Abstract
Background
Congenital diaphragmatic hernia (CDH) remains a significant cause of death in newborns. With advances in neonatal critical care and ventilation strategies, survival in the term infant now exceeds 80% in some centers. Although prematurity is a significant risk factor for morbidity and mortality in most neonatal diseases, its associated risk with infants with CDH has been described poorly. We sought to determine the impact of prematurity on survival using data from the Congenital Diaphragmatic Hernia Registry (CDHR).
Methods
Prospectively collected data from live-born infants with CDH were analyzed from the CDHR from January 1995 to July 2009. Preterm infants were defined as <37 weeks estimated gestational age at birth. Univariate and multivariate logistic regression analysis were performed.
Results
During the study period, 5,069 infants with CDH were entered in the registry. Of the 5,022 infants with gestational age data, there were 3,895 term infants (77.6%) and 1,127 preterm infants (22.4%). Overall survival was 68.7%. A higher percentage of term infants were treated with extracorporeal membrane oxygenation (ECMO) (33% term vs 25.6% preterm). Preterm infants had a greater percentage of chromosomal abnormalities (4% term vs 8.1% preterm) and major cardiac anomalies (6.1% term vs 11.8% preterm). Also, a significantly higher percentage of term infants had repair of the hernia (86.3% term vs 69.4% preterm). Survival for infants that underwent repair was high in both groups (84.6% term vs 77.2% preterm). Survival decreased with decreasing gestational age (73.1% term vs 53.5% preterm). The odds ratio (OR) for death among preterm infants adjusted for patch repair, ECMO, chromosomal abnormalities, and major cardiac anomalies was OR 1.68 (95% confidence interval [CI], 1.34–2.11).
Conclusion
Although outcomes for preterm infants are clearly worse than in the term infant, more than 50% of preterm infants still survived. Preterm infants with CDH remain a high-risk group. Although ECMO may be of limited value in the extremely premature infant with CDH, most preterm infants that live to undergo repair will survive. Prematurity should not be an independent factor in the treatment strategies of infants with CDH.
Survival for neonates born with congenital diaphragmatic hernia CDH has been demonstrated to range from 60% to 90% based on hospital data for survival to discharge.1–3 Overall, survival has improved significantly during the last 30 years and is consistently reported around 65% for all infants.2 However, institutional outcomes still remain highly variable.4–6 These center differences have been attributed to heterogeneity in disease severity and variability in therapeutic strategies among institutions. However, with advances in the management of neonatal respiratory failure including extracorporeal membrane oxygenation (ECMO) and ventilator strategies, survival has improved. As a result, high-risk CDH patients including premature infants are being treated.
Prematurity is the strongest influence on poor outcomes for all neonatal diseases.7 The overall premature birth rate was 12.8% in 2006, which includes a higher proportion in children with CDH.8–10 Levison et al11 found a 30% incidence of prematurity in infants with CDH with a nearly 50% decrease in survival, compared with term infants with CDH (35% vs 64%, respectively; unadjusted odds ratio [OR], 3.45; 95% confidence interval [CI], 1.83–6.50).11 However, it remains unclear whether this decrease in survival is solely attributed to the CDH or other associated factors such as gestational age at delivery.
The purpose of this study was to evaluate infants born with CDH from a large international, multi-center registry. Term and preterm infants were compared with regard to overall survival.
METHODS
Data
The Congenital Diaphragmatic Hernia Study Group (CDHSG) was formed in 1995 to compile data on live-born neonates with CDH to allow assessment of therapies and outcome. Data are collected on all inborn or transferred infants with CDH to form the Congenital Diaphragmatic Hernia Registry (CDHR). The CDHSG is a voluntary collaboration of international tertiary referral centers providing care for CDH patients who provide data to a central registry (participating centers specified in the appendix). The CDHSG registry was approved by the University of Texas School of Medicine at Houston Institutional Review Board (HSC-MS-03-223).
Appendix.
Members of the CDHSG registry
| Hospital | City, state/province | Country |
|---|---|---|
| Arkansas Children’s Hospital | Little Rock, AR | |
| Astrid Lindgren Children’s Hospital | Stockholm | Sweden |
| BC Children’s & Women’s Health Centre | Vancouver, British Columbia | Canada |
| Cardinal Glennon Children’s Hospital | St. Louis, MO | |
| Levine Children’s Hospital | Charlotte, NC | |
| Cedars Sinai Medical Center | Los Angeles, CA | |
| Central Hospital Aichi Prefectural Colony | Kasugai Aichi | Japan |
| Children’s Hospital Medical Center | Boston, MA | |
| Children’s Hospital of Akron | Akron, OH | |
| Children’s Hospital of Austin | Austin, TX | |
| Children’s Hospital of Buffalo | Buffalo, NY | |
| Children’s Hospital of Illinois | Peoria, IL | |
| Children’s Hospital of Los Angeles | Los Angeles, CA | |
| Children’s Hospital of Michigan | Detroit, MI | |
| Children’s Hospital of Oakland | Oakland, CA | |
| Children’s Hospital of Oklahoma | Oklahoma City, OK | |
| Children’s Hospital of Philadelphia | Philadelphia, PA | |
| Children’s Hospital of Wisconsin | Milwaukee, WI | |
| Children’s Hospital Omaha | Omaha, NE | |
| Children’s Hospitals and Clinics | Minneapolis, MN | |
| Children’s Memorial Hermann Hospital | Houston, TX | |
| Children’s Mercy Hospitals & Clinics | Overland Park, KS | |
| Children’s National Medical Center | Washington, DC | |
| Cincinnati Children’s Hospital Medical Center | Cincinnati, OH | |
| Cleveland Clinic Foundation-Children’s Hospital | Cleveland, OH | |
| Cook Children’s Medical Center | Ft. Worth, TX | |
| Duke University Medical Center | Durham, NC | |
| Emory University | Atlanta, GA | |
| Freie Universitat Berlin | Berlin | Germany |
| Golisano Children’s Hospital at Strong | Rochester, NY | |
| Hasbro Children’s Hospital | Providence, RI | |
| Helen DeVos Children’s Hospital | Grand Rapids, MI | |
| Hershey Medical Center | Hershey, PA | |
| James Whitcomb Riley Children’s Hospital | Indianapolis, IN | |
| Kosair Children’s Hospital | Louisville, KY | |
| Le Bonheur Children’s Medical Center | Memphis, TN | |
| Legacy Emanuel Children’s Hospital | Portland, OR | |
| Loma Linda University Children’s Hospital | Loma Linda, CA | |
| Lucile Salter Packard Children’s Hospital | Palo Alto, CA | |
| Lutheran General Hospital | Park Ridge, IL | |
| Massachusetts General Hospital | Boston, MA | |
| Mattel Children’s Hospital at UCLA | Los Angeles, CA | |
| Mayo Clinic | Rochester, MN | |
| Medical College of Georgia | Augusta, GA | |
| Medical College of Virginia | Richmond, VA | |
| Medical University of South Carolina | Charleston, SC | |
| Miami Valley Hospital | Dayton, OH | |
| National Center for Child Health and Development | Tokyo | Japan |
| North Carolina Baptist Hospital | Winston-Salem, NC | |
| Oespedale Pediatrico Bambino Gesu | Rome | Italy |
| Oespedale Riuniti Bergamo | Bergamo | Italy |
| Osaka Medical Center for Maternal and Child Health | Osaka | Japan |
| Osaka University Graduate School of Medicine | Osaka | Japan |
| Phoenix Children’s Hospital | Phoenix, AZ | |
| Primary Children’s Hospital | Salt Lake City, UT | |
| Radboud University Nijmegen Medical Centre | Nijmegen | The Netherlands |
| Rainbow Babies and Children Hospital | Cleveland, OH | |
| Rockford Memorial Children’s Hospital | Rockford, IL | |
| Royal Alexandra Hospital | Edmonton, Alberta | Canada |
| Royal Children’s Hospital Parkville | Victoria | Australia |
| Royal Hospital for Sick Children | Glasgow | Scotland |
| Salesi Children’s Hospital | Ancona | Italy |
| San Diego Children’s Hospital | San Diego, CA | |
| Santa Rosa Children’s Hospital | San Antonio, TX | |
| Shands Children’s Hospital/University of Florida | Gainesville, FL | |
| Sophia Children’s Hospital | Rotterdam | The Netherlands |
| St. Christopher’s Children’s Hospital | Philadelphia, PA | |
| St. Francis Children’s Hospital | Tulsa, OK | |
| St. Joseph’s Hospital and Medical Center | Phoenix, AZ | |
| St. Louis Children’s Hospital | St. Louis, MO | |
| St. Paul Campus Children’s Minneapolis | Minneapolis, MN | |
| Stollery Children’s Hospital | Edmonton, Alberta | Canada |
| Sydney Children’s Hospital | Randwick, New South Wales | Australia |
| T.C. Thompson Hospital | Chattanooga, TN | |
| Texas Children’s Hospital | Houston, TX | |
| The Children’s Hospital of Alabama | Birmingham, AL | |
| The Hospital for Sick Children | Toronto, Ontario | Canada |
| Nationwide Children’s Hospital | Columbus OH | |
| Tulane University Hospital | New Orleans, LA | |
| Universitatsklinikum Mannheim | Mannheim | Germany |
| University Hospital Gasthuisberg | Leuven | Belgium |
| University of California San Diego | San Diego, CA | |
| University of Chicago | Chicago, IL | |
| University of Kentucky Medical Center | Lexington, KY | |
| C.S. Mott Children’s Hospital | Ann Arbor, MI | |
| University of Mississippi Medical Center | Jackson, MS | |
| University of Nebraska Medical Center | Omaha, NE | |
| University of New Mexico Children’s Hospital | Albuquerque, NM | |
| University of North Carolina | Chapel Hill, NC | |
| University of Padua | Padua | Italy |
| University of Puerto Rico Medical Center | San Juan | Puerto Rico |
| University of Texas Medical Branch at Galveston | Galveston, TX | |
| University of Virginia Medical School | Charlottesville, VA | |
| Vanderbilt Children’s Hospital | Nashville, TN | |
| Wilford Hall USAF Medical Center | Lackland AFB, TX | |
| Winnie Palmer Hospital for Women & Babies | Orlando, FL | |
| Yale New Haven Children’s Hospital | New Haven, CT |
Participating centers filed a waiver of consent for data submission or signed a data use agreement for a limited data set. Data include information on delivery and subsequent hospitalization until death or discharge. Because of the registry nature of the data, patients in the CDHR may not have complete data for all variables.
The current study used prospectively collected data from the CDHR from January 1995 to July 2009 from 98 international institutions. Preterm infants were defined as <37 weeks estimated gestational age (GA) at birth. Infants were categorized to preterm and term status with subgrouping of prematurity at 35–36 weeks GA, 33–34 weeks GA, 31–32 weeks GA, 29–30 weeks GA, and ≤28 weeks GA. Patient demographics, birth weight, GA, Apgar scores, associated anomalies, defect size, need for ECMO, treatment details (including surgical timing/approach, need for patch, ventilator management, survival, morbidity (such as gastroesophageal reflux disease, feeding approach, and need for oxygen at 30 days), and duration of stay were collected. Survival was defined as alive at hospital discharge or transfer. Significant associated anomalies included cardiac defects, chromosomal anomalies, and syndromes. Major cardiac anomalies were defined as all cardiac anomalies except patent ductus arteriosus, isolated atrial septal defect, and isolated ventricular septal defects.
Statistical analysis
Clinical variables including death before hospital discharge are reported as percentages and means ± standard deviation. Term and preterm proportions were compared using Chi square analysis with P < .05 was considered statistically significant. Logistic regression was used to evaluate association among variables and death before hospital discharge adjusting for prematurity. Odds ratios were calculated and 95% CIs were generated. The analysis was conducted using STATA 10 (Stata Corp., College Station, TX).
A univariable analysis was performed initially to evaluate the association of each predictor variable with the primary outcome of survival. All independent variables were analyzed, which included patient demographics, status at delivery, treatment and operative data, and associated comorbidities. Statistically significant variables were used in a multivariable logistic regression analysis. These variables were evaluated for their influence on the primary outcome independently as well as in combination for interaction and confounding.
RESULTS
In all, 5,069 live-born infants with CDH were identified from the CDHR. GA data were available in 5,022 patients. Also included were 3,895 term infants (77.6%) and 1,127 preterm infants (22.4%). Most defects were left-sided (81.5%) with 1% bilateral lesions. Preterm infants had a higher percentage of chromosomal anomalies (8.1% vs 4.0%; P < .0001) and major cardiac defects (11.8% vs 6.1%; P < .0001). Descriptive statistics for all variables are shown in Table I.
Table I.
Descriptive statistics of variables
| Term | %* | Preterm | %* | Total | %† | |
|---|---|---|---|---|---|---|
| All patients | 3,451 | 68.7 | 1,571 | 31.3 | 5,022 | |
| Birth weight (kg) | 3.166 ± 0.505 | 2.297 ± 0.624 | ||||
| Gender | ||||||
| Male | 2,310 | 46 | 1,580 | 31.5 | 3,890 | 77.5 |
| Female | 676 | 13.5 | 451 | 9 | 1,127 | 22.5 |
| Race | ||||||
| White | 2,641 | 55.8 | 760 | 16.1 | 3,401 | 71.9 |
| Black | 257 | 5.4 | 110 | 2.3 | 367 | 7.8 |
| Hispanic | 457 | 9.7 | 120 | 2.5 | 577 | 12.2 |
| Asian | 224 | 4.7 | 52 | 1.1 | 276 | 5.8 |
| Native American | 27 | 0.6 | 12 | 0.3 | 39 | 0.8 |
| Other | 57 | 1.2 | 16 | 0.3 | 73 | 1.5 |
| Birth location | ||||||
| Inborn | 1,415 | 28.2 | 523 | 10.4 | 1,938 | 38.6 |
| Outborn | 2,473 | 49.3 | 606 | 12.1 | 3,079 | 61.4 |
| Side | ||||||
| Left | 3,207 | 64.2 | 865 | 17.3 | 4,072 | 81.5 |
| Right | 640 | 12.8 | 237 | 4.7 | 877 | 17.6 |
| Bilateral | 35 | 0.7 | 13 | 0.3 | 48 | 1 |
| Chromosome anomaly | ||||||
| Yes | 152 | 3.1 | 90 | 1.8 | 242 | 4.9 |
| No | 3,693 | 74.5 | 1,021 | 20.6 | 4,714 | 95.1 |
| Major cardiac anomaly | ||||||
| Yes | 235 | 4.7 | 132 | 2.6 | 367 | 7.4 |
| No | 3,633 | 72.8 | 989 | 19.8 | 4,622 | 92.6 |
| ECMO | ||||||
| Yes | 1,287 | 25.6 | 290 | 5.8 | 1577 | 31.3 |
| No | 2,614 | 51.9 | 841 | 16.7 | 3,455 | 68.7 |
| Repaired | ||||||
| Yes | 3,365 | 66.9 | 784 | 15.6 | 4,149 | 82.5 |
| No | 534 | 10.6 | 345 | 6.9 | 879 | 17.5 |
Percent within variable group.
Percent of total patients.
ECMO
Overall, 1,577 infants (31.3%) with CDH underwent ECMO. This included patients who were repaired before, after, or on ECMO as well as nonrepaired infants. ECMO use was less as GA decreased. However, 8 infants (3.7%) at ≤32 weeks GA underwent ECMO with an average birth weight of 2.3 kg. Six of these infants underwent repair and survived. None had chromosomal or major cardiac anomalies.
CDH repair
Repair data were available in 5,028 infants. All repair types were included. Overall, 82.6% of patients underwent repair including 86.3% for term infants. However, the percentage of infants who underwent operative repair decreased significantly with decreasing GA (Table II). Preterm infants had a significantly lower repair rate (69.4%; P < .001).
Table II.
Characteristic and outcome by gestional age groups
| Gestational age (weeks) | Survival
|
CDH repaired
|
Chromosomal anomalies
|
Major cardiac anomalies
|
ECMO
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | Total | n | % | Total | n | % | Total | n | % | Total | n | % | Total | |
| ≤28 | 12 | 31.6 | 38 | 14 | 36.8 | 38 | 2 | 5.3 | 38 | 3 | 7.9 | 38 | 0 | 0.0 | 38 |
| 29–30 | 19 | 33.3 | 57 | 26 | 45.6 | 57 | 8 | 14.3 | 56 | 10 | 18.2 | 55 | 1 | 1.8 | 57 |
| 31–32 | 50 | 42.0 | 119 | 65 | 54.6 | 119 | 14 | 14.9 | 94 | 15 | 12.7 | 118 | 7 | 5.9 | 119 |
| 33–34 | 138 | 51.3 | 269 | 181 | 67.3 | 269 | 28 | 10.7 | 262 | 30 | 11.2 | 267 | 56 | 20.7 | 270 |
| 35–36 | 384 | 59.6 | 644 | 498 | 77.1 | 646 | 38 | 6.0 | 638 | 74 | 11.5 | 643 | 226 | 34.9 | 647 |
| ≥37 | 2,848 | 73.1 | 3,895 | 3,365 | 86.3 | 3,899 | 152 | 4.0 | 3,845 | 235 | 6.1 | 3,868 | 1,287 | 33.0 | 3,901 |
| Overall | 3,451 | 68.7 | 5,022 | 4,149 | 82.6 | 5,028 | 242 | 4.9 | 4,933 | 367 | 7.3 | 4,989 | 1,577 | 31.4 | 5,032 |
Data on primary repair or need for patch were available in 4,112 infants. Primary repair was performed in 2,125 patients (51.7%). Preterm infants had a significantly higher rate of patch repairs (57.9% preterm vs 46.1% term; P < .05).
Survival
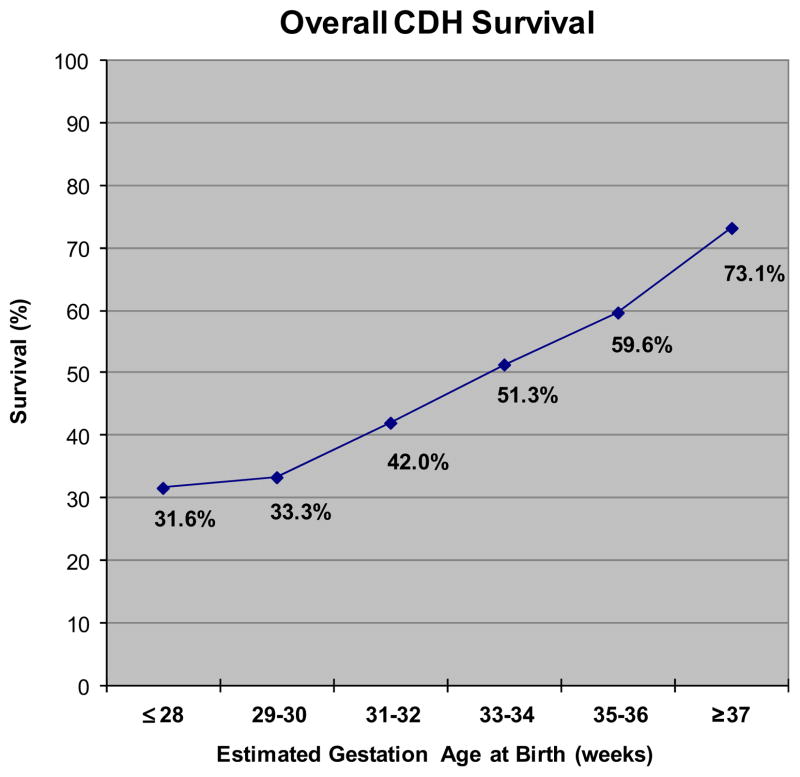
The overall survival for the entire study cohort was 68.7%. Term infants had a significantly higher survival at 73.1% compared with preterm infants at 53.5% (P < .001). As expected, the overall survival rate decreased with decreasing GA (Fig). For infants who underwent repair, overall survival was 83.2%. Although the overall survival was higher in those who underwent repair, pre-term infants who underwent repair still had a significantly lower rate of survival (77.2% vs 84.6%; P < .0001).
Figure.
Overall CDH survival by GA.
All patient variables were evaluated for association with death using univariate analysis. Prematurity, chromosomal anomalies, major cardiac defects, need for patch repair, and need for ECMO were identified as variables that highly correlated with mortality. A multiple logistic regression analysis was performed with these significant variables. The independent, unadjusted odds of death included the following: prematurity (OR, 2.36; 95% CI, 2.06–2.71), chromosomal anomalies (OR, 5.68; 95% CI, 4.28–7.52), major cardiac anomalies (OR, 1.99; 95% CI, 1.80–2.20), patch repair (OR, 9.02; 95% CI, 7.20–11.30), and need for ECMO (OR, 3.13; 95% CI, 2.76–3.55). The adjusted OR for death among preterm infants was 1.68 (95% CI, 1.34–2.11).
DISCUSSION
Despite advances in neonatal critical care, prematurity remains a significant contributor to neonatal mortality in infants with CDH. The incidence of prematurity has increased in the last decade and remains a major cause of mortality in all neonates.12 Although the severity of pulmonary hypoplasia and hypertension are the major determinants of overall survival for infants with CDH, some mortality may be attributed to prematurity because of an increase in associated anomalies. Ninety-five percent of stillborn infants with CDH have an additional major anomaly.13 The impact of prematurity and associated major anomalies on survival among infants with CDH has been described. Compared with those with non–hernia-related anomalies, infants with isolated CDH have a significant survival advantage.14 More than 60% of infants who do not survive the immediate neonatal period have associated anomalies.15 Consequently, successfully managed infants who survive preoperative stabilization and undergo operative repair have less than 10% occurrence of additional anomalies.15
Overall, this study demonstrated 53.5% survival in preterm infants. Although survival decreased with younger gestational ages, infants born after 31 weeks GA still had a survival rate greater than 40%. In general, specific factors that influence mortality remain difficult to delineate, and outcome studies are difficult to interpret because of the tremendous variations in patient disease, management strategies, and operative techniques. Each institution (and even surgeon) is somewhat individualized and maintains certain management preferences, which include ventilation strategies, availability and entry criteria for ECMO, and indications for operative timing. In effort to delineate the impact of prematurity, this study identified significant associated factors that may influence survival including need for ECMO, associated major cardiac anomalies, and chromosomal abnormalities. The analysis demonstrated that prematurity had an increased OR 1.68 (95% CI, 1.34–2.11) for death after adjusting for those significant factors.
A critical factor that influences overall survival is the severity of pulmonary hypoplasia and hypertension. Unfortunately, the CDHR did not start investigating and collecting data with regard to pulmonary hypertension until its third version, which began in January 2007. As a result, these parameters were incomplete for the entire cohort in this study and could not be analyzed specifically. As a surrogate, repair type (either primary repair or patch repair) was used as marker for disease severity. Infants who can undergo a primary repair typically have small defects with mild to minimal pulmonary limitations. Infants with larger defects, such as diaphragmatic agenesis, will invariably be critically ill and require advance therapies such as ECMO. In our cohort, preterm infants had a significant increased rate of patch repair (57.9% preterm vs 46.1% term) with an adjusted OR 9.02 (95% CI, 7.20–11.30) for death.
Important limitations of this study lie in the nature of registry data. As with all outcome studies of CDH, data from the CHDR must be used with caution. A tremendous heterogeneity of disease severity exists within institutions and a wide spectrum of institutions within the CDHSG. As a result, centers may still be limited in their experience with rare, high-risk patients such as preterm infants with major associated anomalies despite being considered a high-volume center. Moderate-risk patients who are repaired at one institution may be considered high risk at another and not be operative candidates. As such, comparison of outcomes among centers should be stratified by disease severity. In our study, the need for patch repair served as a reflection of pulmonary hypertension and hypoplasia severity.
Regardless, the CDHR is a vehicle to collect data from a large number of patients on a rare condition. Such large international registries offer some advantages by ameliorating some institutional biases in patient selection and treatment effects. Registry data remain useful to address broad questions with definable answers. The translation to specific clinical guidelines must be done with caution. Each institution should still recognize their therapeutic limitations. However, even though survival of preterm infants born with CDH is lower as their term counterparts, overall survival is still greater than 50%, with approximately 31% survival of the very preterm infants (≤28 weeks estimated GA). Their high rate of associated anomalies is likely responsible, at least in part, for this increased mortality. Because many of these patients may not be candidates for ECMO based on size alone, survival depends on disease severity, comorbidities, and efficacy of other therapeutic interventions. After adjusting for these factors, preterm infants still have increased odds of death. As a result, prematurity in infants with CDH should not be used a sole parameter to determine initiation of therapy.
Footnotes
The authors are part of the writing committee for the Congenital Diaphragmatic Hernia Study Group. The members of the Congenital Diaphragmatic Hernia Study Group are listed in the appendix.
References
- 1.Boloker J, Bateman DA, Wung JT, Stolar CJ. Congenital diaphragmatic hernia in 120 infants treated consecutively with permissive hypercapnea/spontaneous respiration/elective repair. J Pediatr Surg. 2002;37:357–66. doi: 10.1053/jpsu.2002.30834. [DOI] [PubMed] [Google Scholar]
- 2.Congenital Diaphragmatic Hernia Study Group. Lally KP, Lally PA, Lasky RE, Tibboel D, Jaksic T, et al. Defect size determines survival in infants with congenital diaphragmatic hernia. Pediatrics. 2007;120:e651–7. doi: 10.1542/peds.2006-3040. [DOI] [PubMed] [Google Scholar]
- 3.Langham MR, Jr, Kays DW, Beierle EA, Chen MK, Mullet TC, Rieger K, et al. Twenty years of progress in congenital diaphragmatic hernia at the University of Florida. Am Surg. 2003;69:45–52. [PubMed] [Google Scholar]
- 4.Harrison MR, Adzick NS, Estes JM, Howell LJ. A prospective study of the outcome for fetuses with diaphragmatic hernia. JAMA. 1994;271:382–4. [PubMed] [Google Scholar]
- 5.Jaillard SM, Pierrat V, Dubois A, Truffert P, Lequien P, Wurtz AJ, et al. Outcome at 2 years of infants with congenital diaphragmatic hernia: a population-based study. Ann Thorac Surg. 2003;75:250–6. doi: 10.1016/s0003-4975(02)04278-9. [DOI] [PubMed] [Google Scholar]
- 6.Stege G, Fenton A, Jaffray B. Nihilism in the 1990s: the true mortality of congenital diaphragmatic hernia. Pediatrics. 2003;112:532–5. doi: 10.1542/peds.112.3.532. [DOI] [PubMed] [Google Scholar]
- 7.Patel H, Beeby PJ, Henderson-Smart DJ. Predicting the need for ventilatory support in neonates 30–36 weeks’ gestational age. J Paediatr Child Health. 2003;39:206–9. doi: 10.1046/j.1440-1754.2003.00126.x. [DOI] [PubMed] [Google Scholar]
- 8.Martin JA, Kung HC, Mathews TJ, Hoyert DL, Strobino DM, Guyer B, et al. Annual summary of vital statistics: 2006. Pediatrics. 2008;121:788–801. doi: 10.1542/peds.2007-3753. [DOI] [PubMed] [Google Scholar]
- 9.Congenital Diaphragmatic Hernia Study Group. Lally KP, Lally PA, Langham MR, Hirschl R, Moya FR, et al. Surfactant does not improve survival rate in preterm infants with congenital diaphragmatic hernia. J Pediatr Surg. 2004;39:829–33. doi: 10.1016/j.jpedsurg.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Dott MM, Wong LY, Rasmussen SA. Population-based study of congenital diaphragmatic hernia: risk factors and survival in Metropolitan Atlanta, 1968–1999. Birth Defects Res A Clin Mol Teratol. 2003;67:261–7. doi: 10.1002/bdra.10039. [DOI] [PubMed] [Google Scholar]
- 11.Levison J, Halliday R, Holland AJ, Walker K, Williams G, Shi E, et al. A population-based study of congenital diaphragmatic hernia outcome in New South Wales and the Australian Capital Territory, Australia, 1992–2001. J Pediatr Surg. 2006;41:1049–53. doi: 10.1016/j.jpedsurg.2006.01.073. [DOI] [PubMed] [Google Scholar]
- 12.Draper ES, Zeitlin J, Field DJ, Manktelow BN, Truffert P. Mortality patterns among very preterm babies: a comparative analysis of two European regions in France and England. Arch Dis Child Fetal Neonatal Ed. 2007;92:356–60. doi: 10.1136/adc.2006.097683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butler N, Claireaux AE. Congenital diaphragmatic hernia as a cause of perinatal mortality. Lancet. 1962;1:659–63. doi: 10.1016/s0140-6736(62)92878-7. [DOI] [PubMed] [Google Scholar]
- 14.Winter R, Baraitser M. In the Winter-Baraitser Dysmorphology Database. 2006. [Google Scholar]
- 15.Sweed Y, Puri P. Congenital diaphragmatic hernia: influence of associated malformations on survival. Arch Dis Child. 1993;69:68–70. doi: 10.1136/adc.69.1_spec_no.68. [DOI] [PMC free article] [PubMed] [Google Scholar]