Abstract
Objectives/Hypothesis
Individual speech and language outcomes of deaf children with cochlear implants (CIs) are quite varied. Individual differences in underlying cognitive functions may explain some of this variance. The current study investigated whether behavioral inhibition skills of deaf children were related to performance on a range of audiologic outcome measures.
Design
Retrospective analysis of longitudinal data collected from prelingually and profoundly deaf children who used CIs.
Methods
Behavioral inhibition skills were measured using a visual response delay task that did not require hearing. Speech and language measures were obtained from behavioral tests administered at 1-year intervals of CI use.
Results
Female subjects showed higher response delay scores than males. Performance increased with length of CI use. Younger children showed greater improvement in performance as a function of device use than older children. No other subject variable had a significant effect on response delay score. A series of multiple regression analyses revealed several significant relations between delay task performance and open set word recognition, vocabulary, receptive language, and expressive language scores.
Conclusions
The present results suggest that CI experience affects visual information processing skills of prelingually deaf children. Furthermore, the observed pattern of relations suggests that speech and language processing skills are closely related to the development of response delay skills in prelingually deaf children with CIs. These relations may reflect underlying verbal encoding skills, subvocal rehearsal skills, and verbally mediated self-regulatory skills. Clinically, visual response delay tasks may be useful in assessing behavioral and cognitive development in deaf children after implantation.
Keywords: Cochlear implants, outcomes, response delay, behavioral inhibition, Children, speech perception, language development
Introduction
Numerous studies of audiologic outcomes in deaf children with cochlear implants (CIs) have consistently demonstrated benefit of a CI on the development of oral language abilities such as speech perception, expressive language, vocabulary knowledge, and speech intelligibility.1–3 Despite the impressive results of CIs in many deaf children, the presence of large individual differences in audiologic outcomes of prelingually deaf children remains a challenging clinical and theoretical problem.4 Reported outcomes after cochlear implantation vary from age-appropriate speech and language skills to little benefit other than an awareness of sound. Studies that have examined predictors of CI performance in children have reported effects of early implantation, communication mode, device type, and dynamic range.5 However, a significant portion of outcome variance still remains unexplained by these demographic and medical factors.4
Knutson and his colleagues6 have investigated the relations between several behavioral factors and audiologic outcomes in children with CIs. They tested children 3 years after implantation on the Child Behavior Checklist,7 which measures a variety of behavioral problems from a parental report. The authors reported that “externalizing behaviors” such as attention problems, impulsivity, and aggression were negatively correlated with performance on a range of outcome measures. Their results suggest that a child's ability to control their behavior, particularly when behavioral inhibition was required, was related to the development of oral language skills. The importance of these findings is magnified by a number of earlier studies that have found that deaf children and adults who use sign language appear to be more behaviorally impulsive than their normal-hearing peers.8,9 Therefore, it is important to investigate how behavioral control, language development, and CI use interact during development after implantation.
One cognitive skill that is crucial for analysis and control of behavior, and has received significant attention in the field of developmental neuropsychology, is response delay.10 Response delay requires inhibition of action, maintenance of an inhibited state, and prevention of distraction from extraneous stimuli so that self-directed, goal-oriented behaviors can be accomplished.10 Deficits on response delay measures are found in many children with attention deficit hyperactivity disorder (ADHD).10
Mitchell and Quittner11 examined response delay skills in deaf children with hearing aids and normal hearing children using a task that required children to make repeated key presses separated in time by a specific length. Each time successive key presses were separated by 4 or more seconds, a “point” registered on an LED counter. Over a 6-minute period, children attempted to accumulate as many points as possible but were unaware of the 4-second rule. Mitchell and Quittner found that deaf children's performance on this task was similar to age-matched normal-hearing children. However, the authors could not rule out that access to auditory information played a role in the development of response delay skills. For instance, the authors did not examine whether length of device use, degree of deafness, or any other subject variables had an effect on response delay skills. Furthermore, Mitchell and Quittner did not examine whether scores on speech and language outcome measures were related to response delay skills.
No study to date has examined the response delay skills of profoundly deaf children who use CIs. The present study was designed to determine whether length of CI use or any other subject variables had an effect on response delay skills in this population of children. We also hypothesized that performance on the response delay task would be related to measures of speech and language outcomes in deaf children with CIs.
Methods
Subjects
All participants were part of a large, ongoing, longitudinal study of speech and language development of deaf children with CIs implanted at the Indiana University School of Medicine. A total of 47 prelingually deaf children (profoundly deaf by 2.5 years old) who received CIs by 9 years of age and had been tested on the response delay task were identified. Three of these children were excluded from this study because of clinical evidence of developmental delay. Of the remaining 44 children, none of them had confirmed or suspected diagnoses of motor or cognitive impairments in the charts. All children used Nucleus 22 devices (Cochlear Corp, Englewood, CO). Mean age at implantation was 4.4 years (SD = 1.37) and ranged from 2.2 to 7.5 years old. Mean age of onset was 4.7 months (SD = 8.07) and ranged from 0 to 30 months. Most of the children had unidentified etiologies of deafness (n = 30). Only two children had a verified genetic cause of deafness, and one child was deaf from Cytomegalovirus. Meningitis was the cause of deafness in 11 of the children. Twenty-two children used oral communication methods. These children were immersed in a training and education program that emphasized speaking and listening communication skills. The remaining 22 children used total communication methods. These children were enrolled in education programs that emphasized both spoken and manual English skills. A subset of children (n = 36) had nonverbal IQ scores, from the Wechsler Intelligence Scale for Children III (WISC)12 or the Wechsler Preschool and Primary Scale of Intelligence-Revised (WPPSI),13 in their charts. Mean standardized IQ score was 98.93 (SD = 15.19) and ranged from 69 to 129.
Procedures
The Gordon Diagnostic System is a customized mechanical button box with one blue key located centrally below an LED display and is capable of running a number of neuropsychologic tests.14 The 6-minute Preschool Delay Task, recommended for use in normal-hearing children from 3 to 5 years of age, was used to measure response delay skills.14 The same system was used by Mitchell and Quittner11 in their study of prelingually deaf children with HAs. Children were required to press the button and then wait for some period of time before pressing the button again. Each time the child pressed the button after waiting 4 seconds, he or she received a point. Points were tallied on the display screen. Children were told only that they will receive a point if they “wait long enough,” and they were blind to the 4-second rule. Children received no feedback regarding their performance other than the point counter. The number of points a child received over 6 minutes was divided by the total number of key presses to compute the “error ratio” (ER), which was the primary dependent measure on this task.14 The ER was used as the dependent measure rather than total points so as not to reward children for a higher frequency of presses. The only differences between the task used in the current study and the task used by Mitchell and Quittner11 were length of the delay rule (4 seconds compared with 6 seconds) and length of the task (6 minutes compared with 9 minutes).
The response delay task was administered once every 6 to 12 months from 1 year before implantation to 2 years postimplantation. Test scores were collapsed into one of three intervals of CI use: preimplant, 1 year postimplant, and 2 years postimplant. Not all children were tested at every interval, as is common in clinical populations such as this, creating missing data cells. Missing data usually occurred because children either moved away from the Indianapolis area after implantation and were unable to continue in the study or were too tired to complete all testing procedures attempted on a given day.
Four speech and language outcome measures collected over 6 years of CI use were used in the multivariate regression analyses. Open set speech perception was measured using the Phonetically Balanced Kindergarten (PBK) test.15 Children heard isolated, phonetically balanced, English monosyllabic words and were asked to repeat the words aloud to the examiner. This test was administered using live voice presentation and was scored by word and phonemes correct.
Vocabulary knowledge was assessed with the Peabody Picture Vocabulary Test (PPVT).16 This test used a closed-set, forced-choice format in which each vocabulary item was presented live either orally or using Sign Exact English, depending on the individual child's preferred mode of communication. The child is required to choose from four pictures, one of which correctly corresponded to the meaning of the word.
The Reynell Developmental Language Scales (RDLS), 3rd edition,17 was administered to assess both receptive and expressive language skills. The receptive scales (RDLS-R) measured 10 different skills including word recognition, sentence comprehension, and verbal comprehension of ideational content. The expressive language scales (RDLS-E) assessed skills such as spontaneous production of speech and picture description. Like the PPVT, the RDLS was administered in the child's preferred mode of communication.
Speech intelligibility was assessed using the Beginner's Intelligibility Test (BIT).18 Audio recordings were made of the children in this study who repeated a list of 10 sentences presented to them in the auditory modality by a clinician. These recordings were then played back to three naïve adult listeners who were asked to transcribe what they thought the children were saying. Intelligibility scores were based on the percent number of words correctly transcribed by the adult listeners.
All of the tests were administered in an Otolaryngology clinical setting by licensed health professionals with special training in working with deaf children who used CIs. This retrospective study, and the ongoing prospective longitudinal project from which these data were obtained, was approved by the Human Subjects Institutional Review Board at Indiana University School of Medicine.
Results
To test for effects of the demographic variables on delay ER, we used the SAS (Cary, NC) mixed procedure.19 This statistical method is ideal for analyzing longitudinal data in which each subject was not tested at each time interval. The SAS mixed procedure uses a maximum-likelihood estimation method to create a model without eliminating any participants.20 Scores from subjects tested at two or more time intervals are used to compute an estimation of the mean ER score at each time interval. Scores from all subjects (including those tested at only one interval) are used to compute the variance of the mean ER score at each time interval. With the SAS procedure, systematic selection biases can be minimized by using data from all children, even those who were not tested at each interval, in the test design.
The SAS mixed procedure was used to test for main effects of 10 demographic variables on delay ER score: age of onset of deafness, age at testing, number of active electrodes, nonverbal IQ standard score, pure-tone average (PTA), sex, communication mode, and etiology. Age at implantation could not be analyzed separately from age at test because these two variables were highly correlated (r = 0.77, P < .0001). Because etiology was usually unknown, we constructed two categorical variables to separate subjects into meaningful groups: meningitis versus nonmeningitis and congenital versus acquired. Interval of CI use was included as a repeated measures variable in each model.
We found no significant effect for age of onset, number of electrodes, IQ, PTA, or communication mode on delay ER scores. No significant differences in ER scores were found between children with meningitis history and children without meningitis history, nor were differences found between children with congenital deafness and children with acquired deafness. As illustrated in Figure 1, we found a significant effect for sex on delay ER, with females showing higher mean delay ER scores than males, F (1,33.3) = 6.04, P = .019. The estimated mean ER score was 0.65 for girls and 0.52 for boys. Although age of test barely missed significance, F (1,60) = 3.75, P = .058, we found a significant interaction between age at test and interval of CI use, F (3,54) = 3.23, P = .029. A final mixed model with sex, age at test, and interval of CI use as predictor variables and delay ER score as the dependent variable was computed. In this model, sex, F(1,22.6) = 7.18, P = .014, age at test, F(1,58.9) = 4.09, P = .048, and interval of CI use, F(3,53.2) = 4.35, P = .008, were each significant and independent effects. The interaction between age at test and interval of CI use remained significant in the final model, F(3,54.4) = 3.77, P = .016. All other interactions were nonsignificant.
Fig. 1.
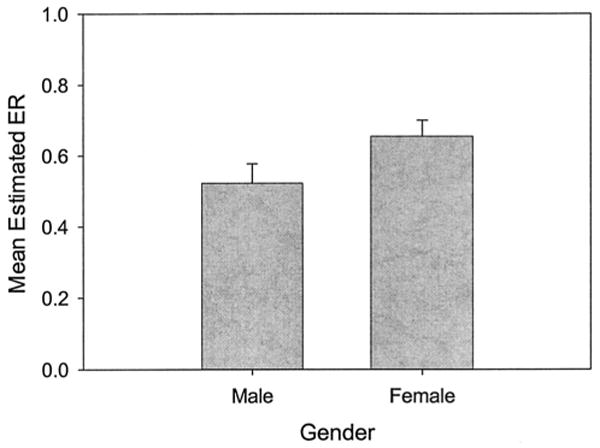
Mean estimated error ratio on the response delay task as a function of gender. Error bars represent SEM. ER = error ratio.
Figure 2 displays the individual ER scores as a function of chronological age at CI activation and length of implant use. This figure illustrates the interaction between age at test and length of CI use detected by the mixed model. At preimplant and 1 year postimplant intervals, delay task performance improved as a function of chronological age at test. However, after 2 years of implant use, ER did not increase with chronological age. The results shown in Figure 2 suggest that children who were younger at test (and younger at time of implantation) showed greater improvement in ER over 2 years of CI use than children who were older at implantation. After 2 years of CI use, younger children appear to catch up to older children in terms of delay task performance.
Fig. 2.
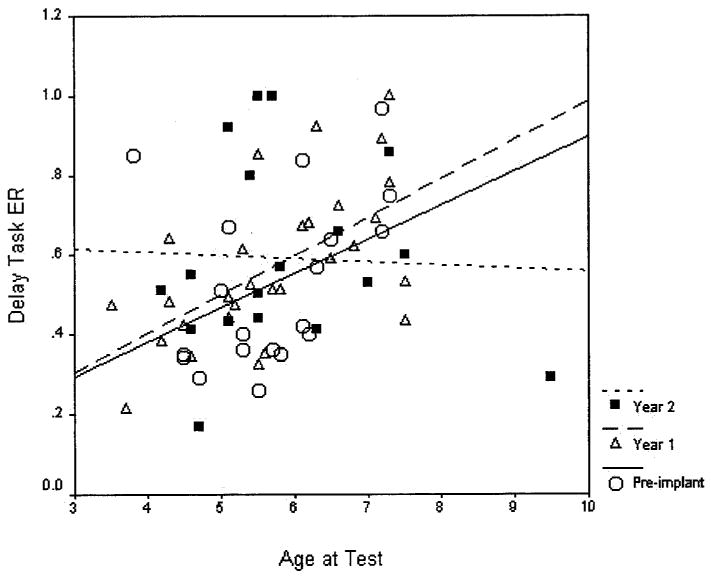
Error ratio on the response delay task as a function of chronological age at testing in years and length of cochlear implant (CI) use. Individual subjects' error ratio (ER) scores are illustrated along with lines of best fit for each interval of CI use.
This interaction should be interpreted with caution, however, because of a possible selection bias in the older children. As mentioned previously, the preschool delay task is clinically recommended for children 3 to 5 years old. For older children, the Gordon Diagnostic System offers a more difficult version of the delay task.14 Some of the older children in this study were unable to complete the harder delay task and, therefore, the easier task was administered to them. Thus, these older children may have been over-represented among our oldest children (particularly the outlier, who was 9.5 years old at testing and had an ER of 0.2).
We conducted a series of stepwise multiple regression analyses to assess the relations between delay task ER and each outcome measure.21 As previously mentioned, individual differences in speech-language outcomes have been show to be related to a number of subject variables. Therefore, in the regression analyses, we included the following predictor variables: age of onset, number of active electrodes, nonverbal IQ standard score, sex, unaided PTA, meningitic (vs. nonmeningitic) etiology, communication mode, chronological age (at delay task testing). Age at implantation was highly correlated with age at testing (r = 0.839, P = .0001). To prevent effects of colinearity from confounding the results, we did not include age at implantation as a predictor variable. Instead, we divided participants into an earlier and later implanted group on the basis of a median split (4.25 years old). This categorical variable was not as strongly correlated with age at testing (r = 0.734, P = .001). All other correlations between predictor variables were low to moderate.
Participants who had delay ER scores at postimplant intervals 1 year, 2 years, or 3 years were included in the regression analysis (n = 37). The interval in which the largest number of subjects had scores was postimplant year 1 (n = 30). Therefore, for those participants who had year 1 scores, we included these in our analyses. For the remaining seven participants, we included scores from either postimplant year 2 (n = 6) or 3 (n = 1). For each participant, the most recent outcome measure was included in the analysis. The postimplant interval in which the measures were obtained ranged from 1 to 6 years postimplant. Length of CI use at the time of outcome measure administration was included as a predictor variable in the multiple regression analyses.
We conducted six stepwise multiple regression analyses, one for each outcome measure. Inclusion and exclusion criteria were P < .05 and P > .10, respectively. Table I summarizes the final regression models for each outcome measure. Length of use was a significant predictor of open set word recognition (PBK-p, PBK-w), vocabulary (PPVT), receptive language (RDLS-rec), expressive language (RDLS-exp), and speech intelligibility (BIT). For all outcome measures, length of CI use was the strongest predictor (betas from 0.583 for PBK-w to 0.931 for PPVT). Oral communication mode was associated with higher open set word recognition, expressive language, and speech intelligibility scores than total communication. Communication mode was a weaker predictor than use (betas from −0.286 for RDLS-exp to −0.480 for PBK-w). A greater number of active electrodes was predictive of greater receptive and expressive language scores. Delay task ER score was a significant predictor of vocabulary and expressive language. In addition, ER was a significant predictor of open set word recognition performance when scored for words but not for phonemes. Although ER was not a significant predictor of BIT score, it approached significance in the final model (P = .07).
TABLE I.
Final Stepwise Regression Models for Speech and Language Outcome Measures.
| Dependent Variable* | Predictor Variables† | Rsq.‡ | Excluded Variables Approaching Significance§ |
|---|---|---|---|
| PPVT | Use: Beta = 0.931, t(1,24) = 7.34, P = .0001 | 0.682 | None |
| ER: Beta = 0.568, t(1,24) = 4.48, P = .00001 | |||
| RDLS-rec | Use: Beta = 0.692, t(1,25) = 5.20, P = .0001 | 0.747 | None |
| ER: Beta = 0.434, t(1,25) = 5.83, P = .0001 | |||
| Elec: Beta = 0.317, t(1,25) = 2.60, P = .016 | |||
| RDLS-exp | Use: Beta = 0.698, t(1,25) = 4.56, P = .0001 | 0.558 | Ons: Beta = −0.227, P = .06 |
| ER: Beta = 0.480, t(1,25) = 3.13, P = .005 | |||
| Elec: Beta = 0.330, t(1,25) = 2.44, P = .023 | |||
| CM: Beta = −0.286, t(1,25) = −2.16, P = .042 | |||
| PBK-p | Use: Beta = 0.653, t(1,21) = 5.18, P = .0001 | 0.651 | None |
| CM: Beta = −0.384, t(1,21) = −3.04, P = .006 | |||
| PBK-w | Use: Beta = 0.583, t(1,21) = 3.82, P = .001 | 0.563 | None |
| CM: Beta = −0.480, t(1,21) = −3.36, P = .003 | |||
| ER: Beta = 0.347, t(1,21) = 2.30, P = .033 | |||
| BIT | Use: Beta = 0.696, t(1,25) = 6.40, P = .0001 | 0.701 | ER: Beta = 0.225, P = .07 |
| CM: Beta = −0.320, t(1,25) = −2.97, P = .007 | |||
| EtM: Beta = −0.310, t(1,25) = −2.84, P = .009 |
PPVT, RDLS scores in age equivalents. PBK, BIT scores in percent correct.
For each predictor, we list standardized coefficient (beta), t value, and P value. Use = length of CI use at outcome test, CM = communication mode (OC = 0, TC = 1), ER = delay ER score, Elec = number of active electrodes, EtM = meningitic vs. nonmeningitic etiology (meningitis assigned a 1, nonmeningitis assigned a 0). Ons = age at onset of profound deafness.
Adjusted R square.
Variables excluded from the final model, but approaching significance (P < .075).
PPVT = Peabody Picture Vocabulary Test; RDLS = Reynell Developmental Language Scale; PBK = Phonetically Balanced Kindergarten test; BIT = Beginner's Intelligibility Test.
Discussion
The present results revealed several new findings regarding the response delay skills of prelingually deaf children who use CIs. First, female participants demonstrated higher performance on the delay task than male participants. This finding is consistent with reports in the ADHD literature that female children with ADHD show lower rates of behavioral inhibition problems compared with males with ADHD.22 However, this finding has not been reported in a population of prelingually deaf children with CIs. Response delay scores also increased with length of CI use. The significant interaction between length of use and chronological age at test suggests that younger implanted children tend to show greater improvement on the delay task then older implanted children.
Why should performance of deaf children on the response delay task improve with CI use? One possibility is that their improvement was caused by practice effects. Although it is impossible to rule this out without comparing our results with a control group of deaf children without CIs, we feel that practice effects are unlikely to have accounted for the effect of CI use for several reasons. First, children practiced the delay task to criterion and demonstrated that they understood the task before testing. Second, at least 1 year separated testing sessions for those children who were tested on the delay task more than once (n = 20).
A second possible explanation for the affect of CI use on delay task performance is that access to sound by way of a CI, and subsequent improvement in speech and language skills, leads to improved delay task performance. The results of the multivariate regression analyses reveal that performance on the delay task was significantly related to scores on open set speech perception, vocabulary, expressive language, and receptive language measures in deaf children with CIs. The strengths of these relationships were comparable with the effect of communication modality. These results suggest that the perceptual and linguistic skills obtained with a CI are also recruited by the response delay task. This is an important finding because it suggests that a CI can lead to improvement on a behavioral task that does not require processing of auditory information.
Several language-related skills such as internal speech, subvocal verbal rehearsal, and counting skills are likely to be used by children during administration of the response delay task. For instance, one possible strategy that a child might use would be to press the button, count to themselves for a few seconds, and then press the button again. This sequence would be repeated until the score counter registered a point, indicating that the child had waited a sufficient length of time. Thereafter, the child would simply count the required number of seconds before each response, thereby maximizing the ER.
Clearly, such a response strategy would require some degree of sophistication in counting and control of internal speech mechanisms which use subvocal verbal rehearsal strategies. Several related findings in the literature suggest that deaf children may not be able to take full advantage of subvocal rehearsal strategies during tasks in which it would be beneficial to do so. For example, Pisoni and Cleary23 measured the immediate memory capacity for spoken lists of digits in normal-hearing children and in deaf children with CIs and reported a main effect of hearing status and an interaction between hearing status and order of recall. Normal-hearing children showed longer digit spans overall compared with deaf children with CIs. When asked to recall the sequence in the same order as presented (forward digit span), the normal-hearing children showed greater forward digit spans compared with the condition that required recall of the sequence in the backward order (backward digit span). The presence of this effect in normal-hearing children was taken as evidence that normal-hearing children were able to benefit substantially from subvocal verbal rehearsal strategies to actively maintain a longer digit sequence in working memory when temporal sequence did not have to be manipulated. In contrast, the deaf children with CIs showed significantly smaller differences in digit span between forward and backward recall conditions, suggesting that these children did not use subvocal verbal rehearsal strategies as efficiently as the normal-hearing children.23
Other studies have found that the atypical recall capacity of deaf children with CIs is not limited to tasks involving lists of auditory stimuli. Indeed, immediate recall of sequences of visual stimuli that can be verbally encoded such as colored lights has also been found to be atypical compared with age-matched, normal-hearing peers.24–26 These findings lend further support to the hypothesis that the working memory skills of deaf children with CIs are atypical. The development of the capacity to rapidly encode, manipulate, and store stimuli that can be represented phonologically or subvocally using verbal rehearsal may be delayed or even disordered as a result of a period of profound deafness before implantation with a CI.23,26
Finally, several recent studies have provided strong converging evidence that subvocal verbal rehearsal abilities are related to open set speech perception, vocabulary knowledge, expressive and receptive language, speech intelligibility, and speaking rates in prelingually deaf children with CIs.23,25,27,28 The present results obtained with the response delay task suggest that subvocal verbal rehearsal abilities are closely tied to other traditional measures of CI benefit. However, relations between delay ER and other process measures known to reflect subvocal rehearsal (such as forward-backward digit span) need to be investigated in future studies of deaf children with CIs.
In summary, the present results reveal several new findings regarding the relations between the cognitive processes involved in a behavioral inhibition task and a range of audiologic outcome measures in prelingually deaf children with CIs. After a period of early auditory deprivation, a CI may lead to improvements on visually based information processing tasks because of improvements in subvocal rehearsal skills. Further work is needed to understand more precisely how early auditory deprivation, and subsequent cochlear implantation, affects performance on the response delay task. For instance, we do not know whether auditory experience with a CI has an effect on behavioral inhibition skills through remodeling of language processes per se or whether there are more specific effects of audition on cortical areas responsible for self-regulatory behavior mediated by prefrontal cortex, anterior and posterior cingulated gyrus, and other brain areas.10,29 Future research on neuropsychologic functions of deaf children who use CIs is needed to answer this and other related questions. Furthermore, neuropsychologic testing of these children may prove useful as a clinical tool in assessing cognitive and linguistic outcomes, and this population of children may benefit from a CI.
Acknowledgments
The authors thank all of the speech/language pathologists and other clinicians who helped in testing the children, Sujuan Gao and Amy Rong QI for their statistical assistance, and to Josh Bradley and Tonya Bergeson-Dana for their comments on an earlier version of this article.
This work was supported by NIH-NIDCD Training Grant T32 DC00012 and NIH-NIDCD Research Grants R01 DC00064, NIH NIDCD R01 DC00423 to Indiana University.
Footnotes
These findings were presented in a podium session at CI 2004, International CI Conference, Indianapolis, IN, May 2004.
Bibliography
- 1.Svirsky M, Robbins A, Kirk K, et al. Language development in profoundly deaf children with cochlear implants. Psych Sci. 2000;11:153–158. doi: 10.1111/1467-9280.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tyler R, Fryauf-Bertschy H, Kelsay D, Gantz B, et al. Speech perception by prelingually deaf children using cochlear implants. Otolaryngol Head Neck Surg. 1997;117:180–187. doi: 10.1016/s0194-5998(97)70172-4. [DOI] [PubMed] [Google Scholar]
- 3.Miyamoto R, et al. Speech intelligibility of children with multichannel cochlear implants. Ann Otol Rhinol Laryngol. 1997;168:35–36. [PubMed] [Google Scholar]
- 4.Sarant J, Blamey P, Dowell R, et al. Variation in speech perception scores among children with cochlear implants. Ear Hear. 2001;22:18–28. doi: 10.1097/00003446-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Geers A, Brenner C, Davidson L. Factors associated with development of speech perception skills in children implanted by age five. Ear Hear. 2003;24:24–35. doi: 10.1097/01.AUD.0000051687.99218.0F. [DOI] [PubMed] [Google Scholar]
- 6.Knutson J, Ehlers S, Wald R, Tyler R. Psychological consequences of pediatric cochlear implant use. Ann Otol Rhinol Laryngol. 2000;185:109–111. doi: 10.1177/0003489400109s1247. [DOI] [PubMed] [Google Scholar]
- 7.Achenbach T, Edelbrock C. Manual for the Child Behavior Checklist-Teacher Report Form. Burlington: University of Vermont, Department of Psychiatry; 1986. [Google Scholar]
- 8.Altshuler K, Deming W, Vollenweider J, et al. Impulsivity and profound early deafness: a cross cultural inquiry. Am Ann Deaf. 1976;121:331–345. [PubMed] [Google Scholar]
- 9.Kelly D, et al. Attention deficits in children and adolescents with hearing loss. A survey. Am J Dis Child. 1993;147:737–741. doi: 10.1001/archpedi.1993.02160310039014. [DOI] [PubMed] [Google Scholar]
- 10.Barkley R. ADHD and the Nature of Self Control. New York: Guilford Press; 1997. [Google Scholar]
- 11.Mitchell T, Quittner A. Multimethod study of attention and behavior problems in hearing-impaired children. J Clin Child Psychol. 1996;25:83–96. [Google Scholar]
- 12.Wechsler D. Wechsler Preschool and Primary Scale of Intelligence-Revised. New York: The Psychological Corporation; 1989. [Google Scholar]
- 13.Wechsler D. Wechsler Intelligence Scale for Children. 3rd. Wilmington, DE: Wide Range, Inc.; 1991. [Google Scholar]
- 14.Gordon M, McClure F, Aylward G. Instruction Manual for the Gordon Diagnostic System (GDS) Model III-R. Dewitt, NY: Gordon Systems, Inc.; 1991. [Google Scholar]
- 15.Haskins H. Evanston, IL: Northwestern University, 1949.
- 16.Dunn L, Dunn L. Peabody Picture Vocabulary Test. 3rd. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- 17.Reynell JK, Huntley M. Reynell Developmental Language Scales. Windsor, UK: NFER-Nelson; 1985. [Google Scholar]
- 18.Osberger M, Robbins A, Todd S, Riley A. Speech intelligibility of children with cochlear implants. Volta Rev. 1994;96:169–180. [Google Scholar]
- 19.Wolfinger R, Chang M. Comparing the SAS GLM and MIXED procedures for repeated measures. Proceedings of the Twentieth Annual SAS Users Group International Conference; Orlando, FL. 1995. [Google Scholar]
- 20.Schafer J, Graham J. Missing data: our view of the state of the art. Psychol Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- 21.Cohen J, Cohen P. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1983. [Google Scholar]
- 22.Gershon J. A meta-analytic review of gender differences in ADHD. J Atten Disord. 2002;5:143–54. doi: 10.1177/108705470200500302. [DOI] [PubMed] [Google Scholar]
- 23.Pisoni D, Cleary M. Measures of working memory span and verbal rehearsal speed in deaf children after cochlear implantation. Ear Hear. 2003;24:106–120. doi: 10.1097/01.AUD.0000051692.05140.8E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cleary M, Pisoni D, Geers A. Some measures of verbal and spatial working memory in eight- and nine-year-old hearing-impaired children with cochlear implants. Ear Hear. 2001;22:395–411. doi: 10.1097/00003446-200110000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dawson P, Busby P, McKay C, Clark G. Short-term auditory memory in children using cochlear implants and its relevance to receptive language. J Speech Lang Hear Res. 2002;45:789–801. doi: 10.1044/1092-4388(2002/064). [DOI] [PubMed] [Google Scholar]
- 26.Pisoni D, Cleary M. Some new findings on learning, memory and cognitive processes in deaf children following cochlear implantation. In: Zeng F, Popper A, Fay R, editors. Cochlear Implants: Auditory Prostheses and Electrical Hearing (Springer Handbook of Auditory Research. Vol. 20. New York: Springer-Verlag; 2004. [Google Scholar]
- 27.Pisoni D, Geers A. Working memory in deaf children with cochlear implants: correlations between digit span and measures of spoken language processing. Ann Otol Rhinol Laryngol. 2000;185:92–93. doi: 10.1177/0003489400109s1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burkholder R, Pisoni D. Speech timing and working memory in profoundly deaf children after cochlear implantation. J Exp Child Psychol. 2003;85:63–88. doi: 10.1016/s0022-0965(03)00033-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubia K, et al. Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neurosci Biobehav Rev. 2000;24:13–19. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]


