Introduction
It has long been known that antigens of the ABO blood group are expressed not only on the surface of red blood cells, but also on cells of other tissues such as the renal parenchyma, at the level of the glomerular capillary endothelium and the distal tubule cells1.
In an ABO-incompatible kidney transplant, the ABO antigens are the target not only of the corresponding natural antibodies (IgM), but also of the immune antibodies (IgG) whose titre can increase abruptly during the hours or days following the transplant. These antibodies, through the activation of complement, are able to trigger the so-called “hyperacute rejection”, characterised by haemorrhagic thrombosis of the transplanted organ with irreversible loss of its function. When this process occurs in the days following the transplant rather that in the immediate post-operative period, it is called “antibody-mediated acute rejection”. This is characterised, from a histological point of view, as inflammatory damage of variable severity of the glomerular and peritubular capillaries and, from a clinical point of view, by severe deterioration of the function of the transplanted organ. The process can be reversed only if treated in the earliest stage of its onset, so the success of the therapeutic intervention is critically dependent on the speed with which the diagnosis is made. This explains the need for precise, regular monitoring for anti-A/B antibodies in the serum of the recipient. The antibody titration can be a valid guide for apheretic treatment, which is aimed at eliminating the anti-A/B antibodies in the pre-transplant period and maintaining a low titre in the post-transplant period.
A recent study1 showed that the expression of these antigens in the renal parenchyma is maximal in subjects with the A1 blood group. Group B subjects have intermediate expression, while group A2 subjects have low expression1; therefore, among all ABO-incompatible transplants, the risk of hyperacute rejection is lowest for A2-incompatible transplants.
Incompatibility for ABO blood group is, therefore, considered an absolute contraindication to kidney transplantation, with the possible exception of transplants from A2 group donors. In at least one quarter of cases, donation of a kidney from a living donor is impractical because of blood group incompatibility2. In these cases, donation from a cadaver is the only feasible option. Unfortunately, because of the limited availability of donors, over the last years the waiting time for a cadaveric transplant has become progressively longer such that it can now exceed 3 years, making this possibility yet more unpredictable for some patients3.
Following the example of the USA, the Netherlands and other European countries4–6, a programme of exchange transplantation from living donors has been activated in Italy3; in this programme two incompatible donor-recipient pairs exchange kidneys in such a way that each recipient receives a compatible kidney. This strategy does, however, have limited possibilities for application unless the programme involves a high number of donor-recipient pairs; furthermore, it is disadvantageous for group O recipients7–9.
The development of new diagnostic and therapeutic instruments has, however, led to the possibility of obtaining identical results from transplants from living ABO-incompatible and ABO-compatible donors10. In fact, already in 1989, drawing on the positive experience gained in Belgium in the 1980s11, the largest programme of renal transplants from living ABO-incompatible donors so far carried out was started in Japan12.
The long-term results of this programme have been excellent12,13. However, the therapeutic protocol, both in Belgium and in Japan, included pre-transplant splenectomy with the aim of reducing the production of antibodies. Splenectomy is a procedure well-recognised to be associated with risks, in some cases severe14, and this has probably dissuaded Transplant Centres outside those countries from using the programme. However, recent studies in the USA gave fresh encouragement to transplants from living ABO-incompatible donors, with the demonstration that equally satisfactory results can be obtained using the monoclonal anti-CD20 antibody, rituximab, instead of splenectomy15,16, or, indeed, without using either of these two interventions17,18. Building on the experience gained in the USA at the beginning of the new century19, the Karolinska Institute in Stockholm designed a new protocol20,21; this protocol produced the best results thus far reported, so it was subsequently adopted, with minor variations, in other European centres22–25. It consists of specific extracorporeal immunoadsorption of the anti-A and anti-B antibodies on GlycoSorb columns (Glicorex Transplantation AB, Lund, Sweden)20,21, a process which is repeated until the “target” titre of IgG isoagglutinins is <1:8 at the time of the transplant. There is, however, still some difference of opinions between the major international transplant centres on what is the ideal target titre of anti-A/B antibodies to reach in the pre-transplant phase in order to prevent hyperacute rejection, with the suggestions ranging from <1/8 to 1/32 depending on different experiences26.
Immunoadsorption on specific immunocolumns, made of a sepharose matrix with specific A or B glycosaccharide ligands designed specifically to bind only anti-A or anti-B antibodies, respectively, is a widely used technique in European transplant centres, thanks to its ease of use, specificity (it removes anti-A and anti-B antibodies and, to a lesser extent, antibodies against other polysaccharide antigens), safety (it does not require replacement of clotting factors, or albumin) and efficacy, reducing the antibody titre by two to four steps in a single session, which is a reduction equivalent to or even greater than that achieved with standard plasmapheresis. The main disadvantage of this procedure is its high cost. Specific immunoadsorption is not yet being used in American and Japanese transplant centres. The former still use standard plasmapheresis, which results in completely non-specific removal of antibodies, not only the anti-A/B, but also anti-HLA, all antimicrobial antibodies, and complement factors. Thus, following this traditional technique it is often necessary to give the patient a transfusion of plasma in order to restore clotting factors and, thereby, reduce the risk of peri-operative haemorrhage. In contrast, with the dual filtration plasmapheresis technique, used in Japan, the fraction of plasma containing clotting factors is re-infused27, although albumin must be replaced. Another technique, used in some centres in the United Kingdom, is selective immunoadsorption of type IgG antibodies through the Therasorb system28. This is another expensive procedure, but its efficacy in removing antibodies is greater than that of standard plasmapheresis. It has the advantage of being able to be used with some success also for HLA-incompatible kidney transplants, but does have some clinical disadvantages, such as the need to use heparin to prevent bleeding, with the consequent increase in the risk of peri-operative bleeding.
Among about 90 patients, reported in the literature up to 2009, who underwent renal transplantation with the selective immunoadsorption technique in the setting of the protocol developed in Sweden, there were only two adverse events: one acute antibody-mediated rejection, which regressed following treatment, and a death from Clostridium difficile bowel infection 4 months after the transplant21,22,24,25. This low incidence of adverse events was not dissimilar to that recorded in the series of patients underoing ABO-compatible transplants. In particular, the incidence of antibody-mediated acute rejection seemed to be even lower than that reported in recent years in the USA18 and in Japan14.
On the basis of this evidence we decided to carry out a kidney transplant from a live ABO-incompatible donor at Parma (Italy).
Case Report
Here we report the case of the first kidney transplant from an ABO-incompatible donor ever carried out in Italy from a living group A1 donor to a group O recipient with a high titre of anti-A1 antibodies (IgG 1:512). The transplant was performed in August 2008 at Parma following a desensitization protocol developed at the Department of Transplant Surgery of the Karolinska Institute in Stockholm (Sweden). This therapeutic protocol, which does not include splenectomy, is based on the following measures: (i) administration of rituximab 375 mg/m2 (as a single dose) 4 weeks prior to the transplant; (ii) selective immunoadsorption to remove the isoagglutinins to reach the target IgG <1:8 in the pre-transplant period, with the sessions performed on days -6, -5, -2, and -1 followed by another three sessions of immunoadsorption after the transplant, on days +2, +5, and +8; (iii) intravenous administration of immunoglobulins 0.5 g/kg (in a single infusion) the day preceding the transplant. Immunosuppressive treatment was started 10 days before the transplant and maintained in the post-transplant period with tacrolimus at doses from 0.15 to 0.20 mg/kg/die, mycophenolate mofetil 1 g x 2/die (with a reduction in the dose 6 weeks after the transplant), and methylprednisolone 16 mg/die (tapered down to 4 mg/die at 3 months after the transplant).
The recipient was a 70-year old male who weighed 67 kg and had blood group O and had been undergoing peritoneal dialysis because of inherited kidney disease for the preceding 6 months. The patient was the youngest of four siblings, the other three having all died of the same disease. The donor of the kidney was the patient’s 59-year old wife, who weighed 55 kg, had blood group A1, was in good health, had no history of significant clinical disorders and had normal renal function. HLA compatibility between the donor and recipient was as follows: two HLA mismatches on locus B, negative cytotoxicity cross-matching for T cells and positive for B cells, and Luminex flow cytofluorimetry negative for donor-specific HLA alloantibodies.
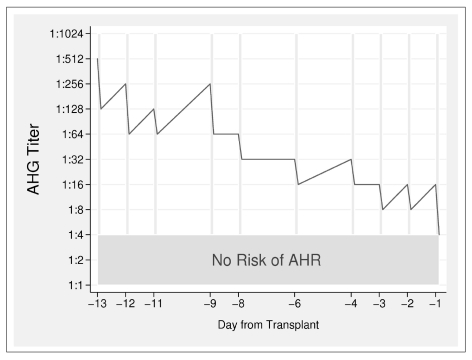
At the time of enrolment, the recipient’s titre of anti-A immune antibodies (IgG) was 1/256, but at the subsequent control before the first immunoadsorption session, the titre had risen to 1:512. In order to reach the desired target of anti-A1 IgG of <1:8 starting from such a high antibody titre, ten sessions of immunoadsorption were necessary prior to the transplant (Figure 1); the three sessions set out in the protocol for the post-transplant period were carried out even if these were perhaps not necessary since the titre remained stable between 1:4 and 1:8 after the transplant. The immunoadsorption procedures were well tolerated by the patient without causing any complications of note. It was necessary to administer calcium gluconate to the patient via an infusion pump in order to avoid hypocalcaemia due to citrate, which is poorly metabolised by patients with renal failure, and to control the blood levels of calcium, potassium and bicarbonate every 2.5–3 L of whole blood processed. At each session, 2.3 to 2.7 plasma volumes were processed at a flow rate of 70 mL/min, using ACD-A at a ratio of 1:14 (1 mL ACD-A/14 mL whole blood processed) to prevent the blood from clotting. The reduction in the antibody titre from before to after the immunoadsorption (two steps with a one step rebound of the titre on the following day) was essentially constant during the last pre-transplant treatments carried out, in accordance with published data29.
Figure 1.
Trend in the anti-IgG titre (red line) during the desensitisation. The x-axis shows the time in relation to the transplant, the y-axis shows the IgG anti-A1 antibody titre. The vertical grey lines represent the sessions of immunoadsorption. The green band at the bottom shows the titre at which the transplant could be performed because the risk of antibody-mediated rejection is virtually nonexistent.
Right up to the time of the transplant, the patient underwent a session of haemodialysis after every session of immunoadsorption in order to maintain bicarbonate, potassium and calcium homeostais. Anti-A1 IgG antibodies were titrated, by testing the patient’s serum against the donor’s red blood cells and in parallel with standard A1 red cells, every day starting from 6 days before the transplant, for about 1 month; the frequency of testing was then gradually reduced until the assays were stopped after 3 months. On the days of the immunoadsorption, the titre was assayed both before and after each apheresis.
The patient’s post-transplant course was uneventful and his renal function was stable despite a persistent anti-A1 IgG antibody titre between 1:4 and 1:8 suggestive of the occurrence of the “accommodation” phenomenon. In further confirmation of this hypothesis, two consecutive biopsies of the graft (on days 21 and 33) did not show signs of subclinical rejection despite showing marked positivity for C4d in the peritubular capillaries (a marker of antibody-mediate activation of complement). At 24 months after the transplant, the patient’s creatininaemia remains stable at about 1.6 mg/dL.
Design and methods
Immunohaematological evaluation
The blood group of both the recipient and donor was typed automatically with Biocards and the Innova Ortho Biovue System; the A subgroup was determined on a plate with anti-lectin serum (Ortho Clinical Diagnostics, UK).
Isohaemagglutinins were titrated using the following methods:
Direct haemagglutination on a OrthoBiovue Neutral card, performed in parallel at every titration of the recipient’s natural IgM anti-A1 antibodies against standard 0.8% RBC (Affirmagen A1) and against the donor’s RBC at 1%. After having filled a set of test-tubes with 200 μL of physiological saline, a series of doubling dilutions (1:2, 1:4, 1:8, etc.) were made of the 200 μL of the serum/plasma under examination in the first test-tube; 200 μL of the mixture obtained were transferred into the next test-tube and so on. From each of these dilutions, 40 μL were taken and placed in the respective columns of the Biocard Ortho Neutral, previously titrated, together with 10 μL of standard A1 RBC or the donor’s RBC at 1%. The cards were incubated at room temperature for 30 minutes, then centrifuged and read. The IgM were of little relevance since they are rapidly reduced by immunoadsorption and, indeed, the titration was interrupted because of the lack of detection of a titre.
Indirect haemagglutination was first carried out with Ortho Biovue monoclonal anti-IgG cards then with Diamed Coombs anti-IgG gel cards, in all cases following neutralisation of the IgM with dithiothreitol in the recipient’s serum/plasma and carried out in parallel with every titration of immune anti-A1 IgG antibodies in the recipient’s serum against standard RBC at 0.8% (Affirmagen A1) and against the donor’s RBC at 1%. In the same way a dilution series was prepared using the previously neutralised serum/plasma. In this case the dilution was started at 1:4 because neutralisation leads per se to a dilution of 1:2. The Diamed-ID method uses 200 μL of neutralised serum/plasma in the first test-tube, but only 100 μL of the mixture are transferred from one dilution test-tube to the next and from every dilution 50 μL are transferred to the respective microcolumn of the Diamed Coombs anti-IgG gel card, together with 25 μL of standard A1 RBC or the donor’s RBC. The card is incubated at 37°C for 15 minutes and then centrifuged and read. The reading of the titre is given by the inverse value of the highest dilution of the serum/plasma which gives an agglutination reaction of 1+. Polyclonal cards (IgG + C3d) were not used since these can increase the titre inappropriately.
Immunoadsorption procedure
The anti-A antibodies were adsorbed onto an Glycosorb ABO (Ditta Glycorex Transplantation AB) immunocolumn set up on a Fresenius Kabi COM.TEC® separator endowed with an Apheresismate informatics system to track the data.
The immunocolumn was extremely simple to prepare, requiring only 500 mL of 0.9% physiological saline for the priming. The flow of the plasma in the immunocolumn had not to exceed 50 mL/min.
The procedure was performed using a double-lumen central venous catheter for dialysis with a flow rate of 70 mL/min. Since citrate is metabolised in part by the kidneys, the risk of citrate-induced hypocalcaemia in patients with renal failure is high and our patient was, therefore, given a continuous infusion of element calcium by pump at velocity of about 8 mmol/hour in order to maintain his ionised calcium >0.90 mmol/L (normal level: 1–1.25 mmol/L). Venous blood-gases were analysed every 3 L of whole blood processed in order to monitor the calcium, potassium and bicarbonate ions in the blood. The risk of rebound hypercalcaemia, due the fact that the citrate is metabolised thus releasing the chelated calcium, was taken into account. However, the haemodialysis performed a few hours after the immunoadsorption corrected any imbalances in calcium, potassium and bicarbonate.
Discussion
Our single case confirms recently published data showing that ABO-incompatible transplants can now give results similar to those of normal ABO-compatible transplants. Our patient’s good outcome was achieved despite some predictors of poor transplant outcome, such as the advanced age of the recipient (70 years), the donor’s blood group (A1) and the recipient’s very high anti-A1 IgG titre (1:512)12,13, considering that only patients with an anti-blood group IgG titre of 1:128 or less can enter the Swedish programme20,21. As reported in the literature, various transplant centres23,30 have applied the transplant protocol in patients with high antibody titres and, despite this, have reached the pre-transplant target by planning an increased number of immunoadsorption or plasma exchange procedures right from the beginning. Also in our case, we considered the possibility of increasing the number of immunoadsorption procedures in accordance with the Swedish protocol which sets out that if the pre-transplantation target antibody titre of <1:8 is not reached, another cycle of immunoadsorption can be attempted, but in the case that this is not successful, the transplant is not performed.
In the immunohaematological management of the transplant of an ABO-incompatible kidney the method used to measure the titre of the antibodies against the donor’s blood group antigens is of critical importance, given that the results can differ greatly depending on the method used31,32. The titration of anti-A/B antibodies has long been carried out in Transfusion Services, particularly for studies of maternal-foetal ABO incompatibility, using different technologies: test-tube assays, microcolumn agglutination systems such as gel-cards (DiaMed -ID MicroTyping System) or glass microbeads (Ortho Biovue System), and cytofluorimetry.
The variety of tests available certainly does not aid standardisation of methods; however, other factors (end-point, technician’s dexterity, reagents used, target RBC) may also be involved in explaining the great variability on the final results of a comparison between different laboratories. In fact, an inter-laboratory study of isohaemagglutinin titres in 30 Japanese institutions, in which the methods normally used were compared, showed that the variation between the minimum and maximum titres ranged from 32-fold for the IgM to 256-fold for the IgG31.
In a comparison of anti-A/B titres in the same sample evaluated in three different European centres, Kumlien et al.32, demonstrated that the difference was frequently three steps if each centre used its chosen titration method, whereas the difference was one step if the same gel method was used by all three centres. Furthermore, in a comparative study at the Johns Hopkins Hospital it was recently shown, in accordance with other studies31,33, that the gel-card technique reduces the variability of the antibody titration results compared to those obtained with the test-tube assays, besides having the advantage of being faster34. In fact, as a result of their study, AuBuchon et al. concluded that to reduce the variability, the reading of the endpoint for the test-tube method should be uniformed to weak+, while greater uniformity for gel cards seems to be given if the end-point is fixed at 1+33. Finally, it has recently been suggested that cytofluorimetry should be used to monitor the titre of isohaemoagglutinins during the transplantation of ABO-incompatible kidney transplants, in order to obtain the most accurate and reproducible results35. However, the simple and economic method of indirect haemagglutination in gel, used at the transplant centre in Stockholm, seems to offer at least the essential guarantee of reproducibility32.
The first titres in our patient were determined with the immunohaematological method in use at the Service of Immunohaematology and Transfusion Medicine of Parma Hospital, that is the Ortho BioVue Card technology. This caused some initial difficulties (as described above) because of the discrepancies found in the expected titres according to a theoretical comparison with the Diamed-ID Micro Typing System method used at Stockholm.
To avoid these technical problems, from about 1 week after the start of desensitisation, only the Diamed -ID method was used to titrate the anti-A1 immune IgG: this strategy led to greater correspondence with the Swedish results and also less variability of results. Furthermore, after the initial anti-A1 (IgG) titration, done by testing the patient’s serum against commercially available, standard RBC (Affirmagen A1), the titration of the recipient’s serum against the donor’s RBC was introduced in parallel for a more correct evaluation of antigen expression in the kidney, because of individual variability in antigen expression.
Finally, another important factor responsible for the variability of titres could be dependent the staff carrying out the tests. Indeed, frequent changes in personnel due to staff rotation could have been the cause of the unexpected increase in anti-A titre of one step, from 1:256 to 1:512, found between our patient’s enrolment in the protocol and the start of desensitisation.
An IgG antibody titre <1:8 at the time of transplantation is associated with an almost inexistent risk of antibody-mediated rejection13,19. Acute antibody-mediated rejection almost always occurs only in the first 2 weeks after the transplant, subsequently becoming increasingly rare because of the establishment of a phenomenon known as “accommodation”36, that is, the acquired resistance of the transplanted organ to the damage mediated by antibodies and by complement. As accommodation develops, the transplanted organ becomes resistant to the detrimental effects of alloantibodies specific for the graft. Experience gained so far seems to indicate that the fundamental requisite for a successful long-term outcome (probably due to the development of accommodation) is the achievement of a low antibody titre at the time of the transplant and in the following 2 weeks36.
A clear disadvantage of ABO-incompatible transplants compared to ABO-compatible ones is that the former are more expensive. However, a recent cost-effectiveness analysis showed that ABO-incompatible transplants are advantageous also from an economic point of view compared to staying on dialysis37. In fact, a formal analysis recently published by the group from the Mayo Clinic38 demonstrated the additional cost of an ABO-incompatible transplant compared to a traditional one is about 38,000 dollars (that is, 72% more than an ABO-compatible transplant). However, if compared with the cost of maintaining a patient on dialysis, an ABO-incompatible transplant is financially advantageous39.
Acknowledgments
We thanks Dr Renzo Mignani (Nephrology Unit, Rimini) whose contribution proved essential for the success of this intervention.
References
- 1.Breimer ME, Molne J, Norden G, et al. Blood group A and B antigen expression in human kidneys correlated to A1/A2/B, Lewis, and secretor status. Transplantation. 2006;82:479. doi: 10.1097/01.tp.0000231697.15817.51. [DOI] [PubMed] [Google Scholar]
- 2.Karpinski M, Knoll G, Cohon A, et al. The impact of accepting living kidney donors with mild hypertension or proteinuria on transplantation rates. Am J Kidney Dis. 2005;47:317–23. doi: 10.1053/j.ajkd.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 3.Report Annuale Centro Nazionale Trapianti 2009. Available at http://www.trapianti.ministerosalute.it.
- 4.Park K, Moon J, Kim S, et al. Exchange donor program in kidney transplantation. Transplantation. 1999;67:336–8. doi: 10.1097/00007890-199901270-00027. [DOI] [PubMed] [Google Scholar]
- 5.Delmonico FL, Morrissey PE, Lipkowitz GS, et al. Donor kidney exchanges. Am J Transplant. 2004;4:1628–34. doi: 10.1111/j.1600-6143.2004.00572.x. [DOI] [PubMed] [Google Scholar]
- 6.de Klerk M, Witvliet MD, Haase-Kromwijk BJ, et al. A highly efficient living donor kidney exchange program for both blood type and crossmatch incompatible donor-recipient combinations. Transplantation. 2006;82:1616–20. doi: 10.1097/01.tp.0000250906.66728.8d. [DOI] [PubMed] [Google Scholar]
- 7.Terasaki PI, Gjertson DW, Cecka JM. Paired kidney exchange is not a solution to ABO incompatibility. Transplantation. 1998;65:291. doi: 10.1097/00007890-199801270-00030. [DOI] [PubMed] [Google Scholar]
- 8.Ross LF, Zenios S. Practical and ethical challenges to paired exchange programs. Am J Transplant. 2004;4:1553–4. doi: 10.1111/j.1600-6143.2004.00574.x. [DOI] [PubMed] [Google Scholar]
- 9.Gentry SE, Segev DL, Montgomery RA. A comparison of populations served by kidney paired donation and list paired donation. Am J Transplant. 2005;5:1914–21. doi: 10.1111/j.1600-6143.2005.00964.x. [DOI] [PubMed] [Google Scholar]
- 10.Genberg H, Kumlien G, Wennberg L, et al. ABO-incompatible kidney transplantation using antigen-specific immunoadsorption and rituximab: a 3-year follow-up. Transplantation. 2008;85:1745–54. doi: 10.1097/TP.0b013e3181726849. [DOI] [PubMed] [Google Scholar]
- 11.Alexandre GPJ, Squifflet JP, De Bruyère M, et al. Splenectomy as a prerequisite for successful human ABO-incompatible renal transplantation. Transplant Proc. 1985;17:138. [Google Scholar]
- 12.Takahashi K, Saito K, Takahara S, et al. Excellent long-term outcome of ABO-incompatible living donor kidney transplantation in Japan. Am J Transplant. 2004;4:1089–96. doi: 10.1111/j.1600-6143.2004.00464.x. [DOI] [PubMed] [Google Scholar]
- 13.Tanabe K, Takahashi K, Sonda K, et al. Long-term results ABO-incompatible living kidney transplantation: a single experience. Transplantation. 1998;65:224–8. doi: 10.1097/00007890-199801270-00014. [DOI] [PubMed] [Google Scholar]
- 14.Ichimaru N, Takahara S. Japan’s experience with living-donor kidney transplantation across ABO barriers. Nat Clin Pract Nephrol. 2008;4:682–92. doi: 10.1038/ncpneph0967. [DOI] [PubMed] [Google Scholar]
- 15.Sonnenday CJ, Warren DS, Cooper M, et al. Plasmapheresis, CMV hyperimmune globulin, and anti-CD-20 allow ABO renal transplantation without splenectomy. Am J Transplant. 2004;4:1315–22. doi: 10.1111/j.1600-6143.2004.00507.x. [DOI] [PubMed] [Google Scholar]
- 16.Gloor JM, Lager DJ, Fidler ME, et al. A comparison of splenectomy versus intensive posttransplant antidonor blood group antibody monitoring without splenectomy in ABO-incompatible kidney transplantation. Transplantation. 2005;80:1572–7. doi: 10.1097/01.tp.0000184622.69708.c1. [DOI] [PubMed] [Google Scholar]
- 17.Segev DL, Simpkins CE, Warren DS, et al. ABO incompatible high-titre renal transplantation without splenectomy or anti-CD20 treatment. Am J Transplant. 2005;5:2570–5. doi: 10.1111/j.1600-6143.2005.01031.x. [DOI] [PubMed] [Google Scholar]
- 18.Montgomery RA, Locke JE, King KE, et al. ABO incompatible renal transplantation: a paradigm ready for broad implementation. Transplantation. 2009;87:1246. doi: 10.1097/TP.0b013e31819f2024. [DOI] [PubMed] [Google Scholar]
- 19.Winters JL, Gloor JM, Pineda AA, et al. Plasma exchange conditioning for ABO-incompatible renal transplantation. J Clin Aphereresis. 2004;19:79–85. doi: 10.1002/jca.20002. [DOI] [PubMed] [Google Scholar]
- 20.Tyden G, Kumlien G, Genberg H, et al. ABO incompatible kidney transplantations without splenectomy, using antigen-specific immunoadsorption and rituximab. Am J Transplant. 2005;5:145–8. doi: 10.1111/j.1600-6143.2004.00653.x. [DOI] [PubMed] [Google Scholar]
- 21.Kumlien G, Ullstrom L, Losvall A, et al. Clinical experience with a new apheresis filter that specifically depletes ABO blood group antibodies. Transfusion. 2006;46:1568–75. doi: 10.1111/j.1537-2995.2006.00927.x. [DOI] [PubMed] [Google Scholar]
- 22.Tyden G, Donauer J, Wadstrom J, et al. Implementation of a protocol for ABO-incompatible kidney transplantation - a three-center experience with 60 consecutive transplantations. Transplantation. 2007;83:1153–5. doi: 10.1097/01.tp.0000262570.18117.55. [DOI] [PubMed] [Google Scholar]
- 23.Wilpert J, Geyer M, Teschner S, et al. ABO-incompatible kidney transplantation- proposal of an intensified apheresis strategy for patients with high initial isoagglutinine titers. J Clin Apheresis. 2007;22:314–22. doi: 10.1002/jca.20153. [DOI] [PubMed] [Google Scholar]
- 24.Oettl T, Halter J, Bachmann A, et al. ABO blood group-incompatible living donor kidney transplantation: a prospective, single-center analysis including serial protocol biopsies. Nephrol Dial Transplant. 2009;24:298–303. doi: 10.1093/ndt/gfn478. [DOI] [PubMed] [Google Scholar]
- 25.Wilpert J, Geyer M, Pisarski P, et al. On demand strategy as an alternative to conventionally scheduled post-transplant immunoadsorptions after ABO-incompatible kidney transplantation. Nephrol Dial Transplant. 2007;22:3048–51. doi: 10.1093/ndt/gfm460. [DOI] [PubMed] [Google Scholar]
- 26.Crew RJ, Ratner LE. ABO-incompatible transplantation: current practice and the decade ahead. Curr Opin Organ Transplant. 2010;15:526–30. doi: 10.1097/MOT.0b013e32833bfbba. [DOI] [PubMed] [Google Scholar]
- 27.Tanabe K. Double-filtration plasmapheresis. Transplantation. 2007;84:S30–S32. doi: 10.1097/01.tp.0000296103.34735.b8. [DOI] [PubMed] [Google Scholar]
- 28.The British Transplantation Society Guidelines for Antibody Incompatible Transplantation, 2006. Available at www.bts.org.uk
- 29.Valli PV, Puga Yung G, Fehr TV, et al. Changes of circulating antibody levels induced by ABO antibody adsorption for ABO-incompatible kidney transplantation. Am J Transplant. 2009;9:1072–80. doi: 10.1111/j.1600-6143.2009.02579.x. [DOI] [PubMed] [Google Scholar]
- 30.Tobian AA, Shirey RS, Montgomery RA, et al. The critical role of plasmapheresis in ABO-incompatible renal transplantation. Transfusion. 2008;48:2453–60. doi: 10.1111/j.1537-2995.2008.01857.x. [DOI] [PubMed] [Google Scholar]
- 31.Tanabe K. Interinstitutional variation in the measurement of anti-A/B antibodies: the Japanese ABO-Incompatible Transplantation Committee Survey. Transplantation. 2007;84:S13–6. doi: 10.1097/01.tp.0000296018.82857.ef. [DOI] [PubMed] [Google Scholar]
- 32.Kumlien G, Wilpert J, Safwenberg J, et al. Comparing the tube test and gel techniques for ABO antibody titration, as performed in three European centers. Transplantation. 2007;84:S17–9. doi: 10.1097/01.tp.0000296019.85986.af. [DOI] [PubMed] [Google Scholar]
- 33.AuBuchon JP, de Widt-Eggen J, Dumont J. Reducing variation in performance of antibody titrations. Vox Sang. 2008;95:57–65. doi: 10.1111/j.1423-0410.2008.01043.x. [DOI] [PubMed] [Google Scholar]
- 34.Shirey RS, Cai W, Montgomery RA, et al. Streamlining ABO antibody titrations for monitoring ABO-incompatible kidney transplants. Transfusion. 2010;50:631–4. doi: 10.1111/j.1537-2995.2009.02478.x. [DOI] [PubMed] [Google Scholar]
- 35.Krishnan NS, Fleetwood P, Higgins RM, et al. Application of flow cytometry to monitor antibody levels in ABO incompatible kidney transplantation. Transplantation. 2008;86:474–7. doi: 10.1097/TP.0b013e31817c4c4c. [DOI] [PubMed] [Google Scholar]
- 36.Park WD Accommodation in ABO-incompatible kidney allografts, a novel mechanism of self-protection against antibody-mediated injury. Am J Transplant. 2003;3:952–60. doi: 10.1034/j.1600-6143.2003.00179.x. [DOI] [PubMed] [Google Scholar]
- 37.Aljeidai AH. Cost analysis of renal transplantation in highly sensitized recipients compared to maintenance hemodialysis. Am J Transplant. 2008;8(S2):180. doi: 10.12659/aot.883698. (Abstr 7) [DOI] [PubMed] [Google Scholar]
- 38.Schwartz J. Complications, resource utilization, and cost of ABO incompatible living donor kidney transplantation Transplantation. 2006;82:155–63. doi: 10.1097/01.tp.0000226152.13584.ae. [DOI] [PubMed] [Google Scholar]
- 39.Schnitzler M. ABO-incompatible living donor transplantation: is it economically “compatible”? Transplantation. 2006;82:168–9. doi: 10.1097/01.tp.0000226242.10027.e7. [DOI] [PubMed] [Google Scholar]