Abstract
Estrogen-mediated regulation of Th1, Th2 and Treg effector functions are well documented but, surprisingly, it is still not known whether estrogen modulates IL-17, a powerful proinflammatory cytokine that plays a pivotal role in several inflammatory and autoimmune diseases. Therefore in the present study, we determined whether estrogen regulates the expression levels of IL-17 in wildtype C57BL/6 mice. By ELISA, ELISPOT and/or flow cytometric analyses, we found that estrogen upregulated the levels of not only IL-17, but also the IL-17-specific transcription factor retinoic acid-related orphan receptor gamma t (RORγt), in activated splenocytes. IL-17 levels were further enhanced by exposure of activated splenocytes to IL-23, particularly in cells from estrogen-treated mice. Exposure of splenocytes to IL-27 or IFN-γ at the time of activation markedly inhibited the levels of IL-17 and RORγt. Interestingly, a delay of 24 hours in exposure of activated splenocytes to IL-27 or IFN-γ decreased IL-17 levels (albeit less profoundly) but not RORγt. These findings imply that the suppressive effects of IL-27 and IFN-γ are more effective prior to the differentiation and commitment of IL-17-secreting cells. Furthermore, inhibition of JAK-2 by AG490 suppressed IL-17 but not RORγt expression suggesting that other transcription factors are also critical in estrogen-mediated upregulation of IL-17.
Keywords: IL-17, estrogen, IL-27, IFN-γ, lupus, RORγt
INTRODUCTION
A recent paradigm shift in inflammation is the discovery of a novel lineage of CD4+ T helper (Th) Th17 cell, which secrete a potent proinflammatory cytokine, IL-17A (referred as IL-17) [1]. IL-17 promotes inflammation by recruiting neutrophils, monocytes, and macrophages to the site of inflammation and also by acting on target cells to stimulate a broad range of strong inflammatory molecules such as CXCL1, 2, 3, 5, 6 [2], IL-6, CXCL8, MCP1 [3]. IL-17 has also been found to cosynergize with TLR ligands, IFN-γ, IL-1β and TNFα to fine-tune inflammatory responses [4]. Additionally, IL-17A has been shown to promote osteoblastogenesis by suppressing leptin in estrogen-deficiency induced bone loss [5]. Recently, a flurry of reports have indicated that proinflammatory IL-17 is involved in various chronic debilitating autoimmune diseases such as SLE, rheumatoid arthritis, psoriasis and multiple sclerosis [6–8]. IL-17 has been shown to increase production of total IgG, anti-dsDNA IgG and IL-6 by peripheral blood mononuclear cells of patients with lupus nephritis [9].
Studies from our laboratory as well other have reported that estrogen, a known immunomodulator, regulates several pro-inflammatory mediators including IFN-γ, MCP-1, MCP-5, Cox-2, iNOS [10–12]. Estrogen-induced upregulation of pro-inflammatory molecules is noteworthy since estrogen has been implicated in many inflammatory autoimmune diseases such as SLE [13]. Although estrogen-induced regulation of Th-1 and Th-2-mediated cytokines and Tregs activation is now well established, to date there are no reports on estrogen regulation of pro-inflammatory Th17 cells [10, 14–16]. Given the importance of IL-17 and estrogen in autoimmune diseases, we wanted to investigate whether estrogen also modulates IL-17 induction in both lupus-prone NZB/W mice and wildtype C57BL/6 mice. Our novel finding in this report is that estrogen promotes IL-17 levels and upregulates IL-17-specific transcription factor, RORγt. Addition of IL-23 upregulates IL-17 induction, however the frequency of IL-17-producing cells remains same. Further we demonstrate that IL-17 levels are inhibited by the addition of IL-27 or IFN-γ and JAK-2 inhibitor. Together, these findings have important implications for understanding and pharmacological manipulation of IL-17-associated and estrogen modulated pathologies.
RESULTS AND DISCUSSION
Estrogen upregulates IL-17 induction in autoimmune mice
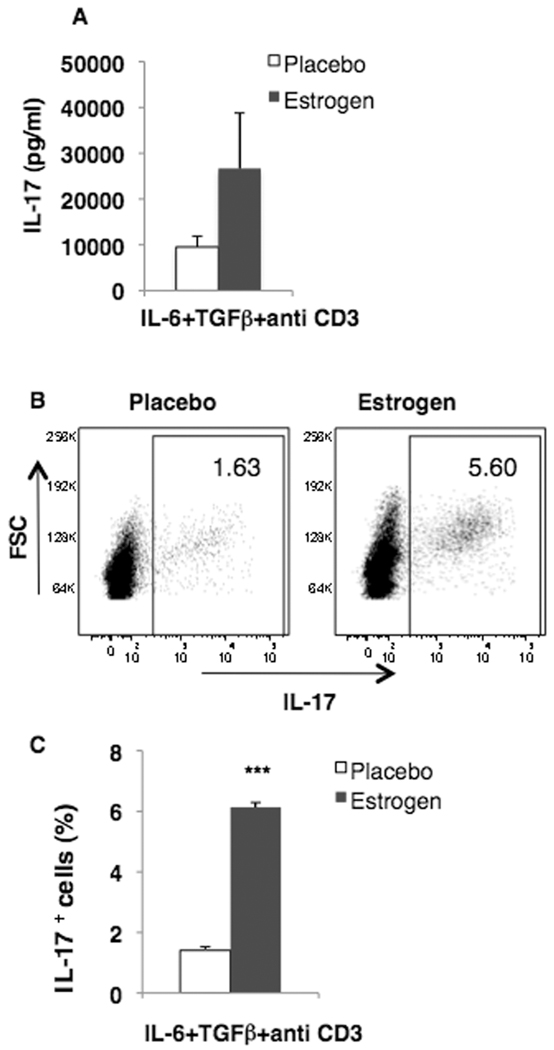
There is growing observation that IL-17 levels and IL-17-secreting cells are increased in SLE patients and in animal models [17–20]. Since estrogen has been shown to promote murine lupus, we hypothesized that estrogen may also promote the induction of IL-17 in lupus-prone mice. Towards this end, splenocytes from estrogen and placebo-treated NZB/W lupus-prone autoimmune mice were stimulated with known IL-17-inducing stimuli (IL-6+TGFβ+antiCD3 antibodies) and IL-17 levels determined in the supernatants collected. As shown in Fig 1A, our preliminary studies suggest that the levels of IL-17 were found to be increased in splenocytes from estrogen-treated (26662.94±12120.27) when compared to placebo-NZB/W mice (8803.77±1352.56 pg/ml; n=7) at 72 hr. The levels of IL-17A in culture supernatants from gonadal-intact mice (9529.56±2372.14 pg/ml; n=5) were similar to that in placebos. Further, flow cytometric analysis also revealed that IL-17-positive cells in estrogen-treated NZB/W mice were increased when compared to placebos in stimulated cells (Fig 1B & C). The numbers of IL-17+ cells/million splenocytes were also higher in estrogen-treated NZB/W mice (61,350±1550) when compared to placebo controls (14,100±1167.4). These initial results suggest that estrogen-treated NZB/W mice have greater propensity to induce IL-17 when compared to placebo-treated mice. The frequency of IL-17+ cells was also increased in unstimulated cells from estrogen-treated NZB/W mice suggesting that estrogen promotes differentiation of IL-17-secreting cells in vivo (data not shown).
Figure 1. IL-17 levels and IL-17+ cells are increased in estrogen-treated autoimmune lupus mice.
Splenic lymphocytes (5×106/ml) from estrogen-and placebo-treated lupus–prone male NZB/W mice were either (A) cultured in the presence of IL-6+TGFβ+antiCD3 antibodies for 72 hr and IL-17 levels in the culture supernatants were analyzed by ELISA or (B, C) were stimulated with IL-6+TGFβ+antiCD3 antibodies for 21 hrs followed by stimulation with PMA (100 ng/ml), ionomycin (2 µg/ml) and brefeldin A (1 µg/ml) for 3 h and stained for intracellular IL-17 expression. (B) Representative flow cytometry plots of IL-17+ cells (percentages indicated). (C) Mean percentage of IL-17+ cells. (A, C) Means ± SEM (estrogen = 2; placebo = 7); *** p<0.001, Tukey-Kramer multiple comparison test.
Estrogen enhances IL-17 levels and intracellular IL-17 positive cells in normal C57BL/6 mice
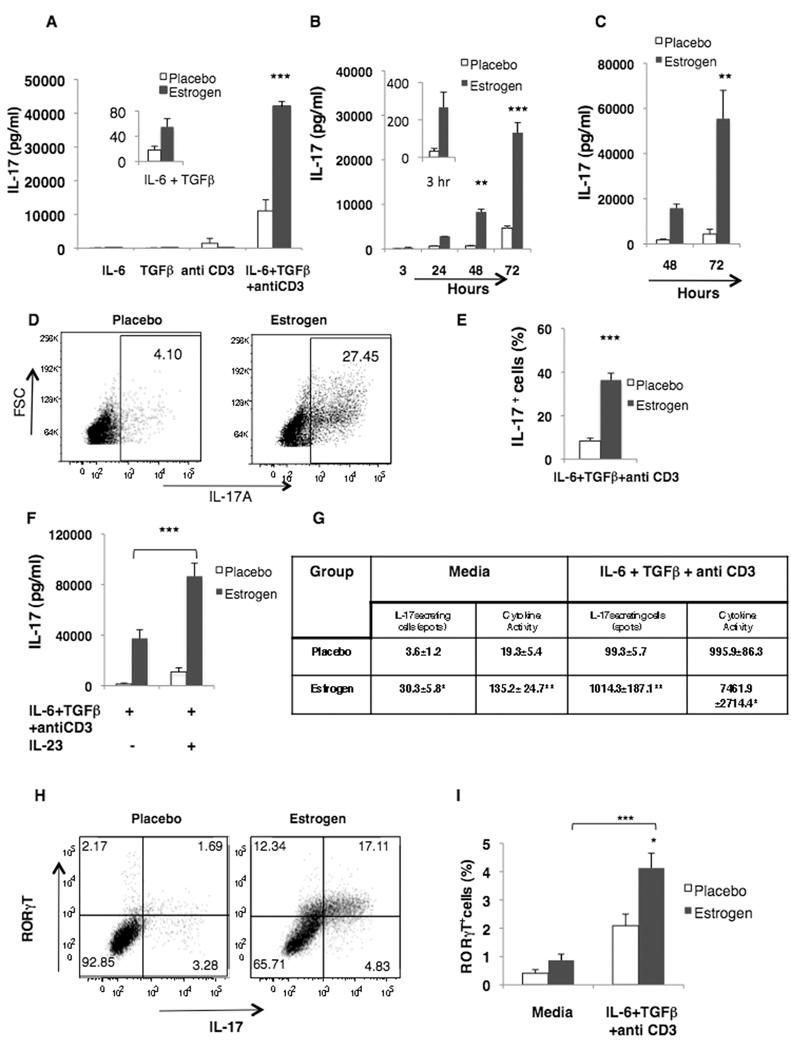
Since estrogen increased IL-17 induction in lupus-prone mice, we next determined whether estrogen could also promote IL-17 in normal mice (C57BL/6). Exposure of cells to IL-6 alone or TGFβ alone did not noticeably induce IL-17 levels. Activation of splenocytes with combination of IL-6 and TGFβ demonstrated low, but detectable levels of IL-17 particularly in cells from estrogen-treated male mice. Impressively, addition of antiCD3 antibody to IL-6 and TGFβ cocktail robustly increased IL-17 levels in cultures from estrogen-treated cells when compared to cells from placebo-treated male mice (Fig 2A). Anti-CD3 antibodies alone induced low levels of IL-17. Kinetics analysis revealed that estrogen promotion of IL-17 induction was evident as early as 3 hr (although not statistically significant) and the levels progressively increased by 72 hr of culture (Fig 2B). Similar studies were performed in female C57BL/6 mice and splenocytes were cultured in presence of IL-17-inducing stimuli for 48 and 72 hr. The levels of IL-17 were significantly increased in estrogen-treated females at 72 hr (Fig 2C). Given that estrogen promoted IL-17 both in males and females, subsequent studies were conducted in gonadectomized male mice. Male C57BL/6 mice were chosen to avoid the confounding effects of endogenous estrogens from extra-gonadal tissues in females. Flow cytometric analyses also showed that estrogen-treated mice have increased IL-17+ cells. Fig 2D is the representative dot plot of IL-17+ cells in placebo- and estrogen-treated mice at 72 hr. The relative numbers of IL-17+ cells and total numbers of IL-17+ cells/million splenocytes were found to be significantly higher in splenocytes from estrogen-treated mice after 72 hr of stimulation with IL-17 inducing stimuli (363,700±31,701) when compared to placebos (83,975±12,658) (Fig 2E). The trends were similar at earlier time points (3 and 24 hr) also however the total percentage of IL-17+ cells was less. Our results differ with Wang et. al. [21], in which estrogen treatment significantly reduced IL-17 induction from MOG (35–55) activated lymphocytes from EAE-induced wildtype mice, however, the same treatment increased estrogen mediated IL-17 induction in EAE-induced PD-1 deficient mice. In addition to the differences in estrogen treatment and levels (they used 2.5 mg slow release pellets for 60 days, which achieved serum estrogen levels 1500–2000 pg/ml that are comparable to pregnancy), there are several notable differences between this study and ours including differences in stimuli (MOG v/s IL-17-inducing stimuli), culture conditions, and autoimmune animal model (EAE v/s lupus). As expected, antigen (MOG) specific IL-17 levels were markedly lower than in our study, where we employed standard IL-17-inducing stimuli. It is thus not surprising that there are differences in IL-17 induction patterns in these two studies. However, both studies suggest that IL-17 is regulated by estrogen.
Figure 2. Estrogen upregulates IL-17 levels, IL-17+ cells and RORγt expression in splenocytes from wildtype mice.
Splenic lymphocytes (5×106/ml) from (A, B) male (placebo=3 and 2; estrogen=3 and 2; representative of three independent experiments) or (C) female C57BL/6 mice (placebo= 3; estrogen=2) were cultured in the presence of either IL-6, TGFβ or anti-CD3 antibody alone or in combination for (A) 72 hr or (B, C) with IL-6 + TGFβ + anti-CD3 antibody for the indicated time points. IL-17 levels in the culture supernatants were determined by ELISA. (D–F) Splenic lymphocytes from estrogen- and placebo-treated wildtype male C57BL/6 mice were stimulated with IL-6 + TGFβ + anti-CD3 antibody for 72 h and (D, E) stained for flow cytometry or (F) were cultured with/without IL-23 for a further 24 h. (D) Representative flow cytometry plots (indicating the percentages) of IL-17+ cells (E) Mean percentage of total IL-17+ cells (placebo=4; estrogen=5; representative of two independent experiment) and (F) IL-17 levels (pg/ml) (placebo=4; estrogen=5). (G–H) Splenic lymphocytes from estrogen- and placebo-treated wildtype male C57BL/6 mice were activated with IL-6+TGFβ+antiCD3 antibody for (G, I) 48 hr and (H) 72 h. (G) The number of IL-17 secreting cells was determined by ELISPOT assay (placebo=3; estrogen=3). (H) Representative flow cytometry plots of RORγt+ IL-17+ cells (percentages indicated; placebo=4; estrogen=5) and (I) Mean percentage (placebo=3; estrogen=3). All data, with the exception of representative plots, are mean ± SEM;* p<0.05; ** p<0.01 and *** p<0.001; (E, and G), two-tailed t-test; (A–C, F, I), Tukey-Kramer multiple comparison test.
Additionally, stimulation of splenocytes with IL-17-inducing stimuli yielded very weak IFN-γ levels (704.994±243.99 pg/ml at 24 hrs). The IFN-γ levels were at least 10–12 fold lesser than what we observe when cells were stimulated with optimal dose (10 µg/ml) of ConA or anti-CD3 antibodies [12] implying the type of stimulation is critical for IFN-γ induction.
Since IL-23 is well documented to be involved in the maintenance and sustenance of IL- 17-producing cells [22], we determined whether IL-23 is also increased in IL-17 inducing conditions. We found that the levels of IL-23 were comparable in placebo- and estrogen-treated mice (data not shown). We next determined whether IL-23 has any effect on IL-17 levels and frequency. We stimulated splenocytes from estrogen- and placebo-treated mice for 72 hr with IL-6+TGFβ+anti CD3 antibodies and added IL-23 and cultured for additional 24 hrs. We found that addition of IL-23 to the culture significantly increased IL-17 induction from estrogen-treated mice (Fig 2F). Interestingly, flow cytometric analysis of IL-17+ cells revealed that there was no increase in the number of IL-17+ cells (data not shown) even though IL-17 levels were increased in supernatants. This suggests that IL-23 promotes secretion of IL-17 levels in estrogen-treated mice.
IL-17-secreting cells are increased in estrogen-treated mice
Since estrogen promoted IL-17 in both male and female wildtype and male autoimmune mice, for detailed analysis of subsequent studies, only wildtype male C57BL/6 mice were utilized. Next we determined whether the increased IL-17 levels in estrogen-treated mice were due to the increased numbers of IL-17 secreting cells. The frequency analysis of IL-17A-secreting cells done by ELISPOT assay confirmed that estrogen increased numbers of IL-17 secreting cell as well as cytokine activity (Fig 2G).
Intracellular expression of RORγt is increased in estrogen treated mice
We next determined whether estrogen also upregulates the expression of RORγt, an IL-17-specific transcription factor. Flow cytometric analysis indicated that the percentage of RORγt+ IL-17+ cells was nearly four times in estrogen-treated mice-when compared to placebo mice at 72 after culture (Fig 2H). Total RORγt expression was also increased in activated splenocytes from estrogen-treated mice when compared to placebos at 48 hr (Fig 2I). This suggests that estrogen-mediated upregulation of IL-17 levels correlates with increased expression of RORγt expression in estrogen-treated cells.
IL-27 and IFN-γ suppresses IL-17 induction
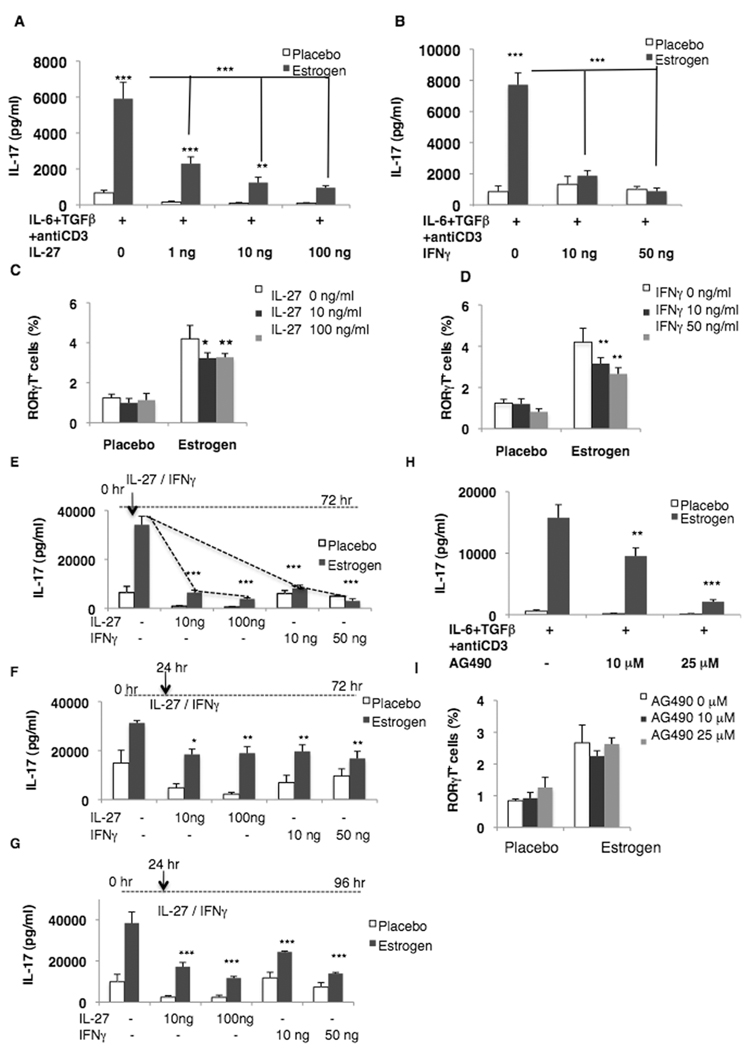
Recent advances in IL-27 biology have shown that IL-27 is not only an initial inducer of Th1 differentiation, but it is also a potent downregulator of cytokines [23, 24]. IL-27 suppress inflammation by: (i) inhibiting IL-17 induction in EAE [23] and/or (ii) inducing Th2 cytokines (e.g. IL-10) [25]. Conversely, IL-27 has also been shown to downregulate regulatory T cells [26]. IFN-γ has also been shown to inhibit the differentiation of naïve CD4 precursors to Th17 cell type [27]. Therefore, we next determined whether estrogen-induced IL-17 could be downregulated by IL-27 or IFN-γ. It was found that IL-27, when added at the time of culture, markedly diminished the induction of IL-17 even at a low dose (1 ng/ml) in both placebo and estrogen-treated mice at 48 hr (Fig 3A). Interestingly, suppression of IL-17 by IL-27 was higher in cells from estrogen-treated mice when compared with placebo-treated mice (e.g. at 72 hr the average inhibition at 10 ng/ml by IL-27 was 79% and 49% in estrogen and placebo treated mice, respectively; data not shown). Interestingly, IFN-γ effectively suppressed IL-17 in cells from estrogen treated mice (Fig 3B) and had minimal suppressive effect in cells from placebo treated mice (at 72 hr the average inhibition at 10 ng/ml of IFN-γ was 76% and 6% in estrogen and placebo treated mice, respectively; data not shown). This may be due to our earlier observations that cells from estrogen treated mice had enhanced IFN-γ-induced responses compared to placebos (e.g. iNOS, MCP-1) [10, 11].
Figure 3. IL-27 and IFN-γ suppress IL-17 induction and RORγt expression.
(A–D) Splenic lymphocytes (5×106/ml) from estrogen- and placebo-treated male C57BL/6 mice were activated with IL-6+TGFβ+anti CD3 antibody in the presence or absence of (A) rIL-27 (placebo=3; estrogen =3; representative of two independent experiment) or (B) rIFN-γ for 48 hr (placebo=4; estrogen=6; representative of two independent experiment), and IL-17 levels determined by ELISA. (C, D) Splenic lymphocytes from estrogen- and placebo-treated male C57BL/6 mice were activated with IL-6+TGFβ+anti CD3 antibody in the presence or absence of (C) rIL-27 or (D) rIFN-γ (placebo=3; estrogen 3) for 48 hrs and the mean percent RORγt+ expression in splenocytes determined by flow cytometry. (E–G) Splenic lymphocytes from estrogen- and placebo-treated male C57BL/6 mice were activated with IL-6+TGFβ+anti CD3 antibody, and rIL-27 or rIFN-γ were added either together with IL-6+TGFβ+anti CD3 antibody stimulation or at the indicated time points. IL-17 levels were measured by ELISA after 72 or 96 hr (placebo=4; estrogen=6). (H) Splenocytes from estrogen- and placebo-treated male C57BL/6 mice were cultured in the presence of the JAK2 inhibitor AG490 for 48 hr and IL-17 levels analyzed (placebo=3; estrogen=5). (I) Splenocytes from estrogen- and placebo-treated male C57BL/6 mice were activated with IL-6+TGFβ+anti CD3 antibody in the presence or absence of AG490 and the percent RORγt+ cells determined after 24 hr (placebo=3; estrogen=4). Data are means ± SEM; * p<0.05, ** p<0.01, *** p<0.001; (A, B, E, and H), Tukey-Kramer test; (C, D, F and G), Student Newman-Keuls test.
Furthermore, presence of IL-27 and IFN-γ in the culture decreased RORγt expression in both estrogen- and placebo-treated splenocytes cultured in presence or absence of either IL-27 or IFN-γ for 48 hrs (Fig 3C and D). This suggests that IL-27 and IFN-γ suppress IL-17 induction by inhibiting RORγt expression. Our findings are in agreement with a recent finding, which suggests that IL-27 inhibits IL-17 induction by suppressing RORγt expression [28]. It is interesting to note that while addition of 100 ng/ml of IL-27 markedly decreased IL-17 levels (83%) (Fig 3A) there was a less dramatic reduction in RORγt (51%) in estrogen treated mice. This implies that other transcription factors (RORα, STAT3; or yet undiscovered) may be involved in IL-17 induction. It is also possible that a modest decrease in RORγt is sufficient to markedly diminish the induction of IL-17.
Interestingly, delaying the addition of IL-27 or IFN-γ after 24 hrs of start of culture did suppress IL-17 induction in 72 and 96 hr (Fig 3F and G) culture. However, the degree of reduction of IL-17 was not as marked as noted when IL-27 or IFN-γ were added at initiation of culture (Fig 3E). Interestingly, the expression of RORγt+ cells was not decreased by delaying the addition of IL-27 and IFN-γ by 24 hr (data not shown). These findings suggest that once the cell is committed to IL-17 secreting cell then the magnitude of inhibitory effect of IL-27 and IFN-γ is lowered as has been reported previously [29]. Impressively, the addition of JAK2 inhibitor AG490 also decreased IL-17 induction (Fig 3H), without modulating the expression of RORγt (Fig 3I). These results further strengthen our view that upstream signaling proteins e.g. JAK2- STAT3 are also critical for IL-17 induction.
CONCLUDING REMARKS
Overall, this is the first study that documents estrogen-treated mice have propensity to induce powerful pro-inflammatory IL-17 in activated splenocytes of mice. Interestingly, estrogen treatment alone (i.e. in absence of stimuli) is not sufficient to induce IL-17 at high levels. However, when appropriately stimulated with IL-17-inducing stimuli, splenocytes from estrogen-treated mice have robust IL-17 induction response. Exposure of cells to IL-23 further enhances IL-17 levels in cells from estrogen-treated mice. This suggests that estrogen exposure pre-sets conditions that favor IL-17 induction upon activation of cells. The estrogen-promotion of IL-17 adds new knowledge as to how this hormone regulates inflammatory conditions. Our studies also show that both IL-27 and IFN-γ can downregulate IL-17, potentially by in part suppressing RORγt expression. These studies have implications to not only a better understanding of estrogen-induced inflammatory cytokines but also provide new possibility of downregulation of this response. Future studies are required to study in detail the signaling events, which favor IL-17 induction in estrogen treated mice.
MATERIALS AND METHODS
Animals
At 4–5 wks of age, male and female wildtype C57BL/6 (Charles River Laboratories) and lupus-prone male NZB/W mice (Jackson Laboratories) were gonadectomized and surgically implanted with silastic capsules containing 17 beta-estradiol (estrogen; 3–5 mg; Sigma-Aldrich) or empty (placebo) implants by standard procedures that have been extensively described in our previous studies [10–12, 30]. These implants are designed to slowly release sustained levels (156–220 pg/ml) of estrogen [11, 30]. Wild type mice were terminated at 2 months. Lupus-prone NZB/W mice were terminated 6 months after estrogen treatment at a time when mice develop lupus (as evidenced by high proteinuria). NZB/W mouse was chosen as an autoimmune susceptible strain since this is a classic model for lupus and the effects of estrogen in promotion of lupus are well established. Since estrogen worsens lupus disease and increases mortality [31], by 6 months of estrogen treatment, we were able to utilize only 2 mice (with the loss of 5) in this particular group for our preliminary experiment. All animal-related procedures were in accordance with Virginia Tech Institutional Animal Care guidelines, and were approved by the Institutional Animal Care and Use Committee. Mice were fed a commercial pellet diet devoid of estrogenic hormones (7013 NIH-31 Modified 6% Mouse/Rat Sterilizable Diet; Harlan-Teklad).
Isolation and culture of Splenic Lymphocytes
IL-17 was induced in splenic lymphocytes (2.5 × 106 cells/ml) by culturing with previously reported [32, 33] recombinant cytokines rIL-6 (20 ng/ml; Ebiosciences) plus TGF-β (3 ng/ml; R&D Systems, Inc., Minneapolis, MN) and anti CD3 antibody (1 µg/ml; Ebiosciences). Control cells were cultured in the absence of these stimuli. In selected experiments, splenocytes were also cultured with rIL-23 (10 ng/ml), rIL-27 (1, 10, 100 ng/ml; Ebiosciences), rIFN-γ (10 and 50 ng/ml; BD PharMingen, San Diego, CA), JAK2 inhibitor AG490 (10, 25 µM) for defined time points. Exposure of cells to the above reagents did not affect the viability of the cells as demonstrated by Alamar Blue assay and 7-AAD-flow cytometric assay (data not shown).
Cytokine ELISA
Protein levels of IL-17 in culture supernatants were determined with IL-17A ELISA kit per manufacturer’s instructions (Ebiosciences) using Vmax microplate reader (Molecular Devices, Sunnyvale, CA).
Flow Cytometric Analysis of Intracellular Expression of IL-17 and RORγt
Percent IL-17 expressing cells and RORγt subset were quantified by flow cytometric analysis. Splenocytes (1×106/100 µl) were cultured for defined time points with additional 3 hr activation with PMA, ionomycin and brefeldin A and then subjected to intracellular staining (antibodies from Ebiosciences) by using BD Cytofix/Cytoperm Kit according to the manufacturers’ instructions. Stained cells were visualized using a FACS Aria flow cytometer (BD Biosciences) and data analyzed using FlowJo version 7 software. Data was expressed as percent IL-17+ or RORγt+ cells.
IL-17 ELISPOT Assay
The numbers of IL-17-secreting cells were determined by using mouse IL-17A ELISPOT kit according to manufacturer’s instructions (Ebiosciences). Splenic lymphocytes (5 × 105/ml) were cultured in presence or absence of IL-6+TGFβ+ antiCD3 antibodies for 48 hr. The spots were counted using automated AID ELISpot plate reader (Autoimmun Diagnostika GmbH, Strassberg, Germany).
Statistical analysis
The significance of differences between placebo and estrogen-treated samples was assessed as indicated using GraphPad InStat version 3.0a for Macintosh (GraphPad Software). The significance level is indicated as asterisk (* for p<0.05; ** for p<0.01 and *** for p<0.001 respectively).
ACKNOWLEDGEMENTS
This work was supported in part by the National Institutes of Health (1 RO1 AI051880-04A1) and Virginia-Maryland Regional College of Veterinary Medicine (VMRCVM) Intramural Research Competition (IRC) Grant (441303) and Lupus Foundation of America. We want to thank Ms. Melissa Makris for flow cytometric analysis and Mr. Peter Jobst, Ms. Connie Kingrea, and the animal care staff.
List of Abbreviations
- ROR
Retinoic acid-related orphan receptor
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
REFERENCES
- 1.Weaver CT. Th17: The ascent of a new effector T-cell subset. European Journal of Immunology. 2009;39:634–640. doi: 10.1002/eji.200939260. [DOI] [PubMed] [Google Scholar]
- 2.Huang F, Kao CY, Wachi S, Thai P, Ryu J, Wu R. Requirement for both JAK-mediated PI3K signaling and ACT1/TRAF6/TAK1-dependent NF-kappaB activation by IL-17A in enhancing cytokine expression in human airway epithelial cells. J Immunol. 2007;179:6504–6513. doi: 10.4049/jimmunol.179.10.6504. [DOI] [PubMed] [Google Scholar]
- 3.Hata K, Andoh A, Shimada M, Fujino S, Bamba S, Araki Y, Okuno T, Fujiyama Y, Bamba T. IL-17 stimulates inflammatory responses via NF-kappaB and MAP kinase pathways in human colonic myofibroblasts. Am J Physiol Gastrointest Liver Physiol. 2002;282:G1035–G1044. doi: 10.1152/ajpgi.00494.2001. [DOI] [PubMed] [Google Scholar]
- 4.Yu JJ, Gaffen SL. Interleukin-17: a novel inflammatory cytokine that bridges innate and adaptive immunity. Front Biosci. 2008;13:170–177. doi: 10.2741/2667. [DOI] [PubMed] [Google Scholar]
- 5.Goswami J, Hernandez-Santos N, Zuniga LA, Gaffen SL. A bone-protective role for IL-17 receptor signaling in ovariectomy-induced bone loss. Eur J Immunol. 2009;39:2831–2839. doi: 10.1002/eji.200939670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol. 2009;129:1339–1350. doi: 10.1038/jid.2009.59. [DOI] [PubMed] [Google Scholar]
- 7.Lubberts E. IL-17/Th17 targeting: on the road to prevent chronic destructive arthritis? cytokine. 2008;41:84–91. doi: 10.1016/j.cyto.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 8.Durelli L, Conti L, Clerico M, Boselli D, Contessa G, Ripellino P, Ferrero B, et al. T-helper 17 cells expand in multiple sclerosis and are inhibited by interferon-beta. Ann Neurol. 2009;65:499–509. doi: 10.1002/ana.21652. [DOI] [PubMed] [Google Scholar]
- 9.Dong G, Ye R, Shi W, Liu S, Wang T, Yang X, Yang N, Yu X. IL-17 induces autoantibody overproduction and peripheral blood mononuclear cell overexpression of IL-6 in lupus nephritis patients. Chin Med J (Engl) 2003;116:543–548. [PubMed] [Google Scholar]
- 10.Karpuzoglu E, Fenaux JB, Phillips RA, Lengi AJ, Elvinger F, Ansar Ahmed S. Estrogen up-regulates inducible nitric oxide synthase, nitric oxide, and cyclooxygenase-2 in splenocytes activated with T cell stimulants: role of interferon-gamma. Endocrinology. 2006;147:662–671. doi: 10.1210/en.2005-0829. [DOI] [PubMed] [Google Scholar]
- 11.Lengi AJ, Phillips RA, Karpuzoglu E, Ansar Ahmed S. Estrogen selectively regulates chemokines in murine splenocytes. J Leukoc Biol. 2007;81:1065–1074. doi: 10.1189/jlb.0606391. [DOI] [PubMed] [Google Scholar]
- 12.Karpuzoglu-Sahin E, Hissong BD, Ansar Ahmed S. Interferon-gamma levels are upregulated by 17-beta-estradiol and diethylstilbestrol. J Reprod Immunol. 2001;52:113–127. doi: 10.1016/s0165-0378(01)00117-6. [DOI] [PubMed] [Google Scholar]
- 13.Ansar Ahmed S, Penhale WJ, Talal N. Sex hormones, immune responses, and autoimmune diseases. Mechanisms of sex hormone action. Am J Pathol. 1985;121:531–551. [PMC free article] [PubMed] [Google Scholar]
- 14.Offner H, Polanczyk M. A potential role for estrogen in experimental autoimmune encephalomyelitis and multiple sclerosis. Ann N Y Acad Sci. 2006;1089:343–372. doi: 10.1196/annals.1386.021. [DOI] [PubMed] [Google Scholar]
- 15.Doria A, Iaccarino L, Sarzi-Puttini P, Ghirardello A, Zampieri S, Arienti S, Cutolo M, Todesco S. Estrogens in pregnancy and systemic lupus erythematosus. Ann N Y Acad Sci. 2006;1069:247–256. doi: 10.1196/annals.1351.022. [DOI] [PubMed] [Google Scholar]
- 16.Hepworth MR, Hardman MJ, Grencis RK. The role of sex hormones in the development of Th2 immunity in a gender-biased model of Trichuris muris infection. Eur J Immunol. 40:406–416. doi: 10.1002/eji.200939589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doreau A, Belot A, Bastid J, Riche B, Trescol-Biemont MC, Ranchin B, Fabien N, et al. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat Immunol. 2009;10:778–785. doi: 10.1038/ni.1741. [DOI] [PubMed] [Google Scholar]
- 18.Kyttaris VC, Zhang Z, Kuchroo VK, Oukka M, Tsokos GC. Cutting edge: IL-23 receptor deficiency prevents the development of lupus nephritis in C57BL/6-lpr/lpr mice. J Immunol. 2010;184:4605–4609. doi: 10.4049/jimmunol.0903595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong CK, Lit LC, Tam LS, Li EK, Wong PT, Lam CW. Hyperproduction of IL-23 and IL-17 in patients with systemic lupus erythematosus: implications for Th17-mediated inflammation in auto-immunity. Clin Immunol. 2008;127:385–393. doi: 10.1016/j.clim.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 20.Yang J, Chu Y, Yang X, Gao D, Zhu L, Wan L, Li M. Th17 and natural Treg cell population dynamics in systemic lupus erythematosus. Arthritis Rheum. 2009;60:1472–1483. doi: 10.1002/art.24499. [DOI] [PubMed] [Google Scholar]
- 21.Wang C, Dehghani B, Li Y, Kaler LJ, Vandenbark AA, Offner H. Oestrogen modulates experimental autoimmune encephalomyelitis and interleukin-17 production via programmed death 1. Immunology. 2009;126:329–335. doi: 10.1111/j.1365-2567.2008.03051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 23.Fitzgerald DC, Ciric B, Touil T, Harle H, Grammatikopolou J, Sarma JD, Gran B, et al. Suppressive Effect of IL-27 on Encephalitogenic Th17 Cells and the Effector Phase of Experimental Autoimmune Encephalomyelitis. J Immunol. 2007;179:3268–3275. doi: 10.4049/jimmunol.179.5.3268. [DOI] [PubMed] [Google Scholar]
- 24.Yoshimura T, Takeda A, Hamano S, Miyazaki Y, Kinjyo I, Ishibashi T, Yoshimura A, Yoshida H. Two-sided roles of IL-27: induction of Th1 differentiation on naive CD4+ T cells versus suppression of proinflammatory cytokine production including IL-23-induced IL-17 on activated CD4+ T cells partially through STAT3-dependent mechanism. J Immunol. 2006;177:5377–5385. doi: 10.4049/jimmunol.177.8.5377. [DOI] [PubMed] [Google Scholar]
- 25.Batten M, Kljavin NM, Li J, Walter MJ, de Sauvage FJ, Ghilardi N. Cutting Edge: IL-27 Is a Potent Inducer of IL-10 but Not FoxP3 in Murine T Cells. J Immunol. 2008;180:2752–2756. doi: 10.4049/jimmunol.180.5.2752. [DOI] [PubMed] [Google Scholar]
- 26.Huber M, Steinwald V, Guralnik A, Brustle A, Kleemann P, Rosenplanter C, Decker T, Lohoff M. IL-27 inhibits the development of regulatory T cells via STAT3. Int Immunol. 2008;20:223–234. doi: 10.1093/intimm/dxm139. [DOI] [PubMed] [Google Scholar]
- 27.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 28.Diveu C, McGeachy MJ, Boniface K, Stumhofer JS, Sathe M, Joyce-Shaikh B, Chen Y, et al. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol. 2009;182:5748–5756. doi: 10.4049/jimmunol.0801162. [DOI] [PubMed] [Google Scholar]
- 29.El-behi M, Ciric B, Yu S, Zhang GX, Fitzgerald DC, Rostami A. Differential effect of IL-27 on developing versus committed Th17 cells. J Immunol. 2009;183:4957–4967. doi: 10.4049/jimmunol.0900735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dai R, Phillips RA, Ansar Ahmed S. Despite inhibition of nuclear localization of NF-kappa B p65, c-Rel, and RelB, 17-beta estradiol up-regulates NF-kappa B signaling in mouse splenocytes: the potential role of Bcl-3. J Immunol. 2007;179:1776–1783. doi: 10.4049/jimmunol.179.3.1776. [DOI] [PubMed] [Google Scholar]
- 31.Roubinian JR, T N, Greenspan JS, Goodman JR, Silteri PK. Effect of castration and sex hormone treatment on survival, anti-nucleic acid antibodies and gromerulonephritis in NZB x NZW F1 mice. Journal of Experimental Medicine. 1978;147:1568. doi: 10.1084/jem.147.6.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 33.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]