Abstract
Background
Due to the emergence of community-associated strains, the prevalence of invasive methicillin-resistant Staphylococcus aureus (MRSA) infections has increased substantially in pediatric patients. A vancomycin AUC0–24/MIC index >400 best predicts treatment outcomes for invasive MRSA infection in adults. Data on whether recommended vancomycin doses in children achieve this break point are lacking.
Objective
This study aimed to assess the likelihood that currently recommended vancomycin doses in children achieve AUC0–24/MIC >400.
Methods
Vancomycin AUC0–24/MIC predictions were conducted across a range of dosages (40–70 mg/kg/d) using a Monte Carlo simulation (n = 5000). AUC0–24 was calculated as daily dose divided by vancomycin clearance, and daily dose was fixed for a given simulation. Three literature-reported estimates in children were used to define vancomycin clearance and its variance. For the MIC distribution of MRSA isolates, susceptibility data were obtained from the University of California, San Francisco Children’s Hospital, San Francisco, California (n = 180; 40% ≤0.5 mg/L; 59% = 1 mg/L; and 1% = 2 mg/L).
Results
Using the recommended empiric dosage of 40 mg/kg/d, 58% to 66% of children were predicted to achieve AUC0–24/MIC >400. Increasing the vancomycin dosage to 60 mg/kg/d substantially increased the likelihood (88%–98%) of achieving this pharmacodynamic target. On sensitivity analysis, a dosage of 40 mg/kg/d was more strongly influenced by small changes in MIC compared with 60 mg/kg/d.
Conclusions
Recommended empiric vancomycin dosing in children (40 mg/kg/d) was not predicted to consistently achieve the pharmacodynamic target of AUC0–24/MIC >400 for invasive MRSA infections. A vancomycin dosage of 60 mg/kg/d was predicted to optimize achievement of this target in children.
Keywords: vancomycin, methicillin-resistant Staphylococcus aureus, pediatrics, pharmacokinetics/pharmacodynamics, Monte Carlo simulation
INTRODUCTION
Vancomycin is the drug of choice for invasive methicillin-resistant Staphylococcus aureus (MRSA) infections in children. Daily doses of 40 mg/kg have been recommended for treatment of non–central nervous system (CNS) MRSA infections.1–4 This dosing recommendation was based on the findings from an early pharmacokinetic study,5 and only a limited number of subsequent studies have reassessed vancomycin pharmacokinetics in children aged >1 year.6–8 With the confirmed increase in invasive MRSA infections in children,9–12 optimization of vancomycin dosing is crucial.
Current recommended pediatric practice uses vancomycin serum troughs to guide therapy.4,13 However, the literature in adults has documented that the vancomycin trough may not be the optimal pharmacokinetic measure for predicting treatment outcomes in invasive MRSA infection. A vancomycin AUC0–24/MIC index is reported to be the best measure in adults, with an AUC0–24/MIC >400 associated with optimal outcomes.14,15 Based on a literature search, vancomycin AUC0–24/MIC has not been studied extensively in children. In a previously published report, the authors modeled AUC0–24/MIC using literature-reported pediatric pharmacokinetic data and MICs for MRSA encountered at the University of California, San Francisco Children’s Hospital, San Francisco, California.16 The analysis predicted that the recommended empiric dosage of 40 mg/kg/d did not achieve AUC0–24/MIC >400 when the MIC was ≥1 mg/L. However, the model did not take into account the inherent interpatient variability in clinical conditions, such as drug clearance and MIC, which may have significantly affected the AUC0–24/MIC predictions. A more detailed assessment of vancomycin pharmacodynamics incorporating variation in these clinical conditions would provide additional insight into an appropriate dosage in children.
The Monte Carlo simulation is a modeling technique that allows the incorporation of biological variation in a model and, via repeated sampling, enables the assessment of the behavior of a model. The simulated data reveal the range of possible outcomes in a patient population, which can be used to determine the probability of a specific outcome.17 The objective of this study was to expand on previous modeling work on vancomycin AUC0–24/MIC in children by assessing the likelihood of recommended vancomycin doses achieving AUC0–24/MIC >400 in children, using Monte Carlo simulation.
METHODS
Patient Population
Simulated patients represent children treated with vancomycin for invasive MRSA infections. Infants aged <1 year were excluded because of their rapid maturational changes in renal function compared with older children.
Vancomycin AUC0–24/MIC Model
Vancomycin AUC0–24/MIC was predicted in children using a standard model. AUC0–24 was calculated as:
AUC0–24/MIC was obtained by dividing the calculated AUC0–24 by the MIC for MRSA isolates. Model parameters were vancomycin daily dose, vancomycin clearance (CL), and MIC for MRSA isolates.
Vancomycin Daily Dose
Multiple pediatric dosing references recommend a vancomycin daily dose of 40 mg/kg for empiric treatment of MRSA infections.1–4 An increased dosage of 60 mg/kg/d has been recommended for CNS infections.1–4 Dosages as high as 70 mg/kg/d have been suggested in certain pediatric cancer populations.6 Consequently, vancomycin daily doses ranging from 40 to 70 mg/kg at 5-mg/kg intervals were assessed. For a given simulation, the daily dosage was fixed.
Vancomycin Clearance
Estimates of vancomycin CL and its variance were obtained from the pediatric literature. Four studies were identified in which vancomycin CL was calculated based on direct measures of vancomycin concentrations in children.5–8 One study did not report the variance of the CL estimate and thus was excluded from simulations.5 The 3 mean (SD) vancomycin CL estimates from the respective studies were 114 (31),6 103 (46),7 and 110 (20) mL/h/kg.8
MIC for MRSA Isolates
The MIC distribution for MRSA used in this simulation was obtained from the results of pediatric cultures at the University of California, San Francisco Children’s Hospital, from July 2007 to June 2008. Vancomycin MIC was determined using a standard microtiter dilution technique with panels made by the University of California, Los Angeles, California, according to Clinical and Laboratory Standards Institute guidelines.18 The distribution of MRSA isolates (n = 180) with MICs ≤0.5, 1, and 2 mg/L were 40%, 59%, and 1%, respectively.
Monte Carlo Simulation
Empiric Coverage
The probability of achieving the pharmacodynamic target of AUC0–24/MIC >400 in children empirically treated with vancomycin for invasive MRSA infections was assessed across vancomycin dosages using the previously described AUC0–24/MIC model and parameter distributions by Monte Carlo simulation. Each CL estimate was assessed separately. Simulations of 5000 patients were conducted for each dosage using Crystal Ball version 11.1.1.1.00 (Oracle Corporation, Redwood Shores, California). For a simulation run, the percentage of patients whose AUC0–24/MIC was >400 was reported, calculated as the total number of patients achieving AUC0–24/MIC >400/5000 · 100.
Sensitivity Analysis
To examine the influence of MIC distribution on the AUC0–24/MIC model and Monte Carlo simulation results, a sensitivity analysis was performed. Monte Carlo simulations were repeatedly conducted as described earlier. However, the MIC distribution was varied to represent a range of conditions between simulations while the dosage was held constant. Daily doses of 40 and 60 mg/kg were used for each sensitivity analysis because they are the 2 most relevant dosage considerations based on current dosing recommendations.1–4 In the first analysis, the sensitivity of the model to a MIC of 1 mg/L was assessed by varying the proportion of isolates with a MIC of 1 mg/L from 0% to 100%, in 5% increments. The proportion with a MIC of 2 mg/L was held constant at 1%, and the proportion with a MIC ≤0.5 mg/L was adjusted so that the overall MIC distribution summed to 100%. The second analysis assessed the influence of increases in the proportion of isolates with a MIC of 2 mg/L varying from 0% to 60%, while holding a MIC ≤0.5 mg/L at 40% and adjusting a MIC of 1 mg/L appropriately.
Known MIC Coverage
To assess AUC0–24/MIC in children with a confirmed MRSA isolate with a known MIC, Monte Carlo simulations were conducted using the same model as mentioned earlier, but with a fixed MIC of 0.5, 1, or 2 mg/L. Daily doses of 40 or 60 mg/kg were assessed.
RESULTS
Empiric Coverage
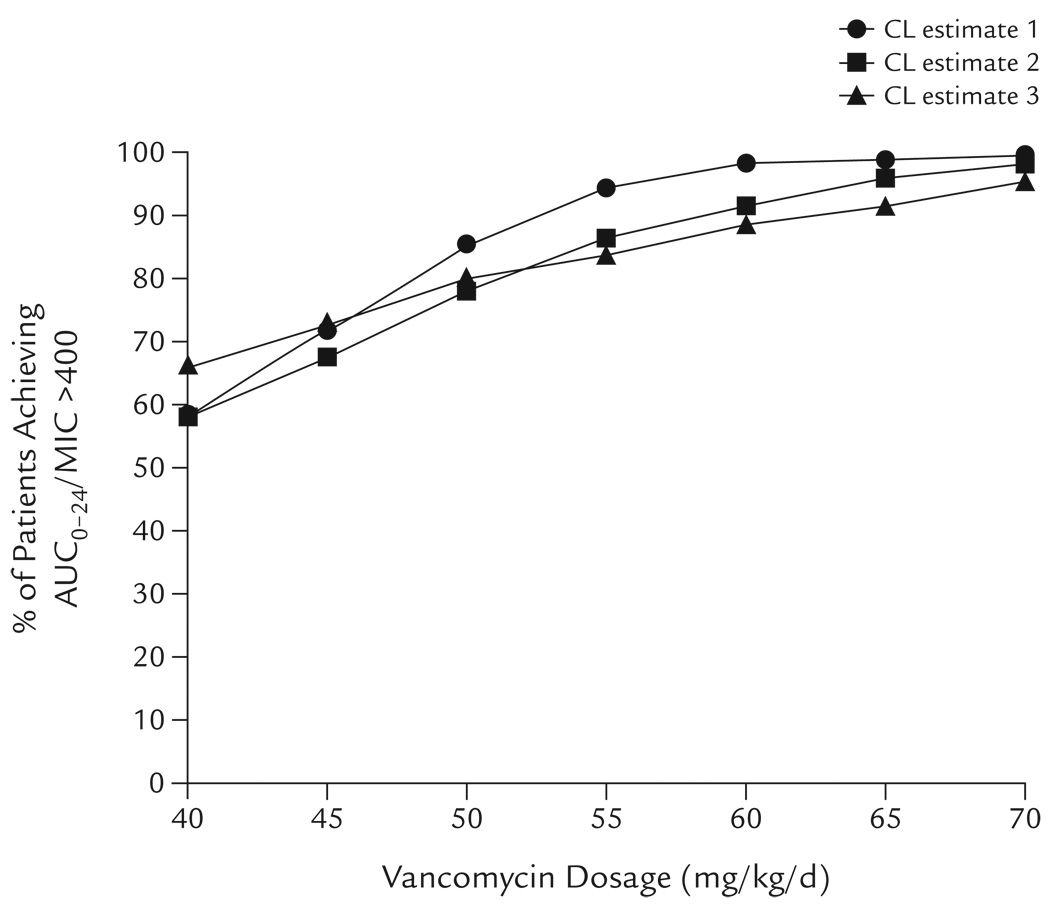
The percentages of children predicted to achieve the pharmacodynamic target of AUC0–24/MIC >400 for vancomycin across a range of dosages (40–70 mg/kg/d) are shown in Figure 1. The results from each CL estimate were in general agreement. With increasing vancomycin daily dose, the percentage of patients predicted to achieve AUC0–24/MIC >400 similarly increased. At 40 mg/kg/d, the percentage predicted to achieve AUC0–24/MIC >400 ranged from 58% to 66%. At 60 mg/kg/d, the percentage predicted to achieve AUC0–24/MIC >400 increased from 88% to 98%. At dosages >60 mg/kg/d, the curve flattened, with only an additional 1% to 7% predicted to achieve AUC0–24/MIC >400 at 70 mg/kg/d compared with 60 mg/kg/d.
Figure 1.
Monte Carlo simulation of the effects of vancomycin dosage on the achievement of AUC0–24/MIC >400 in children receiving vancomycin 40 to 70 mg/kg/d for invasive methicillin-resistant Staphylococcus aureus infection. At each dosage, the percentage of children predicted to achieve AUC0–24/MIC >400 is reported for 3 separate simulations each using a different vancomycin clearance (CL) estimate from the literature.6–8 The same MIC distribution was used for each simulation. See the Methods section for model details.
Sensitivity Analysis
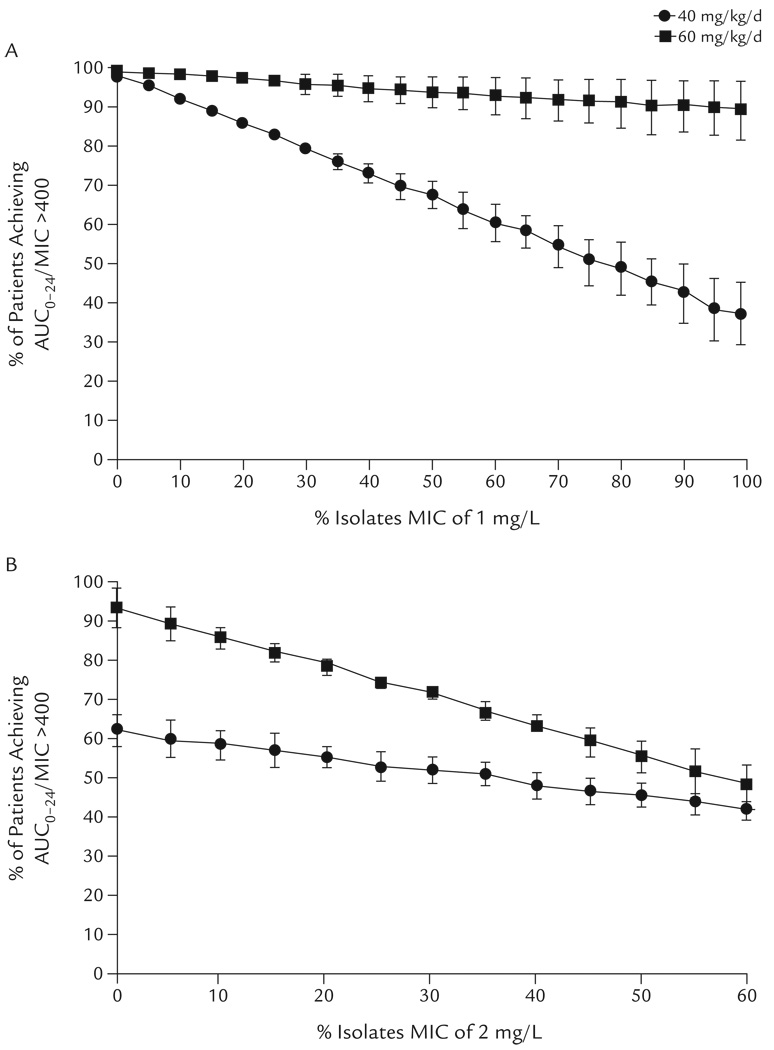
Sensitivity analysis suggested that the model results were strongly influenced by small changes in the MIC distribution when a vancomycin dosage of 40 mg/kg/d was used (Figure 2). For each 10% increase in the proportion of MRSA isolates with a MIC of 1 mg/L, the likelihood of achieving the target AUC0–24/MIC at a dosage of 40 mg/kg/d decreased by a mean of 6% (assuming the proportion with a MIC of 2 mg/L remained constant) (Figure 2A). For example, when 50% of isolates were a MIC of 1 mg/L, AUC0–24/MIC >400 was not achieved in >30% of patients. A dosage of 60 mg/kg/d was much less sensitive to large changes in the proportion of MRSA isolates with a MIC of 1 mg/L compared with a dosage of 40 mg/kg/d. The percentage of children predicted to achieve the target AUC0–24/MIC was >90%, even when the proportion of MRSA isolates with a MIC of 1 mg/L increased to 100%. On the other hand, both dosages were sensitive to changes in the proportion of MRSA isolates with a MIC of 2 mg/L (Figure 2B).
Figure 2.
Monte Carlo simulation of the effects of MIC distribution on the achievement of vancomycin AUC0–24/MIC >400 in children receiving vancomycin 40 or 60 mg/kg/d for invasive methicillin-resistant Staphylococcus aureus infection. (A) The proportion of isolates with a MIC of 1 mg/L varied, while the MIC of 2 mg/L was constant at 1% and the proportion of MIC ≤0.5 mg/L was adjusted so that the overall MIC distribution summed to 100%. (B) The proportion of isolates with a MIC of 2 mg/L was varied, while MIC ≤0.5 mg/L was constant at 40% and the proportion with a MIC of 1 mg/L was adjusted so that the overall MIC distribution summed to 100%. Data points represent the mean (SD) of 3 simulations, each using a different vancomycin clearance estimate.
Known MIC Coverage
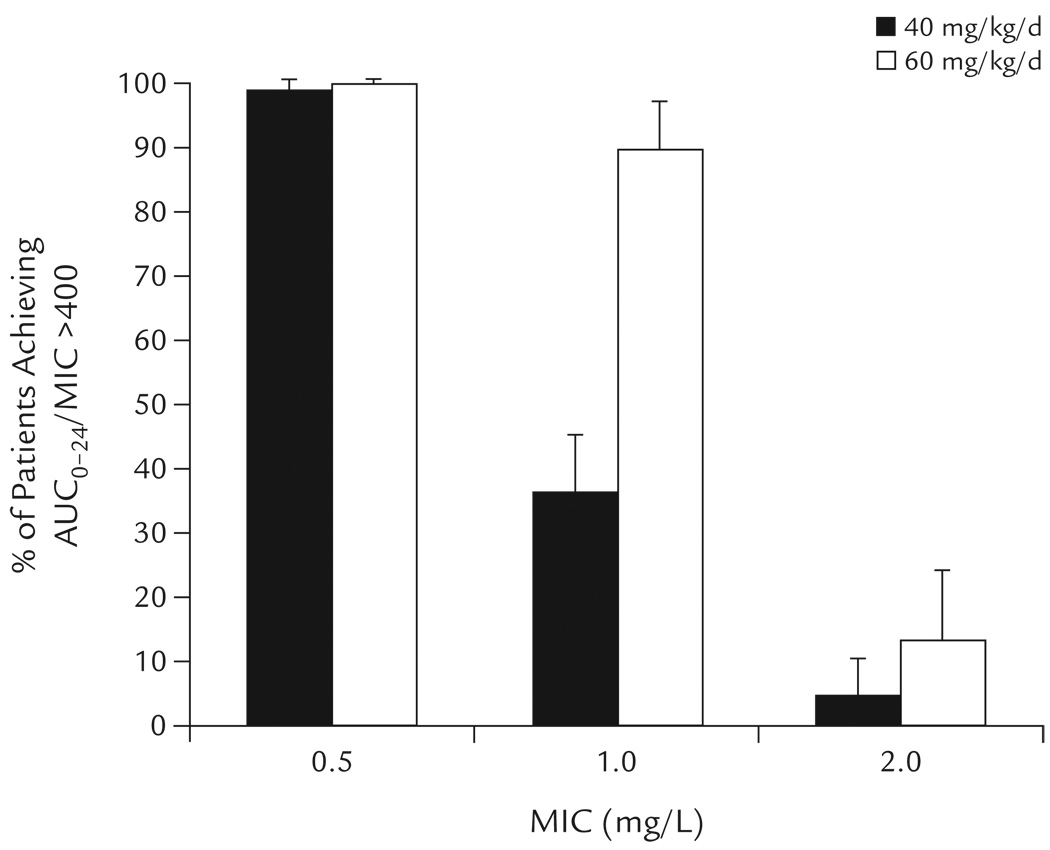
In children with confirmed MRSA and a known MIC, the percentages predicted to achieve AUC0–24/MIC >400 at vancomycin dosages of 40 and 60 mg/kg/d are shown in Figure 3. At a MIC ≤0.5 mg/L, both dosages readily achieved AUC0–24/MIC >400. At a MIC of 1 mg/L, a dosage of 60 mg/kg/d achieved AUC0–24/MIC >400 in 2.4-fold as many children as did a dosage of 40 mg/kg/d. AUC0–24/MIC >400 was achieved in <15% of children for either dosage when the MIC was 2 mg/L.
Figure 3.
Percentages of children predicted to achieve vancomycin AUC0–24/MIC >400 for known MIC at vancomycin dosages of 40 and 60 mg/kg/d for invasive methicillin-resistant Staphylococcus aureus infection. Data points represent the mean (SD) of 3 Monte Carlo simulations, each using a different vancomycin clearance estimate.
DISCUSSION
In this study, the likelihood of achieving the pharmacodynamic target of vancomycin AUC0–24/MIC >400 for the empiric treatment of invasive MRSA infection in children was assessed using Monte Carlo simulation. At the recommended empiric starting dosage of 40 mg/kg/d, 58% to 66% of children were predicted to achieve AUC0–24/MIC >400. Increased vancomycin dosages resulted in a greater likelihood of children achieving AUC0–24/MIC >400. However, at dosing attenuated above 60 mg/kg/d, 88% to 98% of children were predicted to achieve AUC0–24/MIC >400.
The findings are concerning in that up to 42% of children treated for invasive MRSA infections are predicted to not be covered using the recommended empiric vancomycin starting dosage of 40 mg/kg/d. These findings raise the question of whether the current recommended dose of vancomycin is appropriate. The model used in this study suggests that an empiric starting dosage of 60 mg/kg/d is more appropriate; with this dosage, 2% to 12% of children were predicted to not achieve AUC0–24/MIC >400. In addition, a dosage of 60 mg/kg/d was less sensitive to changes in the distribution of MIC of 1 mg/L compared with a dosage of 40 mg/kg/d in terms of the ability to reach AUC0–24/MIC >400 (Figure 2).
Vancomycin is commonly used in pediatrics for the empiric treatment of suspected invasive MRSA infections. Although clinical experience with vancomycin use in children is extensive, studies examining its pharmacokinetics in this population are limited.5–8 Pediatric drug references currently recommend a dosage of 40 mg/kg/d for the empiric coverage of non-CNS MRSA infections.1–4 This dosage was first suggested in 1980 by Schaad et al5 based on the findings from a study of vancomycin in 18 children and a target serum vancomycin trough of <10 mg/L. Subsequent assessments of this dosing guideline have been reported.6,19 One study assessed vancomycin use in 31 infants and children (mean [SD] age, 4.2 [5.1] years) treated for suspected staphylococcal infections using 40 mg/kg/d divided q6h.6 In these patients, ≥1 dosage adjustment was required in 55% of noncancer patients to achieve the goal trough (5–15 mg/L). The final mean daily dose in this group, 50 mg/kg/d, was associated with a mean vancomycin trough of 8 mg/L. Similarly, in the pediatric intensive care unit, in 135 patients with normal renal function (mean age, 5.8 [5.4] years), a mean empiric starting dosage of 47 mg/kg/d resulted in a mean trough of 6 mg/L and required a mean of 1.1 dosage changes per patient to achieve the goal trough (5–10 mg/L).19 At study end, the overall mean daily dose, 61 mg/kg, was associated with a mean vancomycin trough of 8 mg/L.
With the increases in the median vancomycin MIC for MRSA (“MIC creep”20,21) and increasing reports of treatment failures,22 increased goal troughs have been suggested. For example, in adults with invasive MRSA infections, recommendations are for serum vancomycin troughs as high as 15 to 20 mg/L.15,23 In addition, findings in vitro24,25 and in 1 adult26 have suggested that prolonged vancomycin exposure at low concentrations may promote resistance. These findings are of concern considering that the findings from 2 studies in children have suggested that the recommended vancomycin dosage in children, 40 to 45 mg/kg/d, will not regularly achieve vancomycin troughs >10 mg/L.6,19
In addition, dosing guidelines based on vancomycin troughs alone may be misleading in terms of efficacy. Based on the findings from studies in animals and adults, the best measure of vancomycin activity is AUC0–24/MIC.14,15 The achievement of an AUC0–24/MIC >400 in adults has been associated with optimal outcomes for the treatment of invasive MRSA infections.
Previously published work that modeled AUC0–24/MIC in children found that a vancomycin dosage of 60 but not 40 mg/kg/d was associated with a mean AUC0–24/MIC >400 when the MIC of MRSA isolates was 1 mg/L.16 At a MIC of 2 mg/L, neither dosage predicted a mean AUC0–24/MIC >400. These findings are in general agreement with those from the present Monte Carlo simulation. Both used the same underlying structural model and data to make AUC0–24/MIC predictions. The present work expands on the understanding of AUC0–24/MIC in children by incorporating the inherent biologic and epidemiologic variation of the parameters in the model using a Monte Carlo simulation, an empiric method in which repeated random samples are drawn and calculated based on the underlying probability distribution of the parameters. By considering the range of outcomes, a more robust and dynamic picture of the clinical situation is formed compared with the “average” outcome (ie, not all children have the mean vancomycin CL). This methodology allows for a more clinically useful assessment of a dosage or intervention.17
Although this Monte Carlo simulation used a local MIC distribution, the findings can be adapted to reflect distributions at other institutions. The sensitivity analysis predicted coverage across all distributions of MIC of 1 mg/L from 0% to 100% and would allow an institution to predict their individual coverage using its own MIC distribution. Although a MIC of 2 mg/L is considered within the susceptible range, the findings from this simulation suggest that with a MIC of 2 mg/L, <5% of children would reach AUC0–24/MIC >400 at a dosage of 40 mg/kg/d, with little benefit to increased dosing (Figure 3). This predicted poor pharmacodynamic response at a MIC of 2 mg/L is supported by findings from clinical studies in adults, in which patients with an increased MIC (>1 mg/L) had poorer treatment responses.27–29 Therefore, alternatives to vancomycin, such as linezolid or daptomycin, should be considered for the treatment of invasive MRSA infections for isolates with a MIC of 2 mg/L.
Study Limitations
This analysis does not represent new patient data and is inherent to the modeling and simulation approach. Nonetheless, all of the calculations were derived based on data from prior pediatric clinical studies that assessed the pharmacokinetics of vancomycin. The present results also depend significantly on the accuracy of the underlying parameter estimates used in the model. The general agreement of all 3 vancomycin clearance estimates lends credibility to the results. In addition, the sensitivity analysis found possible influences of MIC distribution on the results.
Implementing increased vancomycin dosing is likely to result in higher mean serum concentrations, which raises the concern of potential increased toxicity (eg, nephrotoxicity).15,30,31 However, the dosage proposed (60 mg/kg/d) is currently recommended for CNS infections and has been previously described in the literature.6,19 Based on preliminary data from the University of California, San Francisco Children’s Hospital, this dosage (15 mg/kg IV q6h), now the standard starting dosage in children with normal renal function, has not been found to be associated with any significant increase in the percentage of patients with vancomycin troughs >20 mg/L (unpublished observations, A. Frymoyer et al, 2010). Further studies to determine whether ≥60 mg/kg/d is the optimal dosage to balance efficacy and tolerability are needed. Finally, the importance of AUC0–24/MIC >400 in children has not been studied or verified. Therefore, clinical studies to validate AUC0–24/MIC and outcome in children are necessary. Our modeling results provide initial insight into optimal clinical trial design and dosage selection.
CONCLUSIONS
The findings from this study suggest that the recommended empiric vancomycin dosage in children (40 mg/kg/d) was not predicted to consistently achieve the pharmacodynamic target of AUC0–24/MIC >400 for invasive MRSA infections. A vancomycin dosage of 60 mg/kg/d was predicted to optimize achievement of this target when MIC values are similar to those used in this model. Clinical studies assessing AUC0–24/MIC and clinical outcomes in children are urgently needed.
ACKNOWLEDGMENTS
Research by Dr. Frymoyer was supported by National Institute of General Medical Sciences training grant no. T32 GM07546. Research by Dr. Hersh was supported by National Institute of Child Health and Human Development training grant no. T32 HD044331.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data previously presented at the Pediatric Academic Societies Annual Meeting, May 2–5, 2009, Baltimore, Maryland.
The authors have indicated that they have no other conflicts of interest regarding the content of this article.
REFERENCES
- 1.Vancocin (vancomycin injection) [package insert] Deerfield, Ill: Baxter Healthcare Corporation; 2003. [Google Scholar]
- 2.Robertson J, Shilkofski N, editors. The Harriet Lane Handbook: A Manual for Pediatric House Officers. 17th ed. Philadelphia, Pa: Mosby; 2005. [Google Scholar]
- 3.Taketomo CK, Hodding JH, Kraus DM. Lexi Comp’s Pediatric Dosage Handbook with International Trade Names Index: Including Neonatal Dosing, Drug Administration, & Extemporaneous Preparations (Lexi-Comp’s Drug Reference Handbooks) 14th ed. Hudson, Oh: Lexi-Comp; 2007. [Google Scholar]
- 4.Long SS, Pickering LK, Prober CG, editors. Principles and Practice of Pediatric Infectious Diseases. 3rd ed. Philadelphia, Pa: Churchill Livingstone Elsevier; 2008. [Google Scholar]
- 5.Schaad UB, McCracken GH, Jr, Nelson JD. Clinical pharmacology and efficacy of vancomycin in pediatric patients. J Pediatr. 1980;96:119–126. doi: 10.1016/s0022-3476(80)80347-7. [DOI] [PubMed] [Google Scholar]
- 6.Chang D. Influence of malignancy on the pharmacokinetics of vancomycin in infants and children. Pediatr Infect Dis J. 1995;14:667–673. doi: 10.1097/00006454-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Lamarre P, Lebel D, Ducharme MP. A population pharmacokinetic model for vancomycin in pediatric patients and its predictive value in a naive population. Antimicrob Agents Chemother. 2000;44:278–282. doi: 10.1128/aac.44.2.278-282.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wrishko RE, Levine M, Khoo D, et al. Vancomycin pharmacokinetics and Bayesian estimation in pediatric patients. Ther Drug Monit. 2000;22:522–531. doi: 10.1097/00007691-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Burke RE, Halpern MS, Baron EJ, Gutierrez K. Pediatric and neonatal Staphylococcus aureus bacteremia: Epidemiology, risk factors, and outcome. Infect Control Hosp Epidemiol. 2009;30:636–644. doi: 10.1086/597521. [DOI] [PubMed] [Google Scholar]
- 10.Gerber JS, Coffin SE, Smathers SA, Zaoutis TE. Trends in the incidence of methicillin-resistant Staphylococcus aureus infection in children’s hospitals in the United States. Clin Infect Dis. 2009;49:65–71. doi: 10.1086/599348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan SL, Hulten KG, Gonzalez BE, et al. Three-year surveillance of community-acquired Staphylococcus aureus infections in children. Clin Infect Dis. 2005;40:1785–1791. doi: 10.1086/430312. [DOI] [PubMed] [Google Scholar]
- 12.Purcell K, Fergie J. Epidemic of community-acquired methicillin-resistant Staphylococcus aureus infections: A 14-year study at Driscoll Children’s Hospital. Arch Pediatr Adolesc Med. 2005;159:980–985. doi: 10.1001/archpedi.159.10.980. [DOI] [PubMed] [Google Scholar]
- 13.Ambrose P, Winter ME. Vancomycin. In: Winter ME, editor. Basic Clinical Pharmacokinetics. 4th ed. Philadelphia, Pa: Lippincott Williams & Wilkins; 2004. pp. 451–476. [Google Scholar]
- 14.Moise-Broder PA, Forrest A, Birmingham MC, Schentag JJ. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet. 2004;43:925–942. doi: 10.2165/00003088-200443130-00005. [DOI] [PubMed] [Google Scholar]
- 15.Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: A consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2009;66:82–98. doi: 10.2146/ajhp080434. [published correction appears in Am J Health Syst Pharm. 2009;66:887] [DOI] [PubMed] [Google Scholar]
- 16.Frymoyer A, Hersh AL, Benet LZ, Guglielmo BJ. Current recommended dosing of vancomycin for children with invasive methicillin-resistant Staphylococcus aureus infections is inadequate. Pediatr Infect Dis J. 2009;28:398–402. doi: 10.1097/INF.0b013e3181906e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bradley JS, Dudley MN, Drusano GL. Predicting efficacy of antiinfectives with pharmacodynamics and Monte Carlo simulation. Pediatr Infect Dis J. 2003;22:982–992. doi: 10.1097/01.inf.0000094940.81959.14. [DOI] [PubMed] [Google Scholar]
- 18.Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard. 8th ed. Wayne, Pa: Clinical and Laboratory Standards Institute; 2009. [Google Scholar]
- 19.Glover ML, Cole E, Wolfsdorf J. Vancomycin dosage requirements among pediatric intensive care unit patients with normal renal function. J Crit Care. 2000;15:1–4. doi: 10.1053/jcrc.2000.0150001. [DOI] [PubMed] [Google Scholar]
- 20.Steinkraus G, White R, Friedrich L. Vancomycin MIC creep in non-VISA, vancomycin susceptible clinical MRSA blood isolates from 2001–2005. Program and Abstracts of the 106th Annual Meeting of the American Society for Microbiology; American Society for Microbiology; May 21–25, 2006; Orlando, Fla. Washington, DC. 2006. Abstract A-084. [Google Scholar]
- 21.Wang G, Hindler JF, Ward KW, Bruckner DA. Increased vancomycin MICs for Staphylococcus aureus clinical isolates from a university hospital during a 5-year period. J Clin Microbiol. 2006;44:3883–3886. doi: 10.1128/JCM.01388-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakoulas G, Moellering RC., Jr Increasing antibiotic resistance among methicillin-resistant Staphylococcusaureus aureus strains. Clin Infect Dis. (Suppl 5) 2008:S360–S367. doi: 10.1086/533592. [DOI] [PubMed] [Google Scholar]
- 23.American Thoracic Society/Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 24.Sakoulas G, Eliopoulos GM, Moellering RC, Jr, et al. Staphylococcus aureus accessory gene regulator (agr) group II: Is there a relationship to the development of intermediate-level glycopeptide resistance? J Infect Dis. 2003;187:929–938. doi: 10.1086/368128. [DOI] [PubMed] [Google Scholar]
- 25.Tsuji BT, Rybak MJ, Lau KL, Sakoulas G. Evaluation of accessory gene regulator (agr) group and function in the proclivity towards vancomycin intermediate resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 2007;51:1089–1091. doi: 10.1128/AAC.00671-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakoulas G, Gold HS, Cohen RA, et al. Effects of prolonged vancomycin administration on methicillin-resistant Staphylococcus aureus (MRSA) in a patient with recurrent bacteraemia. J Antimicrob Chemother. 2006;57:699–704. doi: 10.1093/jac/dkl030. [DOI] [PubMed] [Google Scholar]
- 27.Hidayat LK, Hsu DI, Quist R, et al. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: Efficacy and toxicity. Arch Intern Med. 2006;166:2138–2144. doi: 10.1001/archinte.166.19.2138. [DOI] [PubMed] [Google Scholar]
- 28.Sakoulas G, Moise-Broder PA, Schentag J, et al. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol. 2004;42:2398–2402. doi: 10.1128/JCM.42.6.2398-2402.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soriano A, Marco F, Martinez JA, et al. Influence of vancomycin minimum inhibitory concentration on the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2008;46:193–200. doi: 10.1086/524667. [DOI] [PubMed] [Google Scholar]
- 30.Lodise TP, Lomaestro B, Graves J, Drusano GL. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother. 2008;52:1330–1336. doi: 10.1128/AAC.01602-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lodise TP, Patel N, Lomaestro BM, et al. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis. 2009;49:507–514. doi: 10.1086/600884. [DOI] [PubMed] [Google Scholar]