Abstract
Dysfunction of immune systems, including innate and adaptive immunity, is responsible for the immunopathogenesis of systemic lupus erythematosus (SLE). NK cells are a major part of the innate immune system, and diminished populations of NK cells have been reported in SLE patients. However, the mechanisms behind this decrease and the role of NK cells in SLE pathogenesis remain poorly understood. In this study, we found that a deficiency of NK cells, especially CD226+ NK cells, is prominent in patients with active SLE. Meanwhile, expression of the CD226 ligands CD112 and CD155 on plasmacytoid dendritic cells is observed in SLE patients; thus, activation of CD226+ NK cells may be induced by CD226–ligand interactions. Furthermore, IFN-α, which is mainly produced by plasmacytoid dendritic cells, can mediate the activation-induced cell death of NK cells. Therefore, these processes likely contribute to the loss of NK cells in patients with active SLE. Despite the impaired cytotoxicity of peripheral NK cells in human SLE patients and mouse SLE models, we provide evidence that CD226+ NK cells infiltrate the kidneys of predisease MRL-lpr/lpr mice. Kidney-infiltrating NK cells displayed an activated phenotype and a marked ability to produce cytotoxic granules. These results suggest that, before apoptosis, activated NK cells can infiltrate tissues and, to some extent, mediate tissue injury by producing cytotoxic granules and immunoregulatory cytokines.
Systemic lupus erythematosus (SLE) is a chronic multisystem autoimmune disease (1, 2). Although the etiology and pathogenesis of SLE are largely unclear, genetic (3) and environmental factors (4) are thought to contribute to disease development. Furthermore, the adaptive immune system seems to play a central role in the pathogenesis of this disease. The serological hallmarks of SLE are autoantibody production (5) and formation of immune complexes (6). These autoantibody–nuclear Ag immune complexes are thought to participate in SLE pathogenesis, because they damage tissues by triggering inflammation (7, 8). Autoantibody-producing B cells and self-reactive T cells have also been implicated in the pathogenesis of SLE (9–11). In several mouse SLE models, autoimmunity was effectively prevented by inhibiting T cell activation or T–B cell interactions (12, 13). Interestingly, several studies have implied that innate immunity, including complement, has a protective role against the development of SLE, possibly through the clearance of apoptotic debris (14, 15).
NK cells, which are part of the innate immune system, are now described as multifunctional cells. However, whether NK cells contribute to the development of SLE pathology is still unclear. NK cells affect immune responses by killing target cells directly or by producing proinflammatory and regulatory cytokines (16). Furthermore, NK cells have been found, in some cases, to predispose individuals toward autoimmune disease caused by naturally occurring mutations in genes (17). Recent data from several groups suggested that the proportion and absolute number of circulating NK cells are significantly lower in SLE patients (18, 19), but the mechanism at work and its relationship to the pathogenesis of SLE remain unclear.
Substantial evidence indicates that NK cell function relies on receptor–ligand interactions (16). DNAX accessory molecule-1 (CD226) is an activating receptor that is expressed on NK cells and T cells (20). It was demonstrated that CD226+ NK cells play an important role in the recognition of several types of human tumors, such as myeloma, melanoma, and ovarian carcinoma. Results from HIV-1–infected patients indicate that CD226–ligand interactions may contribute to NK cell-mediated recognition and lysis of dendritic cells (DCs) (21). Taken together, these results suggest that CD226+ NK cells contribute to immune surveillance of tumor formation and viral infection. However, the role of CD226+ NK cells in autoimmune disease has not been widely studied. Recent data from rheumatoid arthritis patients indicated that CD226 is expressed on CD4+CD28− T cells and contributes to the activation of this T cell subset (22).
Many SLE patients have elevated levels of serum IFN-α (23–26). Indeed, IFN-α levels are correlated with the activity and severity of SLE (27, 28). Recent evidence suggested that IFN-α is a potent inducer of NK cell activation (29). In this study, we hypothesized that excessive IFN-α in SLE provides an important link between innate and adaptive immunity during disease development. For instance, it was shown to induce DC differentiation in SLE patients (30, 31). Also, prolonged production of IFN-α may lead to the generation of autoimmune T and B cells (26). However, it is still unknown whether NK cell statuses, such as receptor expression and effector functions, are affected by IFN-α in SLE.
In this study, we found that NK cell populations were significantly reduced in SLE patients with active disease, and the majority of NK cells lost were CD226+ NK cells. Additionally, the elevated levels of serum IFN-α in SLE patients could mediate the activation-induced cell death (AICD) of NK cells, contributing to the loss of NK cells in SLE patients with active disease. Moreover, we found evidence that CD226+ NK cells infiltrated the kidneys of predisease MRL/lpr mice. Kidney-infiltrating NK cells acquired an activated phenotype and produced cytotoxic granules and immunoregulatory cytokines, which may induce tissue damage.
Materials and Methods
Patients
Ninety-nine consecutive patients (91 women and 8 men) with SLE from the Department of Rheumatology and Immunology, Anhui Provincial Hospital, were included in the study. All patients fulfilled at least four of the American College of Rheumatology 1997 revised criteria for SLE (32, 33). Disease activity was assessed by the SLE disease activity index (SLEDAI) (34). Among the 99 patients, 75 were diagnosed with active lupus (median age, 32.5 y; range: 13–66 y). Detailed characteristics of the patients with active disease are shown in Supplementary Table I. The mean SLEDAI of these patients was 12.89 (range, 4–30) at the time of blood sampling. The other 24 patients (median age, 33 y; range: 16–51 y), in long-term remission, were enrolled during the follow-up period. Remission was defined by resolution of clinical signs, normalization of laboratory findings, and minimal maintenance therapy. Treatments for the patients with active SLE were dependent on their diagnosis. Briefly, patients were treated with steroids (prednisolone, 1 mg/kg/d) and hydroxychloroquine (200–400 mg/d), alone or in combination. In addition to steroids and antimalarials, patients who were diagnosed with lupus nephritis received cyclophosphamide (600–800 mg once a month) or mycophenolate mofetil (500 mg twice daily) as complementary therapy. Steroids had been discontinued for >1 y in the patients in remission, with the exception of six who had received low-dose prednisone (10–15 mg/d) alone or in combination with antimalarials (hydroxychloroquine, 200 mg/d); no patient received any cytotoxic drugs. Peripheral blood samples, obtained from healthy donors at Hefei blood bank, served as controls. All blood samples were taken after informed consent by the donor and were collected following approval by the Institutional Review Board of the University of Science and Technology of China.
Mice
Female MRL/mp and MRL/lpr mice were purchased from Shanghai Experimental Animal Center, Chinese Science Academy (Shanghai, China). All mice were maintained in a specific pathogen-free microenvironment and received care in compliance with the guidelines outlined in the Guide for the Care and Use of Laboratory Animals. MRL/mp mice were between 8 and 15 wk of age. MRL/lpr mice were used at 9–33 wk of age.
Cell isolation
Human PBMCs from SLE patients and healthy donors were isolated by Ficoll-Hypaque (Solarbio, Beijing) density-gradient centrifugation. After washing twice in PBS, PBMCs were resuspended in complete RPMI 1640 medium consisting of 10% FBS (both from Life Technologies), 100 U/ml penicillin, and 100 µg/ml streptomycin. To isolate mononuclear cells (MNCs), mice were sacrificed, and both kidneys were collected and decapsulated. Tissues were disrupted mechanically and then digested with collagenase D (Sigma) to make single-cell suspensions. After washing, pellets were resuspended in 40% Percoll (GE Healthcare), gently overlaid onto 70% Percoll, and centrifuged at 1260 × g for 30 min at room temperature. Kidney MNCs were isolated from the Percoll interface and washed twice in PBS at 300 × g for 10 min at 4°C. Kidney MNCs were counted using a hemocytometer, and the absolute numbers of NK cells, B cells, and T cells in kidneys were calculated by multiplying the total number of kidney MNCs by the percentage of positive cells determined by flow cytometry. Murine splenic single-cell suspensions were prepared by passing spleens through a 200-gauge stainless steel mesh. Erythrocytes were lysed with RBC lysis buffer (BioLegend), and the remaining cells were washed twice with PBS. The absolute numbers of NK cells, B cells, and T cells in spleens were calculated as above.
Cell staining and flow cytometry
Human PBMCs and mouse splenic and kidney lymphocytes were prepared and stained with mAbs. FcRs were blocked using normal mouse serum or rat serum. Abs to the following Ags were used for staining human PBMCs: CD3 (clone:SK7), Lin-1 (SK7/3G8/SJ25C1/L27/MΦP9/NCAM16.2), CD3 (UCHT1), CD4 (RPA-T4), CD8 (RPA-T8), CD11c (B-ly6), CD16 (3G8), CD19 (HIB19), CD56 (B159), CD69 (FN50), CD85j (GHI/75), CD94 (HP-3D9), CD112 (R2.525), CD123 (7G3), CD158b (CH-L), CD226 (DX11), NKG2D (1D11), HLA-DR (G46-6), and NKp46 (9E2/NKP46) (BD PharMingen); CD155 (300907), NKG2A (131411), and NKG2C (134591) (R&D Systems); and CD158a (EB6), Vβ11 (C21), and Vα24 (C15) (Immunotech). For CD155 staining, cells were stained first with anti-CD155 and allophycocyanin–anti-mouse IgG and then additional staining was performed. For apoptosis detection, PBMCs were harvested and stained with anti-CD56 and anti-CD3 Abs. After two washes in cold binding buffer (10 mM HEPES/NaOH [pH 7.4], 140 mM NaCl, 2.5 mM CaCl2), cells were incubated with Annexin V (BD PharMingen) and propidium iodide (1 µg/ml) for 15 min at room temperature in the dark. Finally, binding buffer was added to a final volume of 400 µl, and flow cytometric analysis was performed. Abs to the following molecules were used to stain murine splenic or kidney lymphocytes: CD3e (145-2C11), CD19 (ID3), CD49b (DX-5), CD69 (H1.2F3) (all from BD PharMingen), and CD226 (10E5) (Biolegend). For intracellular-cytokine assays, after surface Ag staining, cells were fixed, permeabilized, and stained with Alexa Fluor-647–anti-IFN-α (7N4–1) (BD PharMingen), PE–anti-granzyme B (16G6), or allophycocyanin–anti-perforin (eBioMAK-D) (eBioscience). Appropriate isotype controls were used in all experiments to estimate background fluorescence. Stained cells were analyzed using a FACSCalibur flow cytometer (Becton Dickinson), and data were analyzed with WinMDI version 2.9 software.
Intracellular IFN-γ detection
Freshly isolated mouse kidney MNCs (2 × 106/ml) were cultured with RPMI 1640 supplemented with 10% FBS in the presence of PMA (30 ng/ml) and ionomycin (1 µg/ml). One hour later, monensin (10 µg/ml) was added to prevent secretion of the induced cytokines (all of the stimulators were purchased from Sigma). Cells were harvested after culturing for 4 h at 37°C and 5% CO2 and stained with anti-CD3e and anti-DX5 Abs for 30 min at 4°C in the dark. After fixation and permeabilization, cells were stained with anti–IFN-γ (XMG1.2) (BD PharMingen) for 1 h at room temperature in the dark, washed twice with permeabilization buffer, and analyzed by flow cytometry. Appropriate isotype Abs were used as controls for intracellular cytokine detection.
ELISA for cytokine detection
To determine the cytokine levels in SLE patients, plasma samples were collected from all patients and controls and kept at −70°C until use. IFN-α was assayed using a standard sandwich ELISA Kit (PBL Biomedical Laboratories).
In vitro cell culture and stimulation
A total of 1 × 106 human PBMCs was cultured in 24-well plates at 37°C in a 5% CO2 incubator. Cells were incubated in medium alone or with IFN-α (1000 U/ml; PBL Biomedical Laboratories) and then with IL-2 (100 U/ml; Changchun Institute of Biological Products) or IFN-α (1000 U/ml) plus IL-2 (100 U/ml) for 0, 24, 48, or 72 h. In another culture model, for cell activation and CD226 expression detection, PBMCs were cultured in the presence of 20% healthy control plasma or remission patient plasma or the same amount of active patient plasma with or without IFN-α–neutralizing Ab (10 µg/ml; PBL Biomedical Laboratories) for 0, 24, 48, or 72 h. For apoptosis detection, NK cell populations were enriched from whole blood by negative selection using the NK Cell Isolation Kit (Miltenyi Biotec). CD226+ NK cells and CD226− NK cells were also separated by magnetic-bead purification with MACS kits. Briefly, total NK cells were stained with FITC-conjugated CD226 mAb (BD PharMingen). Subsequently, the cells were magnetically labeled with anti-FITC MicroBeads (Miltenyi Biotec). Two isolated cell subsets (2 × 105/ml cells per well) were cultured with plasma samples as described above. Cultured cells were harvested at the indicated time points, and activation and apoptosis of NK cells were analyzed by flow cytometry.
Measuring IFN-inducible gene expression
To assess the expression of genes (PRKR, IFIT1, MX1, and IFI44) that was predominantly induced by type I IFN (IFIGs) (35), 1 × 107 PBMCs from healthy donors were cultured in six-well plates with medium. To study the effect of donor plasma on gene expression, healthy donor PBMCs were stimulated with one of the following additions: IFN-α (1,000 U/ml), 20% plasma from healthy control or SLE patient in remission, or 20% plasma from SLE patient with active disease with or without IFN-α–neutralizing Ab (10 µg/ml). After an incubation of 24 h, the cells were lysed, and total RNA was extracted from each lysate via the phenol/chloroform method using TRIzol reagent (Invitrogen, CA). Cellular RNA (2 µg) was used for cDNA synthesis. Semiquantitative real-time RT-PCR was performed using a SYBR Premix Ex Taq Perfect Real Time Kit (Takara) and a sequence detector (Rotor Gene 3000, Corbett Research). GAPDH was used as a housekeeping gene control. Primer sequences used were as follows: PRKR, 5′-CTT CCA TCT GAC TCA GGT TT-3′ (forward) and 5′-TGC TTC TGA CGG TAT GTA TTA-3′ (reverse); IFIT1, 5′-CTC CTT GGG TTC GTC TAT AAA TTG-3′ (forward) and 5′-AGT CAG CAG CCA GTC TCA G-3′ (reverse); MX1, 5′-TAC CAG GAC TAC GAG ATT G-3′ (forward) and 5′-TGC CAG GAA GGT CTA TTA G-3′ (reverse); IFI44, 5′-CTC GGT GGT TAG CAA TTA TTC CTC-3′ (forward) and 5′-AGC CCA TAG CAT TCG TCT CAG-3′ (reverse); and GAPDH, 5′-GAA GGT GAA GGT CGG AGT C-3′ (forward) and 5′-GAA GAT GGT GAT GGG ATT TC-3′ (reverse).
H&E staining
For histological analysis, 10% formalin-fixed kidney tissue was embedded in paraffin and sectioned at 5 µm. After deparaffinization and dehydration, sections were stained with H&E. Sections were read on a fluorescent microscope (Axio Scope; Carl Zeiss) at ×100 and ×400. Microscopy images were acquired with a Carl Zeiss AxioCam HRc camera and AxioVision Software.
Statistical analysis
Data are expressed as mean ± SEM. One-way ANOVA was used to compare the significant differences among three or more groups, followed by the Bonferroni post hoc test. Analysis was completed using SPSS for Windows (version 10.1; SPSS). Statistical significance was defined as p < 0.05.
Results
Reduced proportion of circulating CD226+ NK cells in SLE patients with active disease
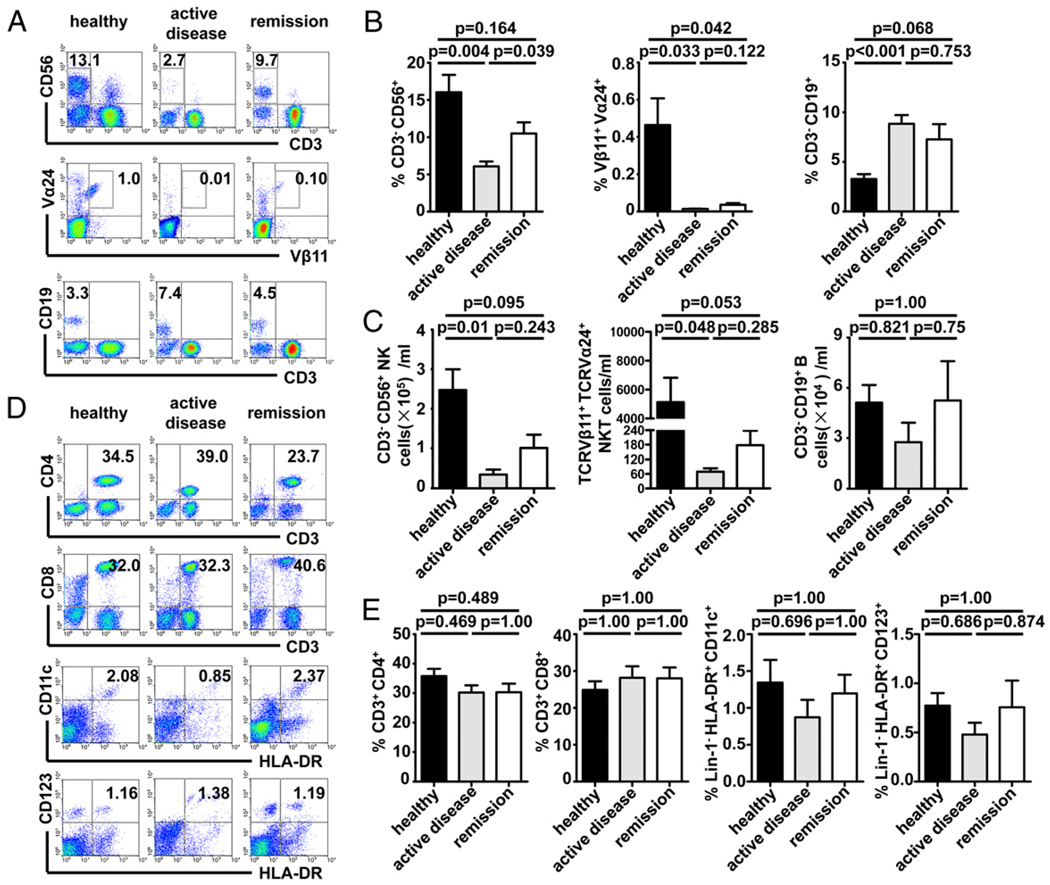
Because the immune state of SLE is poorly understood, involving abnormalities of the innate and adaptive arms of the immune system, we postulated that the frequency of circulating immune effector cells may be significantly altered in SLE patients. To test this hypothesis, the proportions of several lymphocyte subsets in the peripheral blood were determined by flow cytometry. Patients were divided into two groups based on disease activity, which was estimated by the SLEDAI. As shown in Fig. 1A–C, the absolute number and the proportion of CD3−CD56+ NK cells were significantly lower in SLE patients with active disease than in healthy controls and patients in remission. Similarly, the proportion and the absolute number of Vβ11+Vα24+ NKT cells were also significantly lower in patients with active SLE than in healthy controls. We observed a higher proportion of CD3−CD19+ B cells in active SLE patients compared with healthy controls (Fig. 1A, 1B). However, the absolute number of B cells was lower in patients with active SLE than in controls (Fig. 1C), which is consistent with previous reports (36, 37). Additionally, representative examples (Fig. 1D) and cumulative data (Fig. 1E) demonstrated that the proportions of T cells (CD4+ or CD8+), myeloid DCs (MDCs; Lin1−HLA-DR+CD11c+CD123−), and plasmacytoid DCs (PDCs; Lin1−HLA-DR+CD11c−CD123+) were not significantly altered between patients and controls. These data indicated that the proportion and absolute number of circulating NK cells are reduced in SLE patients with active disease, but they are gradually restored to near normal levels in remission.
FIGURE 1.
The frequency of NK cells is reduced in SLE patients with active disease. Freshly isolated PBMCs were stained with anti-CD3, CD11c, CD19, CD56, Vα24, and Vβ11 mAbs and analyzed by flow cytometry. A and D, The relative percentages of NK cells, NKT cells, B cells, CD4+ T cells, CD8+ T, MDCs, and PDCs were analyzed. Representative examples are shown from healthy controls, active SLE, and SLE in remission. C, Numbers of NK cells, NKT cells, and B cells/ml in blood. B and E, Subjects were categorized into groups determined by their SLEDAI. Mean (± SEM) proportion of NK cells, NKT cells, B cells, CD4+ T cells, CD8+ T cells, MDCs, and PDCs are shown for each group. Significance was determined using one-way ANOVA, followed by the Bonferroni post hoc test.
The multiple functions of NK cells, such as natural cytotoxicity and cytokine secretion, can be regulated by interactions between inhibitory and activating families of NK receptors and their respective ligands (16). Because the frequency of NK cells was lower in patients with active SLE disease, we postulated that the expression of receptors on NK cells may be altered in these patients. To test this hypothesis, we compared the expression of a number of representative inhibitory and activating NK cell receptors, such as CD158b, NKG2A, CD94, CD85j, CD69, NKp46, NKG2C, NKG2D, and CD16. With regard to inhibitory receptors, only CD158b showed expression levels significantly lower in active SLE and in remission. With respect to activating receptors, the proportion of NKG2C+ NK cells was lower in remission. Additionally, the proportion of CD16+ NK cells was lower in patients with active disease. Nonetheless, we did not observe any differences between the patient groups and the healthy controls in the expression of NKG2A, CD94, CD85j, CD69, NKp46, and NKG2D by NK cells (data not shown). We next compared CD226 expression on circulating NK cells in SLE patients and healthy controls. Interestingly, the proportion of peripheral CD226+ NK cells was reduced from a peak of 88.36% in healthy controls to 16.19% in active disease (Fig. 2A). Nevertheless, the proportion of CD226+ NK cells returned to near normal levels in remission. Cumulative data for 20 healthy controls, 20 patients with active disease, and 15 remission patients are shown in Fig. 2B; the proportion of CD226+ NK cells was significantly lower in active disease than in healthy controls and patients in remission (p < 0.001 and p < 0.001, respectively). To determine whether a similar phenomenon could be observed in a mouse SLE model, we turned to the MRL/lpr mouse, which has proven valuable in analyses of SLE pathogenesis (38), to assess the proportion of CD226+ NK cells in peripheral circulation. Splenic lymphocytes were isolated from MRL/mp and MRL/lpr mice. As shown in Fig. 2C, the proportion of CD3−DX5+ NK cells was reduced in MRL/lpr mice at 30 wk of age (diseased) compared with MRL/lpr mice at 9 wk of age (predisease) and control MRL/mp mice. Also, the proportion of CD226+ NK cells was lower in diseased MRL/lpr mice compared with predisease MRL/lpr mice and MRL/mp mice (p < 0.001 and p < 0.001, respectively; Fig. 2C, 2D). These data suggested that most of the NK cells lost in active SLE and diseased MRL/lpr mice might be CD226+, as noted in Fig. 2.
FIGURE 2.
Downregulated expression of CD226 on NK cells from SLE patients and MRL/lpr mice with active disease. Freshly isolated PBMCs were stained with anti-CD3, CD56, and CD226 mAbs. A, NK cells were gated (top panel), and expression of CD226 was analyzed (bottom panel) on NK cells from healthy controls, patients with active SLE, and patients in remission. B, Cumulative data are shown for the mean (± SEM) proportion of CD226+ NK cells of healthy controls, patients with active SLE, and patients with SLE in remission. Significance was determined using oneway ANOVA, followed by the Bonferroni post hoc test. C, Freshly isolated mouse splenic lymphocytes were stained with anti-CD3, DX5, and CD226 mAbs. Representative flow cytometric dot plots show the proportion of NK cells (top panel) and the expression of CD226 on NK cells (bottom panel) in an MRL/mp mouse, a diseased MRL/lpr mouse, and a predisease MRL/lpr mouse. D, Cumulative data are shown for the mean (± SEM) proportion of CD226+ NK cells of MRL/mp mice, diseased MRL/lpr mice, and predisease MRL/lpr mice. Significance was determined using one-way ANOVA, followed by the Bonferroni post hoc test.
Dynamic changes in CD226+ NK cell populations in SLE patients are associated with disease activity
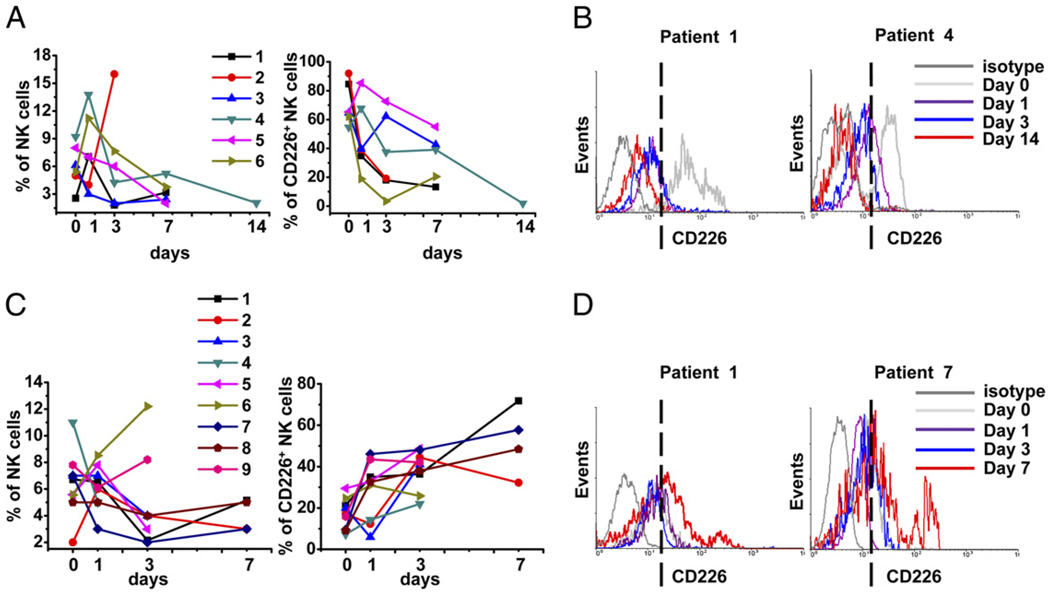
To further assess the status of CD226 expression on NK cells in SLE patients, dynamic observations were made on 15 SLE patients during therapy. These patients were diagnosed with SLE for the first time; they had clinically active disease, as determined by the presence of rash, fever, serositis, or glomerulonephritis. At the first time of blood drawing (day 0), none had received any drugs. During hospitalization, 13 patients were taking prednisolone (1 mg/kg/d) and 2 were taking hydroxychloroquine (200 mg twice daily) in addition to the steroids. None were treated with immunosup-pressive drugs. Blood was drawn for study at the indicated time points during therapy. We were then able to make a dynamic analysis of NK cell proportions and CD226 expression by flow cytometry. As shown in Fig. 3A, the proportion of NK cells was markedly lower in six patients (four received prednisolone, two were treated with prednisolone and hydroxychloroquine) during the exacerbation stage (left panel). Further analysis identified a sustained decrease in CD226+ NK cells in these patients during therapy (right panel). Two representative examples of CD226 expression after gating on CD3−CD56+ NK cells from the six patients are shown in Fig. 3B. By contrast, the conditions of the other nine patients were improved after medication, all of whom received prednisolone as monotherapy, and the NK cell populations were not significantly reduced. Moreover, the proportion of NK cells in patients 6 and 9 increased markedly during therapy (Fig. 3C, left panel). Interestingly, the proportion of CD226+ NK cells was significantly increased in the nine patients during therapy (Fig. 3C, right panel). Two representative examples from the nine patients are shown in Fig. 3D. Taken together, these data suggested that the decreased expression of CD226 on NK cells correlated with disease activity in SLE patients, and therapy might restore the frequency of CD226+ NK cells, at least in some patients.
FIGURE 3.
Dynamic investigation of CD226+ NK cells in SLE patients during therapy. PBMCs were isolated from SLE patients during therapy on the indicated days. A, Proportion of NK cells detected in six patients during the exacerbation stage (left panel). Percentage of CD226+ NK cells of total NK cells in the same six patients (right panel). B, Two representative examples (Patient 1 and Patient 4) are shown from six independent patients; CD3−CD56+ cells were gated, and the percentage of CD226+ NK cells was analyzed. C, Proportion of NK cells in nine patients during remission (left panel). Percentage of CD226+ NK cells of total NK cells in the same nine patients (right panel). D, Two representative examples (Patient 1 and Patient 7) are shown from nine independent patients; CD3−CD56+ cells were gated, and the percentage of CD226+ NK cells was analyzed. The dashed vertical lines indicate the proportion of CD226+ NK cells at the end of treatment.
Expression of the CD226 ligands CD112 and CD155 on DCs in SLE patients
Because CD226 is a surface molecule that transduces activating signals through interactions with its ligands, we next evaluated the expression of two CD226-specific ligands, CD112 and CD155, on circulating PBMCs in SLE patients. As shown in Fig. 4A and 4C, CD112 and CD155 expression on T cells, B cells, and MDCs was detectable (2–5%) in SLE patients and healthy donors. Interestingly, CD226 ligands were mostly expressed on PDCs in SLE patients and healthy controls. CD112 expression levels on PDCs were slightly increased in SLE patients compared with healthy controls, and there were no statistical differences in CD112 and CD155 expression on PDCs between the two groups (Fig. 4B, 4D). Considering that CD226 is highly expressed on NK cells, whereas CD226-specific ligands CD112 and CD155 are substantially expressed on PDCs in SLE patients. It is possible that the interaction between CD226 ligands and CD226 plays a significant role in the pathogenesis of SLE.
FIGURE 4.
Expression of CD112 and CD155 on DCs in SLE patients. Expression of the CD226 ligands CD112 (A) and CD155 (C) was measured by flow cytometry. Expression of the indicated ligands (blue graphs) relative to isotype-matched controls (gray graphs) on T cells, B cells, MDCs, and PDCs was examined. B and D, As in A and C, respectively, cumulative data (mean ± SEM) from CD112+ cells and CD155+ cells in the four cell types are shown.
Activation and apoptosis of CD226+ NK cells are induced by IFN-α from active SLE plasma
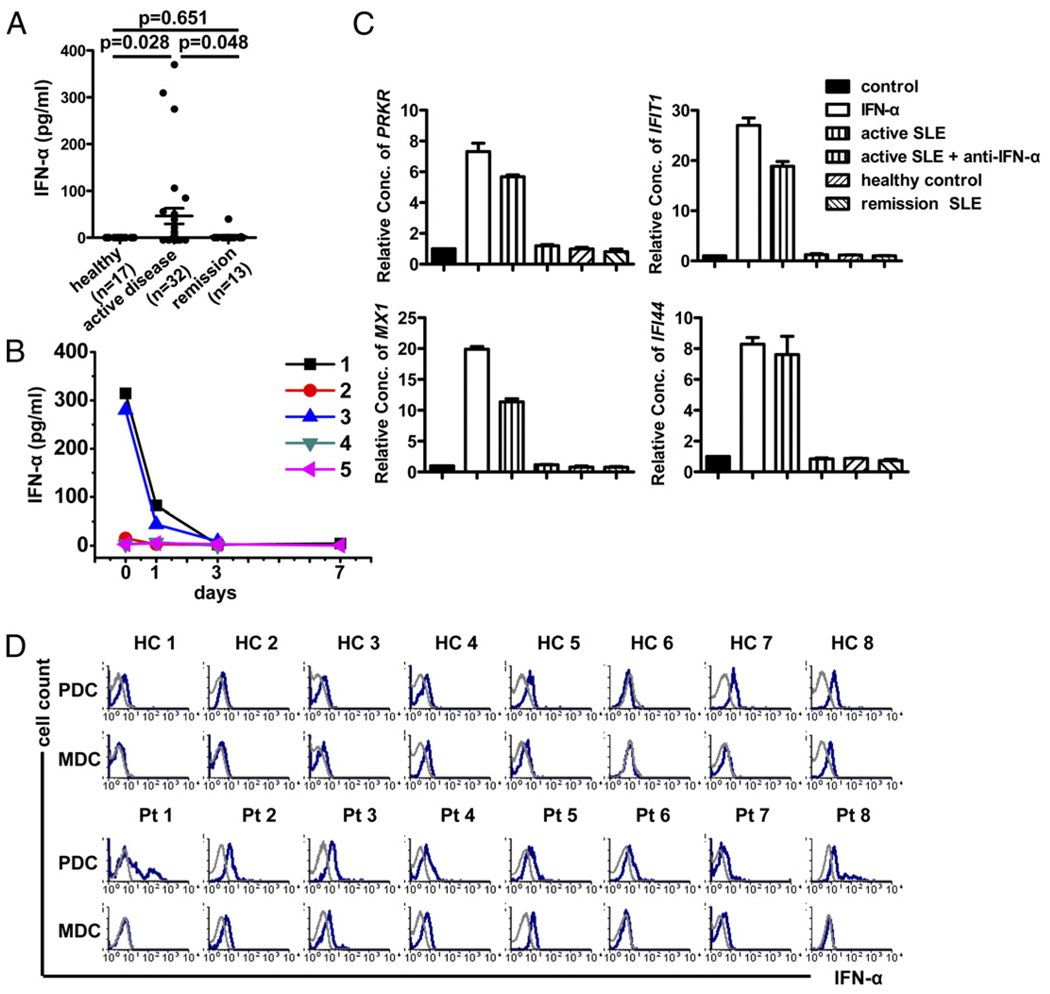
Given that the frequency of NK cells (Fig. 1), especially CD226+ NK cells (Fig. 2), was markedly lower in patients with active SLE, we hypothesized that the status of CD226+ NK cells could be influenced by the cytokine milieu in SLE patients. Therefore, several proinflammatory and immunoregulatory cytokines were measured by ELISA in plasma obtained from SLE patents and healthy controls (data not shown). As shown in Fig. 5A, the levels of IFN-α in plasma samples from patients with active SLE (n = 32) were higher than those in healthy controls (n = 17; p = 0.028) and patients in remission (n = 13; p = 0.048). Moreover, levels of IFN-α were high in three patients with active disease but declined to normal levels after therapy (Fig. 5B).
FIGURE 5.
IFN-α level is elevated in plasma from SLE patients with active disease. A, Plasma level of IFN-α in healthy donors, active SLE, and SLE in remission was quantified by sandwich ELISA. Significance was determined using one-way ANOVA, followed by the Bonferroni post hoc test. B, Plasma was obtained from five SLE patients during therapy, and levels of IFN-α were determined by ELISA. C, To test plasma for functional IFN-α activity, PBMCs from healthy controls were stimulated with IFN-α, plasma from healthy controls or SLE in remission, or plasma from active SLE with or without IFN-α–neutralizing Ab. Relative expression of IFN-α–regulated genes (PRKR, IFIT1, MX1, and IFI44) compared with no stimulation in three independent experiments (mean ± SEM). D, Expression of IFN-α (blue graphs) relative to isotype-matched controls (gray graphs) in MDCs and PDCs obtained from healthy controls and SLE patients was measured by flow cytometry.
To further test the IFN-α activity in plasma from SLE patients, PBMCs from healthy donors were stimulated with different plasma samples, and the relative expression of four IFIGs (PRKR, IFIT1, MX1, and IFI44) in PBMCs was tested after 24 h of incubation. As shown in Fig. 5C, IFIGs were induced by plasma from patients with active disease, but IFIGs were not induced by plasma from healthy controls and patients in remission. Neutralizing Abs to IFN-α inhibited the activity of active SLE plasma. Because IFN-α is produced primarily by PDCs (39–42), we tested IFN-α production by PDCs in SLE patients and healthy controls. Freshly isolated PBMCs were detected without stimuli, and the amount of IFN-α produced by PDCs was analyzed by flow cytometry. As shown in Fig. 5D, IFN-α was detectable from PDCs in SLE patients but not in healthy controls. These data confirmed the elevated levels of IFN-α in SLE observed in earlier studies.
We then postulated that high levels of IFN-α may affect the functions and survival of NK cells in SLE patients. To assess this possibility, we used in vitro experimental models. First, PBMCs isolated from healthy controls were stimulated with IL-2 (100 U/ml), IFN-α (1000 U/ml), or IFN-α plus IL-2. The activation status of NK cells gated from PBMCs was assessed. As seen in Fig. 6A, after 48 h of stimulation with IFN-α, NK cells upregulated the activation marker CD69. Additionally, the CD226+ subset of NK cells was activated. Furthermore, combined treatment with IFN-α and IL-2 induced higher levels of CD69 expression on total NK cells and CD226+ NK cells. By contrast, the expression of CD69 on CD226− NK cells was not upregulated after stimulation. We next investigated whether the plasma factor(s) of SLE patients could induce NK cell activation. PBMCs isolated from healthy controls were stimulated with plasma from healthy controls, SLE patients in remission, or patients with active SLE (plasma levels of IFN-α from active patients were measured by ELISA, concentrations ranged from 13–180 pg/ml). As shown in Fig. 6B, the expression of CD69 on total NK cells and gated CD226+ NK cells was markedly upregulated after 24 h of stimulation with plasma from patients with active SLE. However, the CD226− NK cells were not obviously activated. Meanwhile, to determine whether the activation of NK cells was primarily stimulated by IFN-α from the plasma of patients with active SLE, we added a neutralizing mAb against IFN-α to active SLE plasma. As shown in Fig. 6B, the neutralizing mAb substantially reduced the activation of NK cells and CD226+ NK cells. These data indicated that the high levels of IFN-α in SLE induced an activated phenotype in NK cells, particularly in CD226+ NK cells.
FIGURE 6.
IFN-α or SLE patient plasma activates NK cells from healthy controls in vitro. A, Freshly isolated PBMCs from healthy controls were stimulated with IL-2 (100 U/ml) and then IFN-α (1000 U/ml) or IL-2 (100 U/ml) plus IFN-α (1000 U/ml). Cultured cells were harvested at the indicated time points and analyzed. The ratios of CD69+ NK cells (left panel), CD226+CD69+ NK cells (middle panel), and CD226−CD69+ NK cells (right panel) were analyzed by flow cytometry. One representative example from three independent experiments is shown. B, PBMCs from healthy controls were cultured with medium or four types of plasma, including the plasma from healthy controls, from SLE patients in remission, from active SLE patients, and from active SLE patients with IFN-α–neutralizing Ab. For each type of plasma, three independent samples were adopted repeatedly. Cultured cells were harvested and analyzed as in A. Data are shown as mean ± SEM from three independent experiments. Significance was determined using one-way ANOVA, followed by the Bonferroni post hoc test.
Also, in the two cell-culture experiments, PBMCs isolated from healthy controls were stimulated with IFN-α or active SLE plasma. We found that activation of NK cells (Fig. 6) occurred almost simultaneously with downregulation of CD226 expression on NK cells (Fig. 7A, 7C). We also examined the percentage of apoptotic NK cells in PBMCs during these experiments. After 72 h of stimulation with IFN-α or IFN-α plus IL-2, the proportion of Annexin-V+ NK cells was higher than in controls (Fig. 7B). Furthermore, purified CD226+ and CD226− NK cells were stimulated with SLE plasma for 72 h. As shown in Fig. 7D and 7E, active SLE plasma induced upregulation of Annexin V expression on CD226+ NK cells rather than CD226− NK cells, suggesting that active SLE plasma selectively affects CD226+ NK cells. However, IFN-α blockade was able to reduce the proportion of apoptotic CD226+ NK cells.
FIGURE 7.
Determination of CD226 expression and apoptosis of NK cells after stimulation with IFN-α or SLE patient plasma. A and B, To determine the expression of CD226 on NK cells and detect apoptotic NK cells after stimulation with IFN-α, freshly isolated PBMCs from healthy controls were stimulated with medium or IL-2 and then IFN-α or IL-2 plus IFN-α. Cultured cells were harvested at the indicated time points and analyzed by flow cytometry. One representative example from three independent experiments is shown. PBMCs and purified CD226+ and CD226− NK cells from healthy controls were cultured with medium or four types of plasma, including the plasma from healthy controls, from SLE patients in remission, from active SLE patients, and from active SLE patients with IFN-α–neutralizing Ab. For each type of plasma, three independent samples were adopted repeatedly. The proportion of CD226+ NK cells (C) and apoptosis of CD226+ NK cells (D) and CD226− NK cells (E) were analyzed by flow cytometry. Data are shown as mean ± SEM from three independent experiments. Significance was determined using one-way ANOVA, followed by the Bonferroni post hoc test.
Thus, these experiments confirmed that IFN-α stimulation causes NK cells to undergo activation-induced apoptosis. Interestingly, it is likely that IFN-α predominately downregulates the proportion of CD226+ NK cells. Therefore, the reduced frequency of circulating CD226+ NK cells in patients with active SLE is mirrored in vitro using proper concentrations of IFN-α or active SLE plasma to stimulate the PBMCs.
Activated CD226+ NK cells mediate kidney injury in MRL/lpr mice
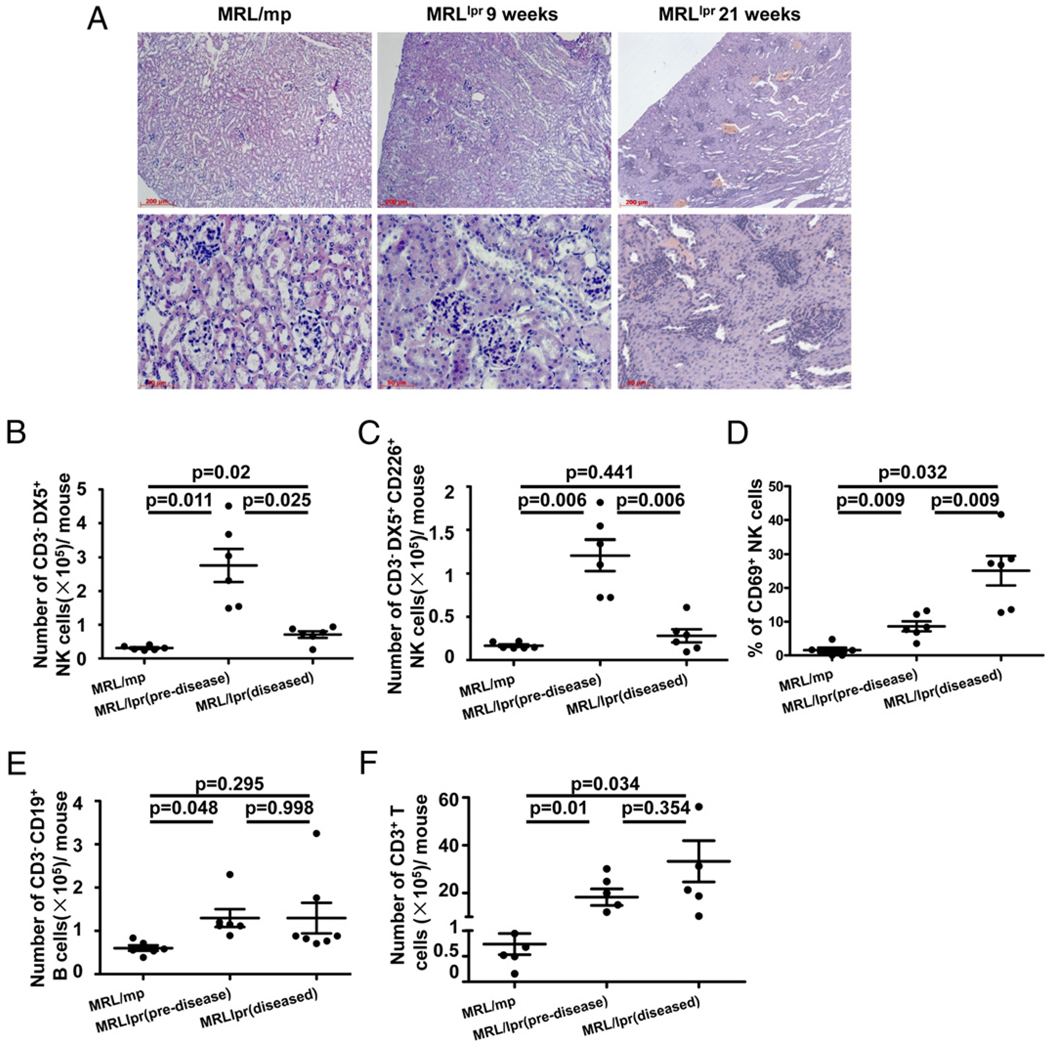
In SLE patients, several organs, including the skin, blood vessels, lungs, and kidneys, are damaged. Therefore, we postulated that CD226+ NK cells, when activated by IFN-α, play a role in tissue damage in SLE. We used the MRL/lpr mouse to test this possibility. Fig. 8A shows representative kidney sections stained with H&E from MRL/mp mice, predisease MRL/lpr mice, and diseased MRL/lpr mice. Cellular infiltration and glomerular lesions were seen in diseased 21-wk-old MRL/lpr mice. By contrast, no sign of inflammation or cellular proliferation was observed in MRL/mp or 9-wk-old MRL/lpr predisease mice. To better characterize the status of infiltrating lymphocytes in the kidneys, absolute numbers of T, B, and NK cells were calculated. The number (Fig. 8F) and proportion (data not shown) of T cells in MRL/lpr mice kidneys (Fig. 8F) and spleens (data not shown) were increased compared with MRL/mp mice. The number of B cells in predisease MRL/lpr mice kidneys was higher than in diseased MRL/lpr and MRL/mp mice (Fig. 8E), but the proportion of B cells was reduced in MRL/lpr mice kidneys compared with MRL/mp mice (data not shown). Although the proportion of NK cells in predisease MRL/lpr mice kidneys was similar to that of MRL/mp mice (data not shown), the absolute number of NK cells was significantly higher in the kidneys of predisease MRL/lpr mice (2.76 ± 0.49 × 105) compared with diseased MRL/lpr mice (0.71 ± 0.09 × 105) and MRL/mp mice (0.31 ± 0.02 × 105) (p = 0.025 and p = 0.011, respectively; Fig. 8B). Furthermore, most of the kidney-infiltrating NK cells in predisease MRL/lpr mice were CD226+ NK cells (Fig. 8C), and CD69 expression on kidney NK cells was expanded from a baseline of ~1.5% in MRL/mp mice to 10–15% in predisease MRL/lpr mice and 20–30% in diseased MRL/lpr mice (p = 0.009 and p = 0.032, respectively; Fig. 8D). Conversely, the number of NK cells was similar in the spleens of MRL/lpr and MRL/mp mice, whereas the proportion of NK cells was lower in the spleens of MRL/lpr mice (data not shown).
FIGURE 8.
Enrichment of NK cell numbers in the kidneys of MRL/lpr mice. A, Representative kidney sections stained with H&E from an MRL/mp mouse, a 9-wk-old MRL/lpr mouse, and a 21-wk-old MRL/lpr mouse. At the time of sacrifice, the kidneys were removed and sectioned before staining with H&E (original magnification ×100 [top row] and ×400 [bottom row]). Kidney MNCs were obtained from MRL/mp and predisease and diseased MRL/lpr mice. Absolute numbers of NK cells (B), CD226+ NK cells (C), B cells (E), and T cells (F) were calculated as detailed in Materials and Methods. Results are expressed as the number of cells per two kidneys. D, NK cells from kidney MNCs in MRL/mp and predisease and diseased MRL/lpr mice were assayed ex vivo for CD69 expression. Results are expressed as the mean ± SEM for each group. Significance was determined using one-way ANOVA, followed by the Bonferroni post hoc test.
We showed that NK cells, especially CD226+ NK cells, infiltrated into predisease MRL/lpr mouse kidneys (Fig. 8B, 8C). In addition to their greater numbers, NK cells in predisease and diseased MRL/lpr kidneys exhibited an enhanced activated phenotype (Fig. 8D). In light of these results, we postulated that these NK cells would be involved in the pathogenesis of renal injury by producing immunoregulatory cytokines and cytotoxic granules. As shown in Fig. 9, NK cells from predisease MRL/lpr kidneys, as well as diseased MRL/lpr kidneys, produced increased amounts of IFN-γ, granzyme B, and perforin compared with NK cells from MRL/mp kidneys. Furthermore, the number of T cells was higher in MRL/lpr kidneys, and activation and production of IFN-γ and granzyme B were enhanced in T cells from MRL/lpr mice compared with MRL/mp mice (data not shown). Taken together, these data suggested that activated CD226+ NK cells are responsible, at least to some extent, for the kidney injury observed in SLE.
FIGURE 9.
Cytotoxic granule and cytokine production by kidney-infiltrating NK cells in MRL/lpr mice. Kidney MNCs isolated from MRL/mp and predisease and diseased MRL/lpr mice were stimulated with PMA + ionomycin for 4 h. A, Representative graphs show IFN-γ, granzyme B, and perforin expressed by kidney NK cells in MRL/mp and predisease and diseased MRL/lpr mice. B, Statistical analysis of the expression of IFN-γ, granzyme B, and perforin in NK cells from MRL/mp and predisease and diseased MRL/lpr mice; means ± SEM are displayed. Significance was determined using one-way ANOVA, followed by the Bonferroni post hoc test.
Discussion
It has long been known that the proportion and absolute number of NK cells are significantly reduced in SLE patients. However, the mechanism underlying this reduction remains unclear. Our data showed, for what we believe is the first time, that the reduced proportion and absolute number of circulating NK cells in SLE patients are due to AICD mainly mediated by IFN-α. Our results indicated that IFN-α levels were high in patients with active disease but were reduced to normal levels in remission. Other cytokines, including IFN-γ, IL-12, and IL-2, did not increase in SLE patients compared with healthy controls (data not shown). It was reported that high levels of IFN-α contribute to the pathogenesis of SLE (26). However, to our knowledge, the role of IFN-α in regulating the frequency of NK cells in SLE has not been studied. In our study, NK cells isolated from healthy controls showed an activated phenotype and high rate of apoptosis after being stimulated by plasma from patients with active SLE. Additionally, this induction could be blocked by a neutralizing IFN-α mAb. Along with the observed reduction in NK cell numbers, the cytotoxicity of NK cells was impaired in SLE patients. Previously published data demonstrated that IFN from SLE patients cannot boost NK cell cytotoxicity ex vivo. Our results extend this finding, showing that although the cytotoxicity of peripheral NK cells was impaired in human SLE patients and mouse SLE models (data not shown), increased expression of CD69 on NK cells could be induced by patient plasma through an IFN-α–dependent mechanism. Furthermore, the kidney-infiltrating NK cells of MRL/lpr mice exhibited an enhanced ability to produce cytotoxic granules and IFN-γ. In addition, abnormal expression of DAP12 molecules may account for the impaired function of NK cells in patients with SLE (43). Interestingly, increased numbers of CD226+ NK cells are present in the kidney of predisease MRL/lpr mice; it is possible that the reduction in the circulating CD226+ NK cells results from these cells becoming activated, leaving the periphery, and infiltrating the kidney to play a role in the disease process. Indeed, we further found a sharp decline in CD226+ NK cells in diseased MRL/lpr mice, which may also be caused by activation-induced apoptosis. Taken together, these results suggested that activated CD226+ NK cells may undergo AICD after infiltrating the kidney and exerting their influence on disease development, which would eventually lead to the decrease in the number of NK cells.
Another interesting finding in this study was the observation that most of the NK cells lost in SLE are CD226+. CD226, an activating receptor and adhesion molecule, is involved in NK cell-mediated lysis of tumor cells from different origins. The expression of CD226 on NK cells is considered to be a key index in several human diseases, including HIV (21) and multiple myeloma (44), but it has not been evaluated in SLE. Our results indicated that the altered proportions of CD226+ NK cells are relevant to disease progression. In general, CD226+ NK cells decline in patients during the exacerbation stage. By contrast, they return to normal levels during remission. Interestingly, a similar phenomenon was observed in mouse SLE models. Moreover, our data suggested that the expression of other receptors, such as the inhibitory receptors NKG2A, CD94, and CD85j and the activating receptors CD69, NKp46, NKG2C, NKG2D, and CD16, on NK cells were not altered in SLE patients. Evidence from recent studies indicated that reduced expression of CD226 increased sensitivity to apoptosis in NKT cells from SLE patients (45). In our cell-culture experiments, plasma from patients with active disease induced enhanced CD226+ NK cell apoptosis via an IFN-α–dependent mechanism. It is likely that the markedly reduced proportion of CD226+ NK cells is related to this process. Thus, similar mechanisms may be involved in the apoptosis of NK cells in SLE patients. Given that dynamic alterations in CD226+ NK cells are relevant to disease activity, we suggest that the frequency of CD226+ NK cells may be used as a clinical index for SLE.
Type I IFNs (IFN-I) can be produced by many cell types in vitro when cells are exposed to different microorganisms. For example, monocyte/macrophages could produce IFN-I in response to influenza and Sendai viruses (46). Also, resident renal cells produced IFN-I in an experimental model of autoantibody-mediated glomerulonephritis (47). However, PDCs, the major source of IFN-α induced by nucleic acid-containing immune complexes, were shown to account for the etiopathogenesis of SLE. Once stimulated by self apoptosis-derived DNA- and/or RNA- or small nuclear ribonucleoprotein particle-containing immune complexes, PDCs migrate from the blood into inflamed sites, including the skin and kidney (39–42). PDCs do not usually produce measurable amounts of IFN-α unless stimulated by microorganisms or their constituents (48). However, our data demonstrated that, without any stimulation, production of IFN-α was detectable in PDCs from patients with active SLE but not from healthy controls. Furthermore, expression of the CD226 ligands CD112 and CD155 on DCs (MDCs and PDCs) was observed in SLE patients. Evidence from previous studies suggested that NK cells are able to modulate the function of DCs, either by promoting their maturation or by killing them (49, 50). For example, engagement of NKp30, an activating receptor on NK cells, results in the activation of cytotoxicity and the production of cytokines, such as IFN-γ and TNF-α (49), which promote DC maturation (50). In contrast, MDCs can activate NK cells via cell–cell contact (51). Furthermore CD226–ligand interactions might be primarily involved in the recognition and killing of DCs (52). We postulate that the CD226–ligand interaction induces the apoptosis of CD226+ NK cells and subsequently downregulates the ability of NK cells to kill PDCs in SLE patients. Thus, PDCs persist in SLE patients, yielding the characteristically sustained IFN-α production observed in SLE, and IFN-α further promotes the activation-induced apoptosis of NK cells. Therefore, we suggest that further investigations aimed at modulating CD226 expression on NK cells in SLE patients may be an attractive avenue of experimental therapeutics.
Once stimulated by IFN-α, NK cells maintain an activated phenotype until apoptosis. Additionally, the cytotoxicity of peripheral NK cells was proved to be impaired in SLE patients. So how do these activated NK cells exert their functions before apoptosis in SLE patients? It was reported in the murine system that peripheral NK cells can migrate into the target organs of autoimmunity (53). In this study, we chose a mouse model that mimics human SLE. Our results indicated that peripheral NK cells in MRL/lpr mice did not produce higher levels of IFN-γ and perforin compared with normal controls, similar to SLE patients. Nevertheless, NK cells were recruited from the periphery into kidneys in predisease MRL/lpr mice, and most of the infiltrating NK cells belonged to the CD226+ subset. Furthermore, kidney-infiltrating NK cells in MRL/lpr mice displayed an activated phenotype, including upregulated expression of CD69 and high production of cytotoxic granules and IFN-γ, which may contribute to inflammation and eventual kidney injury. Therefore, these activated NK cells may infiltrate and damage tissues during the disease process.
Given that IFN-I plays a pivotal role in the etiopathogenesis of SLE, IFN-α might be considered a valuable therapeutic target in SLE. A published phase I trial revealed that the overexpressed IFN-α/β–inducible genes in whole blood and skin lesions were inhibited in the subjects treated with anti–IFN-α mAb. According to our results, the activation of NK cells, as well as tissue injury induced by infiltrating NK cells, might be inhibited by using an IFN-α antagonist in SLE patients. This may play an important role in relieving disease activity and tissue inflammation.
In summary, our results showed that NK cell populations were significantly reduced in patients with active SLE but not in patients in remission or healthy controls. The major NK cell population affected consists of CD226+ NK cells in patients with active disease. It seems that dynamic alterations in CD226+ NK cell populations in patients are relevant to disease activity. Furthermore, we found that, in SLE patients, PDCs sustain the production of IFN-α, and the high levels of IFN-α in active SLE can mediate AICD of NK cells. Meanwhile, the reduction in CD226+ NK cell populations is mediated by increased apoptosis. Additionally, in a mouse model of SLE, CD226+ NK cells infiltrate the kidneys during disease progression and display an activated phenotype. Finally, activated NK cells may damage kidneys to some extent by producing cytotoxic granules and immunoregulatory cytokines.
Supplementary Material
Acknowledgments
This work was supported by the Natural Science Foundation of China (Grants 30730084 and 30721002) and the Ministry of Science and Technology of China (973 Basic Science Project 2007CB512405 and 2009CB522403).
Abbreviations used in this article
- AICD
activation-induced cell death
- DC
dendritic cell
- IFIG
gene induced by type I IFN
- IFN-I
type I IFN
- MDC
myeloid dendritic cell
- MNC
mononuclear cell
- PDC
plasmacytoid dendritic cell
- SLE
systemic lupus erythematosus
- SLEDAI
SLE disease activity index
Footnotes
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Mills JA. Systemic lupus erythematosus. N. Engl. J. Med. 1994;330:1871–1879. doi: 10.1056/NEJM199406303302608. [DOI] [PubMed] [Google Scholar]
- 2.Pisetsky DS, Rönnblom L. Systemic lupus erythematosus: a matter of life and death. Arthritis Rheum. 2009;60:1567–1570. doi: 10.1002/art.24531. [DOI] [PubMed] [Google Scholar]
- 3.Wakeland EK, Liu K, Graham RR, Behrens TW. Delineating the genetic basis of systemic lupus erythematosus. Immunity. 2001;15:397–408. doi: 10.1016/s1074-7613(01)00201-1. [DOI] [PubMed] [Google Scholar]
- 4.Hess EV. Environmental lupus syndromes. Br. J. Rheumatol. 1995;34:597–599. doi: 10.1093/rheumatology/34.7.597. [DOI] [PubMed] [Google Scholar]
- 5.Chen C, Prak EL, Weigert M. Editing disease-associated auto-antibodies. Immunity. 1997;6:97–105. doi: 10.1016/s1074-7613(00)80673-1. [DOI] [PubMed] [Google Scholar]
- 6.Bootsma H, Spronk PE, Hummel EJ, de Boer G, ter Borg EJ, Limburg PC, Kallenberg CG. Anti-double stranded DNA antibodies in systemic lupus erythematosus: detection and clinical relevance of IgM-class antibodies. Scand. J. Rheumatol. 1996;25:352–359. doi: 10.3109/03009749609065646. [DOI] [PubMed] [Google Scholar]
- 7.ter Borg EJ, Horst G, Hummel EJ, Limburg PC, Kallenberg CG. Measurement of increases in anti-double-stranded DNA antibody levels as a predictor of disease exacerbation in systemic lupus erythematosus. A long-term, prospective study. Arthritis Rheum. 1990;33:634–643. doi: 10.1002/art.1780330505. [DOI] [PubMed] [Google Scholar]
- 8.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, Harley JB. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N. Engl. J. Med. 2003;349:1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 9.Shlomchik MJ, Craft JE, Mamula MJ. From T to B and back again: positive feedback in systemic autoimmune disease. Nat. Rev. Immunol. 2001;1:147–153. doi: 10.1038/35100573. [DOI] [PubMed] [Google Scholar]
- 10.Santulli-Marotto S, Retter MW, Gee R, Mamula MJ, Clarke SH. Autoreactive B cell regulation: peripheral induction of developmental arrest by lupus-associated autoantigens. Immunity. 1998;8:209–219. doi: 10.1016/s1074-7613(00)80473-2. [DOI] [PubMed] [Google Scholar]
- 11.Liu X, Wysocki LJ, Manser T. Autoantigen-B cell antigen receptor interactions that regulate expression of B cell antigen receptor Loci. J. Immunol. 2007;178:5035–5047. doi: 10.4049/jimmunol.178.8.5035. [DOI] [PubMed] [Google Scholar]
- 12.Kalled SL, Cutler AH, Datta SK, Thomas DW. Anti-CD40 ligand antibody treatment of SNF1 mice with established nephritis: preservation of kidney function. J. Immunol. 1998;160:2158–2165. [PubMed] [Google Scholar]
- 13.Yan J, Harvey BP, Gee RJ, Shlomchik MJ, Mamula MJ. B cells drive early T cell autoimmunity in vivo prior to dendritic cell-mediated autoantigen presentation. J. Immunol. 2006;177:4481–4487. doi: 10.4049/jimmunol.177.7.4481. [DOI] [PubMed] [Google Scholar]
- 14.Carroll MC. A protective role for innate immunity in systemic lupus erythematosus. Nat. Rev. Immunol. 2004;4:825–831. doi: 10.1038/nri1456. [DOI] [PubMed] [Google Scholar]
- 15.Rönnefarth VM, Erbacher AI, Lamkemeyer T, Madlung J, Nordheim A, Rammensee HG, Decker P. TLR2/TLR4-independent neutrophil activation and recruitment upon endocytosis of nucleosomes reveals a new pathway of innate immunity in systemic lupus erythematosus. J. Immunol. 2006;177:7740–7749. doi: 10.4049/jimmunol.177.11.7740. [DOI] [PubMed] [Google Scholar]
- 16.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat. Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 17.Gilmour KC, Fujii H, Cranston T, Davies EG, Kinnon C, Gaspar HB. Defective expression of the interleukin-2/interleukin-15 receptor beta subunit leads to a natural killer cell-deficient form of severe combined immunodeficiency. Blood. 2001;98:877–879. doi: 10.1182/blood.v98.3.877. [DOI] [PubMed] [Google Scholar]
- 18.Erkeller-Yuksel FM, Lydyard PM, Isenberg DA. Lack of NK cells in lupus patients with renal involvement. Lupus. 1997;6:708–712. doi: 10.1177/096120339700600905. [DOI] [PubMed] [Google Scholar]
- 19.Park YW, Kee SJ, Cho YN, Lee EH, Lee HY, Kim EM, Shin MH, Park JJ, Kim TJ, Lee SS, et al. Impaired differentiation and cytotoxicity of natural killer cells in systemic lupus erythematosus. Arthritis Rheum. 2009;60:1753–1763. doi: 10.1002/art.24556. [DOI] [PubMed] [Google Scholar]
- 20.Shibuya A, Campbell D, Hannum C, Yssel H, Franz-Bacon K, McClanahan T, Kitamura T, Nicholl J, Sutherland GR, Lanier LL, Phillips JH. DNAM-1, a novel adhesion molecule involved in the cytolytic function of T lymphocytes. Immunity. 1996;4:573–581. doi: 10.1016/s1074-7613(00)70060-4. [DOI] [PubMed] [Google Scholar]
- 21.Mavilio D, Lombardo G, Kinter A, Fogli M, La Sala A, Ortolano S, Farschi A, Follmann D, Gregg R, Kovacs C, et al. Characterization of the defective interaction between a subset of natural killer cells and dendritic cells in HIV-1 infection. J. Exp. Med. 2006;203:2339–2350. doi: 10.1084/jem.20060894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fasth AE, Bjo¨rkstro¨m NK, Anthoni M, Malmberg KJ, Malmström V. Activating NK-cell receptors co-stimulate CD4(+)CD28(−) T cells in patients with rheumatoid arthritis. Eur. J. Immunol. 2010;40:378–387. doi: 10.1002/eji.200939399. [DOI] [PubMed] [Google Scholar]
- 23.Ytterberg SR, Schnitzer TJ. Serum interferon levels in patients with systemic lupus erythematosus. Arthritis Rheum. 1982;25:401–406. doi: 10.1002/art.1780250407. [DOI] [PubMed] [Google Scholar]
- 24.Pascual V, Farkas L, Banchereau J. Systemic lupus erythematosus: all roads lead to type I interferons. Curr. Opin. Immunol. 2006;18:676–682. doi: 10.1016/j.coi.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 25.Rönnblom L, Eloranta ML, Alm GV. The type I interferon system in systemic lupus erythematosus. Arthritis Rheum. 2006;54:408–420. doi: 10.1002/art.21571. [DOI] [PubMed] [Google Scholar]
- 26.Rönnblom L, Alm GV. An etiopathogenic role for the type I IFN system in SLE. Trends Immunol. 2001;22:427–431. doi: 10.1016/s1471-4906(01)01955-x. [DOI] [PubMed] [Google Scholar]
- 27.Bengtsson AA, Sturfelt G, Truedsson L, Blomberg J, Alm G, Vallin H, Rönnblom L. Activation of type I interferon system in systemic lupus erythematosus correlates with disease activity but not with antiretroviral antibodies. Lupus. 2000;9:664–671. doi: 10.1191/096120300674499064. [DOI] [PubMed] [Google Scholar]
- 28.Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25:383–392. doi: 10.1016/j.immuni.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 30.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294:1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 31.Banchereau J, Pascual V, Palucka AK. Autoimmunity through cytokine-induced dendritic cell activation. Immunity. 2004;20:539–550. doi: 10.1016/s1074-7613(04)00108-6. [DOI] [PubMed] [Google Scholar]
- 32.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 33.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 34.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH The Committee on Prognosis Studies in SLE. Derivation of the SLEDAI. A disease activity index for lupus patients. Arthritis Rheum. 1992;35:630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 35.Kirou KA, Lee C, George S, Louca K, Papagiannis IG, Peterson MG, Ly N, Woodward RN, Fry KE, Lau AY, et al. Coordinate over-expression of interferon-alpha-induced genes in systemic lupus erythematosus. Arthritis Rheum. 2004;50:3958–3967. doi: 10.1002/art.20798. [DOI] [PubMed] [Google Scholar]
- 36.Odendahl M, Jacobi A, Hansen A, Feist E, Hiepe F, Burmester GR, Lipsky PE, Radbruch A, Dörner T. Disturbed peripheral B lymphocyte homeostasis in systemic lupus erythematosus. J. Immunol. 2000;165:5970–5979. doi: 10.4049/jimmunol.165.10.5970. [DOI] [PubMed] [Google Scholar]
- 37.Arce E, Jackson DG, Gill MA, Bennett LB, Banchereau J, Pascual V. Increased frequency of pre-germinal center B cells and plasma cell precursors in the blood of children with systemic lupus erythematosus. J. Immunol. 2001;167:2361–2369. doi: 10.4049/jimmunol.167.4.2361. [DOI] [PubMed] [Google Scholar]
- 38.Theofilopoulos AN, Dixon FJ. Murine models of systemic lupus erythematosus. Adv. Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- 39.Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, Liu YJ. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 40.Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, Colonna M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 41.Rönnblom L, Alm GV. A pivotal role for the natural interferon alpha-producing cells (plasmacytoid dendritic cells) in the pathogenesis of lupus. J. Exp. Med. 2001;194:F59–F63. doi: 10.1084/jem.194.12.f59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guiducci C, Gong M, Xu Z, Gill M, Chaussabel D, Meeker T, Chan JH, Wright T, Punaro M, Bolland S, et al. TLR recognition of self nucleic acids hampers glucocorticoid activity in lupus. Nature. 2010;465:937–941. doi: 10.1038/nature09102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toyabe S, Kaneko U, Uchiyama M. Decreased DAP12 expression in natural killer lymphocytes from patients with systemic lupus erythematosus is associated with increased transcript mutations. J. Autoimmun. 2004;23:371–378. doi: 10.1016/j.jaut.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 44.El-Sherbiny YM, Meade JL, Holmes TD, McGonagle D, Mackie SL, Morgan AW, Cook G, Feyler S, Richards SJ, Davies FE, et al. The requirement for DNAM-1, NKG2D, and NKp46 in the natural killer cell-mediated killing of myeloma cells. Cancer Res. 2007;67:8444–8449. doi: 10.1158/0008-5472.CAN-06-4230. [DOI] [PubMed] [Google Scholar]
- 45.Tao D, Shangwu L, Qun W, Yan L, Wei J, Junyan L, Feili G, Boquan J, Jinquan T. CD226 expression deficiency causes high sensitivity to apoptosis in NK T cells from patients with systemic lupus erythematosus. J. Immunol. 2005;174:1281–1290. doi: 10.4049/jimmunol.174.3.1281. [DOI] [PubMed] [Google Scholar]
- 46.Sirén J, Pirhonen J, Julkunen I, Matikainen S. IFN-alpha regulates TLR-dependent gene expression of IFN-alpha, IFN-beta, IL-28, and IL-29. J. Immunol. 2005;174:1932–1937. doi: 10.4049/jimmunol.174.4.1932. [DOI] [PubMed] [Google Scholar]
- 47.Fairhurst AM, Xie C, Fu Y, Wang A, Boudreaux C, Zhou XJ, Cibotti R, Coyle A, Connolly JE, Wakeland EK, Mohan C. Type I interferons produced by resident renal cells may promote end-organ disease in autoantibody-mediated glomerulonephritis. J. Immunol. 2009;183:6831–6838. doi: 10.4049/jimmunol.0900742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fitzgerald-Bocarsly P. Human natural interferon-alpha producing cells. Pharmacol. Ther. 1993;60:39–62. doi: 10.1016/0163-7258(93)90021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, Münz C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J. Exp. Med. 2002;195:343–351. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vitale M, Della Chiesa M, Carlomagno S, Pende D, Aricò M, Moretta L, Moretta A. NK-dependent DC maturation is mediated by TNFalpha and IFNgamma released upon engagement of the NKp30 triggering receptor. Blood. 2005;106:566–571. doi: 10.1182/blood-2004-10-4035. [DOI] [PubMed] [Google Scholar]
- 51.Ebihara T, Azuma M, Oshiumi H, Kasamatsu J, Iwabuchi K, Matsumoto K, Saito H, Taniguchi T, Matsumoto M, Seya T. Identification of a polyI:C-inducible membrane protein that participates in dendritic cell-mediated natural killer cell activation. J. Exp. Med. 2010;207:2675–2687. doi: 10.1084/jem.20091573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pende D, Castriconi R, Romagnani P, Spaggiari GM, Marcenaro S, Dondero A, Lazzeri E, Lasagni L, Martini S, Rivera P, et al. Expression of the DNAM-1 ligands, Nectin-2 (CD112) and poliovirus receptor (CD155), on dendritic cells: relevance for natural killer-dendritic cell interaction. Blood. 2006;107:2030–2036. doi: 10.1182/blood-2005-07-2696. [DOI] [PubMed] [Google Scholar]
- 53.Johansson S, Berg L, Hall H, Höglund P. NK cells: elusive players in autoimmunity. Trends Immunol. 2005;26:613–618. doi: 10.1016/j.it.2005.08.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.