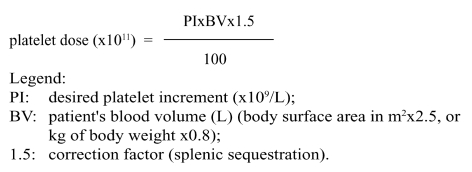 |
ABO/RhD compatibility
The platelet concentrates transfused should be ABO-identical, or at least ABO-compatible, for an efficient yield18,53,54,85,267,284–294.
Adverse reactions
Acute adverse reactions to transfusion therapy with platelets are18:
- non-haemolytic transfusion reactions (usually manifested by shivers, fever and urticaria);
- sepsis, due to bacterial contamination of the blood;
- TRALI.
Transfusion therapy with fresh-frozen plasma
The main indication for transfusion of FFP is correction of deficiencies of clotting factors for which a specific concentrate is not available, in patients with ongoing bleeding18.
In the intra-operative period the transfusion of FFP is indicated to correct congenital deficiencies of clotting factors, for which a specific concentrate is not available, or of multiple clotting factor deficits, when the PT or aPTT, expressed as a ratio is >1.5, in the following circumstances5,17,18,27,59,74,85,91,271,295–306:
- in the presence of acute or chronic liver disease and ongoing bleeding (Grade of recommendation: 1C+)5,27,74,85,296–306;
- in the presence of DIC and active bleeding, together with correction of the underlying cause (Grade of recommendation: 1C+)5,20,74,85,296–306;
- prevention of intra-operative bleeding, in patients with DIC20 and/or acute or chronic liver disease without active bleeding5,27,74,85,296–312 (Grade of recommendation: 2C);
- correction of microvascular bleeding in patients undergoing massive transfusion. If the PT and aPTT cannot be determined within a reasonable time, the FFP can be transfused in any case, in an attempt to halt the bleeding (Grade of recommendation: 1C+)5,27,74,85,91,295–306;
- deficiencies of single clotting factors, in the absence of specific concentrates (for example, factor V deficiency), in the presence of active bleeding or to prevent bleeding, during surgery (Grade of recommendation: 1C+)5,27,74,85,296–306.
Transfusion practice
Method of use
FFP must be thawed between 30 °C and 37 °C in a water bath under continuous agitation or with other appropriate equipment, in order that the temperature can be controlled. After thawing, the plasma must be transfused as soon as possible and in any case within 24 hours, if stored at 4±2 °C52,59. Thawed FFP must not be refrozen (Grade of recommendation: 1C+)59.
Posology
The recommended initial dose of FFP is 10–15 mL/kg of body weight5,59,74,301. The dose of FFP depends in any case on the patient’s clinical situation and laboratory parameters (Grade of recommendation: 1C+)5,59,74,85,301, which can justify the administration of higher doses (up to 30 mL/kg) of FFP20,313–315.
ABO/RhD compatibility
Plasma that is ABO-compatible with the recipient must be used (Grade of recommendation: 1C+)18,53.
FFP can be administered regardless of Rh compatibility; anti-D prophylaxis is not necessary in Rh(D)-negative recipients given Rh(D)-positive FFP (Grade of recommendation: 1C+)18,59.
Inappropriate indications for the transfusion of fresh-frozen plasma
The main inappropriate indication for using FFP in surgical patients is as a blood volume expander18.
Contraindications
Absolute contraindications to the use of FFP are documentated intolerance to plasma or its components and congenital deficiency of immunoglobulin A (IgA) in the presence of anti-IgA antibodies18,59.
Relative contraindications are heart failure and pulmonary oedema.
Adverse reactions to the transfusion of fresh-frozen plasma
The acute adverse reactions to transfusion therapy with FFP are mild allergic reactions (urticaria) or severe, anaphylactic allergic reactions, TRALI, febrile reactions, citrate toxicity and circulatory overload18.
Use of cryoprecipitate and fibrinogen
A specific acquired deficiency of fibrinogen, in the presence of haemorrhage, can be corrected by the transfusion of autologous or allogeneic cryoprecipitate, if available, or of fibrinogen concentrate (not registered in Italy, but can be obtained)20. A dose of 10 units of cryoprecipitate prepared from FFP from whole blood or 3 g of fibrinogen concentrate should raise the plasma fibrinogen by about 1 g/L20,27,316 in an adult person weighing 70 kg and with a plasma volume of about 3,000 mL27.
The response to treatment should be monitored, both clinically and by coagulation tests (PT, aPTT, fibrinogen) (Grade of recommendation: 2C)20.
The Clauss method should be used to determine the fibrinogen concentration (Grade of recommendation: 2C+)20–22.
Persistent, severe hypofibrinogenaemia (<1 g/L) despite treatment with FFP, in the presence of clinically relevant ongoing bleeding and together with correction of the underlying cause, can be treated with cryoprecipitate or fibrinogen concentrate, if available (Grade of recommendation: 2C)20,59,91,317–341.
ABO/RhD compatibility
Fibrinogen can be infused independently of the recipient’s blood group. The same criteria for FFP also apply to cryoprecipitate17,18,53,59.
Side effects and adverse reactions
The acute adverse reactions associated with the administration of cryoprecipitate have mostly been described in case reports and can be attributed to haemolytic reactions caused by anti-A/B antibodies, allergic and febrile reactions, respiratory distress and thrombotic events316.
Allergic and anaphylactic reactions, as well as thrombotic events have also been described following the administration of fibrinogen concentrates337,342,343.
Use of antithrombin concentrates
Almost the only indication for antithrombin (AT), used as replacement therapy, is correction of congenital deficiency in certain circumstances27,344,345. There is no clinical evidence that higher than normal levels of AT guarantee better protection than physiological levels344,346,347.
A recent meta-analysis of the use of AT in critically ill patients did not show any significant effect on a global reduction of mortality, even in subgroups of studies carried out in obstetric or trauma patients346,347; in contrast, it was seen that the use of AT was associated with an increased risk of bleeding.
Acute haemorrhage in the intra-operative period can be associated with an acquired deficiency of AT344; however, the use of AT concentrates in this case is not indicated, even when the levels of AT are considerably below the norm (Grade of recommendation: 2C+)344,346,347.
In the absence of further evidence from randomised, controlled trials, which could demonstrate a favourable effect on clinically relevant end-points, the use of AT cannot be recommended in patients with DIC who are not being contemporaneously also treated with heparin (Grade of recommendation: 1C+)20.
Calculation of the dose of antithrombin to administer
Before starting replacement therapy with the specific concentrate, the amount of functional AT should be assayed (Grade of recommendation: 2C)344.
Given that the administration of 1 IU/kg of weight increases plasma AT activity by 1.5%, the dose to administer can be calculated as follows:
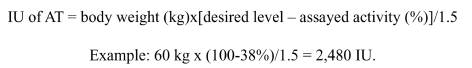 |
The dose and timing of subsequent administrations should be adapted to the plasma activity of AT, monitored every 12–48 hours.
Side effects and adverse reactions
AT infusions are generally well tolerated although allergic type reactions are possible344.
The use of AT concentrates contemporaneously with the administration of heparin increases the risk of haemorrhage and careful clinical and laboratory monitoring is, therefore, necessary in these circumstances (Grade of recommendation: 2C)344.
Blood components for topical use
Fibrin glue
Fibrin glue is a blood component for topical use that has been employed in surgery for more than 20 years348; it is available as a commercial product derived from pools of inactivated plasma, or can be produced from autologous or homologous whole blood or plasma, following protocols for manual or automated production349. The main components are fibrinogen, factor XIII and thrombin (and calcium chloride with or without antifibrinolytics) which can be applied contemporaneously or in succession to the surfaces to be treated. The application of fibrin glue reproduces in situ the final phase of the coagulation cascade through activation of fibrinogen by the thrombin350.
Given its haemostatic potential, this blood component for topical use has been used in cardiovascular, orthopaedic, thoracic, hepatic, splenic and urological surgery with the aim of containing the consumption of blood1,351–354.
Although based on studies of methodologically modest quality, three systematic reviews suggest that fibrin glue can be effective in reducing allogeneic transfusion needs in the peri-operative period134,353,354; however, the adoption of restrictive transfusion protocols reduces this effect134 and greater benefit in terms of saving blood is obtained in the post-operative period353,354.
Recent studies in liver surgery have challenged the role of fibrin glue in reducing intra-operative bleeding in this context and the cost-efficacy ratio of the strategy355,356.
Fibrin glue in heart surgery should only be used in the operations at highest risk (rupture of the external wall of the left ventricle and dissecting aorta)70, because of the possible complications associated with this product357–359.
It has been suggested that fibrin glue could be used, applying it to areas of bleeding parenchyma to facilitate local haemostasis, as a possible complementary therapeutic approach to contain intra-operative consumption of blood, on the basis of local protocols which take into consideration the characteristics of the individual patient (haemorrhagic risk, multiple alloimmunisation), the type of operation, the experience of the surgical and anaesthetic team, as well as the appropriateness of integrating the use of this blood component with other strategies of saving blood, taking into account the cost-efficacy ratio (Grade of recommendation: 2C)1,27,70,134,351–356.
Intra-operative pharmacological strategies for saving blood
Recombinant activated factor VII
The main indications for the use of recombinant activated factor VII (rFVIIa) are peri-operative prophylaxis and the treatment of haemorrhage in patients with haemophilia A or B with inhibitors, in whom replacement treatment with the specific deficient factor is either not possible or not indicated (Grade of recommendation: 2C)360,361 and in patients with acquired haemophilia (Grade of recommendation: 2C+)361, congenital FVII deficiency (Grade of recommendation: 2C+)362–364, or Glanzmann’s thromboasthenia associated with refractariness to platelet transfusion (Grade of recommendation: 2C)18,54,85.
In recent years there has been a notable increase in the use of rFVIIa for the off-label indication of treatment of haemorrhage events due to secondary clotting defects in patients undergoing surgery or those with multiple trauma365, despite the uncertainty of the real risk of thrombotic complications associated with the use of this drug for indications that have not been registered1,110.
The use of rFVIIa, mainly in uncontrolled studies, has been the subject of recommendations, based on the consensus of experts, for the treatment of massive haemorrhage in the fields of surgery, obstetrics and gynaecology366–368.
A recent systematic Cochrane review recommended that rFVIIa be used only in the context of clinical trials, since there is still uncertainty regarding its real efficacy as a haemostatic agent, whether for prophylaxis or for the treatment of major bleeding369. However, records of arterial thromboembolic adverse reactions have recently led the EMEA to contraindicate the use of rFVIIa except for its already approved indications370,371.
Antifibrinolytics
Following the worldwide withdrawal of aprotinin from the market, which occurred on 5 November 2007110, because of the greater mortality associated with the use of this product in heart surgery compared to its lysine analogues (tranexamic acid and epsilon-aminocaproic acid [EACA])372–374, and since EACA has not been marketed in Italy since 2006, tranexamic acid is, currently, the only antifibrinolytic available.
A systematic Cochrane review in 2007 indicated the efficacy of antifibrinolytic agents in reducing the consumption of blood products in major surgery375, finding stronger evidence for tranexamic acid; a subsequent update373 recommended the use of tranexamic acid and AEAC to prevent bleeding in heart surgery.
The use of tranexamic acid and AEAC in orthopaedic surgery (hip and knee replacement) has been shown to be effective in reducing transfusion therapy, without increasing the risk of thromboembolic complications, provided that antithrombotic prophylaxis is given normally; also in this type of surgery, despite the large variability in doses and methods of infusion used in the pre-, intra- and post-operative periods, tranexamic acid was found to be superior to EACA110,375–381.
Tranexamic acid and AEAC have also been used successfully in liver transplants, achieving a reduction in the use of blood without increasing thromboembolic complications382–385; once again, tranexamic acid was found to be superior to EACA384,385.
Tranexamic acid could be a possible complementary pharmacological approach to help to limit intra-operative use of blood during heart surgery, major orthopaedic operations (hip and knee replacements) and liver transplants, on the basis of local protocols taking into consideration the characteristics of the individual patients (haemorrhagic risk, multiple alloimmunisation) and the experience of the surgical and anaesthetic team, provided that normal prophylaxis against venous thromboembolism is given (Grade of recommendation: 2C+)1,2,51,70,110,134,365–371.
Surgical and anaesthesiological techniques of saving blood
The use of surgical techniques and instruments aimed at limiting trauma to tissues and vessels and facilitating local haemostasis is effective in containing intra-operative bleeding (Grade of recommendation: 2C)105,110,155,386–392.
Controlled hypotension (a reduction of systolic blood pressure down to 80–90 mmHg, a reduction in mean blood pressure down to 50–65 mmHg, or a 30% reduction of the mean baseline blood pressure) can be obtained pharmacologically or by spinal or epidural anaesthesia390 and is a commonly used technique which, depending on the experience of the team of anaesthetists, can be used in selected patients to help to limit intra-operative blood loss (Grade of recommendation: 2C)110,393.
A reduction in central venous pressure (1–5 mmHg) can contribute to reducing intra-operative bleeding during liver resection in selected patients and depending on the experience of the surgical and anaesthetic team (Grade of recommendation: 2C)110,394.
Peri-operative hypothermia increases the risk of organ failure, coagulation disorders and intraoperative bleeding95,395–400.
Hypothermia should be prevented, with the purpose of limiting intra-operative bleeding, by prewarming solutions to be infused401,402 and by warming the patient himself (Grade of recommendation: 2C)95,395–400.
Addendum
The process of developing these Recommendations, in conformity with the indications in the methodological manual of the national programme for guidelines (Istituto Superiore di Sanità, Agenzia per i Servizi Sanitari Regionali. Programma Nazionale per le Linee Guida – Manuale Metodologico. Milano, Italia, Arti Grafiche Passoni srl; 2002. Available at: http://www.snlg-iss.it/cms/files/Manuale_PNLG_0.pdf. Last accessed: 03/25/2010), made use of systematic literature reviews and updates of already existing recommendations on the subject.
The methodology used to determine the grades of recommendation drew on that presented at the 2004 Consensus Conference of the American College of Chest Physicians (Guyatt G, Schünemann HJ, Cook D, et al. Applying the grades of recommendation for antithrombotic and thrombolytic therapy. Chest 2004; 126: S179–87).
The recommendations are classified by grades, expressed in Arabic numbers (1, 2), according to their strength, and in letters (A, B, C), reflecting the type of study and evidence provided.
References
- 1.Mannucci PM, Levi M. Prevention and treatment of major blood loss. N Engl J Med. 2007;356:2301–11. doi: 10.1056/NEJMra067742. [DOI] [PubMed] [Google Scholar]
- 2.Lefkou E, Hunt B. Haematological management of obstetric haemorrhage. Obstetrics, Gynaecology and Reproductive Medicine. 2008;18:265–70. [Google Scholar]
- 3.World Health Organization The Prevention and Management of Postpartum Haemorrhage. World Health Organization/Maternal and Child Health 90.7; Report of a Technical Working Group; Geneva. 3–6 July 1989; Geneva: WHO; 1990. [Google Scholar]
- 4.El-Refaey H, Rodeck C. Post-partum haemorrhage: definitions, medical and surgical management. A time for change. Br Med Bull. 2003;67:205–17. doi: 10.1093/bmb/ldg016. [DOI] [PubMed] [Google Scholar]
- 5.American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006;105:198–208. doi: 10.1097/00000542-200607000-00030. [DOI] [PubMed] [Google Scholar]
- 6.Frigo MG, Celleno D, Larciprete G, Veneziani A. Emorragia Ostetrica. In: Celleno D, Frigo MG, editors. Anestesia, Analgesia e Terapia Intensiva in Ostetricia. Roma: Centro d’Informazione e Stampa Universitaria; 2008. pp. 319–42. [Google Scholar]
- 7.Hameed SM, Aird WC. Oxygen delivery. Crit Care Med. 2003;31:S658–S67. doi: 10.1097/01.CCM.0000101910.38567.20. [DOI] [PubMed] [Google Scholar]
- 8.Siegemund M, van Bommel J, Ince C. Assessment of regional tissue oxygenation. Int Care Med. 1999;25:1044–60. doi: 10.1007/s001340051011. [DOI] [PubMed] [Google Scholar]
- 9.Huang YC. Monitoring oxygen delivery in the critically ill. Chest. 2005;128:554S–60S. doi: 10.1378/chest.128.5_suppl_2.554S. [DOI] [PubMed] [Google Scholar]
- 10.Liumbruno G, Bennardello F, Lattanzio A, et al. Recommendations for the transfusion of red blood cells. Blood Transfus. 2009;7:49–64. doi: 10.2450/2008.0020-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hebert PC, Hu LQ, Biro GP. Review of physiologic mechanisms in response to anemia. Can Med Assoc J. 1997;156(Suppl 11):S27–40. [Google Scholar]
- 12.Hebert PC, Van der Linden P, Biro G, Hu LQ. Physiologic aspects of anemia. Crit Care Clin. 2004;20:187–212. doi: 10.1016/j.ccc.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Ellis CG, Jagger J, Sharpe M. The microcirculation as a functional system. Crit Care. 2005;9:S3–8. doi: 10.1186/cc3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallis JP. Nitric oxide and blood: a review. Transfus Med. 2005;15:1–11. doi: 10.1111/j.1365-3148.2005.00542.x. [DOI] [PubMed] [Google Scholar]
- 15.Madjdpour C, Spahn DR, Weiskopf RB. Anemia and perioperative red blood cell transfusion: a matter of tolerance. Crit Care Med. 2006;34(5 Suppl):S102–8. doi: 10.1097/01.CCM.0000214317.26717.73. [DOI] [PubMed] [Google Scholar]
- 16.Pape A, Habler O. Alternatives to allogeneic blood transfusions. Best Pract Res Clin Anaesthesiol. 2007;21:221–39. doi: 10.1016/j.bpa.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Council of Europe . Guide to the Preparation, Use and Quality Assurance of Blood Components Recommendation No R (95) 15 on the Preparation, Use and Quality Assurance of Blood Components. 14th ed. Strasbourg: Council of Europe Press; 2008. [Google Scholar]
- 18.Liumbruno G, Bennardello F, Lattanzio A, et al. Recommendations for the transfusion of plasma and platelets. Blood Transfus. 2009;7:132–50. doi: 10.2450/2009.0005-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Practice guidelines for obstetrical anesthesia: a report by the American Society of Anesthesiologists Task Force on Obstetrical Anesthesia. Anesthesiology. 1999;90:600–11. [PubMed] [Google Scholar]
- 20.Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145:24–33. doi: 10.1111/j.1365-2141.2009.07600.x. [DOI] [PubMed] [Google Scholar]
- 21.Mackie J, Lawrie AS, Kitchen S, et al. A performance evaluation of commercial fibrinogen reference preparations and assays for Clauss and PT-derived fibrinogen. Thromb Haemost. 2002;87:997–1005. [PubMed] [Google Scholar]
- 22.Mackie IJ, Kitchen S, Machin SJ, Lowe GD. Haemostasis and Thrombosis Task Force of the British Committee for Standards in Haematology. Br J Haematol. 2003;121:396–404. doi: 10.1046/j.1365-2141.2003.04256.x. Guidelines on fibrinogen assays. [DOI] [PubMed] [Google Scholar]
- 23.Sehgal LR, Zebala LP, Takagi I, et al. Evaluation of oxygen extraction ratio as a physiologic transfusion trigger in coronary artery bypass graft surgery patients. Transfusion. 2001;41:591–5. doi: 10.1046/j.1537-2995.2001.41050591.x. [DOI] [PubMed] [Google Scholar]
- 24.Spahn DR, Dettori N, Kocian R, Chassot PG. Transfusion in the cardiac patient. Crit Care Clin. 2004;20:269–79. doi: 10.1016/S0749-0704(03)00112-X. [DOI] [PubMed] [Google Scholar]
- 25.Welte M, Hable O. Die Indication zur perioperativen Transfusion von Erythrozyten. Anästh Intensivmed. 2005;3:73–83. [Google Scholar]
- 26.Madjdpour C, Marcucci C, Tissot JD, Spahn DR. Perioperative blood transfusions. Value, risks, and guidelines. Anaesthesist. 2005;54:67–80. doi: 10.1007/s00101-004-0789-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.German Medical Association Cross-Sectional Guidelines for Therapy with Blood Components and Plasma Derivatives. 4th revised edition. 2009. Available at: http://www.bundesaerztekammer.de/downloads/LeitCrossBloodComponents4ed.pdf. Last accessed on 03/25/2010. [DOI] [PMC free article] [PubMed]
- 28.Weiskopf RB, Viele MK, Feiner J, et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA. 1998;279:217–21. doi: 10.1001/jama.279.3.217. [DOI] [PubMed] [Google Scholar]
- 29.Lieberman JA, Weiskopf RB, Kelley SD, et al. Critical oxygen delivery in conscious humans is less than 7.3 ml O2 x kg(-1) x min(-1) Anesthesiology. 2000;92:407–13. doi: 10.1097/00000542-200002000-00022. [DOI] [PubMed] [Google Scholar]
- 30.Ickx BE, Rigolet M, Van Der Linden PJ. Cardiovascular and metabolic response to acute normovolemic anemia. Effects of anesthesia. Anesthesiology. 2000;93:1011–6. doi: 10.1097/00000542-200010000-00024. [DOI] [PubMed] [Google Scholar]
- 31.Weiskopf RB, Feiner J, Hopf H, et al. Heart rate increases linearly in response to acute isovolemic anemia. Transfusion. 2003;43:235–40. doi: 10.1046/j.1537-2995.2003.00302.x. [DOI] [PubMed] [Google Scholar]
- 32.Spahn DR, Leone BJ, Reves JG, Pasch T. Cardiovascular and coronary physiology of acute isovolemic hemodilution: a review of nonoxygencarrying and oxygen-carrying solutions. Anesth Analg. 1994;78:1000–21. doi: 10.1213/00000539-199405000-00029. [DOI] [PubMed] [Google Scholar]
- 33.Spahn DR, Zollinger A, Schlumpf RB, et al. Hemodilution tolerance in elderly patients without known cardiac disease. Anesth Analg. 1996;82:681–6. doi: 10.1097/00000539-199604000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Spahn DR, Seifert B, Pasch T, Schmid ER. Effects of chronic beta-blockade on compensatory mechanisms during acute isovolaemic haemodilution in patients with coronary artery disease. Br J Anaesth. 1997;78:381–5. doi: 10.1093/bja/78.4.381. [DOI] [PubMed] [Google Scholar]
- 35.Jamnicki M, Kocian R, van der Linden P, et al. Acute normovolemic hemodilution: physiology, limitations, and clinical use. J Cardiothorac Vasc Anesth. 2003;17:747–54. doi: 10.1053/j.jvca.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 36.Liumbruno GM, Bennardello F, Lattanzio A, et al. Recommendations for the use of albumin and immunoglobulins. Blood Transfus. 2009;7:216–34. doi: 10.2450/2009.0094-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alderson P, Schierhout G, Roberts I, Bunn F. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev. 2000;2:CD000567. doi: 10.1002/14651858.CD000567. [DOI] [PubMed] [Google Scholar]
- 38.Roberts I, Alderson P, Bunn F, et al. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev. 2004;4:CD000567. doi: 10.1002/14651858.CD000567.pub2. [DOI] [PubMed] [Google Scholar]
- 39.Perel P, Roberts I. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev. 2007;4:CD000567. doi: 10.1002/14651858.CD000567.pub3. [DOI] [PubMed] [Google Scholar]
- 40.Finfer S, Bellomo R, Boyce N, et al. SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247–56. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- 41.Bunn F, Trivedi D, Ashraf S. Colloid solutions for fluid resuscitation. Cochrane Database Syst Rev. 2008;1:CD001319. doi: 10.1002/14651858.CD001319.pub2. [DOI] [PubMed] [Google Scholar]
- 42.Barron ME, Wilkes MM, Navickis RJ. A systematic review of the comparative safety of colloids. Arch Surg. 2004;139:552–63. doi: 10.1001/archsurg.139.5.552. [DOI] [PubMed] [Google Scholar]
- 43.Davidson IJ. Renal impact of fluid management with colloids: a comparative review. Eur J Anaesthesiol. 2006;23:721–38. doi: 10.1017/S0265021506000639. [DOI] [PubMed] [Google Scholar]
- 44.Egli GA, Zollinger A, Seifert B, et al. Effect of progressive haemodilution with hydroxyethyl starch, gelatin and albumin on blood coagulation. Br J Anaesth. 1997;78:684–9. doi: 10.1093/bja/78.6.684. [DOI] [PubMed] [Google Scholar]
- 45.de Jonge E, Levi M. Effects of different plasma substitutes on blood coagulation: a comparative review. Crit Care Med. 2001;29:1261–7. doi: 10.1097/00003246-200106000-00038. [DOI] [PubMed] [Google Scholar]
- 46.Langeron O, Doelberg M, Ang ET, et al. Voluven, a lower substituted novel hydroxyethyl starch (HES 130/0.4), causes fewer effects on coagulation in major orthopedic surgery than HES 200/0.5. Anesth Analg. 2001;92:855–62. doi: 10.1097/00000539-200104000-00011. [DOI] [PubMed] [Google Scholar]
- 47.Boldt J, Haisch G, Suttner S, et al. Effects of a new modified, balanced hydroxyethyl starch preparation (Hextend) on measures of coagulation. Br J Anaesth. 2002;89:722–8. doi: 10.1093/bja/aef242. [DOI] [PubMed] [Google Scholar]
- 48.Strauss RG, Pennell BJ, Stump DC. A randomized, blinded trial comparing the hemostatic effects of pentastarch versus hetastarch. Transfusion. 2002;42:27–36. doi: 10.1046/j.1537-2995.2002.00003.x. [DOI] [PubMed] [Google Scholar]
- 49.Hardy JF, de Moerloose P, Samama M. Groupe d’intérêt en Hémostase Périopératoire. Massive transfusion and coagulopathy: pathophysiology and implications for clinical management. Can J Anaesth. 2006;53(6 Suppl):S40–58. doi: 10.1007/BF03022251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kozek-Langenecker SA, Jungheinrich C, Sauermann W, Van der Linden P. The effects of hydroxyethyl starch 130/0.4 (6%) on blood loss and use of blood products in major surgery: a pooled analysis of randomized clinical trials. Anesth Analg. 2008;107:382–90. doi: 10.1213/ane.0b013e31817e6eac. [DOI] [PubMed] [Google Scholar]
- 51.Searle E, Pavord A, Alfirevic Z. Recombinant factor VIIa and other pro-haemostatic therapies in primary postpartum haemorrhage. Best Pract Res Clin Obstet Gynaecol. 2008;22:1075–88. doi: 10.1016/j.bpobgyn.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 52.Decreto del Ministro della Salute 3 marzo 2005. Caratteristiche e modalità per la donazione del sangue e di emocomponenti. Gazzetta Ufficiale della Repubblica Italiana, n. 85, 13 April 2005.
- 53.Standard di Medicina Trasfusionale. 1st ed. Milan: Edizioni SIMTI; 2007. Società Italiana di Medicina Trasfusionale e Immunoematologia (SIMTI) Edizioni SIMTI. [Google Scholar]
- 54.British Committee for Standards in Haematology Guidelines for the use of platelet transfusions. Br J Haematol. 2003;122:10–23. doi: 10.1046/j.1365-2141.2003.04468.x. [DOI] [PubMed] [Google Scholar]
- 55.Patel IP, Ambinder E, Holland JF, Aldedort LM. In vitro and in vivo comparison of single-donor platelets and multiple-donor pooled platelet transfusions in leukemic patients. Transfusion. 1978;18:116–9. doi: 10.1046/j.1537-2995.1978.18178118554.x. [DOI] [PubMed] [Google Scholar]
- 56.Turner VS, Awker RJ, Mitchell SG, Seymour Mead AM. Paired in vitro and in vivo comparison of apheresis and recovered platelet concentrates stored for five days. J Clin Apher. 1994;9:189–94. doi: 10.1002/jca.2920090309. [DOI] [PubMed] [Google Scholar]
- 57.Heaton WA, Rebulla P, Pappalettera M, Dzik WH. A comparative analysis of different methods for routine blood component preparation. Transfus Med Rev. 1997;11:116–29. doi: 10.1053/tm.1997.0110116. [DOI] [PubMed] [Google Scholar]
- 58.Schiffer AC, Anderson KC, Bennet CL, et al. Platelet transfusion for patients with cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19:1519–38. doi: 10.1200/JCO.2001.19.5.1519. [DOI] [PubMed] [Google Scholar]
- 59.O’Shaughnessy DF, Atterbury C, Bolton Maggs P, et al. British Committee for Standards in Haematology, Blood Transfusion Task Force. Guidelines for the use of fresh-frozen plasma, cryoprecipitate and cryosupernatant. Br J Haematol. 2004;126:11–28. doi: 10.1111/j.1365-2141.2004.04972.x. [DOI] [PubMed] [Google Scholar]
- 60.Ferrari M, Zia S, Valbonesi M, et al. A new technique for hemodilution, preparation of autologous platelet-rich plasma and intraoperative blood salvage in cardiac surgery. Int J Artif Organs. 1987;10:47–50. [PubMed] [Google Scholar]
- 61.Tobe CE, Vocelka C, Sepulvada R, et al. Infusion of autologous platelet rich plasma does not reduce blood loss and product use after coronary artery bypass. A prospective, randomized, blinded study. J Thorac Cardiovasc Surg. 1993;105:1007–14. [PubMed] [Google Scholar]
- 62.Ereth MH, Oliver WC, Jr, Beynen FM, et al. Autologous platelet-rich plasma does not reduce transfusion of homologous blood products in patients undergoing repeat valvular surgery. Anesthesiology. 1993;79:540–7. doi: 10.1097/00000542-199309000-00018. [DOI] [PubMed] [Google Scholar]
- 63.Wong CA, Franklin ML, Wade LD. Coagulation tests, blood loss, and transfusion requirements in plateletrich plasmapheresed versus nonpheresed cardiac surgery patients. Anesth Analg. 1994;78:29–36. doi: 10.1213/00000539-199401000-00007. [DOI] [PubMed] [Google Scholar]
- 64.Shore-Lesserson L, Reich DL, DePerio M, Silvay G. Autologous platelet-rich plasmapheresis: risk versus benefit in repeat cardiac operations. Anesth Analg. 1995;81:229–35. doi: 10.1097/00000539-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 65.Triulzi DJ, Gilmor GD, Ness PM, et al. Efficacy of autologous fresh whole blood or platelet-rich plasma in adult cardiac surgery. Transfusion. 1995;35:627–34. doi: 10.1046/j.1537-2995.1995.35895357892.x. [DOI] [PubMed] [Google Scholar]
- 66.Napier JA, Bruce M, Chapman J, et al. Guidelines for autologous transfusion. II. Perioperative haemodilution and cell salvage. British Committee for Standards in Haematology Blood Transfusion Task Force. Autologous Transfusion Working Party. Br J Anaesth. 1997;78:768–71. doi: 10.1093/bja/78.6.768. [DOI] [PubMed] [Google Scholar]
- 67.Rubens FD, Fergusson D, Wells PS, et al. Platelet-rich plasmapheresis in cardiac surgery: a meta-analysis of the effect on transfusion requirements. J Thorac Cardiovasc Surg. 1998;116:641–7. doi: 10.1016/s0022-5223(98)70172-2. [DOI] [PubMed] [Google Scholar]
- 68.Wajon P, Gibson J, Calcroft R, et al. Intraoperative plateletpheresis and autologous platelet gel do not reduce chest tube drainage or allogeneic blood transfusion after reoperative coronary artery bypass graft. Anesth Analg. 2001;93:536–42. doi: 10.1097/00000539-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 69.Potter PS. Perioperative apheresis. Transfusion. 2004;44(2 Suppl):54S–7S. doi: 10.1111/j.0041-1132.2004.04174.x. [DOI] [PubMed] [Google Scholar]
- 70.Society of Thoracic Surgeons Blood Conservation Guideline Task Force. Ferraris VA, Ferraris SP, Saha SP, et al. Perioperative blood transfusion and blood conservation in cardiac surgery: the Society of Thoracic Surgeons and The Society of Cardiovascular Anesthesiologists clinical practice guideline. Ann Thorac Surg. 2007;83(5 Suppl):S27–86. doi: 10.1016/j.athoracsur.2007.02.099. [DOI] [PubMed] [Google Scholar]
- 71.Wardrop CA, Holland BM, Jones JG. Consensus on red cell transfusion. BMJ. 1995;311:962–3. doi: 10.1136/bmj.311.7011.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Petz LD, Swisher SN, Kleinman S, et al. Clinical Practice of Transfusion Medicine. 3rd ed. New York: Churchill Livingstone; 1996. [Google Scholar]
- 73.Crosby E, Ferguson D, Hume HA, et al. Expert Working Group Guidelines for red blood cell and plasma transfusion for adults and children. Can Med Assoc J. 1997;156(Suppl 11):S1–24. [Google Scholar]
- 74.Calder L, Hebert PC, Carter AO, Graham ID. Review of published recommendations and guidelines for the transfusions of allogeneic red blood cells and plasma. Can Med Assoc J. 1997;156(Suppl 11):S1–8. [Google Scholar]
- 75.Hebert PC, Schweitzer I, Calder L, et al. Review of the clinical practice literature on allogeneic red blood cell transfusion. Can Med Assoc J. 1997;156(Suppl 11):S9–26. [Google Scholar]
- 76.Simon TL, Alverson DC, AuBuchon J, et al. Practice parameter for the use of red blood cell transfusions: developed by the red blood cell administration practice guidelines development task force of the College of American Pathologists. Arch Pathol Lab Med. 1998;122:130–8. [PubMed] [Google Scholar]
- 77.Hebert PC, Wells G, Martin, et al. Variation in red cell transfusion practice in the intensive care unit: a multicentre cohort study. Crit Care. 1999;3:57–63. doi: 10.1186/cc310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–17. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 79.Bracey AW, Radovancevic R, Riggs SA, et al. Lowering the hemoglobin threshold for transfusion in coronary artery bypass procedures: effect on patient outcome. Transfusion. 1999;39:1070–7. doi: 10.1046/j.1537-2995.1999.39101070.x. [DOI] [PubMed] [Google Scholar]
- 80.Murphy MF, Wallington TB, Kelsey P, et al. British Committee for Standards in Haematology, Blood Transfusion Task Force Guidelines for the clinical use of red cell transfusions. Br J Haematol. 2001;113:24–31. doi: 10.1046/j.1365-2141.2001.02701.x. [DOI] [PubMed] [Google Scholar]
- 81.Clinical practice guidelines on the use of blood components (red blood cells, platelets, fresh frozen plasma, cryoprecipitate) Endorsed September 2001. National Health and Medical Research Council, Australasian Society of Blood Transfusion Inc. Available at: http://www.nhmrc.gov.au/publications/synopses/_files/cp78.pdf. Last accessed on 03/25/2010.
- 82.Scottish Intercollegiate Guidelines Network Perioperative blood transfusion for elective surgery. A national clinical guideline; October 2001. Available at: http://www.sign.ac.uk/pdf/sign54.pdf. Last accessed on 03/25/2010.
- 83.Hebert PC, Yetisir E, Martin C, et al. Is a low transfusion threshold safe in critically ill patients with cardiovascular disease? Crit Care Med. 2001;29:227–34. doi: 10.1097/00003246-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 84.Wu WC, Rathore SS, Wang Y, et al. Blood transfusion in elderly patients with acute myocardial infarction. N Engl J Med. 2001;345:1230–6. doi: 10.1056/NEJMoa010615. [DOI] [PubMed] [Google Scholar]
- 85.Miller Y, Bachowski G, Benjamin R, et al. Practice Guidelines for Blood Transfusion. A Compilation from Recent Peer-Reviewed Literature. 2nd ed. American National Red Cross; 2007. Available at: http://www.redcross.org/wwwfiles/Documents/WorkingWiththeRedCross/practiceguidelinesforbloodtrans.pdf. Last accessed on 03/25/2010. [Google Scholar]
- 86.Hebert PC, Fergusson DA. Red blood cell transfusion in critically ill patients. JAMA. 2002;288:1525–6. doi: 10.1001/jama.288.12.1525. [DOI] [PubMed] [Google Scholar]
- 87.Hill SR, Carless PA, Henry DA, et al. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2002;2:CD002042. doi: 10.1002/14651858.CD002042. [DOI] [PubMed] [Google Scholar]
- 88.Carson JL, Hill S, Carless P, et al. Transfusion triggers: a systematic review of the literature. Transfus Med Rev. 2002;16:187–99. doi: 10.1053/tmrv.2002.33461. [DOI] [PubMed] [Google Scholar]
- 89.Vincent JL, Baron JF, Reinhart K, et al. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288:1499–507. doi: 10.1001/jama.288.12.1499. [DOI] [PubMed] [Google Scholar]
- 90.Rao SV, Jollis JG, Harrington RA, et al. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA. 2004;292:1555–62. doi: 10.1001/jama.292.13.1555. [DOI] [PubMed] [Google Scholar]
- 91.Stainsby D, MacLennan S, Thomas D, et al. Guidelines on the management of massive blood loss. Br J Haematol. 2006;135:634–41. doi: 10.1111/j.1365-2141.2006.06355.x. British Committee for Standards in Haematology. [DOI] [PubMed] [Google Scholar]
- 92.McClelland DBL. Clinical transfusion: surgery and critical illness. In: McClelland DBL, editor. Handbook of Transfusion Medicine. London, UK: TSO; 2007. pp. 23–34. [Google Scholar]
- 93.Baele PL, Muylle L, Noens L, et al. Guidelines for the transfusion of red cells. Acta Clin Belg. 2008;63:301–12. doi: 10.1179/acb.2008.060. [DOI] [PubMed] [Google Scholar]
- 94.Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008;36:2667–74. doi: 10.1097/CCM.0b013e3181844677. [DOI] [PubMed] [Google Scholar]
- 95.Briggs C, Guthrie D, Hyde K, et al. British Committee for Standards in Haematology General Haematology Task Force Guidelines for point-of-care testing: haematology. Br J Haematol. 2008;142:904–15. doi: 10.1111/j.1365-2141.2008.07274.x. [DOI] [PubMed] [Google Scholar]
- 96.Practice Guidelines for blood component therapy: A report by the American Society of Anesthesiologists Task Force on Blood Component Therapy. Anesthesiology. 1996;84:732–47. [PubMed] [Google Scholar]
- 97.Valeri CR, Dennis RC, Ragno G, et al. Limitations of the hematocrit level to assess the need for red blood cell transfusion in hypovolemic anemic patients. Transfusion. 2006;46:365–71. doi: 10.1111/j.1537-2995.2006.00730.x. [DOI] [PubMed] [Google Scholar]
- 98.Messmer K, Lewis DH, Sunder-Plassmann L, et al. Acute normovolemic hemodilution. Changes of central hemodynamics and microcirculatory flow in skeletal muscle. Eur Surg Res. 1972;4:55–70. doi: 10.1159/000127600. [DOI] [PubMed] [Google Scholar]
- 99.Laks H, O’Connor NE, Pilon RN, et al. Acute normovolemic hemodilution: effects on hemodynamics, oxygen transport, and lung water in anesthetized man. Surg Forum. 1973;24:201–2. [PubMed] [Google Scholar]
- 100.Bauer H, Pichlmaier H, Ott E, et al. Autotransfusion through acute, preoperative hemodilution: 1st clinical experiences. Langenbecks Arch Chir. 1974;(Suppl):185–9. [PubMed] [Google Scholar]
- 101.Kreimeier U, Messmer K. Perioperative hemodilution. Transfus Apher Sci. 2002;27:59–72. doi: 10.1016/s1473-0502(02)00027-7. [DOI] [PubMed] [Google Scholar]
- 102.Shander A, Rijhwani TS. Acute normovolemic hemodilution. Transfusion. 2004;44(2 Suppl):26S–34S. doi: 10.1111/j.0041-1132.2004.04293.x. [DOI] [PubMed] [Google Scholar]
- 103.Monk TG. Acute normovolemic hemodilution. Anesthesiology Clin N Am. 2005;23:271–81. doi: 10.1016/j.atc.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 104.Catling S. Blood conservation techniques in obstetrics: a UK perspective. Int J Obstet Anesth. 2007;16:241–9. doi: 10.1016/j.ijoa.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 105.Shander A, Goodnough LT. Objectives and limitations of bloodless medical care. Curr Opin Hematol. 2006;13:462–70. doi: 10.1097/01.moh.0000245692.32085.bd. [DOI] [PubMed] [Google Scholar]
- 106.Decreto del Ministro della Sanità 1 settembre 1995. Disciplina dei rapporti tra le strutture pubbliche provviste di servizi trasfusionali e quelle pubbliche e private, accreditate e non accreditate, dotate di frigoemoteche. Gazzetta Ufficiale della Repubblica Italiana, n. 240, 13 October 1995.
- 107.Goodnough LT, Monk TG, Brecher ME. Acute normovolemic hemodilution should replace the preoperative donation of autologous blood as a method of autologous-blood procurement. Transfusion. 1998;38:473–6. doi: 10.1046/j.1537-2995.1998.38598297217.x. [DOI] [PubMed] [Google Scholar]
- 108.Weiskopf RB. Efficacy of acute normovolemic hemodilution assessed as a function of fraction of blood volume lost. Anesthesiology. 2001;94:439–46. doi: 10.1097/00000542-200103000-00013. [DOI] [PubMed] [Google Scholar]
- 109.Billote DB, Abdoue AG, Wixson RL. Comparison of acute normovolemic hemodilution and preoperative autologous blood donation in clinical practice. J Clin Anesth. 2000;12:31–5. doi: 10.1016/s0952-8180(99)00129-4. [DOI] [PubMed] [Google Scholar]
- 110.Cardone D, Klein AA. Perioperative blood conservation. Eur J Anaesthesiol. 2009;26:722–9. doi: 10.1097/EJA.0b013e32832c5280. [DOI] [PubMed] [Google Scholar]
- 111.Kochamba GS, Pfeffer TA, Sintek CF, et al. Intraoperative autotransfusion reduces blood loss after cardiopulmonary bypass. Ann Thorac Surg. 1996;61:900–3. doi: 10.1016/0003-4975(95)01155-2. [DOI] [PubMed] [Google Scholar]
- 112.Helm RE, Klemperer JD, Rosengart TK, et al. Intraoperative autologous blood donation preserves red cell mass but does not decrease postoperative bleeding. Ann Thorac Surg. 1996;62:1431–41. doi: 10.1016/0003-4975(96)00755-2. [DOI] [PubMed] [Google Scholar]
- 113.Olsfanger D, Fredman B, Goldstein B, et al. Acute normovolaemic haemodilution decreases postoperative allogeneic blood transfusion after total knee replacement. Br J Anaesth. 1997;79:317–21. doi: 10.1093/bja/79.3.317. [DOI] [PubMed] [Google Scholar]
- 114.Terada N, Arai Y, Matsuta Y, et al. Acute normovolemic hemodilution for radical prostatectomy: can it replace preoperative autologous blood transfusion? Int J Urol. 2001;8:149–52. doi: 10.1046/j.1442-2042.2001.00272.x. [DOI] [PubMed] [Google Scholar]
- 115.Matot I, Scheinin O, Jurim O, et al. Effectiveness of acute normovolaemic haemodilution to minimize allogeneic blood transfusion in major liver resections. Anesthesiology. 2002;97:794–800. doi: 10.1097/00000542-200210000-00008. [DOI] [PubMed] [Google Scholar]
- 116.Wong JC, Torella F, Haynes SL, et al. Autologous versus allogeneic transfusion in aortic surgery: a multicenter randomized clinical trial. Ann Surg. 2002;235:145–51. doi: 10.1097/00000658-200201000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Habler O, Schwenzer K, Zimmer K, et al. Effects of standardized acute normovolemic hemodilution on intraoperative allogeneic blood transfusion in patients undergoing major maxillofacial surgery. Int J Oral Maxillofac Surg. 2004;33:467–75. doi: 10.1016/j.ijom.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 118.Bennet J, Haynes S, Torella F, et al. Acute normovolemic hemodilution in moderate blood loss surgery: a randomized controlled trial. Transfusion. 2006;46:1097–103. doi: 10.1111/j.1537-2995.2006.00857.x. [DOI] [PubMed] [Google Scholar]
- 119.Takayanagi A, Masumori N, Kobayashi K, et al. Acute normovolemic hemodilution for radical retropubic prostatectomy and radical cystectomy. Urology. 2008;72:401–5. doi: 10.1016/j.urology.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 120.Imai R, Matsumura H, Uchida R, Watanabe K. Perioperative hemodilutional autologous blood transfusion in burn surgery. Int J Care Injured. 2008;39:57–60. doi: 10.1016/j.injury.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 121.Parkin IR, Chiu GA, Schwarz PA, Hodder SC. Acute perioperative normovolaemic haemodilution in major maxillofacial surgery. Br J Oral Maxillofac Surg. 2008;46:387–90. doi: 10.1016/j.bjoms.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 122.Jarnagin WR, Gonen M, Maithel SK, et al. A prospective randomized trial of acute normovolemic hemodilution compared to standard intraoperative management in patients undergoing major hepatic resection. Ann Surg. 2008;248:360–9. doi: 10.1097/SLA.0b013e318184db08. [DOI] [PubMed] [Google Scholar]
- 123.Kahraman S, Altunkaya H, Celebioglu B, et al. The effect of acute normovolemic hemodilution on homologous blood requirements and total estimated red blood cell volume lost. Acta Anaesthesiol Scand. 1997;41:614–7. doi: 10.1111/j.1399-6576.1997.tb04752.x. [DOI] [PubMed] [Google Scholar]
- 124.Hohn L, Schweizer A, Licker M, Morel DR. Absence of beneficial effect of acute normovolemic hemodilution combined with aprotinin on allogeneic blood transfusion requirements in cardiac surgery. Anesthesiology. 2002;96:276–92. doi: 10.1097/00000542-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 125.Svenmarker S, Engstrom KG. The inflammatory response to recycled pericardial suction blood and the influence of cell-saving. Scand Cardiovasc J. 2003;37:158–64. doi: 10.1080/14017430310001465. [DOI] [PubMed] [Google Scholar]
- 126.Bryson GL, Laupacis A, Wells GA. Does acute normovolemic hemodilution reduce perioperative allogeneic transfusion? A meta-analysis. The International Study of Perioperative Transfusion. Anesth Analg. 1998;86:9–15. doi: 10.1213/00000539-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 127.Gillon J, Desmond M, Thomas MJ. Acute normovolemic haemodilution. Transfus Med. 1999;9:259–64. doi: 10.1046/j.1365-3148.1999.00205.x. [DOI] [PubMed] [Google Scholar]
- 128.Segal JB, Blasco-Colmenares E, Norris EJ, Guallar E. Preoperative acute normovolemic hemodilution: a meta-analysis. Transfusion. 2004;44:632–44. doi: 10.1111/j.1537-2995.2004.03353.x. [DOI] [PubMed] [Google Scholar]
- 129.Carless P, Moxey A, O’Connell DO, Henry D. Autologous transfusion techniques: a systematic review of their efficacy. Transfus Med. 2004;14:123–44. doi: 10.1111/j.0958-7578.2004.0489.x. [DOI] [PubMed] [Google Scholar]
- 130.Zohar E, Fredman B, Ellis M, et al. A comparative study of the postoperative allogeneic blood sparing effect of tranexamic acid versus acute normovolemic hemodilution after total knee replacement. Anesth Analg. 1999;89:1382–7. doi: 10.1097/00000539-199912000-00010. [DOI] [PubMed] [Google Scholar]
- 131.Moyes DG, Mistry BD, Conlan AA. Normovolaemic haemodilution using dextran 70 in thoracic surgery. S Afr Med. 1985;67:762–4. [PubMed] [Google Scholar]
- 132.Catoire P, Saada M, Liu N, et al. Effect of preoperative normovolemic hemodilution on left ventricular segmental wall motion during abdominal aortic surgery. Anesth Analg. 1992;75:654–9. doi: 10.1213/00000539-199211000-00002. [DOI] [PubMed] [Google Scholar]
- 133.Welch M, Knight DG, Carr HM, et al. The preservation of renal function by isovolemic hemodilution during aortic operations. J Vasc Surg. 1993;18:858–66. [PubMed] [Google Scholar]
- 134.Davies L, Brown TJ, Haynes S, et al. Costeffectiveness of cell salvage and alternative methods of minimizing perioperative allogeneic blood transfusion: a systematic review and economic model. Health Technol Assess. 2006;10:1–210. doi: 10.3310/hta10440. [DOI] [PubMed] [Google Scholar]
- 135.Waters JH. Red blood cell recovery and reinfusion. Anesthesiology Clin N Am. 2005;23:283–94. doi: 10.1016/j.atc.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 136.Topics in Transfusion Medicine Guidelines for Autologous Blood Collection. Australasian Society of Blood Transfusion. Apr, 2002. Available at: http://www.anzsbt.org.au/publications/documents/2002_Vol9_2.pdf. Last accessed on 03/25/2010.
- 137.Gross JB. Estimating allowable blood loss: corrected for dilution. Anesthesiology. 1983;58:277–80. doi: 10.1097/00000542-198303000-00016. [DOI] [PubMed] [Google Scholar]
- 138.Messmer K. Hemodilution. Surg Clin North Am. 1975;55:659–78. doi: 10.1016/s0039-6109(16)40641-9. [DOI] [PubMed] [Google Scholar]
- 139.Messmer K. Preoperative hemodilution. In: Rossi EC, Simon TL, Moss GS, editors. Principles of Transfusion Medicine. Baltimore, MD: Williams and Wilkins; 1991. pp. 405–9. [Google Scholar]
- 140.American Association of Blood Banks . Intraoperative blood management. In: Waters JH, editor. Perioperative Blood Management A Physician’s Handbook. 1st ed. Bethesda, Maryland: AABB; 2006. pp. 45–76. [Google Scholar]
- 141.American Association of Blood Banks . Autologous blood donation and transfusion. In: Brecher ME, editor. Technical Manual. 15th ed. Bethesda, Maryland: AABB; 2005. pp. 117–38. [Google Scholar]
- 142.Clague CT, Blackshear PL., Jr A low-hemolysis blood aspirator conserves blood during surgery. Biomed Instrum Technol. 1995;29:419–24. [PubMed] [Google Scholar]
- 143.Gregoretti S. Suction-induced hemolysis at various vacuum pressures: implications for intraoperative blood salvage. Transfusion. 1996;36:57–60. doi: 10.1046/j.1537-2995.1996.36196190516.x. [DOI] [PubMed] [Google Scholar]
- 144.Sutera SP. Flow-induced trauma to blood cells. Circ Res. 1977;41:2–8. doi: 10.1161/01.res.41.1.2. [DOI] [PubMed] [Google Scholar]
- 145.Reeder GD. Autotransfusion theory of operation: a review of the physics and hematology. Transfusion. 2004;44(12 Suppl):35S–9S. doi: 10.1111/j.0041-1132.2004.04181.x. [DOI] [PubMed] [Google Scholar]
- 146.Waters JH, Williams B, Yazer MH, Kameneva MV. Modification of suction-induced hemolysis during cell salvage. Anesth Analg. 2007;104:684–7. doi: 10.1213/01.ane.0000255208.96685.2e. [DOI] [PubMed] [Google Scholar]
- 147.Hansen E, Pawlik M. Reasons against the retransfusion of unwashed wound blood. Transfusion. 2004;44(12 Suppl):45S–53S. doi: 10.1111/j.0041-1132.2004.04179.x. [DOI] [PubMed] [Google Scholar]
- 148.Faught C, Wells P, Ferguson D, et al. Adverse effects of methods for minimizing perioperative allogeneic transfusion: a critical review of the literature. Transfus Med Rev. 1998;12:206–25. doi: 10.1016/s0887-7963(98)80061-8. [DOI] [PubMed] [Google Scholar]
- 149.Huet C, Salmi LR, Fergusson D, et al. A meta analysis of the effectiveness of cell salvage to minimize perioperative allogeneic blood transfusion in cardiac and orthopedic surgery. Anesth Analg. 1999;89:861–78. doi: 10.1097/00000539-199910000-00009. [DOI] [PubMed] [Google Scholar]
- 150.Carless PA, Henry DA, Moxey AJ, et al. Cell salvage for minimizing perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2006;4:CD001888. doi: 10.1002/14651858.CD001888.pub2. [DOI] [PubMed] [Google Scholar]
- 151.Reijngoud LW, Pattyn C, De Haan R, et al. Does intraoperative cell salvage remove cobalt and chromium from reinfused blood? J Arthroplasty. 2009;24:1125–9. doi: 10.1016/j.arth.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 152.Dai B, Wang L, Djaiani G, Mazer CD. Continuous and discontinuous cell-washing autotransfusion systems. J Cardiothorac Vasc Anesth. 2004;18:210–7. doi: 10.1053/j.jvca.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 153.Waters JH, Lukanskiene E, Anderson M. Intraoperative blood salvage during cesarean section in a patient with beta thalassemia. Anesth Analg. 2003;97:1808–9. doi: 10.1213/01.ANE.0000087046.91072.E8. [DOI] [PubMed] [Google Scholar]
- 154.Waters JH. Indications and contraindications of cell salvage. Transfusion. 2004;44(12 Suppl):40S–4S. doi: 10.1111/j.0041-1132.2004.04176.x. [DOI] [PubMed] [Google Scholar]
- 155.Gohel MS, Bulbulia RA, Slim FJ, et al. How to approach major surgery where patients refuse blood transfusion (including Jehovah’s Witnesses) Ann R Coll Surg Engl. 2005;87:3–14. doi: 10.1308/1478708051414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang G, Bainbridge D, Martin J, Cheng D. The efficacy of an intraoperative cell saver during cardiac surgery: a meta-analysis of randomized trials. Anesth Analg. 2009;109:320–30. doi: 10.1213/ane.0b013e3181aa084c. [DOI] [PubMed] [Google Scholar]
- 157.Vermeijden WJ, Hagenaars A, van Oeveren W, de Vries J. Do repeated runs of a cell saver device increase the pro-inflammatory properties of washed blood? Eur J Cardiothorac Surg. 2008;34:350–3. doi: 10.1016/j.ejcts.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 158.Lau K, Shah H, Kelleher A, Moat N. Coronary artery surgery: cardiotomy suction or cell salvage? J Cardiothorac Surg. 2007;2:46. doi: 10.1186/1749-8090-2-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.de Haan J, Boonstra PW, Monnik SH, et al. Retransfusion of suctioned blood during cardiopulmonary bypass impairs hemostasis. Ann Thorac Surg. 1995;59:901–7. doi: 10.1016/0003-4975(95)00012-a. [DOI] [PubMed] [Google Scholar]
- 160.Reents W, Babin-Ebell J, Misoph MR, et al. Influence of different autotransfusion devices on the quality of salvaged blood. Ann Thorac Surg. 1999;68:58–62. doi: 10.1016/s0003-4975(99)00472-5. [DOI] [PubMed] [Google Scholar]
- 161.Svenmarker S, Engstrom KG. The inflammatory response to recycled pericardial suction blood and the influence of cell-saving. Scand Cardiovasc J. 2003;37:158–64. doi: 10.1080/14017430310001465. [DOI] [PubMed] [Google Scholar]
- 162.Rother RP, Bell L, Hilmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA. 2005;293:1653–62. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 163.Boonstra PW, van Imhoff GW, Eysman L, et al. Reduced platelet activation and improved hemostasis after controlled cardiotomy suction during clinical membrane oxygenator perfusions. J Thorac Cardiovasc Surg. 1985;89:900–6. [PubMed] [Google Scholar]
- 164.Booke M, Fobker M, Fingerhut D, et al. Fat elimination during intraoperative autotransfusion: an in vitro investigation. Anesth Analg. 1997;85:959–62. doi: 10.1097/00000539-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 165.Booke M, Van Aken H, Storm M, et al. Fat elimination from autologous blood. Anesth Analg. 2001;92:341–3. doi: 10.1097/00000539-200102000-00011. [DOI] [PubMed] [Google Scholar]
- 166.Brooker RF, Brown WR, Moody DM, et al. Cardiotomy suction: a major source of brain lipid emboli during cardiopulmonary bypass. Ann Thorac Surg. 1998;65:1651–5. doi: 10.1016/s0003-4975(98)00289-6. [DOI] [PubMed] [Google Scholar]
- 167.Djaiani G, Fedorko L, Borger MA, et al. Continuousflow cell saver reduces cognitive decline in elderly patients after coronary bypass surgery. Circulation. 2007;116:1888–95. doi: 10.1161/CIRCULATIONAHA.107.698001. [DOI] [PubMed] [Google Scholar]
- 168.Rubens FD, Boodhwani M, Mesana T, et al. Cardiotomy investigators The cardiotomy trial: a randomized, double-blind study to assess the effect of processing of shed blood during cardiopulmonary bypass on transfusion and neurocognitive function. Circulation. 2007;116(Suppl 11):189–97. doi: 10.1161/CIRCULATIONAHA.106.678987. [DOI] [PubMed] [Google Scholar]
- 169.Rosengart TK, DeBois W, O’Hara M, et al. Retrograde autologous priming for cardiopulmonary bypass: a safe and effective means of decreasing hemodilution and transfusion requirements. J Thorac Cardiovasc Surg. 1998;115:426–39. doi: 10.1016/S0022-5223(98)70287-9. [DOI] [PubMed] [Google Scholar]
- 170.Shapira OM, Aldea GS, Treanor PR, et al. Reduction of allogeneic blood transfusions after open-heart operations by lowering cardiopulmonary bypass prime volume. Ann Thorac Surg. 1998;65:724–30. doi: 10.1016/s0003-4975(97)01431-8. [DOI] [PubMed] [Google Scholar]
- 171.Rousou JA, Engleman RM, Flack JE, et al. The ‘Primeless pump’: a novel technique for intraoperative blood conservation. Cardiovasc Surg. 1999;7:228–35. doi: 10.1016/s0967-2109(98)00124-0. [DOI] [PubMed] [Google Scholar]
- 172.Balachandran S, Cross MH, Karthikeyan S, et al. Retrograde autologous priming of the cardiopulmonary bypass circuit reduces blood transfusion after coronary artery surgery. Ann Thorac Surg. 2002;73:1912–8. doi: 10.1016/s0003-4975(02)03513-0. [DOI] [PubMed] [Google Scholar]
- 173.Eising GP, Pfauder M, Niemeyer M, et al. Retrograde autologous priming: is it useful in elective on-pump coronary artery bypass surgery? Ann Thorac Surg. 2003;75:23–7. doi: 10.1016/s0003-4975(02)04099-7. [DOI] [PubMed] [Google Scholar]
- 174.Sobieski MA, Slaughter MS, Hart DE, et al. Prospective study on cardiopulmonary bypass prime reduction and its effects on intraoperative blood product and hemoconcentrator use. Perfusion. 2005;20:31–7. doi: 10.1191/0267659105pf783oa. [DOI] [PubMed] [Google Scholar]
- 175.Murphy GS, Szokol JW, Nitsun M, et al. The failure of retrograde autologous priming of the cardiopulmonary bypass circuit to reduce blood use after cardiac surgical procedures. Anesth Analg. 2004;98:1201–7. doi: 10.1213/01.ane.0000112306.71113.5e. [DOI] [PubMed] [Google Scholar]
- 176.Saczkowski R, Bernier PL, Tchervenkov CI, Arellano R. Retrograde autologous priming and allogeneic blood transfusions: a meta-analysis. Interact Cardiovasc Thorac Surg. 2009;8:373–6. doi: 10.1510/icvts.2008.195354. [DOI] [PubMed] [Google Scholar]
- 177.Sundt TM. Technology insight: randomized trials of off-pump versus on-pump coronary artery bypass surgery. Nat Clin Pract Cardiovasc Med. 2005;2:261–8. doi: 10.1038/ncpcardio0190. [DOI] [PubMed] [Google Scholar]
- 178.Wijeysundera DN, Beattie WS, Djaiani G, et al. Off-pump coronary artery surgery for reducing mortality and morbidity: meta-analysis of randomized and observational studies. J Am Coll Cardiol. 2005;46:872–82. doi: 10.1016/j.jacc.2005.05.064. [DOI] [PubMed] [Google Scholar]
- 179.Khan NE, De Souza A, Mister R, et al. A randomized comparison of off-pump and on-pump multivessel coronary-artery bypass surgery. N Engl J Med. 2004;350:21–8. doi: 10.1056/NEJMoa031282. [DOI] [PubMed] [Google Scholar]
- 180.van Dijk D, Nierich AP, Jansen EW, et al. Early outcome after off-pump versus on-pump coronary bypass surgery: results from a randomized study. Circulation. 2001;104:1761–6. doi: 10.1161/hc4001.097036. [DOI] [PubMed] [Google Scholar]
- 181.Angelini GD, Taylor FC, Reeves BC, Ascione R. Early and midterm outcome after off-pump and onpump surgery in Beating Heart Against Cardioplegic Arrest Studies (BHACAS 1 and 2): a pooled analysis of two randomised controlled trials. Lancet. 2002;359:1194–9. doi: 10.1016/S0140-6736(02)08216-8. [DOI] [PubMed] [Google Scholar]
- 182.Puskas JD, Williams WH, Duke PG, et al. Off-pump coronary artery bypass grafting provides complete revascularization with reduced myocardial injury, transfusion requirements, and length of stay: a prospective randomized comparison of two hundred unselected patients undergoing off-pump versus conventional coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125:797–808. doi: 10.1067/mtc.2003.324. [DOI] [PubMed] [Google Scholar]
- 183.Cheng DC, Bainbridge D, Martin JE, Novick RJ. Does off-pump coronary artery bypass reduce mortality, morbidity, and resource utilization when compared with conventional coronary artery bypass? A metaanalysis of randomized trials. Anesthesiology. 2005;102:188–203. doi: 10.1097/00000542-200501000-00028. [DOI] [PubMed] [Google Scholar]
- 184.Jones RH. Intraoperative crossover: the well-kept surgical secret to apparent surgical success. J Am Coll Cardiol. 2005;45:1529–31. doi: 10.1016/j.jacc.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 185.Henry DA, Carless PA, Moxey AJ, et al. Pre-operative autologous donation for minimizing perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2002;2:CD003602. doi: 10.1002/14651858.CD003602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Forgie MA, Wells PS, Laupacis A, Fergusson D. Preoperative autologous donation decreases allogeneic transfusion but increases exposure to all red blood cell transfusion: results of a metaanalysis. International Study of Perioperative Transfusion (ISPOT) Investigators. Arch Intern Med. 1998;158:610–6. doi: 10.1001/archinte.158.6.610. [DOI] [PubMed] [Google Scholar]
- 187.Coleman D, Stevens AJ, Dodge H, Finch C. Rate of blood regeneration after blood loss. AMA Arch Intern Med. 1953;92:341–9. doi: 10.1001/archinte.1953.00240210045006. [DOI] [PubMed] [Google Scholar]
- 188.Hodges VM, Rainey S, Lappin TR, Maxwell AP. Pathophysiology of anemia and erythrocytosis. Crit Rev Oncol Hematol. 2007;64:139–58. doi: 10.1016/j.critrevonc.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 189.Erslev AJ, Caro J, Miller O, Silver R. Plasma erythropoietin in health and disease. Ann Clin Lab Sci. 1980;10:250–7. [PubMed] [Google Scholar]
- 190.Erslev AJ, Wilson J, Caro J. Erythropoietin titers in anemic, nonuremic patients. J Lab Clin Med. 1987;109:429–33. [PubMed] [Google Scholar]
- 191.Weisbach V, Corbiere C, Strasser E, et al. The variability of compensatory erythropoiesis in repeated autologous blood donation. Transfusion. 2001;41:179–83. doi: 10.1046/j.1537-2995.2001.41020179.x. [DOI] [PubMed] [Google Scholar]
- 192.Singbartl G. Preoperative autologous blood donationpart I. Only two clinical parameters determine efficacy of the autologous predeposit. Minerva Anestesiol. 2007;73:143–51. [PubMed] [Google Scholar]
- 193.Singbartl G, Malgorzata S, Quoss A. Preoperative autologous blood donation—part II. Adapting the predeposit concept to the physiological basics of erythropoiesis improves its efficacy. Minerva Anestesiol. 2007;73:153–60. [PubMed] [Google Scholar]
- 194.Singbartl G, Schreiber J, Singbartl K. Preoperative autologous blood donation versus intraoperative blood salvage: intraindividual analyses and modeling of efficacy in 1103 patients. Transfusion. 2009;49:2374–83. doi: 10.1111/j.1537-2995.2009.02291.x. [DOI] [PubMed] [Google Scholar]
- 195.Bridgens JP, Evans CR, Dobson PM, Hamer AJ. Intraoperative red blood-cell salvage in revision hip surgery. A case-matched study. J Bone Joint Surg Am. 2007;89:270–5. doi: 10.2106/JBJS.F.00492. [DOI] [PubMed] [Google Scholar]
- 196.Tenholder M, Cushner FD. Intraoperative blood management in joint replacement surgery. Orthopedics. 2004;27(6 Suppl):s663–8. doi: 10.3928/0147-7447-20040602-07. [DOI] [PubMed] [Google Scholar]
- 197.Tobias JD. Strategies for minimizing blood loss in orthopedic surgery. Semin Hematol. 2004;41(Suppl 1):145–56. doi: 10.1053/j.seminhematol.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 198.Bess RS, Lenke LG. Blood loss minimization and blood salvage techniques for complex spinal surgery. Neurosurg Clin N Am. 2006;17:227–34. doi: 10.1016/j.nec.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 199.Alvarez GG, Ferguson DA, Neilipovitz DT, Hébert PC. Cell salvage does not minimize perioperative allogeneic blood transfusion in abdominal vascular surgery: a systematic review. Can J Anesth. 2004;51:425–31. doi: 10.1007/BF03018303. [DOI] [PubMed] [Google Scholar]
- 200.Goodnough LT, Monk TG, Sicard G, et al. Intraoperative salvage in patients undergoing elective abdominal aortic aneurysm repair: an analysis of cost and benefit. J Vasc Surg. 1996;24:213–8. doi: 10.1016/s0741-5214(96)70096-4. [DOI] [PubMed] [Google Scholar]
- 201.Healy CF, Doyle M, Egan B, et al. Transfusion requirement and outcomes in patients undergoing abdominal aortic surgery using intra-operative cell salvage. Ir J Med Sci. 2007;176:33–6. doi: 10.1007/s11845-007-0008-z. [DOI] [PubMed] [Google Scholar]
- 202.Marković M, Davidović L, Savić N, et al. Intraoperative cell salvage versus allogeneic transfusion during abdominal aortic surgery: clinical and financial outcomes. Vascular. 2009;17:83–92. doi: 10.2310/6670.2009.00009. [DOI] [PubMed] [Google Scholar]
- 203.Freischlag JA. Intraoperative blood salvage in vascular surgery – worth the effort? Crit Care. 2004;8(Suppl 2):S53–6. doi: 10.1186/cc2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Takagi H, Sekino S, Kato T, et al. Intraoperative autotransfusion in abdominal aortic surgery: metaanalysis of randomized controlled trials. Arch Surg. 2007;142:1098–101. doi: 10.1001/archsurg.142.11.1098. [DOI] [PubMed] [Google Scholar]
- 205.Spark JI, Chetter IC, Kester RC, Scott DJ. Allogeneic versus autologous blood during abdominal aortic aneurysm surgery. Eur J Vasc Endovasc Surg. 1997;14:482–486. doi: 10.1016/s1078-5884(97)80128-1. [DOI] [PubMed] [Google Scholar]
- 206.Clagett GP, Valentine RJ, Jackson MR, et al. A randomized trial of intraoperative autotransfusion during aortic surgery. J Vasc Surg. 1999;29:22–31. doi: 10.1016/s0741-5214(99)70346-0. [DOI] [PubMed] [Google Scholar]
- 207.Patra P, Chaillou P, Bizouarn P. Intraoperative autotransfusion for repair of unruptured aneurysms of the infrarenal abdominal aorta. J Cardiovasc Surg (Torino) 2000;41:407–13. [PubMed] [Google Scholar]
- 208.Hughes LG, Thomas DW, Wareham K, et al. Intraoperative blood salvage in abdominal trauma: a review of 5 years’ experience. Anaesthesia. 2001;56:217–20. doi: 10.1046/j.1365-2044.2001.01832.x. [DOI] [PubMed] [Google Scholar]
- 209.Bowley DM, Barker P, Boffard KD. Intraoperative blood salvage in penetrating abdominal trauma: a randomised, controlled trial. World J Surg. 2006;30:1074–80. doi: 10.1007/s00268-005-0466-2. [DOI] [PubMed] [Google Scholar]
- 210.Kamiyoshihara M, Ibe T, Takeyoshi I. The utility of an autologous blood salvage system in emergency thoracotomy for a hemothorax after chest trauma. Gen Thorac Cardiovasc Surg. 2008;56:222–5. doi: 10.1007/s11748-007-0219-2. [DOI] [PubMed] [Google Scholar]
- 211.Yamada T, Kasamatsu H. Laparoscopic surgery with intraoperative autologous blood transfusion in patients with heavy hemoperitoneum due to ectopic pregnancy. J Am Assoc Gynecol Laparosc. 2000;7:255–6. doi: 10.1016/s1074-3804(00)80051-1. [DOI] [PubMed] [Google Scholar]
- 212.Selo-Ojeme DO. Intraoperative blood salvage and autotransfusion in the management of ruptured ectopic pregnancy: a review. East Afr Med J. 2001;78:465–7. doi: 10.4314/eamj.v78i9.8976. [DOI] [PubMed] [Google Scholar]
- 213.Selo-Ojeme DO, Onwude JL, Onwudiegwu U. Autotransfusion for ruptured ectopic pregnancy. Int J Gynaecol Obstet. 2003;80:103–10. doi: 10.1016/s0020-7292(02)00379-x. [DOI] [PubMed] [Google Scholar]
- 214.Yamada T, Okamoto Y, Kasamatsu H, Mori H. Intraoperative autologous blood transfusion for hemoperitoneum resulting from ectopic pregnancy or ovarian bleeding during laparoscopic surgery. JSLS. 2003;7:97–100. [PMC free article] [PubMed] [Google Scholar]
- 215.Takeda A, Manabe S, Mitsui T, Nakamura H. Management of patients with ectopic pregnancy with massive hemoperitoneum by laparoscopic surgery with intraoperative autologous blood transfusion. J Minim Invasive Gynecol. 2006;13:43–8. doi: 10.1016/j.jmig.2005.09.100. [DOI] [PubMed] [Google Scholar]
- 216.Takeda A, Sakai K, Mitsui T, Nakamura H. Management of ruptured corpus luteum cyst of pregnancy occurring in a 15-year-old girl by laparoscopic surgery with intraoperative autologous blood transfusion. J Pediatr Adolesc Gynecol. 2007;20:97–100. doi: 10.1016/j.jpag.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 217.Selo-Ojeme DO, Feyi-Waboso PA. Salvage autotransfusion versus homologous blood transfusion for ruptured ectopic pregnancy. Int J Gynaecol Obstet. 2007;96:108–11. doi: 10.1016/j.ijgo.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 218.Cataldi S, Bruder N, Dufour H, et al. Intraoperative autologous blood transfusion in intracranial surgery. Neurosurgery. 1997;40:765–71. doi: 10.1097/00006123-199704000-00021. [DOI] [PubMed] [Google Scholar]
- 219.Chanda A, Smith DR, Nanda A. Autotransfusion by cell saver technique in surgery of lumbar and thoracic spinal fusion with instrumentation. J Neurosurg. 2002;96(3 Suppl):298–303. doi: 10.3171/spi.2002.96.3.0298. [DOI] [PubMed] [Google Scholar]
- 220.Gause PR, Siska PA, Westrick ER, et al. Efficacy of intraoperative cell saver in decreasing postoperative blood transfusions in instrumented posterior lumbar fusion patients. Spine (Phila Pa 1976) 2008;33:571–5. doi: 10.1097/BRS.0b013e3181657cc1. [DOI] [PubMed] [Google Scholar]
- 221.Rainaldi MP, Tazzari PL, Scagliarini G, et al. Blood salvage during caesarean section. Br J Anaesth. 1998;80:195–8. doi: 10.1093/bja/80.2.195. [DOI] [PubMed] [Google Scholar]
- 222.Rebarber A, Lonser R, Jackson S, et al. The safety of intraoperative autologous blood collection and autotransfusion during cesarean section. Am J Obstet Gynecol. 1998;179:715–20. doi: 10.1016/s0002-9378(98)70070-5. [DOI] [PubMed] [Google Scholar]
- 223.Allam J, Cox M, Yentis SM. Cell salvage in obstetrics. Int J Obstet Anesth. 2008;17:37–45. doi: 10.1016/j.ijoa.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 224.Clark SL, Hankins GD, Dudley DA, et al. Amniotic fluid embolism: analysis of the national registry. Am J Obstet Gynecol. 1995;172:1158–67. doi: 10.1016/0002-9378(95)91474-9. [DOI] [PubMed] [Google Scholar]
- 225.Fineschi V, Gambassi R, Gherardi M, Turillazzi E. The diagnosis of amniotic fluid embolism: an immunohistochemical study for the quantification of pulmonary mast cell tryptase. Int J Legal Med. 1998;111:238–43. doi: 10.1007/s004140050160. [DOI] [PubMed] [Google Scholar]
- 226.Farrar SC, Gherman RB. Serum tryptase analysis in a woman with amniotic fluid embolism: a case report. J Reprod Med. 2001;46:926–8. [PubMed] [Google Scholar]
- 227.Gist RS, Stafford IP, Leibovitz AB, Beilin Y. Amniotic fluid embolism. Anesth Analg. 2009;108:1599–602. doi: 10.1213/ane.0b013e31819e43a4. [DOI] [PubMed] [Google Scholar]
- 228.Stern K, Goodman HS, Berger M. Experimental isoimmunization to hemo-antigens in man. J Immunol. 1961;97:1245–57. [Google Scholar]
- 229.Fung Kee Fung K, Eason E, Crane J, et al. Prevention of Rh alloimmunization. J Obstet Gynaecol Can. 2003;25:765–73. doi: 10.1016/s1701-2163(16)31006-4. [DOI] [PubMed] [Google Scholar]
- 230.MacKenzie IZ, Roseman F, Findlay J, et al. The kinetics of routine antenatal prophylactic intramuscular injections of polyclonal anti-D immunoglobulin. BJOG. 2006;113:97–101. doi: 10.1111/j.1471-0528.2005.00789.x. [DOI] [PubMed] [Google Scholar]
- 231.Catling SJ, Gioels L. Cell salvage in obstetrics: the time has come. Br J Obstet Gynaecol. 2005;112:131–2. doi: 10.1111/j.1471-0528.2004.00349.x. [DOI] [PubMed] [Google Scholar]
- 232.Catling SJ, Williams S, Fielding AM. Cell salvage in obstetrics: an evaluation of the ability of cell salvage combined with leukocyte depletion filtration to remove amniotic fluid from operative blood loss at caesarean section. Int J Obstet Anesth. 1999;8:79–84. doi: 10.1016/s0959-289x(99)80002-8. [DOI] [PubMed] [Google Scholar]
- 233.Waters JH, Biscotti C, Potter PS, Phillipson E. Amniotic fluid removal during cell salvage in the cesarean section patient. Anesthesiology. 2000;92:1531–6. doi: 10.1097/00000542-200006000-00008. [DOI] [PubMed] [Google Scholar]
- 234.Geoghegan J, Daniels JP, Moore PAS, et al. Cell salvage at caesarean section: the need for an evidencebased approach. BJOG. 2009;116:743–7. doi: 10.1111/j.1471-0528.2009.02129.x. [DOI] [PubMed] [Google Scholar]
- 235.American Society of Anesthesiologists Task Force on Obstetric Anesthesia Practice guidelines for obstetric anesthesia: an updated report by the American Society of Anesthesiologists Task Force on Obstetric Anesthesia. Anesthesiology. 2007;106:843–63. doi: 10.1097/01.anes.0000264744.63275.10. [DOI] [PubMed] [Google Scholar]
- 236.Klimberg I, Sirois R, Wajsman Z, et al. Intraoperative autotransfusion in urologic oncology. Arch Surg. 1986;121:1326–9. doi: 10.1001/archsurg.121.11.1326. [DOI] [PubMed] [Google Scholar]
- 237.Hart OJ, 3rd, Klimberg IW, Wajsman Z, et al. Intraoperative autotransfusion in radical cystectomy for carcinoma of the bladder. Surg Gynecol Obstet. 1989;168:302–6. [PubMed] [Google Scholar]
- 238.Gray Cl, Amling CL, Polston GR, et al. Intraoperative cell salvage in radical retropubic prostatectomy. Urology. 2001;58:740–5. doi: 10.1016/s0090-4295(01)01365-6. [DOI] [PubMed] [Google Scholar]
- 239.Davis M, Sofer M, Gomez-Marin O, et al. The use of cell salvage during radical retropubic prostatectomy: does it influence cancer recurrence? Br J Urol. 2003;91:474–6. doi: 10.1046/j.1464-410x.2003.04129.x. [DOI] [PubMed] [Google Scholar]
- 240.Stoffel JT, Topjian L, Libertino JA. Analysis of peripheral blood for prostate cells after autologous transfusion given during radical prostatectomy. BJU Int. 2005;96:313–5. doi: 10.1111/j.1464-410X.2005.05621.x. [DOI] [PubMed] [Google Scholar]
- 241.Nieder AM, Carmack AJK, Sved PD, et al. Intraoperative cell salvage during radical prostatectomy is not associated with greater biochemical recurrence rate. Urology. 2005;65:730–4. doi: 10.1016/j.urology.2004.10.062. [DOI] [PubMed] [Google Scholar]
- 242.Nieder AM, Manoharan M, Yang Y, Soloway MS. Intraoperative cell salvage during radical cystectomy does not affect long-term survival. Urology. 2007;69:881–4. doi: 10.1016/j.urology.2007.01.060. [DOI] [PubMed] [Google Scholar]
- 243.MacIvor D, Nelson J, Triulzi D. Impact of intraoperative red blood cell salvage on transfusion requirements and outcomes in radical prostatectomy. Transfusion. 2009;49:1431–4. doi: 10.1111/j.1537-2995.2009.02131.x. [DOI] [PubMed] [Google Scholar]
- 244.Nagarsheth NP, Sharma T, Shander A, Awan A. Blood salvage use in gynecologic oncology. Transfusion. 2009;49:2048–53. doi: 10.1111/j.1537-2995.2009.02256.x. [DOI] [PubMed] [Google Scholar]
- 245.Zulim RA, Rocco M, Goodnight JE, et al. Intraoperative autotransfusion in hepatic resection for malignancy. Arch Surg. 1993;128:206–11. doi: 10.1001/archsurg.1993.01420140083013. [DOI] [PubMed] [Google Scholar]
- 246.Liang TB, Li DL, Liang L, et al. Intraoperative blood salvage during liver transplantation in patients with hepatocellular carcinoma: efficiency of leukocyte depletion filters in the removal of tumor cells. Transplantation. 2008;85:863–9. doi: 10.1097/TP.0b013e3181671f2e. [DOI] [PubMed] [Google Scholar]
- 247.Perseghin P, Vigano M, Rocco G, et al. Effectiveness of leukocyte filters in reducing tumor cell contaminations after intraoperative blood salvage in lung cancer patients. Vox Sang. 1997;72:221–4. doi: 10.1046/j.1423-0410.1997.7240221.x. [DOI] [PubMed] [Google Scholar]
- 248.Akchurin RS, Davidov MI, Partigulov SA, et al. Cardiopulmonary bypass and cell-saver technique in combined oncologic and cardiovascular surgery. Artif Organs. 1997;21:763–5. doi: 10.1111/j.1525-1594.1997.tb03738.x. [DOI] [PubMed] [Google Scholar]
- 249.Klimberg IW. Autotransfusion and blood conservation in urologic oncology. Semin Surg Oncol. 1989;5:286–92. doi: 10.1002/ssu.2980050412. [DOI] [PubMed] [Google Scholar]
- 250.Weiss L. Metastatic inefficiency: causes and consequences. Cancer Rev. 1986;3:1–24. [Google Scholar]
- 251.Poli MCC, Villa LL, Colella R, Deheinzelin D. Molecular evidence of tumour cell removal from salvaged blood after irradiation and leukocyte depletion. Transfus Med. 2004;14:151–5. doi: 10.1111/j.0958-7578.2004.00491.x. [DOI] [PubMed] [Google Scholar]
- 252.Catling S, Williams S, Freites O, et al. Use of a leucocyte filter to remove tumour cells from intraoperative cell salvage blood. Anaesthesia. 2008;63:1332–8. doi: 10.1111/j.1365-2044.2008.05637.x. [DOI] [PubMed] [Google Scholar]
- 253.Poli M, Camargo A, Villa L, et al. Intraoperative autologous blood recovery in prostate cancer surgery: in vivo validation using a tumour marker. Vox Sang. 2008;95:308–12. doi: 10.1111/j.1423-0410.2008.01109.x. [DOI] [PubMed] [Google Scholar]
- 254.Hansen E, Hofstädter F, Taeger K. Autologous blood transfusion in tumor operations. Infusionsther Transfusionsmed. 1994;21:337–48. [PubMed] [Google Scholar]
- 255.Hansen E, Knuechel R, Altmeppen J, Taeger K. Blood irradiation for intraoperative autotransfusion in cancer surgery: demonstration of efficient elimination of contaminating tumor cells. Transfusion. 1999;39:608–15. doi: 10.1046/j.1537-2995.1999.39060608.x. [DOI] [PubMed] [Google Scholar]
- 256.Hansen E, Bechmann V, Altmeppen J. Intraoperative blood salvage with irradiation of blood in cancer surgery: answers to current queries. Intensivmed Notfallmed Schmerzther. 2002;37:740–4. doi: 10.1055/s-2002-35917. [DOI] [PubMed] [Google Scholar]
- 257.Hansen E, Bechmann V, Altmeppen J. Intraoperative blood salvage in cancer surgery: safe and effective? Transfus Apher Sci. 2002;27:153–7. doi: 10.1016/s1473-0502(02)00037-x. [DOI] [PubMed] [Google Scholar]
- 258.Sankarankutty AK, Teixera AC, Souza FF, et al. Impact of blood salvage during liver transplantation on reduction in transfusion requirements. Acta Cir Bras. 2006;21(Suppl 1):44–7. doi: 10.1590/s0102-86502006000700011. [DOI] [PubMed] [Google Scholar]
- 259.Phillips SD, Maguire D, Deshpande R, et al. A prospective study investigating the cost effectiveness of intraoperative blood salvage during liver transplantation. Transplantation. 2006;81:536–40. doi: 10.1097/01.tp.0000199318.17013.c5. [DOI] [PubMed] [Google Scholar]
- 260.Massicotte L, Thibeault L, Beaulieu D, et al. Evaluation of cell salvage autotransfusion utility during liver transplantation. HPB (Oxford) 2007;9:52–7. doi: 10.1080/13651820601090596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Feltracco P, Michieletto E, Barbieri S, et al. Microbiologic contamination of intraoperative blood salvaged during liver transplantation. Transplant Proc. 2007;39:1889–91. doi: 10.1016/j.transproceed.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 262.Sugai Y, Sugai K, Fuse A. Current status of bacterial contamination of autologous blood for transfusion. Transfus Apher Sci. 2001;24:255–9. doi: 10.1016/s1473-0502(01)00067-2. [DOI] [PubMed] [Google Scholar]
- 263.Waters JH, Tuohy MJ, Hobson DF, Procop D. Bacterial reduction by cell salvage washing and leukocyte depletion filtration. Anesthesiology. 2003;99:652–5. doi: 10.1097/00000542-200309000-00021. [DOI] [PubMed] [Google Scholar]
- 264.Fong J, Gurewitsch ED, Kump L, Klein R. Clearance of fetal products and subsequent immunoreactivity of blood salvaged at cesarean delivery. Obstet Gynecol. 1999;93:968–72. doi: 10.1016/s0029-7844(98)00559-6. [DOI] [PubMed] [Google Scholar]
- 265.Potter PS, Waters JH, Burger GA, Mraovic B. Application of cell-salvage during cesarean section. Anesthesiology. 1999;90:619–21. doi: 10.1097/00000542-199902000-00038. [DOI] [PubMed] [Google Scholar]
- 266.Williamson KR, Taswell HF. Intraoperative blood salvage: a review. Transfusion. 1991;31:662–75. doi: 10.1046/j.1537-2995.1991.31791368347.x. [DOI] [PubMed] [Google Scholar]
- 267.Bosly A, Muylle L, Noens L, et al. Guidelines for the transfusion of platelets. Acta Clin Belg. 2007;62:36–47. doi: 10.1179/acb.2007.006. [DOI] [PubMed] [Google Scholar]
- 268.Rebulla P. Revisitation of the clinical indications for the transfusion of platelet concentrates. Rev Clin Exp Hematol. 2001;5:288–310. doi: 10.1046/j.1468-0734.2001.00042.x. [DOI] [PubMed] [Google Scholar]
- 269.Tosetto A, Balduini CL, Cattaneo M, et al. Management of bleeding and of invasive procedures in patients with platelet disorders and/or thrombocytopenia: Guidelines of the Italian Society for Haemostasis and Thrombosis (SISET) Thromb Res. 2009;124:e13–8. doi: 10.1016/j.thromres.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 270.Pisciotto PT, Benson K, Hume H, et al. Prophylactic versus therapeutic platelet transfusion practices in hematology and/or oncology patients. Transfusion. 1995;35:498–502. doi: 10.1046/j.1537-2995.1995.35695288769.x. [DOI] [PubMed] [Google Scholar]
- 271.Levi M. Current understanding of disseminated intravascular coagulation. Br J Haematol. 2004;124:567–76. doi: 10.1046/j.1365-2141.2003.04790.x. [DOI] [PubMed] [Google Scholar]
- 272.Hellem AJ, Borchgrevink CF, Ames SB. The role of red cells in haemostasis: the relation between haematocrit, bleeding time and platelet adhesiveness. Br J Haematol. 1961;7:42–50. doi: 10.1111/j.1365-2141.1961.tb00318.x. [DOI] [PubMed] [Google Scholar]
- 273.Livio M, Gotti E, Marchesi D, et al. Uraemic bleeding: role of anemia and beneficial effect of red cell transfusions. Lancet. 1982;2:1013–5. doi: 10.1016/s0140-6736(82)90050-2. [DOI] [PubMed] [Google Scholar]
- 274.Small M, Lowe GD, Cameron E, Forbes CD. Contribution of the haematocrit to the bleeding time. Haemostasis. 1983;13:379–84. doi: 10.1159/000214826. [DOI] [PubMed] [Google Scholar]
- 275.Fernandez F, Goudable C, Sie P, et al. Low haematocrit and prolonged bleeding time in uraemic patients: effect of red cell transfusions. Br J Haematol. 1985;59:139–48. doi: 10.1111/j.1365-2141.1985.tb02974.x. [DOI] [PubMed] [Google Scholar]
- 276.Escolar G, Garrido M, Mazzara R, et al. Experimental basis for the use of red cell transfusion in the management of anemic-thrombocytopenic patients. Transfusion. 1988;28:406–11. doi: 10.1046/j.1537-2995.1988.28588337325.x. [DOI] [PubMed] [Google Scholar]
- 277.Burns ER, Lawrence C. Bleeding time. A guide to its diagnostic and clinical utility. Arch Pathol Lab Med. 1989;113:1219–24. [PubMed] [Google Scholar]
- 278.Ho CH. The hemostatic effect of adequate red cell transfusion in patients with anemia and thrombocytopenia. Transfusion. 1996;36:290. doi: 10.1046/j.1537-2995.1996.36396182154.x. [DOI] [PubMed] [Google Scholar]
- 279.Crowley JP, Metzger JB, Valeri CR. The volume of blood shed during the bleeding time correlates with the peripheral venous hematocrit. Am J Clin Pathol. 1997;108:579–84. doi: 10.1093/ajcp/108.5.579. [DOI] [PubMed] [Google Scholar]
- 280.Valeri CR, Cassidy G, Pivacek LE, et al. Anemiainduced increase in the bleeding time: implications for treatment of nonsurgical blood loss. Transfusion. 2001;41:977–83. doi: 10.1046/j.1537-2995.2001.41080977.x. [DOI] [PubMed] [Google Scholar]
- 281.Eugster M, Reinhart WH. The influence of the hematocrit on primary haemostasis in vitro. Thromb Haemost. 2005;94:1213–8. doi: 10.1160/TH05-06-0424. [DOI] [PubMed] [Google Scholar]
- 282.Webert KE, Cook RJ, Sigouin CS, et al. The risk of bleeding in thrombocytopenic patients with acute myeloid leukaemia. Haematologica. 2006;41:1530–7. [PubMed] [Google Scholar]
- 283.Webert KE, Cook RJ, Couban S, et al. A multicenter pilot-randomized controlled trial of the feasibility of an augmented red blood cell transfusion strategy for patients treated with induction chemotherapy for acute leukemia or stem cell transplantation. Transfusion. 2008;48:81–91. doi: 10.1111/j.1537-2995.2007.01485.x. [DOI] [PubMed] [Google Scholar]
- 284.Standards for Blood Banks and Transfusion Services. 24th ed. Bethesda, MD: American Association of Blood Banks; 2006. [Google Scholar]
- 285.Slichter SJ, Davis K, Enright H, et al. Factors affecting posttransfusion platelet increments, platelet refractoriness, and platelet transfusion intervals in thrombocytopenic patients. Blood. 2005;105:4106–14. doi: 10.1182/blood-2003-08-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Lozano M, Cid J. The clinical implication of platelet transfusions associated with ABO or Rh(D) incompatibility. Transfus Med Rev. 2003;17:57–68. doi: 10.1053/tmrv.2003.50003. [DOI] [PubMed] [Google Scholar]
- 287.Herman JH, King KE. Apheresis platelet transfusion: does ABO matter? [editorial] Transfusion. 2004;44:802–4. doi: 10.1111/j.1537-2995.2004.44601.x. [DOI] [PubMed] [Google Scholar]
- 288.Josephson CD, Mullis NC, Van Demark C, Hillyer CD. Significant numbers of apheresis-derived group O platelet units have “high titer” anti-A/A,B: implications for transfusion policy. Transfusion. 2004;44:805–8. doi: 10.1111/j.1537-2995.2004.03290.x. [DOI] [PubMed] [Google Scholar]
- 289.Tinmouth AT, Semple E, Shehata N, Branch DR. Platelet immunopathology and therapy: a Canadian Blood Services research and development symposium. Transfus Med Rev. 2006;20:294–314. doi: 10.1016/j.tmrv.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 290.Daly PA, Schiffer CA, Aisner J, Wiernik PA. Platelet transfusion therapy. One hour posttransfusion increments are valuable in predicting the need for HLA-matched preparations. JAMA. 1980;243:435–8. doi: 10.1001/jama.243.5.435. [DOI] [PubMed] [Google Scholar]
- 291.O’Connell B, Lee EJ, Schiffer CA. The value of 10-minute post-transfusion platelet counts. Transfusion. 1988;28:66–7. doi: 10.1046/j.1537-2995.1988.28188127957.x. [DOI] [PubMed] [Google Scholar]
- 292.Bishop JF, Matthews JP, McGarth K, et al. Factors influencing 20-hour increments after platelet transfusion. Transfusion. 1991;31:392–6. doi: 10.1046/j.1537-2995.1991.31591263191.x. [DOI] [PubMed] [Google Scholar]
- 293.Contreras M. Diagnosis and treatment of patients refractory to platelet transfusions. Blood Rev. 1998;12:215–21. doi: 10.1016/s0268-960x(98)90001-7. [DOI] [PubMed] [Google Scholar]
- 294.Novotny VM. Prevention and management of platelet transfusion refractoriness. Vox Sang. 1999;76:1–13. doi: 10.1159/000031013. [DOI] [PubMed] [Google Scholar]
- 295.Roback JD, Caldwell S, Carson J, et al. Evidencebased practice guidelines for plasma transfusion. Transfusion. 2010;50:1227–39. doi: 10.1111/j.1537-2995.2010.02632.x. [DOI] [PubMed] [Google Scholar]
- 296.World Health Organisation . The Clinical Use of Blood Handbook. Geneva: WHO; 2005. [Google Scholar]
- 297.NIH - Consensus Conference Fresh-frozen plasma. Indications and risks. JAMA. 1985;253:551–3. [PubMed] [Google Scholar]
- 298.De Backer D, Vandekerckhove B, Stanworth S, et al. Guidelines for the use of fresh frozen plasma. Acta Clin Belg. 2008;63:381–90. doi: 10.1179/acb.2008.079. [DOI] [PubMed] [Google Scholar]
- 299.British Committee for Standards in Haematology Guidelines for the use of fresh frozen plasma. Transfus Med. 1992;2:57–63. doi: 10.1111/j.1365-3148.1992.tb00135.x. [DOI] [PubMed] [Google Scholar]
- 300.Guidelines for the use of fresh-frozen plasma Medical Directors Advisory Committee, National Blood Transfusion Council. S Afr Med J. 1998;88:1344–7. [PubMed] [Google Scholar]
- 301.Marconi M. Italian guidelines for the appropriate use of plasma. Tumori. 2001;87:S14–6. [PubMed] [Google Scholar]
- 302.Agence Française de Sécurité Sanitaire des Produits de Santé Transfusion de plasma frais congelé: produits, indications, méthode général et recommandations. Transf Clin Biol. 2002;9:322–32. doi: 10.1016/s1246-7820(02)00265-3. [DOI] [PubMed] [Google Scholar]
- 303.Hellstern P, Muntean W, Schramm W, et al. Practical guidelines for the clinical use of plasma. Thromb Res. 2002;95:53–7. doi: 10.1016/s0049-3848(02)00153-6. [DOI] [PubMed] [Google Scholar]
- 304.Ortiz P, Mingo A, Lozano M, et al. Guide for transfusion of blood components. Med Clin (Barc) 2005;125:389–96. doi: 10.1157/13079172. [DOI] [PubMed] [Google Scholar]
- 305.Gouezec H, Jego P, Betremieux P, et al. Indications for use of labile blood products and the physiology of blood transfusion in medicine. The French Agency for the Health Safety of Health Products. Transfus Clin Biol. 2005;12:69–76. doi: 10.1016/j.tracli.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 306.Stanworth SJ, Brunskill SJ, Hyde CJ, et al. Appraisal of the evidence for the clinical use of FFP and plasma fractions. Best Pract Res Clin Haematol. 2006;19:67–82. doi: 10.1016/j.beha.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 307.Chee YL, Crawford JC, Watson HG, Greaves M. Guidelines on the assessment of bleeding risk prior to surgery or invasive procedures. Br J Haematol. 2008;140:496–504. doi: 10.1111/j.1365-2141.2007.06968.x. [DOI] [PubMed] [Google Scholar]
- 308.Segal J, Dzik WH. Transfusion Medicine/Hemostasis Clinical Trials Network. Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: an evidencedbased review. Transfusion. 2005;45:1413–25. doi: 10.1111/j.1537-2995.2005.00546.x. [DOI] [PubMed] [Google Scholar]
- 309.Dzik WH. The NHLBI Clinical Trials Network in transfusion medicine and hemostasis: an overview. J Clin Apher. 2006;21:57–9. doi: 10.1002/jca.20092. [DOI] [PubMed] [Google Scholar]
- 310.Stanworth SJ, Brunskill SJ, Hyde CJ, et al. Is fresh frozen plasma clinically effective? A systematic review of randomised controlled trials. Br J Haematol. 2004;126:139–52. doi: 10.1111/j.1365-2141.2004.04973.x. [DOI] [PubMed] [Google Scholar]
- 311.Gajic O, Dzik WH, Toy P. Fresh frozen plasma and platelet transfusion for non bleeding patients in the intensive care unit: benefit or harm? Crit Care Med. 2006;34(Suppl 5):S170–3. doi: 10.1097/01.CCM.0000214288.88308.26. [DOI] [PubMed] [Google Scholar]
- 312.Abdel-Wahab OI, Healy B, Dzik WH. Effect of fresh-frozen plasma transfusion on prothrombin time and bleeding in patients with mild coagulation abnormalities. Transfusion. 2006;46:1279–85. doi: 10.1111/j.1537-2995.2006.00891.x. [DOI] [PubMed] [Google Scholar]
- 313.Chowdhury P, Saayman AG, Paulus U, et al. Efficacy of standard dose and 30 ml/kg fresh frozen plasma in correcting laboratory parameters of haemostasis in critically ill patients. Br J Haematol. 2004;125:69–73. doi: 10.1111/j.1365-2141.2004.04868.x. [DOI] [PubMed] [Google Scholar]
- 314.Santagostino E, Mancuso EM, Morfini M, et al. Solvent/detergent plasma for prevention of bleeding in recessively inherited coagulation disorders: dosing, pharmacokinetics and clinical efficacy. Haematologica. 2006;91:634–9. [PubMed] [Google Scholar]
- 315.Holland LL, Brooks JP. Toward rational fresh frozen plasma transfusion. Am J Clin Pathol. 2006;126:133–9. doi: 10.1309/NQXH-UG7H-ND78-LFFK. [DOI] [PubMed] [Google Scholar]
- 316.Callum JL, Karkouti K, Lin Y. Cryoprecipitate: the current state of knowledge. Transfus Med Rev. 2009;23:177–88. doi: 10.1016/j.tmrv.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 317.Ciavarella D, Reed RL, Counts RB, et al. Clotting factor levels and the risk of diffuse microvascular bleeding in the massively transfused patient. Br J Haematol. 1987;67:365–8. doi: 10.1111/j.1365-2141.1987.tb02359.x. [DOI] [PubMed] [Google Scholar]
- 318.Lundberg GD. Practice parameter for the use of fresh frozen plasma, cryoprecipitate, and platelets: Fresh-Frozen Plasma, Cryoprecipitate, and Platelets Administration Practice Guidelines Development Task Force of the College of American Pathologists. JAMA. 1994;271:777–81. [PubMed] [Google Scholar]
- 319.Ketchum L, Hess JR, Hiippala S. Indications for early fresh frozen plasma, cryoprecipitate, and platelet transfusion in trauma. J Trauma. 2006;60(6 Suppl):S51–8. doi: 10.1097/01.ta.0000199432.88847.0c. [DOI] [PubMed] [Google Scholar]
- 320.Droubatchevskaia N, Wong MP, Chipperfield KM, et al. Guidelines for cryoprecipitate transfusion. BC Med J. 2007;49:441–5. [Google Scholar]
- 321.Hedges SJ, Dehoney SB, Hooper JS, et al. Evidence based treatment recommendations for uremic bleeding. Nat Clin Pract. 2007;3:138–52. doi: 10.1038/ncpneph0421. [DOI] [PubMed] [Google Scholar]
- 322.Mannucci PM, Federici AB, Sirchia G. Hemostasis testing during massive blood replacement: a study of 172 cases. Vox Sang. 1982;42:113–23. doi: 10.1111/j.1423-0410.1982.tb01080.x. [DOI] [PubMed] [Google Scholar]
- 323.Hewson JR, Neame PB, Kumar N, et al. Coagulopathy related to dilution and hypotension during massive transfusion. Crit Care Med. 1985;13:387–91. doi: 10.1097/00003246-198505000-00003. [DOI] [PubMed] [Google Scholar]
- 324.Phillips TF, Soulier G, Wilson RF. Outcome of massive transfusion exceeding two blood volumes in trauma and emergency surgery. J Trauma. 1987;27:903–10. doi: 10.1097/00005373-198708000-00010. [DOI] [PubMed] [Google Scholar]
- 325.Faringer PD, Mullins RJ, Johnson RL, et al. Blood component supplementation during massive transfusion of AS-1 red cells in trauma patients. J Trauma. 1993;34:481–5. doi: 10.1097/00005373-199304000-00002. [DOI] [PubMed] [Google Scholar]
- 326.Cinat ME, Wallace WC, Nastanski F, et al. Improved survival following massive transfusion in patients who have undergone trauma. Arch Surg. 1999;134:964–8. doi: 10.1001/archsurg.134.9.964. [DOI] [PubMed] [Google Scholar]
- 327.Singbartl K, Innerhofer P, Radvan J, et al. Hemostasis and hemodilution: a quantitative mathematical guide for clinical practice. Anesth Analg. 2003;96:929–35. doi: 10.1213/01.ANE.0000052711.68903.5D. [DOI] [PubMed] [Google Scholar]
- 328.Hirshberg A, Dugas M, Banez EI, et al. Minimizing dilutional coagulopathy in exsanguinating hemorrhage: a computer simulation. J Trauma. 2003;54:454–63. doi: 10.1097/01.TA.0000053245.08642.1F. [DOI] [PubMed] [Google Scholar]
- 329.French CJ, Bellomo R, Angus P. Cryoprecipitate for the correction of coagulopathy associated with liver disease. Anaesth Intensive Care. 2003;31:357–61. doi: 10.1177/0310057X0303100403. [DOI] [PubMed] [Google Scholar]
- 330.Ho AM, Karmakar MK, Dion PW. Are we giving enough coagulation factors during major trauma resuscitation? Am J Surg. 2005;190:479–84. doi: 10.1016/j.amjsurg.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 331.Stinger HK, Spinella PC, Perkins JG, et al. The ratio of fibrinogen to red cells transfused affects survival in casualties receiving massive transfusions at an army combat support hospital. J Trauma. 2008;64(2 Suppl):S79–85. doi: 10.1097/TA.0b013e318160a57b. [DOI] [PubMed] [Google Scholar]
- 332.Fenger-Eriksen C, Lindberg-Larsen M, Christensen AQ, et al. Fibrinogen concentrate substitution therapy in patients with massive haemorrhage and low plasma fibrinogen concentrations. Br J Anaesth. 2008;101:769–73. doi: 10.1093/bja/aen270. [DOI] [PubMed] [Google Scholar]
- 333.Fenger-Eriksen C, Anke-Muller E, Hestop J, et al. Thromboelastographic whole blood clot formation after ex vivo addition of plasma substitutes: improvements of the induced coagulopathy with fibrinogen concentrate. Br J Anaesth. 2005;94:324–9. doi: 10.1093/bja/aei052. [DOI] [PubMed] [Google Scholar]
- 334.Fries D, Krismer A, Klinger A, et al. Effect of fibrinogen on reversal of dilutional coagulopathy: a porcine model. Br J Anaesth. 2005;95:171–7. doi: 10.1093/bja/aei160. [DOI] [PubMed] [Google Scholar]
- 335.Heindl B, Delorenzo C, Spannagl M. High dose fibrinogen administration for acute therapy of coagulopathy during massive perioperative transfusion. Anaesthesist. 2005;55:787–90. doi: 10.1007/s00101-005-0865-7. [DOI] [PubMed] [Google Scholar]
- 336.Fries D, Haas T, Klingler A, et al. Efficacy of fibrinogen and prothrombin complex concentrate used to reverse dilutional coagulopathy – a porcine model. Br J Anaesth. 2006;97:460–7. doi: 10.1093/bja/ael191. [DOI] [PubMed] [Google Scholar]
- 337.Spahn DR, Cerny V, Coats TJ, et al. Task Force for Advanced Bleeding Care in Trauma: management of bleeding following major trauma: a European guideline. Crit Care. 2007;11:R17. doi: 10.1186/cc5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Danés AF, Cuenca LG, Bueno SR, et al. Efficacy and tolerability of human fibrinogen concentrate administration to patients with acquired fibrinogen deficiency and active or in high-risk severe bleeding. Vox Sang. 2008;94:221–6. doi: 10.1111/j.1423-0410.2007.01024.x. [DOI] [PubMed] [Google Scholar]
- 339.Weinkove R, Rangarajan S. Fibrinogen concentrate for acquired hypofibrinogenaemic states. Transfus Med. 2008;18:151–7. doi: 10.1111/j.1365-3148.2008.00854.x. [DOI] [PubMed] [Google Scholar]
- 340.Huissoud C, Carrabin N, Audibert F, et al. Bedside assessment of fibrinogen level in postpartum haemorrhage by thromboelastometry. BJOG. 2009;116:1097–102. doi: 10.1111/j.1471-0528.2009.02187.x. [DOI] [PubMed] [Google Scholar]
- 341.Rahe-Meyer N, Pichlmaier M, Haverich A, et al. Bleeding management with fibrinogen concentrate targeting a high-normal plasma fibrinogen level: a pilot study. Br J Anaesth. 2009;102:785–92. doi: 10.1093/bja/aep089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Lak M, Keihani M, Elahi F, et al. Bleeding and thrombosis in 55 patients with inherited afibrinogenaemia. Br J Haematol. 1999;107:204–206. doi: 10.1046/j.1365-2141.1999.01681.x. [DOI] [PubMed] [Google Scholar]
- 343.Kreuz W, Meili E, Peter-Salonen K, et al. Efficacy and tolerability of a pasteurised human fibrinogen concentrate in patients with congenital fibrinogen deficiency. Transfus Apher Sci. 2005;32:247–53. doi: 10.1016/j.transci.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 344.Liumbruno GM, Bennardello F, Lattanzio A, et al. Recommendations for the use of antithrombin concentrates and prothrombin complex concentrates. Blood Transfus. 2009;7:325–34. doi: 10.2450/2009.0116-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Hathaway WE, Goodnight SH., Jr . Malattie dell’Emostasi e Trombosi. Milano: McGraw-Hill Companies Italia; 1994. [Google Scholar]
- 346.Afshari A, Wetterslev J, Brok J, Møller AM. Antithrombin III for critically ill patients. Cochrane Database Syst Rev. 2008;3:CD005370. doi: 10.1002/14651858.CD005370.pub2. [DOI] [PubMed] [Google Scholar]
- 347.Afshari A, Wetterslev J, Brok J, Møller A. Antithrombin III in critically ill patients: systematic review with meta-analysis and trial sequential analysis. BMJ. 2007;335:1248–51. doi: 10.1136/bmj.39398.682500.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Martinowitz U, Saltz R. Fibrin sealant. Curr Opin Hematol. 1996;3:395–402. doi: 10.1097/00062752-199603050-00011. [DOI] [PubMed] [Google Scholar]
- 349.Valbonesi M. Fibrin glues of human origin. Best Pract Res Clin Haematol. 2006;19:191–203. doi: 10.1016/j.beha.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 350.Radosevich M, Goubran HI, Burnouf T. Fibrin sealant: scientific rationale, production methods, properties, and current clinical use. Vox Sang. 1997;72:133–43. doi: 10.1046/j.1423-0410.1997.7230133.x. [DOI] [PubMed] [Google Scholar]
- 351.Sierra DH. Fibrin sealant adhesive systems: a review of their chemistry, material properties and clinical application. J Biomater Appl. 1993;7:309–52. doi: 10.1177/088532829300700402. [DOI] [PubMed] [Google Scholar]
- 352.Mankad PS, Codispoti M. The role of fibrin sealants in hemostasis. Am J Surg. 2001;182(2 Suppl):21S–8S. doi: 10.1016/s0002-9610(01)00773-5. [DOI] [PubMed] [Google Scholar]
- 353.Carless PA, Anthony DM, Henry DA. Systematic review of the use of fibrin sealant to minimize perioperative allogeneic blood transfusion. Br J Surg. 2002;89:695–703. doi: 10.1046/j.1365-2168.2002.02098.x. [DOI] [PubMed] [Google Scholar]
- 354.Carless PA, Henry DA, Anthony DM. Fibrin sealant for minimizing perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2003;2:CD004171. doi: 10.1002/14651858.CD004171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Figueras J, Llado L, Miro M, et al. Application of fibrin glue sealant after hepatectomy does not seem justified. Results of a randomized study in 300 patients. Ann Surg. 2007;245:536–42. doi: 10.1097/01.sla.0000245846.37046.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Berrevoet F, de Hemptinne B. Use of hemostatic agents during liver resection. Dig Surg. 2007;24:288–93. doi: 10.1159/000103660. [DOI] [PubMed] [Google Scholar]
- 357.Birmingham B. TEE diagnosis of mechanical AVR dysfunction associated with biological glue. Anesth Analg. 2001;93:1627–8. doi: 10.1097/00000539-200112000-00075. [DOI] [PubMed] [Google Scholar]
- 358.Bingley JA, Gardner MA, Stafford EG, et al. Late complications of tissue glues in aortic surgery. Ann Thorac Surg. 2000;69:1764–8. doi: 10.1016/s0003-4975(00)01250-9. [DOI] [PubMed] [Google Scholar]
- 359.Kazui T, Washiyama N, Bashar AH, et al. Role of biologic glue repair of proximal aortic dissection in the development of early and midterm redissection of the aortic root. Ann Thorac Surg. 2001;72:509–14. doi: 10.1016/s0003-4975(01)02777-1. [DOI] [PubMed] [Google Scholar]
- 360.Gringeri A. Associazione Italiana dei Centri Emofilia; 2004. Linee guida per la diagnosi ed il trattamento di pazienti con inibitori dei fattori plasmatici della coagulazione. Available at: http://www.aiceonline.it/documenti/LineeGuida/AICE_guida_inib_2004_finale.pdf. Last accessed on 25/03/2010. [Google Scholar]
- 361.Hay CR, Brown S, Collins PW, et al. The diagnosis and management of factor VIII and IX inhibitors: a guideline from the United Kingdom Haemophilia Centre Doctors’ Organisation. Br J Haematol. 2006;133:591–605. doi: 10.1111/j.1365-2141.2006.06087.x. [DOI] [PubMed] [Google Scholar]
- 362.Santagostino E. Linee guida per la terapia sostitutiva dell’emofilia e dei difetti ereditari della coagulazione. Associazione Italiana dei Centri Emofilia; 2003. Available at: http://www.aiceonline.it/documenti/LineeGuida/ITALIA_Coagulopatie.pdf. Last accessed on 03/25/2010. [Google Scholar]
- 363.Bolton-Maggs PH, Perry DJ, Chalmers EA, et al. The rare coagulation disorders: review with guidelines for management from the United Kingdom Haemophilia Centre Doctors’ Organisation. Haemophilia. 2004;10:593–628. doi: 10.1111/j.1365-2516.2004.00944.x. [DOI] [PubMed] [Google Scholar]
- 364.Peyvandi F, Palla R, Menegatti M, Mannucci PM. Introduction. Rare bleeding disorders: general aspects of clinical features, diagnosis, and management. Semin Thromb Hemost. 2009;35:349–55. doi: 10.1055/s-0029-1225757. [DOI] [PubMed] [Google Scholar]
- 365.Ranucci M, Isgrò G, Soro G, et al. Efficacy and safety of recombinant activated factor VII in major surgical procedures. Arch Surg. 2008;143:296–304. doi: 10.1001/archsurg.2007.66. [DOI] [PubMed] [Google Scholar]
- 366.Vincent JL, Rossaint R, Riou B, et al. Recommendations on the use of recombinant activated factor VII as an adjunctive treatment for massive bleeding: a European perspective. Crit Care. 2006;10:R120. doi: 10.1186/cc5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Shander A, Goodnough LT, Ratko T, et al. Consensus recommendations for the off-label use of recombinant human factor VIIa (NovoSeven) therapy. Pharma Ther. 2005;30:644–58. [Google Scholar]
- 368.Welsh A, McLintock C, Gatt S, et al. Guidelines for the use of recombinant activated factor VII in massive obstetric haemorrhage. Aust N Z J Obstet Gynaecol. 2008;48:12–6. doi: 10.1111/j.1479-828X.2007.00823.x. [DOI] [PubMed] [Google Scholar]
- 369.Stanworth SJ, Birchall J, Doree CJ, Hyde C. Recombinant factor VIIa for the prevention and treatment of bleeding in patients without haemophilia. Cochrane Database Syst Rev. 2007;2:CD005011. doi: 10.1002/14651858.CD005011.pub2. [DOI] [PubMed] [Google Scholar]
- 370.European Medicines Agency NovoSeven. European Public Assessment Report. Procedural steps taken and scientific information after the authorization. Changes made after 01/02/2004. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Procedural_steps_taken_and_scientific_information_after_authorisation/human/000074/WC500030872.pdf. Last accessed on 11/01/2010.
- 371.European Medicines Agency NovoSeven. Allegato I. Riassunto delle caratteristiche del prodotto. Available at: http://www.ema.europa.eu/docs/it_IT/document_library/EPAR_-_Product_Information/human/000074/WC500030873.pdf. Last accessed on 11/01/2010.
- 372.Fergusson DA, Hébert PC, Mazer CD, et al. BART Investigators A comparison of aprotinin and lysine analogues in high-risk cardiac surgery. N Engl J Med. 2008;358:2319–31. doi: 10.1056/NEJMoa0802395. [DOI] [PubMed] [Google Scholar]
- 373.Henry D, Carless P, Fergusson D, Laupacis A. The safety of aprotinin and lysine-derived antifibrinolytic drugs in cardiac surgery: a meta-analysis. CMAJ. 2009;180:183–93. doi: 10.1503/cmaj.081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Takagi H, Manabe H, Kawai N, et al. Aprotinin increases mortality as compared with tranexamic acid in cardiac surgery: a meta-analysis of randomized head-to-head trials. Interact Cardiovasc Thorac Surg. 2009;9:98–101. doi: 10.1510/icvts.2008.198325. [DOI] [PubMed] [Google Scholar]
- 375.Henry DA, Carless PA, Moxey AJ, et al. Antifibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2007;4:CD001886. doi: 10.1002/14651858.CD001886.pub2. [DOI] [PubMed] [Google Scholar]
- 376.Hiippala S, Strid L, Wennerstrand M, et al. Tranexamic acid (Cyklokapron) reduces perioperative blood loss associated with total knee arthroplasty. Br J Anaesth. 1995;74:534–7. doi: 10.1093/bja/74.5.534. [DOI] [PubMed] [Google Scholar]
- 377.Hiippala ST, Strid LJ, Wennerstrand MI, et al. Tranexamic acid radically decreases blood loss and transfusions associated with total knee arthroplasty. Anesth Analg. 1997;84:839–44. doi: 10.1097/00000539-199704000-00026. [DOI] [PubMed] [Google Scholar]
- 378.Benoni G, Fredin H. Fibrinolytic inhibition with tranexamic acid reduces blood loss and blood transfusion after knee arthroplasty: a prospective, randomised, double blind study of 86 patients. J Bone Joint Surg Br. 1996;78:434–40. [PubMed] [Google Scholar]
- 379.Zufferey P, Merquiol F, Laporte S, et al. Do antifibrinolytics reduce allogeneic blood transfusion in orthopedic surgery? Anesthesiology. 2006;105:1034–46. doi: 10.1097/00000542-200611000-00026. [DOI] [PubMed] [Google Scholar]
- 380.Levy JH. Pharmacologic methods to reduce perioperative bleeding. Transfusion. 2008;48(1 Suppl):31S–8S. doi: 10.1111/j.1537-2995.2007.01574.x. [DOI] [PubMed] [Google Scholar]
- 381.Kagoma YK, Crowther MA, Douketis J, et al. Use of antifibrinolytic therapy to reduce transfusion in patients undergoing orthopedic surgery: a systematic review of randomized trials. Thromb Res. 2009;123:687–96. doi: 10.1016/j.thromres.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 382.Kang Y, Lewis JH, Navalgund A, et al. Epsilonaminocaproic acid for treatment of fibrinolysis during liver transplantation. Anesthesiology. 1987;66:766–73. [PMC free article] [PubMed] [Google Scholar]
- 383.Boylan JF, Klinck JR, Sandler AN, et al. Tranexamic acid reduces blood loss, transfusion requirements, and coagulation factor use in primary orthotopic liver transplantation. Anesthesiology. 1996;85:1043–8. doi: 10.1097/00000542-199611000-00012. [DOI] [PubMed] [Google Scholar]
- 384.Dalmau A, Sabaté A, Acosta F, et al. Tranexamic acid reduces red cell transfusion better than epsilonaminocaproic acid or placebo in liver transplantation. Anesth Analg. 2000;91:29–34. doi: 10.1097/00000539-200007000-00006. [DOI] [PubMed] [Google Scholar]
- 385.Molenaar IQ, Warnaar N, Groen H, et al. Efficacy and safety of antifibrinolytic drugs in liver transplantation: a systematic review and meta-analysis. Am J Transplant. 2007;7:185–94. doi: 10.1111/j.1600-6143.2006.01591.x. [DOI] [PubMed] [Google Scholar]
- 386.Helm RE, Rosengart TK, Gomez M, et al. Comprehensive multimodality blood conservation: 100 consecutive CABG operations without transfusion. Ann Thorac Surg. 1998;65:125–36. doi: 10.1016/s0003-4975(97)01004-7. [DOI] [PubMed] [Google Scholar]
- 387.Reger TB, Roditski D. Bloodless medicine and surgery for patients having cardiac surgery. Crit Care Nurse. 2001;21:35–44. [PubMed] [Google Scholar]
- 388.Martyn V, Farmer SL, Wren MN, et al. The theory and practice of bloodless surgery. Transfus Apher Sci. 2002;27:29–43. doi: 10.1016/s1473-0502(02)00024-1. [DOI] [PubMed] [Google Scholar]
- 389.Keating EM, Meding JB. Perioperative blood management practices in elective orthopaedic surgery. J Am Acad Orthop Surg. 2002;10:393–400. doi: 10.5435/00124635-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 390.Shander A. Surgery without blood. Crit Care Med. 2003;31(Suppl):S708–14. doi: 10.1097/01.CCM.0000098038.18919.7A. [DOI] [PubMed] [Google Scholar]
- 391.Majeski J. Advances in general and vascular surgical care of Jehovah’s Witnesses. Int Surg. 2000;85:257–65. [PubMed] [Google Scholar]
- 392.Heaton N. Advances and methods in liver surgery: haemostasis. Eur J Gastroenterol Hepatol. 2005;17(Suppl 1):S3–S12. doi: 10.1097/00042737-200504001-00002. [DOI] [PubMed] [Google Scholar]
- 393.Degoute CS. Controlled hypotension: a guide to drug choice. Drugs. 2007;67:1053–76. doi: 10.2165/00003495-200767070-00007. [DOI] [PubMed] [Google Scholar]
- 394.Smyrniotis V, Kostopanagiotou G, Theodoraki K, et al. The role of central venous pressure and type of vascular control in blood loss during major liver resections. Am J Surg. 2004;187:398–402. doi: 10.1016/j.amjsurg.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 395.Iserson KV, Huestis DW. Blood warming: current applications and techniques. Transfusion. 1991;31:558–71. doi: 10.1046/j.1537-2995.1991.31691306256.x. [DOI] [PubMed] [Google Scholar]
- 396.Schmied H, Kurz A, Sessler DI, et al. Mild intraoperative hypothermia increases blood loss and allogeneic transfusion requirements during total hip arthroplasty. Lancet. 1996;347:289–292. doi: 10.1016/s0140-6736(96)90466-3. [DOI] [PubMed] [Google Scholar]
- 397.Schmied H, Schiferer A, Sessler DI, Meznik C. The effects of red-cell scavenging, hemodilution, and active warming on allogeneic blood requirement in patients undergoing hip or knee arthroplasty. Anesth Analg. 1998;86:387–391. doi: 10.1097/00000539-199802000-00032. [DOI] [PubMed] [Google Scholar]
- 398.Winkler M, Akça O, Birkenberg B, et al. Aggressive warming reduces blood loss during hip arthroplasty. Anesth Analg. 2000;91:978–84. doi: 10.1097/00000539-200010000-00039. [DOI] [PubMed] [Google Scholar]
- 399.Kurz A. Thermal care in the perioperative period. Best Pract Res Clin Anaesthesiol. 2008;22:39–62. doi: 10.1016/j.bpa.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 400.Reynolds L, Beckmann J, Kurz A. Perioperative complications of hypothermia. Best Pract Res Clin Anaesthesiol. 2008;22:645–57. doi: 10.1016/j.bpa.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 401.Ohtsuka N, Yamakage M, Chen X, et al. Evaluation of four techniques of warming intravenous fluids. J Anesth. 2002;16:145–9. doi: 10.1007/s005400200010. [DOI] [PubMed] [Google Scholar]
- 402.Satoh J, Yamakage M, Wasaki SI, Namiki A. Performance of three systems for warming intravenous fluids at different flow rates. Anaesth Intensive Care. 2006;34:46–50. doi: 10.1177/0310057X0603400109. [DOI] [PubMed] [Google Scholar]


