Abstract
Acute chorioamnionitis (ACA) is a well-established lesion of the placenta in cases with intra-amniotic infection. In contrast, the clinicopathologic significance of chronic chorioamnionitis (CCA) is unclear. This study was conducted to determine the frequency and severity of CCA in normal pregnancy and various pregnancy complications. Placentas from the following patient groups were studied: 1) term not in labor (TNL; n=100), 2) term in labor (TIL; n=100), 3) preterm labor (PTL; n=100), 4) preterm prelabor rupture of the membranes (PPROM; n=100), 5) preeclampsia at term (TPE; n=100), 6) preterm preeclampsia (PPE; n=100), and 7) small-for-gestational-age at term (SGA; n=100). Amniotic fluid CXCL10 concentration was measured in 64 patients. CXCL9, CXCL10, and CXCL11 mRNA expressions in the chorioamniotic membranes were assessed by real-time quantitative RT-PCR. The frequency of CCA in PTL and PPROM groups was 34% and 39%, respectively, which was higher than those of normal term placentas (TNL 19%, TIL 8%; p<0.05 each). The frequency of CCA in TPE, PPE and SGA groups was 23%, 16%, and 13%, respectively. Concomitant villitis of unknown etiology (VUE) was found in 38.2% and 35.9% of PTL and PPROM cases with CCA, respectively. Interestingly, the median gestational age of preterm CCA cases was higher than that of ACA cases (p<0.05). The median amniotic fluid CXCL10 concentration was higher in cases with CCA than in those without, in both PTL and PPROM groups (p<0.05 and p<0.01, respectively). CXCL9, CXCL10, and CXCL11 mRNA expression in the chorioamniotic membranes was also higher in CCA cases than in those without CCA (p<0.05). We propose that CCA defines a common placental pathologic lesion among the PTL and PPROM groups, especially in cases of late preterm birth. Its association with VUE and the chemokine profile in amniotic fluid suggests an immunological origin, akin to transplantation rejection and graft-versus-host disease in the chorioamniotic membranes.
Keywords: Chorioamnionitis, amniotic fluid, pregnancy, CXCL9, CXCL10, CXCL11
Introduction
Histopathologic examination of human placentas delivered by women with complications of pregnancy often provides valuable and critical information about the mechanisms of disease of the “Great Obstetrical Syndromes”.1,2 Preterm birth affects approximately 500,000 newborns every year in the United States,3 is the leading cause of perinatal morbidity and mortality worldwide, and is a major cause of chronic illness (e.g. cerebral palsy and chronic lung disease).4,5 The cost to society is estimated to be 26 billion dollars per year in the United States alone.6,7 Preterm birth can result from spontaneous preterm labor/preterm prelabor rupture of the membranes or indicated delivery because of maternal (e.g. preeclampsia) or fetal (e.g. intrauterine growth restriction) indications.1
Acute chorioamnionitis (ACA) is the most common lesion reported in the placenta after spontaneous preterm birth. It is a frequent lesion, both in cases with preterm prelabor rupture of membranes (PPROM) or preterm labor with intact membranes (PTL). ACA is due to microbial invasion of the amniotic cavity (MIAC) documented with cultivation or culture-independent methods.8,9 The histopathologic features include amniotropic infiltration by both maternal and fetal neutrophils (which are not normally found) in the chorioamniotic membranes and the umbilical cord.10,11 This amniotropic neutrophil migration is due to gradients of potent neutrophil chemokines, such as IL-8 and CXCL6 (GCP-2), whose concentrations markedly increase in response to MIAC.12,13 The clinical significance of the maternal and fetal inflammatory responses in ACA has been well-characterized, and the severity of the lesions is associated with adverse pregnancy outcomes.14,15 A large body of in vivo and in vitro evidence indicates that the ACA and intra-amniotic infection complex is causally linked to PTL and PPROM.16–19 Normal spontaneous labor at term in the absence of histologic chorioamnionitis also has a molecular signature of acute inflammation, suggesting that inflammation is a phenomenon of both physiologic and pathologic human parturition.20
Chronic chorioamnionitis (CCA) is defined by the infiltration of lymphocytes in the chorioamniotic membranes and the chorionic plate, similar to that of neutrophils in ACA.21 The original description and characterization of this lesion represents a series of seminal contributions.21–23 CCA has been associated with previous spontaneous abortion, intrauterine growth restriction, and preterm birth. It is of interest that CCA is often associated with “chronic villitis of unknown etiology” (VUE). Gersell et al. reported VUE in 64.7% of cases (11/17 CCA cases),22 while Jacques and Qureshi21 reported the co-existence of these lesions in 71% of cases (22/31 CCA cases). However, the clinical significance, underlying pathophysiology, and frequency of CCA have not been clearly defined because the number of cases reported has been limited. The purpose of this study was to determine the frequency and clinical significance of this unique lesion. We conducted a systematic study of 700 cases of patients with normal pregnancies as well as those with PTL, PPROM, preeclampsia, and small-for-gestational-age (SGA) neonates. Since there is an association between CCA and VUE, we postulated that dysregulated expression of anti-angiogenic T cell chemokines CXCL9, CXCL10, and CXCL11 in the amniotic fluid and the chorioamniotic membranes may, in part, be responsible for this lesion.24 Therefore, we performed a histopathological review of the placentas and the chorioamniotic membranes and determined the expression of T cell chemokines in the amniotic fluid (CXCL10) and in the chorioamniotic membranes (CXCL9, CXCL10, CXCL11).
Materials and Methods
Study Population
Placental tissues were collected at the time of delivery. All participating patients provided written informed consent, and the Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services, approved the collection and use of biological materials for research purposes. Using the database of the Bank of Biological Materials of the Perinatology Research Branch of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, we identified 700 cases presenting with the following diagnoses: 1) women not in labor at term without pregnancy complications (TNL; n=100), 2) women in labor at term (TIL; n=100), 3) patients in preterm labor with intact membranes (PTL; n=100), 4) patients with preterm prelabor rupture of the membranes (PPROM; n=100), 5) patients with preeclampsia at term (TPE; n=100), 6) patients with preterm preeclampsia (PPE; n=100), and 7) patients with small-for-gestational-age neonates born at term (SGA; n=100). PTL was defined by the presence of regular uterine contractions with cervical dilation that led to delivery before 37 weeks of gestation. Preeclampsia was defined by both hypertension (systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg on at least two occasions, 4 h to 1 week apart) and proteinuria (≥ 300 mg in a 24-h urine collection or one dipstick measurement ≥ 2+). SGA was defined as less than the 10th percentile in birth weight for gestational age. Amniotic fluid samples (n=64) were obtained by transabdominal amniocentesis from a select group of women who underwent amniocentesis for clinical indications. Samples were kept at −80°C until use in the CXCL10 assay. Extraplacental chorioamniotic membranes (n=59) had been collected in RNAlater (Qiagen, Valencia, CA, USA), and kept at −80 °C until use.
Histopathological Examination
In each case, hematoxylin and eosin (H&E) stained sections of the chorioamniotic membranes roll (n=1), umbilical cord (n=1), and placental disc (n=3) were examined. Pathologists were masked to the clinical diagnosis except for the gestational age at delivery. The diagnosis of CCA was made when lymphocytic infiltration into the chorionic trophoblast layer or chorioamniotic connective tissue was observed. The severity of CCA was scored based upon on two parameters. The extent of inflammation was graded 0 when there was no inflammation, 1 when there were more than two foci of or patchy inflammation, and 2 when diffuse inflammation was present. The stage of inflammation was scored as stage 1 if amniotropic lymphocytic infiltration was limited to the chorionic trophoblast layer sparing the chorioamniotic connective tissue, and stage 2 if lymphocytic infiltration into the chorioamniotic connective tissue was noted. Histopathological screening for other lesions of the placenta was performed according to the diagnostic criteria proposed by the Perinatal Section of the Society for Pediatric Pathology. Such classification encompasses lesions consistent with amniotic fluid infection, maternal vascular underperfusion, and fetal vascular obstruction.10,25,26 The diagnosis of chronic deciduitis with plasma cells was given when lymphoplasmacytic infiltrate was present in the decidua of the basal plate.27
Immunohistochemistry
Immunohistochemistry was performed to identify T cell infiltration by using an antibody against CD3 in all patients with PPROM (n=100). Formalin-fixed, paraffin-embedded, 5-micrometer-thick tissue sections of the chorioamniotic membranes were placed on silanized slides and stained using a Ventana Discovery automatic staining system (Ventana Medical Systems, Tucson, AZ, USA). Immunostaining was performed using mouse monoclonal anti-CD3 antibody (1:50, Novocastra, Newcastle, UK). The Discovery® DAB Map™ Kit (Ventana Medical Systems) was used to detect the chromogen reaction of horseradish peroxidase.
Enzyme-linked Immunosorbent Assay
Amniotic fluid samples obtained by transabdominal amniocentesis were centrifuged at 1,300 × g for 10 min and stored at −80°C until use. The amniotic fluid concentration of CXCL10 was measured by specific enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN, USA), according to manufacturer’s instructions. The sensitivity of the assay was 4.22 pg/ml. The inter-assay coefficient of variation was 5.3%, and the intra-assay coefficient of variation was 3.56%.
Real-Time Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
Chorioamniotic membranes collected in RNAlater from patients with PTL and PPROM were available in 59 cases. Total RNA was isolated using Trizol, and further purified and DNased using an RNeasy mini-column (Qiagen). Reverse transcription reaction was done using SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA, USA) and oligodT primers with 100 ng of total RNA. PCR analyses were performed with TaqMan® Gene Expression Assays (CXCL9, Hs00171065_m1; CXCL10, Hs00171042_m1; CXCL11, Hs00171138_m1; Applied Biosystems, Foster City, CA, USA). RPLP0 was used for normalization. The ABI 7500 FAST Real Time PCR system was used for PCR reactions.
Statistical Analysis
Means and standard deviations were calculated for continuous variables while frequencies and percentages were reported for categorical variables. Unpaired t-tests and chi-square tests were applied for continuous and discrete variables, respectively. Analysis of Variance (ANOVA) was performed to compare differences in the mean expression of CXCL9 mRNA, CXCL10 mRNA, and CXCL11 mRNA in the chorioamniotic membranes according to grade, stage, and severity of CCA. If the overall test was significant, multiple comparisons (post-hoc tests) were performed to determine the source of the difference. Distributions of continuous variables were examined for skewness and normality using Kolmogorov–Smirnov tests. If the data were far from the normality, a generalized linear model using the ranked data was applied. Statistical analyses were performed using SAS Version 9.2 (Statistical Analysis Software, Cary, NC, USA), and all P values were two-sided. P values of less than 0.05 were considered to indicate statistical significance.
Results
Frequency and Severity of Chronic Chorioamnionitis
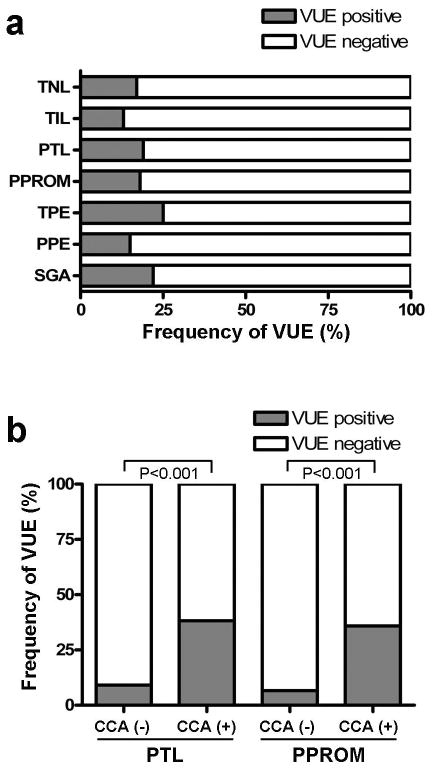
The demographic characteristics and the frequency of CCA in each group are displayed in Table 1. Examples of lymphocytic infiltration limited to the chorionic trophoblast layer (stage 1) and into the chorioamniotic connective tissue (stage 2) are shown in Figure 1. CCA was commonly associated with destruction and thinning of the chorionic trophoblast layer. The frequency of CCA was: 19% (19/100) for women at term not in labor (TNL), 8% (8/100) for women at term in labor (TIL), 34% (34/100) for women in preterm labor with intact membranes (PTL), 39% (39/100) for women with preterm prelabor rupture of the membranes (PPROM), 23% (23/100) for women with preeclampsia at term (TPE), 16% (16/100) for women with preterm preeclampsia (PPE), and 13% (13/100) for women with small-for-gestational-age (SGA) neonates born at term (Figure 2). CCA was more common in PTL and PPROM cases than in TNL (p<0.05 and p<0.01, respectively) or TIL (p<0.001 for each) cases. The frequency of CCA in TIL cases was significantly lower than that in TNL and TPE cases (p<0.05 and p<0.01, respectively). The frequency of CCA in PTL and PPROM cases was significantly higher than that observed in PPE cases (p<0.01 and p<0.001, respectively). Interestingly, PPROM placentas tended to have higher stage CCA compared to PTL cases (p<0.06) although there was no difference in the frequency of CCA between the two groups.
Table 1.
Patient demographics, clinical characteristics, and frequency of choronic chorioamnionitis in each group
| Group | Maternal age (years, mean±s.d.) | Gestational age at delivery (weeks, mean±s.d.) | Birth weight (g, mean±s.d.) | Labor (absent/spontaneous/induced) | Frequency of CCA (%) |
|---|---|---|---|---|---|
| TNL | 29.4 ± 5.9 | 39.5 ± 1.3 | 3449.8 ± 314.4 | 100/0/0 | 19 |
| TIL | 25.5 ± 6.1 | 39.4 ± 1.1 | 3376.0 ± 305.0 | 0/100/0 | 8 |
| PTL | 23.4 ± 6.9 | 33.8 ± 2.7 | 2327.8 ± 615.0 | 0/100/0 | 34 |
| PPROM | 28.4 ± 8.0 | 33.4 ± 2.9 | 2297.8 ± 632.0 | 31/16/53 | 39 |
| TPE | 24.3 ± 6.3 | 38.9 ± 1.2 | 3173.2 ± 500.0 | 10/34/56 | 23 |
| PPE | 27.8 ± 7.6 | 33.5 ± 3.0 | 1906.2 ± 694.3 | 67/0/33 | 16 |
| SGA | 24.2 ± 6.9 | 39.2 ± 1.1 | 2592.5 ± 242.7 | 8/38/54 | 13 |
CCA: chronic chorioamniontis, TNL: term not in labor, TIL: term in labor, PTL: preterm labor, PPROM: preterm prelabor rupture of membranes, TPE: preeclampsia at term, PPE: preterm preeclampsia, SGA: small-for-gestational-age at term.
Figure 1.
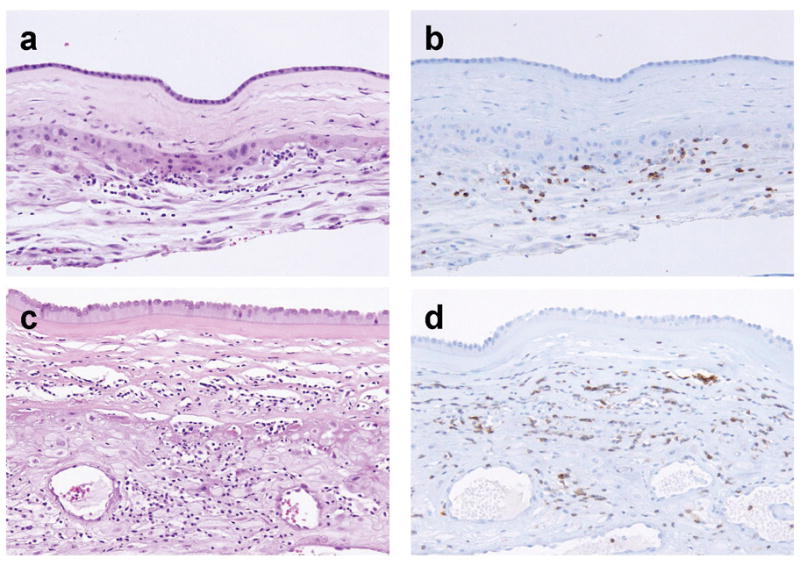
Histological characteristics of chronic chorioamnionitis (CCA). (a, b): Stage 1 inflammation showing infiltration of lymphocytes limited to the chorionic trophoblast layer (a). CD3 immunostaining demonstrates that the majority of these cells are T cells (b). (c, d): Stage 2 inflammation is characterized by infiltration of lymphocytes into the chorioamniotic connective tissue layer (H&E, c), which are largely CD3+ T cells (d).
Figure 2.
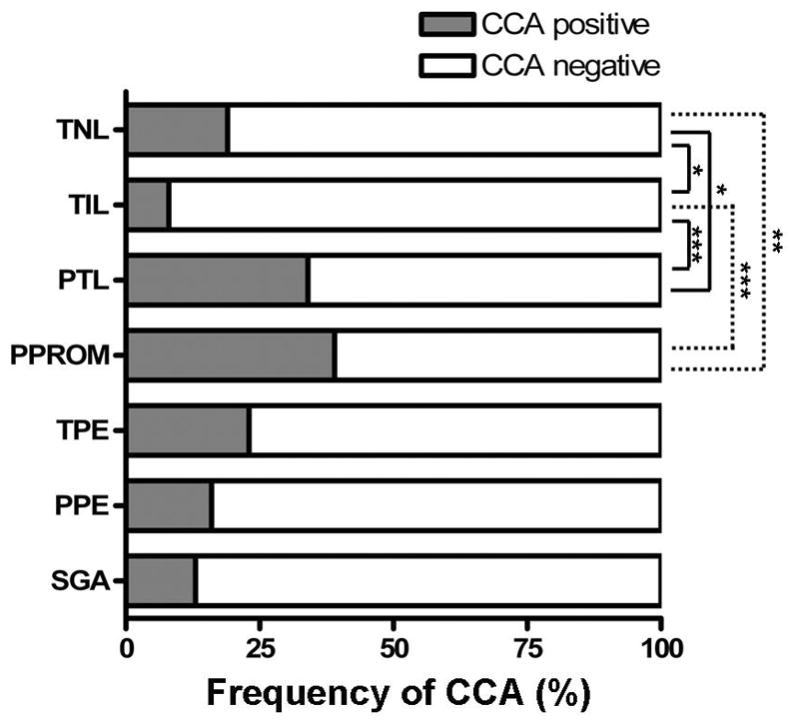
Frequency of chronic chorioamnionitis (CCA) in different obstetrical settings. Frequency of CCA in PTL (34%) and PPROM (39%) groups are significantly higher compared to TNL (19%) and TIL (8%) groups. The difference in frequency between TNL and TIL cases was also statistically significant. The frequency of CCA in each group ranged from 8% to 39%. TNL: term not in labor, TIL: term in labor, PTL: preterm labor, PPROM: preterm prelabor rupture of the membranes, TPE: preeclampsia at term, PPE: preterm preeclampsia, SGA: small-for-gestational-age at term. * p<0.05, **: p<0.01, ***: p<0.001.
Other pathologic changes observed in the placentas of each group are summarized in Table 2. The frequency of VUE among the groups ranged between 13% (PPE group) and 25% (TPE group) (Figure 3a). Placentas with CCA in patients with PTL and PPROM had concomitant VUE in 38.2% and 35.9% of cases, respectively, while VUE was found in only 9.1% and 6.6% of the placentas in the same diagnostic group without CCA (Figure 3b). The frequency of VUE in cases with CCA varied among the groups: 36.8% in TNL, 37.5% in TIL, 43.5% in TPE, 18.8% in PPE, and 30.8% in SGA neonates, Thus, among patients with CCA, the frequency of VUE was high.
Table 2.
Histological findings in each group with and without chronic chorioamnionitis
| Group | Number of cases | Villitis of unknown etiology | Acute chorioamnionitis | Maternal vascular underperfusion | Fetal vascular thrombo-occlusive disease | |
|---|---|---|---|---|---|---|
| TNL | CCA negative | 81 | 10 (12.3%) | 4 (4.9%) | 6 (7.4%) | 0 (0.0%) |
| CCA positive | 19 | 7 (36.8%) | 1 (5.3%) | 1 (5.3%) | 0 (0.0%) | |
| total | 100 | 17 (17%) | 5 (5%) | 7 (7%) | 0 (0.0%) | |
|
| ||||||
| TIL | CCA negative | 92 | 11 (12.0%) | 21 (22.8%) | 3 (3.3%) | 0 (0.0%) |
| CCA positive | 8 | 3 (37.5%) | 1 (12.5%) | 1 (12.5%) | 0 (0.0%) | |
| total | 100 | 14 (14%) | 22 (22%) | 4 (4%) | 0 (0.0%) | |
|
| ||||||
| PTL | CCA negative | 66 | 6 (9.1%) | 20 (30.3%) | 11 (16.7%) | 1 (1.5%) |
| CCA positive | 34 | 13 (38.2%) | 2 (5.9%) | 5 (14.7%) | 1 (2.9%) | |
| total | 100 | 19 (19%) | 22 (22%) | 16 (16%) | 2 (2%) | |
|
| ||||||
| PPROM | CCA negative | 61 | 4 (6.6%) | 12 (19.7%) | 5 (8.2%) | 0 (0.0%) |
| CCA positive | 39 | 14 (35.9%) | 7 (17.9%) | 5 (12.8%) | 1 (2.6%) | |
| total | 100 | 18 (18%) | 19 (19%) | 10 (10%) | 1 (1.0%) | |
|
| ||||||
| TPE | CCA negative | 77 | 15 (19.5%) | 7 (9.1%) | 11 (14.3%) | 1 (1.3%) |
| CCA positive | 23 | 10 (43.5%) | 3 (13.0%) | 8 (34.8%) | 0 (0.0%) | |
| total | 100 | 25 (25%) | 10 (10%) | 19 (19%) | 1 (1.0%) | |
|
| ||||||
| PPE | CCA negative | 84 | 10 (11.9%) | 1 (1.2%) | 53 (63.1%) | 1 (1.2%) |
| CCA positive | 16 | 3 (18.8%) | 0 (0%) | 8 (50.0%) | 0 (0.0%) | |
| total | 100 | 13 (13%) | 1 (1%) | 61 (61%) | 1 (1%) | |
|
| ||||||
| SGA | CCA negative | 87 | 18 (20.7%) | 7 (8.0%) | 14 (16.1%) | 1 (1.1%) |
| CCA positive | 13 | 4 (30.8%) | 0 (0%) | 2 (15.4%) | 1 (7.7%) | |
| total | 100 | 22 (22%) | 7 (7%) | 16 (16%) | 2 (2%) | |
CCA: chronic chorioamniontis, TNL: term not in labor, TIL: term in labor, PTL: preterm labor, PPROM: preterm prelabor rupture of the membranes, TPE: preeclampsia at term, PPE: preterm preeclampsia, SGA: small-for-gestational-age at term.
Figure 3.
Frequency of villitis of unknown etiology (VUE) in each group with chronic chorioamnionitis (CCA). (a) Frequency of VUE in each group ranged from 13% (PPE group) to 25% (TPE group). (b) Concomitant VUE was found in 38.2% and 35.9% of PTL and PPROM cases with CCA, respectively, but in 9.1% and 6.6% of PTL and PPROM cases without CCA, respectively. TNL: Term not in labor, TIL: term in labor, PTL: preterm labor, PPROM: preterm prelabor rupture of the membranes, TPE: preeclampsia at term, PPE: preterm preeclampsia, SGA: small-for-gestational-age at term.
There was also a significant difference in the frequency of chronic deciduitis with plasma cells among the groups (p<0.05). The frequency of chronic deciduitis with plasma cells was 13% for women at term not in labor, 11% for women at term in labor, 22% for women in preterm labor with intact membranes, 25% for women with preterm preeclampsia, and 14% for women with small-for-gestational-age neonates born at term. Its frequency was higher in cases with spontaneous preterm birth (PTL and PPROM) than in those who delivered at term (TNL and TIL) (24% vs. 12%, p<0.005). The frequency was significantly higher in cases with CCA or VUE than in cases without (for CCA: 39% vs. 9%; for VUE: 38% vs. 11%, p<0.001 for each). Chronic deciduitis with plasma cells was largely associated with basal villitis in each placenta as has been described previously.28
Acute chorioamnionitis (ACA) was found in 22% and 19% of PTL and PPROM cases, respectively (see Table 2). Interestingly, the median gestational age at delivery of cases with CCA was significantly higher than that of ACA (median: 33.9 weeks, range: 22.9–36.7 weeks versus median: 32.4 weeks, range: 24.7–34.7 weeks; p<0.05) in PTL and PPROM cases. In nine cases, lesions of ACA and CCA were observed in the same placenta, showing ‘acute on chronic’ chorioamnionitis as previously described.22
Histopathological features consistent with maternal vascular underperfusion or fetal vascular obstruction were also variably found among the groups analyzed (Table 2). The frequency of lesions considered to represent maternal vascular underperfusion were more common in PPE than in PTL and PPROM cases (p<0.001 for each). There was no difference in the frequency of maternal vascular underperfusion or fetal vascular obstruction between cases with and without CCA.
To assess the correlation between the histological evaluation of CCA based on H&E staining and the immunostaining for T cells, we performed immunostaining for CD3 in all placentas of patients with PPROM because CCA was found most frequently in this group. A masked review of CD3 immunoreactivity revealed CCA in 54% of PPROM cases. CD3+ T lymphocytic infiltration which met criteria used in this study was additionally detected in 15 of 61 non-CCA cases classified by histology alone. This was due to differences in microscopic planes from original H&E slides, addition of mild (grade 1/stage 1) cases, grade 1/stage 2 lesions in which T cells were unexpectedly found in the chorioamniotic connective tissue by CD3 immunostaining, and a grade 2/stage 2 lesion in which lymphocytic infiltration was obscure in H&E staining because of the presence of concomitant ACA.
Amniotic Fluid CXCL10 Concentration and CXCL9, CXCL10, CXCL11 mRNA Expressions in the Chorioamniotic Membranes
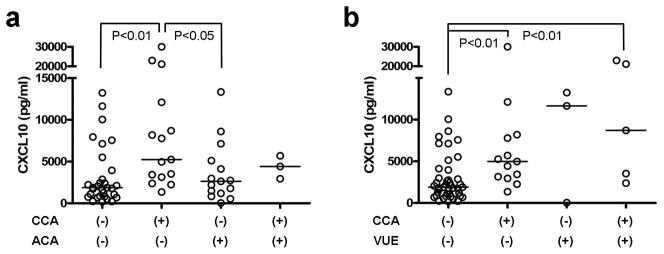
Since CCA shows an amniotropic T cell migration pattern, we postulated that the concentrations of amniotic fluid T cell chemokines CXCL9, CXCL10, and CXCL11 would be higher in CCA cases as placental expression of those chemokines was increased in VUE cases.24 Amniotic fluid samples obtained by amniocentesis within 4 weeks before delivery were available in 64 PTL and PPROM cases. Comparisons between cases with and without CCA demonstrated that the cases with CCA had significantly higher amniotic fluid CXCL10 concentration (p<0.01) (Figure 4a). There was a relationship between the severity of CCA and the amniotic fluid CXCL10 concentration among grade1/stage 1, grade 2/stage1 or grade 1/stage 2, and grade 2/stage 2 cases. This clearly showed that the severity of CCA relates to the concentration of amniotic fluid CXCL10 (p<0.001, R=0.446). On the other hand, amniotic fluid CXCL10 concentration was not significantly different in ACA, while it seemed to be elevated in VUE cases, although the number of cases with isolated VUE without CCA (n=3) was insufficient for meaningful statistical comparison (Figure 4b).
Figure 4.
Comparisons of amniotic fluid CXCL10 according to the presence of chronic chorioamnionitis (CCA), acute chorioamnionitis (ACA), and villitis of unknown etiology (VUE). (a) While CCA cases have significantly higher amniotic fluid CXCL10 concentration, it is not elevated in cases with ACA. (b) CCA cases with concomitant VUE have higher amniotic fluid CXCL10 concentrations compared to cases without inflammation.
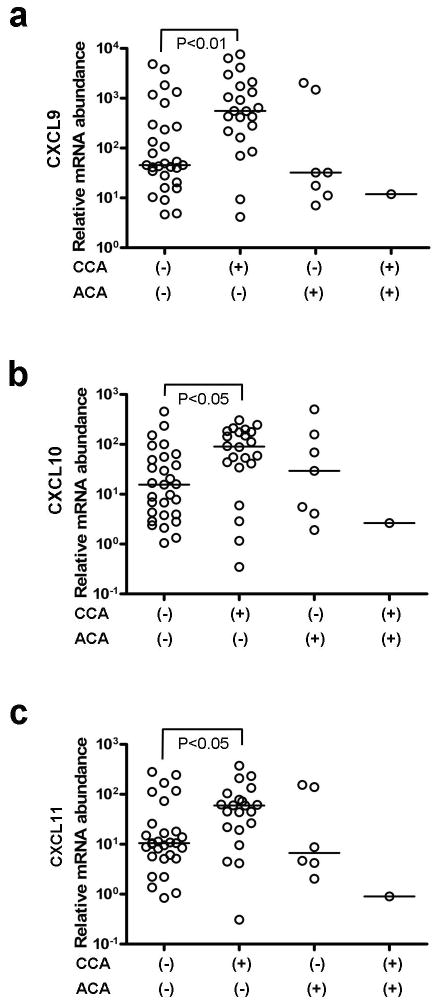
In a previous analysis of the human amnion transcriptome, we found mRNA expression of CXCL9, CXCL10, and CXCL11.29 As the chorioamniotic membranes are potential sources of amniotic fluid CXCL9, CXCL10, and CXCL11, we tested whether mRNA expression of these chemokines in the chorioamniotic membranes changed in CCA. qRT-PCR analysis showed mRNA expression of these three chemokines was significantly higher in the chorioamniotic membranes with CCA than in those without CCA in PTL and PPROM cases. The median mRNA expressions of CXCL9, CXCL10, and CXCL11 in CCA cases were 2.64 fold, 1.69 fold, and 1.74 fold higher than in cases without CCA (p<0.01, p<0.05, and p<0.05, respectively) (Figures 5a, 5b, and 5c). However, mRNA expressions of these chemokines did not increase with ACA.
Figure 5.
mRNA expressions of CXCL9, CXCL10, and CXCL11 in the chorioamniotic membranes. CXCL9 (a), CXCL10 (b), and CXCL11 (c) mRNA expressions are significantly higher in chronic chorioamnionitis (CCA) positive cases, but not in acute chorioamnionitis (ACA) positive cases when compared to cases without inflammation.
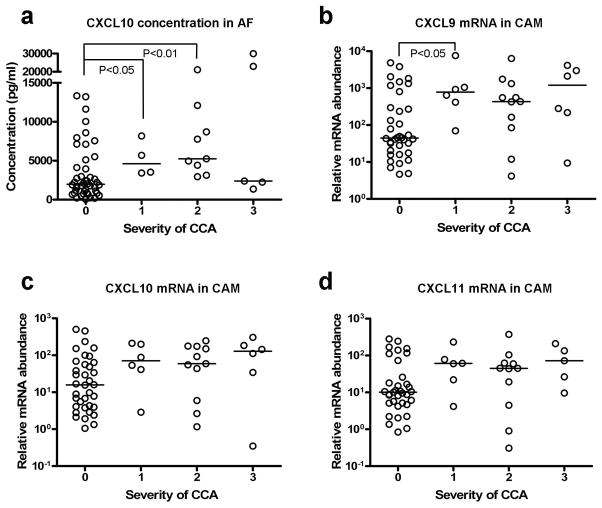
On further examination of amniotic fluid CXCL10 concentration and mRNA expressions of CXCL9, CXCL10, and CXCL11 in the chorioamniotic membranes according to the severity of CCA, we found that even histologically mild CCA had significantly higher amniotic fluid CXCL10 concentration (p<0.05) and higher expression of CXCL9 mRNA (p<0.05) in the chorioamniotic membranes than in those without CCA (Figures 6a, 6b, 6c, and 6d).
Figure 6.
Comparisons of amniotic fluid (AF) CXCL10 concentration (a) and mRNA expressions of CXCL9 (b), CXCL10 (c), and CXCL11 (d) in the chorioamniotic membranes (CAM) according to the severity of chronic chorioamnionitis (CCA). Severity of CCA was graded as 1: grade 1/stage 1 inflammation, 2: grade 1/stage 2 or grade 2/stage 1 inflammation, and 3: grade 2/stage 2 inflammation. The comparisons reveal that even mild CCA is associated with significantly increased amniotic fluid CXCL10 concentration and CXCL9 mRNA expression in the chorioamniotic membranes.
Discussion
The primary finding of this study is that CCA is a common histologic lesion of the placenta of patients with preterm labor with intact membranes and preterm prelabor rupture of the membranes. Moreover, the changes of amniotic fluid CXCL10 concentration and the common association with VUE indicate that CCA is not simply a locally restricted histologic phenomenon. The CCA and elevated amniotic fluid T cell chemokine CXCL10 complex could be considered a counterpart of the acute chorioamnionitis (ACA) and elevated neutrophil chemokines (IL-8, CXCL6) complex, which is due to ascending intra-amniotic infection.12,13 The median gestational age of CCA in PTL and PPROM groups is higher than that of ACA. This is important because the pathologic basis for spontaneous preterm labor and PPROM resulting in “late preterm (near-term) delivery” has remained a mystery.7,30 Our data suggest that this is an important lesion which accounts for the most frequent type of all spontaneous preterm deliveries. This association is novel.
In contrast to ACA following microbial invasion of the amniotic cavity, the underlying etiology of CCA is unknown. Subclinical intra-amniotic infection by microorganisms (such as viruses) is clearly one possibility. Several studies focusing on the viral load of amniotic fluid using PCR techniques have reported variable detection rates for viruses, such as cytomegalovirus, parvovirus, and adenovirus.31,32 Miller et al. even detected viral genomes in 40 of 686 cases of second trimester genetic amniocentesis obtained from asymptomatic patients.33 However, meticulous evaluation for potential viral, bacterial, and protozoal pathogens did not reveal any specific infectious agents in a previous analysis of 17 cases of CCA.22 Jacques and Qureshi also pointed out that the pathology of villitis lesions was not consistent with the typical presentation found in villitis of infectious origins, such as cytomegalovirus, toxoplasmosis, syphilis, and herpes simplex virus.21 Therefore, the frequent coexistence of CCA and VUE noted in previous studies (and documented in the current study) strongly suggests that these two pathologic lesions are likely to have a non-infectious origin. We propose, based on the change in the chemokine profile of amniotic fluid and the association with VUE, an immunological etiology. Specifically, we postulate that CCA is akin to combined allograft rejection and graft-versus-host disease (GVHD) in the chorioamniotic membranes as previously suggested for VUE.24 The essential pathology of VUE is the interaction between infiltrating maternal T cells and fetal placental resident macrophages (Hofbauer cells) in the fetal chorionic villi.34,35 We think that the pathophysiology of CCA is basically identical, given that the cells involved include maternal T cells, fetal chorionic trophoblasts, Hofbauer cells, and myofibroblasts in the chorioamniotic connective tissue.
Multiple etiologies such as infection, uteroplacental ischemia, uterine over-distension, and abnormal allograft rejection are thought to be associated with preterm birth.2,36 Therefore, preterm parturition itself has a syndromic nature.2 Ascending intrauterine infection and uteroplacental ischemia are typical histopathological alterations of the placenta found in major subsets of preterm birth.10,26,37,38 Acute chorioamnionitis is the pathologic expression of intra-amniotic infection. Histological features compatible with inadequate, superficial placentation and subsequent underperfusion of the placenta characterize another group of preterm birth, including preeclampsia and intrauterine growth restriction before the gestational age of 28 weeks.39 Our observations clearly indicate that CCA comprises a major group, a definable placental phenotype associated with preterm birth. Given that this lesion is observed at a higher gestational age at birth than others, we postulate that this lesion is a major feature in late preterm (near-term) birth. We found the trajectory of the frequency of CCA - decreasing with advancing gestation - particularly intriguing. VUE is considered a lesion of term (not preterm) placentas;28,40 therefore, this lesion cannot account for the pathology of late preterm (near-term) birth. The underlying pathologic lesion of late preterm birth is of major healthcare importance because these are the most common types of all preterm births, and infants have an increased rate of mortality, respiratory distress, infection, and behavioral problems despite being born so close to term.41,42
A unique finding of this study is a surprisingly high frequency of CCA in each group. It seems that the detection of mild CCA lesions (which are likely to be under-diagnosed in routine surgical pathology practice) is primarily responsible for this observation. Gersell et al22 provided meticulous and comprehensive descriptions of the pathology of chronic chorioamnionitis based on the types of leukocytic infiltrates (lymphocytic, lymphoplasmacytic, and neutrophilic) and the anatomical regions (chorion along, amnion and chorion, and chorionic plate) involved. Jacques and Qureshi,21 in their analysis of CCA cases, applied a grading system to describe the extent of inflammation (1 +, few scattered foci; 2 +, up to half of the membrane roll involved; and 3 +, more than half of the membrane roll involved). Our criteria for the grading and staging of inflammation reflect the principles used in both of the studies, the primary difference being the inclusion of stage 1 lesions in which inflammatory infiltrates are confined to the chorionic trophoblast layer. Therefore, the pathologic significance of mild CCA could be a subject of debate if it were based on morphologic grounds alone; however, the clear changes in amniotic fluid concentrations of CXCL10 and increased CXCL9, CXCL10, and CXCL11 mRNA expression in the chorioamniotic membranes lends strong support for its biological significance, even in mild cases. We argue that this lesion reflects a change in the profile of intra-amniotic chemokines. This situation is analogous to elevation of amniotic fluid IL-6 in even mild ACA.43
Of note, two recent studies reported the presence of chronic inflammation and chronic deciduitis in substantial proportions of preterm cases. Goldenberg et al found chronic inflammation involving any site of the placenta (free fetal membranes, chorionic plate, and decidua basalis) in 20.9% and 13.4% of indicated and spontaneous preterm birth cases, respectively.44 Based on the pathologic descriptions in this report, chronic deciduitis of the basal plate27 and CCA are thought to be included in the analysis. Edmondson et al compared the pathologic lesions in 39 cases of idiopathic preterm labor without clinical chorioamnionitis with a gestational age-matched control group and found chronic deciduitis with plasma cells in 41% of cases and in 15% of controls, respectively.45 The findings in the present study showing an association between chronic deciduitis with plasma cells and preterm birth cases seem to be consistent with previous observations. Collectively, all these observations suggest that a large proportion of preterm births have a non-infectious immunologic component.
Another intriguing observation in this study is the relatively high frequency of VUE in PTL (19 %) and PPROM (18 %) cases. This indicates that, despite the general notion that VUE is largely a lesion found in term placentas, the occurrence of VUE in preterm cases can vary depending on the cohorts analyzed. A study of 539 preterm and 214 term placentas also found chronic villitis in 17% of preterm deliveries without umbilical vasculitis.46 CCA and VUE seem to be different histologic manifestations of the same pathologic process, and thereby result in different clinical consequences depending on the anatomical regions of the placenta involved. Since inflammation of the chorioamniotic membranes is present in both physiologic (term) and pathologic (preterm) parturition,8,20 CCA is expected to have a more profound impact than VUE involving the villous part of the placenta in spontaneous preterm birth. A higher stage of CCA in PPROM cases than in PTL cases and a higher frequency of CCA in patients at term not in labor rather than term in labor represent other intriguing observations requiring further investigation.
CXCL9, CXCL10, and CXCL11 belong to a family of CXC chemokines which are involved in the migration of CXCR3+ activated T lymphocytes, and which also have anti-angiogenic activity.47 Increased expression of these chemokines has been described as one of the major changes taking place in either organ transplantation rejection or GVHD.48–51 Higher mRNA expressions of CXCL9, CXCL10, and CXCL11 in the chorioamniotic membranes of CCA cases are quite consistent with our expectations. Pathologic up-regulation of CXC chemokines for CXCR3+ cells in the chorioamniotic membranes would lead to increased amniotic fluid concentration, and eventually, development of CCA by stimulating amniotropic maternal T cell migration. A limitation of this study is that we did not measure amniotic fluid CXCL9 and CXCL11 concentrations. This was due to limited volumes of amniotic fluid available for immunoassay. However, it is noteworthy that CXCL11 concentration in the amniotic fluid was found to have predictive value for preterm birth in an analysis of 312 second trimester transabdominal amniocentesis samples, although the placental pathology of the cases was not studied.52 We also found six cases among PTL and PPROM cases without CCA that had very high amniotic fluid CXCL10 concentrations. Re-examination of these select cases with additional CD3 immunostaining revealed ACA in one case and T cell infiltration consistent with CCA in the other four cases.
In conclusion, the findings reported herein provide strong support for the view that CCA and increased intra-amniotic CXCL10 concentrations are an additional and important clue that may explain what has been called, thus far, “idiopathic preterm births”. We consider that untimely birth is the result of a pathologic process, and that CCA is a major immunopathologic lesion of preterm birth in which even histologically mild but amniotropic T cell inflammation is associated with a derangement of the intra-amniotic chemokine environment. Future studies of the mechanisms responsible for the perturbed T cell chemokine regulation in the amniotic fluid and the chorioamniotic membranes are urgently needed to unveil the fundamental cause of this lesion as well as a major fraction of spontaneous preterm birth.
Acknowledgments
This work was supported in part by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS. The authors are grateful to the patients who agreed to participate in our studies and to the nurses, laboratory staff and clinicians who made this work possible.
Footnotes
Disclosure/Conflict of Interest
The authors declare no conflict of interest.
Reference List
- 1.Faye-Petersen OM. The placenta in preterm birth. J Clin Pathol. 2008;61:1261–1275. doi: 10.1136/jcp.2008.055244. [DOI] [PubMed] [Google Scholar]
- 2.Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. Bjog. 2006;113:17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davidoff MJ, Dias T, Damus K, et al. Changes in the gestational age distribution among U.S. singleton births: impact on rates of late preterm birth, 1992 to 2002. Semin Perinatol. 2006;30:8–15. doi: 10.1053/j.semperi.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Hagberg H, Mallard C, Jacobsson B, et al. Role of cytokines in preterm labour and brain injury. Bjog. 2005;112:16–18. doi: 10.1111/j.1471-0528.2005.00578.x. [DOI] [PubMed] [Google Scholar]
- 5.Romero R, Gotsch F, Pineles B, et al. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev. 2007;65:S194–S202. doi: 10.1111/j.1753-4887.2007.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 6.Russell RB, Green NS, Steiner CA, et al. Cost of hospitalization for preterm and low birth weight infants in the United States. Pediatrics. 2007;120:e1–e9. doi: 10.1542/peds.2006-2386. [DOI] [PubMed] [Google Scholar]
- 7.Raju TN. Epidemiology of late preterm (near-term) births. Clin Perinatol. 2006;33:751–763. doi: 10.1016/j.clp.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Romero R, Espinoza J, Goncalves LF, et al. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiGiulio DB, Romero R, Amogan HP, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One. 2008;3:e3056. doi: 10.1371/journal.pone.0003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redline RW, Faye-Petersen O, Heller D, et al. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2003;6:435–448. doi: 10.1007/s10024-003-7070-y. [DOI] [PubMed] [Google Scholar]
- 11.Salafia CM, Weigl C, Silberman L, et al. The prevalence and distribution of acute placental inflammation in uncomplicated term pregnancies. Obstet Gynecol. 1989;73:383–389. [PubMed] [Google Scholar]
- 12.Cherouny PH, Pankuch GA, Romero R, et al. Neutrophil attractant/activating peptide-1/interleukin-8: association with histologic chorioamnionitis, preterm delivery, and bioactive amniotic fluid leukoattractants. Am J Obstet Gynecol. 1993;169:1299–1303. doi: 10.1016/0002-9378(93)90297-v. [DOI] [PubMed] [Google Scholar]
- 13.Mittal P, Romero R, Kusanovic JP, et al. CXCL6 (granulocyte chemotactic protein-2): a novel chemokine involved in the innate immune response of the amniotic cavity. Am J Reprod Immunol. 2008;60:246–257. doi: 10.1111/j.1600-0897.2008.00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoon BH, Park CW, Chaiworapongsa T, et al. Intrauterine infection and the development of cerebral palsy. Bjog. 2003;110:124–127. doi: 10.1016/s1470-0328(03)00063-6. [DOI] [PubMed] [Google Scholar]
- 15.Gotsch F, Romero R, Kusanovic JP, et al. The fetal inflammatory response syndrome. Clin Obstet Gynecol. 2007;50:652–683. doi: 10.1097/GRF.0b013e31811ebef6. [DOI] [PubMed] [Google Scholar]
- 16.Novy MJ, Duffy L, Axthelm MK, et al. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reprod Sci. 2009;16:56–70. doi: 10.1177/1933719108325508. [DOI] [PubMed] [Google Scholar]
- 17.Sadowsky DW, Adams KM, Gravett MG, et al. Preterm labor is induced by intraamniotic infusions of interleukin-1beta and tumor necrosis factor-alpha but not by interleukin-6 or interleukin-8 in a nonhuman primate model. Am J Obstet Gynecol. 2006;195:1578–1589. doi: 10.1016/j.ajog.2006.06.072. [DOI] [PubMed] [Google Scholar]
- 18.Maymon E, Romero R, Pacora P, et al. A role for the 72 kDa gelatinase (MMP-2) and its inhibitor (TIMP-2) in human parturition, premature rupture of membranes and intraamniotic infection. J Perinat Med. 2001;29:308–316. doi: 10.1515/JPM.2001.044. [DOI] [PubMed] [Google Scholar]
- 19.Chen FC, Sarioglu N, Buscher U, et al. Lipopolysaccharide binding protein in the early diagnosis of intraamniotic infection of pregnant women with premature rupture of the membranes. J Perinat Med. 2009;37:135–139. doi: 10.1515/JPM.2009.004. [DOI] [PubMed] [Google Scholar]
- 20.Haddad R, Tromp G, Kuivaniemi H, et al. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am J Obstet Gynecol. 2006;195:394, e1–24. doi: 10.1016/j.ajog.2005.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacques SM, Qureshi F. Chronic chorioamnionitis: a clinicopathologic and immunohistochemical study. Hum Pathol. 1998;29:1457–1461. doi: 10.1016/s0046-8177(98)90016-8. [DOI] [PubMed] [Google Scholar]
- 22.Gersell DJ, Phillips NJ, Beckerman K, et al. Chronic chorioamnionitis: a clinicopathologic study of 17 cases. Int J Gynecol Pathol. 1991;10:217–229. [PubMed] [Google Scholar]
- 23.Gersell DJ. Chronic villitis, chronic chorioamnionitis, and maternal floor infarction. Semin Diagn Pathol. 1993;10:251–266. [PubMed] [Google Scholar]
- 24.Kim MJ, Romero R, Kim CJ, et al. Villitis of unknown etiology is associated with a distinct pattern of chemokine up-regulation in the feto-maternal and placental compartments: implications for conjoint maternal allograft rejection and maternal anti-fetal graft-versus-host disease. J Immunol. 2009;182:3919–3927. doi: 10.4049/jimmunol.0803834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Redline RW, Ariel I, Baergen RN, et al. Fetal vascular obstructive lesions: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2004;7:443–452. doi: 10.1007/s10024-004-2020-x. [DOI] [PubMed] [Google Scholar]
- 26.Redline RW, Boyd T, Campbell V, et al. Maternal vascular underperfusion: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2004;7:237–249. doi: 10.1007/s10024-003-8083-2. [DOI] [PubMed] [Google Scholar]
- 27.Khong TY, Bendon RW, Qureshi F, et al. Chronic deciduitis in the placental basal plate: definition and interobserver reliability. Hum Pathol. 2000;31:292–295. doi: 10.1016/s0046-8177(00)80241-5. [DOI] [PubMed] [Google Scholar]
- 28.Redline RW. Villitis of unknown etiology: noninfectious chronic villitis in the placenta. Hum Pathol. 2007;38:1439–1446. doi: 10.1016/j.humpath.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 29.Han YM, Romero R, Kim JS, et al. Region-specific gene expression profiling: novel evidence for biological heterogeneity of the human amnion. Biol Reprod. 2008;79:954–961. doi: 10.1095/biolreprod.108.069260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raju TN, Higgins RD, Stark AR, et al. Optimizing care and outcome for late-preterm (near-term) infants: a summary of the workshop sponsored by the National Institute of Child Health and Human Development. Pediatrics. 2006;118:1207–1214. doi: 10.1542/peds.2006-0018. [DOI] [PubMed] [Google Scholar]
- 31.Wenstrom KD, Andrews WW, Bowles NE, et al. Intrauterine viral infection at the time of second trimester genetic amniocentesis. Obstet Gynecol. 1998;92:420–424. doi: 10.1016/s0029-7844(98)00210-5. [DOI] [PubMed] [Google Scholar]
- 32.Liesnard C, Donner C, Brancart F, et al. Prenatal diagnosis of congenital cytomegalovirus infection: prospective study of 237 pregnancies at risk. Obstet Gynecol. 2000;95:881–888. doi: 10.1016/s0029-7844(99)00657-2. [DOI] [PubMed] [Google Scholar]
- 33.Miller JL, Harman C, Weiner C, et al. Perinatal outcomes after second trimester detection of amniotic fluid viral genome in asymptomatic patients. J Perinat Med. 2009;37:140–143. doi: 10.1515/JPM.2009.027. [DOI] [PubMed] [Google Scholar]
- 34.Myerson D, Parkin RK, Benirschke K, et al. The pathogenesis of villitis of unknown etiology: analysis with a new conjoint immunohistochemistry-in situ hybridization procedure to identify specific maternal and fetal cells. Pediatr Dev Pathol. 2006;9:257–265. doi: 10.2350/08-05-0103.1. [DOI] [PubMed] [Google Scholar]
- 35.Kim JS, Romero R, Kim MR, et al. Involvement of Hofbauer cells and maternal T cells in villitis of unknown aetiology. Histopathology. 2008;52:457–464. doi: 10.1111/j.1365-2559.2008.02964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldenberg RL, Culhane JF, Iams JD, et al. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arias F, Rodriquez L, Rayne SC, et al. Maternal placental vasculopathy and infection: two distinct subgroups among patients with preterm labor and preterm ruptured membranes. Am J Obstet Gynecol. 1993;168:585–591. doi: 10.1016/0002-9378(93)90499-9. [DOI] [PubMed] [Google Scholar]
- 38.Arias F, Victoria A, Cho K, et al. Placental histology and clinical characteristics of patients with preterm premature rupture of membranes. Obstet Gynecol. 1997;89:265–271. doi: 10.1016/S0029-7844(96)00451-6. [DOI] [PubMed] [Google Scholar]
- 39.McElrath TF, Hecht JL, Dammann O, et al. Pregnancy disorders that lead to delivery before the 28th week of gestation: an epidemiologic approach to classification. Am J Epidemiol. 2008;168:980–989. doi: 10.1093/aje/kwn202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Becroft DM, Thompson JM, Mitchell EA, et al. Placental villitis of unknown origin: epidemiologic associations. Am J Obstet Gynecol. 2005;192:264–271. doi: 10.1016/j.ajog.2004.06.062. [DOI] [PubMed] [Google Scholar]
- 41.Raju TN. The problem of late-preterm (near-term) births: a workshop summary. Pediatr Res. 2006;60:775–776. doi: 10.1203/01.pdr.0000246074.73342.1e. [DOI] [PubMed] [Google Scholar]
- 42.Gray RF, Indurkhya A, McCormick MC, et al. Prevalence, stability, and predictors of clinically significant behavior problems in low birth weight children at 3, 5, and 8 years of age. Pediatrics. 2004;114:736–743. doi: 10.1542/peds.2003-1150-L. [DOI] [PubMed] [Google Scholar]
- 43.Yoon BH, Romero R, Kim CJ, et al. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol. 1995;172:960–970. doi: 10.1016/0002-9378(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 44.Goldenberg RL, Andrews WW, Faye-Petersen O, et al. The Alabama Preterm Birth Project: placental histology in recurrent spontaneous and indicated preterm birth. Am J Obstet Gynecol. 2006;195:792–796. doi: 10.1016/j.ajog.2006.05.050. [DOI] [PubMed] [Google Scholar]
- 45.Edmondson N, Bocking A, Machin G, et al. The prevalence of chronic deciduitis in cases of preterm labor without clinical chorioamnionitis. Pediatr Dev Pathol. 2009;12:16–21. doi: 10.2350/07-04-0270.1. [DOI] [PubMed] [Google Scholar]
- 46.Salafia CM, Vogel CA, Vintzileos AM, et al. Placental pathologic findings in preterm birth. Am J Obstet Gynecol. 1991;165:934–938. doi: 10.1016/0002-9378(91)90443-u. [DOI] [PubMed] [Google Scholar]
- 47.Romagnani P, Lasagni L, Annunziato F, et al. CXC chemokines: the regulatory link between inflammation and angiogenesis. Trends Immunol. 2004;25:201–209. doi: 10.1016/j.it.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 48.Panzer U, Reinking RR, Steinmetz OM, et al. CXCR3 and CCR5 positive T-cell recruitment in acute human renal allograft rejection. Transplantation. 2004;78:1341–1350. doi: 10.1097/01.tp.0000140483.59664.64. [DOI] [PubMed] [Google Scholar]
- 49.Tan J, Zhou G. Chemokine receptors and transplantation. Cell Mol Immunol. 2005;2:343–349. [PubMed] [Google Scholar]
- 50.Wysocki CA, Panoskaltsis-Mortari A, Blazar BR, et al. Leukocyte migration and graft-versus-host disease. Blood. 2005;105:4191–4199. doi: 10.1182/blood-2004-12-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piper KP, Horlock C, Curnow SJ, et al. CXCL10-CXCR3 interactions play an important role in the pathogenesis of acute graft-versus-host disease in the skin following allogeneic stem-cell transplantation. Blood. 2007;110:3827–3832. doi: 10.1182/blood-2006-12-061408. [DOI] [PubMed] [Google Scholar]
- 52.Malamitsi-Puchner A, Vrachnis N, Samoli E, et al. Elevated second trimester amniotic fluid interferon gamma-inducible T-cell alpha chemoattractant concentrations as a possible predictor of preterm birth. J Soc Gynecol Investig. 2006;13:25–29. doi: 10.1016/j.jsgi.2005.09.008. [DOI] [PubMed] [Google Scholar]