Abstract
Transient electrical impulses are conventionally used to elicit physiological responses in excitable tissues. While electrical stimulation has many advantages, it requires an electrode-tissue interface, exhibits relatively low spatial selectivity and always produces a “stimulus artifact”. Recently, it has been shown that pulsed, low-energy infrared laser light can evoke nerve, muscle and sensory responses similar to those induced by traditional electrical stimulation in a contact-free, damage-free, artifact-free and spatially selective manner. However, the effect of transient infrared laser light on neurotransmission in the CNS is still largely unknown. Here, we tested the effect of infrared laser light on GABAergic neurotransmission. We recorded spontaneous inhibitory postsynaptic currents (sIPSCs) from cultured rat cortical neurons prior to and after infrared laser stimulation. Using transient infrared laser light, we either stimulated the neuronal soma that had axonal projections to the recorded neuron or directly stimulated the axons that projected to the recorded neuron. Optical stimulation led to enhanced amplitude, decreased decay time constant and increased frequency of sIPSCs. These alterations of sIPSC properties produced by optical stimulation were specifically mediated by GABAA receptors and caused by the transient laser light per se since no exogenous substances such as caged compounds were used. These data show that optical stimulation using transient infrared laser light can alter GABAergic neurotransmission and demonstrate that it may be an alternative approach to electrical stimulation in studying GABAergic function.
Keywords: Infrared laser, Optical stimulation, Neuron, GABAergic inhibition, GABAA receptors, IPSCs
Introduction
Transient electrical impulses are conventionally used to evoke physiological responses in excitable tissues (Merrill et al., 2005), and electrical stimulation is widely used for electrophysiological neurotransmission studies. By varying the electrical parameters such as current (mA), voltage (V), pulse width (ms) and stimulus rate (Hz), a variety of stimulation paradigms can be used to manipulate neurotransmission (Merrill et al., 2005). Although electrical stimulation has many advantages and has been utilized as the gold standard for neural stimulation for over a century, this technique has several limitations (Wells et al., 2007b). First, it requires an electrode-tissue interface to deliver the electrical energy and thus can cause tissue damage. Second, the current spreads beyond the electrode, resulting in a relatively poor spatial selectivity. Finally, electrical stimulation always produces a “stimulus artifact” that interferes with recordings adjacent to the stimulating site. Recently, it was shown that optical stimulation using pulsed, low-energy infrared laser light generated from a tunable free electron laser source or a solid state Holmium:YAG laser (λ = 2.12 μm) effectively overcomes many of the limits of electrical stimulation in a peripheral nerve-muscle preparation (Wells et al., 2005, 2007b). When an optimal radiant exposure was utilized, stimulation using infrared laser light produced compound nerve and/or muscle potentials similar to those induced by electrical stimulation in peripheral nerves (Wells et al., 2005; Izzo et al., 2006). The optical stimulation approach exhibits several advantages to electrical stimulation. It does not require an electrode-tissue interface (contact-free and damage-free). It also does not produce a stimulus artifact, thus allowing for recordings at the area adjacent to the stimulating site. In addition, it can activate specific target nerve fibers without affecting adjacent fibers, thus providing relatively high spatial selectivity (Izzo et al., 2006; Wells et al., 2007b). Since optical stimulation using transient infrared laser light induces physiological responses similar to those evoked by electrical stimulation in peripheral nerves, it has the potential to be an alternative approach to studying neurotransmission.
Inhibition in the brain is mediated primarily by the neurotransmitter γ-aminobutyric acid (GABA) (Olsen and Macdonald, 2002; Beleboni et al., 2004), which is used as a primary neurotransmitter by 30–40% of all CNS neurons (Beleboni et al., 2004). Once GABA is released, it can activate ionotropic GABAA receptors as well as metabotropic GABAB receptors. GABAA receptors mediate the majority of fast inhibition in the brain (Olsen and Macdonald, 2002; Beleboni et al., 2004), and activation of GABAA receptors results in two types of GABAergic inhibition, phasic and tonic. While tonic inhibition is generated by continuous activation of extrasynaptic GABAA receptors by low ambient concentration of GABA, phasic inhibition, mediated by inhibitory postsynaptic currents (IPSCs), is produced by brief exposure of postsynaptic GABAA receptors to high concentration of GABA released from presynaptic terminals (Farrant and Nusser, 2005). Since the effect of optical stimulation using transient pulses of infrared laser light on neurotransmission in the CNS is largely unknown, in this study, we determined the effect of laser light stimulation on GABAergic neurotransmission, the major inhibitory system in the brain that is involved in many neurological and psychiatric disorders, by examining the effects of pulsed infrared laser light on the properties of spontaneous inhibitory postsynaptic currents (sIPSCs).
Materials and Methods
Culture of cortical neurons
Pregnant Sprague-Dawley rats were anesthetized by isoflurane, and embryonic 18 day old pups were removed. The cerebral cortices were dissected and transferred to a Petri dish containing Hank’s Balanced Salt Solution (Sigma-Aldrich, St. Louis, MO). The cortices were then minced and transferred to a tube containing trypsin (0.6 mg/ml). After incubation for 30 min at room temperature, the cortices were transferred to a tube containing 2 ml NeurobasalA/B27 (Invitrogen, Grand Island, NY). The cortices were triturated, and the dissociated neurons in the supernatant were plated at a density of ~ 1×104 cells/cm2 on coverslips coated with poly-L-ornithine. The neurons were first maintained in Dulbecco’s Modified Eagle Medium (Invitrogen) supplemented with 8% fetal bovine serum, 8% F12 nutrient mixture, 80 μM glutamine (Invitrogen), 20 IU/ml penicillin/streptomycin (Life Technologies, Grand Island, NY). Cytosine arabinoside (Ara C, 5.5 μM, Sigma-Aldrich) was added to the culture 48 hours after plating. After incubating in Ara C for 48 hours, the media was then changed to NeurobasalA/B27 (Invitrogen) supplemented with penicillin/streptomycin (20 IU/ml, Life Technologies). The neuronal cultures were maintained at 37°C in an incubator with 5% CO2/95% air. Media was partially (50%) replaced every 2–3 days. Electrophysiological recordings were performed in the third week after dissociation. Studies were performed in accordance with the National Institutes of Health for the Care and Use of Laboratory Animals guidelines, and the experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Vanderbilt University School of Medicine.
Electrophysiological recordings
Whole-cell recordings were performed at room temperature on cultured rat cortical neurons bathed in the external solution (see below for ion composition of all solutions). Glutamate receptors were blocked by 6-cyano-7-nitroquinoxaline-2,3-dione disodium salt (CNQX, 10 μM) and DL-2-amino-5-phosphopentanoic acid (AP5, 40 μM). GABAB receptors were blocked by CGP52432 (1 μM). The recording electrodes were filled with internal solution and had resistance of ~ 2.0 MΩ. Pulsed, infrared laser light (λ = ~ 1.85 μm) generated from a Capella R-1850 Infrared Nerve Stimulator (Aculight, Bothell, WA) was delivered via an optical fiber (200 μm in diameter), which was immersed in external solution during recordings. The lower edge of the optical fiber tip was positioned at about the same level as the recording electrode tip, and the angle of the optical fiber related to the dish bottom was kept stable (Supplementary Figure 1) so that the energy delivered to different neurons was comparable. The Infrared Nerve Stimulator could generate laser light with different energy levels (radiant exposures, J/cm2), pulse repetition rates (Hz) and pulse widths (ms). The laser energy delivered was measured using a J50LP-1 energy detector (Molectron, Portland, OR) at the tip of optical fiber without immersion in the recording solution. The spontaneous inhibitory postsynaptic currents (sIPSCs) were recorded from one neuron when optical stimulation was applied either to a neuronal soma that had axonal projections to the recorded neuron or directly to the axons that projected to the recorded neuron. Currents were filtered at 2 kHz and recorded with a 200A patch clamp amplifier (Molecular Devices, Foster City, CA) and Digidata 1322 series interface (Molecular Devices). Voltage was clamped at −60 mV during recordings. Series resistance was not compensated. However, it was checked frequently throughout the experiment, and recordings were terminated when series resistance increased by >20%.
Temperature determination
The temperature alteration evoked by infrared laser light at the site of stimulation was determined using a Flir ThermoVision A20M infrared camera (Trek Equipment, Sausalito, CA). Given that heat may rapidly spread to the solution when neurons are immersed in the recording solution, we removed most of the solution so that there was only a thin film of solution between the optic fiber tip and the neurons. The temperature change was measured ~ 5 minutes after laser stimulation was turned on.
Data analysis and statistics
Detection of individual sIPSCs was performed offline using the Mini Analysis program (Synaptosoft, Fort Lee, NJ). The detection threshold was set at 15 pA. The events within the sIPSC area <50 fC (these events were too small to be considered sIPSCs) were excluded from analysis. For each neuron, sIPSC kinetics were analyzed on averaged events that were aligned by rise times. At least 100 events were averaged for each neuron at each recording condition. The decay of the average sIPSCs was fitted using the formula Σane(−t/τn), where “a” denoted the relative amplitude of the exponential component, “τ” represented the time constant and “n” was the number of exponential components. Average sIPSCs were generally fitted by two exponential components, and a weighted decay time constant (decay τw) in the form of (a1*τ1 + a2*τ2)/(a1 + a2) was used to compare the rate of decay prior to and in the presence of optical stimulation, where “a1” and “a2” were the relative amplitudes of the exponential components at time zero. Overlapping sIPSCs were excluded from average sIPSC analysis but were included in sIPSC frequency analysis. Paired Student’s t-test was used to compare the sIPSC properties prior to and after optical stimulation.
Results
The properties of sIPSCs were altered by optical stimulation
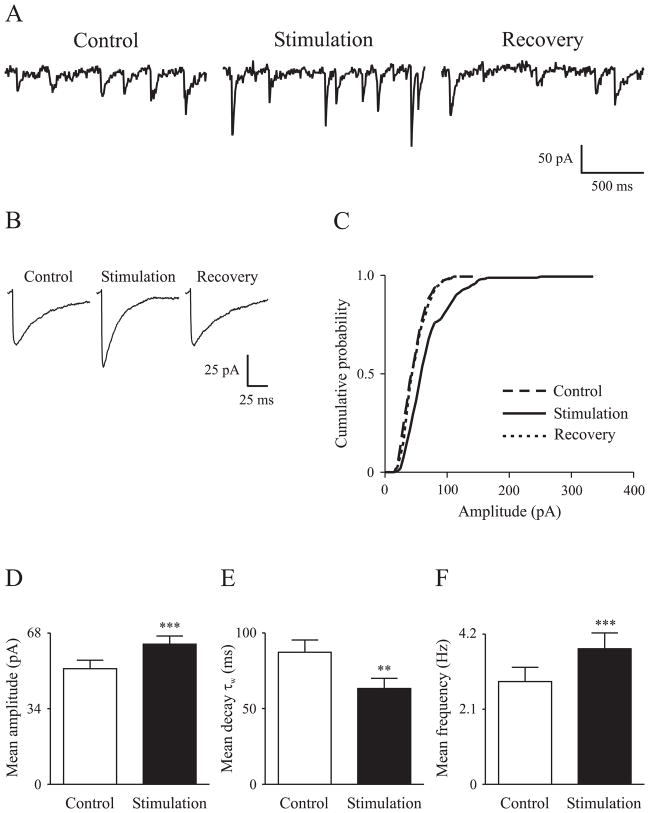
GABAergic neurotransmission prior to and in the presence of transient infrared laser light stimulation was studied in cultured rat cortical neurons. The sIPSCs were recorded in the presence of 40 μM AP5, 10 μM CNQX and 1 μM CGP52432 to block glutamate and GABAB receptors. Optical stimulation (pulse width, 2 ms; repetition rate, 30 Hz) at 0.36 J/cm2 (~ 0.1 mJ/pulse; output measured from the fiber without immersion in the solution) was applied either to neuronal somas that had axonal projections to the recorded neurons or directly to the axons that projected to recorded neurons. The sIPSC responses recorded using these two stimulation paradigms were similar, and thus the data were pooled. Stimulation with infrared laser light at 30 Hz and 0.36 J/cm2 for ~ 5 minutes (so that enough events could be collected for analysis) increased sIPSC frequency, enhanced sIPSC amplitude and accelerated sIPSC decay (Figure 1A, B, C). The effects on sIPSCs were instantaneous upon onset of the laser stimulation. Optical stimulation augmented the mean sIPSC amplitude from 52.0 ± 3.8 pA to 63.1 ± 3.6 pA (n = 18, p<0.001) (Figure 1D). The mean weighted decay time constant (τw) was reduced by optical stimulation (87.3 ± 8.1 ms vs. 63.4 ± 6.6 ms, p<0.01) (Figure 1E). The sIPSC frequency was increased from 2.9 ± 0.4 Hz to 3.8 ± 0.4 Hz (p<0.001) (Figure 1F).
Figure 1. Alteration of sIPSC properties by optical stimulation.
A, Raw sIPSC traces prior to (control), in the presence of (stimulation) and 10 minutes after (recovery) optical stimulation. B, Average sIPSCs to show the amplitude and decay changes due to optical stimulation. C, Cumulative probability plot to show the enhancement (rightward shift) of sIPSC amplitude by optical stimulation. D, E, F, Bar graphs to show the significant changes in mean sIPSC amplitude, weighted decay time constant (decay τw) and frequency prior to and in the presence of optical stimulation, respectively. Error bars denote SEM. Laser light parameters: radiant exposure, 0.36 J/cm2; repetition rate, 30 Hz; pulse width, 2 ms.
** Significantly different from control at p<0.01; *** p<0.001
Alteration of sIPSC properties was specifically mediated by GABAA receptors
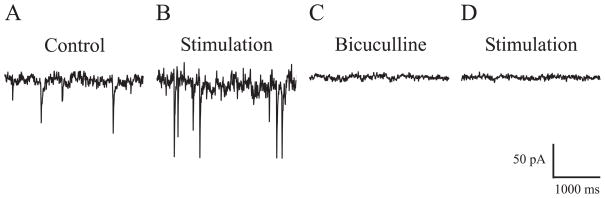
To demonstrate that the laser-induced IPSC alterations were mediated via GABAA receptors and not through other receptors or channels such as heat-sensitive transient receptor potential (TRP) channels (Ramsey et al., 2006), the GABAA receptor antagonist bicuculline methiodide (40 μM) was applied to the recording solution to block GABAA receptor function (n = 5). Optical stimulation (radiant exposure, 0.36 J/cm2; repetition rate, 30 Hz; pulse width, 2 ms) altered IPSC properties as compared with control (Figure 2A, B). After the stimulation was stopped, application of bicuculline resulted in a complete blockade of sIPSCs (Figure 2C). Then, in the presence of bicuculline, optical stimulation was applied again for 4–5 minutes, but this did not evoke any responses (Figure 2D).
Figure 2. Alteration of sIPSC properties specifically mediated by GABAA receptors.
Compared with control (A), optical stimulation (at the same laser parameters as in Figure 1) altered sIPSC properties (B). In the same neuron, bicuculline (40 μM) completely blocked sIPSCs (C), and optical stimulation for ~ 4 minutes in the presence of bicuculline did not evoke additional responses (D).
Discussion
In the present study, we report that optical stimulation using transient infrared laser light alters GABAergic neurotransmission. We demonstrated that optical stimulation augmented the amplitude, accelerated the decay and increased the frequency of sIPSCs. We also demonstrated that the alteration of sIPSC properties by optical stimulation was specifically mediated by GABAA receptors.
Stimulation of GABAergic neurotransmission by transient infrared laser light
Laser light has been used to study neuronal function. Activation of synaptic neurotransmission occurs in a sub-millisecond time scale. Photoactivation of caged compounds including caged GABA by continuous laser light (λ = ~ 310 nm) has been used as a high temporal and spatial resolution technique to study the fast kinetics of neurotransmission (Wieboldt et al., 1994). In recent years, several groups have worked to enable continuous laser light (λ = ~ 300–600 nm) to induce neural activity all with the purpose of achieving temporally and spatially precise control over the activation process. Most of these efforts have been aimed at genetically engineering light-activatable ion channel switches in combination with transgenic approaches to introduce these switches in animals (Zhang et al., 2007). While this approach is particularly useful for animal studies that may lead to new insights into disease pathogenesis and treatment or to monitor treatment efficacies, direct translation to treatment of human diseases faces the same obstacles of any gene therapy approach, namely, how to deliver the engineered light-activatable switches to human cells in the intact body.
Alternatively, with infrared laser stimulation, pulsed optical energy is delivered directly to neurons which absorb the light owing to endogenous chromophores, predominantly water which has a broad absorption band centered around λ = 1.93 μm. Previous studies in the peripheral nervous system found that the direct neural activation using pulsed infrared laser relied on a photothermal effect (Wells et al., 2007a). In the current study, we observed that optical stimulation increased the frequency of sIPSCs, indicating enhanced presynaptic GABA release. Occurrence of sIPSCs was not synchronized with laser pulses, which is fundamentally different from previous studies in peripheral nervous system in which action potentials were pulse-locked (one laser pulse resulting one action potential) with laser stimulation (Izzo et al., 2006; Wells et al., 2007b). Interestingly, at the energy level that was used for electrophysiological recordings, we determined that there was ~ 1°C increase in temperature at the site of stimulation (data not shown). It is possible that the transient heat generated by infrared laser light may have stimulated vesicular neurotransmitter release. Infrared laser light has been demonstrated to stimulate cholinergic transmission in peripheral nerves (Wells et al., 2007b), and it has been reported that chronic stimulation using infrared laser light can alter the levels of multiple amino acid neurotransmitters including glutamate and GABA in the brain (Ahmed et al., 2008). Therefore, it is likely that optical stimulation using infrared laser light may have pre- and/or postsynaptic actions and also alter the function of other types of neurotransmission such as glutamatergic neurotransmission. Further experiments are needed to explore this possibility.
Potential application of the optical stimulation in basic research and clinical treatment
Our studies demonstrated that optical stimulation using infrared laser light can be used in basic electrophysiology research as alternative stimulation modality since it exhibits several advantages over electrical stimulation. For example, optical stimulation under many conditions can avoid damage and/or toxicity caused by the necessity of electrical stimulation with an electrode-tissue interface. Optical stimulation produces no stimulus artifact, allowing for recordings adjacent to the stimulating site. In addition, optical stimulation exhibits high spatial selectivity. Theoretically, the minimum spot size is determined by the diffraction limit of light (roughly λ/2) and can be delivered to neural targets via optical fibers as small as 4 μm (Wells et al., 2007a).
As the major inhibitory system in the brain, GABAergic neurotransmission plays a critical role in the pathophysiology of many neurological disorders such as epilepsy (Macdonald et al., 2006). Increasing evidence suggests that neural stimulation using electrical impulses is a promising new avenue for treatment of epilepsy (Pollo and Villemure, 2007). Development of implanted optical stimulation devices using optical fibers may overcome some of the shortcomings of electrical stimulation such as tissue toxicity related to implanted electrodes (Merrill et al., 2005). Furthermore, taking advantage of high spatial selectivity, optical stimulation can be delivered via optical fibers to epileptogenic zone (specific cortical layers or pathways) for control of epilepsy (Pollo and Villemure, 2007). In addition, implantation of electrical neurostimulators prevents patients from receiving magnetic resonance imaging (MRI) studies, since MRI scanners can induce significant current through the electrodes causing tissue injury (Gleason et al., 1992). Optical stimulation on the other hand is not susceptible to electromagnetic field induction of current when delivered through an optical conduit. Compared with electrical wire commonly used in implantable neurostimulators, MRI safety concerns would be significantly reduced when using optical fibers.
Supplementary Material
Acknowledgments
The authors thank Li Ding for excellent technical assistance and Alex Caruthers for data analysis. We also thank Jonathan Cayce and Mark Mackanos for their help in estimating the temperature changes evoked by infrared laser light at the site of stimulation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed NA, Radwan NM, Ibrahim KM, Khedr ME, El Aziz MA, Khadrawy YA. Effect of three different intensities of infrared laser energy on the levels of amino acid neurotransmitters in the cortex and hippocampus of rat brain. Photomed Laser Surg. 2008;26:479–488. doi: 10.1089/pho.2007.2190. [DOI] [PubMed] [Google Scholar]
- Beleboni RO, Carolino RO, Pizzo AB, Castellan-Baldan L, Coutinho-Netto J, dos Santos WF, Coimbra NC. Pharmacological and biochemical aspects of GABAergic neurotransmission: Pathological and neuropsychobiological relationships. Cell Mol Neurobiol. 2004;24:707–728. doi: 10.1007/s10571-004-6913-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: Phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Gleason CA, Kaula NF, Hricak H, Schmidt RA, Tanagho EA. The effect of magnetic resonance imagers on implanted neurostimulators. Pacing Clin Electrophysiol. 1992;15:81–94. doi: 10.1111/j.1540-8159.1992.tb02904.x. [DOI] [PubMed] [Google Scholar]
- Izzo AD, Richter CP, Jansen ED, Walsh JT., Jr Laser stimulation of the auditory nerve. Lasers Surg Med. 2006;38:745–753. doi: 10.1002/lsm.20358. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Kang JQ, Gallagher MJ, Feng HJ. GABAA receptor mutations associated with generalized epilepsies. Adv Pharmacol. 2006;54:147–169. doi: 10.1016/s1054-3589(06)54007-4. [DOI] [PubMed] [Google Scholar]
- Merrill DR, Bikson M, Jefferys JG. Electrical stimulation of excitable tissue: Design of efficacious and safe protocols. J Neurosci Methods. 2005;141:171–198. doi: 10.1016/j.jneumeth.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Macdonald RL. GABAA receptor complex: Structure and function. In: Egebjerg J, Schousboe A, Krogsgaard-Larsen P, editors. Glutamate and GABA receptors and transporters: Structure, function and pharmacology. Taylor and Francis; London: 2002. pp. 202–235. [Google Scholar]
- Pollo C, Villemure JG. Rationale, mechanisms of efficacy, anatomical targets and future prospects of electrical deep brain stimulation for epilepsy. Acta Neurochir Suppl. 2007;97:311–320. doi: 10.1007/978-3-211-33081-4_34. [DOI] [PubMed] [Google Scholar]
- Ramsey S, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- Wells J, Kao C, Jansen ED, Konrad P, Mahadevan-Jansen A. Application of infrared light for in vivo neural stimulation. J Biomed Opt. 2005;10:064003. doi: 10.1117/1.2121772. [DOI] [PubMed] [Google Scholar]
- Wells J, Kao C, Konrad P, Milner T, Kim J, Mahadevan-Jansen A, Jansen ED. Biophysical mechanisms of transient optical stimulation of peripheral nerve. Biophys J. 2007a;93:2567–2580. doi: 10.1529/biophysj.107.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells J, Konrad P, Kao C, Jansen ED, Mahadevan-Jansen A. Pulsed laser versus electrical energy for peripheral nerve stimulation. J Neurosci Methods. 2007b;163:326–337. doi: 10.1016/j.jneumeth.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieboldt R, Ramesh D, Carpenter BK, Hess GP. Synthesis and photochemistry of photolabile derivatives of γ-aminobutyric acid receptor in the millisecond time region. Biochemistry. 1994;33:1526–1533. doi: 10.1021/bi00172a032. [DOI] [PubMed] [Google Scholar]
- Zhang F, Aravanis AM, Adamantidis A, de Lecea L, Deisseroth K. Circuit-breakers: Optical technologies for probing neural signals and systems. Nat Rev Neurosci. 2007;8:577–581. doi: 10.1038/nrn2192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.