Abstract
Background
Interleukin-13 Receptor α2 (IL-13Rα2) is a tumor-associated antigen and target for cancer therapy. Since IL-13Rα2 is heterogeneously overexpressed in a variety of human cancers, it would be highly desirable to uniformly upregulate IL-13Rα2 expression in tumors for optimal targeting.
Methods
We examined epigenetic regulation of IL-13Rα2 in a murine model of human pancreatic cancer by Bisulfite-PCR, sequencing for DNA methylation and chromatin immunoprecipitation for histone modification. Reverse transcription-PCR was performed for examining changes in IL-13Rα2 mRNA expression after treatment with histone deacetylase (HDAC) and c-jun inhibitors. In vitro cytotoxicity assays and in vivo testing in animal tumor models were performed to determine whether HDAC inhibitors could enhance anti-tumor effects of IL-13-PE in pancreatic cancer. Mice harboring subcutaneous tumors were treated with HDAC inhibitors systemically and IL-13-PE intratumorally.
Results
We found that CpG sites in IL-13Rα2 promoter region were not methylated in all pancreatic cancer cell lines studied including IL-13Rα2-positive and IL-13Rα2-negative cell lines and normal cells. On the other hand, histones at IL-13Rα2 promoter region were highly-acetylated in IL-13Rα2-positive but much less in receptor-negative pancreatic cancer cell lines. When cells were treated with HDAC inhibitors, not only histone acetylation but also IL-13Rα2 expression was dramatically enhanced in receptor-negative pancreatic cancer cells. In contrast, HDAC inhibition did not increase IL-13Rα2 in normal cell lines. In addition, c-jun in IL-13Rα2-positive cells was expressed at higher level than in negative cells. Two types of c-jun inhibitors prevented increase of IL-13Rα2 by HDAC inhibitors. HDAC inhibitors dramatically sensitized cancer cells to immunotoxin in the cytotoxicity assay in vitro and increased IL-13Rα2 in the tumors subcutaneously implanted in the immunodeficient animals but not in normal mice tissues. Combination therapy with HDAC inhibitors and immunotoxin synergistically inhibited growth of not only IL-13Rα2-positive but also IL-13Rα2-negative tumors.
Conclusions
We have identified a novel function of histone modification in the regulation of IL-13Rα2 in pancreatic cancer cell lines in vitro and in vivo. HDAC inhibition provides a novel opportunity in designing combinatorial therapeutic approaches not only in combination with IL-13-PE but with other immunotoxins for therapy of pancreatic cancer and other cancers.
Introduction
Interleukin-13 Receptor α2 (IL-13Rα2) is a high affinity receptor for the Th2 derived cytokine IL-13 and a known cancer testis antigen [1,2]. IL-13Rα2 is over expressed in a variety of human cancers including malignant glioma, head and neck cancer, Kaposi's sarcoma, renal cell carcinoma, and ovarian carcinoma [3-7]. We have demonstrated previously that IL-13Rα2 can be effectively targeted by a recombinant immunotoxin, consisting of IL-13 and truncated pseudomonas exotoxin (IL-13-PE) [8-11]. IL-13-PE is highly cytotoxic to tumor cells in vitro and in vivo that express high levels of IL-13Rα2 [12]. Several phase I and II clinical trials, and one phase III clinical trial, evaluating the safety, tolerability, and efficacy of this agent have been completed in patients with recurrent glioblastoma multiforme [13,14]. Most recently, we have demonstrated expression of IL-13Rα2 in human pancreatic ductal adenocarcinoma [15]. Seventy-one percent of pancreatic tumors overexpressed IL-13Rα2 chain. Pancreatic tumors were also successfully targeted by IL-13-PE in an animal model of human cancer [15,16]. Thus, IL-13Rα2 is currently being assessed as a cancer therapy in a variety of preclinical and clinical trials [4,17,18]
The significance of IL-13Rα2 expression in cancer is not known and the mechanism of its upregulation is still not clear. Epigenetic mechanisms such as DNA methylation and histone modification are known to be involved in many disease pathogenesis including cancer [19]. DNA methylation occurs on cytosines that are followed by guanines (CpG dinucleotides) and is usually associated with gene silencing [20]. Histones are modified at several different amino acid residues and with many different modifications including methylation, acetylation, phosphorylation and ubiquitination. Some lysine residues can either be methylated or acetylated, and there are three different possibilities for each methylated site [21]. Histone modification can be transiently altered by the cell environment [22]. Mainly, gene expression is activated by histone acetylation and decreased by methylation. Histone acetylation induced by histone acetyltransferase (HAT) is associated with gene transcription, while histone hypoacetylation induced by histone deacetylase (HDAC) is associated with gene silencing [23].
HDAC inhibition results in increased acetylation in histones and causes over expression of some genes. HDAC inhibitors are grouped into various classes based on their structures [24]. Trichostatin A (TSA), suberoylanilide hydroxamic acid (SAHA), and sodium butyrate (NaB) are commonly studied HDAC inhibitors. These inhibitors induce cell growth arrest and apoptosis in a broad spectrum of transformed cells [25]. Because of these characteristics, HDAC inhibitors are being tested in the clinic for cancer therapy. Two HDAC inhibitors, SAHA and Romidepsin, are licensed by FDA for the treatment of cutaneous T-cell lymphoma [26].
In the present study, we have examined the epigenetic regulation of the IL-13Rα2 gene in pancreatic cancer cell lines and investigated whether the IL-13Rα2 gene can be modulated by epigenetic mechanisms. We have also examined the effect of HDAC inhibitors on IL-13Rα2 expression. We demonstrate for the first time that three different HDAC inhibitors dramatically upregulate IL-13Rα2 in pancreatic cancer cell lines expressing no or low levels of IL-13Rα2. These inhibitors also modestly upregulated IL-13Rα2 in cells expressing higher levels of IL-13Rα2. More importantly, HDAC inhibitors sensitized pancreatic tumor cells to IL-13-PE and mediated enhanced sensitivity even though these cells did not naturally express IL-13Rα2. A combination therapy of HDAC inhibitors and IL-13-PE demonstrated a pronounced anti-tumor effect in human tumor bearing immunodeficient mice indicating a synergistic impact on tumor response. Thus, a novel combination of HDAC inhibitors and IL-13-PE may have a prominent role in pancreatic cancer or other cancer therapies in the clinic.
Materials and methods
Cell culture and reagents
Pancreatic cancer cell lines and human umbilical vein endothelial cell line (HUVEC) were obtained from the American Type Culture Collection (Manassas, VA). Human normal gingival fibroblasts (HGF) was obtained from Sciencell (San Diego, CA) and human pancreatic ductal epithelial cells (HPE) from Cell Systems (Kirkland, WA). Renal cell carcinoma (PM-RCC) cell line was developed in our laboratory [4]. Recombinant IL-13-PE was produced and purified in our laboratory [9,11,27]. Trichostatin A (TSA), sodium butyrate (NaB) and SP600125 were purchased from Sigma-Aldrich (St. Louis, MO). SR11302 was purchased from Tocris Bioscience (Ellisville, MO). Suberoylanilide Hydroxamic Acid (SAHA) was purchased from Selleck (Houston, TX).
Reverse transcription-PCR
Quantitative reverse transcription-PCR (qRT-PCR) and RT-PCR were performed as described previously [28,29] using a SYBR 1 reagent kit (Bio-Rad, Hercules, CA). Mouse IL-13Rα2 and β-actin primers were purchased from QIAGEN (Valencia, CA). Gene expression was normalized to β-actin before the fold change in gene expression was determined.
Chromatin immunoprecipitation (ChIP) assays
ChIP assays were performed using a ChIP assay kit (Millipore, Billerica, MA). To cross-link DNA with chromatin, 1 × 106 cells were incubated for 5 min in 1% formaldehyde at 37°C. The cells were harvested, washed with phosphate buffered saline (PBS), resuspended in lysis buffer and 200-1000 bp fragments of DNA from chromatin were prepared as recommended by the manufacturer. One hundredth of the resultant solution was used as an internal control. The remainder was immunoprecipitated for 16 hours at 4°C using anti-acetylated histone H3 and anti-acetylated histone H4 antibodies (Millipore, Billerica, MA). The precipitated immune complexes were recovered using protein A-agarose, and then purified using QIAamp DNA mini kit (QIAGEN). Samples were analyzed by qPCR to determine a ratio of histone acetylation at the IL-13Rα2 promoter site using propriety primers Hs04516601_cn for IL-13Rα2 gene and RNase P/TERT reference copy number primers after following the manufacturer's instructions (Applied Biosystems, Foster City, CA).
Bisulfite-PCR and sequencing
Bisulfite sequencing was performed using CpGenome Fast DNA Modification Kit (Millipore, Billerica, MA). Briefly, 1 μg of genome DNA was incubated for 16 hours at 50°C with sodium bisulfite solution. The modified DNA was purified by DNA binding column. The promoter region of IL-13Rα2 gene was amplified by PCR using specific primer pairs, FW: 5'-TTGGGGAGAAAGAGAGATTTG-3', and BW: 5'-CAAACTTACCCCACCCAAAA-3'. The PCR products were cloned into pCR2.1 vector using a TOPO-cloning KIT (Invitrogen, Carlsbad, CA) and sequenced using an ABI377 automated sequencer. At least 10 clones were sequenced for each cell line.
AP-1 activation assay
Nuclear extracts from cell lines were collected using the Transfactor Extract Kit (Active Motif, Carlsbad, CA) and tested for DNA binding activity using the AP-1 family TransAM Kit (Active Motif) according to the manufacturer's instructions [28].
Immunohistochemistry (IHC) and Immunocytochemistry (ICC)
Expression of human and mouse IL-13Rα2 protein in pancreatic cancer cell lines and mouse organs was observed by indirect immunofluorescence-immunostaining as described previously [28,30] using anti-mouse monoclonal and anti-human IL-13Rα2 polyclonal antibodies (R&D, Minneapolis, MN). Tissue samples were fixed in 10% formalin solution for IHC and human cells were fixed by 4% paraformaldehyde (PFA) for ICC. The nucleus was counterstained by DAPI.
IL-13Rα2 gene knockdown by RNA interference
Retrovirus-mediated RNA interference was performed using the pSuper RNAi system (Oligoengine, Seattle, WA) following the manufacturer's instructions as described previously [16,28].
Protein synthesis inhibition assay
In vitro cytotoxic activity of IL-13 cytotoxin (IL-13-PE) was measured by the inhibition of protein synthesis as described earlier [11]. All assays were performed in quadruplicate and data are shown as mean ± SD.
Tumor xenograft studies
Panc-1 and ASPC-1 cells (2 × 106) were injected s.c. in the left flank of female athymic nude mice. From day 4 after tumor implantation, 5 mg/kg TSA was subcutaneously (s.c.) injected every alternative days or 25 mg/kg SAHA were intraperitoneally (i.p.) injected daily for 14 days. From day 5, 50 or 100 μg/kg IL-13-PE or PBS/0.2% human serum albumin (vehicle) were intratumorally (i.t.) injected daily for 14 days. Mice body weight and tumor size was measured every 4-7 days from day 4. Measurement was continued until more than one tumor reached 20 mm in diameter in each group. Their appearances were observed through out the entire experiment for detecting toxic side effects from the treatment. Animal studies were conducted under an approved protocol in accordance with the principles and procedures outlined in the NIH Guide for the Care and Use of Laboratory Animals.
Statistical analysis
The data were analyzed for statistical significance using Student's t test for comparison between two groups and ANOVA among more than two groups. All experiments including the animal model were repeated at least twice.
Results
IL-13Rα2 expression in pancreatic cancer cell lines
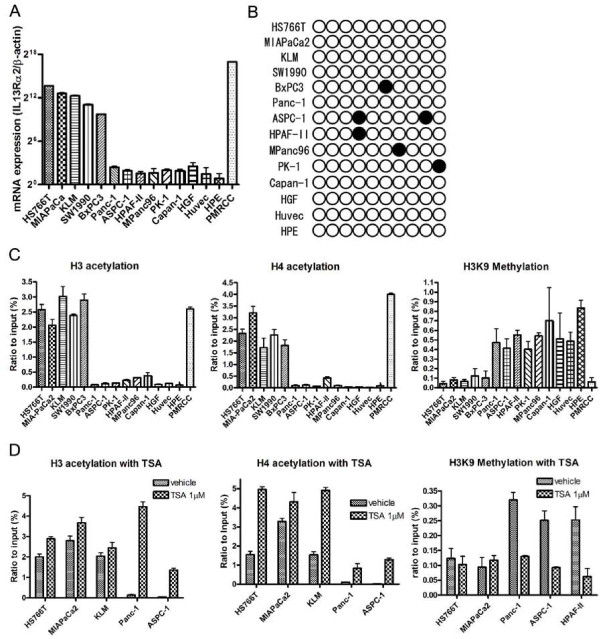
Eleven pancreatic cancer cell lines and three types of normal cell lines (fibroblast, umbilical vein endothelial cells and pancreatic ductal epithelial cells) were examined for IL-13Rα2 expression. qRT-PCR analysis identified five pancreatic cancer cell lines (HS766T, MIAPaCa2, KLM, SW1990 and BxPC3), which expressed high levels of IL-13Rα2 mRNA, and six cell lines (Panc-1, ASPC-1, HPAF-II, Mpanc96, PK-1 and Capan-1) expressed low levels IL-13Rα2 mRNA (negative cell line) (Figure 1A). All three normal cell lines showed extremely low levels of IL-13Rα2 mRNA. We also examined IL-13Rα2 protein expression in these cell lines by flow-cytometric analysis using monoclonal antibody to IL-13Rα2. These results essentially corroborated the mRNA results (data not shown) [15,31].
Figure 1.
IL-13Rα2 expression in pancreatic cancer and normal cell lines and DNA methylation and Histone modification of IL-13Rα2 promoter. A, qRT-PCR for IL-13Rα2 expression in pancreatic cancer and normal cell lines was performed. Data shown is ratio of human IL-13Rα2/β-actin expression and multiplied by 222 for convenience. Bars, SD of triplicate determinations. B, Bisulfite-sequencing of IL-13Rα2 promoter. Only one CpG site is present within the IL-13Rα2 promoter region. Methylated and unmethylated alleles are shown as solid and open circles, respectively. C, Acetylation and methylation status of histones H3 and H4 in pancreatic cancer and normal cell lines. The region around the IL-13Rα2 promoter was amplified by qPCR after ChIP using anti-acetylated histone H3 and H4 antibody and anti-methylated H3K9. Results were standardized by amplification of the IL-13Rα2 promoter using DNA before precipitation (Input). D, Acetylation and methylation status of histones H3 and H4 after incubation with TSA. Cells were incubated with 1 μM TSA or vehicle for 24 hours and fixed by 1% PFA. Results were standardized using DNA before precipitation.
Mutation analysis of IL-13Rα2 cDNA
We investigated whether there were gene sequence changes in the IL-13Rα2 gene by performing sequencing of IL-13Rα2 cDNA. However, no mutations were detected in any pancreatic cancer cell lines studied (data not shown).
DNA methylation in IL-13Rα2 promoter
We next examined any epigenetic changes in IL-13Rα2 gene. Since there is only one CpG site in the IL-13Rα2 promoter region, we examined DNA methylation at this site [32]. We picked more than 10 independent clones for analysis. In at least 80% of the clones tested from all cell lines including three normal cell lines, no methylation was detected (Figure 1B). As a control, we also studied DNA methylation of other CpG sites located ~100 bases upstream from the IL-13Rα2 promoter region. In contrast to the CpG in the IL-13Rα2 promoter region, the distant CpG site showed methylation in all cell lines (Supplementary Figure 1).
Regulation of histone acetylation and methylation in IL-13Rα2 promoter region
We also examined histone acetylation of the IL-13Rα2 promoter region using a chromatin-immunoprecipitation technique (ChIP). In all IL-13Rα2-positive pancreatic cell lines, histone H3 was highly acetylated compared to IL-13Rα2-negative and normal cell lines (Figure 1C). Similar acetylation results were observed for histone H4. In sharp contrast, the methylation status at the H3K9 site, which is a site for transcriptional repression, was high in IL-13Rα2-negative cell lines compared to IL-13Rα2-positive cell lines (Figure 1C).
Next, we examined the effect of histone acetylation inhibition by HDAC inhibitors on IL-13Rα2 expression. When pancreatic cancer lines expressing undetectable levels of IL-13Rα2 were treated with TSA, histone H3 and H4 acetylation was dramatically increased. TSA also increased acetylation in pancreatic cancer cells expressing high levels of IL-13Rα2 but this increase was less dramatic (Figure 1D). In contrast, TSA caused a significant decrease in H3K9 methylation in pancreatic cancer cells with undetectable levels of IL-13Rα2 expression but no change in high IL-13Rα2 expressing cell lines (Figure 1D).
Histone deacetylation inhibition increases IL-13Rα2 expression in pancreatic cancer cell lines
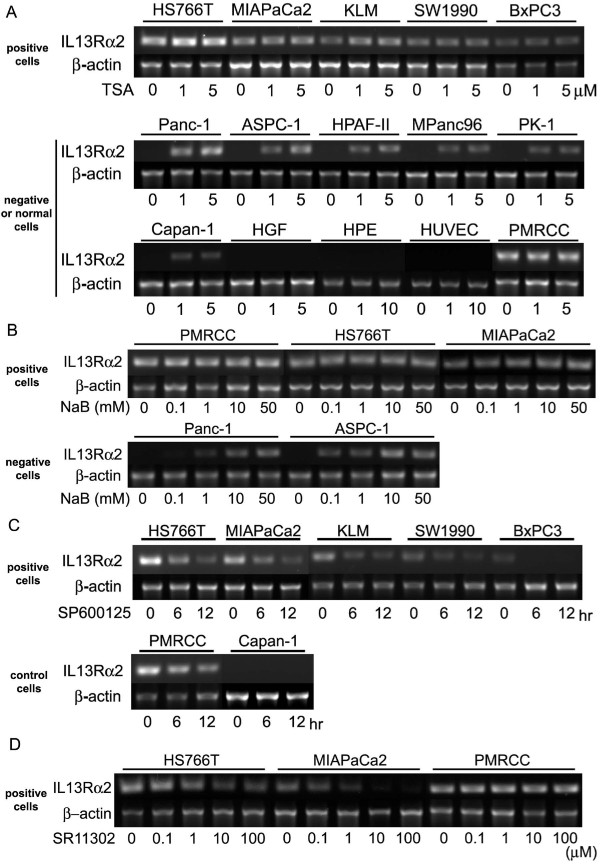
As the relationship between histone acetylation and IL-13Rα2 expression levels was observed, we tested whether HDAC inhibitors can modulate IL-13Rα2 expression in pancreatic cancer cell lines. Interestingly, similar to histone acetylation, TSA treatment resulted in increased IL-13Rα2 mRNA expression in pancreatic cancer cell lines that normally have undetectable levels of IL-13Rα2 expression, while no changes were seen in cells expressing high levels of IL-13Rα2 mRNA or normal cell lines (Figure 2A). Similar results were obtained with another HDAC inhibitor, sodium butyrate (NaB) (Figure 2B).
Figure 2.
Regulation of IL-13Rα2 expression by HDAC and AP-1 inhibitors. A, Conventional RT-PCR of IL-13Rα2 mRNA after incubation with TSA. Cells were incubated with 1 or 5 μM TSA for 24 hours and total RNA was extracted. PM-RCC cells were used as a positive control. β-actin is shown as a reference gene. B, Conventional RT-PCR of IL-13Rα2 after incubation with NaB. Cells were incubated with 0 - 50 mM NaB for 24 hours and total RNA extracted. C, Conventional RT-PCR of IL-13Rα2 gene after incubation with SP600125. Cells were incubated with 10 μM SP600125 for 6 or 12 hours and total RNA extracted. D, Conventional RT-PCR of IL-13Rα2 after incubation with AP-1 inhibitor, SR11302. Cells were incubated with 0 - 100 μM SR11302 for 12 hours and total RNA extracted.
Role of AP-1 transcription factor activity in IL-13Rα2 regulation in pancreatic cancer cell lines
To determine the mechanism of the differential effect of HDAC inhibition in cells expressing undetectable levels of IL-13Rα2, we examined whether the transcription factor (AP-1) is activated in these cell lines as reported by Wu et al. [32]. We found that pancreatic cancer cell lines that highly express IL-13Rα2 (HS766T, MIAPaCa2, and KLM), and those which express undetectable levels (Panc-1 and ASPC-1), both show high c-jun activity (Supplementary Figure 2A). In contrast, normal cell lines showed low c-jun activity. We did not observe any significant differences in c-Fos activity, another AP-1 member (Supplementary Figure 2B) between cancer and normal cell lines.
Interestingly, when high IL-13Rα2-expressing cells were treated with the c-jun N-terminal kinase inhibitor, SP600125, IL-13Rα2 expression decreased (Figure 2C), whereas SP600125 had no effect on cells expressing undetectable levels of IL-13Rα2. Another pan-AP-1 inhibitor, SR11302, also decreased IL-13Rα2 expression in IL-13Rα2 expressing cell lines in a concentration-dependent manner (Figure 2D). The effects of TSA and SP600125 on IL-13Rα2 protein expression in pancreatic cancer cells were also analyzed by IHC. IL-13Rα2 protein levels were also found to increase in the presence of TSA and decrease in the presence of SP600125. In addition, SP600125 prevented the increase of IL-13Rα2 protein by TSA (Figure 3A).
Figure 3.
Modulation of IL-13Rα2 protein by HDAC and AP-1 inhibitors and stability of IL-13Rα2 expression. A, ICC of IL-13Rα2 after incubation with TSA and SP600125 is shown. Cells were incubated with 1 μM TSA and/or 10 μM SP600125 for 24 hours and fixed by 4% PFA. IL-13Rα2 was visualized by Alexa488. Recovery of IL-13Rα2 expression after incubation with TSA (B) and SP600125 (C). Cells were incubated with 1 μM TSA or SP600125 for 24 hours or 12 hours, respectively and then inhibitors were removed by replacing with new medium without TSA for 1-5 days or SP600125 for 12-48 hours. IL-13Rα2 gene expression was determined by conventional RT-PCR.
Stability of upregulated IL-13Rα2 expression by HDAC inhibitor
We examined the stability of upregulated IL-13Rα2 expression in IL-13Rα2-expressing and negative pancreatic cancer cell lines when treated with HDAC inhibitor. After treatment with TSA and SP600125 for 24 hours, the drugs were removed and cell culture was continued. IL-13Rα2 expression was still elevated 3 days after TSA removal in IL-13Rα2 undetectable cell lines (Figure 3B). In contrast, in IL-13Rα2 positive cell lines, IL-13Rα2 expression returned to pre-treatment levels within 24 hours following SP600125 removal (Figure 3C).
HDAC inhibition increases IL-13 induced matrix metalloproteinases via IL-13Rα2 upregulation
As we have shown that IL-13 can upregulate Matrix metalloproteinases (MMPs) expression in IL-13Rα2 expressing pancreatic cancer cell lines [28], we investigated the impact of IL-13Rα2 upregulation by HDAC inhibitors by examining IL-13 induced MMPs expression. TSA treatment increased mRNA expression for MMPs through upregulation of IL-13Rα2 after treatment with IL-13 in two IL-13Rα2 negative cell lines (Figure 4A). Interestingly, when IL-13 signaling was blocked by an inhibitor of the AP-1 pathway (SP600125), it prevented the increase in MMPs expression by TSA. Thus, MMPs expression showed a positive correlation with IL-13Rα2 expression in IL-13 treated cells.
Figure 4.
HDAC inhibitor inhibits MMPs expression activated by IL-13 through induction of IL-13Rα2. A, Conventional RT-PCR for expression of MMPs was performed after cells were incubated with 1 μM TSA and/or 10 μM SP600125 for 24 hours. Twenty-two hours prior to harvesting cells, IL-13 was added to the cultured medium and total RNA extracted. β-actin is shown as a reference gene. B, MMPs expression in IL-13Rα2 knock-down (α2KD) cells incubated with TSA. Mock and α2KD cells were treated with TSA and IL-13 same as in panel B.
To confirm whether TSA increased MMPs expression as a result of IL-13Rα2 induction, we conducted a knock-down of the IL-13Rα2 gene using two different sequences of siRNA in Panc-1 and ASPC-1 cell lines. MMPs expression was suppressed in IL-13Rα2 knock-down cells treated with TSA (Figure 4B).
HDAC inhibition increases the anti-cancer effect of IL-13-PE targeting IL-13Rα2 in vitro and in vivo
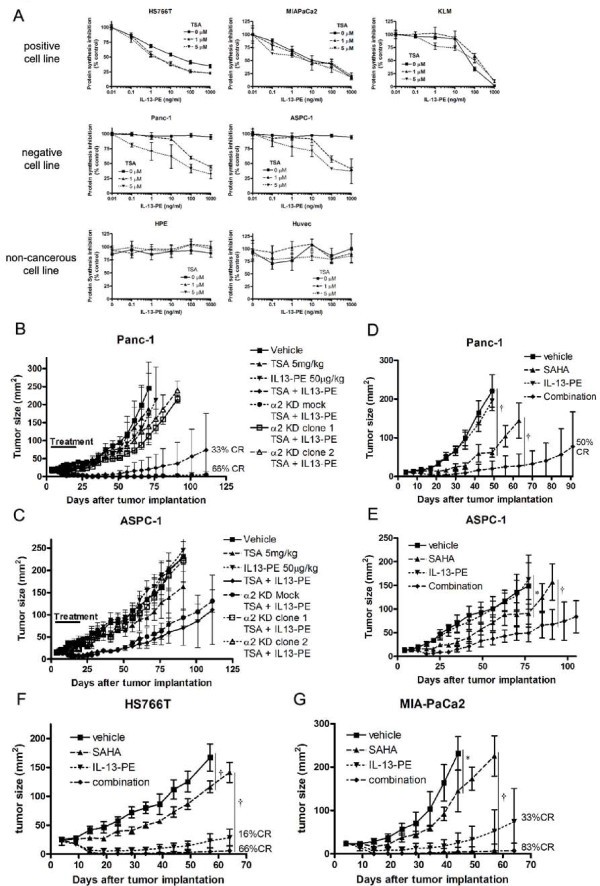
As HDAC inhibition increased IL-13Rα2 expression in IL-13Rα2-negative but not in normal cell lines, we examined whether HDAC inhibition enhanced the anti-cancer effect of IL-13-PE in IL-13Rα2-negative pancreatic cancer cell lines. The anti-cancer effect of IL-13-PE was evaluated using a protein synthesis inhibition assay in vitro (Figure 5A). IL-13-PE inhibited protein synthesis in IL-13Rα2-positive cancer cells (IC50 between 10 and 50 ng/ml) without TSA, but not in IL-13Rα2-negative cancer cells nor normal cells (IC50 > 1000 ng/ml). TSA treatment enhanced the cytotoxicity of IL-13-PE in IL-13Rα2-negative cancer cells (IC50 40-50 ng/ml with 5 μM TSA), but not in normal cells (IC50 > 1000 ng/ml with 5 μM TSA).
Figure 5.
HDAC inhibitors induce anti tumor effect of IL-13Rα2 targeted immmunotoxin IL13-PE in IL-13Rα2-negative pancreatic cancer cell lines. A, Cytotoxicity assay was performed in IL-13Rα2-negative and -positive pancreatic cancer and normal cell lines. Cells were pre-treated with 0 - 5 μM TSA for 24 hours and then treated with 0 - 1000 ng/ml IL-13-PE for 20 hours in leucine-free medium. Protein synthesis was evaluated by H3-leucine incorporation. Percentage cytotoxicity was calculated with no treatment control as 100%. B and C, Regression of IL-13Rα2-negative pancreatic tumors (Panc-1 and ASPC-1) treated with 5 mg/kg TSA and/or 100 μg/kg IL-13-PE as described in methods. Mock combination means tumors were mock transected with control vector and treated with HDAC inhibitors and IL-13-PE in vivo. D and E, Regression of IL-13Rα2-negative pancreatic tumors treated with SAHA and/or IL-13-PE. Mice were treated daily with i.p. injection of SAHA (25 mg/kg) from day 4 after tumor implantation for two weeks followed by i.t. injection of IL-13-PE (100 μg/kg) from day 5 for two weeks. F and G, Regression of IL-13Rα2-posotive pancreatic tumors (HS766T and MIA-PaCa2) treated with SAHA and/or IL-13-PE. The schedule of treatment was similar as in panel D and E. Statistical significances are shown by *: P < 0.05, †: P < 0.001.
We next examined the enhancement of the anti-cancer effect of IL-13-PE by HDAC inhibition in xenograft mouse models of human cancer. IL-13Rα2-negative pancreatic cancer cell lines (Panc-1 and ASPC-1) were implanted in the flanks of immunodeficient mice and treated with two different HDAC inhibitors, TSA and SAHA followed by IL-13-PE immunotoxin. Neither TSA nor IL-13-PE alone affected the tumor growth, but when combined, a dramatic inhibition of tumor growth was observed (Figure 5B and 5C). In contrast, when IL-13Rα2 was knocked-down prior to TSA therapy, the anti-tumor effect of combination of TSA and IL-13-PE was completely eliminated compared to mock vector transfected tumors, which showed dramatic tumor response (Figure 5B).
A second HDAC inhibitor, SAHA, itself showed some anti-cancer effect in two tumor models (Figure 5D and 5E). However, when mice were treated with SAHA followed by IL-13-PE, a significant decrease in tumor size was observed. In addition, 50% of mice showed complete elimination of their tumors in combination group.
Next, we evaluated anti-cancer effect of combination of SAHA and IL-13-PE in IL-13Rα2-positive pancreatic cancer model (HS766T and MIA-PaCa2). We observed that IL-13-PE could significantly decrease tumor size in both IL-13Rα2-positive tumors (Figure 5F and 5G). But when combined with SAHA, IL-13-PE not only decreased tumor size but also completely eliminated tumors in 66 to 83% of mice. These data suggest that SAHA can enhance anti-cancer effect of IL-13-PE even in IL-13Rα2-positive pancreatic cancers.
We monitored the body weight of mice and their general condition throughout the experimental period and detected no adverse effects caused by the treatment (data not shown). In addition, we observed no organ toxicity in vital organs such as the liver, brain, lung, kidney, pancreas and spleen of IL-13-PE and HDAC inhibitor-treated mice evaluated by histological examination (Supplementary Figure 3)
HDAC inhibitor significantly increased IL-13Rα2 in the pancreatic tumors implanted in the mice but not in mice organs
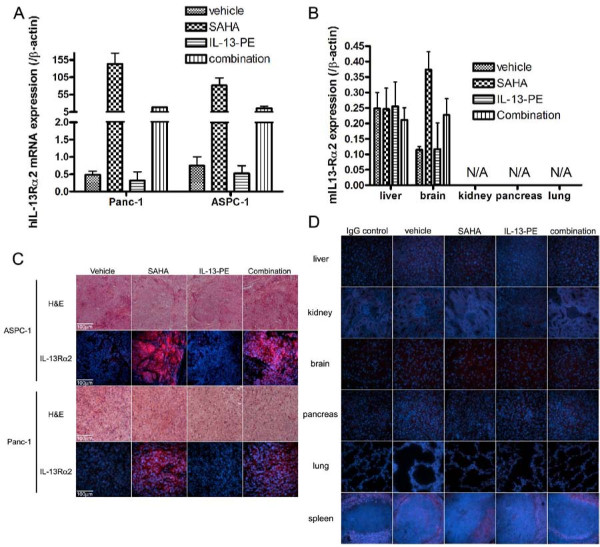
After SAHA and IL-13-PE treatment, implanted tumors and mice organs (liver, brain, pancreas, kidney, spleen and lung) were harvested and IL-13Rα2 expression was examined at mRNA and protein levels. Human IL-13Rα2 mRNA was significantly increased in tumors in both SAHA treated mice (Figure 6A) and TSA treated mice (Supplementary Figure 4). IL-13-PE treatment had no effect by itself but in combination with SAHA, a significant decrease in IL-13Rα2 expression was observed. In contrast, none of the organs except brain showed a modest increase in mouse IL-13Rα2 mRNA expression (Figure 6B).
Figure 6.
IL-13Rα2 expression is upregulated in pancreatic tumors but not in organs of mice after treatment with HDAC inhibitor, SAHA. A, qRT-PCR of human IL-13Rα2 in implanted pancreatic tumors after SAHA and IL-13-PE treatment. Tumors were harvested next day after IL-13-PE treatment and total RNA extracted. Data shown is ratio of human IL-13Rα2/β-actin expression and multiplied by 1000 for convenience. Bars, SD of triplicate determinations. B, qRT-PCR of mouse IL-13Rα2 in mice organs after SAHA and IL-13-PE treatment. Tissues were harvested at the same time point as in panel A and total RNA extracted. Data shown is ratio of mouse IL-13Rα2/β-actin expression and multiplied by 100 for convenience. C, IHC of human IL-13Rα2 in implanted pancreatic tumors after SAHA and IL-13-PE treatment. D, IHC of mouse IL-13Rα2 in mice organs after SAHA and IL-13-PE treatment. Liver, brain, kidney, pancreas, lung and spleen were fixed for immunostaining of mouse IL-13Rα2 as visualized by Alexa555. Nucleus was counterstained by DAPI.
We also examined IL-13Rα2 protein expression by IHC. Similar to mRNA results, human IL-13Rα2 was dramatically increased in tumors from SAHA treated mice and when combined with IL-13-PE, a decrease in IL-13Rα2 expression was observed (Figure 6C). In normal tissues, mouse IL-13Rα2 was not detected or levels were below the detection limit of the assay in all organs examined (Figure 6D).
Discussion
We demonstrate for the first time that IL-13Rα2, a tumor antigen, is highly susceptible to epigenetic modulation in pancreatic cancer cell lines. Interestingly, DNA methylation and histone acetylation were differentially regulated in cells overexpressing or not overexpressing IL-13Rα2. Histones (H3 and H4) were highly acetylated at the promoter region of IL-13Rα2 in IL-13Rα2-positive pancreatic cancer cell lines, but not in IL-13Rα2-negative cell lines. In contrast, histones in IL-13Rα2-negative pancreatic cell lines and normal cell lines were highly methylated, but not in IL-13Rα2 positive cell lines. The reason for the differential histone acetylation and methylation is not known but appears to correlate with IL-13Rα2 expression and may be responsible for variability of IL-13Rα2 expression in cancer cells.
The role of histone acetylation was explored further using histone deacetylase (HDAC) inhibitors. Interestingly, in the presence of HDAC inhibitors (TSA and NaB), IL-13Rα2 expression was significantly induced in IL-13Rα2-negative cell lines whose histones were not acetylated compared to IL-13Rα2-positive cell lines in which histones were acetylated. The mechanism of differential IL-13Rα2 regulation was examined. IL-13 signals through IL-13Rα2 via the AP-1 pathway and inactivation of this pathway by JNK and AP-1 inhibition suppressed IL-13Rα2 expression in IL-13Rα2-positive cell lines. Additionally, inactivation of the AP-1 pathway also suppressed induction of IL-13Rα2 by HDAC inhibitors in IL-13Rα2-negative cell lines. In accordance, Wu et al. have reported the importance of c-jun, which is a member of AP-1 transcription factor, in IL-13Rα2 expression [32]. These observations indicate a strong correlation between transcription factor and histone acetylation in the IL-13Rα2 at the promoter region.
The significance of IL-13Rα2 upregulation by HDAC inhibitors was examined. As expected, IL-13 induced STAT6 phosphorylation in IL-13Rα2-negative pancreatic cancer cell lines (Supplementary Figure 5). Interestingly, TSA increased IL-13Rα2 expression, but suppressed STAT6 phosphorylation induced by IL-13 treatment. The suppression of STAT6 phosphorylation by TSA was inhibited by IL-13Rα2 RNAi indicating that IL-13Rα2 is directly involved in this counter-regulation (data not shown). Similarly, as expected, IL-13 did not induce MMPs expression in IL-13Rα2-negative pancreatic cancer cell lines [28]. However, when cells were treated with TSA, IL-13 could increase MMP-9, 12 and 14 mRNA as IL-13Rα2 expression was upregulated. In contrast, MMPs were not induced by TSA when IL-13Rα2 was knocked-down by RNAi or IL-13 signaling was inhibited by JNK inhibitor.
We took advantage of upregulation of IL-13Rα2 in pancreatic cancer cell lines and hypothesized that HDAC inhibitors may enhance the sensitivity of IL-13 receptor-targeted immunotoxin, IL-13-PE, in pancreatic cancers. We have previously demonstrated that IL-13-PE is a powerful anti-cancer agent, causing regression of IL-13Rα2-positive human tumors derived from variety of human cancers including pancreatic cancer [15,16]. However, for efficacy, these tumors must express high levels of IL-13Rα2. Since cancer is a heterogeneous disease, drug-induced upregulation of IL-13Rα2 could be used in cancers expressing even low levels of IL-13 α2 to enhance the intensity of the immunotoxin anti-cancer response. Indeed, we demonstrate that pre-treatment of tumor cell lines in vitro with TSA enhanced their sensitivity to IL-13-PE and made IL-13Rα2-negative cell lines extremely sensitive to IL-13-PE. In contrast, TSA treatment did not sensitize normal epithelial cell lines, thus providing a therapeutic advantage of targeting tumors but not normal tissues. Consequently, the use of HDAC inhibitors may open a new avenue of treating pancreatic cancer when combined with IL-13-PE. It is possible that HDAC inhibitors may also sensitize tumors to other immunotoxins targeting different antigens or cell surface receptors.
The reason why normal epithelial cells are not sensitized to IL-13-PE by TSA is not clear. Epithelial cells exhibit a similar histone modification pattern to IL-13Rα2-negative pancreatic cancer cell lines but, IL-13Rα2 is not upregulated in normal epithelial cells by HDAC inhibitors. This may be because normal cell lines show no c-jun activity, while IL-13Rα2-negative pancreatic cancer cell lines show a 2-6 fold increase in c-jun activity indicating that TSA induction of high levels of IL-13Rα2 is dependent on the AP-1/c-jun pathway.
We also demonstrate that HDAC inhibitors when combined with IL-13-PE cause more dramatic tumor responses than those caused by either agent alone in two pancreatic cancer models. Pancreatic cancers in situ were not sensitive to IL-13-PE as they do not naturally express IL-13Rα2 and TSA or SAHA alone showed only modest to moderate anti-tumor effects. However, when TSA or SAHA were combined with IL13-PE a dramatic inhibition of tumor growth was observed. In agreement with our observations, HDAC inhibition has been reported in combination therapies for other types of cancer. Combination therapy of SAHA and retinoic acid has been examined for resistant acute promyelocytic leukemia in which SAHA enhanced the anti-cancer effect of retinoic acid [33]. Another HDAC inhibitor, LAQ824, is reported to be effective in combination with adoptive T-cell transfer therapy against mouse model of melanoma [34]. These authors hypothesized that LAQ824 increases the tumor-associated antigen expression enhancing the anti-tumor effectiveness of T cell therapy.
It is important to note that while HDAC inhibition enhanced the remarkable anti-cancer effects of IL-13-PE in pancreatic cancer models in vivo by upregulating IL-13Rα2 in the tumors, no significant upregulation of IL-13Rα2 expression was observed in any vital organs. In addition, no detectable histological changes were observed in any vital organs. Although IL-13-PE was injected locally, our findings confirm that this novel combination therapeutic approach is safe. Future studies will examine systemic administration of IL-13-PE in combination with HDAC inhibitors in syngenic animal tumor models. Taken together, our results provide support for testing this novel combination in the clinic for the therapy of human cancer including pancreatic cancer for which no therapeutic options are currently available.
Abbreviations
IL-13Rα2: interleukin 13 receptor alpha 2; IL-13-PE: interleukin 13 pseudomonas exotoxin.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
Conceived and designed the experiments: TF, BHJ, RKP. Performed the experiments: TF. Analyzed the data: TF. Wrote the paper: TF, BHJ, RKP.
All authors have read and approved the final manuscript.
Supplementary Material
Figure S1: DNA methylation status of upstream sequences from IL-13Rα2 promoter site. DNA methylation status was examined by bisulfite-sequencing at the CpG site located about 100 bases upstream from IL-13Rα2 promoter region. Methylated and unmethylated alleles are shown as solid and open circles, respectively.
Figure S2: AP-1 transcription factor activity in pancreatic cancer cell lines. c-jun (A) and c-Fos (B) activity in pancreatic cancer and normal cell lines. Protein samples were extracted from nuclear fraction. AP-1 activity was measured by ELISA.
Figure S3: Histological finding of vital organs in SAHA and IL-13-PE treated mice. Tissue specimens were obtained from mice liver, kidney, spleen, pancreas, brain and lung in each group of SAHA and IL-13-PE treated experiment (day 19) for hematoxylin and eosin staining.
Figure S4: IL-13Rα2 expression is upregulated in pancreatic tumors after treatment with TSA. qRT-PCR of human IL-13Rα2 in implanted human pancreatic tumors, Panc-1 (A) and ASPC-1 (B) after TSA and IL-13-PE treatment. Tumors were harvested next day after IL-13-PE treatment ended and total RNA was extracted. Data shown is ratio of human IL-13Rα2/β-actin expression. Bars, SD of triplicate determinations.
Figure S5: HDAC inhibitor inhibits IL-13 induced STAT6 activation through induction of IL-13Rα2. Western blotting of phospho- and total STAT6 after incubation of cells with TSA and/or SP600125. Cells were incubated with 1 μM TSA and/or 10 μM SP600125 for 24 hours. Fifteen minutes before harvest, IL-13 was added to the culture medium. Protein samples were prepared from nuclear compartment and separated by electrophoresis.
Contributor Information
Toshio Fujisawa, Email: toshio.fujisawa@fda.hhs.gov.
Bharat H Joshi, Email: bharat.joshi@fda.hhs.gov.
Raj K Puri, Email: raj.puri@fda.hhs.gov.
Acknowledgements
We thank Drs. Brenton McCright and John Thomas for reviewing the manuscript and Dr. Takashi Furusawa from National Cancer Institute, protein section and members of Tumor Vaccines and Biotechnology Branch, Division of Cellular and Gene Therapies, Center for Biologics Evaluation and Research and for their suggestions.
References
- Kawakami K, Terabe M, Kawakami M, Berzofsky JA, Puri RK. Characterization of a novel human tumor antigen interleukin-13 receptor alpha2 chain. Cancer Res. 2006;66:4434–4442. doi: 10.1158/0008-5472.CAN-05-1265. [DOI] [PubMed] [Google Scholar]
- Scanlan MJ, Gure AO, Jungbluth AA, Old LJ, Chen YT. Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunol Rev. 2002;188:22–32. doi: 10.1034/j.1600-065X.2002.18803.x. [DOI] [PubMed] [Google Scholar]
- Husain SR, Joshi BH, Puri RK. Interleukin-13 receptor as a unique target for anti-glioblastoma therapy. Int J Cancer. 2001;92:168–175. doi: 10.1002/1097-0215(200102)9999:9999<::AID-IJC1182>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Puri RK, Leland P, Obiri NI, Husain SR, Kreitman RJ, Haas GP, Pastan I, Debinski W. Targeting of interleukin-13 receptor on human renal cell carcinoma cells by a recombinant chimeric protein composed of interleukin-13 and a truncated form of Pseudomonas exotoxin A (PE38QQR) Blood. 1996;87:4333–4339. [PubMed] [Google Scholar]
- Kawakami K, Husain SR, Kawakami M, Puri RK. Improved anti-tumor activity and safety of interleukin-13 receptor targeted cytotoxin by systemic continuous administration in head and neck cancer xenograft model. Mol Med. 2002;8:487–494. [PMC free article] [PubMed] [Google Scholar]
- Husain SR, Obiri NI, Gill P, Zheng T, Pastan I, Debinski W, Puri RK. Receptor for interleukin 13 on AIDS-associated Kaposi's sarcoma cells serves as a new target for a potent Pseudomonas exotoxin-based chimeric toxin protein. Clin Cancer Res. 1997;3:151–156. [PubMed] [Google Scholar]
- Kioi M, Kawakami M, Shimamura T, Husain SR, Puri RK. Interleukin-13 receptor alpha2 chain: a potential biomarker and molecular target for ovarian cancer therapy. Cancer. 2006;107:1407–1418. doi: 10.1002/cncr.22134. [DOI] [PubMed] [Google Scholar]
- Debinski W, Obiri NI, Powers SK, Pastan I, Puri RK. Human glioma cells overexpress receptors for interleukin 13 and are extremely sensitive to a novel chimeric protein composed of interleukin 13 and pseudomonas exotoxin. Clin Cancer Res. 1995;1:1253–1258. [PubMed] [Google Scholar]
- Debinski W, Obiri NI, Pastan I, Puri RK. A novel chimeric protein composed of interleukin 13 and Pseudomonas exotoxin is highly cytotoxic to human carcinoma cells expressing receptors for interleukin 13 and interleukin 4. J Biol Chem. 1995;270:16775–16780. doi: 10.1074/jbc.270.28.16775. [DOI] [PubMed] [Google Scholar]
- Joshi BH, Kawakami K, Leland P, Puri RK. Heterogeneity in interleukin-13 receptor expression and subunit structure in squamous cell carcinoma of head and neck: differential sensitivity to chimeric fusion proteins comprised of interleukin-13 and a mutated form of Pseudomonas exotoxin. Clin Cancer Res. 2002;8:1948–1956. [PubMed] [Google Scholar]
- Joshi BH, Puri RK. Optimization of expression and purification of two biologically active chimeric fusion proteins that consist of human interleukin-13 and Pseudomonas exotoxin in Escherichia coli. Protein Expr Purif. 2005;39:189–198. doi: 10.1016/j.pep.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Kawakami K, Kawakami M, Kioi M, Husain SR, Puri RK. Distribution kinetics of targeted cytotoxin in glioma by bolus or convection-enhanced delivery in a murine model. J Neurosurg. 2004;101:1004–1011. doi: 10.3171/jns.2004.101.6.1004. [DOI] [PubMed] [Google Scholar]
- Joshi BH, Hogaboam C, Dover P, Husain SR, Puri RK. Role of interleukin-13 in cancer, pulmonary fibrosis, and other T(H)2-type diseases. Vitam Horm. 2006;74:479–504. doi: 10.1016/S0083-6729(06)74019-5. [DOI] [PubMed] [Google Scholar]
- Kunwar S, Prados MD, Chang SM, Berger MS, Lang FF, Piepmeier JM, Sampson JH, Ram Z, Gutin PH, Gibbons RD. et al. Direct intracerebral delivery of cintredekin besudotox (IL13-PE38QQR) in recurrent malignant glioma: a report by the Cintredekin Besudotox Intraparenchymal Study Group. J Clin Oncol. 2007;25:837–844. doi: 10.1200/JCO.2006.08.1117. [DOI] [PubMed] [Google Scholar]
- Shimamura T, Fujisawa T, Husain SR, Joshi B, Puri RK. Interleukin 13 mediates signal transduction through interleukin 13 receptor alpha2 in pancreatic ductal adenocarcinoma: role of IL-13 Pseudomonas exotoxin in pancreatic cancer therapy. Clin Cancer Res. 2009;16:577–586. doi: 10.1158/1078-0432.CCR-09-2015. [DOI] [PubMed] [Google Scholar]
- Fujisawa T, Nakashima H, Nakajima A, Joshi BH, Puri RK. Targeting IL-13Ralpha2 in human pancreatic ductal adenocarcinoma with combination therapy of IL-13-PE and gemcitabine. Int J Cancer. 2010. [DOI] [PubMed]
- Allen C, Paraskevakou G, Iankov I, Giannini C, Schroeder M, Sarkaria J, Puri RK, Russell SJ, Galanis E. Interleukin-13 displaying retargeted oncolytic measles virus strains have significant activity against gliomas with improved specificity. Mol Ther. 2008;16:1556–1564. doi: 10.1038/mt.2008.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlon KS, Brown C, Cooper LJ, Raubitschek A, Forman SJ, Jensen MC. Specific recognition and killing of glioblastoma multiforme by interleukin 13-zetakine redirected cytolytic T cells. Cancer Res. 2004;64:9160–9166. doi: 10.1158/0008-5472.CAN-04-0454. [DOI] [PubMed] [Google Scholar]
- Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429:457–463. doi: 10.1038/nature02625. [DOI] [PubMed] [Google Scholar]
- Urdinguio RG, Sanchez-Mut JV, Esteller M. Epigenetic mechanisms in neurological diseases: genes, syndromes, and therapies. Lancet Neurol. 2009;8:1056–1072. doi: 10.1016/S1474-4422(09)70262-5. [DOI] [PubMed] [Google Scholar]
- Lennartsson A, Ekwall K. Histone modification patterns and epigenetic codes. Biochim Biophys Acta. 2009;1790:863–868. doi: 10.1016/j.bbagen.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Bird A. Perceptions of epigenetics. Nature. 2007;447:396–398. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- Eberharter A, Becker PB. Histone acetylation: a switch between repressive and permissive chromatin. Second in review series on chromatin dynamics. EMBO Rep. 2002;3:224–229. doi: 10.1093/embo-reports/kvf053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly WK, Marks PA. Drug insight: Histone deacetylase inhibitors--development of the new targeted anticancer agent suberoylanilide hydroxamic acid. Nat Clin Pract Oncol. 2005;2:150–157. doi: 10.1038/ncponc0106. [DOI] [PubMed] [Google Scholar]
- Marks PA, Jiang X. Histone deacetylase inhibitors in programmed cell death and cancer therapy. Cell Cycle. 2005;4:549–551. doi: 10.4161/cc.4.4.1564. [DOI] [PubMed] [Google Scholar]
- Duvic M, Talpur R, Ni X, Zhang C, Hazarika P, Kelly C, Chiao JH, Reilly JF, Ricker JL, Richon VM, Frankel SR. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL) Blood. 2007;109:31–39. doi: 10.1182/blood-2006-06-025999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi BH, Husain SR, Puri RK. Preclinical studies with IL-13PE38QQR for therapy of malignant glioma. Drug News Perspect. 2000;13:599–605. doi: 10.1358/dnp.2000.13.10.858450. [DOI] [PubMed] [Google Scholar]
- Fujisawa T, Joshi B, Nakajima A, Puri RK. A novel role of interleukin-13 receptor alpha2 in pancreatic cancer invasion and metastasis. Cancer Res. 2009;69:8678–8685. doi: 10.1158/0008-5472.CAN-09-2100. [DOI] [PubMed] [Google Scholar]
- Joshi BH, Leland P, Calvo A, Green JE, Puri RK. Human adrenomedullin up-regulates interleukin-13 receptor alpha2 chain in prostate cancer in vitro and in vivo: a novel approach to sensitize prostate cancer to anticancer therapy. Cancer Res. 2008;68:9311–9317. doi: 10.1158/0008-5472.CAN-08-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi BH, Plautz GE, Puri RK. Interleukin-13 receptor alpha chain: a novel tumor-associated transmembrane protein in primary explants of human malignant gliomas. Cancer Res. 2000;60:1168–1172. [PubMed] [Google Scholar]
- Kawakami K, Kawakami M, Husain SR, Puri RK. Potent antitumor activity of IL-13 cytotoxin in human pancreatic tumors engineered to express IL-13 receptor alpha2 chain in vivo. Gene Ther. 2003;10:1116–1128. doi: 10.1038/sj.gt.3301956. [DOI] [PubMed] [Google Scholar]
- Wu AH, Low WC. Molecular cloning and identification of the human interleukin 13 alpha 2 receptor (IL-13Ra2) promoter. Neuro Oncol. 2003;5:179–187. doi: 10.1215/S1152851702000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He LZ, Tolentino T, Grayson P, Zhong S, Warrell RP Jr, Rifkind RA, Marks PA, Richon VM, Pandolfi PP. Histone deacetylase inhibitors induce remission in transgenic models of therapy-resistant acute promyelocytic leukemia. J Clin Invest. 2001;108:1321–1330. doi: 10.1172/JCI11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo DD, Prins RM, Begley JL, Donahue TR, Morris LF, Bruhn KW, de la Rocha P, Yang MY, Mok S, Garban HJ. et al. Enhanced antitumor activity induced by adoptive T-cell transfer and adjunctive use of the histone deacetylase inhibitor LAQ824. Cancer Res. 2009;69:8693–8699. doi: 10.1158/0008-5472.CAN-09-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: DNA methylation status of upstream sequences from IL-13Rα2 promoter site. DNA methylation status was examined by bisulfite-sequencing at the CpG site located about 100 bases upstream from IL-13Rα2 promoter region. Methylated and unmethylated alleles are shown as solid and open circles, respectively.
Figure S2: AP-1 transcription factor activity in pancreatic cancer cell lines. c-jun (A) and c-Fos (B) activity in pancreatic cancer and normal cell lines. Protein samples were extracted from nuclear fraction. AP-1 activity was measured by ELISA.
Figure S3: Histological finding of vital organs in SAHA and IL-13-PE treated mice. Tissue specimens were obtained from mice liver, kidney, spleen, pancreas, brain and lung in each group of SAHA and IL-13-PE treated experiment (day 19) for hematoxylin and eosin staining.
Figure S4: IL-13Rα2 expression is upregulated in pancreatic tumors after treatment with TSA. qRT-PCR of human IL-13Rα2 in implanted human pancreatic tumors, Panc-1 (A) and ASPC-1 (B) after TSA and IL-13-PE treatment. Tumors were harvested next day after IL-13-PE treatment ended and total RNA was extracted. Data shown is ratio of human IL-13Rα2/β-actin expression. Bars, SD of triplicate determinations.
Figure S5: HDAC inhibitor inhibits IL-13 induced STAT6 activation through induction of IL-13Rα2. Western blotting of phospho- and total STAT6 after incubation of cells with TSA and/or SP600125. Cells were incubated with 1 μM TSA and/or 10 μM SP600125 for 24 hours. Fifteen minutes before harvest, IL-13 was added to the culture medium. Protein samples were prepared from nuclear compartment and separated by electrophoresis.