Abstract
In the mid-1980s, iron oxide nanoparticles were developed as contrast agents for diagnostic imaging. In the last two decades, established methods to label cells with superparamagnetic iron oxides (SPIOs) have been developed to aid in targeted delivery and tracking of stem cell therapies. The surge in cellular therapy clinical trials for cardiovascular applications has seen a similar rise in the number of preclinical animal studies of SPIO-labeled stem cells in an effort to understand the mechanisms of cardiovascular regenerative therapy and stem cell biodistribution. The adoption of a limited number of methods of direct labeling of stem cells with SPIOs is due in large part to the desire to rapidly translate these techniques to clinical trials. In this review, we will outline the most commonly adopted methods for iron oxide labeling of stem cells for cardiovascular applications and describe strategies for magnetic resonance imaging (MRI) of magnetically labeled cells in the heart.
Keywords: Magnetic resonance imaging (MRI), Stem cells, Superparamagnetic iron oxide (SPIO), Cellular labeling, Cell imaging, Transfection, Electroporation
1. Introduction
Magnetic resonance imaging (MRI) provides many desirable features for cell tracking and delivery. MRI offers the interactivity of X-ray interventional techniques without exposing the patient or cells to ionizing radiation. Moreover, the high spatial resolution and exquisite soft tissue detail of MRI are superior to X-ray cardiac interventional methods, which can only provide information about the lumen of the heart or vessels in combination with iodinated contrast agents. In addition, MRI allows noninvasive, serial imaging for dynamic tracking of cell migration and engraftment (1–12).
Although there are many MRI cellular labeling methods, direct cellular labeling with superparamagnetic iron oxide (SPIO) contrast agents have been mostly widely used for many reasons (13–15). The lack of unique surface markers for stem cells that are retained with stem cell differentiation down a cardiac lineage has limited the feasibility of receptor-based labeling methods. In addition, direct cell labeling methods are relatively simple, fast, and inexpensive. Several clinically approved formulations of SPIO-based contrast agents are available that have been used for cell labeling in a variety of diseases. Toxicity of these agents is low, since the SPIO nanoparticles that are released from dying cells can be degraded in the normal iron recycling pathways. Compared to gadolinium-based contrast agents, SPIOs become more effective upon cell internalization due to particle clustering and, thereby, create large “blooming” hypointensities on standard clinical MRI scanners. While SPIOs are not internalized natively by nonphagocytic cells, simple methods to induce internalization and uptake have been developed and tested in a variety of stem cells (2, 4, 11, 16–18). One of the most common methods is “magnetofection” – a method where transfection agents (TAs) are used to coat SPIOs to encourage endocytosis of the SPIO–TA complex (13, 14). Concentrations of 2–10 pg iron/cell can be achieved after 12–48 h incubation in vitro (13). After exogenous labeling of stem cells, the SPIOs are stably maintained in endosomes and have been imaged for several months after delivery to the heart (3, 5, 9, 18).
Magnetoelectroporation (MEP) is another common method of SPIO cellular labeling. Magnetoelectroporation uses small pulsed voltages to encourage endocytosis of SPIOs (19). No transfection agents are needed, which may aid in more rapid clinical translation. In addition, millions of cells can be labeled in seconds using magnetoelectroporation, which may be important in certain cell lines that are altered by culturing in vivo. Furthermore, for cardiac cellular delivery, magnetoelectroporation may prove to be the method of choice where cellular delivery cannot be delayed by 24–48 h after an acute cardiac event.
Both magnetofection and magnetoelectroporation can be used to label cells with a variety of contrast agents. A recent study performing a head-to-head comparison of magnetofection and MEP demonstrated preserved cell viability and proliferation in embryonic stem cells by both techniques (20). However, cardiac differentiation of embryonic stem cells was most attenuated by MEP and iron uptake was greatest with magnetofection (20). Detailed methods using these techniques to label stem cells for cardiovascular applications using SPIO contrast agents will be given in this review.
2. Materials
2.1. Cell Culture and Preparation
Stem cell media, suitable for cell origin and requirements, for example, MEM alpha supplemented with 10% fetal bovine serum (FBS, HyClone, Logan, UT, USA) and 1% antibiotic/antimycotic containing penicillin, streptomycin, and amphotericin B (Gibco/Invitrogen, Grand Island, NY, USA) for mesenchymal stem cells.
10 mM Phosphate Buffered Saline (PBS) (1×), pH = 7.4.
Trypsin (0.5 g/L) with ethylenediaminetetraacetic acid (EDTA 0.2 g/L) (Gibco/Invitrogen, Grand Island, NY, USA), warmed to 37°C in a water bath.
2.2. Labeling with Transfection Agents
TAs are highly charged molecules that will form complexes with iron oxide particles through electrostatic interactions. There are several classes of these agents, but the most convenient and commonly utilized labeling methods are those based on commercially available TAs including dendrimers, such as Superfect, poly-l-lysine (PLL), Lipofectamin, and FUGENE. Iron oxide magnetic nanoparticles are used in conjunction with TAs to label cells and can be distinguished primarily based on the size of the nanoparticles. For brevity, we list several formulations that are approved or under development by major pharmaceutical concerns.
-
Iron oxide contrast agents:
Commercially available ferumoxides are Feridex (Berlex Laboratories Inc., Wayne, NJ, USA) or Endorem (Guerbet SA, Paris, France). Ferumoxide stock solution contains 11.2 mgFe/mL with particles approximately 80–150 nm in diameter (21). Ferumoxide stock solution should be stored at 4°C; do not freeze! Feridex is an FDA-approved liver contrast agent since 1996. In Europe, this compound is registered under name Endorem. Both agents contain a dextran coating to minimize clumping.
Ferucarbotran (Resovist, Bayer Schering Pharma AG, Berlin, Germany) is an SPIO composed of a colloidal solution of iron oxide nanoparticles coated with carboxydextran. It is currently used for the detection and characterization of focal liver tumor lesions and approved for clinical use in the European, Australian, and Japanese markets (see Note 1).
Ferumoxtran (Sinerem, Guerbet SA, Paris, France or Combidex, AMAG Pharmaceuticals Inc., Cambridge, MA, USA) is a member of the ultrasmall superparamagnetic iron oxide (USPIO) class of contrast agents with a median diameter <50 nm. Due to the smaller diameter, these particles will not be filtered by the reticuloendothelial system as quickly as SPIOs when injected intravenously. Thus, they tend to accumulate in lymph nodes and are used to distinguish normal from metastatic nodes (see Note 2).
-
Transfection agents:
PLL (PLL, Sigma, St Louis, MO, USA) as the polyamine PLL hydrobromide with molecular weight of 388,199 Daltons (catalog number P-1524) is the most commonly used TA. A stock solution of PLL at concentration of 1.5 mg/mL should be stored in −20°C.
Protamine sulfate (American Pharmaceuticals Partner, Schaumburg, IL, USA), which is a drug used clinically to reverse the effects of heparin therapy, is another commonly used TA. It is available in bottles at a concentration of 10 mg/mL.
2.3. Magnetoelectro-poration
Ferumoxide stock solution (see Subheading 2.2).
Electroporation cuvettes, 0.4 mm gap (Gene Pulser BioRad, Hercules, CA). It is important to deliver electrical pulses with the proper field strength and duration. The exact pulse delivery will be dependent on the type of cells that will be labeled. Mammalian cells typically require field strengths up to 6.15 kV/cm, which can be obtained using the 0.4 cm cuvette.
BTX electroporation system (Harvard Apparatus, Holliston, MA, see Fig. 1).
Culture media and 10 mM PBS as in Subheading 2.1.
Fig. 1.
BTX electroporation system (a) that can be used for magnetoelectroporation. This system may be operated by a switch (b) or foot pedal (not shown).
2.4. Magnetic Resonance Imaging
Clinical MRI scanner equipped with surface coils to image the heart.
MRI-compatible ECG leads and monitoring equipment.
3. Methods
There are two commonly used iron oxide labeling methods: (1) labeling with transfection agents, for example, PLL (22) or protamine sulfate (21); and (2) Magnetoelectroporation (19, 23). Prior to labeling, the cells must be prepared in a clean, appropriate environment. The cell can be frozen and thawed immediately prior to labeling, but viability is improved if the cells are brought back into culture before labeling.
3.1. Cell Culture and Preparation
When the stem cells approach confluence in a culture dish/flask, remove the old media and wash the monolayer once or twice with PBS.
Remove PBS with a pipette and add a minimal volume (∼1 mL for a T-75 flask) of prewarmed trypsin.
Incubate the cells in trypsin for at 37°C in humidified, enriched in 5% CO2 air, then check microscopically to determine whether the monolayer of cells is lifting off the culture dish after ∼2-3 minutes.
When single cell suspension has been obtained, add ∼10 mL of complete media and transfer the cell suspension to a sterile 15 mL conical tube and spin the cells on tabletop centrifuge (∼600 × g for 10 min).
Discard the supernatant carefully, so as not to disturb the cell pellet and resuspend the cells in complete media for counting.
Once the number and concentration of cells has been determined, reseed the cells. For example, MSCs typically will be reseeded into a fresh T-75 flask at a concentration of ∼2 × 105 cells/mL.
3.2. Labeling with Transfection Agent
Each combination of TA and (U)SPIOs should be carefully titrated and optimized, since too low concentrations may not lead to sufficient cellular uptake, whereas too high concentrations may induce precipitates (see Note 3) or may be cytotoxic
3.2.1. Labeling with (U) SPIOs–PLL Complexes
Allow the cells to grow to 80–90% confluence of the culture dish surface area.
While maintaining clean suitable environment for cell culture, prepare complete media appropriate for the stem cell type at the standard volume needed for regular cell growth. Add (U) SPIOs to media solution to obtain final concentration of 25 μg Fe/mL (13, 14, 24) and mix the solution for few seconds with intermittent hand shaking to obtain homogenous solution.
Add PLL to achieve final concentration of 375 ng/mL (2, 24). When using Sigma stock solution (as described in Subheading 2.2), add 0.25 μL of this stock per 1 mL of the ferumoxide/media mixture. Mix the solution shaking gently and incubate for 30–60 min with occasional gentle hand mixing.
After the (U)SPIO–PLL media solution is fully mixed, discard the old media from the cells, wash the monolayer with PBS, and add the media containing (U)SPIO–PLL complex to the culture flask. Incubate the cells with this solution overnight, for example, approximately 16–18 h at 37°C in air enriched with 5% CO2 (see Note 4).
After overnight incubation, remove the media containing (U) SPIO–PLL complexes, rinse the cells with warm PBS, trypsinize, and collect for counting and administration. The lack of species specificity of SPIO–PLL labeling is shown for MSCs in Fig. 2.
Fig. 2.
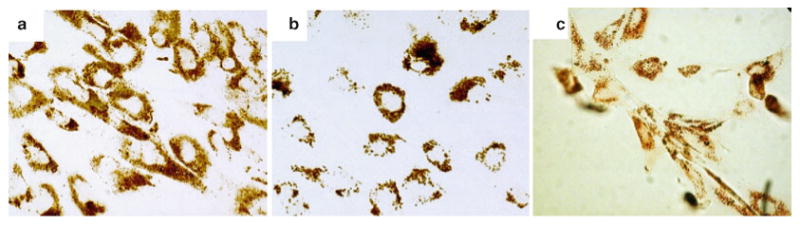
Mesenchymal stem cells (MSCs) using ferumoxides–PLL. Cells were labeled for 48 h (a, b) or 24 h (c) with 25 mg Fe/ml Feridex and 375 ng/ml PLL. DAB-enhanced Prussian blue stain of labeled human (a), porcine (b), and canine (c) MSCs show an efficient intracellular uptake of particles into endosomes that is nonspecific across species. (Adapted from Bulte and Kraitchman (5) with permission.)
3.2.2. Cell Labeling with Protamine sulfate complexes
Dilute protamine sulfate in distilled water to the concentration of 1 mg/mL.
Combine (U)SPIOs with appropriate serum-free media based on the cell type to obtain a concentration of 100 μg Fe/mL. For example, add 9 μL of ferumoxide formulation for every 1 mL of media for MSCs.
Add protamine sulfate to the (U)SPIO solution to obtain its concentration of 4.5–6 μg/mL (25–27) and hand shake the solution periodically over 5–10 min.
After 5–10 min, add an equal volume of standard cell culture media with a double concentration of serum to create final ferumoxide concentration of 50 μg/mL.
Replace old media in cell culture with newly created media with (U)SPIO–protamine sulfate complexes and incubate with cells overnight.
After overnight incubation, remove the media containing (U) SPIO–protamine sulfate complexes, rinse the cells with warm PBS, trypsinize, and collect for counting and administration.
3.3. Magnetoelectro-poration
Remove media and wash the cells with PBS.
Trypsinize and count the cells. After counting, spin the cells on tabletop centrifuge (∼600 × g for 10 min for MSCs) and wash with PBS.
Resuspend the cells in 10 mM sterile PBS at the density of 1.5 × 106 cells/mL (see Note 5) and transfer to sterile electroporation cuvette(s). While cell suspensions <1 mL/cuvette may be used, care must be taken to ensure that the cuvettes' electrodes are entirely covered by the cell suspension. For example, using the BTX apparatus and 0.4 mm gap electrooration cuvettes, the total volume of cell suspension mixed with (U)SPIOs cannot be smaller than 700 μL.
Add (U)SPIOs to obtain a final concentration (after mixing with cell suspension) of 2,000 μg Fe/mL. For example, mix 130 μL of ferumoxides with 600 μL of cell suspension to obtain a final electroporation mix volume equal to 730 μL (see Note 6).
Using BTX electroporation system, electroporate cells using the following conditions: 50 V pulse strength; 5 ms pulse duration; and 20 pulses in intervals of 100 ms (see Note 7).
Leave the cuvettes in the holder for 1 min, transfer to ice, and let them to rest for 5 min to allow for membrane recovery.
Remove the small top layer of foam and transfer cells to 50 mL conical tube containing culture media. Leave the tube with cells on ice for at least 15–20 min (see Note 8).
Wash the cells twice with PBS. Spin the cells in media on tabletop centrifuge (∼600 × g for 10 min).
Pipette off the supernatant, resuspend MSCs in fresh, sterile 10 mM PBS, and spin again. Repeat steps 8 and 9 and then proceed to step 10.
Discard the supernatant and resuspend cell pellet in 1 mL (or other desired amount) of PBS. Count the cells and dilute to final concentration for administration.
3.4. Cardiac Magnetic Resonance Imaging
(U)SPIO-labeled stem cells can be delivered in several ways including direct visualization during open-chest procedures, intracoronary administration using conventional angiographic catheters, and transmyocardially using specialized catheters for delivery of therapeutics to the myocardium. High spatial resolution T2*-weighted images will depict the labeled cells as hypointensities. Because these hypointensities can be hard to distinguish from other hypointensities, such as calcified plaque or metallic objects like stents, off-resonant imaging techniques have been developed to portray the magnetic susceptibilities from iron-labeled cells as hyperintense signal (28–32).
3.4.1. T2*-Weighted Imaging
For high-resolution imaging, images are acquired over multiple cardiac cycles using ECG-gating. Motion artifacts from breathing are suppressed using either navigator echo techniques or breath-holding.
While T2*-weighting can be obtained using several imaging techniques, gradient echo imaging with an extended echo time (TE) that does not degrade cardiac images appears to provide the best compromise in image quality on clinical scanners (see Fig. 3) (2).
Typical gradient echo imaging parameters are: 6 ms repetition time (TR); 1.6 ms TE; 20° flip angle; 512 × 512 image matrix; 5–8 mm slice thickness (ST): 32 kHz bandwidth (BW); and 2–4 number of signal averages (NSA). Images are acquired in the standard short or long axis planes to cover the extent of the left ventricle.
Fig. 3.
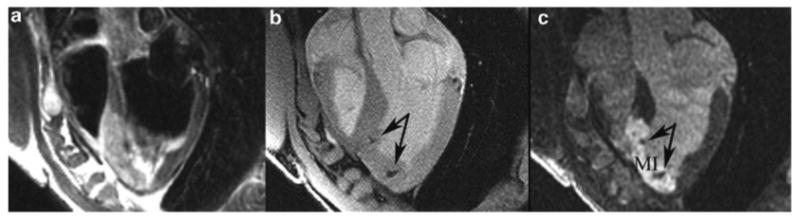
Representative hypointensities (arrows) from ferumoxide–PLL-labeled mesenchymal stem cell in long-axis MRIs of an infarcted swine using fast spin echo image (a), fast gradient echo (b), and delayed contrast-enhanced MRI (c), which demonstrates infarcted myocardium as hyperintensities. Labeled-MSCs were delivered transmyocardially using a specialized MRI-compatible injection catheter. (Adapted from Kraitchman et al. (2) with permission.)
3.4.2. Off-Resonance Imaging
There are several types of off-resonance imaging techniques that have been used to image (U)SPIOs. One method uses a spectral excitation in combination with spin echo imaging, which is probably not well-suited for cardiac applications (29). Another method, GRadient echo Acquisition for Superparamagnetic particles/suscePtibility (GRASP), modifies the refocusing pulses to create positive contrast from iron-labeled cells (30, 33). Background suppression using this technique is excellent. A third method, Inversion-Recovery with ON-resonant water suppression (IRON), uses frequency-selective prepulses to suppress the water signal leaving positive contrast from iron-labeled cells (31). While not providing as much background suppression as the GRASP method, IRON MRI provides the flexibility to be combined with either spin echo or gradient echo techniques as well as two-dimensional single plane or three-dimensional volume acquisitions. Recently, it has been demonstrated that these off-resonance imaging techniques will not benefit from field strengths >4.7 T (34). Thus, these techniques are ideal for use on currently available clinical scanners.
As with T2*-weighted imaging, ECG-gating and respiratory gating or breath holds are used to suspend cardiac and respiratory motion, respectively.
Typical imaging parameters for three dimensional fast spin echo IRON imaging at 3 T are: 2 ms TR; 11.6 ms TE; 24 echo train length (ETL): 11.6 ms interecho spacing; 170 Hz bandwidth water suppression; 95° iron saturation pulse. In the heart, fat suppression is recommended. Depending on the number of (U)SPIO-labeled cells per voxel and the image resolution, the off-resonant positive contrast will appear as hyperintense areas surrounding the cells and with a typical dipole appearance (see Fig. 4). The volume of the hyperintensities can be measured to determine a relative concentration of labeled cells.
Fig. 4.
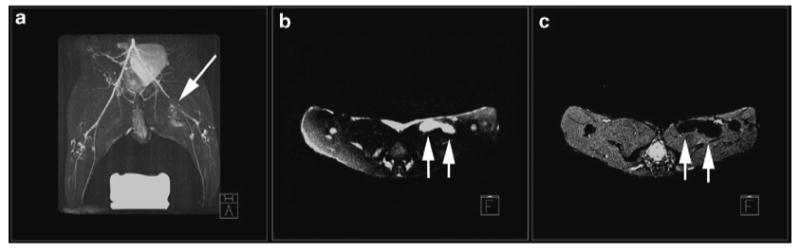
A maximum intensity projection of a 3D T2-prepared MR angiogram (a) acquired on a 3 T MR clinical scanner in a rabbit model of peripheral arterial disease. Mesenchymal stem cells (MSCs) that were labeled using magnetoelectroporation with ferumoxides were injected into the medial thigh. Two injection sites imaged immediately after injection are shown with IRON MRI (arrows, b) and conventional gradient echo imaging (arrows, c). (Adapted from Kraitchman and Bulte (32) with permission.)
Fig. 5.
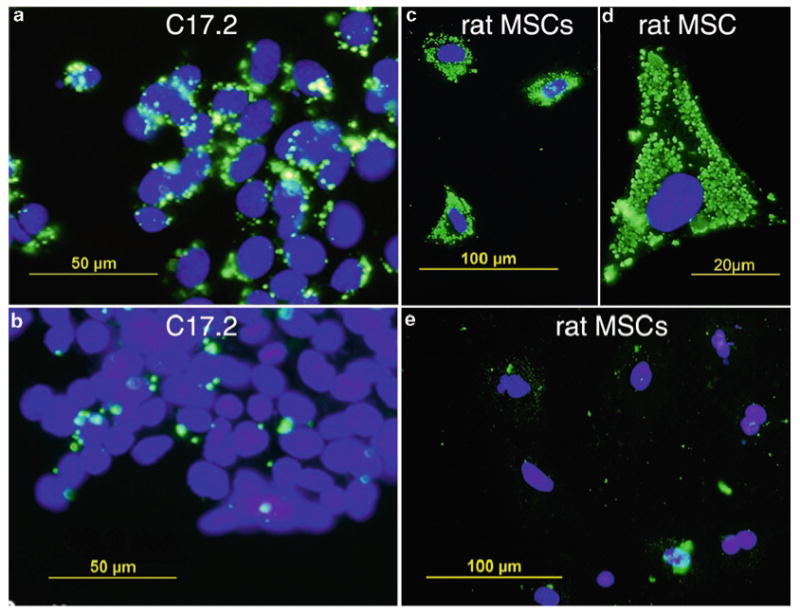
Iron uptake by MEP-treated cells. C17.2 mouse neural stem cells (a, b) and rat mesenchymal stem cells (c–e) were incubated with ferumoxides (2 mg Fe/mL) with (a, c, d) and without (b, e) magnetoelectroporation (MEP). Only MEP-treated cells show significant ferumoxide uptake as assessed by antidextran immunofluorescent staining (green). The higher magnification in (d) demonstrates ferumoxide-containing clusters with a measured diameter of 830 ± 350 nm. (From Walczak et al. (19) with permission.)
Fig. 6.
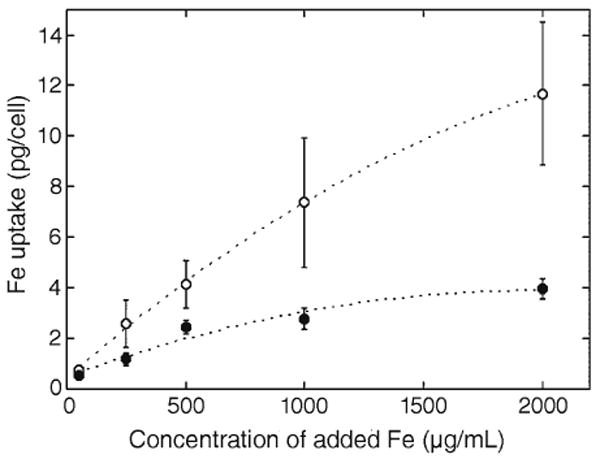
Iron content of MEP-labeled rat MSCs (open circles) and C17.2 cells (closed circles). The amount of cellular iron uptake increases with the added amount of ferumoxides. Rat MSCs, which have a larger cytoplasmic volume, contain more iron than smaller C17.2 cells. (From Walczak et al. (19) with permission.)
Footnotes
Because ferucarbotrans are smaller than ferumoxides, the ratio of SPIO to cationic transfection agents may need to be adjusted. This may explain why unpublished data have suggested that aggregates using ferucarbotrans are more common. However, Politi and coworkers were able to more efficiently label neural precursor cells with ferucarbotrans than ferumoxides or USPIOs (35)
While USPIO labeling has been performed in cardiovascular applications (36), a few reports suggest that cell labeling with SPIOs is more efficient than USPIOs (35, 37, 38).
Cahill et al. filtered ferumoxide–PLL complexes using a 0.2 μm mesh to reduce aggregates that would otherwise form during incubation with muscle stem cell transplants in media (39).
If overnight labeling of cells is not possible, cells can be also efficiently labeled in 2 h by using serum-free media with ferumoxide–PLL complexes formation and cell nourishment (22).
If cell density per cuvette exceeds 5 × 106, cell clumping can occur during magnetoelectroporation procedure. Thus, using less than 2 × 106 cells per cuvette is recommended to avoid cell clumping (19).
Iron uptake will be determined in part by of the type of cells. Cells with more cytoplasmic volume can be labeled with a larger amount of SPIOs (see Fig. 5). In addition, increasing the SPIO concentration can enhance intracellular iron uptake during magnetoelectroporation. Walczak and coworkers have demonstrated a correlation between concentrations ranging from 250 to 2,000 of Fe μg/mL and cellular iron uptake (see Fig. 6) (19).
Augmenting the number of pulses with a lower voltage (40 mV) can also enhance intracellular iron uptake by magnetoelectroporation (19). Iron uptake quantity by rat MSCs sufficient for MR imaging (more than 2 pg of iron per cell) were achieved even with the small amount of 250 of Fe μg/mL (19). However, labeling with small amounts of iron may not permit cell tracking if the cells proliferate and, thereby, dilute the label.
Our studies in MSCs have found that incubation in media on ice after magnetoelectroporation increases cell viability. We would speculate that this “rest” period allows the membrane to recover prior to exposure to a harsh environment in vivo.
References
- 1.Weissleder R, Cheng HC, Bogdanova A, Bogdanov A., Jr Magnetically labeled cells can be detected by MR imaging. J Magn Reson Imaging. 1997;7(1):258–63. doi: 10.1002/jmri.1880070140. [DOI] [PubMed] [Google Scholar]
- 2.Kraitchman DL, Heldman AW, Atalar E, et al. In vivo magnetic resonance imaging of mesenchymal stem cells in myocardial infarction. Circulation. 2003;107(18):2290–3. doi: 10.1161/01.CIR.0000070931.62772.4E. [DOI] [PubMed] [Google Scholar]
- 3.Hill JM, Dick AJ, Raman VK, et al. Serial cardiac magnetic resonance imaging of injected mesenchymal stem cells. Circulation. 2003;108(8):1009–14. doi: 10.1161/01.CIR.0000084537.66419.7A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garot J, Unterseeh T, Teiger E, et al. Magnetic resonance imaging of targeted catheter-based implantation of myogenic precursor cells into infarcted left ventricular myocardium. J Am Coll Cardiol. 2003;41(10):1841–6. doi: 10.1016/s0735-1097(03)00414-5. [DOI] [PubMed] [Google Scholar]
- 5.Bulte JW, Kraitchman DL. Monitoring cell therapy using iron oxide MR contrast agents. Curr Pharm Biotechnol. 2004;5(6):567–84. doi: 10.2174/1389201043376526. [DOI] [PubMed] [Google Scholar]
- 6.Rickers C, Gallegos R, Seethamraju RT, et al. Applications of magnetic resonance imaging for cardiac stem cell therapy. J Interv Cardiol. 2004;17(1):37–46. doi: 10.1111/j.1540-8183.2004.01712.x. [DOI] [PubMed] [Google Scholar]
- 7.Kustermann E, Roell W, Breitbach M, et al. Stem cell implantation in ischemic mouse heart: a high-resolution magnetic resonance imaging investigation. NMR Biomed. 2005;18(6):362–70. doi: 10.1002/nbm.967. [DOI] [PubMed] [Google Scholar]
- 8.de Vries IJ, Lesterhuis WJ, Barentsz JO, et al. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat Biotechnol. 2005;23(11):1407–13. doi: 10.1038/nbt1154. [DOI] [PubMed] [Google Scholar]
- 9.Stuckey DJ, Carr CA, Martin-Rendon E, et al. Iron particles for noninvasive monitoring of bone marrow stromal cell engraftment into, and isolation of viable engrafted donor cells from, the heart. Stem Cells. 2006;24(8):1968–75. doi: 10.1634/stemcells.2006-0074. [DOI] [PubMed] [Google Scholar]
- 10.Amado LC, Schuleri KH, Saliaris AP, et al. Multimodality noninvasive imaging demonstrates in vivo cardiac regeneration after mesenchymal stem cell therapy. J Am Coll Cardiol. 2006;48(10):2116–24. doi: 10.1016/j.jacc.2006.06.073. [DOI] [PubMed] [Google Scholar]
- 11.Ebert SN, Taylor DG, Nguyen HL, et al. Noninvasive tracking of cardiac embryonic stem cells in vivo using magnetic resonance imaging techniques. Stem Cells. 2007;25(11):2936–44. doi: 10.1634/stemcells.2007-0216. [DOI] [PubMed] [Google Scholar]
- 12.Arai T, Kofidis T, Bulte JW, et al. Dual in vivo magnetic resonance evaluation of magnetically labeled mouse embryonic stem cells and cardiac function at 1.5 t. Magn Reson Med. 2006;55(1):203–9. doi: 10.1002/mrm.20702. [DOI] [PubMed] [Google Scholar]
- 13.Frank JA, Miller BR, Arbab AS, et al. Clinically applicable labeling of mammalian and stem cells by combining superparamagnetic iron oxides and transfection agents. Radiology. 2003;228:480–7. doi: 10.1148/radiol.2281020638. [DOI] [PubMed] [Google Scholar]
- 14.Frank JA, Zywicke H, Jordan EK, et al. Magnetic intracellular labeling of mammalian cells by combining (FDA-approved) super-paramagnetic iron oxide MR contrast agents and commonly used transfection agents. Acad Radiol. 2002;9:S484–S7. doi: 10.1016/s1076-6332(03)80271-4. [DOI] [PubMed] [Google Scholar]
- 15.Kalish H, Arbab AS, Miller BR, et al. Combination of transfection agents and magnetic resonance contrast agents for cellular imaging: relationship between relaxivities, electrostatic forces, and chemical composition. Magn Reson Med. 2003;50(2):275–82. doi: 10.1002/mrm.10556. [DOI] [PubMed] [Google Scholar]
- 16.Cahill KS, Germain S, Byrne BJ, Walter GA. Non-invasive analysis of myoblast transplants in rodent cardiac muscle. Int J Cardiovasc Imaging. 2004;20(6):593–8. doi: 10.1007/s10554-004-3902-8. [DOI] [PubMed] [Google Scholar]
- 17.Tallheden T, Nannmark U, Lorentzon M, et al. In vivo MR imaging of magnetically labeled human embryonic stem cells. Life Sci. 2006;79(10):999–1006. doi: 10.1016/j.lfs.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 18.Bulte JW, Kraitchman DL. Iron oxide MR contrast agents for molecular and cellular imaging. NMR Biomed. 2004;17(7):484–99. doi: 10.1002/nbm.924. [DOI] [PubMed] [Google Scholar]
- 19.Walczak P, Kedziorek D, Gilad AA, Lin S, Bulte JW. Instant MR labeling of stem cells using magnetoelectroporation. Magn Reson Med. 2005;54(4):769–74. doi: 10.1002/mrm.20701. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki Y, Zhang S, Kundu P, Yeung AC, Robbins RC, Yang PC. In vitro comparison of the biological effects of three transfection methods for magnetically labeling mouse embryonic stem cells with ferumoxides. Magn Reson Med. 2007;57(6):1173–9. doi: 10.1002/mrm.21219. [DOI] [PubMed] [Google Scholar]
- 21.Arbab AS, Yocum GT, Kalish H, et al. Efficient magnetic cell labeling with protamine sulfate complexed to ferumoxides for cellular MRI. Blood. 2004;104(4):1217–23. doi: 10.1182/blood-2004-02-0655. [DOI] [PubMed] [Google Scholar]
- 22.Bulte JW, Arbab AS, Douglas T, Frank JA. Preparation of magnetically labeled cells for cell tracking by magnetic resonance imaging. Methods Enzymol. 2004;386:275–99. doi: 10.1016/S0076-6879(04)86013-0. [DOI] [PubMed] [Google Scholar]
- 23.Walczak P, Ruiz-Cabello J, Kedziorek DA, et al. Magnetoelectroporation: improved labeling of neural stem cells and leukocytes for cellular magnetic resonance imaging using a single FDA-approved agent. Nanomedicine. 2006;2(2):89–94. doi: 10.1016/j.nano.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 24.Kostura L, Kraitchman DL, Mackay AM, Pittenger MF, Bulte JW. Feridex labeling of mesenchymal stem cells inhibits chondrogenesis but not adipogenesis or osteogenesis. NMR Biomed. 2004;17(7):513–7. doi: 10.1002/nbm.925. [DOI] [PubMed] [Google Scholar]
- 25.Janic B, Iskander AS, Rad AM, Soltanian-Zadeh H, Arbab AS. Effects of ferumoxides-protamine sulfate labeling on immunomodulatory characteristics of macrophage-like THP-1 cells. PLoS One. 2008;3(6):e2499. doi: 10.1371/journal.pone.0002499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rad AM, Janic B, Iskander AS, Soltanian-Zadeh H, Arbab AS. Measurement of quantity of iron in magnetically labeled cells: comparison among different UV/VIS spectrometric methods. Biotechniques. 2007;43(5):627–8. 30, 32 passim. doi: 10.2144/000112599. [DOI] [PubMed] [Google Scholar]
- 27.Pawelczyk E, Arbab AS, Pandit S, Hu E, Frank JA. Expression of transferrin receptor and ferritin following ferumoxides-protamine sulfate labeling of cells: implications for cellular magnetic resonance imaging. NMR Biomed. 2006;19(5):581–92. doi: 10.1002/nbm.1038. [DOI] [PubMed] [Google Scholar]
- 28.Seppenwoolde JH, Viergever MA, Bakker CJ. Passive tracking exploiting local signal conservation: the white marker phenomenon. Magn Reson Med. 2003;50(4):784–90. doi: 10.1002/mrm.10574. [DOI] [PubMed] [Google Scholar]
- 29.Cunningham CH, Arai T, Yang PC, McConnell MV, Pauly JM, Conolly SM. Positive contrast magnetic resonance imaging of cells labeled with magnetic nanoparticles. Magn Reson Med. 2005;53(5):999–1005. doi: 10.1002/mrm.20477. [DOI] [PubMed] [Google Scholar]
- 30.Mani V, Saebo KC, Itskovich V, Samber DD, Fayad ZA. GRadient echo Acquisition for Superparamagnetic particles with Positive contrast (GRASP): Sequence characterization in membrane and glass superparamagnetic iron oxide phantoms at 1.5 T and 3 T. Magn Reson Med. 2006;55:126–35. doi: 10.1002/mrm.20739. [DOI] [PubMed] [Google Scholar]
- 31.Stuber M, Gilson WD, Schär M, et al. Positive contrast visualization of iron oxide-labeled stem cells using inversion recovery with ON-resonant water suppression (IRON) Magn Reson Med. 2007;58:1072–7. doi: 10.1002/mrm.21399. [DOI] [PubMed] [Google Scholar]
- 32.Kraitchman DL, Bulte JW. Imaging of stem cells using MRI. Basic Res Cardiol. 2008;103(2):105–13. doi: 10.1007/s00395-008-0704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mani V, Briley-Saebo KC, Hyafil F, Itskovich V, Fayad ZA. Positive magnetic resonance signal enhancement from ferritin using a GRASP (GRE acquisition for superparamagnetic particles) sequence: ex vivo and in vivo study. J Cardiovasc Magn Reson. 2006;8(1):49–50. [Google Scholar]
- 34.Farrar CT, Dai G, Novikov M, et al. Impact of field strength and iron oxide nanoparticle concentration on the linearity and diagnostic accuracy of off-resonance imaging. NMR Biomed. 2008;21(5):453–63. doi: 10.1002/nbm.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Politi LS, Bacigaluppi M, Brambilla E, et al. Magnetic-resonance-based tracking and quantification of intravenously injected neural stem cell accumulation in the brains of mice with experimental multiple sclerosis. Stem Cells. 2007;25(10):2583–92. doi: 10.1634/stemcells.2007-0037. [DOI] [PubMed] [Google Scholar]
- 36.Nelson GN, Roh JD, Mirensky TL, et al. Initial evaluation of the use of USPIO cell labeling and noninvasive MR monitoring of human tissue-engineered vascular grafts in vivo. FASEB J. 2008;22(11):3888–95. doi: 10.1096/fj.08-107367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oude Engberink RD, van der Pol SM, Dopp EA, de Vries HE, Blezer EL. Comparison of SPIO and USPIO for in vitro labeling of human monocytes: MR detection and cell function. Radiology. 2007;243(2):467–74. doi: 10.1148/radiol.2432060120. [DOI] [PubMed] [Google Scholar]
- 38.Sun R, Dittrich J, Le-Huu M, et al. Physical and biological characterization of superparamagnetic iron oxide- and ultrasmall superparamagnetic iron oxide-labeled cells: a comparison. Invest Radiol. 2005;40(8):504–13. doi: 10.1097/01.rli.0000162925.26703.3a. [DOI] [PubMed] [Google Scholar]
- 39.Cahill KS, Gaidosh G, Huard J, Silver X, Byrne BJ, Walter GA. Noninvasive monitoring and tracking of muscle stem cell transplants. Transplantation. 2004;78(11):1626–33. doi: 10.1097/01.tp.0000145528.51525.8b. [DOI] [PubMed] [Google Scholar]


