Abstract
It is well accepted that osteoblasts respond to fluid shear stress (FSS) depending on the loading magnitude, rate, and temporal profiles. Although in vivo observations demonstrated that bone mineral density changes as the training intensity gradually increases/decreases, whether osteoblasts perceive such slow temporal changes in the strength of stimulation remains unclear. In this study, we hypothesized that osteoblasts can detect and respond differentially to the temporal gradients of FSS. In specific, we hypothesized that when the temporal FSS gradient is high enough, i) the increasing FSS inhibits the osteoblastic potential in supporting osteoclastogenesis and enhances the osteoblastic anabolic responses; ii) on the other hand, the deceasing FSS would have opposite effects on osteoclastogenesis and anabolic responses. To test the hypotheses, stepwise varying FSS was applied on primary osteoblasts and osteogenic and resorption markers were analyzed. The cells were subjected to FSS increasing from 5, 10, to 15 or decreasing from 15, 10, to 5 dyn/cm2 at a step of 5 dyn/cm2 for either 6 or 12 hours. In a subset experiment, the cells were stimulated with stepwise increasing or decreasing FSS at a higher step (10 dyn/cm2) for 12 hours. Our results showed that, with the step of 5 dyn/cm2, the stepwise increasing FSS inhibited the osteoclastogenesis with a 3- to 4-fold decrease in RANKL/OPG gene expression versus static controls, while the stepwise decreasing FSS increased RANKL/OPG ratio by 2- to 2.5-fold versus static controls. Both increasing and decreasing FSS enhanced alkaline phosphatase expression and calcium deposition by 1.0- to 1.8 fold versus static controls. For a higher FSS temporal gradient (three steps of 10 dyn/cm2 over 12 hour stimulation), the increasing FSS enhanced the expression of alkaline phosphatase expression and calcium deposition by 1.3 fold, while the decreasing FSS slightly inhibited them by -10% compared with static controls. Taken together, our results suggested that osteoblasts can detect the slow temporal gradients of FSS and respond differentially in a dose-dependent manner, which may account for the observed bone mineral density changes in response to the gradual increasing/decreasing exercise in vivo. The stepwise FSS can be a useful model to study bone cell responses to long-term mechanical usage or disuse. These studies will complement the short-term studies and provide additional clinically relevant insights on bone adaptation.
Keywords: RANKL, OPG, alkaline phosphatase (ALP), calcium deposition, FSS temporal gradient
Introduction
It is well accepted that bone adapts its mass and structure under cues from its mechanical environment [1-5]. Increasing loading associated with progressive exercise increases bone mass and mineral density, especially under high-impact loading [6]. In contrast, decreasing loading associated with reduced physical activity such as bed rest and spaceflight results in pronounced bone loss [7, 8]. To investigate the cellular and molecular mechanisms underlying these in vivo observations, the sensitivities and responses of bone cells to various mechanical stimuli have been studied extensively in vitro using fluid shear stress (FSS) stimulation [9-16]. Variety of flow regimens have been applied to bone cells, including the unidirectional steady FSS with varying magnitudes, dynamic pulsatile or oscillatory FSS with varying frequencies (usually ≥ 0.5 Hz) and loading waveforms (triangle, sinusoidal or square waveforms) [16]. To mimic the intermittent nature of the in vivo loading patterns, rest periods were inserted in some studies [17, 18]. These in vitro studies convincingly demonstrate that bone cells can detect and respond to dynamic mechanical stimuli. However, the temporal gradients of the FSS applied in these studies were typically high within individual flow cycles (on the order of 10-20 dyn/cm2 per sec), but the peak FSS did not vary among cycles. Whether bone cells can detect and respond differentially to the FSS increase or decrease over a longer period of hours or even days has not been addressed yet.
Bone appears to have “memory” of its loading history. Continuous loading seems to be less potent in bone formation than rest-inserted loading regimens [19, 20]: it fails to further increase bone mass after initial response [21]. In addition, in vitro studies showed that bone cells responded differently to unidirectional, pulsatile, or oscillatory FSS [16], suggesting that bone cells is capable to perceive the temporal patterns of FSS stimulation, although continuous stimulation tend to desensitize the cells as shown in decreased intracellular calcium response [22] and production of nitric oxide [23] and prostaglandin E(2) [24]. The sensitivity was recovered by inserting rest periods during FSS sessions [18]. A recent study showed that pretreatment of MC3T3-E1 cells with a gradual rise in FSS abrogated the effects of loading frequency and magnitude on cells [25]. Taken together, the existing evidence strongly suggests that bone cells respond to rapid changes in FSS magnitude and rate [26] but with decreasing sensitivity to steady stimulation. However, the lower limit of the temporal gradients of FSS that the cells can detect and respond to is not fully understood.
In this study, we hypothesized that osteoblasts can detect and respond differentially to the temporal gradients of FSS. In specific, we hypothesized that when the temporal FSS gradient is high enough, i) the increasing FSS inhibits the osteoblastic potential in supporting osteoclastogenesis and enhances the osteoblastic anabolic responses; ii) on the other hand, the deceasing FSS would have an opposite effects on osteoclastogenesis and anabolic responses. To test the hypotheses, stepwise varying FSS was applied on primary osteoblasts and bone formation and resorption markers were analyzed. The cells were subjected to FSS increasing from 5, 10, to 15 or decreasing from 15, 10, to 5 dyn/cm2 at a step of 5 dyn/cm2 for either 6 or 12 hours. In a subset experiment, the cells were stimulated with increasing or decreasing stepwise FSS in three steps at a higher step (10 dyn/cm2) for total 12 hours. Our results suggested that osteoblasts can detect the slow temporal gradients of FSS and respond differentially in a dose-dependent manner, which may account for the observed bone mineral density changes in response to the gradual increasing/decreasing exercise in vivo. The stepwise FSS can be a useful model to study bone cell responses to long-term mechanical usage or disuse. These studies will complement the short-term studies and provide additional clinically relevant insights on bone adaptation.
Materials and methods
Cell culture
Murine primary osteoblasts were harvested from calvariae of newborn Wistar rat (2∼3 days old) followed a previously published protocol [27]. Although cell lines are commonly used in mechanotransduction studies, we preferred to use primary cells due to their close resemblance to in vivo osteoblasts. Briefly, calvariae were stripped of periosteum and surrounding soft tissues before minced into small pieces (∼0.5×0.5 cm in dimension) aseptically. The bone pieces were cultured in medium containing DMEM/F12, penicillin (100 U/ml), streptomycin (100 mg/ml) and 0.5% l-glutamine (Hyclone), supplemented with 10% heat-inactivated fetal calf serum (Hyclone) in a 95% air-5% CO2 humidified environment at 37°C for about one week, during which osteoblasts migrated from the bone pieces and attached to the bottoms of cell culture flasks. The initial osteoblasts were digested with 0.25% trypin, purified to remove fibroblasts according to their difference in attaching on culture flasks and subcultured. Cells used in all experiments of this study were in passage #2 or #3, whose osteoblastic phenotype was confirmed by von Kossa staining and expression of the important osteoblastic markers such as collagen I, osteocalcin, and alkaline phosphatase.
FSS Application
Cells (2×105) were seeded on serum coated glass slides (7.5 × 2.5 cm in dimension) and subjected to FSS after 2 days' culture to reach about 100% confluency through a home-made parallel-plate flow chamber as described previously [12]. In our preliminary tests, serum starvation was found to result in cell detachment from the slides during FSS stimulation. In this study the cells were cultured in the presence of 10% serum before they were exposed to FSS stimulation. A peristaltic pump (Longer Peristaltic Pump, Hebei, China) was used to drive the flow and the rotation speed of the pump was set to achieve the desired FSS magnitude according to the equation (τw =6ηQ/WH2), where τw is the FSS, η is the medium viscosity, Q is the flow rate, W and H are the width and height of the flow chamber, respectively. Since the parameters of η, W and H were fixed in the system, FSS was directly controlled by adjusting the flow rate driven by the pump. The cells were exposed to the same culture medium during the FSS stimulation. Except the pump, the flow system including the cells, flow chamber and medium reservoir was placed inside an incubator.
Experimental Groups
To study the effects of the increasing and decreasing FSS on the osteoblastic potential to support osteoclastogenesis and osteogenesis, five experimental groups were used: two groups were subjected to a stepwise increasing FSS (
 5-10-15 dyn/cm2) with a 5 dyn/cm2 increase every 2 or 4 hours for a total stimulation of 6 or 12 hours; the other two groups were subjected to a stepwise decreasing fluid shear (
5-10-15 dyn/cm2) with a 5 dyn/cm2 increase every 2 or 4 hours for a total stimulation of 6 or 12 hours; the other two groups were subjected to a stepwise decreasing fluid shear (
 15-10-5 dyn/cm2) with a 5 dyn/cm2 decrease every 2 or 4 hours for a total stimulation of 6 or 12 hours; In parallel, the last group was statically incubated as a control. The increasing and decreasing FSS levels were manually adjusted within 2-3 sec between levels. The flow experiments were repeated 4 times, and half of the cells were analyzed for bone formation indices and the other half for osteoclastogenesis analysis (detailed below).
15-10-5 dyn/cm2) with a 5 dyn/cm2 decrease every 2 or 4 hours for a total stimulation of 6 or 12 hours; In parallel, the last group was statically incubated as a control. The increasing and decreasing FSS levels were manually adjusted within 2-3 sec between levels. The flow experiments were repeated 4 times, and half of the cells were analyzed for bone formation indices and the other half for osteoclastogenesis analysis (detailed below).
Because the above experiments with the step of 5 dyn/cm2 FSS did not show difference in osteogenesis between the increasing and decreasing FSS groups, we performed a subset experiment using a larger step of FSS with three additional experimental groups. One group was subjected to a stepwise increasing FSS (
 10-20-30 dyn/cm2) with a 10 dyn/cm2 increment every 4 hours for 12 hours; the other group was subjected to a stepwise decreasing FSS (
10-20-30 dyn/cm2) with a 10 dyn/cm2 increment every 4 hours for 12 hours; the other group was subjected to a stepwise decreasing FSS (
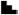 30-20-10 dyn/cm2) with a 10 dyn/cm2 decrease every 4 hours for 12 hours. In parallel, static incubated cells were used as controls. This experiment was repeated three times, and only the osteogenesis indices were analyzed.
30-20-10 dyn/cm2) with a 10 dyn/cm2 decrease every 4 hours for 12 hours. In parallel, static incubated cells were used as controls. This experiment was repeated three times, and only the osteogenesis indices were analyzed.
RANKL and OPG gene expression
Although the main function of osteoblasts is involved in bone formation, recent studies found that osteoblasts express nuclear factor kappa B (NF-κB) ligand (RANKL) and osteoprotegerin (OPG), the two major regulators of the differentiation and survival of osteoclasts [28]. A decrease in the RANKL/OPG ratio in osteoblast inhibited osteoclastogenesis and thus favored bone formation [29]. In the present study, to qualify the effects of the stepwise varying FSS on the osteoblastic potential to support osteoclastogenensis, we tested the RANKL and OPG gene expression in osteoblasts. Immediately after the FSS simulation as described in the preceding section, total cell RNA was extracted using a guanidine thiocyanate/phenol method as recommended by the manufacturer (RNAsol, Qbiogene, France). RNA (2μg) was reverse transcribed to cDNA in a 20 μL reaction mixture containing 1μL (200 U/μl) transcriptor reverse transcriptase (TaKaRa Biotechnology Co., Dalian, China). Real-time PCR reactions were performed in a mixture of 25 μL in the presence of specific primer pairs, which were designed using published gene sequences (PubMed, NCBI Entrez Nucleotide Database) and synthesized by Yingjun Biotechnology Co (Shanghai, China). The following primer pairs were used:
| OPG: | forward 5′-ACAGCTGGCACACCAGTGAT-3′ |
| reverse 5′-ATCCTCTCTACACTCTCGGC-3′ | |
| RANKL: | forward 5′-CCATCGGGTTCCCATAAAGTCAGT-3′ |
| reverse 5′-AAAGCCCCAAAGTACGTCGCATCT-3′ | |
| β-actin: | forward 5′- ATA TCG CTG CGC TGG TCG TC -3′ |
| reverse 5′- AGG ATG GCG TGA GGG AGA GC -3′ |
β-actin was used as an internal control. PCR conditions were 30 seconds at 94°C, 30 seconds at 58°C, and 30 seconds at 72°C for 35 cycles using a Gene Amp PCR system 2400 (Perkin Elmer, Waltham, MA, USA). Aliquots (10 μL) from PCR product were electrophoresed on a 2% agarose gel stained with ethidium bromide (Sigma, St. Louis, MO, USA). The intensity of amplified bands was analyzed with the MAC BAS software. Each RNA sample was analyzed in triplicate and mRNA levels of OPG and RANKL were normalized with β-actin.
ALP Activity and Calcium Deposition Assays
During bone formation osteoblasts produce osteoid mainly composed of Type I collagen and subsequently mineralized to form hard bone tissue. Alkaline phosphatase (ALP) is an enzyme produced by osteoblasts and involved in bone mineralization by facilitating calcium deposition into the bone matrix [30]. Therefore, ALP activity and calcium deposition are generally regarded as osteogenic indicators, which have been found to be upregulated under FSS stimulation [31, 32]. To quantify the effects of the stepwise varying FSS on osteogenesis, ALP and calcium deposition were tested. Cells were collected after exposure of the stepwise increasing or decreasing FSS. One half of the collected cells were used for ALP activity and total protein assay. Cells were lysed with 500 μL of 0.05% Triton X-100 (Fluka, St. Louis, MO, USA) in PBS on ice and centrifuged at 5000 rmp for 5 min to recover the lysate. Aliquot of lysate (50 μL) was used for assessing the ALP activity, which was determined kinetically by monitoring the conversion of p-nitrophenyl phosphate to p-nitrophenol using a commercial kit (Biosino biotechnology and Science, Beijing, China). The absorbance at 405 nm was recorded. Another aliquot of lysate (20μL) was used for assessing total protein content, which was quantified by bicinchoninic acid (BCA) colorimetric detection using a commercial kit (Boao biotechnnology, Beijing, China). The absorbance at 562 nm was recorded. The rest of collected cells were used for calcium deposition assay. One mL of 0.1 M HCl was added into the cell pellet and vibrated overnight at 4°C. The supernatant was collected by centrifuge at 5000 rpm for 5 min. Aliquot of 30 μL supernatant was used for calcium deposition assay using methylthymol blue conjugation method. The absorbance at 570 nm was recorded. All the absorbance tests were performed in triplicate using a BIORAD 680 microplate reader. For each sample, the ALP activity and calcium deposition were normalized with the total protein.
Data Processing and Statistical Analysis
The mRNA expression of RANKL and OPG, the ratio of RANKL/OPG, ALP activity, and calcium deposition were normalized with either β-actin or the total protein. The changes of these measures of the FSS groups relative to those of static controls were reported and compared using Student's t-tests (two-tailed) for paired samples. Statistical significance was set to a p-value less than 0.05.
Results
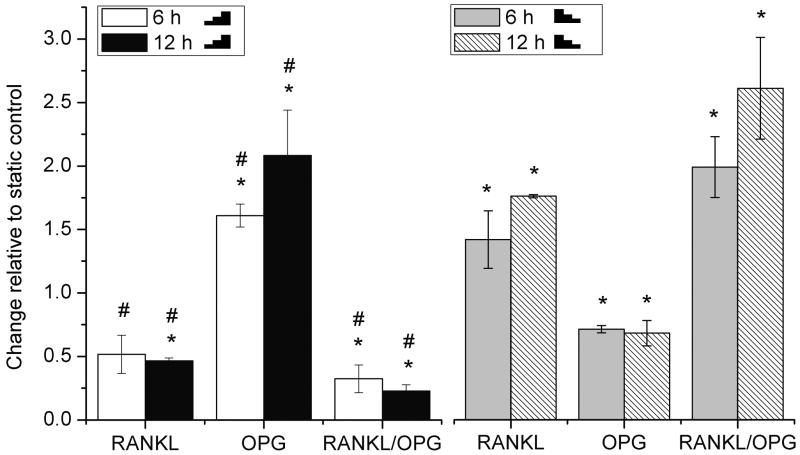
RANKL and OPG mRNA were conversely regulated by stepwise increasing and decreasing FSS
Compared with static controls, application of the stepwise increasing FSS (
 5-10-15 dyn/cm2) for 6 and 12 hours significantly decreased the osteoblastic gene expression of RANKL by 2-fold and increased OPG by 1.5- and 2-fold, respectively, resulting in a significant 3- and 4-fold reduction of the RANKL/OPG ratio (Fig. 1). In contrast, application of the stepwise decreasing FSS (
5-10-15 dyn/cm2) for 6 and 12 hours significantly decreased the osteoblastic gene expression of RANKL by 2-fold and increased OPG by 1.5- and 2-fold, respectively, resulting in a significant 3- and 4-fold reduction of the RANKL/OPG ratio (Fig. 1). In contrast, application of the stepwise decreasing FSS (
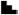 15-10-5 dyn/cm2) for 6 and 12 hours significantly increased RANKL by 1.4- and 1.8-fold and decreased OPG by -25%, resulting in a 2- and 2.5-fold increase of the RANKL/OPG ratio, respectively (Fig. 1). No significant difference was detected between the 6 and 12 hour stimulations using either increasing or decreasing FSS profile.
15-10-5 dyn/cm2) for 6 and 12 hours significantly increased RANKL by 1.4- and 1.8-fold and decreased OPG by -25%, resulting in a 2- and 2.5-fold increase of the RANKL/OPG ratio, respectively (Fig. 1). No significant difference was detected between the 6 and 12 hour stimulations using either increasing or decreasing FSS profile.
Fig. 1.
The RANKL and OPG gene expression and RANKL/OPG ratio examined in rat primary osteoblasts after 6 or 12 h stimulation with stepwise increasing (
 5-10-15 dyn/cm2) or decreasing (
5-10-15 dyn/cm2) or decreasing (
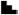 15-10-5 dyn/cm2) FSS relative to static control (=1). The RANKL and OPG mRNA were normalized with β-actin. To indicate significant difference between pair comparison, the following symbals are used: * FSS vs the static control; # stepwise increasing FSS vs decreasing FSS for the same stimulation period (6 or 12h). There was no difference between 6 h and 12 h stimulation using either increasing or decreasing FSS.
15-10-5 dyn/cm2) FSS relative to static control (=1). The RANKL and OPG mRNA were normalized with β-actin. To indicate significant difference between pair comparison, the following symbals are used: * FSS vs the static control; # stepwise increasing FSS vs decreasing FSS for the same stimulation period (6 or 12h). There was no difference between 6 h and 12 h stimulation using either increasing or decreasing FSS.
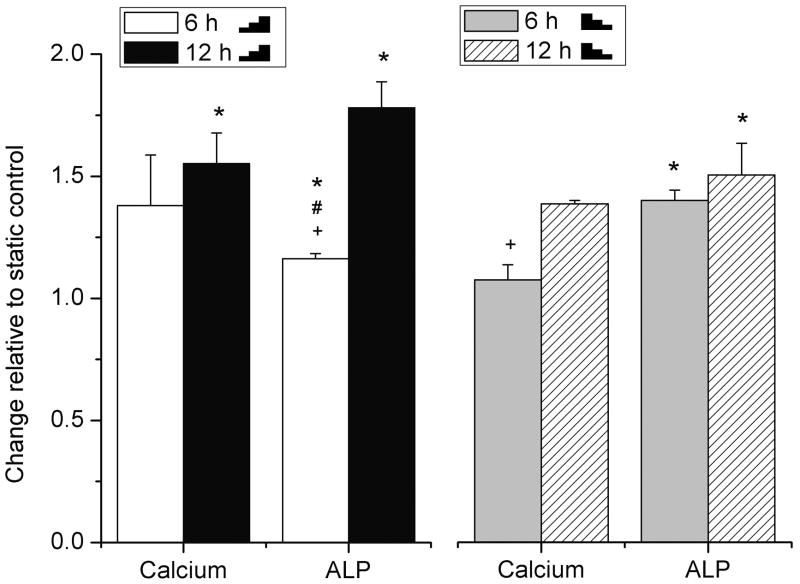
ALP activity and calcium deposition were dependent on the step of FSS
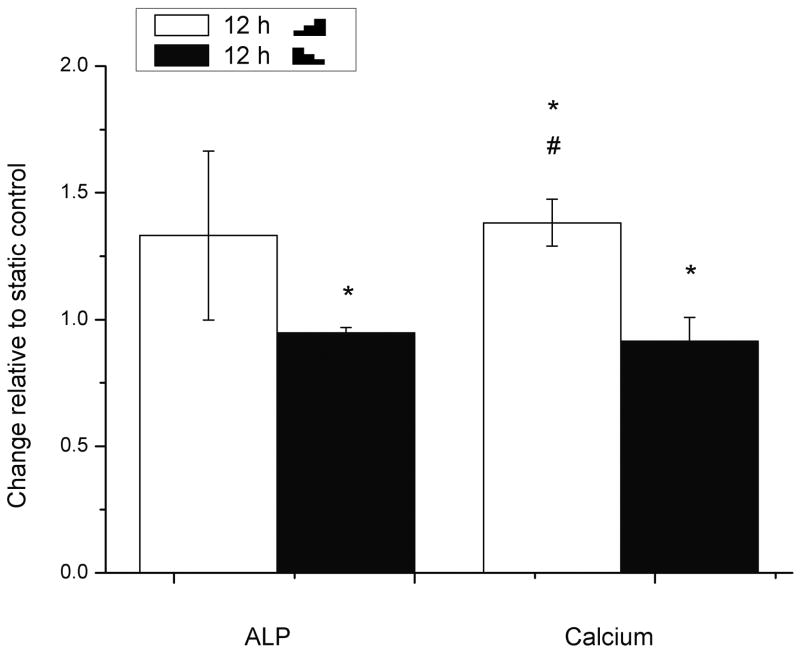
In contrast to RANKL/OPG, the osteoblastic ALP activity and calcium deposition were upregulated by 1.0- to 1.8-fold in all sheared groups (with the step of 5 dyn/cm2) compared with static controls and did not show a consistent dependence on the temporal gradients of the FSS (Fig. 2). Longer stimulation of FSS (12 h vs. 6 h) appeared to have the same or greater effect in ALP activity and calcium deposition no matter whether the FSS was increased or decreased (Fig. 2). We suspected that the gradient of the FSS used in the above experiments (5 dyn/cm2) was too small to be detected by the cells. Therefore, we repeated the experiment with an increased step of FSS (10 dyn/cm2) and found that compared with the static controls, application of the stepwise increasing FSS (
 10-20-30 dyn/cm2) over 12 h enhanced the osteoblastic ALP activity and calcium deposition by 1.3 fold, while application of stepwise decreasing FSS (
10-20-30 dyn/cm2) over 12 h enhanced the osteoblastic ALP activity and calcium deposition by 1.3 fold, while application of stepwise decreasing FSS (
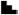 30-20-10 dyn/cm2) slightly (but not significantly) inhibited the ALP activity and calcium deposition by -10% (Fig. 3). There was a significant difference in calcium deposition between the increasing and decreasing FSS profiles.
30-20-10 dyn/cm2) slightly (but not significantly) inhibited the ALP activity and calcium deposition by -10% (Fig. 3). There was a significant difference in calcium deposition between the increasing and decreasing FSS profiles.
Fig. 2.
The ALP activity and calcium deposition examined in rat primary osteoblasts after 6 or 12 h stimulation with increasing (
 5-10-15 dyn/cm2) or decreasing (
5-10-15 dyn/cm2) or decreasing (
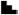 15-10-5 dyn/cm2) FSS relative to static control (=1). The cacium and ALP were normalized with total protein of cells. To indicate significant difference between pair comparison, the following symbals are used: * FSS vs the static control; # stepwise increasing FSS vs decreasing FSS for the same stimulation period (6 or 12h); + 6 h vs 12 h of the same increasing/decreasing FSS pattern.
15-10-5 dyn/cm2) FSS relative to static control (=1). The cacium and ALP were normalized with total protein of cells. To indicate significant difference between pair comparison, the following symbals are used: * FSS vs the static control; # stepwise increasing FSS vs decreasing FSS for the same stimulation period (6 or 12h); + 6 h vs 12 h of the same increasing/decreasing FSS pattern.
Fig. 3.
The ALP activity and calcium deposition examined in rat primary osteoblasts after 12 h stimulation with either increasing (
 10-20-30 dyn/cm2) or decreasing (
10-20-30 dyn/cm2) or decreasing (
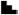 30-20-10 dyn/cm2) FSS relative to static control (=1). The cacium and ALP were normalized with total protein of cells. To indicate significant difference between pair comparison, the following symbals are used: * FSS vs the static control; # stepwise increasing FSS vs decreasing FSS.
30-20-10 dyn/cm2) FSS relative to static control (=1). The cacium and ALP were normalized with total protein of cells. To indicate significant difference between pair comparison, the following symbals are used: * FSS vs the static control; # stepwise increasing FSS vs decreasing FSS.
Discussion
Although bone adaptation to mechanical usage is well accepted, the mechanisms driving this dynamic process remain poorly understood. Frost proposed the concept of “mechanostat” to explain bone formation or resorption based on the magnitude of tissue strain [33]. Later experimental evidence showed much more complicated adaptive behaviors of bone: its formation/resorption processes are triggered by alteration of loading patterns and the set-point of switching from bone formation to bone resorption is not static as Frost initially proposed. Numerous in vitro studies (summarized in Table 1) have established that osteoblasts can detect and respond not only the magnitude but also the temporal gradients of FSS. Two questions remain unknown: i) whether osteoblasts can detect and respond differentially to the temporal increase or decrease in the FSS magnitude as expected in the in vivo cases when the training intensity is gradually increased or decreased; ii) If yes, what is the lower limit of the temporal gradients of FSS that bone cells can detect and respond. In this investigation, we attempted to address these questions by varying the FSS magnitude over a relatively long period time.
Table 1. Review of osteoblastic responses to various flow patterns.
| Flow types/Shear stress details | Responses | Year | Reference |
|---|---|---|---|
| Unidirectional steady flow | |||
| 4.3dyn/cm2, 0.5-15min | cAMP increased up to 12 fold, which was mediated by prostaglandin. | 1990 | Reich [9] |
| 6 or 24dyn/cm2, 5min-8 h | PGE2 increased 40% after 5 min and 9- to 20-fold in 8 h, higher shear resulted more increase. 24 dyn/cm2 increased IP3 up to 2 h and partially due to PKC activation and mediated by PGE2; | 1991 | Reich [31] |
| 18, 35, 70dynes/cm2 | [Ca2+]i responses (peak amplitude and number of responsive cells) increased in a manner dependent on FSS magnitude; | 1995 | Hung [12] |
| 6dyn/cm2, up to 12h | NO (in NOS form) increased and sustained for 12 h; | 1996 | Johnson [45] |
| 4dyn/cm2, up to 8h | ALP decreased compared with static control; | 1997 | Hillsley [41] |
| 17–20dyn/cm2, up to 24h | mRNA and protein of TGF-β1 increased after 3 and 24 h via cation channel. No mRNA expression of PDGF-A, IGF-I, IGF-II, or IL-6 was detected; | 1998 | Sakai [46] |
| 12dyn/cm2, 1h | Actin cytoskeleton Reorganization, recruitment of β1-integrins and α-actinin to focal adhesions, mRNA and protein of Cox-2 and c-fos was increased. | 1998 | Pavalko[15] |
| 12dyn/cm2, up to 3h | The process required IP3-mediated [Ca2+]i release; | 2000 | Chen[22] |
| 0.25-26dyn/cm2, up to 6h | Transient FSS associated with the onset of flow stimulated ∼ 4-fold increase in NO release compared with steady flow; | 1999 | McAllister [47] |
| 10dyn/cm2, 24h | Increased IL-11 (mediated by prostaglandin) and stimulated bone remodeling; | 1999 | Sakai [48] |
| 10dyn/cm2, 4 or 4.5h | Induction of Cox-2 expression was via a PKA, not PKC signaling pathway | 2002 | Wadhwa [49] |
| 5 or 20dyn/cm2, 1-3h | Disrupted junctional protein distribution and decreased intercellular coupling | 2003 | Thi [34]. |
| 12dyn/cm2, 4 or 6h | Inhibited TNF-α induced apoptosis via PI3-kinase activation and caspase-3 inhibition; | 2003 | Pavalko[50] |
| 20dyn/cm2, 30min | Increased proliferation, ALP activity, phosphorylation of ERK and expression of integrin-β1, which may involve ERK, NO synthase, Cox, and PTX pathway; NF-κB translocation was essential for shear induced Cox-2 increase, which | 2003 | Kapur [39] |
| 12dyn/cm2, up to 2h | was dependent on [Ca2+]i release; | 2003 | Chen [51] |
| 10dyn/cm2, 90min | The induction of Cox-2 and PGE release did not require intact microfilaments or microtubules; | 2004 | Norvell [42] |
| 10dyn/cm2, 10min | Plasma membrane cholesterol was essential for mechanotransduction; | 2004 | Ferraro [52] |
| 10dyn/cm2, up to 5h | Fibronectin-induced formation of focal adhesions promoted FSS-induced PGE2 release and upregulation of Cox-2 protein; | 2004 | Ponik [35] |
| 1-63×10-5dyn/cm2, up to 48h | Increased proliferation, ALP, and fibronectin via stimulation of PEG2 and TGF-β1; | 2004 | Liegibel [53] |
| 10dyn/cm2, 1h | Modulated the activity of GSK-3β and β-catenin, which regulated Cox-2 expression; | 2004 | Norvell [54] |
| 12dyn/cm2, up to15min | Induced ATP release was Ca2+ dependent and mediated PGE2 release via P2 receptor activation [43]; | 2005 | Genetos |
| 10 or 20dyn/cm2, 2h | Increased cell stiffness; | 2007 | Jaasma [55] |
| pulsatile cycles (0.8-10dyn/cm2, 0.5Hz, 60s) and laminal flow (14min, 0.8dyn/cm2, 8 or 12h) | Increased collagen I and MMP-1, 3 gene expression depended on the integrity of microtubule but not on actin filaments; | 2007 | Myers [56] |
| 12dyn/cm2, up to 60min | The induced ERK1/2 phosphorylation requires Ca2+-dependent ATP release, which was mediated through P2Y7, not P2Y2 [44]; | 2008 | Liu D |
| 12dyn/cm2, up to 24h | Integrin-β1 played predominant roles for shear-induced signaling and gene expression. Integrin-β1/Shc association led to the activation of ERK; | 2008 | Lee[57] |
| 4, 8, 16dynes/cm2, 30min | Increased PGE2 was mediated by Cx43-hemichannel that adapted to mechanical loading; | 2008 | Siller-Jackson [58] |
| 20dyn/cm2, 2h | Increased vimentin and cross-linking proteins (α-actinin and filamin); | 2008 | Jackson [36] |
| 12dyn/cm2, up to 20min stepwise increasing/decreasing (5-10-15, or 15-10-5 dyn/cm2), 6 or 12 h | Type II cGMP-dependent protein kinase mediates mechanotransduction; RANKL/OPG ratio decreased with stepwise increasing stimulation and increased with decreasing shear. ALP and calcium deposition increased unless the step change was increased to 10dyn./cm2. | 2009 | Rangaswami [59] The present study |
|
| |||
| Pulsatile flow | |||
| 0-15dyn/cm2(average5), rectified half sine wave, up to 24h | ALP mRNA decreased compared with static control and steady flow of 4 dyn/cm2, but no change in mRNA of collagen and osteopontin; | 1996 | Hillsley [41] |
| 6±5dyn/cm2, 3Hz, up to 48h, (0.6, 6)±5dyn/cm2, 0.3Hz, 7days | PEG2 increased shortly, 7 days' increased proliferation, ALP but not mineralization compared with static control; | 2001 | Nauman [32] |
| 12dyn/cm2, 1Hz, square wave, 15min | Proliferation increased more than the same level of steady flow (a smooth 30s ramped increase from 0 to 12 dyn/cm2, sustained steady shear for 7 min, and a smooth 30s ramped decrease 30s) via ERK1/2 and Rb protein; | 2002 | Jiang [61] |
| 3.9 dyn/cm2 (3 Hz) or 6.4 dyn/cm2 (5 Hz), 10min | Increased alignment of stress fibers. The increased NO release was inhibited by disrupting actin while the increased PGE2 was inhibited by disrupting either the actin or microtubule [60]; | 2005 | McGarry JG |
| 10dyn/cm2, 5Hz, 30min | Cbfa1/Runx2 was necessary for maximal induction of Cox-2; | 2006 | Mehrotra [62] |
| 10dyn/cm2, 5Hz, 1h | Sustained induction of RANKL expression after stopping FSS was dependent on PKA and ERK signaling pathways; | 2006 | Mehrotra [37] |
| 5dyn/cm2, 1Hz, 5h | Upregulated vascular endothelial growth factor gene expression; | 2007 | Thi [63] |
|
| |||
| Oscillatory flow | |||
| 20dyn/cm2, 0.5 or 1.0 or 2.0Hz, 4min | Increased [Ca2+]i and the response decreased with the increase of frequency. The [Ca2+]i response decreases in the order of pulsatile (0-2 Pa), steady (2 Pa) and oscillatory flow (+/-2Pa). | 1998 | Jacobs [16] |
| 20dyn/cm2, 1Hz, up to 2h | [Ca2+]i released and OPN gene expression increased via ERK1/2 and p38 activation; | 2001 | You [64] |
| 1.9, 4.7, 9.3dyn/cm2, 1Hz, 1- 2h | Inhibited TNF-α induced NF-κ B activation via an IKAPPA B kinase; | 2001 | Kurokouchi [65] |
| 20dyn/cm2, 2Hz, up to 15min | Refractory periods between the [Ca2+]i oscillation. Multiple low-magnitude oscillations of [Ca2+]I existed during continuous flow. | 2003 | Donahue [66] |
| Up to 20dyn/cm2, 1Hz, up to 1h | Sufficient nutrient supply or waste removal is needed for the response to oscillating fluid flow induced shear stress; | 2003 | Donahue[67] |
| 10 or 20dyn/cm2, 1Hz, up to 1h | Insertion of short-term rest periods resulted in multiple[Ca2+]i responses, increases in [Ca2+]i magnitudes and overall responding cells and OPN mRNA, but not PGE2; | 2005 | Batra [18] |
| 10dyn/cm2, 1Hz, 1h | Decreased ratio of RANKL/OPG; | 2006 | Kim [29] |
| 11dyn/cm2, 0.5Hz, 1, 5, 24h | Delayed in stress fiber formation and alignment, but similar temporal effects on induction of Cox-2 and OPN protein expression compared with unidirectional flow (8 dyn/cm2); | 2007 | Ponik [40] |
| 10-12dyn/cm2, 0.5Hz, up to 4h | FAK was important for FSS-induced mechanotransduction; | 2009 | Young [68] |
| 20dyn/cm2, 1Hz, sine wave, 2h | Gap junctions are involved in the mechanosignaling process. | 2009 | Jekir [69] |
To test our hypothesis that osteoblasts can respond differentially to the stepwise increasing/decreasing shear stress over a relative long period of time, we applied “step-up” and “step-down” FSS train tests on osteoblasts. In contrast to most in vitro studies where the cells were subjected to relatively steady flow conditions (such as the constant FSS magnitude in the unidirectional FSS studies, or constant peak-to-peak FSS amplitudes in pulsatile or oscillatory studies that are summarized and referenced in Table 1), we stepwise increased the magnitude of the FSS from 5, 10 to 15 dyn/cm2 or from 10, 20 to 30 dyn/cm2 or stepwise decreased the flow stimulation over a total period of 6 or 12 h. Anabolic responses have been demonstrated for the unidirectional flow at such stress levels for 2 to 4 h in our preliminary study (data not shown) and previous studies [31, 34-36]. The results from the step-up and step-down experiments are quite intriguing: the osteoblastic RANKL/OPG expression ratio, an index of the potential of osteoblasts to support osteoclastogenesis, was differentially regulated. We found that increasing FSS in three steps of 5 dyn/cm2 over a time period of 6 or 12 h resulted in decreased osteoclastogenesis potentials, which was compatible with a previous study, where 30 min, 1 h and 2 h of 1 Hz oscillatory flow with a peak FSS of 10 dyn/cm2 decreased the ratio of RANKL/OPG relative to static control [29]. Interestingly, decreasing FSS at the same rate (15-10-5 dyn/cm2) showed increased RANKL/OPG ratio and thus enhanced osteoclastogenesis even with the presence of loading (≥ 5 dyn/cm2) over 6 or 12 h. Although there was a previous report on increasing RANKL at the stopping of flow stimulation [37], our result was the first cellular observation to demonstrate that osteoblasts respond differentially to stepwise increasing FSS versus decreasing FSS. This may offer a potential explanation of the in vivo observation that declining physical activity is associated with decreased bone mass [38].
On the other hands, the osteoblastic ALP activity and calcium deposition (markers of osteogenesis) were not as sensitive to the stepwise varying FSS as the RANKL/OPG ratio (Figs. 2 and 3). Both the increasing and decreasing FSS with three steps of 5 dyn/cm2 enhanced ALP activity and calcium deposition, consistent with previous studies with uniform shear stresses [39]. However, when we increased and decreased FSS at a higher step (10 dyn/cm2), we observed declining ALP activity and calcium deposition with decreasing FSS stimulation and an opposite effects caused by the increasing FSS stimulation. The results suggested that the anabolic responses of osteoblasts can be modulated by the gradients of FSS, but to a less degree compared with the osteoblastic RANKL/OPG response. Bacabac et al found that a gradually increased shear stress, which eliminated the initial stress-kick and decreased the shear stress step, strongly decreased the NO response of bone cells [25]. That study suggested that osteoblasts were sensitive to the rate of applied FSS, which was consistent with our results of RANKL/OPG, which increased with stepwise decreasing FSS and decreased with stepwise increasing FSS. However, we did not observe a consistent relationship between osteoblasts' ALP activity and calcium deposition with the gradients of FSS: at a lower step of 5 dyn/cm2, ALP and calcium deposition were upregulated regardless whether the stepwise increasing or decreasing FSS was applied; at a higher step of 10 dyn/cm2, these activities were sensitive to the gradient of the applied FSS. The differences between the two studies may due to the different duration of the FSS stimulation (hours vs. minutes) and different outcome measures (ALP and calcium vs. NO). In an in vivo study [70], adult rat ulna was subjected to cyclic end compression at 2 Hz, 360 cycles per day, 5 days per week, for 15 weeks. The peak load was applied in one of the three regimens: i) stepwise decreasing (13.5-11.3-9.0N); ii) stepwise increasing (9.0-11.3-13.5N); or iii) constant peak 11.3N. The periosteal bone formation rate was found to be in the order of group i) > group iii) > group ii). In contrast, our study showed that decreasing FSS (30-20-10dyn/cm2) for 12 hr resulted in decreased ALP and calcium deposition. The reason for the discrepancy between the two studies is not clear. One should note the differences in the two studies such as the testing system (in vivo vs. in vitro) and testing period (15 week vs. 12 h). The strain induced by the peak load of 13.5N was not reported in the paper. If the strain was high enough to induce microcracks in the stepwise decreasing loading group, an injury response might have been initiated, which may have overwhelmed the regular bone remodeling responses in the following steps.
The mechanisms responsible for the osteoblastic responses under stepwise varying FSS are not clear and require further studies. It is generally accepted that bone cells respond to the onset of loading (FSS) by cytoskeletal reorganization and the resulting self-stiffening is the adaptive strategy that the cells to regain equilibrium in the presence of mechanical force (Table 1 and references therein). The formation of stress fibers appears to be a fast cellular response (within 15 min after onset of the loading) that is essential for downstream changes in gene and protein expression. Whether the cytoskeletal organization was involved in the differential responses to the stepwise increasing or decreasing stimulation need to be examined in future studies. The other question concerned the exact signal that triggered the cells' differential responses. Although we believe that the global FSS gradient over the entire stimulation period (6 or 12 h) account for the observed differential RANKL/OPG expression to the increasing and decreasing FSS, whether the ramp rate associated with the step-up or step-down (5 or 10 dym/cm2 change over 2 to 3 s) contributed to the differential responses was not clear. Due to the limitation of the system, we applied stepwise increasing and decreasing FSS, resulting in discontinuity in the FSS gradient profile. A gradual modulation of the FSS magnitude will be needed to address these questions in future investigations.
Additional limitations were present in this study. Osteocytes are believed to be the major mechanosensors and transducers in bone [11]. However, primary osteoblasts were used in current study because i) osteoblasts are precursors of osteocytes and both are sensitive to FSS, ii) we like to use primary cells that are closer to in vivo conditions, and iii) primary osteoblasts are easier to obtain than primary osteocytes. The findings from primary osteoblasts have to be confirmed in osteocytes in future studies. Another limitation was that we utilized unidirectional flow pattern. Future studies should utilize oscillatory fluid flow, which is more physiological. Previous studies showed that osteoblasts responded differently to unidirectional and oscillatory shear stress (Table 1 and references therein). The stress fiber formation was more prominent and faster under unidirectional steady fluid flow compared to oscillatory fluid [16, 40, 41]. However, both flow patterns induced similar cyclooxygenase-2 and osteopontin protein expression [40, 42]. Pulsatile FSS induced the least amount of alkaline phosphatase compared to unidirectional FSS and stationary control [41]. The fraction of cells displaying intracellular calcium responses and the amplitude of the calcium waves were shown to decrease in the order of pulsatile, unidirectional steady, and oscillatory FSS [16]. We expect similar differential responses to the stepwise increasing and decreasing FSS with oscillatory flow pattern; further examination is needed to confirm our speculation. One more limitation was the relative short loading period. Although we adopted loading periods up to 12 hours to mimic the in vivo conditions with slowly increasing or decreasing physical activities, this loading period was still quite short (hours in the cellular stimulation versus days or months for the gradual change of in vivo loading). The flow period was limited due to technical constrains such as potential contaminations as well as concerns of over-confluence of the cells during long-term studies. Lastly, the RANKL/OPG expression was detected at the mRNA gene level, not the protein level. In the future work, we plan to shear the cells for 24 hours or longer, or leave the cells in static medium for additional times, which may allow detect the changes in protein levels.
In summary, we have tested the osteoblastic responses (RANKL, OPG, ALP and calcium deposition) under a novel stepwise increasing/decreasing FSS stimulation in this preliminary investigation. We found that primary osteoblasts were able to detect the gradient of FSS and respond to the stimulations differentially. In specific, our results showed that, with the step of 5 dyn/cm2, the stepwise increasing FSS inhibited the osteoclastogenesis with a 3- to 4-fold decrease in RANKL/OPG gene expression versus static controls, while the stepwise decreasing FSS increased RANKL/OPG ratio by 2- to 2.5-fold versus static controls. Both increasing and decreasing FSS promoted alkaline phosphatase expression and calcium deposition by 1.0- to 1.8 fold versus static controls. For a higher FSS temporal gradient (the step of 10 dyn/cm2 over 12 hour stimulation), the increasing FSS enhanced the expression of alkaline phosphatase expression and calcium deposition by 1.3 fold, while the decreasing FSS slightly inhibited them by -10% compared with static controls. Taken together, our results suggested that with an adequate temporal gradient, the stepwise increasing FSS increases the bone formation and decreases bone resorption, while the stepwise decreasing FSS results in opposite effects. These findings may explain the in vivo bone mineral density changes in response to the gradual increase/decrease in the strength of exercise. The stepwise FSS can be a useful model to study bone cell responses to long-term mechanical usage or disuse. These studies will complement the short-term studies and provide additional clinically relevant insights on bone adaptation.
Acknowledgments
This study was supported by grants from NSF of China (10972243), STC of Chongqing, China (2007BB5167), Project 111 of China (B0602), and NIH of the USA (R01AR054385).
References
- 1.Suva LJ, Gaddy D, Perrien DS, Thomas RL, Findlay DM. Regulation of bone mass by mechanical loading: microarchitecture and genetics. Curr Osteoporos Rep. 2005;3:46–51. doi: 10.1007/s11914-005-0003-0. [DOI] [PubMed] [Google Scholar]
- 2.Turner CH, Pavalko FM. Mechanotransduction and functional response of the skeleton to physical stress: the mechanisms and mechanics of bone adaptation. J Orthop Sci. 1998;3:346–55. doi: 10.1007/s007760050064. [DOI] [PubMed] [Google Scholar]
- 3.Judex S, Zernicke RF. High-impact exercise and growing bone: relation between high strain rates and enhanced bone formation. J Appl Physiol. 2000;88:2183–91. doi: 10.1152/jappl.2000.88.6.2183. [DOI] [PubMed] [Google Scholar]
- 4.Mosley JR. Osteoporosis and bone functional adaptation: mechanobiological regulation of bone architecture in growing and adult bone, a review. J Rehabil Res Dev. 2000;37:189–99. [PubMed] [Google Scholar]
- 5.Skerry TM. The response of bone to mechanical loading and disuse: fundamental principles and influences on osteoblast/osteocyte homeostasis. Arch Biochem Biophys. 2008;473:117–23. doi: 10.1016/j.abb.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 6.Heinonen A, Oja P, Kannus P, Sievanen H, Haapasalo H, Manttari A, Vuori I. Bone mineral density in female athletes representing sports with different loading characteristics of the skeleton. Bone. 1995;17:197–203. doi: 10.1016/8756-3282(95)00151-3. [DOI] [PubMed] [Google Scholar]
- 7.Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res. 2004;19:1006–12. doi: 10.1359/JBMR.040307. [DOI] [PubMed] [Google Scholar]
- 8.Tervo T, Nordstrom P, Neovius M, Nordstrom A. Reduced physical activity corresponds with greater bone loss at the trabecular than the cortical bone sites in men. Bone. 2009 doi: 10.1016/j.bone.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Reich KM, Gay CV, Frangos JA. Fluid shear stress as a mediator of osteoblast cyclic adenosine monophosphate production. J Cell Physiol. 1990;143:100–4. doi: 10.1002/jcp.1041430113. [DOI] [PubMed] [Google Scholar]
- 10.Burger EH, Klein-Nulend J, Veldhuijzen JP. Modulation of osteogenesis in fetal bone rudiments by mechanical stress in vitro. J Biomech. 1991;24(Suppl 1):101–9. doi: 10.1016/0021-9290(91)90381-v. [DOI] [PubMed] [Google Scholar]
- 11.Weinbaum S, Cowin SC, Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech. 1994;27:339–60. doi: 10.1016/0021-9290(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 12.Hung CT, Pollack SR, Reilly TM, Brighton CT. Real-time calcium response of cultured bone cells to fluid flow. Clin Orthop Relat Res. 1995:256–69. [PubMed] [Google Scholar]
- 13.Klein-Nulend J, Semeins CM, Ajubi NE, Nijweide PJ, Burger EH. Pulsating fluid flow increases nitric oxide (NO) synthesis by osteocytes but not periosteal fibroblasts--correlation with prostaglandin upregulation. Biochem Biophys Res Commun. 1995;217:640–8. doi: 10.1006/bbrc.1995.2822. [DOI] [PubMed] [Google Scholar]
- 14.Smalt R, Mitchell FT, Howard RL, Chambers TJ. Induction of NO and prostaglandin E2 in osteoblasts by wall-shear stress but not mechanical strain. Am J Physiol. 1997;273:E751–8. doi: 10.1152/ajpendo.1997.273.4.E751. [DOI] [PubMed] [Google Scholar]
- 15.Pavalko FM, Chen NX, Turner CH, Burr DB, Atkinson S, Hsieh YF, Qiu J, Duncan RL. Fluid shear-induced mechanical signaling in MC3T3-E1 osteoblasts requires cytoskeleton-integrin interactions. Am J Physiol. 1998;275:C1591–601. [PubMed] [Google Scholar]
- 16.Jacobs CR, Yellowley CE, Davis BR, Zhou Z, Cimbala JM, Donahue HJ. Differential effect of steady versus oscillating flow on bone cells. J Biomech. 1998;31:969–76. doi: 10.1016/s0021-9290(98)00114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srinivasan S, Weimer DA, Agans SC, Bain SD, Gross TS. Low-magnitude mechanical loading becomes osteogenic when rest is inserted between each load cycle. J Bone Miner Res. 2002;17:1613–20. doi: 10.1359/jbmr.2002.17.9.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Batra NN, Li YJ, Yellowley CE, You L, Malone AM, Kim CH, Jacobs CR. Effects of short-term recovery periods on fluid-induced signaling in osteoblastic cells. J Biomech. 2005;38:1909–17. doi: 10.1016/j.jbiomech.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Robling AG, Hinant FM, Burr DB, Turner CH. Shorter, more frequent mechanical loading sessions enhance bone mass. Med Sci Sports Exerc. 2002;34:196–202. doi: 10.1097/00005768-200202000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Robling AG, Hinant FM, Burr DB, Turner CH. Improved bone structure and strength after long-term mechanical loading is greatest if loading is separated into short bouts. J Bone Miner Res. 2002;17:1545–54. doi: 10.1359/jbmr.2002.17.8.1545. [DOI] [PubMed] [Google Scholar]
- 21.Saxon LK, Robling AG, Alam I, Turner CH. Mechanosensitivity of the rat skeleton decreases after a long period of loading, but is improved with time off. Bone. 2005;36:454–64. doi: 10.1016/j.bone.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Chen NX, Ryder KD, Pavalko FM, Turner CH, Burr DB, Qiu J, Duncan RL. Ca(2+) regulates fluid shear-induced cytoskeletal reorganization and gene expression in osteoblasts. Am J Physiol Cell Physiol. 2000;278:C989–97. doi: 10.1152/ajpcell.2000.278.5.C989. [DOI] [PubMed] [Google Scholar]
- 23.Klein-Nulend J, Helfrich MH, Sterck JG, MacPherson H, Joldersma M, Ralston SH, Semeins CM, Burger EH. Nitric oxide response to shear stress by human bone cell cultures is endothelial nitric oxide synthase dependent. Biochem Biophys Res Commun. 1998;250:108–14. doi: 10.1006/bbrc.1998.9270. [DOI] [PubMed] [Google Scholar]
- 24.Kreke MR, Huckle WR, Goldstein AS. Fluid flow stimulates expression of osteopontin and bone sialoprotein by bone marrow stromal cells in a temporally dependent manner. Bone. 2005;36:1047–55. doi: 10.1016/j.bone.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Bacabac RG, Smit TH, Mullender MG, Van Loon JJ, Klein-Nulend J. Initial stress-kick is required for fluid shear stress-induced rate dependent activation of bone cells. Ann Biomed Eng. 2005;33:104–10. doi: 10.1007/s10439-005-8968-5. [DOI] [PubMed] [Google Scholar]
- 26.Donahue SW, Jacobs CR, Donahue HJ. Flow-induced calcium oscillations in rat osteoblasts are age, loading frequency, and shear stress dependent. Am J Physiol Cell Physiol. 2001;281:C1635–41. doi: 10.1152/ajpcell.2001.281.5.C1635. [DOI] [PubMed] [Google Scholar]
- 27.Ecarot-Charrier B, Glorieux FH, van der Rest M, Pereira G. Osteoblasts isolated from mouse calvaria initiate matrix mineralization in culture. J Cell Biol. 1983;96:639–43. doi: 10.1083/jcb.96.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–23. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 29.Kim CH, You L, Yellowley CE, Jacobs CR. Oscillatory fluid flow-induced shear stress decreases osteoclastogenesis through RANKL and OPG signaling. Bone. 2006;39:1043–7. doi: 10.1016/j.bone.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 30.Christenson RH. Biochemical markers of bone metabolism: an overview. Clin Biochem. 1997;30:573–93. doi: 10.1016/s0009-9120(97)00113-6. [DOI] [PubMed] [Google Scholar]
- 31.Reich KM, Frangos JA. Effect of flow on prostaglandin E2 and inositol trisphosphate levels in osteoblasts. Am J Physiol. 1991;261:C428–32. doi: 10.1152/ajpcell.1991.261.3.C428. [DOI] [PubMed] [Google Scholar]
- 32.Nauman EA, Satcher RL, Keaveny TM, Halloran BP, Bikle DD. Osteoblasts respond to pulsatile fluid flow with short-term increases in PGE(2) but no change in mineralization. J Appl Physiol. 2001;90:1849–54. doi: 10.1152/jappl.2001.90.5.1849. [DOI] [PubMed] [Google Scholar]
- 33.Frost HM. Bone “mass” and the “mechanostat”: a proposal. Anat Rec. 1987;219:1–9. doi: 10.1002/ar.1092190104. [DOI] [PubMed] [Google Scholar]
- 34.Thi MM, Kojima T, Cowin SC, Weinbaum S, Spray DC. Fluid shear stress remodels expression and function of junctional proteins in cultured bone cells. Am J Physiol Cell Physiol. 2003;284:C389–403. doi: 10.1152/ajpcell.00052.2002. [DOI] [PubMed] [Google Scholar]
- 35.Ponik SM, Pavalko FM. Formation of focal adhesions on fibronectin promotes fluid shear stress induction of COX-2 and PGE2 release in MC3T3-E1 osteoblasts. J Appl Physiol. 2004;97:135–42. doi: 10.1152/japplphysiol.01260.2003. [DOI] [PubMed] [Google Scholar]
- 36.Jackson WM, Jaasma MJ, Tang RY, Keaveny TM. Mechanical loading by fluid shear is sufficient to alter the cytoskeletal composition of osteoblastic cells. Am J Physiol Cell Physiol. 2008;295:C1007–15. doi: 10.1152/ajpcell.00509.2007. [DOI] [PubMed] [Google Scholar]
- 37.Mehrotra M, Saegusa M, Wadhwa S, Voznesensky O, Peterson D, Pilbeam C. Fluid flow induces Rankl expression in primary murine calvarial osteoblasts. J Cell Biochem. 2006;98:1271–83. doi: 10.1002/jcb.20864. [DOI] [PubMed] [Google Scholar]
- 38.Vuori I, Heinonen A, Sievanen H, Kannus P, Pasanen M, Oja P. Effects of unilateral strength training and detraining on bone mineral density and content in young women: a study of mechanical loading and deloading on human bones. Calcif Tissue Int. 1994;55:59–67. doi: 10.1007/BF00310170. [DOI] [PubMed] [Google Scholar]
- 39.Kapur S, Baylink DJ, Lau KH. Fluid flow shear stress stimulates human osteoblast proliferation and differentiation through multiple interacting and competing signal transduction pathways. Bone. 2003;32:241–51. doi: 10.1016/s8756-3282(02)00979-1. [DOI] [PubMed] [Google Scholar]
- 40.Ponik SM, Triplett JW, Pavalko FM. Osteoblasts and osteocytes respond differently to oscillatory and unidirectional fluid flow profiles. J Cell Biochem. 2007;100:794–807. doi: 10.1002/jcb.21089. [DOI] [PubMed] [Google Scholar]
- 41.Hillsley MV, Frangos JA. Alkaline phosphatase in osteoblasts is down-regulated by pulsatile fluid flow. Calcif Tissue Int. 1997;60:48–53. doi: 10.1007/s002239900185. [DOI] [PubMed] [Google Scholar]
- 42.Norvell SM, Ponik SM, Bowen DK, Gerard R, Pavalko FM. Fluid shear stress induction of COX-2 protein and prostaglandin release in cultured MC3T3-E1 osteoblasts does not require intact microfilaments or microtubules. J Appl Physiol. 2004;96:957–66. doi: 10.1152/japplphysiol.00869.2003. [DOI] [PubMed] [Google Scholar]
- 43.Genetos DC, Geist DJ, Liu D, Donahue HJ, Duncan RL. Fluid shear-induced ATP secretion mediates prostaglandin release in MC3T3-E1 osteoblasts. J Bone Miner Res. 2005;20:41–9. doi: 10.1359/JBMR.041009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu D, Genetos DC, Shao Y, Geist DJ, Li J, Ke HZ, Turner CH, Duncan RL. Activation of extracellular-signal regulated kinase (ERK1/2) by fluid shear is Ca(2+)- and ATP-dependent in MC3T3-E1 osteoblasts. Bone. 2008;42:644–52. doi: 10.1016/j.bone.2007.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson DL, McAllister TN, Frangos JA. Fluid flow stimulates rapid and continuous release of nitric oxide in osteoblasts. Am J Physiol. 1996;271:E205–8. doi: 10.1152/ajpendo.1996.271.1.E205. [DOI] [PubMed] [Google Scholar]
- 46.Sakai K, Mohtai M, Iwamoto Y. Fluid shear stress increases transforming growth factor beta 1 expression in human osteoblast-like cells: modulation by cation channel blockades. Calcif Tissue Int. 1998;63:515–20. doi: 10.1007/s002239900567. [DOI] [PubMed] [Google Scholar]
- 47.McAllister TN, Frangos JA. Steady and transient fluid shear stress stimulate NO release in osteoblasts through distinct biochemical pathways. J Bone Miner Res. 1999;14:930–6. doi: 10.1359/jbmr.1999.14.6.930. [DOI] [PubMed] [Google Scholar]
- 48.Sakai K, Mohtai M, Shida J, Harimaya K, Benvenuti S, Brandi ML, Kukita T, Iwamoto Y. Fluid shear stress increases interleukin-11 expression in human osteoblast-like cells: its role in osteoclast induction. J Bone Miner Res. 1999;14:2089–98. doi: 10.1359/jbmr.1999.14.12.2089. [DOI] [PubMed] [Google Scholar]
- 49.Wadhwa S, Choudhary S, Voznesensky M, Epstein M, Raisz L, Pilbeam C. Fluid flow induces COX-2 expression in MC3T3-E1 osteoblasts via a PKA signaling pathway. Biochem Biophys Res Commun. 2002;297:46–51. doi: 10.1016/s0006-291x(02)02124-1. [DOI] [PubMed] [Google Scholar]
- 50.Pavalko FM, Gerard RL, Ponik SM, Gallagher PJ, Jin Y, Norvell SM. Fluid shear stress inhibits TNF-alpha-induced apoptosis in osteoblasts: a role for fluid shear stress-induced activation of PI3-kinase and inhibition of caspase-3. J Cell Physiol. 2003;194:194–205. doi: 10.1002/jcp.10221. [DOI] [PubMed] [Google Scholar]
- 51.Chen NX, Geist DJ, Genetos DC, Pavalko FM, Duncan RL. Fluid shear-induced NFkappaB translocation in osteoblasts is mediated by intracellular calcium release. Bone. 2003;33:399–410. doi: 10.1016/s8756-3282(03)00159-5. [DOI] [PubMed] [Google Scholar]
- 52.Ferraro JT, Daneshmand M, Bizios R, Rizzo V. Depletion of plasma membrane cholesterol dampens hydrostatic pressure and shear stress-induced mechanotransduction pathways in osteoblast cultures. Am J Physiol Cell Physiol. 2004;286:C831–9. doi: 10.1152/ajpcell.00224.2003. [DOI] [PubMed] [Google Scholar]
- 53.Liegibel UM, Sommer U, Bundschuh B, Schweizer B, Hilscher U, Lieder A, Nawroth P, Kasperk C. Fluid shear of low magnitude increases growth and expression of TGFbeta1 and adhesion molecules in human bone cells in vitro. Exp Clin Endocrinol Diabetes. 2004;112:356–63. doi: 10.1055/s-2004-821014. [DOI] [PubMed] [Google Scholar]
- 54.Norvell SM, Alvarez M, Bidwell JP, Pavalko FM. Fluid shear stress induces beta-catenin signaling in osteoblasts. Calcif Tissue Int. 2004;75:396–404. doi: 10.1007/s00223-004-0213-y. [DOI] [PubMed] [Google Scholar]
- 55.Jaasma MJ, Jackson WM, Tang RY, Keaveny TM. Adaptation of cellular mechanical behavior to mechanical loading for osteoblastic cells. J Biomech. 2007;40:1938–45. doi: 10.1016/j.jbiomech.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 56.Myers KA, Rattner JB, Shrive NG, Hart DA. Osteoblast-like cells and fluid flow: cytoskeleton-dependent shear sensitivity. Biochem Biophys Res Commun. 2007;364:214–9. doi: 10.1016/j.bbrc.2007.09.109. [DOI] [PubMed] [Google Scholar]
- 57.Lee DY, Yeh CR, Chang SF, Lee PL, Chien S, Cheng CK, Chiu JJ. Integrin-mediated expression of bone formation-related genes in osteoblast-like cells in response to fluid shear stress: roles of extracellular matrix, Shc, and mitogen-activated protein kinase. J Bone Miner Res. 2008;23:1140–9. doi: 10.1359/jbmr.080302. [DOI] [PubMed] [Google Scholar]
- 58.Siller-Jackson AJ, Burra S, Gu S, Xia X, Bonewald LF, Sprague E, Jiang JX. Adaptation of connexin 43-hemichannel prostaglandin release to mechanical loading. J Biol Chem. 2008;283:26374–82. doi: 10.1074/jbc.M803136200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rangaswami H, Marathe N, Zhuang S, Chen Y, Yeh JC, Frangos JA, Boss GR, Pilz RB. Type II cGMP-dependent protein kinase mediates osteoblast mechanotransduction. J Biol Chem. 2009;284:14796–808. doi: 10.1074/jbc.M806486200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McGarry JG, Klein-Nulend J, Prendergast PJ. The effect of cytoskeletal disruption on pulsatile fluid flow-induced nitric oxide and prostaglandin E2 release in osteocytes and osteoblasts. Biochem Biophys Res Commun. 2005;330:341–8. doi: 10.1016/j.bbrc.2005.02.175. [DOI] [PubMed] [Google Scholar]
- 61.Jiang GL, White CR, Stevens HY, Frangos JA. Temporal gradients in shear stimulate osteoblastic proliferation via ERK1/2 and retinoblastoma protein. Am J Physiol Endocrinol Metab. 2002;283:E383–9. doi: 10.1152/ajpendo.00547.2001. [DOI] [PubMed] [Google Scholar]
- 62.Mehrotra M, Saegusa M, Voznesensky O, Pilbeam C. Role of Cbfa1/Runx2 in the fluid shear stress induction of COX-2 in osteoblasts. Biochem Biophys Res Commun. 2006;341:1225–30. doi: 10.1016/j.bbrc.2006.01.084. [DOI] [PubMed] [Google Scholar]
- 63.Thi MM, Iacobas DA, Iacobas S, Spray DC. Fluid shear stress upregulates vascular endothelial growth factor gene expression in osteoblasts. Ann N Y Acad Sci. 2007;1117:73–81. doi: 10.1196/annals.1402.020. [DOI] [PubMed] [Google Scholar]
- 64.You J, Reilly GC, Zhen X, Yellowley CE, Chen Q, Donahue HJ, Jacobs CR. Osteopontin gene regulation by oscillatory fluid flow via intracellular calcium mobilization and activation of mitogen-activated protein kinase in MC3T3-E1 osteoblasts. J Biol Chem. 2001;276:13365–71. doi: 10.1074/jbc.M009846200. [DOI] [PubMed] [Google Scholar]
- 65.Kurokouchi K, Jacobs CR, Donahue HJ. Oscillating fluid flow inhibits TNF-alpha -induced NF-kappa B activation via an Ikappa B kinase pathway in osteoblast-like UMR106 cells. J Biol Chem. 2001;276:13499–504. doi: 10.1074/jbc.M003795200. [DOI] [PubMed] [Google Scholar]
- 66.Donahue SW, Donahue HJ, Jacobs CR. Osteoblastic cells have refractory periods for fluid-flow-induced intracellular calcium oscillations for short bouts of flow and display multiple low-magnitude oscillations during long-term flow. J Biomech. 2003;36:35–43. doi: 10.1016/s0021-9290(02)00318-4. [DOI] [PubMed] [Google Scholar]
- 67.Donahue TL, Haut TR, Yellowley CE, Donahue HJ, Jacobs CR. Mechanosensitivity of bone cells to oscillating fluid flow induced shear stress may be modulated by chemotransport. J Biomech. 2003;36:1363–71. doi: 10.1016/s0021-9290(03)00118-0. [DOI] [PubMed] [Google Scholar]
- 68.Young SR, Gerard-O'Riley R, Kim JB, Pavalko FM. Focal adhesion kinase is important for fluid shear stress-induced mechanotransduction in osteoblasts. J Bone Miner Res. 2009;24:411–24. doi: 10.1359/JBMR.081102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jekir MG, Donahue HJ. Gap junctions and osteoblast-like cell gene expression in response to fluid flow. J Biomech Eng. 2009;131:011005. doi: 10.1115/1.3005201. [DOI] [PubMed] [Google Scholar]
- 70.Schriefer JL, Warden SJ, Saxon LK, Robling AG, Turner CH. Cellular accommodation and the response of bone to mechanical loading. J Biomech. 2005;38:1838–45. doi: 10.1016/j.jbiomech.2004.08.017. [DOI] [PubMed] [Google Scholar]