Abstract
In the brain, the mu-opioid receptor (MOR) activates neural nitric oxide synthase (nNOS) through the PI3K/Akt pathway. The resulting nitric oxide (NO) enhances the function of the glutamate N-methyl-d-aspartate receptor (NMDAR)/calcium and calmodulin-dependent serine/threonine kinase (CaMKII), which subsequently diminishes MOR signaling strength. Because the ERK1/2 cascade is implicated in opioid tolerance, we analyzed the role of morphine-generated NO in this negative regulation. We found that NO-released endogenous zinc ions recruit the Ras/Raf-1/ERK1/2 cassette to histidine triad nucleotide-binding protein 1 (HINT1). A-Raf and B-Raf showed little or no MOR association. The zinc ions bridge the Raf-1 cysteine-rich domain (CRD) with HINT1 at the MOR C-terminus. Morphine also recruits PKCγ via NO/zinc to the MOR-HINT1 complex. Both Raf-1 and PKCγ CRDs bind simultaneously to HINT1, enabling PKCγ to enhance Raf-1 function to intensify MEK/ERK1/2 activation. Thus, through attached HINT1, the MOR facilitates the cross-talk of two NO- and zinc-regulated signal-transduction pathways, PKC/Src and Raf-1/ERK1/2, implicated in the negative control of morphine effects. This study reveals new aspects of ERK1/2 regulation by the MOR without requiring the transactivation of a receptor tyrosine kinase. Antioxid. Redox Signal. 14, 2413–2425.
Introduction
Opioids are probably the most effective drugs in attenuating nociceptive perception, and they are the analgesics of choice to relieve intense chronic pain. These substances exert this positive action by binding to specific mu, delta, and kappa receptors in the nervous system, members of the GPCR (G protein–coupled receptor) family. Pharmacologic studies performed on genetically modified mice indicate that morphine and its derivatives induce antinociception through the mu-opioid receptor (MOR) (13), which is coupled to Gi, Go, Gz, and Gq/11 proteins, and that activation of this receptor regulates diverse effectors, such as inwardly rectifying potassium channels; voltage-activated calcium channels; adenylyl cyclase; phospholipases; the MAPK cascade; the PI3K/Akt/nNOS pathway; the N-methyl-d-aspartate glutamate receptor (NMDAR)/CaMKII cascade; and so on (36). Unfortunately, repeated administration of opioid analgesic drugs provokes a progressive decrease in their potency (tolerance), higher doses then being required to obtain the expected outcome, with the risk of augmenting the adverse effects and of establishing physical dependence.
The most common mechanism that produces GPCR desensitization involves the phosphorylation of intracellular residues by G-protein receptor kinases (GRKs), which is followed by arrestin binding and subsequent internalization of the receptor molecule. However, although opioids such as etorphine and [d-Ala2, N-Me-Phe4, and Gly5-ol]-encephalin (DAMGO) stimulate efficient internalization of the MOR, strong tolerance to morphine analgesia develops but without receptor internalization (9, 31). A series of studies revealed that the activity of the NMDAR/NO/CaMKII cascade in neural tissue is mostly responsible for the development of tolerance to morphine antinociception (9). This negative regulation of MOR-mediated effects by NMDAR provides the basis for the clinical efficacy of NMDAR antagonists in preventing, and even rescuing, morphine analgesia from tolerance (14).
A series of signaling proteins participate in this MOR-NMDAR cross-talk, including HINT1, a member of the histidine triad (HIT) protein family. HINT1 is a 126-amino acid protein of about 14 kDa that exists as a homodimer (23). HINT1 binds to the C terminus of the MOR (33), and it connects this receptor to GTPase activating proteins (GAPs) of receptor-activated GαGTP subunits, the RGS17(Z2) and RGS20(Z1) proteins (33). On morphine challenge, this HINT1/RGSZ complex recruits inactive PKCγ to the MOR C terminus, a process mediated by zinc ions (32). The HINT1 protomer contains a row of three histidines that are separated by hydrophobic amino acids, which are crucial for its zinc-dependent interaction with the cysteine-rich domain (CRD) of the PKCγ C1 regulatory domain (32). The free zinc ions required are initially provided by the morphine-induced activation of the PI3K/Akt/nNOS pathway (36) and the subsequent action of NO on intracellular zinc stores (18). At late postmorphine intervals, MOR-induced NMDAR activation facilitates the translocation of zinc ions from the presynaptic terminal into the postsynaptic neuron, contributing to the recruitment of PKCγ to the MOR environment (22, 36). The PKCγ activates the tyrosine kinase Src (35), and both PKC and Src kinases produce the sustained potentiation of NMDAR calcium currents (2, 35). Subsequently, the activation of CaMKII is essential for the NMDAR-dependent development of tolerance to the analgesic effects of morphine (9, 37). In addition, the production of morphine analgesic tolerance also requires ERK1/2 activation (3, 29), which probably contributes to the sensitization to pain by increasing the activity of glutamate NMDAR (11).
Interestingly, some functional and structural analogies exist between PKCγ and the Raf kinase that acts upstream of ERK1/2. Raf kinases are ubiquitous serine/threonine kinases that play critical roles in mediating the activation of the ERK1/2 cascade by the small G protein, p21 Ras (25).
The C1 regulatory domain of all PKC isozymes is a CRD that is organized as a tandem C1A and C1B repeat, coordinating the binding of zinc as well as that of phorbol esters or diacylglycerol (DAG) (17, 27). CRD motifs also are present in the regulatory region of the Raf kinases (26). All Raf kinases possess three highly conserved regions, designated C1, C2, and C3 (20), whereas the catalytic domain of Raf kinase is in the C-terminal half of the protein (C3). The amino terminus of Raf contains two conserved regions (C1 and C2) that negatively regulate the kinase domain. The C1 region consists of two distinct structural modules called the Ras-binding domain (RBD) and the CRD. The CRD contains two zinc-finger motifs resembling the C1 domain of the conventional protein kinase C. It is notable that the Raf CRD does not bind DAG, even though it does interact with phosphatidylserine (10).
Therefore, morphine-induced activation of PKC and ERK1/2 is essential for the NMDAR-dependent development of tolerance to the analgesic effects of this opioid. Since the initial report associating PKC with MOR-dependent enhancement of NMDAR function (2), other elements have been identified that bridge the gap between both receptors, as well as NO redox signaling (32, 35, 36). With respect to the ERK1/2 pathway, its activation promotes the surface expression of AMPAR-containing GluR1/GluR2L subunits, which enhance NMDAR function by long-term potentiation (LTP) of the postsynapse (50). However, little is known about the molecular processes implicated in the morphine-evoked activation of this MAPK. Because PKC increases the activity of the ERK1/2 cascade by acting directly on Raf kinases (16), we investigated whether the HINT1 complex can act as an adaptor coupling the PKC and Raf kinase pathways under the regulation of the MOR. We also examined the influence of the zinc metal ion in this process, and of the nNOS/NO cascade as the physiologic source of these ions.
Materials and Methods
Zinc-microfluorescence imaging in CD1 mouse striatal slices
Transverse 200-μm brain slices were prepared according to standard procedures. The slices were placed on the thermostatically heated stage of an inverted microscope and bathed in artificial cerebrospinal fluid: 124 NaCl, 1.75 KCl, 1.3 MgSO4, 2.4 CaCl2, 1.25 KH2PO4, 26 NaHCO3, and 10 dextrose (mM), pH 7.4. The temperature inside the piezo-electrically controlled incubation chamber (Life Imaging Services, Basel, Switzerland) was held at 32°C in an atmosphere of 95% O2/5% CO2.
For intracellular Zn2+ imaging, the slices were preloaded with 50 μM cellular permeable Newport Green DCF diacetate (N7991; Invitrogen, Carlsbad, CA), 0.1% pluronic acid (P3000MP, Invitrogen), and 0.5% dimethyl sulfoxide for 1 h, as described in a previous study (22). Saline or morphine (10 μM) was added to the wells. The MOR antagonist naloxone was added (30 μM) 10 min before the morphine, and the nNOS inhibitor L-NNA (100 μM) was added 20 min before the morphine. Images were obtained with confocal microscopy by using a Leica DMIII 6000 CS confocal fluorescence microscope equipped with a TCS SP5 scanning laser (excitation, 488 nm; emission, 498–520 nm) through a 10×0.4 HC PL APO objective. The images shown in Fig. 4 were collected after 30 min.
FIG. 4.
Morphine induces the release of endogenous zinc by a MOR and nNOS mechanism. Mouse brain coronal slices were preloaded with 50 μM cell-permeable Newport Green diacetate for 1 h. One hemisection was treated with morphine (10 μM), whereas the other hemisection served as a control. Zinc-related fluorescent images were taken through a 10×0.4 HC PL APO objective (excitation, 488; emission, 498–520). Striatal (interaural, 3.58 mm; bregma, −0.22 mm) and PAG (interaural, 0.88 mm; bregma, −2.92 mm) regions are shown. Baseline vehicle (NG), morphine treated (NG + MPH), naloxone (30 μM) treated (NG + MPH + NX), and L-NNA (100 μM) treated (NG + MPH + LNNA). Images were transformed to indexed color and presented in grey and in pseudocolor. Scale: 500 μm. The assay was repeated 3 times on brain coronal slices derived from different mice, and the results were comparable. Representative images are shown.
Preparation and solubilization of PAG synaptoneurosome-enriched fraction
MOR immunoprecipitation and co-precipitation of associated proteins
Procedures involving mice strictly followed the guidelines of the European Community for the Care and Use of Laboratory Animals (Council Directive 86/609/EEC) and Spanish Law (RD 1201/2005) regulating animal research. The experimental protocols were reviewed and approved by the Committee for Animal Experimentation at the CSIC.
Experimental tissue was obtained from male albino CD1 mice (Charles River, Barcelona, Spain) weighing 22–27 g. For the immunoprecipitation studies, the periaqueductal gray matter (PAG) from eight mice were pooled for each postmorphine interval. The assays were repeated at least twice on samples obtained from different mice that had received an identical opioid treatment and that had been killed at the same interval after opioid administration. The methods used to prepare the PAG synaptosomal fraction have been described elsewhere (31, 32). The affinity-purified IgGs against the extracellular domains, N terminus (NT), and second external loop (2EL) of murine MOR were labeled with biotin (Pierce 21217 and 21339). The MOR was immunoprecipitated from solubilized membranes, as described in earlier work (31, 37). The immunoprecipitated proteins were resolved with SDS/polyacrylamide gel electrophoresis (PAGE) in 10 cm×10 cm×1.5-mm gel slabs (7–14% total acrylamide concentration, 2.6% bisacrylamide cross-linker concentration). The PAG structures from eight mice (corresponding to an interval after morphine) yielded enough immunoprecipitated MOR and associated proteins to load approximately four gel lanes. The separated proteins were then transferred onto 0.2-μm PVDF membranes and probed with the selected antibodies in DecaProbe chambers (PR 150; Hoefer-GE, Barcelona, Spain).
Spectrophotometric detection of zinc released from PAG synaptosomes
Samples (0.5 ml total volume) were prepared by adding 100 μl of PAG membrane suspension, vehicle, or NO donor to 400 μl of Hepes buffer [25 mM; pH 7.8; treated with Chelex-100 resin (BioRad)]. Calibration samples were prepared from ZnCl2 (100 mM) solution (Sigma 39059) and Hepes buffer. For blanks, the metal solution was substituted with Chelex-100–treated water. Complexation was initiated by the addition of the zinc chelator Zincon (Sigma 96440) (stock solution, 1.6 mM in NaOH, 1 M) to reach a final concentration of 40 μM. Absorbance (600 nm) was recorded after 20 min of sample incubation at room temperature (RT) on a BioChrom Ultrospec 2100 spectrophotometer (Cambridge, UK).
Detection of signaling proteins
Western blots were probed with the following antibodies: affinity-purified IgG raised against extracellular domains of murine MOR (aa NT 2-16, 2EL 208-216) (1:3,000); anti-PKCγ (1:1,000; Abcam ab4145); anti-Raf-1 (1:1,000; BD Biosciences 610152); anti-A-Raf (1:1,000; BD 610074); anti-B-Raf (1:1,000; BD 612375); anti-Ras (1:1,000; BD 610001); anti-Rap1 (1:1,000; BD 610195); anti-pan ERK (1:5,000; BD 610124); anti-phospho-ERK (T202+Y204) (1:5,000; ab24157); anti-RasGRP2 (1:1,000; aa 591-604 IREEEVQTVEDGVF); anti-RasGRP3 (1:1,000; aa 94-107 GLIRMTEEFREVAS); and nNOS (1:1,000; Santa Cruz sc-1025). All antibodies were diluted in TBS+0.05% Tween 20 (TTBS) and incubated with polyvinylidene difluoride (PVDF) membranes (BioRad 162-0176) overnight at 6°C. The primary antibodies were detected with the corresponding secondary antibodies conjugated to horseradish peroxidase and diluted to 1:10,000. Antibody binding was visualized with the ECL Plus Western Blotting Detection System (RPN2132; Amersham Biosciences), and chemiluminescence was recorded with the ChemiImager IS-5500 system (Alpha Innotech, San Leandro, CA). Densitometry was performed by using Quantity One Software (BioRad) and expressed as the integrated volume (average optical density of the pixels within the object area/mm2).
Studies conducted with recombinant proteins
The interaction of HINT1 (200 nM) with either PKCγ (100 nM) or Raf-1 (100 nM) (Abnova H00005894) was studied. A similar assay was conducted to analyze the interaction between HINT1 and the catalytic domain of PKCγ (Calbiochem 539513) or Raf-1 (GenScript Z02210). The interaction of HINT1 with H-Ras (Calbiochem 553329) also was evaluated. Zinc was removed from recombinant proteins by 60 min of incubation at room temperature in a buffer containing 10 mM Hepes, pH 7.5; 150 mM NaCl; 5 mM EDTA; 200 μM DTT, and 1 mM N,N,N′,N′-tetrakis(2-pyridylmethyl) ethylenediamine (TPEN; Fluka WA16827). The buffer was then exchanged with 10 mM Hepes, pH 7.5, 150 mM NaCl, and proteins were concentrated in centrifugal filter devices (10-kDa nominal molecular mass limit; Amicon Microcon YM-10 42407, Millipore). After zinc removal, proteins were incubated either alone (negative control) or together with the GST-tagged protein in 400 μl buffer of 10 mM HEPES, pH 7.4, 150 mM NaCl, and 0.005% Tween 20 for 30 min at room temperature. After incubation, 40 μl of glutathione sepharose (GE 17 0756 01) was added, and pellets were obtained by centrifugation and washed 3 times, solubilized in 2×Laemmli buffer, and analyzed with Western blotting.
The possible mutual interference of Raf-1 and PKCγ for their binding to HINT1 protein was studied. The incubation of recombinant HINT (200 nM) with GST-Raf-1 was carried out with increasing amounts of PKCγ for 30 min at room temperature (10 mM Hepes, pH 7.4, 150 mM NaCl, 100 μM zinc).
In vitro effect of zinc on the recruitment of signaling proteins to MOR: CRD alteration, zinc chelator, and NO generators
The effect of increasing concentrations of zinc on the association of a series of signaling proteins with MOR was studied in PAG synaptosomal membranes. Zinc chloride (Puratronic, Alfa Aesar 231-592-0) was added, together with biotinylated anti-MOR IgGs, and the samples were incubated for 4 h at 4°C. In another set of assays, the time-course for 3 μM zinc to produce Raf-1 and PKCγ association with the PAG MOR was studied during 30 min at RT in the absence and presence of TPEN. Subsequently, zinc was removed with centrifugation, and PAG synaptosomal membranes were solubilized in Nonidet P-40 buffer, as previously described (31, 37). The solubilized membranes were incubated overnight at 4°C with approximately 3 μg of biotin (Pierce 21217 and 21339) conjugated primary antibody (affinity-purified IgGs) raised against the murine MOR. The MOR-associated proteins were then separated with SDS-PAGE chromatography and analyzed with Western blots. The PKC inhibitor calphostin C, which binds to and alters the structure of the CRD (Merck-Calbiochem 208725), was added 1 h before the zinc chloride. The NO generators (S)-nitroso-N-acetylpenicillamine (SNAP; Tocris Bioscience 0598) and (±)-(E)-ethyl-2-[(E)-hydroxyimino]-5-nitro-3-hexeneamide (NOR-3 or FK409; Merck-Calbiochem 489530) were incubated with the samples overnight and with the anti-MOR IgGs at 4°C. SNAP and NOR-3 also were studied for 1 h at RT. The procedure continued as described earlier. The heavy metal ion chelator TPEN (Fluka WA16827) was added to the solubilized samples together with the zinc chloride.
Animals, intracerebroventricular injection, and evaluation of antinociception
Male albino CD-1 mice weighing 22–25 g were housed and used strictly in accordance with the guidelines of the European Community for the Care and Use of Laboratory Animals (Council Directive 86/609/EEC). The response of the animals to nociceptive stimuli and the influence of morphine sulfate (Merck, Darmstadt, Germany) were determined by the warm-water (52°C) tail-flick test. Baseline latencies ranged from 1.5 to 2.0 s, and they were not significantly affected by the inhibitor l-NG-nitroarginine (L-NNA; Tocris 0664) or its solvent. A cut-off time of 10 s was used to minimize the risk of tissue damage. Antinociception was expressed as a percentage of the maximum possible effect (MPE=100×[test latency-baseline latency]/[cutoff time-baseline latency]). Groups of eight mice were lightly anesthetized with ether and received 10 nmol morphine sulfate (Merck, Darmstadt, Germany) (in a volume of 4 μl) injected into the lateral ventricle. Antinociception was assessed at different time intervals thereafter. Data are expressed as the mean±SEM.
A mouse knockout 129SvJ strain with targeted disruption of HINT1 (40) was generously supplied by I.B. Weinstein and J.B. Wang. The genotype was confirmed with PCR analysis of DNA extracted from tail biopsies.
Statistical significance
ANOVA, followed by the Student–Newman–Keuls test (SigmaStat; SPSS Science Software, Erkrath, Germany) was performed, and significance was defined as p<0.05.
Results
Morphine stimulates Raf-1 binding to the MOR
The ICV administration of morphine produces a dose- and time-dependent antinociceptive effect that reaches a maximum at about 30 min after its injection. The administration of 3 nmol and 10 nmol of the opioid led to peak analgesic effects of about 50% and 80% of the maximum analgesic effect that is detected in the thermal tail-flick test (cutoff time of 10 s). A remnant effect of about 30% is observed 90 min after injection of the higher dose, and postopioid analgesia was no longer detected at 3 h (Fig. 1B).
FIG. 1.
Morphine recruits Raf-1and PKCγ to the MOR. (A) The primary structure of Raf-1 and of conventional PKC (α, β, γ). Raf-1: C1–C3 conserved regions; the N-terminal regulatory region contains C1 and C2; the C1 region consists of two subregions: the Ras-binding domain (RBD) and the cysteine-rich domain (CRD); the C-terminal C3 region corresponds to the catalytic domain. PKC: C1–C4 conserved regions; the N-terminus contains the regulatory region with the pseudo substrate (PS); C1 contains tandem C1A and C1B cysteine-rich domains (CRDs) that bind zinc and phorbol esters/diacylglycerol; C2 binds phosphatidylserine and calcium; the C-terminal region contains the ATP-binding site (C3) and the kinase domain (C4) that binds the substrate. In both kinases, the N-terminal regulatory domain regulates (inhibits) the activity of the C-terminal catalytic domain. (B) Morphine (3 nmol and 10 nmol) was injected ICV into mice. Data are the analgesic time course determined by the tail-flick test. Each data point is the mean±SEM, n=8 mice. *Significantly different from the preopioid control interval (0 min), p<0.05. (C) Ex vivo molecular studies: After 10 nmol ICV morphine, groups of eight mice were killed at the intervals indicated; their PAG were pooled and used for MOR immunoprecipitation and co-precipitation of PKCγ, Raf-1, and B-Raf. Immunosignals are shown relative to 0 min (animals not receiving the opioid were attributed an arbitrary value of 1). The nNOS inhibitor L-NNA (7 nmol) was injected ICV 30 min before morphine. Open bars, control mice; solid bars, mice receiving L-NNA. Immunoprecipitated MOR signals are the loading control. Each bar is the mean of three assays performed on PAG samples obtained from independent groups of eight mice each. Data are expressed as mean±SEM. *Significantly different from the group 0 min, p<0.05. Inset: Immunosignals for PKCγ (78 kDa), Raf-1 (74 kDa), B-Raf (95 kDa), and A-Raf (68 kDa) determined in PAG membranes with the antibodies and dilutions used to detect MOR-associated proteins.
The MOR in the midbrain PAG is rather superficial to the aqueduct and is reached by ICV-injected opioids to produce supraspinal antinociception. In this study, the mice were injected with 10 nmol morphine, after which groups of eight animals were killed at different time points of the analgesic time course, and PAG synaptosomal membranes were obtained for the ex vivo determinations. Morphine, during its analgesic time course, promotes MOR-PKCγ association. With the cessation of analgesia, this association, although reduced, still persisted for several hours (Fig. 1C) (32). The CRD-containing Raf-1 serine and threonine kinase also responded to morphine, increasing its association with the MOR. The A-Raf and B-Raf isoforms, although present in PAG synaptosomes, are seldom associated with MOR in response to morphine.
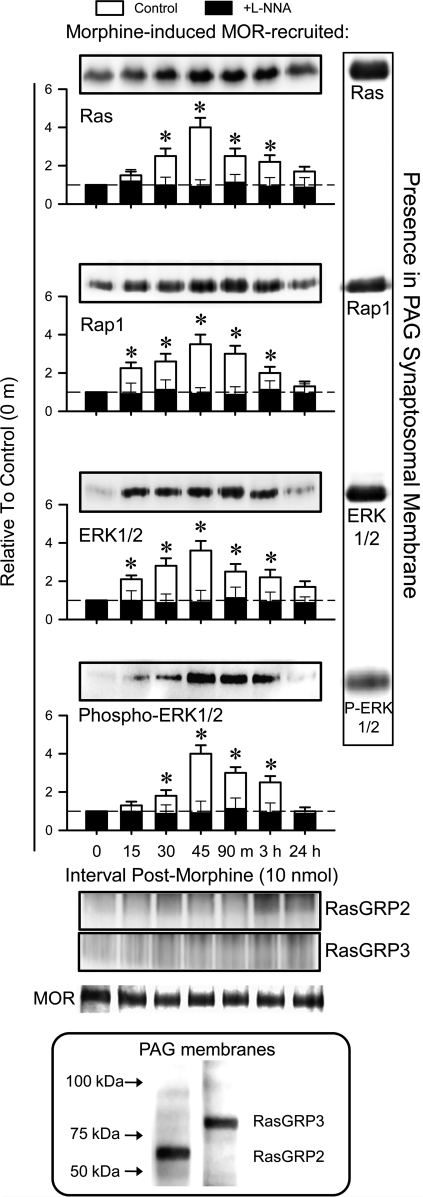
Morphine also increased the MOR association with the regulators and effectors of Raf-1 activity, such as Ras, Rap1, and ERK1/2. During the time course of morphine antinociception, MOR-associated ERK1/2 became progressively phosphorylated, suggesting its activation in this compartment (Fig. 2). All of these proteins reached and left the MOR with kinetics similar to that of Raf-1, suggesting that they were recruited as a functional complex.
FIG. 2.
Morphine recruits Ras and Rap1, as well as ERK1/2, to the MOR: effect of nNOS inhibition. ICV injection of 10 nmol morphine to mice was followed by ex vivo analysis of the MOR association with Ras, Rap1, ERK1/2, and activated ERK1/2 in the midbrain PAG structure. Open bars, control mice; solid bars, mice receiving L-NNA. Insets: PAG synaptosomal Ras (21 kDa), Rap1 (21 kDa), ERK1/2 (42–44 kDa), activated ERK1/2, RasGRP2 (69 kDa), and RasGRP3 (78 kDa). Details are as in the legend to Fig. 1.
The morphine-induced MOR-PKCγ association depends on nNOS-provided free zinc ions (32). Accordingly, in vivo administration of L-NNA, an nNOS inhibitor, prevented morphine from stimulating this MOR-PKCγ association. This treatment also avoided MOR association with Ras, Rap1, Raf-1, and ERK1/2 (Figs. 1 and 2). Comparable results were obtained with in vivo administration of another nNOS inhibitor, NG-nitro-l-arginine methyl ester (L-NAME, 25 nmol per mouse) (not shown). Thus, NO is required for morphine to recruit these proteins into the MOR environment. The GEF activators of Ras that contain DAG-binding CRD (30), RasGRP2 and RasGRP3, are present in mouse PAG synaptosomes. However, morphine failed to promote their association with the MOR (Fig. 2).
HINT1 mediates the binding of Raf-1 to the MOR C-terminus
The MOR-associated HINT1 protein recruits several signaling proteins to morphine-activated MORs [e.g., Akt, nNOS, and PKCγ (32, 33, 36)], and this protein is also essential in the MOR association with Ras/Raf-1/ERK1/2. Raf-1 and PKCγ are present in PAG synaptosomes from mice in which the HINT1 gene has been disrupted. However, in those mice, morphine failed to stimulate the MOR association with Raf-1 (Fig. 3), Ras, Rap1, or ERK1/2 (data not shown).
FIG. 3.
MOR-associated HINT1 mediates the morphine-induced binding of Raf-1. HINT1 (+/+) and (−/−) 129 SvJ mice received ICV 10 nmol morphine. At intervals after injection, groups of four mice were killed, and their PAGs were pooled for the study of MOR association with Raf-1 and PKCγ. The assays were repeated twice. Representative blots are shown. Further details are in the legend to Fig. 1. Diagram: relevance of free zinc in Raf-1 and PKCγ CRD binding to HINT1 homodimer at the MOR C-terminus.
Morphine-induced recruitment of Raf-1 to MOR: role of the nNOS/NO/zinc pathway and of the Raf-1 CRD
Morphine activates nNOS via the MOR/PI3K/Akt pathway (36) and promotes PKCγ-HINT1 association through free zinc ions (32). We set up an experiment to gain evidence that morphine promotes the NO-mediated release of zinc from endogenous stores. Thus, the application of morphine to mouse brain slices increased fluorescent signals associated with the increase of free zinc ions. This effect was reduced by the antagonist of MOR naloxone and also by the inhibition of nNOS activity (Fig. 4). The effects of ICV morphine on MOR-associated proteins persisted during the ex vivo analysis procedure, which requires approximately 24 h at 4°C, thus indicating their stability.
The role of zinc ions in these protein interactions was then assayed in membranes derived from PAG synaptosomes. Initially, the zinc ions were incubated with the membranes for 4 h at the same temperature as that used for the ex vivo assays, 4°C. Free zinc ions were then removed by centrifugation and washing, the membranes were solubilised, and the MORs were immunoprecipitated. We observed that the addition of zinc increased the association of MOR, not only with PKCγ but also with Raf-1. This was observed over a wide range of zinc ion concentrations, from mid-nanomolar concentrations to a maximum at low-micromolar concentrations (Fig. 5A). To optimize these immunosignals over those of the controls obtained without added zinc, this part of the study was carried out by using micromolar concentrations of the metal ion. In doing so, we observed that zinc also recruited Ras, Rap1, and ERK1/2 to the MOR environment, probably forming a preexisting complex with Raf-1 (Fig. 5B). Afterward, the time course to promote MOR-Raf-1 association was determined at 22°C in 3 μM zinc. In these conditions, zinc promoted the association of Raf-1 and PKCγ with MOR in the first 5 min of incubation, and these associations were maximal after about 15 min. As expected, the heavy-metal chelator TPEN blocked such associations (Fig. 5C). Interestingly, 15 min after the start of the incubation, the buffering/muffling capacity of the tissue reduced the concentration of free zinc to almost one third [after centrifugation of the samples, the supernatant was recovered and analyzed with Zincon (34), as described in Methods]. These data indicate that zinc promoted the associations described as soon as zinc reached the target proteins. Afterward, free zinc diminished greatly, but the established associations persisted during the solubilization and analysis procedure. Therefore, in vitro assays appeared to reproduce reasonably the qualitative effects of morphine on the MOR-Raf-1(PKCγ) association observed in the ex vivo analysis.
FIG. 5.
Effect of zinc on the in vitro association of the Raf-1/ERK1/2 cassette with MOR. (A, B) PAG synaptosomes were incubated with zinc chloride for 4 h at 4°C. (C) The time course for the effect of 3 μM zinc on MOR association with PKCγ and Raf-1; incubation was conducted at room temperature with and without 100 μM TPEN. At the end of the procedure, free zinc was removed by three runs of centrifugation washing. Afterward, MOR was immunoprecipitated, and associated proteins were detected with the corresponding antibodies. The study was repeated 3 times on different PAG preparations. Data are expressed relative to their control (no zinc, arbitrary value of 1) as mean±SEM. Equal loading was determined by the MOR signals. *Significantly different from the control group (no zinc), p<0.05.
Over the range of zinc concentrations studied, nNOS (Fig. 5B) and Akt (not shown) were not recruited to the MOR. Therefore, the micromolar zinc concentrations used apparently produced no MOR aggregation, unspecific recruitment of proteins to MOR, or interference with IgG binding to the MOR. High micromolar zinc concentrations reduced the MOR association with PKCγ, Raf-1, and Raf-1–complexed proteins. It is then possible that these concentrations promote the arrival of third-partner proteins to displace PKCγ and the Raf-1/ERK1/2 cassette from their association with the MOR.
Zinc ions complex the sulfydryl group in cysteine with the imidazolyl nitrogen in histidine, as well as with the carboxyl and carbonyl oxygens of glutamic and aspartic acids (24). To confirm that the Raf-1 CRD was involved in its interaction with HINT1 at the MOR, we used calphostin C, which reacts with clusters of cysteines, such as those of the PKC C1 regulatory domain, and alters their structure (12). The presence of this compound prevented the zinc-mediated association of PKCγ and Raf-1 to the MOR-HINT1 complex (Fig. 6A). This was also true for Ras, Rap1, and ERK1/2. PKC inhibitors not affecting the integrity of the C1 CRD, such as chelerythrine or Gö7874, did not prevent the in vitro zinc-mediated MOR-Raf-1 association (not shown). The presence of TPEN reduced the formation of the zinc-dependent MOR–Raf-1 association (Fig. 6B), as well as that of Ras, Rap1, and ERK1/2 (data not shown). Calphostin and TPEN exhibit almost no dissociating activity on preformed Raf-1-MOR (PKCγ-MOR) complexes (Fig. 6C). Thus, their use in the in vitro assays mostly prevents the zinc-promoted association of target proteins, whereas these compounds produce a minor effect on the firmly established interactions.
FIG. 6.
Effect of calphostin and TPEN on zinc-dependent MOR- Raf-1 association. (A) The effect of the CRD modifier calphostin C was studied on the in vitro zinc-mediated recruitment of Raf-1 and PKCγ to MOR. Calphostin C was added to PAG synaptosomes 1 h before zinc chloride, and the incubation was continued for 4 h at 4°C. (B) The heavy-metal ion chelator TPEN prevents zinc from facilitating Raf-1 and PKCγ binding to the MOR. (C) Effect of TPEN, 100 μM, and calphostin, 50 nM, on the basal (no zinc added) association of Raf-1 and PKCγ with MOR in PAG membranes. Equal loading was determined by the MOR signals. *Significantly different from the control group, p<0.05. Details are as in the legend to Fig. 5.
The NO generators SNAP and NOR3 at the studied concentrations of 100 μM released about 6 to 8 nmoles of free zinc ions per milligram of PAG membrane protein (Fig. 7). With about 0.8 mg protein in a 0.5-ml assay, the final free zinc concentration reached the low micromolar range. Thus, zinc content reasonably agrees with that described for this ion in brain tissue (45). The NO donors increased MOR-Raf-1 and MOR-PKCγ associations and failed to recruit nNOS to MOR (Fig. 8). This effect was observed at long intervals (24 h) and also at short intervals (1 h) of SNAP/NOR-3 incubation with the PAG membranes. The co-incubation of TPEN prevented formation of the NO-induced Raf-1- and PKCγ-MOR associations. Therefore, in PAG synaptosomal membranes, the source of zinc ions required for the MOR–Raf-1 interaction was mobilized by NO.
FIG. 7.
SNAP and NOR-3 release endogenous zinc from PAG synaptosome membranes. SNAP and NOR-3 (100 μM) were added to PAG membranes. The assays were conducted at RT. Upper panel: Calibration curve for Zincon detection of Zn2+. Middle panel: Time course for SNAP-induced release of endogenous zinc. Lower panel: NO donors (100 μM) were incubated with PAG membranes for 30 min. Zinc release was monitored by its complexation with the zinc chelator Zincon. The absorbance (600 nm) was recorded at RT on a BioChrom Ultrospec 2100 spectrophotometer (Cambridge, UK). Data are expressed as the mean±SEM of three independent assays. *Significantly different from the control group (without NO donor), p<0.05.
FIG. 8.
NO provides the zinc ions required for MOR association with Raf-1 and PKCγ. SNAP and NOR3 were incubated with PAG membranes either for 1 h at RT or for 24 h at 4°C. The NO donors (100 μM) were incubated alone or with TPEN (100 μM). Subsequently, the influence of these treatments on the MOR association with Raf-1, PKCγ, and nNOS proteins was evaluated. The data in each column are expressed as the mean±SEM of three independent assays. MOR signals served to control loading. *Significantly different from the control group, p<0.05.
Zinc ions support the simultaneous and direct binding of Raf-1 and PKCγ CRDs to HINT1 protein
In vivo, zinc ions mediate the association of Raf-1 and PKCγ with HINT1. By using recombinant proteins, we analyzed Raf-1 and PKCγ association with the HINT1 protein and the role of zinc in those interactions. First, the recombinant proteins were incubated with the heavy-metal chelator TPEN to remove any metal content. Subsequently, Raf-1 and PKCγ showed a zinc-dependent association with the HINT1 protein. The removal of their respective regulatory domains where the CRD resides prevented the catalytic domains of these kinases from binding to the HINT1 protein. Moreover, recombinant Ras failed to bind to the HINT1 protein, and this was explored with Ras loaded with GDP or GTPγS or in the presence of zinc (Fig. 9A). Conversely, PKCγ displayed a lack of binding to Raf-1 in the presence and absence of zinc. However, both proteins were co-precipitated simultaneously by HINT1. Increasing concentrations of PKCγ did not noticeably affect Raf-1 binding to the HINT1 protein (Fig. 9B). Therefore, HINT1 stabilizes the simultaneous zinc-dependent binding of PKCγ and Raf-1 CRDs. The formation of this ternary complex could be of relevance in the cross-regulation of PKCγ- and Raf-1–mediated signaling pathways.
FIG. 9.
Raf-1 and PKCγ CRD bind via zinc to HINT1. Formation of a ternary complex. (A) Interaction of recombinant proteins HINT1, PKCγ, and Raf-1. The HINT1 protomer was used at 200 nM, whereas PKCγ and Raf-1 [whole sequence and catalytic domain (cd)] were used at 100 nM. After TPEN zinc removal, proteins were incubated alone (negative control) or with the GST-tagged protein. The influence of added zinc was evaluated. After incubation, glutathione sepharose (GS) was added to the incubation mixture; the proteins were resolved by SDS-PAGE chromatography and analyzed with Western blotting. A similar study of association was conducted between the recombinant proteins Ras and HINT1. (B) HINT1 mediates the formation of a ternary complex with Raf-1 and PKCγ. After TPEN zinc removal, the whole sequences of Raf-1 and PKCγ showed no interaction with or without added zinc. The binding of Raf-1 to the HINT1 protein was not reduced, whereas the HINT1-PKCγ association increased.
Discussion
This study demonstrates that the neural MOR internalizes morphine signals by regulating nNOS/NO and through the oxidative release of zinc ions from intracellular stores, probably from cysteine-rich metallothioneins (18, 24). With NO, morphine increases the availability of neuronal free zinc (36) to the levels required to recruit PKCγ and Raf-1 kinases to the control of the MOR. Thus, these zinc-containing kinases behave as redox sensors in which zinc binding and release controls their binding with the HINT1 homodimer at the MOR C terminus. PKCγ and Raf-1 do not compete for NO- and zinc-dependent binding to the HINT1 protein, suggesting their assembly into a complex in which they could influence each others' activity. In this respect, morphine promotes the sustained potentiation of NMDAR function through the activation of PKCy/Src (35) and by the activation of the Ras/Raf-1/MEK/ERK1/2 signaling cascade (38, 50). Thus, their concurrence under the control of the MOR could constitute the checkpoint whereby PKC acts on Raf-1, bringing about ERK1/2-mediated long-term potentiation of the NMDAR-CaMKII function, which, in turn, operates as a negative feedback reducing MOR signalling (41). Thus, the morphine-activated Akt/nNOS/NO pathway works upstream of PKC/Src and Raf-1/ERK1/2 pathways, providing the free zinc ions necessary to foment their cross-talk at the HINT1 protein. The discovery of this regulatory mechanism help us to understand better the steps between morphine and the activation of the ERK1/2, and it fills some of the gaps in our knowledge of NMDAR-mediated morphine analgesic tolerance.
ICV morphine recruits proteins of the MAPK cascade to the MOR [i.e., Raf-1, Ras, Rap1, and ERK1/2]. All of these regulatory proteins associate with and dissociate from MOR with similar kinetics, suggesting that they work as a functional complex. Our results indicate that Raf-1 CRD associates directly with the HINT1 protein and that it is essential to bring the ERK1/2 cascade under regulation of the MOR. Certainly, guanine nucleotide exchange factors (RasGEF) containing CRD, such as RasGRP, could also bind to the MOR-HINT1 complex in a zinc-dependent manner. However, RasGEF and Raf-1 share the binding site at RasGTP (1, 43), and for RasGTP to bind Raf-1, it must first dissociate from the GEF. Consequently, Ras activators cannot mediate Raf-1/ERK1/2 translocation to the MOR-HINT1 complex. Moreover, morphine did not promote RasGRP2/3 binding to MOR. Ras itself was also discarded because RasGDP/GTP displays no binding to the HINT1 protein. The Raf isoforms A-Raf and B-Raf differ from Raf-1 in the corresponding N-termini sequences (28). These differences could account for morphine mostly inducing the recruitment of Raf-1 to the MOR.
Structurally, Raf-1 kinase exhibits considerable similarity to conventional protein kinase C (see Fig. 1). Both proteins contain a regulatory amino-terminus and a carboxy-terminal catalytic domain. Additionally, a similar CRD is present within the regulatory domain of each protein, and both bind zinc ions (27, 44). Raf-1 CRD must contain structurally essential bound zinc ions to support RasGTP binding. However, this CRD does not interact with DAG or phorbol esters (10). The Raf-1 N-terminus locks the kinase in an inactive conformation and contains two conserved regions, the RBD and the CRD. The RBD domain supports binding to active, GTP-bound Ras (6). In addition to its RBD at the N-terminus, the CRD also plays a role in the binding of active RasGTP to Raf-1. This binding facilitates Raf-1 translocation from the cytoplasm to the plasma membrane (20) and releases the Raf-1 C3 catalytic domain from its negative regulatory interaction with the RBD-CRD regions (46).
The NO appears to be essential in the MOR-regulation of ERK1/2 signaling. In neurons, NO is the major mediator of calcium-dependent activation of Ras (48). However, Ras binding to Raf-1 is insufficient by itself for Raf-1 activation. It is postulated that after Ras binding, Raf-1 must undergo further modifications to become activated. These include tyrosine, serine/threonine phosphorylation, phospholipid binding, and interaction with other proteins (20). NO also stimulates the zinc-mediated recruitment of Raf-1 to HINT1 at the MOR C terminus, bringing the kinase under close regulation by PKCγ and probably by Src as well. Morphine-activated PKCγ can regulate Raf-1 by phosphorylation, particularly at C3 serine497 and serine499 (20), greatly increasing its activity (16). Moreover, MOR-recruited PKCγ also activates Src (35), which, in turn, could also regulate Raf-1 acting on C2 tyrosines340/341 (20).
At this stage, zinc and the HINT1 protein emerge as key regulators of ERK1/2 activity by the MOR but without the need for receptor tyrosine kinase transactivation. Zinc plays a dynamic role in the regulation of PKC activity, allowing the kinase to be turned on or off (17). DAG release from PKC exposes a zinc-binding site in the C1 CRD. Zinc can now bridge the inactive kinase to the HINT1-MOR complex at the plasma membrane (32). The binding of phorbol esters/DAG to C1 CRD PKCγ releases zinc and activates the kinase. This step would promote PKCγ segregation from the MOR (17, 32). Analogous to that described for PKC, binding of RasGTP to the Raf-1 zinc finger disrupts the zinc-mediated intramolecular binding of CRD to the catalytic domain and commences the initial steps of Raf-1 activation (20). In case this action also releases zinc, morphine-activated nNOS/NO would provide free zinc ions to recruit Raf-1 effectively to the MOR. There, activation of Raf-1 by PKC/Src phosphorylation could account for its segregation.
The HINT1 homodimer (23) supports the simultaneous binding of inactive PKCγ and Raf-1. This ternary complex provides a mechanism for the cross-talk of two signal-transduction pathways under regulation of the MOR. There exists a functional parallelism between what is found here for HINT1 and the 14-3-3 proteins. Both are homodimers, and the 14-3-3 also acts as an adaptor, bringing together a series of proteins, Raf-1 and PKC included (42). Under 14-3-3 regulation, Raf-1 and PKC are stabilized as inactive. PKC activation leads to a complex dissociation, suggesting that 14-3-3 is a transient mediator of Raf-1 phosphorylation and activation by PKC (4, 42). The 14-3-3 ζ protein binds to Raf-1 by C2 P-serine259 and C3 P-serine621, and like HINT1, it binds the CRD as well (4). In the in vitro assays, HINT1 differs from 14-3-3 in that PKCγ and Raf-1 binding to HINT1 does not require serine phosphorylation and depends on the presence of free zinc ions. In the frame of this regulation, Rap1 should diminish the ERK1/2 activation step at the HINT1/Raf-1/PKCγ ternary complex by reducing Ras binding to Raf-1.
A series of reports indicate that, whereas experimentally increased zinc levels activate PI3K/Akt (19, 21) and ERK1/2 pathways (39), overexpression of PTEN has opposite effects (47). PTEN is a lipid phosphatase that removes the product of PI3K, PIP3, which is required to translocate and activate Akt at the cell membrane. Zinc and PTEN are linked in that high zinc concentrations directly inhibit PTEN and then facilitate the PI3K/Akt/nNOS/NO pathway. NO can directly activate Ras (48), and our results indicate that the NO-evoked oxidative release of zinc ions contributes to the Ras/Raf-1–mediated activation of ERK1/2. It has been reported that decreases in cellular zinc bring about low levels of ERK1/2 phosphorylation (49). Therefore, the activity of the Ras-ERK1/2 signaling pathway can be influenced by the Akt/nNOS-mediated production of NO and free zinc ions. Thus, high zinc concentrations acting on PTEN could simultaneously affect both signaling pathways.
It is estimated that, in presynaptic vesicles of certain glutamatergic terminals, free or chelatable zinc can reach millimolar concentrations. After physiologic stimulation, zinc ions are co-released with glutamate from presynaptic terminals, reaching high micromolar concentrations in the synaptic cleft (15). Moreover, some of these zinc ions are rapidly translocated into postsynaptic neurons through divalent cation permeable glutamate-activated channels, where they transiently reach high concentrations, mostly in the postsynaptic density region (22, 45). This activity confers a neurotransmitter role for the presynaptically released zinc ions, with the singularity of reaching targets inside the postsynaptic neuron. The cytosolic increase in zinc produced by its neuronal translocation, or caused by nNOS/NO acting on intracellular stores, would be expected to influence signaling events (8). Thus, besides the MOR/PI3K/AkT/nNOS/NO route, the coexistence of MOR and NMDAR at postsynaptic sites in PAG neurons would facilitate the access of permeated zinc ions that could regulate PKCγ and Raf-1 binding to HINT1 (36). Nonetheless, tight control of zinc is required to maintain the redox homeostasis, and it must be rapidly buffered to reestablish the high picomolar/low nanomolar concentrations typically found in neurons (5, 7, 24). Certainly, the zinc concentrations used in the in vitro assays do not necessarily reproduce the physiologic conditions responsible for NO- and zinc-dependent recruitment of PKCγ and Raf-1 to MOR-HINT1 complex. It is more likely that such effects are achieved at mid-nanomolar concentrations of zinc. However, the use of low micromolar concentrations in vitro permitted better immunodetection and the pharmacologic characterization of the signaling proteins under study.
In summary, morphine promotes the recruitment of the Ras/Raf-1/ERK1/2 signaling cassette, and of PKCγ, to the HINT1-MOR complex. The CRDs in the N terminal regulatory region of the Raf-1 and PKCγ serine/threonine kinases are those implicated in their zinc-dependent binding to HINT1 protein. The homodimeric structure of HINT1 supports the simultaneous binding of these kinases, enabling the cross-talk of these two signaling pathways under the regulation of the MOR. In this compartment, PKCγ exerts a fine degree of control over Raf-1 and the subsequent activation of ERK1/2. The morphine-activated MOR/PI3K/Akt/nNOS pathway provides the NO necessary to release the free zinc ions implicated in the binding of PKCγ and Raf-1 to the MOR–HINT1 complex. Thus, our data unveil a redox mechanism by which the MOR, via NO and zinc ions, regulates the ERK1/2 cascade in neural cells.
Abbreviations Used
- CRD
cysteine-rich domain
- DAG
diacylglycerol
- ERK
extracellular signal-regulated kinase
- GPCR
G protein–coupled receptor
- HINT1
histidine triad nucleotide-binding protein 1
- ICV
intracerebroventricular
- MAPK
mitogen-activated protein kinase
- MOR
mu opioid receptor
- NMDAR
N-methyl-d-aspartate receptor
- nNos
neural nitric oxide synthase
- NO
nitric oxide
- NOR-3
(±)-(E)-ethyl-2-[(E)-hydroxyimino]-5-nitro-3-hexeneamide
- PAG
periaqueductal gray matter
- PKC
protein kinase C
- Rap/Ras
low-molecular-weight G proteins
- RBD
Ras-binding domain
- SNAP
(S)-nitroso-N-acetylpenicillamine
- Src/Fyn
nonreceptor tyrosine kinase
- TPEN
N,N,N′,N′-tetrakis(2-pyridylmethyl) ethylenediamine
Acknowledgments
This research was supported by the Instituto de Salud Carlos III PI08-0417 (PSB) and PS09/00332 (JG). MRM is currently supported by a CIBERSAM contract. ETM is a predoctoral fellow supported by the Spanish Ministry of Science and Innovation. We thank Concha Bailón and Beatriz Fraile for their excellent technical assistance.
Author Disclosure Statement
The authors declare that, except for income received from our primary employer, “Ministerio de Ciencia y Tecnología,” no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service, and no personal financial holdings could be perceived as constituting a potential conflict of interest.
References
- 1.Buday L. Downward J. Many faces of Ras activation. Biochim Biophys Acta. 2008;1786:178–187. doi: 10.1016/j.bbcan.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 2.Chen L. Huang LY. Sustained potentiation of NMDA receptor-mediated glutamate responses through activation of protein kinase C by a μ opioid. Neuron. 1991;7:319–326. doi: 10.1016/0896-6273(91)90270-a. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y. Sommer C. The role of mitogen-activated protein kinase (MAPK) in morphine tolerance and dependence. Mol Neurobiol. 2009;40:101–107. doi: 10.1007/s12035-009-8074-z. [DOI] [PubMed] [Google Scholar]
- 4.Clark GJ. Drugan JK. Rossman KL. Carpenter JW. Rogers-Graham K. Fu H. Der CJ. Campbell SL. 14-3-3 zeta negatively regulates raf-1 activity by interactions with the Raf-1 cysteine-rich domain. J Biol Chem. 1997;272:20990–20993. doi: 10.1074/jbc.272.34.20990. [DOI] [PubMed] [Google Scholar]
- 5.Colvin RA. Holmes WR. Fontaine CP. Maret W. Cytosolic zinc buffering and muffling: their role in intracellular zinc homeostasis. Metallomics. 2010;2:306–317. doi: 10.1039/b926662c. [DOI] [PubMed] [Google Scholar]
- 6.Daub M. Jockel J. Quack T. Weber CK. Schmitz F. Rapp UR. Wittinghofer A. Block C. The RafC1 cysteine-rich domain contains multiple distinct regulatory epitopes which control Ras-dependent Raf activation. Mol Cell Biol. 1998;18:6698–6710. doi: 10.1128/mcb.18.11.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erickson JC. Hollopeter G. Thomas SA. Froelick GJ. Palmiter RD. Disruption of the metallothionein-III gene in mice: analysis of brain zinc, behavior, and neuron vulnerability to metals, aging, and seizures. J Neurosci. 1997;17:1271–1281. doi: 10.1523/JNEUROSCI.17-04-01271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frederickson CJ. Koh JY. Bush AI. The neurobiology of zinc in health and disease. Nat Rev Neurosci. 2005;6:449–462. doi: 10.1038/nrn1671. [DOI] [PubMed] [Google Scholar]
- 9.Garzón J. Rodríguez-Muñoz M. Sánchez-Blázquez P. Do pharmacological approaches that prevent opioid tolerance target different elements in the same regulatory machinery? Curr Drug Abuse Rev. 2008;1:222–238. doi: 10.2174/1874473710801020222. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh S. Xie WQ. Quest AF. Mabrouk GM. Strum JC. Bell RM. The cysteine-rich region of raf-1 kinase contains zinc, translocates to liposomes, and is adjacent to a segment that binds GTP-ras. J Biol Chem. 1994;269:10000–10007. [PubMed] [Google Scholar]
- 11.Ji RR. Gereau RW. Malcangio M. Strichartz GR. MAP kinase and pain. Brain Res Rev. 2009;60:135–148. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kazanietz MG. Lewin NE. Bruns JD. Blumberg PM. Characterization of the cysteine-rich region of the Caenorhabditis elegans protein Unc-13 as a high affinity phorbol ester receptor: analysis of ligand-binding interactions, lipid cofactor requirements, and inhibitor sensitivity. J Biol Chem. 1995;270:10777–10783. doi: 10.1074/jbc.270.18.10777. [DOI] [PubMed] [Google Scholar]
- 13.Kieffer BL. Gaveriaux-Ruff C. Exploring the opioid system by gene knockout. Prog Neurobiol. 2002;66:285–306. doi: 10.1016/s0301-0082(02)00008-4. [DOI] [PubMed] [Google Scholar]
- 14.Kissin I. Bright CA. Bradley EL., Jr The effect of ketamine on opioid-induced acute tolerance: can it explain reduction of opioid consumption with ketamine-opioid analgesic combinations? Anesth Analg. 2000;91:1483–1488. doi: 10.1097/00000539-200012000-00035. [DOI] [PubMed] [Google Scholar]
- 15.Knott AB. Bossy-Wetzel E. Nitric oxide in health and disease of the nervous system. Antioxid Redox Signal. 2009;11:541–554. doi: 10.1089/ars.2008.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolch W. Heidecker G. Kochs G. Hummel R. Vahidi H. Mischak H. Finkenzeller G. Marme D. Rapp UR. Protein kinase C alpha activates RAF-1 by direct phosphorylation. Nature. 1993;364:249–252. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- 17.Korichneva I. Zinc dynamics in the myocardial redox signaling network. Antioxid Redox Signal. 2006;8:1707–1721. doi: 10.1089/ars.2006.8.1707. [DOI] [PubMed] [Google Scholar]
- 18.Kroncke KD. Zinc finger proteins as molecular targets for nitric oxide-mediated gene regulation. Antioxid Redox Signal. 2001;3:565–575. doi: 10.1089/15230860152542934. [DOI] [PubMed] [Google Scholar]
- 19.Kroncke KD. Klotz LO. Zinc fingers as biologic redox switches? Antioxid Redox Signal. 2009;11:1015–1027. doi: 10.1089/ARS.2008.2269. [DOI] [PubMed] [Google Scholar]
- 20.Leicht DT. Balan V. Kaplun A. Singh-Gupta V. Kaplun L. Dobson M. Tzivion G. Raf kinases: function, regulation and role in human cancer. Biochim Biophys Acta. 2007;1773:1196–1212. doi: 10.1016/j.bbamcr.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leslie NR. The redox regulation of PI 3-kinase-dependent signaling. Antioxid Redox Signal. 2006;8:1765–1774. doi: 10.1089/ars.2006.8.1765. [DOI] [PubMed] [Google Scholar]
- 22.Li Y. Hough CJ. Suh SW. Sarvey JM. Frederickson CJ. Rapid translocation of Zn(2+) from presynaptic terminals into postsynaptic hippocampal neurons after physiological stimulation. J Neurophysiol. 2001;86:2597–2604. doi: 10.1152/jn.2001.86.5.2597. [DOI] [PubMed] [Google Scholar]
- 23.Lima CD. Klein MG. Weinstein IB. Hendrickson WA. Three-dimensional structure of human protein kinase C interacting protein 1, a member of the HIT family of proteins. Proc Natl Acad Sci U S A. 1996;93:5357–5362. doi: 10.1073/pnas.93.11.5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maret W. Zinc coordination environments in proteins as redox sensors and signal transducers. Antioxid Redox Signal. 2006;8:1419–1441. doi: 10.1089/ars.2006.8.1419. [DOI] [PubMed] [Google Scholar]
- 25.Moodie SA. Willumsen BM. Weber MJ. Wolfman A. Complexes of Ras.GTP with Raf-1 and mitogen-activated protein kinase kinase. Science. 1993;260:1658–1661. doi: 10.1126/science.8503013. [DOI] [PubMed] [Google Scholar]
- 26.Mott HR. Carpenter JW. Zhong S. Ghosh S. Bell RM. Campbell SL. The solution structure of the Raf-1 cysteine-rich domain: a novel ras and phospholipid binding site. Proc Natl Acad Sci U S A. 1996;93:8312–8317. doi: 10.1073/pnas.93.16.8312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newton AC. Protein kinase C: structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem Rev. 2001;101:2353–2364. doi: 10.1021/cr0002801. [DOI] [PubMed] [Google Scholar]
- 28.Okada T. Hu CD. Jin TG. Kariya K. Yamawaki-Kataoka Y. Kataoka T. The strength of interaction at the Raf cysteine-rich domain is a critical determinant of response of Raf to Ras family small GTPases. Mol Cell Biol. 1999;19:6057–6064. doi: 10.1128/mcb.19.9.6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polakiewicz RD. Schieferl SM. Dorner LF. Kansra V. Comb MJ. A mitogen-activated protein kinase pathway is required for mu-opioid receptor desensitization. J Biol Chem. 1998;273:12402–12406. doi: 10.1074/jbc.273.20.12402. [DOI] [PubMed] [Google Scholar]
- 30.Quilliam LA. Rebhun JF. Castro AF. A growing family of guanine nucleotide exchange factors is responsible for activation of Ras-family GTPases. Prog Nucleic Acid Res Mol Biol. 2002;71:391–444. doi: 10.1016/s0079-6603(02)71047-7. [DOI] [PubMed] [Google Scholar]
- 31.Rodríguez-Muñoz M. de la Torre-Madríd E. Sánchez-Blázquez P. Garzón J. Morphine induces endocytosis of neuronal mu-opioid receptors through the sustained transfer of Galpha subunits to RGSZ2 proteins. Mol Pain. 2007;3:19. doi: 10.1186/1744-8069-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodríguez-Muñoz M. de lT-M. Sánchez-Blázquez P. Wang JB. Garzón J. NMDAR-nNOS generated zinc recruits PKCgamma to the HINT1-RGS17 complex bound to the C terminus of Mu-opioid receptors. Cell Signal. 2008;20:1855–1864. doi: 10.1016/j.cellsig.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 33.Rodríguez-Muñoz M. Sánchez-Blázquez P. Vicente-Sánchez A. Bailón C. Martín-Aznar B. Garzón J. The histidine triad nucleotide-binding protein 1 supports mu-opioid receptor-glutamate NMDA receptor cross-regulation. Cell Mol Life Sci. 2010 doi: 10.1007/s00018-010-0598-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sabel CE. Neureuther JM. Siemann S. A spectrophotometric method for the determination of zinc, copper, and cobalt ions in metalloproteins using Zincon. Anal Biochem. 2010;397:218–226. doi: 10.1016/j.ab.2009.10.037. [DOI] [PubMed] [Google Scholar]
- 35.Sánchez-Blázquez P. Rodríguez-Muñoz M. de la Torre-Madríd E. Garzón J. Brain-specific Gαz interacts with Src tyrosine kinase to regulate Mu-opioid receptor-NMDAR signaling pathway. Cell Signal. 2009;21:1444–1454. doi: 10.1016/j.cellsig.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Sánchez-Blázquez P. Rodríguez-Muñoz M. Garzón J. Mu-opioid receptors transiently activate the Akt-nNOS pathway to produce sustained potentiation of PKC-mediated NMDAR-CaMKII signaling. PLoS One. 2010;5:e11278. doi: 10.1371/journal.pone.0011278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sánchez-Blázquez P. Rodríguez-Muñoz M. Montero C. de la Torre-Madrid E. Garzón J. Calcium/calmodulin-dependent protein kinase II supports morphine antinociceptive tolerance by phosphorylation of glycosylated phosducin-like protein. Neuropharmacology. 2008;54:319–330. doi: 10.1016/j.neuropharm.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt H. Schulz S. Klutzny M. Koch T. Handel M. Hollt V. Involvement of mitogen-activated protein kinase in agonist-induced phosphorylation of the mu-opioid receptor in HEK 293 cells. J Neurochem. 2000;74:414–422. doi: 10.1046/j.1471-4159.2000.0740414.x. [DOI] [PubMed] [Google Scholar]
- 39.Seo SR. Chong SA. Lee SI. Sung JY. Ahn YS. Chung KC. Seo JT. Zn2+ -induced ERK activation mediated by reactive oxygen species causes cell death in differentiated PC12 cells. J Neurochem. 2001;78:600–610. doi: 10.1046/j.1471-4159.2001.00438.x. [DOI] [PubMed] [Google Scholar]
- 40.Su T. Suzui M. Wang L. Lin CS. Xing WQ. Weinstein IB. Deletion of histidine triad nucleotide-binding protein 1/PKC-interacting protein in mice enhances cell growth and carcinogenesis. Proc Natl Acad Sci U S A. 2003;100:7824–7829. doi: 10.1073/pnas.1332160100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trujillo KA. Akil H. Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science. 1991;251:85–87. doi: 10.1126/science.1824728. [DOI] [PubMed] [Google Scholar]
- 42.Van Der Hoeven PC. Van Der Wal JC. Ruurs P. Van Dijk MC. Van BJ. 14-3-3 isotypes facilitate coupling of protein kinase C-zeta to Raf-1: negative regulation by 14-3-3 phosphorylation. Biochem J. 2000;345:297–306. doi: 10.1042/0264-6021:3450297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vetter IR. Wittinghofer A. The guanine nucleotide-binding switch in three dimensions. Science. 2001;294:1299–1304. doi: 10.1126/science.1062023. [DOI] [PubMed] [Google Scholar]
- 44.Warne PH. Viciana PR. Downward J. Direct interaction of Ras and the amino-terminal region of Raf-1 in vitro. Nature. 1993;364:352–355. doi: 10.1038/364352a0. [DOI] [PubMed] [Google Scholar]
- 45.Weiss JH. Sensi SL. Koh JY. Zn(2+): a novel ionic mediator of neural injury in brain disease. Trends Pharmacol Sci. 2000;21:395–401. doi: 10.1016/s0165-6147(00)01541-8. [DOI] [PubMed] [Google Scholar]
- 46.Winkler DG. Cutler RE., Jr Drugan JK. Campbell S. Morrison DK. Cooper JA. Identification of residues in the cysteine-rich domain of Raf-1 that control Ras binding and Raf-1 activity. J Biol Chem. 1998;273:21578–21584. doi: 10.1074/jbc.273.34.21578. [DOI] [PubMed] [Google Scholar]
- 47.Wu W. Wang X. Zhang W. Reed W. Samet JM. Whang YE. Ghio AJ. Zinc-induced PTEN protein degradation through the proteasome pathway in human airway epithelial cells. J Biol Chem. 2003;278:28258–28263. doi: 10.1074/jbc.M303318200. [DOI] [PubMed] [Google Scholar]
- 48.Yun HY. Gonzalez-Zulueta M. Dawson VL. Dawson TM. Nitric oxide mediates N-methyl-D-aspartate receptor-induced activation of p21ras. Proc Natl Acad Sci U S A. 1998;95:5773–5778. doi: 10.1073/pnas.95.10.5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zago MP. Mackenzie GG. Adamo AM. Keen CL. Oteiza PI. Differential modulation of MAP kinases by zinc deficiency in IMR-32 cells: role of H(2)O(2) Antioxid Redox Signal. 2005;7:1773–1782. doi: 10.1089/ars.2005.7.1773. [DOI] [PubMed] [Google Scholar]
- 50.Zhu JJ. Qin Y. Zhao M. Van AL. Malinow R. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell. 2002;110:443–455. doi: 10.1016/s0092-8674(02)00897-8. [DOI] [PubMed] [Google Scholar]