Abstract
Phocein is a widely expressed, highly conserved intracellular protein of 225 amino acids, the sequence of which has limited homology to the ς subunits from clathrin adaptor complexes and contains an additional stretch bearing a putative SH3-binding domain. This sequence is evolutionarily very conserved (80% identity between Drosophila melanogaster and human). Phocein was discovered by a yeast two-hybrid screen using striatin as a bait. Striatin, SG2NA, and zinedin, the three mammalian members of the striatin family, are multimodular, WD-repeat, and calmodulin-binding proteins. The interaction of phocein with striatin, SG2NA, and zinedin was validated in vitro by coimmunoprecipitation and pull-down experiments. Fractionation of brain and HeLa cells showed that phocein is associated with membranes, as well as present in the cytosol where it behaves as a protein complex. The molecular interaction between SG2NA and phocein was confirmed by their in vivo colocalization, as observed in HeLa cells where antibodies directed against either phocein or SG2NA immunostained the Golgi complex. A 2-min brefeldin A treatment of HeLa cells induced the redistribution of both proteins. Immunocytochemical studies of adult rat brain sections showed that phocein reactivity, present in many types of neurons, is strictly somato-dendritic and extends down to spines, just as do striatin and SG2NA.
INTRODUCTION
Neurons have unique structural and functional polarity: they extend a single, usually long and thin axon and numerous shorter, thicker dendrites (Dotti et al., 1988; Craig and Banker, 1994). Information gathered and processed by the dendrites flow through the axons to the synapses. The structure and composition of axons and dendrites are quite different, particularly concerning their respective plasma membranes, cytoskeleton (Gunning et al., 1998; Baas, 1999), and proteins involved in vesicular traffic, such as motor proteins (Foletti et al., 1999; Burack et al., 2000). Striatin is a neuronal, intracellular protein strictly expressed in the somato-dendritic compartment, including spines, of subsets of neurons: thus, it can be considered as a marker of neuronal polarity (Castets et al., 1996; Kachidian et al., 1998; Salin et al., 1998). Found in the cytosol as well as associated with membranes, striatin is endowed with protein-protein association modules as diverse as a caveolin-binding motif, a coiled-coil structure, a Ca2+-calmodulin–binding amphiphilic helix, and a WD-repeat domain (Castets et al., 1996; Bartoli et al., 1998, 1999b; Moqrich et al., 1998). Striatin down-regulation in embryonic motoneurons leads to the blockade of dendritic, but not axonal, growth, indicating that it may play a role in the establishment of polarity within developing neurons (Bartoli et al., 1999a). The physiological effect elicited by down-regulating this quantitatively minor cellular component suggests that striatin lies at a signaling crossroad and cannot be bypassed. Owing to these multiple functional domains, we hypothesized that striatin might be a scaffold allowing establishment, in a Ca2+-dependent manner, of multiprotein complexes specific to soma and dendrites.
To validate such a hypothesis, we searched for potential interactors of striatin, and by means of the two-hybrid strategy, we identified a novel, intracellular, 26-kDa protein, phocein (named after the Greek founders of the port of Marseille). Because we recently showed that two proteins, SG2NA and zinedin, share with striatin identical protein-protein association modules (Castets et al., 2000), we looked for their possible interaction with phocein, which, indeed, was verified by in vitro experiments.
The sequence of phocein was found to contain stretches of homology with the ς subunits of clathrin adaptor complexes. Clathrin, a coat protein, is linked to different types of vesicles by various sets of adaptor protein complexes (AP), heterotetramers comprising two heavy chains or adaptins and two light chains, a μ and a ς chain (Schmid, 1997; Kirchhausen, 1999). AP-1 occurs on the Golgi complex, whereas AP-2 is found at the plasma membrane. Other clathrin adaptor complexes have been recently discovered, such as AP-3 (Dell'Angelica et al., 1997; Simpson et al., 1997) and AP-4 (Dell'Angelica et al., 1999).
The data presented in this study show that phocein, although mostly present on the Golgi complex in unpolarized HeLa cells, is seen within adult rat brain neurons from the perinuclear area down to the smallest dendritic branches.
MATERIALS AND METHODS
Two-Hybrid Assay
A fusion protein comprising the LexA DNA-binding domain and striatin was used as a bait to search for fusion proteins expressed by a construct containing a rat brain cDNA library (Matchmaker; Clontech, Palo Alto, CA) and the activation domain of Gal4 (Dagher and Filhol-Cochet, 1997). A full-length striatin insert (Castets et al., 1996) was ligated into the pLex 11 vector (a gift from M.C. Dagher, Commissariat à l'Energie Atomique, Grenoble, France) in-frame with the LexA DNA-binding domain, yielding plasmid pLex-stri. L40 yeast strain cells grown in minimal medium were transformed with pLex-stri, using the lithium acetate method (Gietz et al., 1992). The Lex-stri fusion protein was stably expressed in L40 cells, as verified by immunoblotting using anti-striatin antibodies ( Castets et al., 1996). L40 cells expressing Lex-stri were transformed with the plasmid library. From 5 × 106 colonies obtained 5 d after cotransfection, 63 colonies were His+. They were tested for β-galactosidase (β-gal) activity by a color filter assay using the substrate 5-bromo-4-chloro-3-indolyl-d-galactoside. Plasmids from the 58 His+ Lac Z+ colonies were prepared according to the method of Kimmel and Berger (1987). After electroporation in Escherichia coli HB101 cells of Leu− phenotype, the selected library plasmids were rescued by complementing the Leu− phenotype on minimal medium. The 58 selected colonies were accounted for by only two plasmids, encoding inserts of 1.7 and 2.5 kilobases (kb), respectively, named pGAD 10-phocein-1.7 and pGAD 10-phocein-2.5. Inserts were sequenced using the specific primers of pGAD10 and primers designed by a “gene walking” strategy (ESGS, Evry, France). The 1.7-kb sequence was fully included in the 2.5-kb sequence, which contained an open reading frame (ORF) of 678 bp, encoding phocein, preceded by 7 bp and followed by a 3′-noncoding sequence ending by a poly-A stretch (phocein sequence, −7 to +2506).
Northern Blots
Total RNA from various rat tissues were purified using TRIZOL (Life Technologies, Grand Island, NY). Each RNA (10 μg) was electrophoresed on 1% agarose-6% formaldehyde gels and transferred on Nytran-plus membranes (Schleicher and Schuell, Keene, NH). A 659-bp phocein probe (nucleotides −7 to +652) was obtained by digesting pGAD10-phocein-2.5 with EcoRI. Phocein and actin probes were labeled by random priming with [α32P]dCTP. The blots were hybridized overnight at 42°C in the presence of formamide. After hybridization, the membranes were washed several times at 50°C in 0.1× SSC and 0.1% SDS. Membranes were exposed at −70°C with amplifying screens, using Fuji (Tokyo, Japan) films.
Production of a GST-Phocein Fusion Protein and Obtaining Antibodies
A 2-kb BamHI/BglII fragment of the selected library plasmid was subcloned in a pGEX4T-3 vector (Amersham Pharmacia Biotech, Arlington Heights, IL), yielding pGST-phocein encoding the full-length phocein sequence in-frame with that of glutathione S-transferase (GST). E. coli JM109 cells were transformed and, upon induction by 0.1 mM isopropyl β-d-thiogalactoside, expressed high levels of GST-phocein (52 kDa). The cells were lysed, and the fusion protein contained in the soluble fraction was purified on glutathione-Sepharose. Two rabbits were immunized with the purified fusion protein according to published procedures (60–120 μg per injection). Antisera were tested on Western blots of purified GST-phocein and rat brain subfractions. Anti-GST-phocein antibodies were affinity purified either on strips of blots of GST-phocein or on a GST-phocein affinity resin (obtained by coupling 3 mg of GST-phocein to 1 ml of CNBr-activated Sepharose 4B (Amersham Pharmacia Biotech). The blots or resin were incubated for a few hours with the anti-phocein serum and washed, and the antibodies were eluted with 0.1 M glycine-HCl buffer, pH 2.5. The antibody solution was adjusted to pH 7.5. It was mixed with 50% glycerol and 0.1% bovine serum albumin (BSA) and kept at −20°C.
Coimmunoprecipitation and Pull-Down Assays
Rat brain homogenates were fractionated, using buffers containing either 0.1 mM Ca2+ or 1 mM EDTA, into cytosol and a 100,000 × g pellet, containing membranes (the detergent-soluble fraction) and cytoskeleton (the detergent-insoluble fraction) (Bartoli et al., 1998). Protein was determined by the microBCA method (Pierce, Rockford, IL). For immunoprecipitation assays, brain fractions were precleared by incubation at 4°C for at least 1 h with preimmune serum-coated Pansorbin cells (Calbiochem, San Diego, Ca; 1 ml of cell suspension for 10 mg of brain protein).
For coimmunoprecipitation assays, batches of 200 μl of a 10% suspension of washed Pansorbin cells preincubated in 1% BSA-containing Tris saline buffer (TBS buffer; 50 mM Tris-HCl, pH 7.4, and 150 mM NaCl) were incubated with 140 μg of rabbit preimmune immunoglobulins or affinity-purified anti-phocein, anti-striatin, anti-SG2NA, and anti-zinedin antibodies, in the presence of 0.1% BSA (Castets et al., 2000) for at least 4 h at 4°C with gentle agitation. The Pansorbin cells were washed in TBS several times. Precleared brain cytosol (0.5 ml, ∼1 mg) or the Lubrol-soluble brain fraction was added and incubated overnight at 4°C with gentle agitation. Pansorbin cells were washed four times (two first washes with 50 mM Tris-HCl, pH 7.4, 500 mM NaCl, 1 mM EDTA, and 0.5% Nonidet P-40; two last washes with 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, and 0.05% deoxycholate) and boiled for 5 min in Laemmli sample buffer. The solubilized proteins were electrophoresed on 8, 10, or 15% polyacrylamide-SDS gels and transferred onto Protran membranes (Schleicher and Schuell). The antibodies used for revelation were the anti-striatin serum 75 (1:2000; Castets et al., 1996) and affinity-purified anti-phocein, anti-SG2NA, and anti-zinedin antibodies (all at 0.2–0.3 μg/ml). The ECL procedure (Pierce) was used.
For pull-down assays, 2.5 μg GST or purified GST-phocein were incubated with 40 μl of 50% glutathione-sepharose for 2 h at 4° in TBs containing 0.1% BSA. After three washes in TBS, 200 μl (about 400 μg protein) of rat brain cytosol or lubrol-soluble fraction were added to the resin and incubated overnight at 4° with gentle agitation. After extensive washes with TBS, the resin pellets were treated as above.
Immunofluorescence Studies and Fractionation of HeLa cells: Brefeldin A (BFA) Treatment
HeLa and Hep-2 cells (American Type Culture Collection, Manassas, VA) were grown in Eagle's medium modified by Dulbecco (DMEM), supplemented with 10% fetal calf serum, 2 mM l-glutamine, penicillin, and streptomycin (GIBCO-BRL, Grand Island, NY). For treatment with BFA (Sigma, St. Louis, MO), HeLa cells grown on coverslips were incubated for 1, 2, and 10 min at 37°C with 5 μg/ml BFA in DMEM or with a 1:2000 dilution of ethanol in DMEM as a control. Cells were then fixed and processed for immunofluorescence.
For immunofluorescence studies, cells grown on coverslips were washed in phosphate saline buffer (PBS), fixed in a solution containing 3.7% paraformaldehyde and 30 mM sucrose, for 30 min at 4°C. The cells were washed once in PBS and, after quenching for 10 min, were washed in PBS containing 50 mM NH4Cl and washed again in PBS supplemented with 1 mg/ml BSA. The cells were incubated with primary antibodies in permeabilization buffer A (PBS containing 1 mg/ml BSA and 0.05% saponin or 0.1% Triton X-100) for 45 min at room temperature. After two washes in buffer A, the cells were incubated for 45 min at room temperature in buffer A containing the labeled secondary antibody. After two washes in buffer A and one in PBS, the cells were mounted on microscope slides in 100 mM Tris-HCl buffer, pH 8.5, containing 100 mg/ml Mowiol (Calbiochem) and 25% (vol/vol) glycerol. The antibodies used were a mouse monoclonal antibody CTR433 (a gift of M. Bornens, Institut Curie, Paris, France); a mouse monoclonal antibody raised against clathrin light chains (American Type Culture Collection, CON-1); a mouse monoclonal antibody raised against γ-adaptin (Sigma, A 4200); preimmune rabbit immunoglobulins (Sigma); Texas Red-conjugated goat anti-mouse immunoglobulins and Alexa 488-conjugated goat anti-rabbit immunoglobulins (Molecular Probes, Eugene, OR); Cy5-conjugated goat anti-mouse immunoglobulins (Amersham-Pharmacia-Biotech).
Subcellular fractionation was performed according to the method of Monneron and d'Alayer (1978) with modifications. Briefly, confluent cells from 10 dishes (10 cm in diameter) were washed in TSM (50 mM Tris-HCl, pH 7.4, buffer, containing 150 mM NaCl, 5 mM MgCl2, and inhibitors of proteases), homogenized in 2.3 M sucrose at room temperature with a motor-driven glass-Teflon Potter homogenizer, and adjusted with cold TSM to 40% sucrose using a refractometer. The homogenate was layered above a 1.8-ml 50% sucrose-TSM cushion and overlayered with TSM buffer in 12-ml polyallomer tubes. The gradients were centrifuged at 120,000 × g for 2 h at 4°C. The white membrane fraction termed G obtained at the interface of 40% sucrose-TNM buffer and the yellow fraction termed M obtained at the interface of homogenate-50% sucrose were collected and washed. The nuclear pellet and the cytosol were saved. All fractions were normalized for protein and analyzed on 15 and 7% polyacrylamide-SDS gels, and the proteins were transferred to nitrocellulose.
Immunohistochemical Study of Rat Brain Sections
Adult Wistar rats were deeply anesthetized using a mixture of 0.5 ml of ketamine (50 mg/ml, Rhône-Mérieux, Lyon, France) and 0.37 ml of xylazine (2 mg/kg, Bayer, Elkhart, IN). They were transcardially perfused with 400 ml of 0.1 M phosphate buffer, pH 7.4, containing 4% paraformaldehyde. The brain and adrenal glands were removed and postfixed in the same solution. Vibratome sections, 30–40 μm, were cut and processed for immunocytochemistry at the optical level, using the immunoperoxidase method as described previously (Bernard et al., 1997). Briefly, sections were preincubated in PBS containing 10% normal goat serum and incubated for 15 h at room temperature in a solution containing the primary antibodies. Primary antibodies were affinity-purified anti-phocein antibodies (0.1 to 0.5 μg/ml in PBS containing 1% normal goat serum). Control antibodies were unimmunized rabbit antibodies (Sigma), and solutions of affinity-purified anti-phocein antibodies preadsorbed on the blotted phocein fusion-protein. The sections were washed, incubated for 1.5 h in biotinylated goat anti-rabbit antibodies (1:200; Vector Laboratories, Burlingame, CA), washed, incubated for 1 h in an avidin-biotin-peroxidase solution, and washed. Peroxidase was revealed by using 3,3′-diaminobenzidine in the presence of 0.01% H202, with nickel salt enhancement. Low-magnification light microscopy images were acquired with a light microscope (Nikon, France S.A., Champigny-Sur-Marne, France) coupled to a 3-chip charge-coupled device camera linked to a computer (Power Mac; Macintosh). The acquired image dimensions, 768 × 576 pixels, were reduced to obtain a final resolution of 300 dots per inch. High-magnification images were obtained from scanned photomicrographs (1200 × 1200 pixels). An image-editing software (Adobe Photoshop; Adobe Systems, Mountain View, CA) was used to create montages, which were printed on a dye sublimation printer. Only contrast and brightness of images were adjusted digitally.
Miscellaneous
In Vitro Transcription Translation Assay.
A phocein-encoding plasmid pcDNA 3-phocein was obtained by inserting the phocein insert contained in pGAD 10-phocein-2.5 into the NotI site of plasmid pcDNA 3 (Invitrogen, San Diego, CA). Transcription-translation in vitro assays were performed using the TNT T7 system (Promega, Madison, WI) and pcDNA3-phocein, in the presence of [S35]methionine (1000 Ci/mmol; ICN, Costa Mesa, CA).
Gel Filtration.
Brain cytosol (3.7 mg of protein in 3 ml of TBS containing 1 mM EDTA) was performed on a Biogel A 5m column (1.9 × 85 cm; calibrated as indicated by d'Alayer et al., 1983). Calibrating proteins were porcine thyroglobulin (TG; r = 9.8 nm), β-gal (r = 8.2 nm), catalase (cat; r = 5.2 nm), and alcohol dehydrogenase (ADH; r = 4.6 nm).
Sucrose Gradients.
Brain cytosol (400 μl, 0.6 mg of protein) was layered on 11-ml sucrose gradients, 15–45%, and centrifuged for 16 h at 105.000 × g at 4°C. After centrifugation, 21 fractions (450 μl each) were collected, starting from the bottom of the gradient. Calibrating proteins were ADH (7.4 S), cat (11.3 S), apoferritin (17.2 S), and TG (19 S).
RESULTS
Identification of Phocein and Domain Prediction
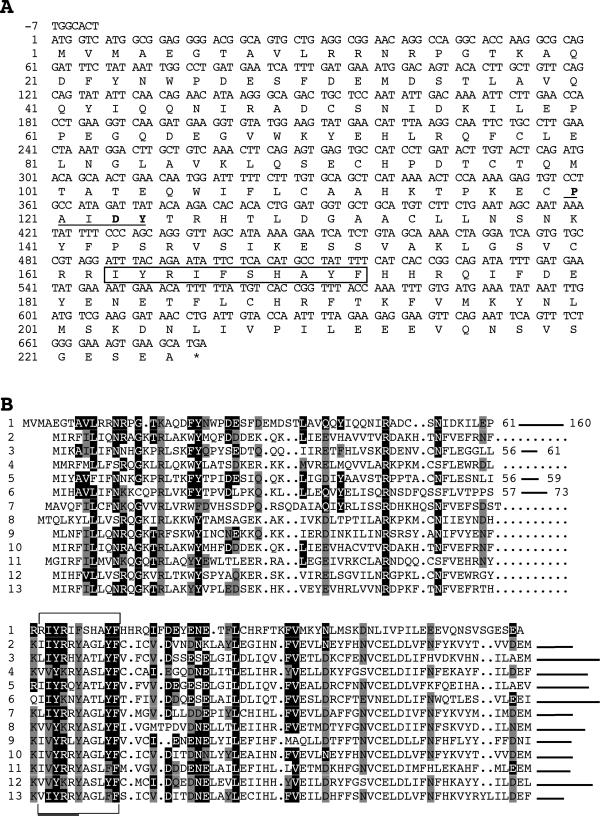
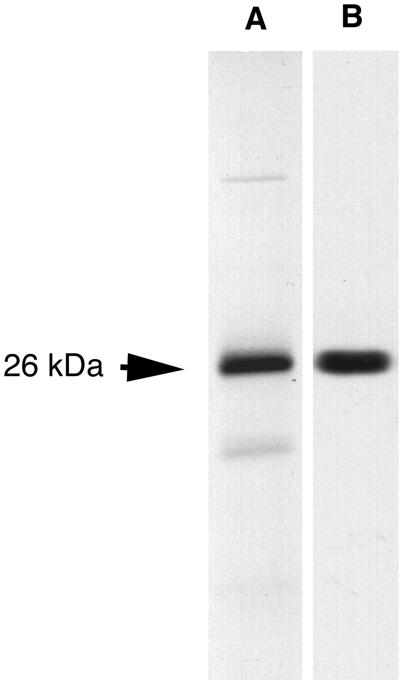
A yeast two-hybrid screen of a rat brain library conducted with a LexA-striatin fusion protein yielded a clone containing an insert of 2.5 kb encoding a 225-amino acid (aa) ORF. The corresponding protein, of 26 kDa theoretical molecular weight, has been named phocein (Figure 1A). The ATG codon lies within a classical eukaryotic translation start sequence. An in vitro transcription-translation–coupled assay showed that the plasmid encoding phocein directs the synthesis of a protein of 26 kDa, the predicted molecular mass (Figure 2, lane A; in lane B, brain cytosol present on the same blot was revealed by anti-phocein affinity-purified antibodies). Two polyadenylation signals are present in the 3′-untranslated sequence at nucleotides 923 and 2456 (Baillat and Castets, unpublished results). A BLASTN 2 search resulted in several matches (Altschul et al., 1997). One, a mouse cDNA named 2C4D (970 bp, accession number U01138) is 98% identical with phocein cDNA (Temeles et al., 1994). However, a 1-bp frameshift with respect to the phocein sequence, due to a sequencing error, results in a shorter ORF with a different C-terminal deduced protein sequence. Several other matches occurred in human cDNAs and genomic sequences, some of which contain the entire phocein gene, encoding protein sequences 100% identical with the rat sequence. Some of these sequences localize to chromosome 11. Search in expressed sequence tag databases allowed us to predict the correct mouse cDNA sequence, encoding a putative protein of 26 kDa, 100% identical with the rat sequence, and we were able to establish that there are several stop codons upstream of the translation start ATG. The Drosophila melanogaster gene encoding phocein is now known (chromosome II; accession number AAF57380) and encodes a protein 223 aa long that is 80% identical to the human sequence. In the worm Caenorhabditis elegans chromosome III, a sequence (AAB59173) encodes a predicted protein sequence of 223 aa, which is 67% identical to the human sequence.
Figure 1.
(A) Nucleotide (EMBL accession number AJ132008) and predicted aa sequences of the phocein cDNA. Nucleotides are positively numbered from the first base of the putative initiation codon, in the 5′ to 3′ direction. The aa sequence, in single-letter code, is shown below the nucleotide sequence. The putative SH3-binding domain is underlined. The clathrin adaptor complexes small chain signature of the ς subunit of adaptor complexes is boxed. (B) Alignment of phocein (lane 1) with various ς subunits of adaptor complexes, according to ProDom alignment PD003841. The aa in black boxes are identical in phocein and the ς subunits; the aa in gray boxes are homologous. Numbers 1–13 refer to the following proteins, identified by their accession numbers: 1, phocein, AJ132008 (rat); 2–13: ς subunits; 2, P53680 (human); 3, Q92572 (human); 4, Q00382 (mouse); 5, Q09905 (Schizosaccharomyces pombe); 6, P47064 (Saccharomyces cerevisiae); 7, Q00381 (S. cerevisiae); 8, P35181 (S. cerevisiae); 9, O96254 (Plasmodium falciparum); 10, Q19123 (C. elegans); 11, O82201 (Arabidopsis thaliana); 12, O23685 (A. thaliana); 13, O50016 (maize).
Figure 2.
Phocein synthesized in vitro and native phocein migrate at the same apparent molecular mass in SDS-gels. (A) Phocein synthesized in vitro using pcDNA3-phocein and an in vitro transcription-translation assay. Autoradiogram. (B). Western blot of rat brain cytosol revealed by affinity-purified anti-phocein antibodies.
In addition to finding orthologues of phocein in different species by a Blast search, a Proscan search (Bairoch et al., 1997) was conducted and revealed an interesting homology (78% identity) with the “clathrin adaptor complexes small chain signature” of the ς subunits (I, L, V, M) (I, L, V, M)YRxxxxLYF (Prosite PS00989) (Figure 1, boxed). We compared the phocein sequence with the ProDom multialignment of the adaptor complex ς subunits (12 known protein sequences, excluding orthologues) and found several stretches of conserved aa, identically spaced, in the amino- and carboxy-terminal regions of phocein (Figure 1B).
Between the two blocks containing homologous stretches, phocein displays a 100-aa–long stretch (aa 61–160) that has no counterpart in ς subunits and contains a putative SH3-binding domain (aa 120–124, underlined in Figure 1A) of the type PxxDY recently described by Mongiovi et al. (1999). Several putative phosphorylation sites exist (four for casein kinase II: aa 29–32, 52–55, 101–104, 115–118; three for protein kinase C: aa 115–117, 138–140, 147–149; one for protein kinase A: aa 149–152). Finally, it may be interesting to note that phocein and the yeast protein mob1 share 22% identity and 47% homology over a stretch of 185 aa (Luca and Winey, 1998). Mob1p is implicated in the onset of septum formation in Schizosaccharomyces pombe (Salimova et al., 2000). The aa implied in the identities and homologies seen between phocein and, respectively, the yeast ς subunits and mob1 are not particularly overlapping.
Phocein Is Expressed in Many Tissues
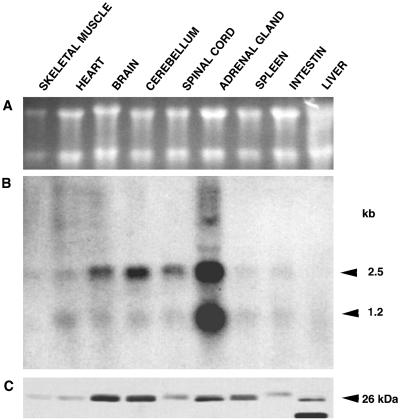
Multitissue rat Northern blots (Figure 3 A) were analyzed with a phocein probe (Figure 3B). Phocein transcripts were prominent in cerebellum, brain, spinal cord, and especially the adrenal gland. Two transcripts of 2.5 and 1.2 kb were present, the 2.5-kb transcript being more abundant. The presence of two transcripts could be explained by the fact that in rat there are two polyadenylation sites. Blots exposed for a longer time also revealed phocein transcripts in muscle, heart, and several other tissues.
Figure 3.
Expression of phocein. (A) Northern blot made with total RNA from different rat tissues (10 μg/lane); ethidium bromide staining of the blot region corresponding to rRNAs. (B) This Northern blot was analyzed with a 659-bp phocein probe (nucleotides −7 to +652). Sizes of the transcripts are indicated on the right. (C) Multitissue Western blot (120 μg of cytosolic protein/lane) revealed with affinity-purified anti-phocein antibodies (0.5 μg/ml). The molecular mass of phocein is indicated on the right.
Rabbit antibodies were raised against a GST–whole-length phocein fusion protein. They were affinity purified and used to follow phocein expression at the protein level. As shown in Figure 3C, cytosolic phocein was abundant in brain and cerebellum, in agreement with the Northern blot data, and in the adrenal gland. However, the amount of cytosolic phocein in this gland was not as high as would be expected from the abundance of the transcripts; the adrenal gland also contained a sizable amount of particulate phocein, but other tissues did also. In spleen, on the contrary, the amount of protein was larger than expected from the Northern blots. Intestinal phocein migrated with a slightly different apparent molecular mass than in other tissues; furthermore, one additional abundant protein of slightly lower apparent molecular mass was immunolabeled in liver.
Brain Phocein Is Both Cytosolic and Associated with Membranes
Brain fractionation showed that phocein is distributed both in the cytosol (40%) and in the particulate, detergent-soluble fraction (60%). Phocein was wholly solubilized from a 100,000 × g pellet using nonionic detergents such as Lubrol-PX or zwitterionic detergents such as 3-([3-cholamidopropyl]dimethylammonio)-2-hydroxy-1-propanesulfonate. Striatin and the other proteins of its family, zinedin and SG2NA, behave in the same way (Castets et al., 1996, 2000; Bartoli et al., 1998).
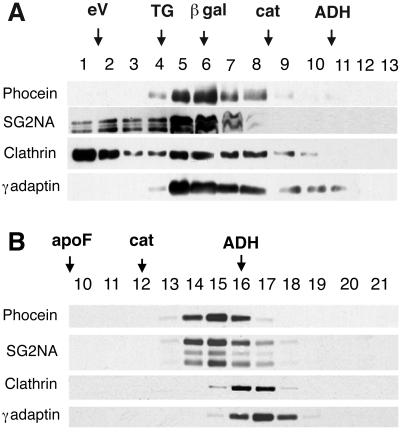
Data from gel filtration experiments and sucrose gradient centrifugations showed that brain cytosolic phocein was present in fractions corresponding to proteins or protein complexes of larger size than expected for each monomer. Gel filtration showed that, although some cytosolic phocein eluted in fractions compatible with the expected Stokes radius (∼4 nm if globular), most of it eluted in fractions of much larger Stoke's radii, 7–10 nm (Figure 4 A), similar, for instance, to values obtained for clathrin adaptor complexes (7.0 for AP-1 and AP-2, 8.5 for AP-3, 6.5 nm for AP-4; Dell'Angelica et al., 1999) (see the γ-adaptin lane in Figure 4A). SG2NA was found in the same fractions. In addition, small amounts of phocein and SG2NA were detected in the excluded volume (Figure 4A, excluded volume eV: fractions 1 and 2), indicating the presence of phocein within bulky complexes. Sucrose gradient experiments again indicated that both phocein and SG2NA were present in fractions containing proteins with an S value of 8.5 ± 0.5 (Figure 4B). Thus, both phocein and SG2NA behaved as protein complexes.
Figure 4.
Gel filtration and sucrose gradients. (A) Cytosolic brain proteins were gel filtered as described in MATERIALS AND METHODS. Fractions 1 and 2 are the excluded volume eV. (B) Cytosolic brain proteins were overlaid on sucrose gradients as described. Aliquots of the fractions were analyzed by Western blotting and revealed with the indicated antibodies. Calibrating proteins are indicated by arrows above the fraction numbers: porcine thyroglobulin, TG; β-galactosidase, β-gal; apoferritin, apo F; catalase, cat; and alcohol dehydrogenase, ADH.
Phocein Interacts with All Members of the Striatin Family
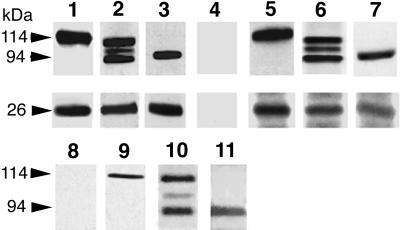
The two-hybrid strategy in yeast demonstrated that phocein directly interacts with striatin. Coimmunoprecipitation and pull-down assays using brain fractions were performed to confirm this interaction in vitro and to see whether phocein also interacts with zinedin and SG2NA, which share the same protein-protein association modules. Striatin (Figure 5, lane 1, top), SG2NA (Figure 5, lane 2, top), and zinedin (Figure 5, lane 3, top) contained in rat brain cytosol were coimmunoprecipitated along with phocein by anti-phocein antibodies (Figure 5, lanes 1–3, bottom) but not by rabbit unimmunized antibodies (Figure 5, lane 4). Coimmunoprecipitation of these proteins also occurred when detergent-solubilized membranes were used (Baillat and Monneron, unpublished results). Conversely, phocein from rat brain cytosol (Figure 5, lanes 5–7, bottom) or from solubilized brain membranes (Baillat and Monneron, unpublished results) was coimmunoprecipitated along with striatin (Figure 5, lane 5, top), SG2NA (Figure 5, lane 6, top), and zinedin (Figure 5, lane 7 top), using the respective affinity-purified antibodies, whereas striatin, SG2NA, and zinedin were not detected when Pansorbin cells coated with control antibodies were used (as in Figure 5, lane 4).
Figure 5.
Phocein and the proteins of the striatin family coimmunoprecipitate and pull down each other. Coimmunoprecipitation, lanes 1–7. Pansorbin cells, coated with preimmune immunoglobulins (lane 4) and affinity-purified anti-phocein antibodies (lanes 1–3) were incubated with rat brain cytosol. The washed pellets were analyzed by Western blots, using anti-striatin (lane 1, top), anti-SG2NA (lane 2, top), and anti-zinedin (lane 3, top) affinity-purified antibodies and anti-phocein purified antibodies (0.5 μg/ml; bottom part of the blot, lanes 1–3). Washed Pansorbin cells, coated with anti-striatin (lane 5), anti-SG2NA (lane 6), and anti-zinedin (lane 7) affinity-purified antibodies, were incubated with rat brain cytosol. The washed pellets were analyzed by Western blots. The blot was revealed by the anti-striatin serum (lane 5, top; no. 75, 1:2000), anti-SG2NA antibodies (0.2 μg/ml; lane 6, top), and anti-zinedin antibodies (lane 7, top; 1 μg/ml) and by affinity-purified anti-phocein antibodies (lanes 5–7, bottom). The ECL procedure was used. Pull-down experiments, lanes 8–11. Washed GST-coated beads (lane 8) and GST-phocein–coated beads (lanes 9–11) were incubated with brain cytosol and analyzed by Western blotting. The blots were revealed by an anti-striatin antiserum (no. 75, 1:2000; lane 9), affinity-purified anti-SG2NA (lane 10), anti-zinedin (lane 11) antibodies, and all three antibodies (lane 8). ECL procedure. The molecular masses of the proteins are indicated on the left (arrows).
In pull-down experiments, in which glutathione-Sepharose beads saturated with GST-phocein or with GST were incubated with rat brain cytosol or solubilized membranes, endogenous striatin was retained on GST-phocein–coated beads (Figure 5, lane 9), as well as SG2NA (Figure 5, lane 10) and zinedin (Figure 5, lane 11), but not on GST–coated beads (Figure 5, lane 8).
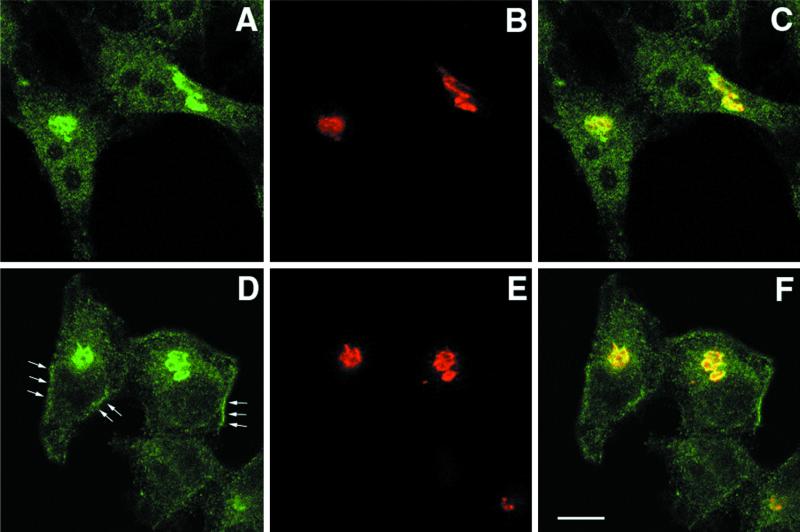
Phocein and SG2NA Colocalize over the Golgi Area of HeLa Cells
Because subcellular localization of proteins can be conveniently studied in HeLa and Hep-2 cells, which are amenable to treatment with various drugs, we studied the localization of phocein and SG2NA in the two cell lines. SG2NA was expressed in both, whereas striatin was not, a fact consistent with the restricted expression of the latter protein to a few species of neurons (Castets et al., 1996). Because the results obtained with the two cell lines were very similar, only the results obtained with HeLa cells are shown. As shown in Figure 6, antibodies against phocein (A) and SG2NA (D) strongly revealed a seemingly tubular, reticulated, juxtanuclear network that appeared to be the Golgi apparatus. The staining observed with either antibody was considered to be specific because it was not observed with preimmune immunoglobulins or with antibodies raised against striatin or antibodies preincubated with the respective antigens (Baude and Monneron, unpublished results). The presumptive Golgi labeling was confirmed by the use of the CTR433 antibody, which recognizes a medial Golgi resident protein (Figure 6, B and E) (Jasmin et al., 1989). The colocalization of phocein and SG2NA with the CTR433-immunolabeled protein was indicated by classical immunofluorescence (Benmerah, unpublished results) and confirmed by combined confocal images, showing yellow staining of the Golgi apparatus in the combined images (Figure 6, C and F). Phocein and SG2NA only partially colocalized with clathrin and AP-1 (γ-adaptin) at the level of the trans-Golgi network (Benmerah, unpublished results). The use of confocal microscopy showed that, close to some areas of the plasma membrane, bright signals from SG2NA were observed (Figure 6, D, arrows). Such labeling was not seen in the case of phocein.
Figure 6.
Phocein and SG2NA localize to the Golgi apparatus in HeLa cells. HeLa cells were fixed, permeabilized, and processed for immunofluorescence microscopy using rabbit, affinity-purified anti-phocein (A) and anti-SG2NA antibodies (D) and a mouse monoclonal antibody raised against CTR433 (B and E), revealed by an Alexa 488-labeled antibody raised against rabbit immunoglobulins and a Cy5-labeled antibody raised against mouse immunoglobulins. Cells were observed under a confocal microscope. Medial optical cuts of representative cells are shown (C and F). Areas of colocalization appear yellow in the computer-generated composite image. Bar, 10 μm.
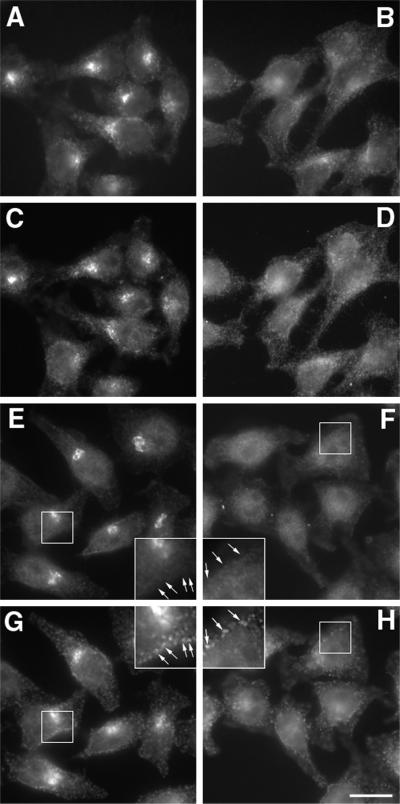
Phocein Association with the Golgi Complex Is Sensitive to BFA
Golgi-associated clathrin coats containing the adaptor complex AP-1 are dispersed away from Golgi membranes by BFA, whereas plasma membrane-associated clathrin coats containing the adaptor complex AP-2 are not modified by BFA (Klausner et al., 1992; Robinson and Kreis, 1992). When HeLa cells were treated for 2 min with BFA, AP-1 became dispersed throughout the cell (Figure 7, compare A and B). A shorter time (1 min) lead to little effect on AP-1 localization (Benmerah, unpublished results). Phocein was dispersed in exactly the same way as was AP-1 (Figure 7, 2-min treatment, compare C and D; Benmerah, unpublished results). With respect to the BFA treatment, SG2NA behaved just as did phocein and AP-1 (Benmerah, unpublished results). The effect of BFA was specific of Golgi-associated coats because a 10-min treatment had no effect on plasma membrane-associated clathrin coats, as shown by the fact that the peripheral punctate clathrin staining seen at the plasma membrane was not affected (Figure 7, compare insets in G and H). Phocein was not detected where coated pits were observed (Figure 7, compare insets in F and H).
Figure 7.
Phocein is mislocalized to cytosol in BFA-treated cells. Ethanol (A, C, E, and G)- and BFA (B, D, F, and H)-treated cells (A–D, 2-min treatment; E–H, 10-min treatment) were fixed and processed for immunofluorescence microscopy using an anti-γ-adaptin monoclonal antibody (100.3; A and B), the polyclonal, affinity-purified anti-phocein antibodies (C–F), and an anti-clathrin monoclonal antibody (CON-1; G and H), revealed by a Texas Red-labeled antibody raised against mouse immunoglobulins and an Alexa 488-labeled antibody raised against rabbit immunoglobulins. Cells were observed under an epifluorescence microscope attached to a cooled charge-coupled device camera. The same field is shown in A and C, in B and D, in E and G, and in F and H. Note that in G clathrin coats are seen over the Golgi area as well as at the plasma membrane, whereas in H, the only clathrin coats left are localized at the plasma membrane. From E to H, insets show a higher magnification (2×) of the portions of cells included in squares. Bar, 15 μm (30 μm in the insets).
Next, we investigated whether phocein actually binds membranes in HeLa cells. Subfractionation of lysed HeLa cells on sucrose gradients yielded, in addition to nuclei, three different fractions: 1) floated, light membranes, originating from plasma membrane, smooth endoplasmic reticulum (ER), and Golgi; 2) cytosol; and 3) heavy membranes (mitochondria, some nuclear membranes, lysosomes, and cytoskeleton) (% protein content of the fractions: 5, 30, 65) (Monneron and d'Alayer, 1978). Phocein, present only in the cytosol and the light membrane fraction, was threefold enriched in the latter, because the phocein ratio in the light membrane fraction versus cytosol was approximately 1:2 (Monneron, unpublished results). Because the light, floated membrane fraction had been thoroughly washed before analysis, the enrichment of phocein in this fraction indicated that phocein binds membranes. The light membrane fraction also contained a sizable amount of SG2NA and almost all the caveolin and most of the γ-adaptin (the heavy chain of AP-1) present in the cell lysate.
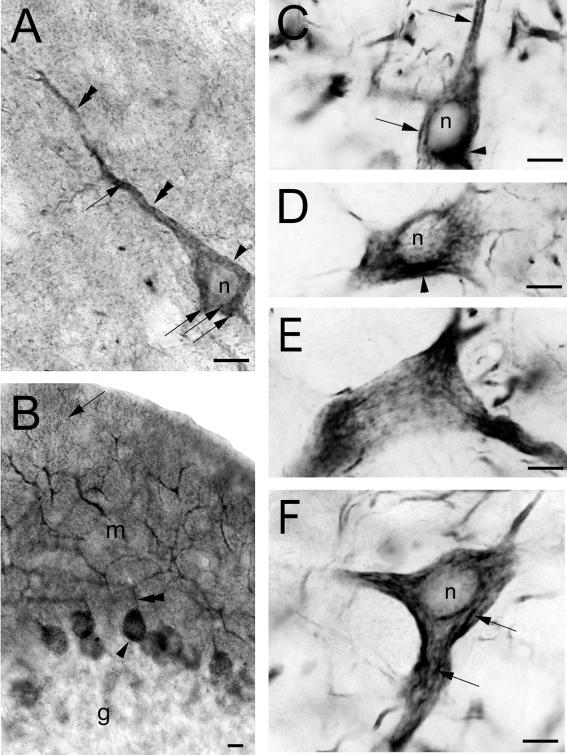
In the CNS, Phocein Immunoreactivity Occurs in Neurons and Is Somato-dendritic
Immunocytochemistry at the optical level was applied to rat brain, cerebellum, brain stem, and adrenal gland sections using affinity-purified antibodies. Immunoreactivity for phocein was present throughout the rat brain (all cortical layers, including both pyramidal cells, Figure 8A, and nonpyramidal cells; amygdaloid, septal, habenular, and thalamic complexes; hippocampus, all layers; caudate-putamen), cerebellum (essentially molecular layer and Purkinje cell bodies, glomeruli, and some Golgi cells, Figure 8 B; deep cerebellar nuclei), and brainstem. The strongest labeling occurred in the motor nuclei of cranial nerves in the pons and the bulb (Figure 8, C–F).
Figure 8.
Somato-dendritic localization of phocein in neurons. (A) Phocein immunoreactivity is restricted to the soma (arrowheads) and dendrites (double arrowheads) of cortical pyramidal cells. The intracytoplasmic labeling present in the soma and apical dendrite is granular (arrows). (B) Phocein labeling is present in the soma (arrowhead) and all branches of the dendritic arborization of cerebellar Purkinje cells, from the large proximal dendrites (double arrowhead) to the most distal, thin dendrites (arrow). (C–F) Intracytoplasmic, vermiculated labeling (arrows in C and F) is visible in the soma and emerging proximal dendrites (E) of neurons in the spinal trigeminal nucleus (C and D), in the bulbar reticular nucleus (E), and in the red nucleus (F). A labeled area close to the nuclei may correspond to a Golgi apparatus (C and D). In D, a network reminiscent of the ER is labeled. Note that nuclei (n) are devoid of staining. Bars, 10 μm.
In all the examined brain structures, immunoreactivity for phocein was present only in neurons, not in glial cells. The labeling was intracytoplasmic, restricted to cell bodies and dendrites (Figure 8, A-F). Importantly, phocein was excluded from axons, just as were striatin and SG2NA. Nuclei were unlabeled (Figure 8, A–F). In the soma (Figure 8, A–D and F), the labeling was particulate and often reticulated, suggesting staining of the ER. Perinuclear staining was usual, probably due to the staining of the Golgi apparatus (Figure 8, A, C, and D). Labeling was vermiculated all along the dendrites (Figure 9, A and E: proximal dendrites). Preliminary ultrastructural studies of the cerebellum molecular layer indicate that phocein is present within spines, associated with membrane profiles (Y. Bailly, unpublished data).
In addition to brain structures, the adrenal gland was studied. The medulla strongly reacted with anti-phocein antibodies, whereas the cortex was much less stained (Monneron, unpublished results). Phocein and SG2NA distributions in this gland are thus comparable.
Control experiments showed that 1) the omission of anti-phocein antibody resulted in the removal of all staining; 2) no staining was obtained when either preimmune sera or rabbit control immunoglobulins were used as primary antibodies; and 3) preabsorption of the anti-phocein antibodies with blotted GST-phocein resulted in the absence of staining.
DISCUSSION
The rationale to identify the partners of striatin was that major cellular and physiological effects had been observed following striatin down-regulation (Bartoli et al., 1999a). Indeed, striatin, an intracellular, neuronal protein endowed with multiple protein-protein interaction domains, has a very peculiar localization, being restricted to CNS structures primarily related to locomotor activity (Castets et al., 1996; Bartoli et al. 1998, 1999a,b; Moqrich et al., 1998). Furthermore, it displays a polar subcellular distribution, being strictly localized to the somato-dendritic compartment (Castets et al., 1996; Kachidian et al., 1998; Salin et al., 1998;). On the one hand, the transient blockade of striatin synthesis in embryonic motoneurons resulted in the severe impairment of dendritic growth. On the other hand, striatin down-regulation in live rats led to a decrease in locomotor activity that paralleled the decrease of striatin in striata (Bartoli et al., 1998). To define the molecular role of striatin, we used a yeast two-hybrid strategy to search for partners and identified phocein, a 225-aa intracellular protein. Validation of the interaction between phocein and striatin by in vitro experiments was straightforward. The respective interactions of phocein with SG2NA and zinedin, which belong to the striatin family (Castets et al., 2000), were also demonstrated.
Fractionation of brain homogenate shows that phocein is distributed, in approximately equal amounts, in the cytosol, where it behaves as a protein complex, and in the membrane fraction. The proteins belonging to the striatin family are identically distributed (Castets et al., 2000). Because the sequence of phocein lacks a region that could account for a transmembrane segment, as well as properly located motifs required for prenylation and myristoylation, phocein is likely to be associated with membranes through its interaction with striatin, zinedin, and SG2NA. The latter proteins are themselves not transmembrane proteins, but their association with membranes is robust, because it withstands high ionic strength and alkaline treatments (Monneron, unpublished results).
An interesting finding was the sequence homology between the N- and C-terminal domains of phocein and the ς subunits of adaptor proteins, the two homologous phocein domains being separated by an additional, intervening stretch containing a putative SH3-binding motif. The sequence homology between phocein and ς subunits led us to hypothesize that phocein might be a component of a novel coat, quite different from known adaptors. Immunolocalization studies of phocein in cultured cells (HeLa and Hep-2 cells) indeed showed that phocein is conspicuous over the Golgi area, where it colocalizes with SG2NA. To be recruited onto the Golgi complex, AP-1 and AP-3 need the intervention of ADP-ribosylation factors (Arfs), at variance with the AP-2 complex at the plasma membrane. Treatment of cells with BFA, which inhibits the activity of Arf exchange factors, results in the dispersion of Arf-dependent coats and, within minutes, in the fragmentation of the Golgi complex, which is cycled back to the ER (Donaldson et al., 1992; Klausner et al., 1992; Robinson and Kreis, 1992; Dell'Angelica et al., 1997). Noticeably, the various classes of Arf exchange factors display variable sensitivities to BFA (Chardin and McCormick, 1999). We show here that, in HeLa cells, phocein and SG2NA follow the fate of AP-1 upon BFA treatment, an indication that their sensitivity to BFA is comparable to that of known Golgi-associated coat complexes. However, in addition to coats, Arf1 activation also results in the assembly onto Golgi membranes of several cytosolic proteins, such as actin, ankyrin, and the βIΣ* species of spectrin; the recruitement of these proteins is prevented by BFA (De Matteis and Morrow, 1998).
Although the localization of phocein to the Golgi complex in HeLa cells and its sensitivity to BFA are compatible with the hypothesis of a role for phocein in vesicular traffic, at the moment we are lacking functional data to support that idea. The distribution of phocein within polarized cells such as neurons neither confirms nor denies this hypothesis. Within adult brain neurons, phocein is not confined to the Golgi complex. As seen in rat brain sections, phocein immunolabeling, although it filling the soma, is found within dendrites down to the most distal and tenuous branches and spines. Noticeably, the axons are unstained. Striatin, SG2NA, and phocein thus share exactly the same subcellular distribution. Such polar distributions are quite important to stress, because, if the hypothesis of a relationship between phocein and vesicular traffic holds true, no neuronal coats or proteins involved in vesicular traffic have been, to our knowledge, demonstrated to be restricted to either axons or dendrites, with the exception of EEA1 (Wilson et al., 2000).
It is noteworthy to recall that phocein is highly conserved throughout the animal kingdom: the phocein orthologues found in D. melanogaster and C. elegans are unusually conserved (80% identity between fly and human, 67% between worm and human). Studies of phocein in such species should therefore help determine its function, which appears to be a very conserved cellular process.
ACKNOWLEDGMENTS
The pertinent suggestions and comments from Pietro De Camilli have been very helpful. We also thank Domenica Borgese, Bruno Goud, André LeBivic, and John Bergeron for critical reading of the manuscript. We thank Michel Bornens for supplying the CTR433 antibody, Yann Goureau for expert assistance in confocal microscopy, and Yves Colette for supplying HeLa cells. This work was supported by Centre National de la Recherche Scientifique and by grants from the Association pour la Recherche sur le Cancer (ARC 9318 to A.M. and F.C. and ARC 9679 to A. Benmerah) and Association Française contre les Myopathies (AFM FRN 210/6481). Abdelaziz Moqrich was supported by the Association pour la Recherche sur le Cancer (1999) and by the Lilly Foundation (2000).
Abbreviations used:
- β-gal
β-galactosidase
- aa
amino acid
- ADH
alcohol dehydrogenase
- AP
adaptor protein complexes
- Arf
ADP-ribosylation factor
- BFA
brefeldin A
- BSA
bovine serum albumin
- cat
catalase
- DMEM
Eagle's medium modified by Dulbecco
- ER
endoplasmic reticulum
- GST
glutathione S-transferase
- kb
kilobases
- ORF
open reading frame
- PBS
phosphate saline buffer
- TBS
Tris saline buffer
- TG
thyroglobulin
- TSM
Tris saline-Mg2+ buffer
REFERENCES
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST, and PSI-BLAST. a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW. Microtubules and neuronal polarity: lessons from mitosis. Neuron. 1999;22:23–31. doi: 10.1016/s0896-6273(00)80675-3. [DOI] [PubMed] [Google Scholar]
- Bairoch A, Bucher P, Hoffmann K. The PROSITE database: its status in 1997. Nucleic Acids Res. 1997;25:317–322. doi: 10.1093/nar/25.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli M, Gaillard S, Monneron A. European Congress of Cell Biology Book of Abstracts. Bologna, Italy: T. Pozan; 1999b. pp. 136–137. [Google Scholar]
- Bartoli M, Monneron A, Ladant D. Interaction of calmodulin with striatin, a WD-repeat protein present in neuronal dendritic spines. J Biol Chem. 1998;273:22248–22253. doi: 10.1074/jbc.273.35.22248. [DOI] [PubMed] [Google Scholar]
- Bartoli M, Ternaux JP, Forni C, Portalier P, Salin P, Amalric M, Monneron A. Down-regulation of striatin, a neuronal calmodulin-binding protein, impairs rat locomotor activity. J Neurobiol. 1999a;40:234–243. [PubMed] [Google Scholar]
- Bernard V, Somogyi P, Bolam JP. Cellular, subcellular, and subsynaptic distribution of AMPA-type glutamate receptor subunits in the neostriatum of the rat. J Neurosci. 1997;17:819–828. doi: 10.1523/JNEUROSCI.17-02-00819.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burack MA, Silverman MA, Banker G. The role of selective transport in neuronal protein sorting. Neuron. 2000;26:465–472. doi: 10.1016/s0896-6273(00)81178-2. [DOI] [PubMed] [Google Scholar]
- Castets F, Bartoli M, Barnier JV, Baillat G, Salin P, Moqrich A, Bourgeois JP, Denizot F, Rougon G, Calothy G, Monneron A. A novel calmodulin-binding protein, belonging to the WD-repeat family, is localized in dendrites of a subset of CNS neurons. J Cell Biol. 1996;134:1051–1062. doi: 10.1083/jcb.134.4.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castets F, Rakitina T, Gaillard S, Moqrich A, Mattei M-G, Monneron A. Zinedin, SG2NA and striatin are calmodulin-binding, WD-repeat proteins principally expressed in brain. J Biol Chem. 2000;275:19970–19977. doi: 10.1074/jbc.M909782199. [DOI] [PubMed] [Google Scholar]
- Chardin P, McCormick F. Brefeldin A: the advantage of being uncompetitive. Cell. 1999;97:153–155. doi: 10.1016/s0092-8674(00)80724-2. [DOI] [PubMed] [Google Scholar]
- Craig AM, Banker G. Neuronal polarity. Annu Rev Neurosci. 1994;17:267–310. doi: 10.1146/annurev.ne.17.030194.001411. [DOI] [PubMed] [Google Scholar]
- Dagher MC, Filhol-Cochet O. Making hybrids of two-hybrid systems. BioTechniques. 1997;22:916–922. doi: 10.2144/97225st05. [DOI] [PubMed] [Google Scholar]
- d'Alayer J, Berthillier G, Monneron A. Structure of brain adenylate cyclase: proteolysis-dependent modifications. Biochemistry. 1983;22:3948–3953. doi: 10.1021/bi00285a034. [DOI] [PubMed] [Google Scholar]
- Dell'Angelica EC, Mullins C, Bonifacino JS. AP-4, a novel protein complex related to clathrin adaptors. J Biol Chem. 1999;274:7278–7285. doi: 10.1074/jbc.274.11.7278. [DOI] [PubMed] [Google Scholar]
- Dell'Angelica EC, Ohno H, Ooi CE, Rabinovich E, Roche KW, Bonifacino JS. AP-3, an adaptor-like protein complex with ubiquitous expression. EMBO J. 1997;16:917–928. doi: 10.1093/emboj/16.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis MA, Morrow JS. The role of ankyrin and spectrin in membrane transport and domain formation. Curr Opin Cell Biol. 1998;10:542–549. doi: 10.1016/s0955-0674(98)80071-9. [DOI] [PubMed] [Google Scholar]
- Donaldson JG, Finazzi D, Klausner RD. Brefeldin A inhibits the Golgi membrane-catalyzed exchange of guanine nucleotide onto ARF protein. Nature. 1992;360:350–352. doi: 10.1038/360350a0. [DOI] [PubMed] [Google Scholar]
- Dotti CG, Sullivan CA, Banker G. The establishment of polarity by hippocampal neurons in culture. J Neurosci. 1988;8:1454–1468. doi: 10.1523/JNEUROSCI.08-04-01454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foletti DL, Prekeris R, Scheller RH. Generation and maintenance of neuronal polarity: mechanisms of transport and targeting. Neuron. 1999;23:641–644. doi: 10.1016/s0896-6273(01)80022-2. [DOI] [PubMed] [Google Scholar]
- Gietz D, St. Jean A, Woods RA, Schiestl RH. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 1992;20:1425. doi: 10.1093/nar/20.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning P, Hardeman E, Jeffrey P, Weinberger R. Creating intracellular structural domains: spatial segregation of actin and tropomyosin isoforms in neurons. BioEssays, 1998;20:892–900. doi: 10.1002/(SICI)1521-1878(199811)20:11<892::AID-BIES4>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Jasmin BJ, Cartaud J, Bornens M, Changeux JP. Golgi apparatus in chick skeletal muscle: changes in its distribution during end-plate development and after denervation. Proc Natl Acad Sci USA. 1989;86:7218–7222. doi: 10.1073/pnas.86.18.7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachidian P, Vuillet J, Bartoli M, Castets F, Nieoullon A, Kerkerian-Le Goff L. Relationships between striatin-containing neurons and cortical or thalamic afferent fibers in the rat striatum: an ultrastructural study by dual labeling. Neuroscience. 1998;85:111–122. doi: 10.1016/s0306-4522(97)00593-9. [DOI] [PubMed] [Google Scholar]
- Kimmel AR, Berger SL. Preparation of cDNA and the generation of cDNA libraries: overview. Methods Enzymol. 1987;152:307–316. doi: 10.1016/0076-6879(87)52035-3. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T. Adaptors for clathrin-mediated traffic. Annu Rev Cell Dev Biol. 1999;15:705–732. doi: 10.1146/annurev.cellbio.15.1.705. [DOI] [PubMed] [Google Scholar]
- Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca FC, Winey M. MOB1, an essential yeast gene required for completion of mitosis and maintenance of ploidy. Mol Biol Cell. 1998;9:29–46. doi: 10.1091/mbc.9.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongiovi AM, Romano PR, Panni S, Mendoza M, Wong WT, Musacchio A, Cesareni G, Di Fiore PP. A novel peptide-SH3 interaction. EMBO J. 1999;18:5300–5309. doi: 10.1093/emboj/18.19.5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monneron A, d'Alayer J. Isolation of plasma and nuclear membranes of thymocytes. I. Enzymatic composition and ultrastructure. J Cell Biol. 1978;77:211–231. doi: 10.1083/jcb.77.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moqrich A, Mattei MG, Bartoli M, Rakitina T, Baillat G, Monneron A, Castets F. Cloning of human striatin cDNA (STRN), gene mapping to 2p22–p21, and preferential expression in brain. Genomics. 1998;51:136–139. doi: 10.1006/geno.1998.5342. [DOI] [PubMed] [Google Scholar]
- Robinson MS, Kreis TE. Recruitment of coat proteins onto Golgi membranes in intact and permeabilized cells: effects of brefeldin A and G protein activators. Cell. 1992;69:129–138. doi: 10.1016/0092-8674(92)90124-u. [DOI] [PubMed] [Google Scholar]
- Salimova E, Sohrmann M, Fournier N, Simanis V. The S. pombe orthologue of the S. cerevisiae mob1 gene is essential and functions in signaling the onset of septum formation. J Cell Sci. 2000;113:1695–1704. doi: 10.1242/jcs.113.10.1695. [DOI] [PubMed] [Google Scholar]
- Salin P, Kachidian P, Bartoli M, Castets F. Distribution of striatin, a newly identified calmodulin-binding protein in the rat brain: an in situ hybridization and immunocytochemical study. J Comp Neurol. 1998;397:41–59. [PubMed] [Google Scholar]
- Schmid SL. Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu Rev Biochem. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- Simpson F, Peden AA, Christopoulou L, Robinson MS. Characterization of the adaptor-related protein complex, AP-3. J Cell Biol. 1997;137:835–845. doi: 10.1083/jcb.137.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temeles GL, Ram PT, Rothstein JL, Schultz RM. Expression patterns of novel genes during mouse preimplantation embryogenesis. Mol Reprod Dev. 1994;37:121–129. doi: 10.1002/mrd.1080370202. [DOI] [PubMed] [Google Scholar]
- Wilson JM, de Hoop M, Zorzi N, Toh B, Dotti CG, Parton RG. EEA1, a tethering protein of the early sorting endosome, shows a polarized distribution in hippocampal neurons, epithelial cells and fibroblasts. Mol Biol Cell. 2000;11:2657–2671. doi: 10.1091/mbc.11.8.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]