Abstract
Old arguments that free O2 must have been available at Earth's surface prior to the origin of photosynthesis have been revived by a new study that shows that aerobic respiration can occur at dissolved oxygen concentrations much lower than had previously been thought, perhaps as low as 0.05 nM, which corresponds to a partial pressure for O2 of about 4 × 10−8 bar. We used numerical models to study whether such O2 concentrations might have been provided by atmospheric photochemistry. Results show that disproportionation of H2O2 near the surface might have yielded enough O2 to satisfy this constraint. Alternatively, poleward transport of O2 from the equatorial stratosphere into the polar night region, followed by downward transport in the polar vortex, may have brought O2 directly to the surface. Thus, our calculations indicate that this “early respiration” hypothesis might be physically reasonable. Key Words: Early Earth—Oxygen—Respiration—Tracer transport—General circulation. Astrobiology 11, 293–302.
1. Introduction
Over the past 40 years, both biologists (Schwartz and Dayhoff, 1978; Castresana et al., 1994; Pereira et al., 2001; Ducluzeau et al., 2008; Brochier-Armanet et al., 2009) and paleontologists (Cloud, 1972; Schopf, 1975; Towe, 1978, 1988, 1990, 1996) have argued that free O2 must have been available, at least in small quantities, prior to the origin of oxygenic photosynthesis. These arguments have been considered highly speculative because free O2 is generally considered to have been absent near Earth's surface prior to the origin of oxygenic photosynthesis (Walker, 1977; Kasting, 1993). Indeed, significant amounts of free O2 did not appear in the atmosphere until about 2.4 Ga (Holland, 1994, 2006; Farquhar et al., 2000). Before this major rise of atmospheric oxygen, even photosynthetically produced O2 would have existed only locally within surface water and in short-lived plumes of gas that escaped into the otherwise anoxic atmosphere (Kasting, 1992; Pavlov et al., 2001).
The arguments that some free oxygen must have existed before photosynthesis are almost all biologically based. Cloud (1972) pointed out that early O2 producers would have needed chemical electron acceptors to protect themselves against the O2 they created. He suggested that ferrous iron in the early oceans could have provided such a sink. However, the O2 would have been produced internally within cells, whereas the ferrous iron was external, so it is not obvious that this solution would have worked. Schopf (1975) recognized this and suggested that early photosynthesizers must have had at least rudimentary mechanisms for coping with oxygen. Towe (1978, 1988, 1996) elaborated on this idea by examining the evolutionary history of specific enzymes for O2 protection, notably the superoxide dismutases, and arguing that they must have evolved at a very early stage.
Molecular phylogenists have added to the strength of these arguments over the years. Based on an early composite protein/rRNA evolutionary tree, Schwartz and Dayhoff (1978) reasoned that aerobic respiration must have preceded oxygenic photosynthesis because of its wide distribution among different organisms. Their analysis included genes for different cytochrome oxidases, which are key enzymes in aerobic metabolism. More recent studies (Castresana et al., 1994; Pereira et al., 2001; Ducluzeau et al., 2008; Brochier-Armanet et al., 2009) have examined the history of cytochrome oxidases in increasing detail. The current consensus is that at least some members of this enzyme family had a monophyletic origin that preceded the origin of oxygenic photosynthesis. Nitric oxide reduction, that is, oxidation of organic matter using NO (the key step in denitrification), may also have been an early evolutionary invention (Castresana and Saraste, 1995). Whether aerobic respiration or nitric oxide reduction came first, and whether one of these metabolisms evolved from the other, remains unresolved (Hendriks et al., 2000; Pereira et al., 2001; Ducluzeau et al., 2008).
Now, a new study (Stolper et al., 2010) has shown that some modern aerobes can respire at extremely low O2 levels—much lower than previously thought. The prevailing wisdom has been that organisms switch from aerobic respiration to anaerobic forms of metabolism (primarily fermentation) below the Pasteur point of ∼0.01 times the present atmospheric level of O2. The solubility of O2 in pure water at 25°C is 1.3 × 10−3 mol/L/bar (Sander, 2009), so this corresponds to a dissolved O2 concentration of 2.7 μM. (O2 solubility in seawater is about 20% lower.) By contrast, the new study shows that the common bacterium E. coli can grow at dissolved O2 concentrations of 3 nM or lower, or about 1000 times lower than previously believed. The new work was made possible by the development of O2-sensing electrodes that are accurate down to 3–5 nM concentrations. (Note of caution: The observed lower limit for respiration in these studies is also the lower limit of accuracy for the electrodes. This suggests that additional confirmation of these results is needed.) A theoretical extrapolation to smaller organisms suggests that a primitive aerobe, only 0.5 μm in diameter, might be able to respire at dissolved O2 concentrations of 0.05 nM (Stolper et al., 2010). In terms of equivalent atmospheric partial pressure, this lower limit is equivalent to pO2 = 4 × 10−8 bar, or about 10−7 times the present atmospheric level. These limits are summarized in Table 1, where they are compared to calculated O2 and H2O2 sources that might have been available prior to the origin of photosynthesis. The calculations are described below.
Table 1.
Measured and Calculated Limits on Respiration and O2/H2O2 Availability
| Limit | [O2] nM* | pO2 (bar) |
|---|---|---|
| Observed lower growth limit | 3 | 2 × 10−6 |
| Extrapolated lower growth limit† | 0.05 | 4 × 10−8 |
| Average surface O2 (2-bar CO2 model) | 8 × 10−6 | 6 × 10−12 |
| Transient surface O2 (equinox model) | 7 × 10−4 | 5 × 10−10 |
| Transient surface O2 (winter solstice model) | 3 × 10−3 | 2 × 10−9 |
| Maximum transient surface O2 | 4 | 3 × 10−6 |
| Limit | [H2O2] nM | pH2O2 (bar) |
|---|---|---|
| Average surface H2O2 (2-bar CO2 model) | 0.16 | 2 × 10−15 |
Henry's Law constants at 25°C:
O2: 1.3 × 10−3 mol/L/bar
H2O2: 8.2 × 104 mol/L/bar
1 nM = 10−9 mol/L.
For 0.5 μm diameter cells (Stolper et al., 2010).
Although we ourselves have previously argued that free O2 was scarce prior to the origin of oxygenic photosynthesis, the new work by Stolper et al. (2010) has prompted us to reexamine our assumptions. For example, photochemical models do indeed predict that some free O2, along with photochemically generated oxidants such as hydrogen peroxide (H2O2) and sulfate (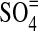 ), should have been generated in small quantities (see, e.g., Kasting et al., 1984; McKay and Hartman, 1991; Liang et al., 2006; Segura et al., 2007). And much larger concentrations of free O2, up to 0.1% mixing ratio, should have existed at high altitudes, where it was produced by CO2 photolysis (Kasting et al., 1984; Liang et al., 2006; Segura et al., 2007). The question then is could any of this high-altitude O2 have made its way down to the surface before it was photochemically destroyed? Our modeling work addresses this question.
), should have been generated in small quantities (see, e.g., Kasting et al., 1984; McKay and Hartman, 1991; Liang et al., 2006; Segura et al., 2007). And much larger concentrations of free O2, up to 0.1% mixing ratio, should have existed at high altitudes, where it was produced by CO2 photolysis (Kasting et al., 1984; Liang et al., 2006; Segura et al., 2007). The question then is could any of this high-altitude O2 have made its way down to the surface before it was photochemically destroyed? Our modeling work addresses this question.
2. Photochemical Production of O2 and H2O2
Oxygen should have been produced abiotically in the sunlit portions of the early stratosphere. Following the atmospheric photochemistry explored by Kasting et al. (1979), Kasting and Walker (1981), and Kasting et al. (1984), the production of O2 in the stratosphere begins with the photolysis of CO2, which is by far the most abundant oxygen-bearing species in these model atmospheres:
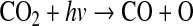 |
(1) |
Here, ν is frequency of the photon and h is Planck's constant. The products of Reaction 1 then combine to form O2:
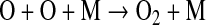 |
(2) |
where M is a third molecule, required to carry off the excess energy of the collision. O2 can also be produced in the troposphere by Reaction 1, along with
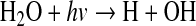 |
(3) |
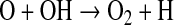 |
(4) |
Little of this O2 makes its way down to the surface, though, because it is destroyed almost immediately by reaction sequences such as
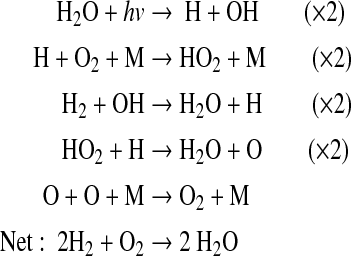 |
(5) |
along with
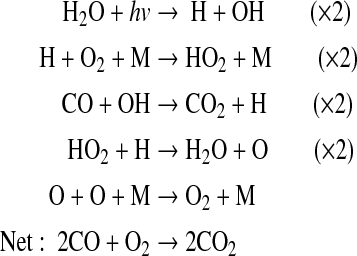 |
(6) |
The formyl radical, HCO, also contributes to O2 destruction by the reactions
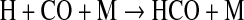 |
(7) |
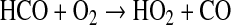 |
(8) |
Once converted to HO2, O2 can readily react with other species by reactions such as those shown in sequences (5) and (6). The net effect of this tropospheric photochemistry is to recombine O2 with H2 and CO through reactions that are catalyzed by the by-products of H2O photolysis.
Other reduced gases, CH4 for example, may also have served as O2 sinks on early Earth. CH4 should have become abundant once methanogens evolved (Walker, 1977; Kharecha et al., 2005). But CH4 would have been produced at the expense of H2 by the reaction
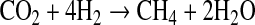 |
(9) |
Alternatively, CH4 could have been produced by fermentation of organic matter, followed by production of methane from by-products such as acetate. But much of this organic matter itself would presumably have been generated from H2-based anoxygenic photosynthesis (Kharecha et al., 2005). Thus, for our purposes here, it is sufficient to think of H2 as a surrogate for all such reduced gases.
Because of catalytic cycles such as (5) and (6), the O2 content of the early atmosphere would have depended inversely on the H2 and CO concentrations. The H2 mixing ratio of the early atmosphere should have been determined by the balance between volcanic outgassing and escape of hydrogen to space (Walker, 1977; Kasting and Catling, 2003) and is expected to have been of the order of 100–1000 ppmv for H2 outgassing rates of (0.7–7) × 1012 mol/yr. These outgassing rates bracket the range considered by Holland (2002) and Canfield et al. (2006).
2.1. Model scenarios
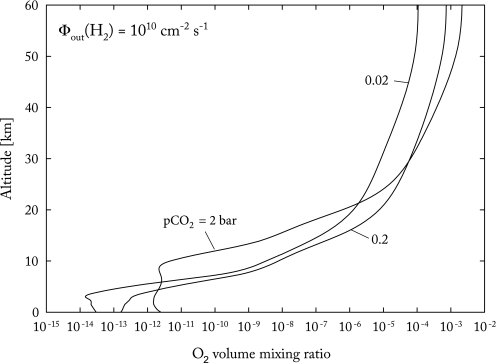
Vertical profiles of O2 in the early atmosphere have been calculated by a number of different authors (e.g., Kasting et al., 1979, 1984; Pavlov et al., 2001; Segura et al., 2007). We used the model of Segura et al. (2007) to calculate O2 mixing ratio profiles for three different atmospheric CO2 partial pressures: 0.02, 0.2, and 2 bar (Fig. 1). These choices of pCO2 are meant to span the range of plausible CO2 concentrations during the Late Hadean/Early Archean, ∼4.0–3.0 Ga. At the two lower CO2 concentrations, the atmospheric surface pressure was taken to be 1 bar, and the surface temperature was set equal to 278 K. For the 0.2-bar atmosphere, that surface temperature would be appropriate for 4.0 Ga and 75 percent of current solar luminosity (Kasting, 1990). The 0.02-bar CO2 atmosphere would have needed help from other greenhouse gases to reach this surface temperature during this time period. For the 2-bar CO2 calculation, the surface pressure was taken to be 2.8 bar, and the surface temperature was set at 317 K, which is again consistent with conditions at 4.0 Ga (Kasting, 1990). No new one-dimensional climate simulations were performed for this study. The eddy diffusion profile in the latter case was changed to be self-consistent with this warmer, thicker troposphere by increasing the vertical range over which high (105 cm2 s−1) eddy coefficients applied (Kasting, 1990).
FIG. 1.
Vertical profiles of O2 volume mixing ratio at CO2 partial pressures of 0.02, 0.2, and 2.0 bar. These profiles are calculated with a one-dimensional photochemical model with an H2 outgassing rate Φout(H2) = 1010 cm−2 s−1.
The hydrogen outgassing rate in each model was assumed to be 1 × 1010 H2 molecules cm−2 s−1, or about 2.7 × 1012 mol/yr. This is close to the H2 outgassing rate (2.4 × 1012 mol/yr) estimated by Holland (2009). This produced atmospheric H2 mixing ratios close to 300 ppmv in all three cases. Some sensitivity studies, described below, were also performed with an outgassing rate that was 10 times smaller. SO2 outgassing was fixed at 3.5 × 108 cm−2 s−1, or ∼9.4 × 1010 mol/yr, following Ono et al. (2003). CO outgassing (which is smaller than H2 outgassing) was neglected. CH4 was held to negligible values. NO was assumed to have been produced by lightning at a rate of 5.85 × 108 cm−2 s−1, or ∼1.6 × 1011 mol/yr for the 0.2-bar CO2 case. O2 production was 2.5 times higher. All lightning production rates were scaled to a value of 2.7 × 1011 mol NO/yr for modern Earth, which is slightly higher than the value of 2.1 × 1011 mol/yr estimated by Martin et al. (2002).
2.2. Predicted O2 concentrations
Figure 1 shows that the concentration of O2 in the model atmosphere increases with pCO2, as expected. The O2 mixing ratio in the stratosphere, near 50 km, reaches a maximum of about 2 × 10−3 in the high-CO2 case. This, of course, would be more than enough to sustain aerobic respiration were the same O2 concentration to exist at the surface. The O2 mixing ratio at the surface, however, is 9 orders of magnitude lower, about 2 × 10−12, because it is being consumed by Reactions 5–9 above. Indeed, the little O2 that exists near the surface is sustained by lightning in this model, which breaks down CO2 according to
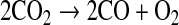 |
(10) |
But this surface O2 concentration is still far too low to sustain respiration, even in the most optimistic case defined by Stolper et al. (2010). Given that the total surface pressure is 2.8 bar in this model, pO2 near the surface is ∼6 × 10−12 bar. This is still almost 4 orders of magnitude lower than the limit for respiration (Table 1).
2.3. Production of H2O2
A second photochemically produced oxidant that has received previous attention (Kasting et al., 1984; McKay and Hartman, 1991; Liang et al., 2006) is hydrogen peroxide, H2O2. H2O2 is produced from the by-products of H2O photolysis,
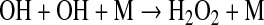 |
(11) |
which can yield O2 by the disproportionation reaction
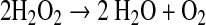 |
(12) |
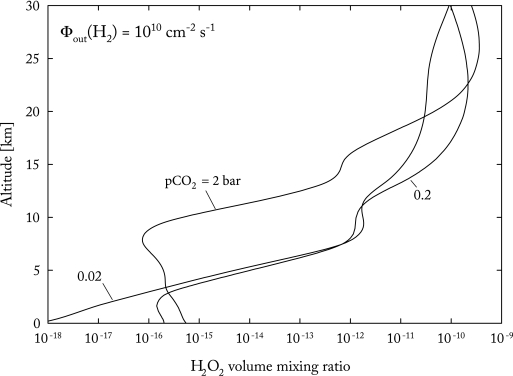
This type of reaction is slow in the gas phase but could be catalyzed in solution by contact with mineral surfaces. Concentrations of H2O2 in the troposphere are also very low for the three cases studied (Fig. 2); however, the solubility of H2O2 is much higher than that of O2, about 8.2 × 104 mol/L/bar at 25°C (Sander, 2009). For the 2-bar CO2 case, the predicted partial pressure of H2O2 at the surface is about 2 × 10−15 bar, so the dissolved H2O2 concentration is 1.6 × 10−10 mol/L. A similar ground-level H2O2 partial pressure is obtained for the 0.2-bar CO2 case if the H2 outgassing rate is divided by 10 (which remains within the plausible range of outgassing rates). Dividing by 2 to account for the stoichiometry of Reaction 13 gives an equivalent dissolved O2 concentration of 0.08 nM, just above the lower limit for respiration extrapolated by Stolper et al. (2010) for small cells. So, if their calculated threshold is correct, and if H2O2 was converted to O2 and taken up by organisms before it could react with something else, then this is a possible mechanism for fueling aerobic respiration prior to the invention of oxygenic photosynthesis.
FIG. 2.
Vertical profiles of H2O2 volume mixing ratio for the three model atmospheres shown in Fig. 1.
We should note that McKay and Hartman (1991) put forward a hypothesis similar to the one presented here, that is, they argued that the presence of photochemically produced H2O2 allowed early organisms to develop defenses against oxygen toxicity. They used numbers from a photochemical model study by Kasting et al. (1985). The quoted flux of H2O2 to the surface was ∼1010 cm−2 s−1, and the maximum dissolved H2O2 concentration in surface water, as calculated from a simple box model, was 5 × 10−8 mol/L. That value is about 300 times higher than the one estimated here. But the Kasting et al. (1985) model was designed to simulate relatively oxygenated, Proterozoic atmospheres, not the anoxic Archean atmospheres considered here. Our calculated H2O2 surface flux (mostly from H2O2 dissolved in rainwater) in the present 0.2-bar CO2 model is of the order of 106 cm−2 s−1, which is about 104 times less than estimated by McKay and Hartman. With our more modest numbers, H2O2 appears to be just marginally capable of fueling early respiration.
3. Transport of O2 by the Brewer-Dobson Circulation
An alternative way of getting O2 to the surface would be to transport it downwards from the stratosphere in the polar night region where it was protected against photochemical destruction. The calculations shown in Figs. 1 and 2 cannot account for this mechanism because our one-dimensional photochemical model assumes global average conditions, with the sun always shining. O2 is destroyed within seconds or minutes when it reaches the troposphere in the presence of ultraviolet (UV) photons. In the real, modern atmosphere, ozone (O3) is produced photochemically in the stratosphere and is transported poleward and downward by the Brewer-Dobson circulation (Brewer, 1949; Holton et al., 1995; Callaghan and Salby, 2002; Austin and Li, 2006). This suggests a mechanism by which photochemically produced O2 could have been transported to the surface of early Earth.
Exchange of air between the stratosphere and troposphere then occurs through a variety of processes, most of which are dominant in regions outside the tropics. In the absence of physical features (such as topography), some mass exchange occurs by diffusion, while some occurs by a mechanism known as “tropopause folding” (Reed, 1955; Danielsen, 1968; Shapiro, 1980; Wei, 1987; Lamarque and Hess, 1994). Although the time-mean tropopause is approximately symmetric about the equator, tropopause structure in regions with large vertical shear and strong meridional temperature gradients can fold and thereby transport stratospheric air downward (Holton et al., 1995). Such events commonly occur in conjunction with cyclogenesis (i.e., the development of low-pressure circulation systems in the atmosphere). Part of this transport is also described by the influence of breaking waves above the tropopause according to the downward control principle (Haynes et al., 1991). Topographic features also play an important role in troposphere-stratosphere exchange by generating planetary-scale waves that propagate into the stratosphere and then break to induce the mixing of air (McIntyre and Palmer, 1983; Randel et al., 1993). Finally, the stratosphere in the winter hemisphere cools because of the strong equator-to-pole temperature gradient, which intensifies the Brewer-Dobson circulation and causes the formation of a polar vortex. Air that flows from the stratosphere through the polar vortex can mix with air in the troposphere either directly (Bowman, 1993; Mizuta and Yoden, 2001) or from the interaction of planetary waves with the vortex (Waugh, 1993).
3.1. Three-dimensional (3-D) tracer model
We investigated the downward transport of stratospheric oxygen by modeling O2 as a passive atmospheric tracer in an idealized atmospheric general circulation model (GCM). Our GCM was described by Frierson et al. (2006) and Haqq-Misra (2010) and includes a spectral dynamical core (with T42 resolution), gray radiative transfer, latent heat release, and a diffusive boundary layer scheme. This provides a way to estimate the amount of stratospheric O2 that could reach the surface without the need for an elaborate coupled climate-chemistry model, which would be a far more demanding computational task. Here, we conceptually account for the photochemical loss of O2 in the troposphere; however, our model contains no actual atmospheric chemistry and should be considered only as a proof of concept for downward oxygen transport on early Earth. We note that surface oxygen does not need to be well mixed because early respiring organisms may have thrived in oxygen oases that only covered a fraction of the surface.
We represent O2 as a passive tracer that follows the flow determined in our model by solving an advection-diffusion equation. We release this passive tracer instantaneously into the stratosphere of a GCM configuration that has already reached a statistically steady state. By doing so, we simulate photochemical production of O2 at a single moment in time so that we can observe the amount of tracer that reaches the surface.
Our tracer ξ = ξ(t,p,φ,λ) is a function of time t, pressure p, latitude φ, and longitude λ. It is given an initial distribution centered on the equator with the form
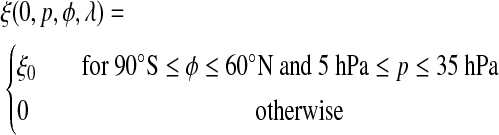 |
(13) |
In terms of altitude, the tracer release area corresponds to a range of about 21–28 km in the modern atmosphere. We set the unitless tracer concentration to a constant value ξ0 in this region. Because we specify no other sources or sinks of ξ, we do not include reaction rates for O2 production and destruction. However, a low-oxygen early Earth atmosphere would be susceptible to O2 loss from water vapor photolysis in the troposphere, so any stratospherically produced O2 that is transported to the surface will therefore have a lifetime limited by its exposure to sunlight. The longest residence time of such an O2 molecule would occur in the polar night, where some regions reside in perpetual darkness for up to 6 months. UV photons would be absent or scarce during this season and allow O2 to accumulate. In the summertime, though, we assume that O2 could not accumulate in the troposphere because it would be destroyed in the presence of direct sunlight. Thus, we only model the concentration of ξ over a period of 180 days when loss of O2 would be minimal. Shielding by organic haze could conceivably extend the region where downwelling O2 might survive in the troposphere (Pavlov et al., 2001; Wolf and Toon, 2010); however, examining this possibility would require a more elaborate model.
Transport of stratospheric tracer to the surface was explored under eight different climate conditions in order to examine the various processes that contribute to stratosphere-troposphere mass exchange. [See Haqq-Misra (2010) for detailed descriptions of these eight climate states.] The first two states consider idealized dry and moist circulations (which we refer to as dry 3-D and moist 3-D) that approximate the structure of the observed atmosphere. In these cases, exchange of tracer between the stratosphere and troposphere occurs through diffusion across the tropopause as well as tropopause folding events generated by baroclinically unstable waves. We next add realistic topographic data to dry 3-D and moist 3-D to generate planetary waves that can propagate into the stratosphere and strengthen the Brewer-Dobson circulation to increase tracer transport. (Realistic mountains and land-water masks are generated by interpolating from a one-sixth degree U.S. Navy mean topography and percent water data set. These data are limited to changes in surface geopotential height only and do not include soil temperature, moisture, or other surface properties.) Transport in the winter hemisphere is also enhanced by a stronger Brewer-Dobson circulation and accompanies the formation of a polar night vortex, so we add a seasonal cycle to the model to calculate dry and moist 3-D states with seasons. Finally, we combine these mechanisms to calculate dry 3-D and moist 3-D states that include both topography and seasons in order to maximize the downward transport of tracer.
3.2. Tracer model results
Initially, we performed calculations for models that lacked a seasonal cycle. The tracer ξ was released into the stratosphere at some initial time, t0, and its subsequent evolution was followed for 180 model days. At this point, we calculated the fraction of tracer, ξ/ξ0, that had reached the surface. Our calculations show that tracer transport in the dry 3-D case is greater than in moist 3-D because poleward transport by the stratospheric Brewer-Dobson circulation is stronger in the absence of moisture. (In general, meridional circulations are weaker in moist atmospheres because the condensation of water vapor in ascending air causes a decrease in the lapse rate—the vertical rate of cooling—so that air parcels rise more slowly.) This produces a maximum polar tracer abundance of ξ/ξ0 ≈ 3 × 10−6 for dry 3-D and ξ/ξ0 ≈ 2 × 10−6 for moist 3-D. When we include realistic topography, planetary waves are generated that propagate into the stratosphere and provide a modest increase in surface tracer. Here, we find that our dry and moist simulations with topography give us maximum polar tracer abundances of ξ/ξ0 ≈ 9 × 10−6 and ξ/ξ0 ≈ 5 × 10−6, respectively.
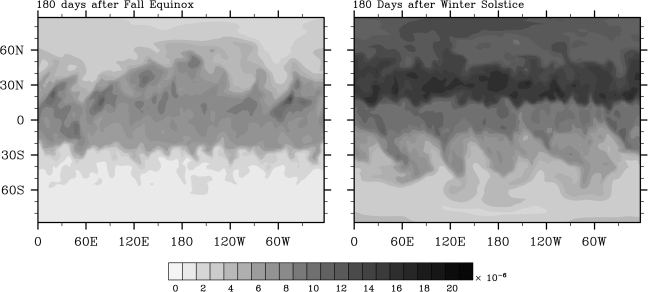
The more interesting, and more realistic, cases are those in which a seasonal cycle is included. The seasonal cycle enhances downward transport by inciting a polar vortex in the winter hemisphere and also provides a region of darkness in which downwelling stratospheric O2 might survive. The most efficient downward transport occurs when both topography and seasons are included, because in this case topographic planetary waves are generated in the winter hemisphere where surface westerly winds are strong. Results from two initial simulations with a dry model atmosphere are shown in Fig. 3. In the left-hand panel, the simulation begins at northern autumnal equinox, while the right-hand panel shows a simulation that began at northern winter solstice. The two panels show relative tracer abundance ξ/ξ0 at the surface after 180 days of evolution. In both cases, the tracer tends to accumulate toward the equator, but our winter solstice case shows an increase of about a factor of 10 in surface tracer abundance at the northern pole.
FIG. 3.
Fraction of tracer ξ/ξ0 at the surface 180 days after northern autumnal equinox (left panel) and northern winter solstice (right panel). Both simulations assume dry conditions and include topography.
We first consider the results for the equinox simulation (left-hand panel). This simulation may be the most applicable to our O2 transport problem, because the North Pole would remain shaded throughout the calculation. In this case, the maximum tracer abundance in the northern polar region is about ξ/ξ0 ≈ 5 × 10−6. To calculate a surface O2 concentration, this number must be multiplied by the O2 mixing ratio at the height at which the tracer was released, roughly 20–30 km. According to Fig. 1, the O2 mixing ratio at these altitudes is about 10−4 for both the 0.2-bar and 2-bar CO2 simulations. Hence, the calculated ground-level O2 partial pressure is about 5 × 10−10 bar. (This number should correspond to the 0.2-bar CO2 case, as the dynamical transport calculation is not valid for atmospheres with surface pressures greater than 1 bar.) This pO2 value is about 1000 times greater than the average surface pO2 calculated for the 0.2-bar CO2 case (Fig. 1), but it remains a factor of 100 lower than the lowest limit for respiration estimated by Stolper et al. (2010; see Table 1). So we see that this particular simulation does not succeed in explaining the early appearance of respiration.
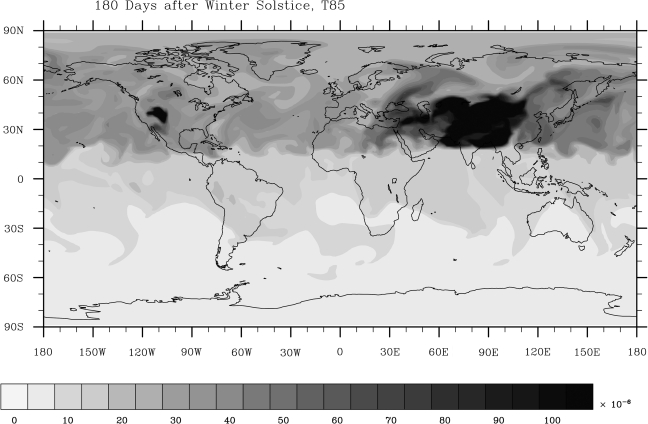
These results may be overly pessimistic, however. The simulation that begins at winter solstice predicts high-latitude tracer levels that are about 4 times higher, ξ/ξ0 ≈ 2 × 10−5 (Fig. 3, right-hand panel). Furthermore, tracer transport in our model can be shown to depend on spatial (spectral) resolution. When we doubled the spectral resolution of our winter solstice model (to T85), the relative tracer abundance increased locally to as much as 3 × 10−4 (Fig. 4). The largest accumulations occurred near large mountain ranges such as the Himalayan and Rocky mountains. The reason for this accumulation can be understood in terms of the mechanism of Taylor columns (e.g., Hogg, 1973; Huppert, 1975). Much of this tracer occupies a region over land, where it could not dissolve into seawater, but some of it extends into the Bay of Bengal, the Arabian Sea, and perhaps even the Great Salt Lake. Of course, we do not know what topographic features were present on early Earth, but it appears that large mountain ranges can cause surface tracer to accumulate to concentrations from 10 to 50 times greater than the average hemispheric abundance. If these mountain ranges existed in the polar regions where O2 could have been protected from sunlight, then lakes or seas within these regions might have been exposed to relatively high surface O2 concentrations.
FIG. 4.
Fraction of tracer ξ/ξ0 at the surface 180 days after northern winter solstice under dry conditions with doubled spectral resolution.
Abundances of O2 in the stratosphere may also be underestimated in the above calculations. The O2 mixing ratio continues to increase above 30 km in all our photochemical simulations, reaching values as high as 2 × 10−3 in the 2-bar CO2 case (Fig. 1). Stratospheric O2 could have been enhanced by another factor of 3 or more by the increased UV flux from the young Sun (Segura et al., 2007). (The calculations shown here assume present-day solar UV fluxes.) If our tracer model had extended higher into the stratosphere, some of this higher-O2 air may have been brought down to the surface as well. An approximate upper limit on surface O2 can be estimated by multiplying the maximum surface tracer abundance from our transport model, 3 × 10−4, by the O2 concentration at 50 km in the 2-bar CO2, high-UV case from Segura et al. (2007), ∼10−2. This yields a surface pO2 value of 3 × 10−6 bar and a corresponding dissolved O2 concentration of 4 nM. The latter value exceeds the observed threshold for aerobic growth in E. coli (Stolper et al., 2010). Although this upper limit is admittedly optimistic, it demonstrates that this mechanism might also have allowed aerobic organisms to evolve in an otherwise anoxic world.
Further discussion should be added to the above calculations because of the uncertain conditions of early Earth. In particular, planetary formation models predict that younger planets rotate faster, and it has been suggested that early Earth rotated with a day as short as 14 to 18 hours (e.g., Walker and Zahnle, 1986; Williams, 2000). Because the vertical penetration depth of the overturning circulation is proportional to rotation rate (Hoskins et al., 1985), a faster rotator will be more effective at downward transport. As a result, downward transport may be somewhat enhanced on a rapidly rotating planet. Additionally, deviations in the axial tilt of Earth would change the latitudinal distribution of sunlight so that the wintertime Brewer-Dobson circulation strengthens as obliquity increases and weakens as obliquity decreases. The continental configuration of early Earth is also unknown, which means that topographically generated planetary waves could have been more active or less active in the past, depending on the size and position of the continents. For all three of these parameters, we have chosen present-day Earth values because of the uncertainty in the conditions of early Earth. Our model calculations can therefore be understood as a demonstration of an atmospheric transport mechanism that can be scaled appropriately as we continue to learn about the early history of Earth.
4. Discussion
All the “successful” simulations for delivery of either O2 or H2O2 to Earth's surface require high-CO2 early atmospheres. This is because high CO2 concentrations lead to greater production of O2 in the stratosphere from photolysis (Reactions 1 and 2). Additionally, high CO2 creates a warmer atmosphere by way of its greenhouse effect, which increases the concentrations of H2O and its photolysis by-product, OH, that is instrumental in forming H2O2 (Reaction 11).
Recently published estimates of paleo-CO2 concentration, however, suggest that it may have been very low. Rosing et al. (2010) estimated an upper limit of 1 × 10−3 bar, or 3 times the present atmospheric level, based on an analysis of banded iron formations. According to these authors, CO2 partial pressures higher than this value would have caused all the magnetite to be quantitatively converted to siderite, and this of course is not observed. If Rosing et al. are correct, then CO2 concentrations must have been low during the entire time between 1.8 and 3.8 Ga when banded iron formations are known to have formed. However, the Rosing et al. (2010) paper is controversial, and several critical comments (not yet published) have been submitted regarding their conclusions. We hypothesize that conversion of magnetite to siderite was limited by the supply of organic matter, not by atmospheric or dissolved CO2. Support for this hypothesis is provided by the presence of isotopically light carbon in the siderite, which indicates that organic matter was involved in its formation (Becker and Clayton, 1972; Heimann et al., 2010). So, this particular argument for low CO2 does not appear to be valid.
Other published estimates for pCO2 in the Archean are also much lower than we require here. Rye et al. (1995) estimated that pCO2 was less than 0.03 bar at 2.8 Ga, based on an analysis of paleosols. Their estimate, however, has been criticized by Sheldon (2006). Sheldon argued that the thermodynamic data they used were out of date and that if their analysis were repeated it would predict pCO2 values lower than today, which is not at all consistent with the need for higher greenhouse warming in the past (Kasting, 1987; von Paris et al., 2008). Sheldon's own analysis of paleosols also led to low values, ∼0.008 bar, but this estimate applies to the time around 2.2 Ga. Furthermore, his analysis assumed that every CO2 molecule that enters the soil reacts with silicate minerals. If this is not true, then atmospheric CO2 partial pressures could have been much higher than he calculated. Thus, there is nothing to preclude high CO2 concentrations during the early to mid-Archean, which is presumably when respiration first evolved.
Theoreticians have made different predictions for CO2 partial pressures on early Earth, with Walker (1985) favoring values as high as 10 bar prior to the origin of the continents and Sleep and Zahnle (2001) favoring values not much higher than today. We favor pCO2 values ≥0.2 bar prior to the origin of methanogenesis, as this is approximately the amount of CO2 that was needed to keep the surface from freezing in the absence of additional greenhouse warming from CH4 (Kasting, 1987; Haqq-Misra et al., 2008). So, we consider the CO2 partial pressures assumed here to be plausible for the time period we are considering.
5. Conclusions
Trace amounts of oxidants, including free O2 and H2O2, could have been supplied to organisms living on Earth's surface prior to the origin of oxygenic photosynthesis. If the threshold for aerobic respiration (0.05 nM dissolved O2) estimated by Stolper et al. (2010) is correct, then these trace oxidant levels might have permitted the early evolution of respiration. Production of both O2 and H2O2 is enhanced in warm, dense atmospheres that are rich in CO2.
Although the availability of H2O2 at the surface is reasonably well estimated by the models employed here, the problem of stratospheric O2 delivery deserves more detailed treatment. A fully coupled chemistry-climate GCM would allow for a more robust treatment of this problem by providing accurate temporal and spatial variations in O2 concentration and by allowing the inclusion of phenomena such as the presence of UV-screening organic haze.
Acknowledgments
We are grateful to Steven Feldstein for numerous discussions and insights regarding atmospheric dynamics and troposphere-stratosphere exchange. We also thank two anonymous reviewers for several suggestions that helped to strengthen this paper.
Author Disclosure Statement
No competing financial interests exist.
Abbreviation
GCM, general circulation model.
References
- Austin J. Li F. On the relationship between the strength of the Brewer-Dobson circulation and the age of stratospheric air. Geophys Res Lett. 2006. p. 33. [DOI]
- Becker R.H. Clayton R.N. Carbon isotopic evidence for origin of a banded iron-formation in Western Australia. Geochim Cosmochim Acta. 1972;36:577–595. [Google Scholar]
- Bowman K.P. Large-scale isentropic mixing properties of the Antarctic polar vortex from analyzed winds. J Geophys Res. 1993;98:13–23. [Google Scholar]
- Brewer A.W. Evidence for a world circulation provided by the measurements of helium and water vapour distribution in the stratosphere. Quarterly Journal of the Royal Meteorological Society. 1949;75:351–363. [Google Scholar]
- Brochier-Armanet C. Talla E. Gribaldo S. The multiple evolutionary histories of dioxygen reductases: implications for the origin and evolution of aerobic respiration. Mol Biol Evol. 2009;26:285–297. doi: 10.1093/molbev/msn246. [DOI] [PubMed] [Google Scholar]
- Callaghan P.F. Salby M.L. Three-dimensionality and forcing of the Brewer–Dobson circulation. Journal of the Atmospheric Sciences. 2002;59:976–991. [Google Scholar]
- Canfield D.E. Rosing M.T. Bjerrum C. Early anaerobic metabolisms. Philos Trans R Soc Lond B Biol Sci. 2006;361:1819–1836. doi: 10.1098/rstb.2006.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castresana J. Saraste M. Evolution of energetic metabolism: The respiration-early hypothesis. Trends Biochem Sci. 1995;20:443–448. doi: 10.1016/s0968-0004(00)89098-2. [DOI] [PubMed] [Google Scholar]
- Castresana J. Lübben M. Saraste M. Higgins D.G. Evolution of cytochrome oxidase, an enzyme older than atmospheric oxygen. EMBO J. 1994;13:2516–2525. doi: 10.1002/j.1460-2075.1994.tb06541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloud P.E. A working model of the primitive Earth. Am J Sci. 1972;272:537–548. [Google Scholar]
- Danielsen E.F. Stratospheric-tropospheric exchange based on radioactivity, ozone and potential vorticity. Journal of the Atmospheric Sciences. 1968;25:502–518. [Google Scholar]
- Ducluzeau A.L. Ouchane S. Nitschke W. The cbb3 oxidases are an ancient innovation of the domain Bacteria. Mol Biol Evol. 2008;25:1158–1166. doi: 10.1093/molbev/msn062. [DOI] [PubMed] [Google Scholar]
- Farquhar J. Bao H.M. Thiemens M. Atmospheric influence of Earth's earliest sulfur cycle. Science. 2000;289:756–758. doi: 10.1126/science.289.5480.756. [DOI] [PubMed] [Google Scholar]
- Frierson D.M.W. Held I.M. Zurita-Gotor P. A gray-radiation aquaplanet moist GCM. Part I: static stability and eddy scale. Journal of the Atmospheric Sciences. 2006;63:2548–2566. [Google Scholar]
- Haqq-Misra J. Ph.D. thesis, The Pennsylvania State University, University Park. 2010. A dynamical hierarchy for the general circulation. [Google Scholar]
- Haqq-Misra J.D. Domagal-Goldman S.D. Kasting P.J. Kasting J.F. A revised, hazy methane greenhouse for the Archean Earth. Astrobiology. 2008;8:1127–1137. doi: 10.1089/ast.2007.0197. [DOI] [PubMed] [Google Scholar]
- Haynes P.H. McIntyre M.E. Shepherd T.G. Marks C.J. Shine K.P. On the “downward control” of extratropical diabatic circulations by eddy-induced mean zonal forces. Journal of the Atmospheric Sciences. 1991;48:651–679. [Google Scholar]
- Heimann A. Johnson C.M. Beard B.L. Valley J.W. Roden E.E. Spicuzza M.J. Beukes N.J. Fe, C, and O isotope compositions of banded iron formation carbonates demonstrate a major role for dissimilatory iron reduction in ∼2.5 Ga marine environments. Earth Planet Sci Lett. 2010;294:8–18. [Google Scholar]
- Hendriks J. Oubrie A. Castresana J. Urbani A. Gemeinhardt S. Saraste M. Nitric oxide reductases in bacteria. Biochim Biophys Acta. 2000;1459:266–273. doi: 10.1016/s0005-2728(00)00161-4. [DOI] [PubMed] [Google Scholar]
- Hogg N.G. On the stratified Taylor column. J Fluid Mech. 1973;58:517–537. [Google Scholar]
- Holland H.D. Early Proterozoic atmospheric change. In: Bengtson S., editor. Early Life on Earth. Columbia University Press; New York: 1994. pp. 237–244. [Google Scholar]
- Holland H.D. Volcanic gases, black smokers, and the Great Oxidation Event. Geochim Cosmochim Acta. 2002;66:3811–3826. [Google Scholar]
- Holland H.D. The oxygenation of the atmosphere and oceans. Philos Trans R Soc Lond B Biol Sci. 2006;361:903–915. doi: 10.1098/rstb.2006.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland H.D. Why the atmosphere became oxygenated: a proposal. Geochim Cosmochim Acta. 2009;73:5241–5255. [Google Scholar]
- Holton J.R. Haynes P.H. McIntyre M.E. Douglass A.R. Rood R.B. Pfister L. Stratosphere-troposphere exchange. Rev Geophys. 1995;33:403–439. [Google Scholar]
- Hoskins B.J. McIntyre M.E. Robertson A.W. On the use and significance of isentropic potential vorticity maps. Quarterly Journal of the Royal Meteorological Society. 1985;111:877–946. [Google Scholar]
- Huppert H.E. Some remarks on the initiation of inertial Taylor columns. J Fluid Mech. 1975;67:397–412. [Google Scholar]
- Kasting J.F. Theoretical constraints on oxygen and carbon dioxide concentrations in the Precambrian atmosphere. Precambrian Res. 1987;34:205–229. doi: 10.1016/0301-9268(87)90001-5. [DOI] [PubMed] [Google Scholar]
- Kasting J.F. Bolide impacts and the oxidation state of carbon in the Earth's early atmosphere. Orig Life Evol Biosph. 1990;20:199–231. doi: 10.1007/BF01808105. [DOI] [PubMed] [Google Scholar]
- Kasting J.F. Proterozoic climates: the effect of changing atmospheric carbon dioxide concentrations. In: Schopf J.W., editor; Klein C., editor. The Proterozoic Biosphere: A Multidisciplinary Study. Cambridge University Press; Cambridge: 1992. pp. 165–168. [Google Scholar]
- Kasting J.F. Earth's early atmosphere. Science. 1993;259:920–926. doi: 10.1126/science.11536547. [DOI] [PubMed] [Google Scholar]
- Kasting J.F. Catling D. Evolution of a habitable planet. Annu Rev Astron Astrophys. 2003;41:429–463. [Google Scholar]
- Kasting J.F. Walker J.C.G. Limits on oxygen concentration in the prebiological atmosphere and the rate of abiotic fixation of nitrogen. J Geophys Res. 1981;86:1147–1158. [Google Scholar]
- Kasting J.F. Liu S.C. Donahue T.M. Oxygen levels in the prebiological atmosphere. J Geophys Res. 1979;84:3097–3107. [Google Scholar]
- Kasting J.F. Pollack J.B. Crisp D. Effects of high CO2 levels on surface temperature and atmospheric oxidation state of the early Earth. J Atmos Chem. 1984;1:403–428. doi: 10.1007/BF00053803. [DOI] [PubMed] [Google Scholar]
- Kasting J.F. Holland H.D. Pinto J.P. Oxidant abundances in rainwater and the evolution of atmospheric oxygen. J Geophys Res. 1985;90:10497–10510. doi: 10.1029/jd090id06p10497. [DOI] [PubMed] [Google Scholar]
- Kharecha P. Kasting J.F. Siefert J.L. A coupled atmosphere-ecosystem model of the early Archean Earth. Geobiology. 2005;3:53–76. [Google Scholar]
- Lamarque J.-F. Hess P.G. Cross-tropopause mass exchange and potential vorticity budget in a simulated tropopause folding. Journal of the Atmospheric Sciences. 1994;51:2246–2269. [Google Scholar]
- Liang M.C. Hartman H. Kopp R.E. Kirschvink J.L. Yung Y.L. Production of hydrogen peroxide in the atmosphere of a Snowball Earth and the origin of oxygenic photosynthesis. Proc Natl Acad Sci USA. 2006;103:18896–18899. doi: 10.1073/pnas.0608839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R.V. Chance K. Jacob D.J. Kurosu T.P. Spurr R.J.D. Bucsela E. Gleason J.F. Palmer P.I. Bey I. Fiore A.M. An improved retrieval of tropospheric nitrogen dioxide from GOME. J Geophys Res. 2002. p. 107. [DOI]
- McIntyre M.E. Palmer T.N. Breaking planetary waves in the stratosphere. Nature. 1983;305:593–600. [Google Scholar]
- McKay C.P. Hartman H. Hydrogen-peroxide and the evolution of oxygenic photosynthesis. Orig Life Evol Biosph. 1991;21:157–163. doi: 10.1007/BF01809444. [DOI] [PubMed] [Google Scholar]
- Mizuta R. Yoden S. Chaotic mixing and transport barriers in an idealized stratospheric polar vortex. Journal of the Atmospheric Sciences. 2001;58:2616–2629. [Google Scholar]
- Ono S. Eigenbrode J.L. Pavlov A.A. Kharecha P. Rumble D., III Kasting J.F. Freeman K.H. New insights into Archean sulfur cycle from mass-independent sulfur isotope records. Earth Planet Sci Lett. 2003;213:15–30. [Google Scholar]
- Pavlov A.A. Brown L.L. Kasting J.F. UV shielding of NH3 and O2 by organic hazes in the Archean atmosphere. J Geophys Res. 2001;106:23267–23287. [Google Scholar]
- Pereira M.M. Santana M. Teixeira M. A novel scenario for the evolution of haem-copper oxygen reductases. Biochim Biophys Acta. 2001;1505:185–208. doi: 10.1016/s0005-2728(01)00169-4. [DOI] [PubMed] [Google Scholar]
- Randel W.J. Gille J.C. Roche A.E. Kumer J.B. Mergenthaler J.L. Waters J.W. Fishbein E.F. Lahoz W.A. Stratospheric transport from the tropics to middle latitudes by planetary-wave mixing. Nature. 1993;365:533–535. [Google Scholar]
- Reed R.J. A study of a characteristic type of upper-level frontogenesis. Journal of Meteorology. 1955;12:226–237. [Google Scholar]
- Rosing M.T. Bird D.K. Sleep N.H. Bjerrum C.J. No climate paradox under the faint early Sun. Nature. 2010;464:744–747. doi: 10.1038/nature08955. [DOI] [PubMed] [Google Scholar]
- Rye R. Kuo P.H. Holland H.D. Atmospheric carbon dioxide concentrations before 2.2 billion years ago. Nature. 1995;378:603–605. doi: 10.1038/378603a0. [DOI] [PubMed] [Google Scholar]
- Sander R. Henry's Law constants. In: Lindstrom P.J., editor; Mallards W.G., editor. NIST Chemistry WebBook. National Institute of Standards and Technology; Gaithersburg, MD: 2009. NIST Standard Reference Database Number 69. [Google Scholar]
- Schopf J.W. Precambrian paleobiology: problems and perspectives. Annu Rev Earth Planet Sci. 1975;3:213–249. [Google Scholar]
- Schwartz R.M. Dayhoff M.O. Origins of prokaryotes, eukaryotes, mitochondria, and chloroplasts. Science. 1978;199:395–403. doi: 10.1126/science.202030. [DOI] [PubMed] [Google Scholar]
- Segura A. Meadows V. Kasting J. Crisp D. Cohen M. Abiotic formation of O2 and O3 in high-CO2 terrestrial atmospheres. Astron Astrophys. 2007;472:665–680. [Google Scholar]
- Shapiro M.A. Turbulent mixing within tropopause folds as a mechanism for the exchange of chemical constituents between the stratosphere and troposphere. Journal of the Atmospheric Sciences. 1980;37:994–1004. [Google Scholar]
- Sheldon N.D. Precambrian paleosols and atmospheric CO2 levels. Precambrian Res. 2006;147:148–155. [Google Scholar]
- Sleep N.H. Zahnle K. Carbon dioxide cycling and implications for climate on ancient Earth. J Geophys Res. 2001;106:1373–1399. [Google Scholar]
- Stolper D.A. Revsbech N.P. Canfield D.E. Aerobic growth at nanomolar oxygen concentrations. Proc Natl Acad Sci USA. 2010;107:18755–18760. doi: 10.1073/pnas.1013435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towe K.M. Early Precambrian oxygen: a case against photosynthesis. Nature. 1978;274:657–661. [Google Scholar]
- Towe K.M. Biochemical arguments for early O2. In: Broadheads T.W., editor. Molecular Evolution and the Fossil Record. University of Tennesee; Knoxville: 1988. pp. 114–129. [Google Scholar]
- Towe K.M. Aerobic respiration in the Archaean? Nature. 1990;348:54–56. doi: 10.1038/348054a0. [DOI] [PubMed] [Google Scholar]
- Towe K.M. Environmental oxygen conditions during the origin and early evolution of life. Adv Space Res. 1996;18:7–15. [Google Scholar]
- von Paris P. Rauer H. Grenfell J.L. Patzer B. Hedelt P. Stracke B. Trautmann T. Schreier F. Warming the early earth: CO2 reconsidered. Planet Space Sci. 2008;56:1244–1259. [Google Scholar]
- Walker J.C.G. Evolution of the Atmosphere. MacMillan; New York: 1977. [Google Scholar]
- Walker J.C.G. Carbon dioxide on the early Earth. Orig Life Evol Biosph. 1985;16:117–127. doi: 10.1007/BF01809466. [DOI] [PubMed] [Google Scholar]
- Walker J.C.G. Zahnle K.J. Lunar nodal tide and distance to the Moon during the Precambrian. Nature. 1986;320:600–602. doi: 10.1038/320600a0. [DOI] [PubMed] [Google Scholar]
- Waugh D.W. Subtropical stratospheric mixing linked to disturbances in the polar vortices. Nature. 1993;365:535–537. [Google Scholar]
- Wei M.-Y. A new formulation of the exchange of mass and trace constituents between the stratosphere and troposphere. Journal of the Atmospheric Sciences. 1987;44:3079–3086. [Google Scholar]
- Williams G.E. Geological constraints on the Precambrian history of Earth's rotation and the Moon's orbit. Rev Geophys. 2000;38:37–59. [Google Scholar]
- Wolf E.T. Toon O.B. Fractal organic hazes provided an ultraviolet shield for early Earth. Science. 2010;328:1266–1268. doi: 10.1126/science.1183260. [DOI] [PubMed] [Google Scholar]