Abstract
The cardiorenal syndrome is a clinical and pathophysiological entity defined as the concomitant presence of renal and cardiovascular dysfunction. In patients with severe sepsis and septic shock, acute cardiovascular, and renal derangements are common, that is, the septic cardiorenal syndrome. The aim of this paper is to describe the pathophysiology and clinical features of septic cardiorenal syndrome in light of the actual clinical and experimental evidence. In particular, the importance of systemic and intrarenal endothelial dysfunction, alterations of kidney perfusion, and myocardial function, organ “crosstalk” and ubiquitous inflammatory injury have been extensively reviewed in light of their role in cardiorenal syndrome etiology. Treatment includes early and targeted optimization of hemodynamics to reverse systemic hypotension and restore urinary output. In case of persistent renal impairment, renal replacement therapy may be used to remove cytokines and restore renal function.
1. Introduction and Definitions
The cardiorenal syndromes (CRSs) are relatively new clinical and pathophysiological entities which have been defined as the concomitant presence of renal and cardiovascular dysfunction [1]. According to Ronco and colleagues, five subtypes of the syndrome exist [2]. Type 1 CRS is defined as acute renal failure secondary to an abrupt worsening of cardiac function, for example, cardiogenic shock or acute congestive heart failure. Type 2 CRS describes a progressive and permanent chronic kidney dysfunction which is caused by chronic worsening in cardiac function, for example, chronic congestive heart failure. Type 3 CRS consists of an acute cardiac dysfunction (e.g., heart failure, arrhythmia, and ischemia) secondary to an abrupt worsening of renal function (e.g., acute kidney ischemia or glomerulonephritis). Type 4 CRS describes a state of chronic kidney disease (e.g., chronic glomerular disease) causing a decreased cardiac function, cardiac hypertrophy, and/or increased risk of adverse cardiovascular events. Type 5 CRS reflects concomitant cardiac and renal dysfunctions in the setting of a systemic condition which primarily affect both organs (e.g., diabetes mellitus and sepsis) [2].
The simultaneous presence of acute cardiovascular and renal alterations in septic patients is defined as septic cardiorenal syndrome. Cardiac and renal dysfunctions are often part of the clinical picture of severe sepsis and septic shock [3]. Following classification of Ronco, sepsis may represent an acute cause of Type 5 cardiorenal syndrome [2].
Renal dysfunction can be observed during severe sepsis and is part of the clinical picture of septic shock and multiple organ failure [1]. Acute renal failure is defined as an acute worsening of renal function based on increasing levels of serum creatinine or reduced urinary output [4]. Following RIFLE criteria, acute kidney injury (AKI) ranges from minor alterations in renal function to indication for renal replacement therapy [5]. AKI is common among critically ill patients, and sepsis and septic shock account for more than 50% of cases [6–8]. As suggested by Bellomo et al., sepsis-induced inflammatory injury of microvessels, hypotension and hypoperfusion during septic shock may play a causative role on development of AKI [2].
Moreover, a high proportion of septic patients develop left ventricular systolic impairment, either with or without involvement of other organs [9]. Cardiac dysfunction in sepsis is characterized by decreased contractility, impaired ventricular response to fluid therapy, and, in some patients, progressive ventricular dilatation. Current data support a complex underlying pathophysiology with a host of potential pathways leading to myocardial depression [10]. This is a well-described but poorly understood phenomenon in which microvascular alterations, autonomic dysregulation, metabolic changes and inflammatory signalling have all previously been hypothesized as potential mechanism for cardiac dysfunction [11].
Despite several studies investigate the incidence of AKI in sepsis or pathophysiology of septic cardiomyopathy, data are lacking about concomitant renal and cardiac injury in severe sepsis or septic shock. The purpose of this paper is to review pathophysiology and clinical aspects of septic cardiorenal syndrome in light of the actual clinical and experimental evidence.
2. Epidemiology
Incidence of sepsis in Europe is 350 new cases on 100.000 inhabitants per year [12] and its prevalence is high among all hospitalised patients (one–third) and, mostly, among those admitted to ICUs. Indeed, 10%–15% of all patients admitted to ICUs develop septic shock [13].
Moreover, numerous studies have shown septic AKI to be highly common among the critically ill, ranging from 16% to 41% [14, 15] of patients with severe sepsis and septic shock [16]. Patients with septic AKI are often older, have a higher prevalence of comorbidity and are more severely ill than those with nonseptic AKI [17].
On the other side, myocardial dysfunction may occur in up to 20% of patients with septic shock. Patients with myocardial dysfunction have significantly higher mortality (70%) compared to septic patients without cardiovascular impairment (20%) [18]. Biomarkers such as cardiac troponin T and I have been studied in sepsis. Elevations in cardiac troponin T and I correlate with the presence of left ventricular systolic dysfunction [19–21] and 30–80% of patients with severe sepsis and septic shock show NSTEMI on ECG with serum troponin values above the normal range. Furthermore, levels of cardiac troponin also correlate with duration of hypotension and intensity of vasopressor support in patients with septic shock [22, 23]. The potential role of B-type natriuretic peptide (BNP) as a biomarker has also been evaluated in septic patients. Recent studies have shown increased levels of BNP in patients with severe sepsis and septic shock [24]. Levels of BNP correlate with the degree of myocardial dysfunction and mortality [10]. More recently, echocardiography has been utilized to define heart dysfunction in severe sepsis and septic shock. In a longitudinal study with transthoracic echocardiography in septic shock patients, left ventricular ejection fraction was significantly depressed in all patients [25], resulting in severe reductions in left ventricular stroke volume. Of interest, these abnormalities were more pronounced in survivors than in nonsurvivors.
3. Hemodynamic Alterations
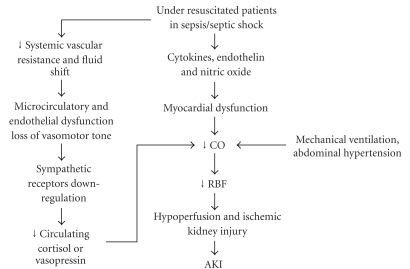
Type 1 cardiorenal syndrome is defined as an acute cardiac dysfunction which leads to acute renal failure, that is, acute cardiorenal syndrome [1]. Traditionally, septic AKI has been seen as the consequence of renal hypoperfusion and reduced renal blood flow, that is, an ischemic kidney injury, which occurs during severe sepsis, septic shock or multiple organ failure [26]. During early phases of septic shock, and in underresuscitated patients, systemic vasodilation and fluid shift reduce cardiac preload, thus reducing cardiac output. This may decrease renal blood flow (RBF) [27] and reduce glomerular filtration rate, leading to prerenal azotemia [26]. If renal hypoperfusion continues, ischemic injury to kidneys occurs, and AKI develops (see Figure 1).
Figure 1.
Hemodynamic alteration in underresuscitated sepsis patients (see text for details).
Sepsis and septic shock are also characterized by a variable degree of myocardial dysfunction, which is linked to multiple factors. Experimental studies on laboratory animals show the role of mediators such as cytokines, endothelin [28], and nitric oxide [29] on myocardial cells and mitochondrial dysfunction [30] as possible mechanisms involved in this phenomenon [10]. Moreover, ventilation with positive end-expiratory pressure required by patients with severe sepsis or septic shock may contribute to intrarenal hemodynamic alterations. In a prospective experimental study on humans, Jacob et al. [31] evaluated the effects of increasing intrathoracic pressure with positive end-expiratory pressure on renal blood flow. High values of PEEP were associated to decreased mean arterial pressures, cardiac output and urinary output. PEEP-induced decrease in urinary output was correlated to renal perfusion pressure decrease. Furthermore, as demonstrated by Peng et al. [32] in bacteremic dogs, the presence of intrabdominal hypertension can adversely affect cardiac output and contribute to renal hypoperfusion (see Figure 1).
As a consequence of all these alterations, renal blood flow, and oxygen delivery decrease [33]. There is evidence that early goal directed hemodynamic optimization has positive effects on survival of septic patients, and restoring and maintaining good organ perfusion and oxygenation may account for this effect [34]. To preserve or restore renal function, a judicious, targeted use of fluids and vasopressors is recommended [35]. Recovery of renal function and diuresis herald a general improvement in systemic oxygen delivery and consumption which is of good prognostic value [26].
4. Microvascular Alterations
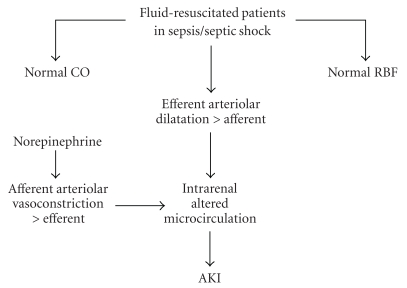
Sepsis-induced alterations of microcirculation are ubiquitous and are linked to both cardiovascular and renal failure [36]. Several studies [37–39] suggest that systemic vasodilation leads to reduced tissue oxygen delivery (DO2), with progressive mitochondrial dysfunction/disruption and cytopathic hypoxia, which can cause organ failure [40]. In early phases of severe sepsis/septic shock, reduction in renal blood flow is associated to arterial hypotension, fluid shift, hypovolemia, and low cardiac output, that is, ischemic AKI (see above). Old experimental studies showed that renal blood flow was reduced in endotoxemic rats [41]. However, in fluid-resuscitated septic patients with AKI, cardiac output is normal or high and glomerular filtration rate can be low despite normal or high renal blood flow [26] (see Figure 2). This is because glomerular filtration rate is related to glomerular filtration pressure, which relies on the balance between afferent and efferent arteriolar tone. During sepsis glomerular efferent arteriola dilates more than afferent, thus reducing glomerular filtration pressure [42] (see Figure 2). Vasopressors, such norepinephrine, are employed to treat arterial hypotension during septic shock [3]. Besides increasing renal blood flow through a restored renal perfusion pressure, norepinephrine increases glomerular filtration rate acting on the afferent-efferent arteriolar tone, with a more intense vasoconstrictive effect on efferent arteriola [43]. In experimental models of septic shock in ewes, Langenberg at al. suggest that recovery from AKI has been associated to an increase in renal vascular resistance [44]. While a judicious use of vasopressors may contribute to restore glomerular filtration pressure and renal function, overzealous use of norepinephrine may also lead to afferent arteriolar vasoconstriction which reduces glomerular blood flow and filtration pressure. Moreover, the increased circulating level of catecholamines, which is part of the neurohormonal stress response to sepsis, results in sustained angiotensin II release, which can adversely affect renal perfusion [45]. All these effects contribute to cause and maintain renal dysfunction.
Figure 2.
Hemodynamic alteration in fluid-resuscitated sepsis patients (see text for details).
5. Organ “Crosstalk”
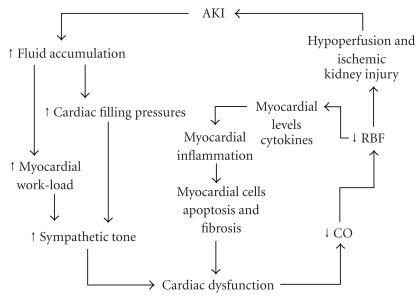
Type 3 cardiorenal syndrome is defined as AKI leading to acute cardiac dysfunction, that is, acute renocardiac syndrome [1]. Indeed, a marked left ventricular dilatation has been shown in experimental models of bilateral renal ischemia in mouse [46]. Three mechanisms may be involved, that is, fluid overload, myocardial inflammation, and reduced cytokines clearance.
During sepsis, renal hypoperfusion brings to progressive worsening fluid accumulation which can adversely impact on myocardial function, further decreasing cardiac output and renal blood flow, and initiating a vicious cycle between renal and cardiovascular dysfunction. Cardiac filling pressures increase, as does myocardial work load and oxygen consumption [27]. Sympathetic burden on cardiovascular system can be already high due to neurohormonal response to stress and use of vasopressors. Thus, acute cardiac dysfunction can precipitate, with further reduction in renal blood flow (see Figure 3).
Figure 3.
Organ “crosstalk” (see text for details).
The ischemic injury to kidneys may contribute to “long distance” organ damage in sepsis [47]. During ischemic renal injury in mouse, Kelly demonstrated increased myocardial levels of mRNA for TNF-alpha, IL-1, and ICAM-1, resulting in increased leukocyte infiltration and activation [46]. The same inflammatory damage could occur during sepsis-induced ischemic renal injury and lead to myocardial cells apoptosis and fibrosis [46], with progressive myocardial dysfunction. Indeed, sepsis-associated myocardial dysfunction can be prevented by anti-TNF-alpha antibodies or receptor antagonists [46] and cytokines removal by the mean of high volume hemofiltration has shown beneficial effects on cardiac function and hemodynamics in septic patients [48]. Knotek et al. showed the effect of TNF neutralization on renal function by a TNF-soluble receptor in the endotoxemic mice, demonstrating the role of TNF in the early renal dysfunction (16 h) [49].
Finally, AKI itself can result in reduced clearance of systemic, circulating cytokines, which can worsen myocardial inflammatory injury. Expressions of cytokines and leukocyte adhesion molecules, and expression of membrane ion and water-channel protein in distant organs, including the cardiovascular systems, are altered during AKI [50].
6. Organ Inflammation
Type 5 cardiorenal syndrome is defined as a systemic insult which leads to concomitant renal and cardiac dysfunction [1]. An inflammatory pathogenesis can be a common key feature for both the kidneys and cardiovascular system during sepsis, leading to cell ultrastructural alterations and organ dysfunction [47, 51]. In a prospective observational study on 1836 hospitalized patients with community-acquired pneumonia, Murugan at al. demonstrated that renal injury and AKI associated to pneumonia recognize an inflammatory pathogenesis [52]. In this paper, outcome of renal injured patients was strictly related to IL-6 plasma concentration [52]. Endotoxin mediated release of TNF-α may affect simultaneously kidneys and cardiovascular system [53]. In the endotoxemic mice, Knotek et al. suggested that TNF-alpha can be also released by glomerular mesangial cells in response to Gram-negative endotoxin and act promoting leukocyte migration and activation in renal tissue, thus inducing septic AKI [49]. In an experimental model of cultured human proximal tubular cells, Jo et al. demonstrated that endotoxin, TNF-α and other pro-inflammatory cytokines induced apoptosis of renal tubular cells [54].
Inflammation has a well-defined role in inducing hypotension in septic patients [55]. Proinflammatory cytokines, such as TNF-α, IL-1 and IL-6, may also induce myocardial inhibition [28–30]. Sepsis-induced release of nitric oxide and increased production of peroxynitrite also depress myocardial function. Tavernier et al. studied contractile function of cardiac myocytes isolated 12 h after induction of endotoxemia in rats. Authors demonstrated that cardiomyocytes from LPS-injected rats had depressed twitch shortening compared with control cells and that contractile depression was unaffected by inhibitors of nitric oxide synthase [56]. Moreover, in a retrospective analysis of human autoptic specimens, Kooy et al. demonstrated the formation of peroxynitrite within the myocardium during sepsis, suggesting a role for peroxynitrite in inflammation-associated myocardial dysfunction [57]. On the other hand, renal hypoperfusion during sepsis-induced low cardiac output state leads to myocardial inflammation, apoptosis, and fibrosis (see above) [46].
7. Changes in Microvascular Permeability
Sepsis induced inflammatory response causes diffuse alteration in microcirculation [58]. Microcirculatory dysfunction contributes to altered tissue perfusion and oxygen delivery/consumption, thus contributing to septic shock and renal failure, that is, type 5 CRS [2]. Enhanced endothelial expression of leukocyte adhesion molecules and alteration of endothelial cells contacts can increase microvascular permeability, thus leading to extravascular fluid shift, fluid overload, hypovolemia, reduced venous return, and low cardiac output. Interstitial edema further reduces oxygen delivery to tissues, and fluid overload is an independent risk factor for mortality among septic patients with AKI [59]. At renal level, increased expression of adhesion molecules is associated to enhanced leukocytes migration, which may lead to endothelial cells injury and detachment, as shown by Paller during experimental renal ischemic injury in rats [60]. Altered glomerular permeability results in microalbuminuria [61].
Glycocalix is a thin (0.5–1.2 μm) molecular structure which lies beneath capillary endothelial cells and regulates capillary flow, leukocytes adhesion and migration, platelets adhesion and coagulation [62]. It is important in regulating capillary permeability. Several studies suggest that glycocalix disruption may contribute to increased permeability, both in systemic and renal microcirculation [63, 64], increasing leukostasis, microthrombosis, fluid shift, and interstitial edema. This leads to reduced oxygen delivery to tissues and organ failure [65].
8. Clinical Features
Septic cardiorenal syndrome is a clinical diagnosis. Its definition implies concomitant presence of acute hemodynamic and renal dysfunction in a patient with sepsis. Sepsis is defined by two or more signs among tachycardia, tachypnea, leukocitosis/leukopenia, and fever/hypothermia [3]. In severe sepsis one acute organ dysfunction is present, usually cardiovascular or renal. Arterial hypotension, an arterial systolic pressure below 95 mmHg, or 40 mmHg below the usual in previously hypertensive patients, is typically observed when hemodynamic dysfunction becomes manifest [3]. Myocardial dysfunction may be present as well, with reduced myocardial contractility and left ventricular ejection fraction [66]. Serum cardiac troponins and B-type natriuretic peptide may be elevated as they are sensitive and specific biomarkers of myocardial damage [67]. As suggested by Ammann et al., in septic, critically ill patients, serum troponin I levels may increase in absence of coronary artery disease, as a marker of myocardial dysfunction, and its levels correlate with mortality rates [68, 69]. However, when AKI is present, serum troponin may be elevated due to underlying renal dysfunction [70], as demonstrated by Musso and Colleagues in patients with chronic renal failure [71].
Typically, reduced urine output and increased serum creatinine are considered as clinical signs of acute renal failure in clinical practice [72], and they were included in RIFLE criteria [6, 7]. However, serum creatinine lacks of sensitivity since its plasma levels rise only after half of the renal function is lost [73]. Alternative biomarkers for AKI include serum interleukin-18 and urinary kidney injury molecule-1, cystatin-C, and beta-2 microglobulin [74]. In an observational cohort study, Soni et al. demonstrated that neutrophil gelatinase associated lipocallin (NGAL) acted as a sensitive biomarker for AKI, particularly for septic AKI [72]. In a series of 143 critically ill children, serum NGAL was a sensitive marker for AKI during systemic inflammatory response syndrome (SIRS) and septic shock [75]. Its plasma level correlated with severity of the syndrome and showed some specificity for septic shock [75]. Recently, Bagshaw et al., in a prospective observational study, demonstrated that patients with septic AKI had higher levels of plasma and urine NGAL compared to those with nonseptic AKI [76]. Interestingly, NGAL, with interleukin-1 receptor antagonist and protein C, was recently included among plasma biomarkers which could allow an early diagnosis of septic shock and multiple organ failure in patients admitted to emergency department with suspected sepsis [77].
9. Treatment
Removal of infective source, antibiotic therapy and supportive care are all indicated in presence of sepsis-associated cardiovascular and renal dysfunction [3]. Early hemodynamic optimization was efficacious in reducing mortality among critically ill septic patients [34]. Fluids are administered to restore intravenous volume and vasopressors or inotropic drugs are infused to revert systemic vasodilation and myocardial depression. Their use should be targeted to specific and clinical end points, such as mean arterial pressure or central venous oxygen saturation [78]. Increased venous return and increased myocardial contractility lead to increased cardiac output. This may contribute to improved renal blood perfusion and glomerular filtration, thus restoring urinary output [79]. Loop diuretics, such furosemide, are often used to increase and/or maintain urinary output during septic AKI, but their efficacy has been questioned and their use may be detrimental on renal function [76]. If oliguria is present, fluid administration should be judicious as volume overload and tissue edema may develop, contributing to impaired lung function and tissue oxygenation [79]. Once systemic cardiovascular optimization has been obtained, a shift towards more restrictive fluid administration strategies has been advocated to reduce AKI associated complications [76]. When renal function is persistently reduced despite hemodynamic optimization, continuous renal replacement therapy (CRRT) is indicated [80]. Worsening serum creatinine levels, volume overload, metabolic acidosis, and electrolytic alterations usually mandate CRRT. CRRT, and particularly high volume venovenous hemofiltration, may also modulate inflammatory response during sepsis, acting through cytokine removal or adsorption, even though the exact mechanism is still debated [81]. However, definite evidence of CRRT for nonrenal indications is still lacking, venovenous hemofiltration can improve hemodynamics and revert sepsis-associated hypotension [82]. Thus, in patients with septic cardiorenal syndrome, CRRT may not be only supportive, but also contribute to reverse common causative factors.
10. Conclusions
Cardiorenal syndrome is common among patients with severe sepsis and septic shock. Pathogenesis is related to multiple factors affecting both the heart and kidneys, including shock related renal hypoperfusion, systemic and intrarenal vasodilation, ubiquitous inflammatory injury to tissues, endothelial dysfunction, and altered capillary permeability. Injured kidneys can further impair myocardial function, thus contributing to maintain shock and organ hypoperfusion. Early and targeted optimization of hemodynamics is indicated to reverse systemic hypotension and to restore urinary output. In case of persistent renal impairment, venovenous hemofiltration may be used to remove cytokines and restore renal function.
References
- 1.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. Journal of the American College of Cardiology. 2008;52(19):1527–1539. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 2.Ronco C. Cardiorenal syndromes: definition and classification. Contributions to Nephrology. 2010;164:33–38. doi: 10.1159/000313718. [DOI] [PubMed] [Google Scholar]
- 3.Srisawat N, Hoste EEA, Kellum JA. Modern classification of acute kidney injury. Blood Purification. 2010;29(3):300–307. doi: 10.1159/000280099. [DOI] [PubMed] [Google Scholar]
- 4.Kellum JA, Bellomo R, Ronco C. Definition and classification of acute kidney injury. Nephron—Clinical Practice. 2008;109(4):c182–c187. doi: 10.1159/000142926. [DOI] [PubMed] [Google Scholar]
- 5.Bagshaw SM, George C, Dinu I, Bellomo R. A multi-centre evaluation of the RIFLE criteria for early acute kidney injury in critically ill patients. Nephrology Dialysis Transplantation. 2008;23(4):1203–1210. doi: 10.1093/ndt/gfm744. [DOI] [PubMed] [Google Scholar]
- 6.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Critical Care. 2004;8(4):R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. Journal of the American Medical Association. 2005;294(7):813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 8.Ronco C, Cruza DN, Ronco F. Cardiorenal syndromes. Current Opinion in Critical Care. 2009;15(5):384–391. doi: 10.1097/MCC.0b013e32832e971b. [DOI] [PubMed] [Google Scholar]
- 9.Sado D, Greaves K. Myocardial perfusion echocardiography: a novel use in the diagnosis of sepsis-induced left ventricular systolic impairment on the intensive care unit. European Journal of Echocardiography. 2011;12(1):81–84. doi: 10.1093/ejechocard/jeq093. [DOI] [PubMed] [Google Scholar]
- 10.Zanotti-Cavazzonia SL, Hollenberg SM. Cardiac dysfunction in severe sepsis and septic shock. Current Opinion in Critical Care. 2009;15(5):392–397. doi: 10.1097/MCC.0b013e3283307a4e. [DOI] [PubMed] [Google Scholar]
- 11.Rudiger A, Singer M. Mechanisms of sepsis-induced cardiac dysfunction. Critical Care Medicine. 2007;35(6):1599–1608. doi: 10.1097/01.CCM.0000266683.64081.02. [DOI] [PubMed] [Google Scholar]
- 12.Esteban A, Frutos-Vivar F, Ferguson ND, et al. Sepsis incidence and outcome: contrasting the intensive care unit with the hospital ward. Critical Care Medicine. 2007;35(5):1284–1289. doi: 10.1097/01.CCM.0000260960.94300.DE. [DOI] [PubMed] [Google Scholar]
- 13.Brun-Buisson C. The epidemiology of the systemic inflammatory response. Intensive Care Medicine. 2000;26(1):S64–S74. doi: 10.1007/s001340051121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoste EAJ, Lameire NH, Vanholder RC, Benoit DD, Decruyenaere JMA, Colardyn FA. Acute renal failure in patients with sepsis in a surgical ICU: predictive factors, incidence, comorbidity, and outcome. Journal of the American Society of Nephrology. 2003;14(4):1022–1030. doi: 10.1097/01.asn.0000059863.48590.e9. [DOI] [PubMed] [Google Scholar]
- 15.Oppert M, Engel C, Brunkhorst FM, et al. Acute renal failure in patients with severe sepsis and septic shock—a significant independent risk factor for mortality: results from the German Prevalence Study. Nephrology Dialysis Transplantation. 2008;23(3):904–909. doi: 10.1093/ndt/gfm610. [DOI] [PubMed] [Google Scholar]
- 16.Lopes JA, Jorge S, Resina C, et al. Acute kidney injury in patients with sepsis: a contemporary analysis. International Journal of Infectious Diseases. 2009;13(2):176–181. doi: 10.1016/j.ijid.2008.05.1231. [DOI] [PubMed] [Google Scholar]
- 17.Parmar A, Langenberg C, Wan L, May CN, Bellomo R, Bagshaw SM. Epidemiology of septic acute kidney injury. Current Drug Targets. 2009;10(12):1169–1178. doi: 10.2174/138945009789753183. [DOI] [PubMed] [Google Scholar]
- 18.Blanco J, Muriel-Bombín A, Sagredo V, et al. Incidence, organ dysfunction and mortality in severe sepsis: a Spanish multicentre study. Critical Care. 2008;12(6, article R158) doi: 10.1186/cc7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandes CJ, Jr., Akamine N, Knobel E. Cardiac troponin: a new serum marker of myocardial injury in sepsis. Intensive Care Medicine. 1999;25(10):1165–1168. doi: 10.1007/s001340051030. [DOI] [PubMed] [Google Scholar]
- 20.Mehta NJ, Khan IA, Gupta V, Jani K, Gowda RM, Smith PR. Cardiac troponin I predicts myocardial dysfunction and adverse outcome in septic shock. International Journal of Cardiology. 2004;95(1):13–17. doi: 10.1016/j.ijcard.2003.02.005. [DOI] [PubMed] [Google Scholar]
- 21.ver Elst KM, Spapen HD, Nguyen DN, Garbar C, Huyghens LP, Gorus FK. Cardiac troponins I and T are biological markers of left ventricular dysfunction in septic shock. Clinical Chemistry. 2000;46(5):650–657. [PubMed] [Google Scholar]
- 22.Arlati S, Casella GP, Lanzani M, et al. Myocardial necrosis in ICU patients with acute non-cardiac disease: a prospective study. Intensive Care Medicine. 2000;26(1):31–37. doi: 10.1007/s001340050008. [DOI] [PubMed] [Google Scholar]
- 23.Turner A, Tsamitros M, Bellomo R. Myocardial cell injury in septic shock. Critical Care Medicine. 1999;27(9):1775–1780. doi: 10.1097/00003246-199909000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Rivers EP, McCord J, Otero R, Jacobsen G, Loomba M. Clinical utility of B-type natriuretic peptide in early severe sepsis and septic shock. Journal of Intensive Care Medicine. 2007;22(6):363–373. doi: 10.1177/0885066607307523. [DOI] [PubMed] [Google Scholar]
- 25.Jardin F, Fourme T, Page B, et al. Persistent preload defect in severe sepsis despite fluid loading: a longitudinal echocardiographic study in patients with septic shock. Chest. 1999;116(5):1354–1359. doi: 10.1378/chest.116.5.1354. [DOI] [PubMed] [Google Scholar]
- 26.Zanotti Cavazzoni SL, Dellinger RP. Hemodynamic optimization of sepsis-induced tissue hypoperfusion. Critical Care. 2006;10(3, article S2) doi: 10.1186/cc4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siegenthaler N, Giraud R, Piriou V, Romand JA, Bendjelid K. Microcirculatory alterations in critically ill patients: pathophysiology, monitoring and treatments. Annales Francaises d’Anesthesie et de Reanimation. 2010;29(2):135–144. doi: 10.1016/j.annfar.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 28.Mink SN, Kasian K, Jacobs H, Cheng ZQ, Light RB. N,N′-diacetylchitobiose, an inhibitor of lysozyme, reverses myocardial depression and lessens norepinephrine requirements in Escherichia coli sepsis in dogs. Shock. 2008;29(6):681–687. doi: 10.1097/shk.0b013e31815816c3. [DOI] [PubMed] [Google Scholar]
- 29.Ichinose F, Buys ES, Neilan TG, et al. Cardiomyocyte-specific overexpression of nitric oxide synthase 3 prevents myocardial dysfunction in murine models of septic shock. Circulation Research. 2007;100(1):130–139. doi: 10.1161/01.RES.0000253888.09574.7a. [DOI] [PubMed] [Google Scholar]
- 30.Larche J, Lancel S, Hassoun SM, et al. Inhibition of mitochondrial permeability transition prevents sepsis-induced myocardial dysfunction and mortality. Journal of the American College of Cardiology. 2006;48(2):377–385. doi: 10.1016/j.jacc.2006.02.069. [DOI] [PubMed] [Google Scholar]
- 31.Jacob LP, Chazalet JJA, Payen DM, et al. Renal hemodynamic and functional effect of PEEP ventilation in human renal transplantations. American Journal of Respiratory and Critical Care Medicine. 1995;152(1):103–107. doi: 10.1164/ajrccm.152.1.7599806. [DOI] [PubMed] [Google Scholar]
- 32.Peng ZY, Critchley LA, Joynt GM, Gruber PC, Jenkins CR, Ho AMH. Effects of norepinephrine during intra-abdominal hypertension on renal blood flow in bacteremic dogs. Critical Care Medicine. 2008;36(3):834–841. doi: 10.1097/CCM.0B013E31816439FB. [DOI] [PubMed] [Google Scholar]
- 33.Abraham E, Singer M. Mechanisms of sepsis-induced organ dysfunction. Critical Care Medicine. 2007;35(10):2408–2416. doi: 10.1097/01.ccm.0000282072.56245.91. [DOI] [PubMed] [Google Scholar]
- 34.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. New England Journal of Medicine. 2001;345(19):1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 35.Dellinger RP, Levy MM, Carlet JM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Critical Care Medicine. 2008;36(1):296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 36.Langenberg C, Bellomo R, May C, Wan L, Egi M, Morgera S. Renal blood flow in sepsis. Critical Care. 2005;9(4):R363–R374. doi: 10.1186/cc3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsuda N, Hattori Y. Vascular biology in sepsis: pathophysiological and therapeutic significance of vascular dysfunction. Journal of Smooth Muscle Research. 2007;43(4):117–137. doi: 10.1540/jsmr.43.117. [DOI] [PubMed] [Google Scholar]
- 38.Jones AE, Puskarich MA. Sepsis-induced tissue hypoperfusion. Critical Care Clinics. 2009;25(4):769–779. doi: 10.1016/j.ccc.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 39.Exline MC, Crouser ED. Mitochondrial mechanisms of sepsis-induced organ failure: mitochondria in sepsis. Frontiers in Bioscience. 2008;13(13):5030–5041. doi: 10.2741/3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brealey D, Karyampudi S, Jacques TS, et al. Mitochondrial dysfunction in a long-term rodent model of sepsis and organ failure. American Journal of Physiology—Regulatory Integrative and Comparative Physiology. 2004;286(3):R491–R497. doi: 10.1152/ajpregu.00432.2003. [DOI] [PubMed] [Google Scholar]
- 41.Kikeri D, Pennell JP, Hwang KH, et al. Endotoxemic acute renal failure in awake rats. American Journal of Physiology—Renal Fluid and Electrolyte Physiology. 1986;250(6, part 2):F1098–F1106. doi: 10.1152/ajprenal.1986.250.6.F1098. [DOI] [PubMed] [Google Scholar]
- 42.Morelli A, Ricci Z, Bellomo R, et al. Prophylactic fenoldopam for renal protection in sepsis: a randomized, double-blind, placebo-controlled pilot trial. Critical Care Medicine. 2005;33(11):2451–2456. doi: 10.1097/01.ccm.0000186413.04875.ef. [DOI] [PubMed] [Google Scholar]
- 43.Bellomo R, Wan L, May C. Vasoactive drugs and acute kidney injury. Critical Care Medicine. 2008;36(4):S179–S186. doi: 10.1097/CCM.0b013e318169167f. [DOI] [PubMed] [Google Scholar]
- 44.Langenberg C, Wan L, Egi M, May CN, Bellomo R. Renal blood flow and function during recovery from experimental septic acute kidney injury. Intensive Care Medicine. 2007;33(9):1614–1618. doi: 10.1007/s00134-007-0734-8. [DOI] [PubMed] [Google Scholar]
- 45.Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. New England Journal of Medicine. 1999;341(8):577–585. doi: 10.1056/NEJM199908193410806. [DOI] [PubMed] [Google Scholar]
- 46.Wen X, Murugan R, Peng Z, Kellum JA. Pathophysiology of acute kidney injury: a new perspective. Contributions to Nephrology. 2010;165:39–45. doi: 10.1159/000313743. [DOI] [PubMed] [Google Scholar]
- 47.Li X, Hassoun HT, Santora R, Rabb H. Organ crosstalk: the role of the kidney. Current Opinion in Critical Care. 2009;15(6):481–487. doi: 10.1097/MCC.0b013e328332f69e. [DOI] [PubMed] [Google Scholar]
- 48.Ronco C, Piccinni P, Kellum J. Rationale of extracorporeal removal of endotoxin in sepsis: theory, timing and technique. Contributions to Nephrology. 2010;167:25–34. doi: 10.1159/000315916. [DOI] [PubMed] [Google Scholar]
- 49.Knotek M, Rogachev B, Wang W, et al. Endotoxemic renal failure in mice: role of tumor necrosis factor independent of inducible nitric oxide synthase. Kidney International. 2001;59(6):2243–2249. doi: 10.1046/j.1523-1755.2001.00740.x. [DOI] [PubMed] [Google Scholar]
- 50.Feltes CM, van Eyk J, Rabb H. Distant-organ changes after acute kidney injury. Nephron—Physiology. 2008;109(4):p80–p84. doi: 10.1159/000142940. [DOI] [PubMed] [Google Scholar]
- 51.Fink MP, Delude RL. Epithelial barrier dysfunction: a unifying theme to explain the pathogenesis of multiple organ dysfunction at the cellular level. Critical Care Clinics. 2005;21(2):177–196. doi: 10.1016/j.ccc.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 52.Murugan R, Karajala-Subramanyam V, Lee M, et al. Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney International. 2010;77(6):527–535. doi: 10.1038/ki.2009.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wan L, Bagshaw SM, Langenberg C, Saotome T, May C, Bellomo R. Pathophysiology of septic acute kidney injury: what do we really know? Critical Care Medicine. 2008;36(4):S198–S203. doi: 10.1097/CCM.0b013e318168ccd5. [DOI] [PubMed] [Google Scholar]
- 54.Jo SK, Lee SY, Han SY, et al. α-melanocyte Stimulating Hormone (MSH) decreases cyclosporine a induced apoptosis in cultured human proximal tubular cells. Journal of Korean Medical Science. 2001;16(5):603–609. doi: 10.3346/jkms.2001.16.5.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mohammed I, Nonas SA. Mechanisms, detection, and potential management of microcirculatory disturbances in sepsis. Critical Care Clinics. 2010;26(2):393–408. doi: 10.1016/j.ccc.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 56.Tavernier B, Li JM, El-Omar MM, et al. Cardiac contractile impairment associated with increased phosphorylation of troponin I in endotoxemic rats. FASEB Journal. 2001;15(2):294–296. doi: 10.1096/fj.00-0433fje. [DOI] [PubMed] [Google Scholar]
- 57.Kooy NW, Lewis SJ, Royall JA, Ye YZ, Kelly DR, Beckman JS. Extensive tyrosine nitration in human myocardial inflammation: evidence for the presence of peroxynitrite. Critical Care Medicine. 1997;25(5):812–819. doi: 10.1097/00003246-199705000-00017. [DOI] [PubMed] [Google Scholar]
- 58.Balestra GM, Legrand M, Ince C. Microcirculation and mitochondria in sepsis: getting out of breath. Current Opinion in Anaesthesiology. 2009;22(2):184–190. doi: 10.1097/ACO.0b013e328328d31a. [DOI] [PubMed] [Google Scholar]
- 59.Payen D, de Pont AC, Sakr Y, Spies C, Reinhart K, Vincent JL. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Critical Care. 2008;12(3):p. R74. doi: 10.1186/cc6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paller MS. Effect of neutrophil depletion on ischemic renal injury in the rat. Journal of Laboratory and Clinical Medicine. 1989;113(3):379–386. [PubMed] [Google Scholar]
- 61.Basu S, Bhattacharya M, Chatterjee TK, Chaudhuri S, Todi SK, Majumdar A. Microalbuminuria: a novel biomarker of sepsis. Indian Journal of Critical Care Medicine. 2010;14(1):22–28. doi: 10.4103/0972-5229.63034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aird WC. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood. 2003;101(10):3765–3777. doi: 10.1182/blood-2002-06-1887. [DOI] [PubMed] [Google Scholar]
- 63.Adamson RH. Permeability of frog mesenteric capillaries after partial pronase digestion of the endothelial glycocalyx. Journal of Physiology. 1990;428:1–13. doi: 10.1113/jphysiol.1990.sp018197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rehm M, Zahler S, Lötsch M, et al. Endothelial glycocalyx as an additional barrier determining extravasation of 6% hydroxyethyl starch or 5% albumin solutions in the coronary vascular bed. Anesthesiology. 2004;100(5):1211–1223. doi: 10.1097/00000542-200405000-00025. [DOI] [PubMed] [Google Scholar]
- 65.Chappell D, Westphal M, Jacob M. The impact of the glycocalyx on microcirculatory oxygen distribution in critical illness. Current Opinion in Anaesthesiology. 2009;22(2):155–162. doi: 10.1097/ACO.0b013e328328d1b6. [DOI] [PubMed] [Google Scholar]
- 66.Hunter JD, Doddi M. Sepsis and the heart. British Journal of Anaesthesia. 2010;104(1):3–11. doi: 10.1093/bja/aep339. [DOI] [PubMed] [Google Scholar]
- 67.Maeder M, Fehr T, Rickli H, Ammann P. Sepsis-associated myocardial dysfunction: diagnostic and prognostic impact of cardiac troponins and natriuretic peptides. Chest. 2006;129(5):1349–1366. doi: 10.1378/chest.129.5.1349. [DOI] [PubMed] [Google Scholar]
- 68.Ammann P, Fehr T, Minder E, Günter C, Bertel O. Elevation of troponin I in sepsis and septic shock. Intensive Care Medicine. 2001;27(6):965–969. doi: 10.1007/s001340100920. [DOI] [PubMed] [Google Scholar]
- 69.Annane PD, Bellissant PE, Cavaillon JM. Septic shock. Lancet. 2005;365(9453):63–78. doi: 10.1016/S0140-6736(04)17667-8. [DOI] [PubMed] [Google Scholar]
- 70.Wong CK, White HD. Implications of the new definition of myocardial infarction. Postgraduate Medical Journal. 2005;81(959):552–555. doi: 10.1136/pgmj.2005.035071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Musso P, Cox I, Vidano E, Zambon D, Panteghini M. Cardiac troponin elevations in chronic renal failure: prevalence and clinical significance. Clinical Biochemistry. 1999;32(2):125–130. doi: 10.1016/s0009-9120(98)00089-7. [DOI] [PubMed] [Google Scholar]
- 72.Soni SS, Ronco C, Katz N, Cruz DN. Early diagnosis of acute kidney injury: the promise of novel biomarkers. Blood Purification. 2009;28(3):165–174. doi: 10.1159/000227785. [DOI] [PubMed] [Google Scholar]
- 73.Bellomo R, Kellum JA, Ronco C. Defining acute renal failure: physiological principles. Intensive Care Medicine. 2004;30(1):33–37. doi: 10.1007/s00134-003-2078-3. [DOI] [PubMed] [Google Scholar]
- 74.Lisowska-Myjak B. Serum and urinary biomarkers of acute kidney injury. Blood Purification. 2010;29(4):357–365. doi: 10.1159/000309421. [DOI] [PubMed] [Google Scholar]
- 75.Wheeler DS, Devarajan P, Ma Q, et al. Serum neutrophil gelatinase-associated lipocalin (NGAL) as a marker of acute kidney injury in critically ill children with septic shock. Critical Care Medicine. 2008;36(4):1297–1303. doi: 10.1097/CCM.0b013e318169245a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bagshaw SM, Bennett M, Haase M, et al. Plasma and urine neutrophil gelatinase-associated lipocalin in septic versus non-septic acute kidney injury in critical illness. Intensive Care Medicine. 2010;36(3):452–461. doi: 10.1007/s00134-009-1724-9. [DOI] [PubMed] [Google Scholar]
- 77.Shapiro NI, Trzeciak S, Hollander JE, et al. A prospective, multicenter derivation of a biomarker panel to assess risk of organ dysfunction, shock, and death in emergency department patients with suspected sepsis. Critical Care Medicine. 2009;37(1):96–104. doi: 10.1097/CCM.0b013e318192fd9d. [DOI] [PubMed] [Google Scholar]
- 78.Hollenberg SM. Inotrope and vasopressor therapy of septic shock. Critical Care Clinics. 2009;25(4):781–802. doi: 10.1016/j.ccc.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 79.Prowle JR, Echeverri JE, Ligabo EV, Ronco C, Bellomo R. Fluid balance and acute kidney injury. Nature Reviews Nephrology. 2010;6(2):107–115. doi: 10.1038/nrneph.2009.213. [DOI] [PubMed] [Google Scholar]
- 80.Joannidis M. Continuous renal replacement therapy in sepsis and multisystem organ failure. Seminars in Dialysis. 2009;22(2):160–164. doi: 10.1111/j.1525-139X.2008.00552.x. [DOI] [PubMed] [Google Scholar]
- 81.Honoré PM, Joannes-Boyau O, Gressens B. Blood and plasma treatments: the rationale of high-volume hemofiltration. Contributions to Nephrology. 2007;156:387–395. doi: 10.1159/000102129. [DOI] [PubMed] [Google Scholar]
- 82.Oda S, Sadahiro T, Hirayama Y, et al. Non-renal indications for continuous renal replacement therapy: current status in Japan. Contributions to Nephrology. 2010;166:47–53. doi: 10.1159/000314851. [DOI] [PubMed] [Google Scholar]