Abstract
Background
Preterm delivery has a variety of causes, with each of these presumably carrying its own mortality risk. To the extent that they add to the risk of mortality, the various pathologic factors triggering preterm delivery will confound the causal contribution of gestational age to mortality, inflating the observed rates of gestational-age-specific mortality. We have previously estimated that about half of the mortality of US preterm singletons may be due to unmeasured pathologies that increase mortality risk and also cause preterm birth. In this paper, we examine the impact that rare factors may have, at least in theory, on preterm mortality.
Methods
We constructed a simple model of gestational-age specific mortality, in which we arbitrarily selected a function to represent the mortality due to immaturity alone (“baseline” risk). We then added “unmeasured” confounding factors that cause mortality and also cause preterm birth. This construct allowed us to calculate, in simple scenarios, the proportion of preterm mortality that could be caused by unmeasured confounding.
Results
We found that rare pathologies with moderate-to-strong effects can substantially contribute to preterm mortality. The presence of such rare factors can also produce an intersection of gestational-age-specific mortality curves when stratifying by known risk factors.
Conclusions
It is possible that a few relatively rare factors may account for a large fraction of preterm mortality. The search for such factors should be a primary focus of future research on preterm delivery.
Preterm birth has been described as a “syndrome”,1 given that several paths, mostly pathological and often unmeasured, can lead to early birth.1–9 This heterogeneity of causation implies that the mortality risk faced by preterm babies will also be heterogeneous: mortality depends on both the processes leading to preterm birth and the level of fetal maturity achieved at the time of birth (and on the interaction of these). Despite the clinical evidence for such heterogeneity, epidemiologists generally lack the information necessary to address this biologic complexity. Preterm birth is thus often examined either as a single dichotomous outcome or as clinical categories that are unlikely to reflect homogeneous etiologic entities.6 A full understanding of preterm birth has been hampered by the difficulties inherent in addressing this complexity. The heterogeneity of preterm births also results in confounding of the causal contribution of gestational age (as a proxy of immaturity) to mortality. This leads to gestationalage-specific mortality rates that overestimate the “true” effect of gestational age (and that exaggerate the projected benefits of preventing early birth).
The above argument is analogous to that made for the relation between low birth weight and mortality. It has been shown that, at least in principle, rare factors with large effects could produce a substantial gradient of mortality with lower birth weights even if birth weight itself had no causal effect.10–11 It is almost certain, however, that preterm delivery (which results in the birth of a physiologically immature baby) has a direct causal effect on mortality. We have previously estimated that immaturity alone may contribute only about half the neonatal mortality of singleton babies born preterm, leaving the rest to the pathologies that cause both preterm delivery and elevated mortality.12
Here we present a simple model to explore the extent to which rare factors that increase mortality and cause early delivery might contribute to preterm mortality. Given that the causal effect of gestational age on mortality is unknown (and unknowable, for all practical purposes), we do not attempt to reproduce empirical gestational-age-specific mortality curves. Instead, we arbitrarily choose a function to represent the set of “baseline” week-specific mortality rates (the mortality rates that would be observed if healthy fetuses were randomly delivered). Starting with this function and a few simple assumptions, we add hypothetical “unmeasured” confounding factors (pathologies) and calculate the proportion contributed by these factors to preterm mortality. We further show how the hypothesized conditions can produce an intersection of gestational-age-specific mortality curves, as seen, for example, when comparing the mortality of singletons and twins, or of babies with and without preeclampsia. 9,13–15
How uncontrolled confounding can distort the causal effect of gestational age on neonatal mortality
We expand the model proposed by Basso et al for birth weight10–11 to allow for an underlying causal effect of gestational age on neonatal mortality. Conditions of the model follow.
All babies are assigned a “target” gestation (i.e. a natural duration of pregnancy in the absence of any perturbing factor). The distribution of target gestation is assumed to be normal, with μ = 280 days and σ = 10 days.
- We define a baseline mortality function that represents the risk of neonatal death faced by a healthy fetus if it were randomly delivered at a given gestational week from 24 to 45 weeks. The risk is expressed by the following quadratic function (Figure 1):
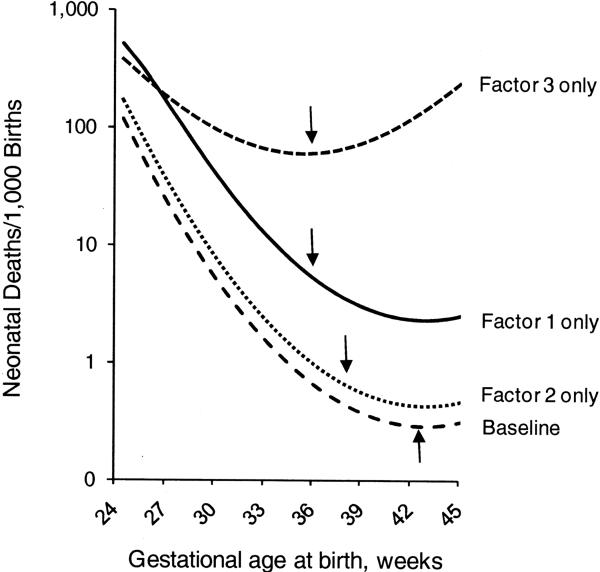
where p(M) represents the probability of death z, and the week of gestation at birth in standardized units, with 0 = 40 weeks (shown in weeks in figures and tables). The coefficients a, b, and c are, respectively, −8, −0.15, and 0.036. (Mortality rates are calculated at mid-week, from 24.5 to 45.5 weeks).(1) We introduce three “pathologies” as confounding factors. Factor 1 affects only 0.6% of births but has strong effects, shortening gestation by 50 days (thus increasing mortality due to immaturity), and also directly increasing mortality with an odds ratio (OR) of 8.0. Factor 2 is more common but weaker: it affects 4% of births, shortens gestation by 35 days, and increases mortality with an OR of 1.5. Factor 3 is similar to Factor 1, but with a direct effect on mortality that varies with gestational age (starting with an OR of 4.0 at 24 weeks, and increasing by 30% for each additional week that the fetus remains in utero). Figure 1 shows the week-specific mortality risk of babies with no pathology (baseline), babies with only Factor 1, babies with only Factor 2, and babies with only Factor 3. (The arrows indicate the gestational age by which approximately 99% of the babies in each group are born).
We consider the effects of these unmeasured pathologies two at the time (the weak factor and either of the strong factors; Figure 2). These pathologies are modeled to occur and act independently of each other, with no interaction between immaturity and pathology. At each gestational week, the observed mortality rates reflect a mixture of healthy babies (whose mortality risk is represented by the baseline) plus some proportion of babies with the unmeasured pathologies. Because these groups differ in their distribution of gestational ages at birth, the specific mix of healthy and pathological births varies across gestation. This varying mix strongly affects the observed mortality at a given week.
Figure 1.
The relation between gestational age at birth, mortality, and Factors 1 (or 3) and Factor 2. Arrows represent the gestational age by which approximately 99% of babies affected by that factor (or with no factor) have been born.
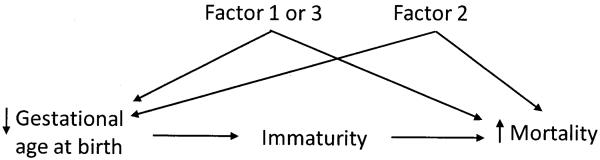
Figure 2.
Gestational-age-specific mortality among babies with no pathological factor (baseline), with only Factor 1, only Factor 2, and only Factor 3. Arrows indicate the (approximate) gestational age by which 99% of babies are born. (Babies with more than one factor are not represented).
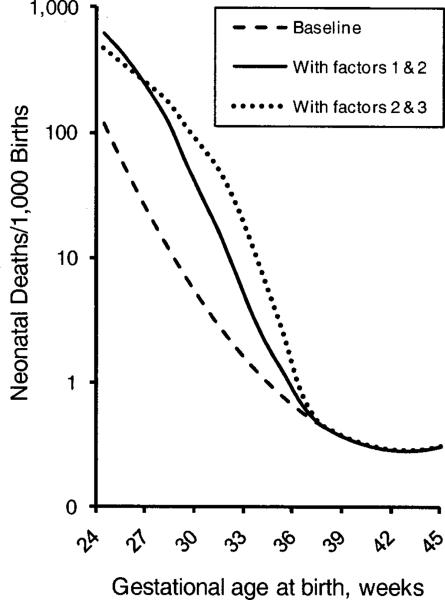
Figure 3 shows the gestational-age-specific mortality in three scenarios: no unmeasured pathologies (baseline only); baseline population mixed with babies with Factor 1 and Factor 2; and baseline population mixed with babies with Factor 2 and Factor 3. (It is possible for a given baby to be independently affected by both factors.) With the addition of unmeasured confounders, the preterm baseline rate becomes unobservable. The unmeasured confounders substantially increase the mortality risk at any given preterm week (e.g., at week 24.5, the mortality risk goes from 12% without Factors 1 and 2 to 62% with them). These confounders also raise the rate of both preterm birth (from 1.8% to 6.0%) and total mortality (from 0.35 to 0.54 per 1000 births). The introduction of Factor 3 instead of Factor 1 yields an overall mortality of 0.85 per 1000 and also results in a substantial change of shape of the mortality curve.
Figure 3.
Baseline mortality and mortality in the presence of Factor 1 and Factor 2 (solid line) and in the presence of Factor 2 and Factor 3 (dotted line). In this representation, the pathological factors are unmeasured and babies with and without factors are mixed in the same curve.
The contribution of pathology to total and preterm mortality
The introduction of confounding factors increases mortality in two ways. One is via a direct effect (determined by the odds ratio). The other is indirect, through earlier delivery. To determine the proportion of mortality due to the direct effect of pathology, we first calculated total mortality in populations of babies exposed to the effect of one or more of the unmeasured factors. Next, we removed the effect on direct mortality of the pathologic factor (setting its OR to 1.0) and calculated its indirect contribution to mortality through earlier birth only. One minus the ratio of indirect mortality to total mortality yields the proportion of mortality due to the direct effect alone. We performed this calculation for each of the three factors individually and in all combinations (Table 1). We repeated the calculations for mortality among preterm births only.
Table 1.
Proportion of direct mortality (due to pathology) in selected scenarios (non-T babies).a
| % Frequency of factor | Preterm birth | Total mortality (per 1000) | Indirect mortality (per 1000) | % direct mortality (due to pathology) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Factor 1 Shift:b −50 OR = 8.0 | Factor 2 Shift:b −35 OR = 1.5 | Factor 3 Shift:b −50 OR = 4.0 (1.3)c | % | All infants | Preterm infants | All infants | Preterm infants | All infants | Preterm infants |
| 0.0 | 0.0 | 0.0 | 1.8 | 0.35 | 0.63 | ||||
| 0.6 | 0.0 | 0.0 | 2.4 | 0.45 | 4.93 | 0.36 | 1.03 | 19.1 | 79.2 |
| 0.0 | 4.0 | 0.0 | 5.4 | 0.40 | 1.29 | 0.38 | 0.92 | 5.0 | 28.5 |
| 0.0 | 0.0 | 0.6 | 2.4 | 0.72 | 17.30 | 0.36 | 1.03 | 49.6 | 94.1 |
| 0.6 | 4.0 | 0.0 | 6.0 | 0.54 | 3.58 | 0.39 | 1.12 | 26.7 | 68.6 |
| 0.0 | 4.0 | 0.6 | 6.0 | 0.80 | 8.11 | 0.39 | 1.12 | 50.8 | 86.1 |
| 0.6 | 4.0 | 0.6 | 6.5 | 0.96 | 9.89 | 0.41 | 1.32 | 57.1 | 86.7 |
| 0.3 | 0.0 | 0.0 | 2.1 | 0.40 | 3.11 | 0.36 | 0.86 | 10.7 | 72.5 |
| 0.0 | 0.0 | 0.3 | 2.1 | 0.56 | 11.53 | 0.36 | 0.86 | 36.2 | 92.6 |
| 0.3 | 4.0 | 0.0 | 5.7 | 0.47 | 2.49 | 0.39 | 1.03 | 17.5 | 58.8 |
| 0.0 | 4.0 | 0.3 | 5.7 | 0.60 | 4.88 | 0.39 | 1.03 | 35.6 | 78.9 |
| 0.3 | 4.0 | 0.3 | 6.0 | 0.67 | 5.93 | 0.39 | 1.13 | 41.5 | 80.9 |
| 0.0 | 8.0 | 0.0 | 9.0 | 0.44 | 1.41 | 0.40 | 0.97 | 9.0 | 30.8 |
| 0.6 | 8.0 | 0.0 | 9.5 | 0.62 | 3.27 | 0.42 | 1.15 | 32.1 | 64.9 |
| 0.0 | 8.0 | 0.6 | 9.5 | 0.88 | 5.97 | 0.42 | 1.15 | 51.8 | 80.8 |
| 0.6 | 8.0 | 0.6 | 10.1 | 1.08 | 7.65 | 0.45 | 1.32 | 58.7 | 82.8 |
The proportion of mortality due to pathology is calculated by subtracting the mortality when all ORs are 1.0 from the observed mortality calculated with the original OR, divided by the observed mortality (and multiplied by 100). The estimates in the table are based on mortality rates calculated at the mid-point of each gestational week in the interval 24.5–45.5. The proportion of preterm birth is estimated based on the cumulative proportion of babies born from 24.0 to 37.0 weeks.
Number of days that gestation is shortened.
The quantity in parenthesis reflects by how much the OR increases at each additional week that the fetus remains in utero.
Total and preterm mortality in the absence of unmeasured confounding is shown in the first row. Adding Factor 1 (row 2) increases mortality, particularly among preterm babies. Its direct effect explains 19% of total mortality and 79% of the mortality among preterm babies (Factor 1 affects 25% of preterm births and virtually all births before 33 weeks). Factor 2 alone (row 3) contributes a much smaller portion to preterm and total mortality, despite the fact that it affects 68% of preterm births (and virtually all births before 33 weeks). The smaller impact of Factor 2 on mortality reflects its modest odds ratio. The effect of Factor 3 alone (row 4) is stronger than that of Factor 1, due to its rapidly increasing OR (the proportion of preterm babies with Factor 3 is the same as for Factor 1). In the combinations that include Factor 2 and either Factor 1 or Factor 3 (row 5 and 6), the proportion of mortality due to pathology is substantial (with Factors 1 or 3 each contributing about 10% of all preterm babies and 47% of babies born before 33 weeks). When all three factors are present (row 7), pathology alone accounts for 58% of total mortality and 87% of preterm mortality. We explored the sensitivity of these estimates to the prevalence of the factors, first by halving the prevalence of Factors 1 and 3, and then by doubling the prevalence of Factor 2. In both situations, more than half of preterm mortality is caused by the direct effect of one or both of the rare but dangerous factors. Additional tables in the eAppendix (http://links.lww.com) show how these proportions vary when modifying the effect of the pathologies on mortality (eTable 1) or on gestational age (eTable 2). The contribution of Factors 1 and 3 to the mortality of preterm infants falls below 50% in some of the scenarios where the OR was halved, but never below 39%. The factors' relative contribution to overall mortality was, however, substantially reduced in most of these scenarios.
How unmeasured confounding can lead to intersecting gestational-age-specific mortality curves
We now add T, a measured exposure that shortens gestation by 20 days (similar to the mean difference in gestational age between singletons and twins). This shift results in a preterm birth rate of 46% among T babies (compared with <2% in the non-T population). In the absence of any other factor that causes both preterm birth and mortality (and ignoring the possibility that T reduces mortality), there are two possibilities for the effect of T on mortality:
-
A)
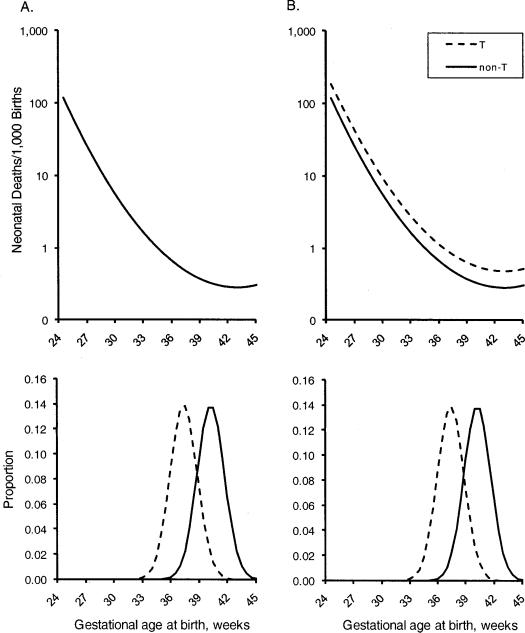
T has no direct effect on the risk of death. In this case, the gestational-age-specific mortality curves of non-T and T babies will (by definition) be identical (Figure 4A). Even so, the observed total mortality of T babies will be increased because more T babies are born early (the observed OR of mortality among T births is 1.61 compared with non-T births).
-
B)
T directly increases mortality. We assign T an OR of 1.7, applied to the baseline risk of immaturity. This results in the mortality for T babies being higher than that of non-T babies at each gestational age (Figure 4B). The observed OR of mortality due to T is now 2.74, combining the direct effect of T on mortality with immaturity.
Figure 4.
Distribution of “target” gestations and baseline mortality for non-T and T babies.
A. T has no direct effect on mortality
B. T increases mortality with an OR of 1.7
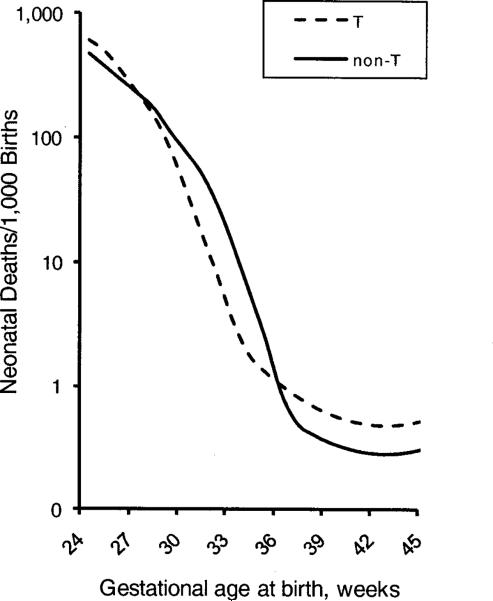
Consider scenario B. If we now add the unmeasured Factors 2 and 3 (which affect T and non-T babies equally, so that all factors act independently of each other), the mortality curves will intersect (Figure 5). This happens because, at any given preterm week, Factors 2 and 3 are more prevalent in non-T than in T babies. (The curves would also intersect if we added Factor 1 instead of Factor 3.)
Figure 5.
Week-specific mortality of T and non-T babies in the presence of two unmeasured factors (Factors 2 and 3), when T increases baseline mortality by an OR of 1.7 at each gestational age.
For intersection to occur, it is necessary for at least one of the unmeasured factors to confer a direct mortality risk higher than that conferred by T. Under these conditions, non-T babies will have higher mortality at certain preterm gestational ages because their early birth will more frequently have been caused by a more severe underlying cause than T (Factor 3). (If T did not directly increase mortality, as in scenario A, the intersection would be even more pronounced.) Table 2 provides the composition of T and non-T births at three specified times. For example, among births on day 238, 85% of T babies have no additional pathology. In contrast, 99% of non-T babies have Factor 2 or Factor 3. (A detailed discussion of the table is provided in the eAppendix, http://links.lww.com)
Table 2.
Proportion of babies born with factors 2 or 3 (or no factor) on three selected days of gestation.
| “Target” gestation (day)a | Proportion % | OR | Mortality rate (per 1,000) | Week-specific OR of T | ||
|---|---|---|---|---|---|---|
| Births at start of week 27 (day 189) | ||||||
| Non-T | No Factor 2 or 3 | 189 | 0.00 | 1.00 | 19.62 | |
| Only Factor 2 | 224 | 0.00 | 1.50 | 29.14 | ||
| Only Factor 3 | 239 | 0.77 | 8.79 | 149.56 | ||
| Factors 2 and 3 | 274 | 99.23 | 13.18 | 208.73 | ||
| Combined | 208.27 | |||||
| T | No Factor 2 or 3 | 209 | 0.00 | 1.70 | 32.90 | |
| Only Factor 2 | 244 | 8.49 | 2.55 | 48.55 | ||
| Only Factor 3 | 259 | 81.78 | 14.94 | 230.16 | ||
| Factors 2 and 3 | 294 | 9.73 | 22.41 | 309.61 | ||
| Combined | 222.46 | 1.09 | ||||
| Births at start of week 34 (day 238) | ||||||
| Non-T | No Factor 2 or 3 | 238 | 0.48 | 1.00 | 1.02 | |
| Only Factor 2 | 273 | 88.48 | 1.50 | 1.53 | ||
| Only Factor 3 | 288 | 11.03 | 55.14 | 53.26 | ||
| Factors 2 and 3 | 323 | 0.00 | 82.72 | 77.81 | ||
| Combined | 7.23 | |||||
| T | No Factor 2 or 3 | 258 | 85.48 | 1.70 | 1.73 | |
| Only Factor 2 | 293 | 14.43 | 2.55 | 2.59 | ||
| Only Factor 3 | 308 | 0.09 | 93.74 | 87.28 | ||
| Factors 2 and 3 | 343 | 0.00 | 140.62 | 125.45 | ||
| Combined | 1.93 | 0.27 | ||||
| Births at start of week 38 (day 266) | ||||||
| Non-T | No Factor 2 or 3 | 266 | 98.98 | 1.00 | 0.32 | |
| Only Factor 2 | 301 | 1.02 | 1.50 | 0.48 | ||
| Only Factor 3 | 316 | 0.00 | 1126.79 | 265.72 | ||
| Factors 2 and 3 | 351 | 0.00 | 1690.18 | 351.84 | ||
| Combined | 0.33 | |||||
| T | No Factor 2 or 3 | 286 | 100.00 | 1.70 | 1.73 | |
| Only Factor 2 | 321 | 0.00 | 2.55 | 2.59 | ||
| Only Factor 3 | 336 | 0.00 | 1915.54 | 87.28 | ||
| Factors 2 and 3 | 371 | 0.00 | 2873.31 | 125.45 | ||
| Combined | 0.55 | 1.67 | ||||
Target gestation is the time at which the babies would have been born, if T, factor2, or factor 3 had not intervened to shorten gestation.
Discussion
Pathologic factors that reduce duration of gestation and increase mortality risk can profoundly affect gestational-age-specific mortality risk, producing a much higher empirical mortality risk at early gestations than that caused by gestational age. Factors with a strong direct effect on mortality can, in theory, account for most of the mortality of preterm babies, even when those factors are rare (<1%). Thus, pathology (directly or through interaction with immaturity) could easily be a much more important determinant of preterm mortality than early birth – even when affecting a minority of all preterm births.
We based our model on an arbitrary “baseline” mortality curve and simple assumptions about confounding factors. We did not try to reproduce an empirical mortality curve, because we do not know the true baseline effect of immaturity on mortality or the true effects of putative confounding factors that might cause preterm birth and mortality. The scenarios presented here merely provide a proof of principle.
Our previous estimate that pathology contributed almost half of preterm mortality was based on empirical data (US babies born between 1995 and 2002), after identifying preterm babies that could be considered relatively “healthy.” Given that such data did not allow identification of truly “healthy” preterm births, we considered this proportion to be an underestimate of the true contribution of preterm pathology.12 The theoretical scenarios presented here support this conclusion.
To some extent, the situation with gestational age mirrors what has been shown for birth weight,10 where a rare, potent, and unmeasured confounder was able to replicate the observed relation of birth weight with mortality when birth weight was assumed to have no direct effect on mortality. The important difference is that gestational age is allowed to have a causal effect on mortality (through immaturity). Still, the underlying principles are the same. In both the birth-weight and gestational-age examples, mortality curves can intersect as a consequence of unmeasured factors,11 a manifestation of collider-stratification bias.16–17
Jobe18 argues that certain pathologies leading to preterm birth may in themselves accelerate lung maturation of very preterm babies, thus making their survival chances better than those of a healthy baby born at the same gestational age. Stress, selective survival, or other mechanisms19–24 have been proposed to explain how high-risk babies may have better survival than “normal” babies when born at low gestational ages.9,13–15 Our explanation (of unmeasured confounders being more frequent among preterm births) is supported by a recent paper showing that babies delivered electively between 34 and 36 weeks had lower rates of ventilation use, sepsis, and neonatal-intensive-care unit admissions than spontaneous births.25 Elective deliveries presumably represent the relatively healthy fetuses still in utero, while babies delivered prematurely (by either an indicated medical intervention or spontaneously) are delivered because of a recognized or unrecognized problem. Our explanation is consistent with the empirical evidence and does not require the underlying causal effect of gestational age to vary between the low- and high-risk groups. It is likely, however, that a variety of mechanisms shape the risk among groups of preterm babies, with the distribution of these varying across gestational ages.
We have not considered unmeasured confounding factors that increase gestational age and also increase mortality (anencephaly, for example, acts in this way26). Such confounding may explain at least part of the increased mortality of post-term babies, and could be incorporated into the model.
A further limitation is the arbitrariness of our parameters. The completely deterministic nature of the model (and the fact that we include only 2 or 3 factors) can result in oddly shaped curves or in curves that intersect several times across the gestational-age spectrum. (This is the case, for example, when adding only factors 1 and 3).
We allowed Factor 3 to increase its effect on mortality with longer gestation. This is intended to represent a uterine environment that grows progressively more hostile—a plausible scenario,27 and often the basis for obstetric intervention. However, the manner in which we implemented this is unrealistic, as is the fact that we did not allow synergy between pathology and immaturity. We have elected this approach for the sake of simplicity and to allow virtually exact calculations. Furthermore, we believe that the principles illustrated here would hold in a more complex setting.
The heterogeneity of preterm birth is now widely recognized.1–9 It is not unreasonable to expect that different causes of preterm birth would have varying effects on mortality. If a substantial portion of preterm mortality (and perhaps morbidity) is due not to immaturity per se, but to the direct effects of the pathological causes of preterm birth, the conventional cut-off of gestational age at <37 weeks may be inadequate for identifying the pathologies that account for most of preterm mortality. Focusing on very early preterm births may be more productive, as most (if not all) of those babies would have pathologic factors. Depending on the strength of their direct effects on mortality, the risk factors associated with preterm delivery as usually defined may be of limited importance. Even among the very preterm, there may not be just a single cause of early birth: several mechanisms may act in concert. (For example, intra-amniotic infection is more frequent among very early births,28 but it may not be the only cause of early delivery of such infants). It would, perhaps, be more feasible to identify individual causes among moderate preterm births (say, 32 to 34 weeks), and then assess whether combinations of these are responsible for the very early births.
If various mechanisms can cause preterm birth, this has other implications for research. For example, some studies consider outcomes for a specific group of very preterm babies (e.g., those born after preeclampsia) compared with other very preterm babies (born due to other, often unspecified, causes). The underlying –and untested- assumption is that none of the other causes of preterm birth independently causes the outcome in question. If this assumption is false (and we suggest it may well be), no etiologic interpretation of these studies would be possible.
The complexity of preterm delivery may present frustrating problems, but it is by addressing these problems that we may be able to identify the mechanisms associated with the highest morbidity and mortality among preterm babies. Considering more detailed clinical information on pregnancy and on the clinical course of preterm infants could lead to the description of new subtypes of preterm births that are vulnerable to the worst outcomes.
It is well known that confounding is a major obstacle to establishing causal links. Still, the fact that immaturity itself plays such a plausible causal role in mortality risk has deflected attention from the extent to which confounding might be present with preterm birth. Our models suggest that this confounding is likely much stronger than has been appreciated, probably stronger than in many other situations (although probably not more than the confounding of birth weight). We have explored only a limited number of simple scenarios in a deterministic model with a few independently acting factors. Reality is much more complicated. The relative contribution of pathology depends on the overall set of causes of preterm birth and on the underlying “true” effect of immaturity—both of which elude our observation. Nonetheless, it appears that, if the factors causing preterm birth also have a moderate-to-strong effect on mortality, they could play an important—and as yet unrecognized—role in preterm mortality.
Supplementary Material
Acknowledgment
We thank Clarice R Weinberg and Anne Marie Jukic for their comments on an earlier version of this manuscript.
Financial Support This research was supported in part by the Intramural program of the NIH, National Institute of Environmental Health Sciences (Z01 ES044003).
Footnotes
Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Romero R, Mazor M, Munoz H, et al. The preterm labor syndrome. Ann N Y Acad Sci. 1994;734:414–29. doi: 10.1111/j.1749-6632.1994.tb21771.x. [DOI] [PubMed] [Google Scholar]
- 2.Klebanoff MA, Schoendorf KC. Invited commentary: what's so bad about curves crossing anyway? Am J Epidemiol. 2004;160:211–2. doi: 10.1093/aje/kwh203. discussion 215–6. [DOI] [PubMed] [Google Scholar]
- 3.Gotsch F, Gotsch F, Romero R, et al. The preterm parturition syndrome and its implications for understanding the biology, risk assessment, diagnosis, treatment and prevention of preterm birth. J Matern Fetal Neonatal Med. 2009;22(Suppl 2):5–23. doi: 10.1080/14767050902860690. [DOI] [PubMed] [Google Scholar]
- 4.Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. Bjog. 2006;113(Suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savitz DA. Invited commentary: disaggregating preterm birth to determine etiology. Am J Epidemiol. 2008;168:990–2. doi: 10.1093/aje/kwn193. discussion 993–4. [DOI] [PubMed] [Google Scholar]
- 6.Klebanoff MA, Shiono PH. Top down, bottom up and inside out: reflections on preterm birth. Paediatr Perinat Epidemiol. 1995;9:125–9. doi: 10.1111/j.1365-3016.1995.tb00126.x. [DOI] [PubMed] [Google Scholar]
- 7.Kelly R, Holzman C, Senagore P, et al. Placental vascular pathology findings and pathways to preterm delivery. Am J Epidemiol. 2009;170:148–58. doi: 10.1093/aje/kwp131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McElrath TF, Hecht JL, Dammann O, et al. Pregnancy disorders that lead to delivery before the 28th week of gestation: an epidemiologic approach to classification. Am J Epidemiol. 2008;168:980–9. doi: 10.1093/aje/kwn202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lie RT. Invited commentary: intersecting perinatal mortality curves by gestational age-are appearances deceiving? Am J Epidemiol. 2000;152:1117–9. doi: 10.1093/aje/152.12.1117. discussion 1120. [DOI] [PubMed] [Google Scholar]
- 10.Basso O, Wilcox AJ, Weinberg CR. Birth weight and mortality: causality or confounding? Am J Epidemiol. 2006;164:303–11. doi: 10.1093/aje/kwj237. [DOI] [PubMed] [Google Scholar]
- 11.Basso O, Wilcox AJ. Intersecting birth weight-specific mortality curves: solving the riddle. Am J Epidemiol. 2009;169:787–97. doi: 10.1093/aje/kwp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basso O, Wilcox A. Mortality risk among preterm babies: immaturity versus underlying pathology. Epidemiology. 2010;21:521–7. doi: 10.1097/EDE.0b013e3181debe5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen XK, Wen SW, Smith G, et al. Pregnancy-induced hypertension is associated with lower infant mortality in preterm singletons. Bjog. 2006;113:544–51. doi: 10.1111/j.1471-0528.2006.00898.x. [DOI] [PubMed] [Google Scholar]
- 14.Chen XK, Wen SW, Smith G, et al. New-onset hypertension in late pregnancy is associated with lower fetal and infant mortality in preterm twins. Hypertens Pregnancy. 2006;25:205–15. doi: 10.1080/10641950600912984. [DOI] [PubMed] [Google Scholar]
- 15.Cheung YB, Yip P, Karlberg J. Mortality of twins and singletons by gestational age: a varying-coefficient approach. Am J Epidemiol. 2000;152:1107–16. doi: 10.1093/aje/152.12.1107. [DOI] [PubMed] [Google Scholar]
- 16.Greenland S. Quantifying biases in causal models: classical confounding vs collider-stratification bias. Epidemiology. 2003;14:300–6. [PubMed] [Google Scholar]
- 17.Whitcomb BW, Schisterman EF, Perkins NJ, Platt RW. Quantification of collider-stratification bias and the birthweight paradox. Paediatr Perinat Epidemiol. 2009;23:394–402. doi: 10.1111/j.1365-3016.2009.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jobe AH. Lung maturation: the survival miracle of very low birth weight infants. Pediatr Neonatol. 2010;51:7–13. doi: 10.1016/S1875-9572(10)60003-4. [DOI] [PubMed] [Google Scholar]
- 19.Fung G, Bawden K, Chow P, Yu V. Chorioamnionitis and outcome in extremely preterm infants. Ann Acad Med Singapore. 2003;32:305–10. [PubMed] [Google Scholar]
- 20.Bustos R, Kulovich MV, Gluck L, et al. Significance of phosphatidylglycerol in amniotic fluid in complicated pregnancies. Am J Obstet Gynecol. 1979;133:899–903. doi: 10.1016/0002-9378(79)90309-0. [DOI] [PubMed] [Google Scholar]
- 21.Yoon JJ, Kohl S, Harper RG. The relationship between maternal hypertensive disease of pregnancy and the incidence of idiopathic respiratory distress syndrome. Pediatrics. 1980;65:735–9. [PubMed] [Google Scholar]
- 22.Ersch J, Fauchere JC, Bucher HU, et al. The pulmonary paradox in premature infants: in-utero infected lungs do better than those with accelerated maturation. J Perinat Med. 2004;32:84–9. doi: 10.1515/JPM.2004.016. [DOI] [PubMed] [Google Scholar]
- 23.Kramer MS, Platt RW, Wen SW, et al. A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics. 2001;108:E35. doi: 10.1542/peds.108.2.e35. [DOI] [PubMed] [Google Scholar]
- 24.Joseph KS. Commentary: exegesis of effect modification - biological or spurious? Paediatr Perinat Epidemiol. 2009;23:417–20. doi: 10.1111/j.1365-3016.2009.01051.x. author reply 421–3. [DOI] [PubMed] [Google Scholar]
- 25.Bailit JL, Gregory KD, Reddy UM, et al. Maternal and neonatal outcomes by labor onset type and gestational age. Am J Obstet Gynecol. 2010;202:245 e1–245 e12. doi: 10.1016/j.ajog.2010.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson AB, Laurence KM, Turnbull AC. The relationship in anencephaly between the size of the adrenal cortex and the length of gestation. J Obstet Gynaecol Br Commonw. 1969;76:196–9. doi: 10.1111/j.1471-0528.1969.tb05821.x. [DOI] [PubMed] [Google Scholar]
- 27.Doyle LW. Antenatal progesterone to prevent preterm birth. Lancet. 2009;373:2000–2. doi: 10.1016/S0140-6736(09)61077-1. [DOI] [PubMed] [Google Scholar]
- 28.Andrews WW, Hauth JC, Goldenberg RL. Infection and preterm birth. Am J Perinatol. 2000;17:357–65. doi: 10.1055/s-2000-13448. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.