Structured abstract
Purpose of review
To discuss recent HIV epidemic models examining the transmission of antiretroviral (ARV) drug resistance.
Recent findings
A relatively small number of recent transmission models have investigated ARV resistance in the context of therapeutic, combined ART (cART); ARV-vaginal microbicides (ARV-VMB); and oral pre-exposure prophylaxis (PrEP). Models of cART use have highlighted potential concerns about future resistance transmission, particularly in resource-constrained settings, and have emphasized the benefits of viral load monitoring in limiting resistance spread. PrEP models have concluded that inadvertent use by HIV-infected individuals could increase resistance prevalence, and that risk compensation by PrEP users could limit their beneficial effects on HIV transmission. ARV-VMB models have demonstrated that while resistance can reduce prophylactic effectiveness in preventing HIV acquisition of female ARV-VMB users, it may concomitantly benefit users' male partners if the resistant strains that female users acquire are less transmissible than wild-type strains. The models have examined the balance between these two factors at the population level.
Summary
Recent HIV transmission models have adopted a wide assortment of structures and assumptions to explore drug resistance in the context of different ARV interventions in various settings. There is a need for future work emphasizing the simultaneous effects of multiple ARV interventions, as well as the public health impact of resistance, not just its prevalence.
Keywords: HIV, mathematical modeling, antiretroviral, resistance, pre-exposure prophylaxis (PrEP), microbicides
Introduction
As scale-up of combined antiretroviral therapy (cART) in developing countries continues, more attention is being given to the possible consequences of antiretroviral (ARV) use on HIV transmission. Of particular interest are rates of drug resistance emergence and transmission in resource-constrained settings. Acquired (or secondary) resistance may emerge within an individual treated with cART due to treatment pressure. The transmission of such resistant strains to others is referred to as transmitted (or primary) resistance (TDR).
Various mathematical models with different assumptions, parameter values and complexity levels have been used to try to predict the population-level impact of various forms of ARV use (1): for therapeutic benefit (cART (2-9)), prevention of mother-to-child transmission (PMTCT (10)) and pre-exposure prophylaxis (PrEP) (administered either orally (11, 12) or as topical vaginal microbicides (ARV-VMB) (13, 14)). Early models generally focused on the public health benefits of ARV interventions, ignoring the risk of resistance emergence and transmission. More recently, ARV resistance has become a focus in itself (8, 11), as its potential to jeopardize the scale-up of cART in resource-limited settings with few treatment options has become of increasing concern (15).
The earliest models of cART focused on men who have sex with men (MSM) and intravenous drug user populations in industrialized settings (3, 4, 16, 17), generally predicting high levels of resistance in the long-term (as high as 26%-83% with high coverage and/or under pessimistic scenarios (4, 16)), at least partly due to relatively early treatment initiation (the “hit early, hit hard” approach). Later, increased cART usage in resource-limited settings was paralleled by greater numbers of models predicting its impact in these regions (2, 18). These models tended to predict relatively low levels of TDR in the short-term, often due to assumptions that cART coverage would remain low because of resource constraints and treatment guidelines advising relatively late treatment initiation (2, 18). However, longer-term predictions were more varied due to differences in parameter assumptions and model structure, such as the infectiousness of resistant strains, how quickly resistance emerged, and whether resistant strains could revert to wild-type.
This review focuses on recent modeling work involving ARV TDR using population-based HIV transmission models. We do not cover within-host models (19-21) or linear (Markov, cohort or decision-analysis) models because they do not explicitly model HIV transmission (22-25). We also exclude ARV resistance in the context of PMTCT and post-exposure prophylaxis because, while several cohort models have been published (10, 23, 26, 27), we know of no published HIV transmission models.
Therapeutic, combination ART (cART)
While resistance has been of concern in high-income countries since the introduction of cART (4, 16, 17), the large number of treatment options for patients in these settings means that drug resistance is generally a lesser threat to patients' prognoses. In contrast, the limited treatment options for cART patients in low-income countries mean that regimens must be preserved, and therefore resistance is a bigger concern. Additionally, recent modeling work exploring universal test and treat for HIV prevention (28, 29) raises new questions regarding cART-related resistance.
cART-based HIV prevention works on the assumption that ARVs decrease HIV infectiousness by decreasing viral load (29-31). How transmissibility (sometimes described as “viral fitness”) would change for resistant strains is unclear and challenging to measure. Recent models have generally assumed that resistant strains are not as infectious as wild-type (7-9, 11, 14), but it is unclear if this is a reasonable assumption. The extensive literature on resistance in industrialized countries may not be readily generalizable to resource-poor settings, where cART usage follows different patterns, absolute numbers on treatment are generally larger and monitoring is less intense. For modeling wild-type as well as resistant infectiousness, many models (e.g. (9)) have relied on the relationship between viral load and infectiousness estimated in Rakai, Uganda (30, 32). However, transmissibility of resistant strains may not have a predictable association with patients' viral load, and viral fitness may change rapidly, as the fast turnover of virus and lack of HIV proof-reading mechanism may result in compensatory mutations that restore transmissibility over time (33), producing a “transmissibility spectrum” at the population-level. For present cART coverage levels and potential future increases, there is a need to examine the extent to which resistance could undermine a) the effectiveness of cART in delaying HIV disease progression (an assessment of which is beyond the scope of this article); and b) its use for preventing HIV spread.
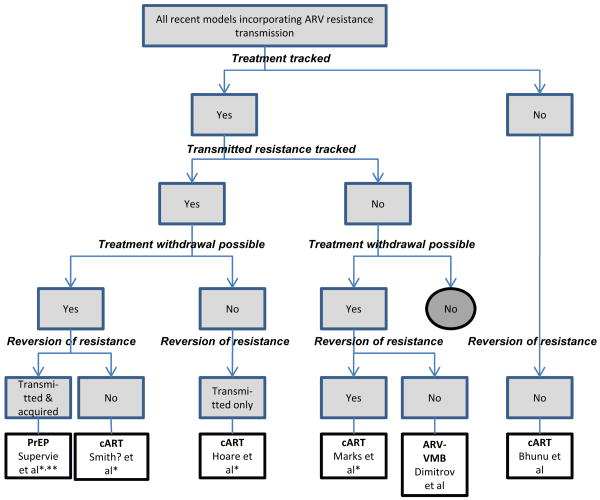
Recent cART models incorporating resistance transmission have been of two basic types: a) theoretical (examining the impact of model structure and assumptions on different resistance outcomes) (6, 8); and b) setting-specific (7, 9). We briefly describe characteristics of recent ARV-resistance transmission models in table 1 and summarize key resistance assumptions of each in Figure 1.
Table.
Attributes of recent (2009 onwards) mathematical model studies incorporating the impact of antiretroviral drug resistance.
| Author | Setting | Structure | Objective | Resistance-related assumptions* | Outcomes |
|---|---|---|---|---|---|
| Therapeutic cART | |||||
| Smith? et al 2010 (9) | MSM, San Francisco, US | Deterministic, multiple disease stages, specific resistant phenotypes based on ARV classes | Trace the evolutionary history of ARV resistance in San Francisco and predict future dynamics | 7 resistance categories (single, dual or triple class). Withdrawal from treatment is possible but no reversion to wild-type or loss of mutations to any ARV class. | cART has prevented 1° resistance reaching >15% in San Francisco. However patterns continue to evolve; 60% of currently circulating resistant strains can cause self-sustaining epidemics. A wave of NNRTI-resistant strains will emerge over the next 5 years. |
| Marks et al 2010 (8) | Theoretical (based on an MSM transmission model (4)) | Stochastic version based on deterministic model (4) (similar to Figure 1a) | “Thought experiment”: how chance events affect the emergence of resistance | Allows reversion from resistant to wild-type HIV infection upon cessation of cART. No explicit differentiation of 1° and 2° resistance. | Variability in 1° resistance prevalence within an epidemic is large when numbers of new infections are small (<200/y) but diminishes rapidly with higher numbers |
| Bhunu et al 2009 (6) | Theoretical | Deterministic, HIV and AIDS stages | Mathematical analysis of competing wild-type and resistant strains | A fraction of those initiating cART immediately develop resistance; the remainder are transferred from the AIDS to HIV compartment. No compartments distinguish treated from untreated individuals. | Qualitative analysis involving analytic solutions including stability analysis. Wild-type and resistant strains would co-exist, rather than being driven to extinction, whenever the reproduction numbers** >1. Resistance increases with increasing ARV use. |
| Hoare et al 2010 (7) | Southeast Asia (Thailand as example) | Deterministic, multiple disease stages, separation of those with 1° and 2° resistance | Impact of universal one-regimen cART on ARV resistance in Southeast Asian settings | Those with 1° resistance may revert to wild-type when untreated but resistance redevelops upon treatment. | Without monitoring, ∼24% new infections could include resistance mutations after 10 years. |
| Antiretroviral microbicides (ARV-VMB) or Pre-exposure prophylaxis (PrEP) | |||||
| Abbas et al 2010 (abstract) (12) | Sub-Saharan Africa, heterosexual | Age, gender, sexual activity and disease stage stratified (likely deterministic compartmental but not specified). Allows drug discontinuation. | PrEP, optimistic and pessimistic scenarios determined by parameter values | Optimistic (pessimistic): 75% (25%) PrEP effectiveness, 60% (15%) at-risk population use PrEP, 5% (25%) already infected inadvertently use PrEP | Optimistic (pessimistic): 2.5% (40%) resistance prevalence after 10 years. Inadvertent PrEP use by previously-infected people is the major determinant of resistance prevalence from PrEP. |
| Dimitrov et al 2010 (14) | Heterosexual populations, low-or middle-income settings | Deterministic, HIV and AIDS stages, gender | Population-level benefits of ARV-VMB by gender | Resistance transmission but no reversion. No explicit differentiation of 1° and 2° resistance. | Women are more likely than men to benefit from ARV-VMB use. A substantial male advantage only occurs if risk of resistance developing is high and HIV-positive women use the ARV-VMB indefinitely (because when resistance develops it reduces risk of female-to-male HIV transmission). |
| Supervie et al 2010 (11) | MSM, San Francisco, US | Deterministic, multiple disease stages, ARV use as cART as well as PrEP, separation of those with 1° and 2° resistance | Predicting public health impact of PrEP | Assumes individuals are HIV tested before being prescribed PrEP. Allows PrEP cessation and reversion to wild-type. “Fairly high” prevalence of 1° resistance when PrEP introduced. “Resistance” is the same for PrEP and cART. | “Paradox” that PrEP could increase proportion of new infections that are ARV resistant, even though absolute numbers decrease, if PrEP is effective (efficacy >30%, relative efficacy against resistant strains >0.2 but <1). If there is risk compensation, PrEP could significantly increase 1° resistance; if not, it is likely to decrease. |
Resistant strains were assumed to be less infectious than wild-type and unless stated, risk compensation was not considered.
The reproductive number is the number of secondary (onward) infections transmitted by one infected individual.
1° and 2° - primary (transmitted) and secondary (acquired) resistance, respectively.
NNRTIs – Non-nucleoside reverse transcriptase inhibitors; MSM – men who have sex with men
Figure 1.
Categorization tree for recently published (2009 onwards) HIV transmission models incorporating ARV resistance. All included models involve transmission of resistant and wild-type HIV strains, with all models but Bhunu et al following the structure of Figure 2a (6).) “Treatment” refers to all forms of ARV therapy or prophylaxis: cART, PrEP or ARV-VMB. “Transmitted resistance tracked” refers to tracking TDR and acquired resistance separately. Model assumptions and/or details: * there is a cost of resistance, in terms of reduced infectiousness of resistant strains; ** investigation of the potential effect of risk compensation. Abbas et al is not included in this figure as full details of their model are not available from the conference abstract (12).
Marks et al conducted a “thought experiment” to explore variabilities in TDR expected due to stochasticity (chance events) alone (8). They found that stochasticity can produce substantial variability within an epidemic (i.e. over time) when HIV incidence is low (<200 new infections annually) but this stochastic variability reduces as the number of incident cases grows. Across epidemics, TDR again showed substantial variability for small but not large numbers of incident cases if treatment was introduced after endemic equilibrium was established, but stochastic variation could persist in settings with earlier treatment introduction even with more than 10,000 incident cases annually. These results suggest that sound interpretation of temporal trends in TDR prevalence within a given setting require repeated surveys including hundreds of new infections, and that stochastic models can also help explain differences in TDR across settings.
Bhunu et al also conducted a theoretical analysis, examining conditions under which wild-type and resistant HIV strains can co-exist in settings with cART (6). As expected intuitively, they showed that either or both strains will die out if their respective reproductive numbers (R0, the average number of infections that each infected individual transmits to others over their entire infectious lifespan) are less than the threshold value of 1, and that both strains will co-exist if both reproductive numbers are above 1. They also report that increasing treatment rates increases the prevalence of both wild-type and resistant HIV (due to increased life expectancy in treated individuals), but that AIDS cases will decrease. While this study helps our theoretical understanding, its basic assumptions are markedly different from other models (Figure 1), making comparisons difficult. For example, Bhunu et al do not seem to adopt the common assumption that HIV infectiousness decreases with cART.
A recent model of the HIV epidemic among MSM in San Francisco explicitly modeled seven strains with single, double or triple class resistance to the three principal ARV classes (9). The authors calibrated the model to HIV prevalence in 1987 before making future predictions. Their results suggested that 60% of resistant strains currently circulating can cause self-sustaining epidemics, presenting a significant challenge to universal test and treat approaches. In particular, they predicted that NNRTI-resistant strains are likely to increase in prevalence substantially over the next 5 years, a finding that could have serious implications for cART in low-income countries, where most first-line regimens have an NNRTI backbone.
Modeling of the heterosexual HIV epidemic in Thailand involved a simpler resistance scheme, assuming that a single triple-ARV regimen is the only feasible option for Southeast Asian heterosexual populations (7). Universal cART access resulted in ∼24% of new infections being ARV-resistant after 10 years if patients were not monitored for treatment failure. However, only a minority of treatment-naïve individuals (≤1%) were expected to have detectable resistant virus, as it was assumed that transient reversion of resistant strains (where individuals continue to carry a minority resistant strain in absence of treatment) was possible for individuals with TDR. This suggests that resistance could remain hidden, only to re-emerge when the selective pressure of cART is applied. Finally, the authors found that viral load testing every two years, followed by switches to permanently effective, second-line therapy among those with virologic failure, reduced prevalence of TDR by more than 50% compared to no testing. The benefits of viral load monitoring increased with more frequent testing.
Antiretroviral vaginal microbicides (ARV-VMB) and pre-exposure prophylaxis (PrEP)
Preliminary studies suggesting that PrEP with ARVs could prevent transmission (34, 35) have been supported by a recent study reporting a 44% reduction in HIV incidence using a dual ARV drug PrEP regimen (36). However, the population-level impact of resistance due to PrEP is difficult to predict and concerns have been expressed regarding resistance emergence among PrEP users with undiagnosed acute HIV infection (37). Inadvertent PrEP/ARV-VMB use by HIV-positive individuals and the risk of systemic absorption of ARV-VMB may be key drivers in resistance development. While prophylactic effectiveness against HIV strains resistant to a particular PrEP drug will be limited, these drug-resistant strains will likely have reduced fitness relative to wild-type HIV, resulting in lower viral loads and/or infectiousness (38, 39). That is, the risk of HIV acquisition by PrEP users may increase in the context of circulating resistance, but the risk of subsequent transmission may decrease. The net result of drug resistance in the context of PrEP/ARV-VMB interventions will depend on the balance between these factors.
Topical ARV-VMB
In a recent modeling study of ARV-VMB use, Dimitrov et al (14) analyzed a wide range of scenarios (related to coverage and speed of scale-up, proportions of HIV-positive women ceasing ARV-VMB use due to control measures, and risk of systemic ARV absorption) under various epidemic conditions for developing countries. Assuming rapid roll-out and no control measures, the median fraction of infections averted (males and females combined) over 10 years varied between 16-23% and 22-28%, and resistance prevalence varied between 2%-3% and 17-20% for low- and high-risk (of systemic absorption of ARV-VMB) products, respectively. Scenarios involving high-risk ARV-VMB and low levels of HIV-positive females ceasing ARV-VMB use prevented the largest number of new HIV infections and produced the highest resistance prevalence. Limiting ARV-VMB use by HIV-positive women drastically reduced resistance levels, by up to 60% in some scenarios.
In comparison, an earlier model by Wilson et al predicted median levels of resistance among women of 5% and 22% for low-risk and high-risk ARV-VMB respectively and somewhat lower fractions of new infections prevented over 10 years (median 7% and 8% in females and males respectively for low-risk ARV-VMB, with corresponding values of 11% and 14% for high-risk ARV-VMB) (13). Despite these differences, the results of both studies highlight the importance of establishing surveillance systems for regular management and monitoring of ARV-VMB users.
Oral Pre-exposure prophylaxis (PrEP)
Two recent modeling studies have examined PrEP-related resistance transmission (11, 12). A model of HIV transmission among MSM in San Francisco concluded that PrEP interventions could prevent 20%-75% of new cases over 10 years (depending on PrEP efficacy and coverage), but that risk compensation could substantially compromise these reductions (11). Additionally, the model predicted that TDR prevalence could increase to levels exceeding 60% if coverage and efficacy are high. Another model focusing on sub-Saharan Africa (for which only a conference abstract is available) produced rather different estimates: overall resistance prevalence (transmitted plus acquired) was 40% under low coverage and efficacy (scenario 1), with substantially lower resistance prevalence (2.5%) at higher levels of efficacy and coverage (scenario 2) (12). As increasing coverage is expected to increase resistance, we hypothesize from the limited information provided in the abstract that their findings of an opposite trend may largely be due to confounding introduced by the choice of scenarios investigated. More specifically, the fraction of HIV-positive individuals who inadvertently used PrEP (one of the most important drivers of resistance prevalence in their study) was set to 25% in scenario 1 and only 5% in scenario 2. Nevertheless, these apparent discrepancies suggest that further work is needed to understand PrEP-related resistance in carefully selected scenarios.
Levels of model complexity
The resistance transmission models discussed above exhibit different levels of complexity (Figure 2). While theoretical papers, which aim to gain analytical insights (6) or identify the sensitivity of results to model structure (8) remain simple, studies predicting quantitative effects have tended to adopt more complexity, with more careful parameterization (7, 9, 11, 14). While the theoretical papers do not provide specific, quantitative predictions, they provide a necessary understanding of how model structure and assumptions affect results. The majority of resistance transmission models have assumed homogeneous risk behavior within populations (e.g. (7, 9, 11)), yet behavioral heterogeneity increases realism and is likely to influence model predictions (40).
Figure 2.
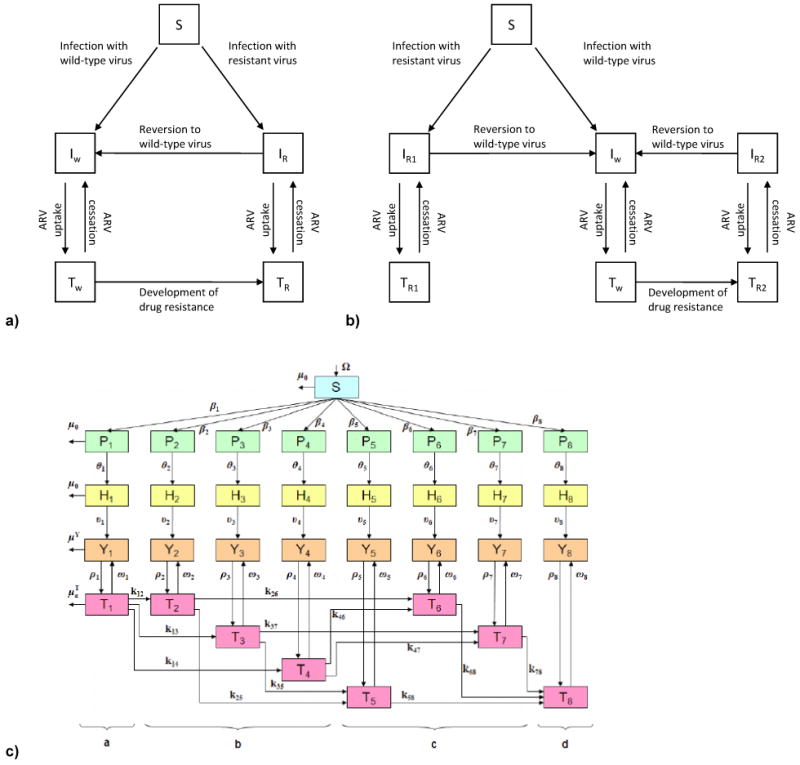
a) Basic structure of deterministic HIV transmission models in the context of drug resistance. The main compartments are: susceptible (S), infected with wild-type virus and untreated (Iw), infected with drug-resistant virus and untreated (IR), infected with wild-type virus and receiving ARVs (Tw), and infected with drug-resistant virus (TR) and receiving ARVs. Not included in the diagram are stages of infection, specific resistance categories to particular drug classes, and other modifications included in some models. (For simplicity, model entry and exit rates are omitted. Figure adapted from Marks et al (8) but a similar structure for resistance transmission has been used for many cART models (4, 11).) b) Change to model structure to separate individuals infected with HIV with primary (transmitted, R1) resistance and those initially infected with wild-type HIV who acquire resistance as a result of treatment: secondary (acquired, R2) ARV resistance. Similar structure to that used by Hoare et al (7). c) Structure of recent model of HIV transmission among MSM in San Francisco, US, with 33 compartments: schematic taken from Smith? et al (9). Compartments are: susceptible (S), HIV-infected with primary infection (P), infected not eligible for cART (H), cART eligible but not yet treated (Y) and treated (T). Strains are grouped into eight categories (subscripts) based upon resistance to ARV drug classes (further details in publication). PrEP models will differ because ARVs will be taken by susceptible (S) individuals, but the issues and concepts discussed here are similar.
Figure 2a adapted from Marks et al [8], 2b is adapted from Hoare et al [7] and figure 2c is reproduced with permission from Smith et al [9].
The required degree of model complexity depends on the context of a given research question. Figures 2a and 2b demonstrate that only models that explicitly separate individuals with TDR and acquired resistance can specify different rates of reversion to wild-type. This distinction can be important, as those with acquired resistance have mixed infections of resistant and wild-type strains, and thus if treatment pressure is removed, reversion is likely to occur more quickly than in those with TDR. This level of realism may be particularly important for models allowing cART uptake at earlier stages of infection or high rates of treatment withdrawal, because there is more opportunity for reversion.
While separating individuals with TDR from those with acquired resistance seems relatively straightforward, complexity increases and calculations can become unwieldy when stages of infection or other types of heterogeneity are also included. Figure 2c shows the structure of a recent model which stratifies resistance by individual ARV drug classes and resistance type (9). Incorporation of treatment withdrawal and reversion to wild-type within such a framework would substantially increase the computational complexity. The model was used for a retrospective analysis of the epidemic in San Francisco where, aside from the very early days when a “hit early, hit hard” approach was adopted, cART has generally been administered relatively late in infection. Therefore, levels of treatment withdrawal are likely to have been low (and patients would progress rapidly to AIDS upon cessation) and thus reversion may not be relevant enough to necessitate the additional complexity. In contrast, models of universal test and treat probably require treatment withdrawal categories and differential reversion rates by resistance type (TDR and acquired) because individuals will frequently stop cART earlier in infection, and would therefore be at substantial risk of transmitting HIV.
The public health impact of resistance
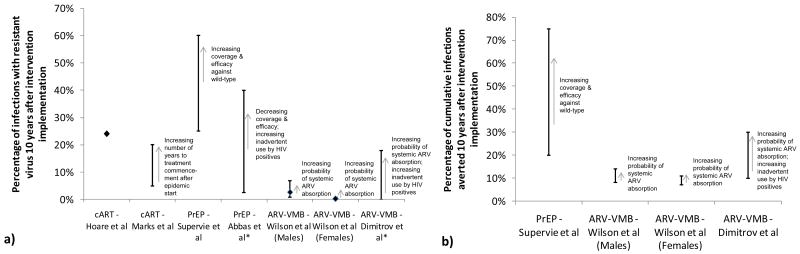
Predictions of the potential severity of drug resistance vary between studies, not only due to different model assumptions, but also because of different time horizons and different choices of outcome: for example, only a few studies chose the percentage of infections averted as an outcome, and while some studies report the overall prevalence of resistance (12, 14), others distinguish between acquired and transmitted resistance (7, 8, 11). Supervie et al point out that there may be a “paradox” when exploring different outcomes; for example, the proportion of new infections that are resistant increased over time in their study, but the actual number of new resistant infections (and all new infections) decreased (11). Comparing Figures 3a and 3b highlights the conundrum that interventions generating higher levels of resistance also tend to prevent more infections (13, 14). This tension between outcomes raises the question whether meaningful benefits from ARV prevention interventions will inevitably lead to high resistance amongst those infections which are not averted. These findings also highlight the need to identify which combination of outcomes give the fullest epidemiological picture and which ones are most relevant for public health officials and policy makers, particularly as more models exploring ARV resistance become available.
Figure 3.
a) Model-estimated prevalence of resistance ten years after intervention implementation. Estimates represent TDR (resistance prevalence of new infections 10 years after intervention implementation (7, 8, 11); Wilson et al's estimates represent cumulative proportion of new infections resistant over 10 years (13)); except * studies (12, 14) which estimate prevalence of resistance for all HIV infections. Hoare et al (Southeast Asia, heterosexual population) give a point estimate (in main text (7)); Marks et al (men who have sex with men, high-income settings) give a range ∼5%-20% (shown as error bars, taken from their Figures 2b-c simulation results of mean outcomes plus one standard deviation) from a stochastic model assuming treatment commencement 20 years and 30 years into the epidemic, respectively (8); Supervie et al (men who have sex with men, San Francisco, US) give a range of values in their Figure 3c (shown as error bars) based on varying levels of PrEP coverage (40-100%) and efficacy against wild-type strains (30-90%), in the absence of risk compensation (11); Abbas et al (sub-Saharan Africa heterosexual population) give resistance prevalence estimates assuming optimistic (40%) and pessimistic (2.5%) values, shown as error bars, for model parameters (with limited information available from the conference abstract – outcome is stated as drug resistance prevalence rather than explicitly stated as TDR (12)). Wilson et al (heterosexual population, setting not specified) give ranges stratified by gender (error bars represent interquartile ranges); shown on graph are estimates for ARV-VMB with high risk of systemic absorption; low-risk ARV-VMB give lower resistance estimates (data not shown) (13). Dimitrov et al (heterosexual population, developing countries) give resistance prevalence estimates (Figure 5c) (14). b) Model estimates of percentage of cumulative infections averted 10 years after intervention implementation, where available.
Ultimately, we wish to maximize the health gains provided by ARV drugs, which may include their use in preventing further HIV spread, but not at the risk of jeopardizing HIV patients' prognoses. Specifically, we need improved understanding of how TDR prevalence will affect the incidence-reducing potential of different ARV-based HIV prevention interventions, as well as the therapeutic efficacy of cART. Monitoring of cART program performance is crucial to help identify resistance emergence and transmission, through initiatives such as WHO HIVResNet (41). Transmission models can help in designing the most appropriate monitoring strategies, looking at monitoring frequency (7) and type (clinical or laboratory testing; CD4 count and viral load testing) (2) for cART and monitoring HIV acquisition among ARV-VMB and PrEP users (14). Models can also allow us to understand other issues which will be crucial for designing HIV treatment and prevention programs, such as the prevalence of minority resistance and its likely rate of emergence as majority resistance under cART (7).
HIVResNet was designed to determine when newly acquired TDR reaches 5% and 15% thresholds within a population, and yet we do not know how these levels relate to performance of first-line cART regimens. At what level of TDR must we think about changing first-line cART regimens, and over what timeframe? As the public health impact of resistance involves both HIV treatment and prevention, we require models which are larger in scope, incorporating multiple ARV interventions simultaneously (particularly cART and PrEP/ARV-VMB) in order to explore the potential synergies of these initiatives and the role that resistance plays amongst them.
Conclusion
A relatively small number of recent ARV resistance transmission models have examined different ARV interventions in different settings, using different assumptions and model structures (Figure 1). Model results have varied quite substantially with respect to predicted levels of resistance and effects on HIV incidence, suggesting that a solid understanding of feasible coverage and effectiveness levels, likely degrees of risk compensation, and other major forces are needed to refine model predictions and meaningfully interpret results for a given setting. As the momentum behind newer prevention strategies such as universal test-and-treat grows, there should be more exploration of the ways in which resistance transmission may jeopardize their clinical and public-health benefits. Future studies should look, in particular, at the synergies and redundancies (42) in ARV interventions (cART, PrEP, ARV-VMB, PMTCT), especially where the same ARV drugs are used or there is substantial cross-resistance between drugs. We need more exploration of multiple lines of cART and ARV resistance in order to identify when it is most appropriate to switch an individual to second-line therapy in terms of outcomes relevant to public health, and when a change in first-line regimen is warranted.
Above and beyond the modelers' remit, we need more empirical data from surveillance initiatives such as HIVResNet, particularly from settings where cART use is in its infancy, as patterns of ARV resistance may differ from those observed in high-income countries. These data will feed into models to predict future ARV use and how best to preserve their lifespan, especially because alternative ARVs are still prohibitively expensive for most resource-constrained settings. We therefore need more confidence in what level of TDR constitutes a real concern and could begin to reverse the benefits that ARVs have provided over the last 15 years. In conclusion, all model results suggest that in order to preserve the positive benefits of current and future ARV-based prevention tools, the potential risk associated with their wide scale use cannot be overlooked.
Acknowledgments
This work was supported by the Wellcome Trust [GR082623MA to RFB] and National Institutes of Health [NIAID R01 AI83059 to KP]. We thank the UK Medical Research Council (MRC) for Centre funding.
Footnotes
We declare that we have no conflict of interest.
References
- 1.Baggaley RF, Ferguson NM, Garnett GP. The epidemiological impact of antiretroviral use predicted by mathematical models: a review. Emerg Themes Epidemiol. 2005 Sep 10;2:9. doi: 10.1186/1742-7622-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baggaley RF, Garnett GP, Ferguson NM. Modelling the impact of antiretroviral use in resource-poor settings. PLoS Med. 2006 Apr;3(4):e124. doi: 10.1371/journal.pmed.0030124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blower SM, Aschenbach AN, Gershengorn HB, Kahn JO. Predicting the unpredictable: transmission of drug-resistant HIV. Nat Med. 2001 Sep;7(9):1016–20. doi: 10.1038/nm0901-1016. [DOI] [PubMed] [Google Scholar]
- 4.Blower SM, Gershengorn HB, Grant RM. A tale of two futures: HIV and antiretroviral therapy in San Francisco. Science. 2000 Jan 28;287(5453):650–4. doi: 10.1126/science.287.5453.650. [DOI] [PubMed] [Google Scholar]
- 5.Nagelkerke NJ, Jha P, de Vlas SJ, Korenromp EL, Moses S, Blanchard JF, et al. Modelling HIV/AIDS epidemics in Botswana and India: impact of interventions to prevent transmission. Bull World Health Organ. 2002;80(2):89–96. [PMC free article] [PubMed] [Google Scholar]
- 6.Bhunu CP, Garira W, Magombedze G. Mathematical analysis of a two strain HIV/AIDS model with antiretroviral treatment. Acta Biotheor. 2009 Sep;57(3):361–81. doi: 10.1007/s10441-009-9080-2. [DOI] [PubMed] [Google Scholar]
- 7.Hoare A, Kerr SJ, Ruxrungtham K, Ananworanich J, Law MG, Cooper DA, et al. Hidden drug resistant HIV to emerge in the era of universal treatment access in Southeast Asia. PLoS One. 2010;5(6):e10981. doi: 10.1371/journal.pone.0010981. * This paper describes a numerical analysis of an HIV transmission model parameterized to represent epidemics in Southeast Asia (using Thailand as an example), with one cART treatment regimen. It highlights the importance of detecting virological failure and procuring access to second-line therapies to reduce the prevalence of TDR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marks AJ, Pillay D, McLean AR. The effect of intrinsic stochasticity on transmitted HIV drug resistance patterns. J Theor Biol. 2010 Jan 7;262(1):1–13. doi: 10.1016/j.jtbi.2009.09.017. ** A very interesting theoretical study examining how chance events (stochasticity) affect the emergence and spread of ARV drug resistance, with very relevant implications for the surveillance of drug resistance. Stochasticity is important in small populations (such as infected drug-resistant individuals) or low transmission settings, yet there has been little work with stochastic models to investigate resistance spread. [DOI] [PubMed] [Google Scholar]
- 9.Smith? RJ, Okano JT, Kahn JS, Bodine EN, Blower S. Evolutionary dynamics of complex networks of HIV drug-resistant strains: the case of San Francisco. Science. 2010 Feb 5;327(5966):697–701. doi: 10.1126/science.1180556. ** This paper is one of the first to model resistance to ARV drug classes separately. After using the model to trace the evolutionary history of ARV resistance among MSM in San Francisco, the authors predict that drug-resistant strains could sustain the epidemic and threaten the effectiveness of test and treat strategies in this setting. [DOI] [PubMed] [Google Scholar]
- 10.Westreich D, Eron J, Behets F, Horst C, Van Rie A. Survival in women exposed to single-dose nevirapine for prevention of mother-to-child transmission of HIV: a stochastic model. J Infect Dis. 2007 Mar 15;195(6):837–46. doi: 10.1086/511276. [DOI] [PubMed] [Google Scholar]
- 11.Supervie V, Garcia-Lerma JG, Heneine W, Blower S. HIV, transmitted drug resistance, and the paradox of preexposure prophylaxis. Proc Natl Acad Sci U S A. 2010 Jul 6;107(27):12381–6. doi: 10.1073/pnas.1006061107. * This paper involves modeling resistance outcomes for PrEP under various conditions of PrEP efficacy and relative fitness of ARV-resistant HIV strains. It is one of the few modeling analyses to investigate the potential increase in resistance transmission resulting from possible risk compensation in the population. It also highlights that choice of outcome is important: while the proportion of all new HIV transmission events that are resistant may increase, the actual number of these events may be falling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbas UL, Hood G, Wetzel A, Mellors JW. Emergence and spread of HIV drug resistance arising from roll out of antiretroviral pre-exposure prophylaxis (PrEP). MIcrobicides M2010 Conference; May 22-25; Pittburgh, Pennsylvania, USA. 2010. Abstract 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson DP, Coplan PM, Wainberg MA, Blower SM. The paradoxical effects of using antiretroviral-based microbicides to control HIV epidemics. Proc Natl Acad Sci U S A. 2008 Jul 15;105(28):9835–40. doi: 10.1073/pnas.0711813105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dimitrov DT, Masse B, Boily MC. Who will benefit from a wide-scale introduction of vaginal microbicides in developing countries? Statistical Communications in Infectious Diseases. 2010;2(1) doi: 10.2202/1948-4690.1012. Art. 4. * This paper contributes to the current debate regarding the benefit each gender will receive by the wide-scale introduction of ARV-VMB, and how this benefit may be affected by the emergence of drug resistance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett DE, Bertagnolio S, Sutherland D, Gilks CF. The World Health Organization's global strategy for prevention and assessment of HIV drug resistance. Antivir Ther. 2008;13 2:1–13. [PubMed] [Google Scholar]
- 16.Zaric GS, Brandeau ML, Bayoumi AM, Owens DK. The effects of protease inhibitors on the spread of HIV and the development of drug-resistance HIV strains: a simulation study. Simulation. 1998;71(4):262–75. [Google Scholar]
- 17.Tchetgen E, Kaplan EH, Friedland GH. Public health consequences of screening patients for adherence to highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2001 Feb 1;26(2):118–29. doi: 10.1097/00042560-200102010-00003. [DOI] [PubMed] [Google Scholar]
- 18.Vardavas R, Blower S. The emergence of HIV transmitted resistance in Botswana: “when will the WHO detection threshold be exceeded?”. PLoS One. 2007;2(1):e152. doi: 10.1371/journal.pone.0000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gadhamsetty S, Dixit NM. Estimating frequencies of minority nevirapine-resistant strains in chronically HIV-1-infected individuals naive to nevirapine by using stochastic simulations and a mathematical model. J Virol. 2010 Oct;84(19):10230–40. doi: 10.1128/JVI.01010-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McLean AR, Nowak MA. Competition between zidovudine-sensitive and zidovudine-resistant strains of HIV. AIDS. 1992 Jan;6(1):71–9. doi: 10.1097/00002030-199201000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Ribeiro RM, Bonhoeffer S, Nowak MA. The frequency of resistant mutant virus before antiviral therapy. AIDS. 1998 Mar 26;12(5):461–5. doi: 10.1097/00002030-199805000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Walensky RP, Weinstein MC, Yazdanpanah Y, Losina E, Mercincavage LM, Toure S, et al. HIV drug resistance surveillance for prioritizing treatment in resource-limited settings. AIDS. 2007 May 11;21(8):973–82. doi: 10.1097/QAD.0b013e328011ec53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holmes CB, Zheng H, Martinson NA, Freedberg KA, Walensky RP. Optimizing treatment for HIV-infected South African women exposed to single-dose nevirapine: balancing efficacy and cost. Clin Infect Dis. 2006 Jun 15;42(12):1772–80. doi: 10.1086/504382. [DOI] [PubMed] [Google Scholar]
- 24.Paltiel AD, Freedberg KA, Scott CA, Schackman BR, Losina E, Wang B, et al. HIV preexposure prophylaxis in the United States: impact on lifetime infection risk, clinical outcomes, and cost-effectiveness. Clin Infect Dis. 2009 Mar 15;48(6):806–15. doi: 10.1086/597095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van de Vijver DA, Derdelinckx I, Boucher CA. Circulating HIV type 1 drug resistance will have limited impact on the effectiveness of preexposure prophylaxis among young women in Zimbabwe. J Infect Dis. 2009 May 1;199(9):1310–7. doi: 10.1086/597804. [DOI] [PubMed] [Google Scholar]
- 26.Pinkerton SD, Holtgrave DR, Bloom FR. Cost-effectiveness of post-exposure prophylaxis following sexual exposure to HIV. AIDS. 1998 Jun 18;12(9):1067–78. [PubMed] [Google Scholar]
- 27.Bassett IV, Freedberg KA, Walensky RP. Two drugs or three? Balancing efficacy, toxicity, and resistance in postexposure prophylaxis for occupational exposure to HIV. Clin Infect Dis. 2004 Aug 1;39(3):395–401. doi: 10.1086/422459. [DOI] [PubMed] [Google Scholar]
- 28.Dodd PJ, Garnett GP, Hallett TB. Examining the promise of HIV elimination by ‘test and treat’ in hyperendemic settings. AIDS. 2010 Mar 13;24(5):729–35. doi: 10.1097/QAD.0b013e32833433fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009 Jan 3;373(9657):48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 30.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000 Mar 30;342(13):921–9. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 31.Cohen MS, Gay C, Kashuba AD, Blower S, Paxton L. Narrative review: antiretroviral therapy to prevent the sexual transmission of HIV-1. Ann Intern Med. 2007 Apr 17;146(8):591–601. doi: 10.7326/0003-4819-146-8-200704170-00010. [DOI] [PubMed] [Google Scholar]
- 32.Gray RH, Wawer MJ, Brookmeyer R, Sewankambo NK, Serwadda D, Wabwire-Mangen F, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001 Apr 14;357(9263):1149–53. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 33.Nijhuis M, Schuurman R, de Jong D, Erickson J, Gustchina E, Albert J, et al. Increased fitness of drug resistant HIV-1 protease as a result of acquisition of compensatory mutations during suboptimal therapy. AIDS. 1999 Dec 3;13(17):2349–59. doi: 10.1097/00002030-199912030-00006. [DOI] [PubMed] [Google Scholar]
- 34.Derdelinckx I, Wainberg MA, Lange JM, Hill A, Halima Y, Boucher CA. Criteria for drugs used in pre-exposure prophylaxis trials against HIV infection. PLoS Med. 2006 Nov;3(11):e454. doi: 10.1371/journal.pmed.0030454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peterson L, Taylor D, Roddy R, Belai G, Phillips P, Nanda K, et al. Tenofovir disoproxil fumarate for prevention of HIV infection in women: a phase 2, double-blind, randomized, placebo-controlled trial. PLoS Clin Trials. 2007;2(5):e27. doi: 10.1371/journal.pctr.0020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure Chemoprophylaxis for HIV Prevention in Men Who Have Sex with Men. N Engl J Med. Nov 23; doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michael NL. Oral Preexposure Prophylaxis for HIV - Another Arrow in the Quiver? N Engl J Med. Nov 23; doi: 10.1056/NEJMe1012929. [DOI] [PubMed] [Google Scholar]
- 38.Goudsmit J, De Ronde A, Ho DD, Perelson AS. Human immunodeficiency virus fitness in vivo: calculations based on a single zidovudine resistance mutation at codon 215 of reverse transcriptase. J Virol. 1996 Aug;70(8):5662–4. doi: 10.1128/jvi.70.8.5662-5664.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leigh Brown AJ, Frost SD, Mathews WC, Dawson K, Hellmann NS, Daar ES, et al. Transmission fitness of drug-resistant human immunodeficiency virus and the prevalence of resistance in the antiretroviral-treated population. J Infect Dis. 2003 Feb 15;187(4):683–6. doi: 10.1086/367989. [DOI] [PubMed] [Google Scholar]
- 40.Vickerman P, Watts C, Delany S, Alary M, Rees H, Heise L. The importance of context: model projections on how microbicide impact could be affected by the underlying epidemiologic and behavioral situation in 2 African settings. Sex Transm Dis. 2006 Jun;33(6):397–405. doi: 10.1097/01.olq.0000218974.77208.cc. [DOI] [PubMed] [Google Scholar]
- 41.Bennett DE, Myatt M, Bertagnolio S, Sutherland D, Gilks CF. Recommendations for surveillance of transmitted HIV drug resistance in countries scaling up antiretroviral treatment. Antivir Ther. 2008;13 2:25–36. [PubMed] [Google Scholar]
- 42.Dodd PJ, White PJ, Garnett GP. Notions of synergy for combinations of interventions against infectious diseases in heterogeneously mixing populations. Math Biosci. 2010 doi: 10.1016/j.mbs.2010.06.004. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]